Abstract
The microbiome plays an important role in a wide variety of skin disorders. Not only is the skin microbiome altered, but also surprisingly many skin diseases are accompanied by an altered gut microbiome. The microbiome is a key regulator for the immune system, as it aims to maintain homeostasis by communicating with tissues and organs in a bidirectional manner. Hence, dysbiosis in the skin and/or gut microbiome is associated with an altered immune response, promoting the development of skin diseases, such as atopic dermatitis, psoriasis, acne vulgaris, dandruff, and even skin cancer. Here, we focus on the associations between the microbiome, diet, metabolites, and immune responses in skin pathologies. This review describes an exhaustive list of common skin conditions with associated dysbiosis in the skin microbiome as well as the current body of evidence on gut microbiome dysbiosis, dietary links, and their interplay with skin conditions. An enhanced understanding of the local skin and gut microbiome including the underlying mechanisms is necessary to shed light on the microbial involvement in human skin diseases and to develop new therapeutic approaches.
Keywords: skin microbiome, gut dysbiosis, atopic dermatitis, acne vulgaris, psoriasis, dandruff, skin cancer, rosacea, wound healing, dietary, probiotics
1. Introduction
The skin epidermis, along with its appendage structures, such as sweat and sebaceous glands, provide a total skin surface of about 25 m and is one of the largest epithelial surfaces for interaction with microbes [1]. The skin is a first-line barrier from the outer environment, continuously interacting with it. The gastrointestinal (GI) tract is one of the largest interfaces (30 m) between the host and its environment [2]. About 60 tons of food is estimated to pass through the gut in a lifetime, all of which have a big impact on human health [3]. Both the gut and skin are immensely immersed with microbiota as it is estimated that the skin has about 10 microbial cells while the gut accounts for 10 microbial cells [4,5]. The microbiota point to the assemblage of specific microorganisms that are present within a defined environment. The emergence of next-generation sequencing in the past decade has provided unprecedented insights into microbiome composition, both on skin and in the gut. The microbiome refers to the genomes present in a certain environment, meaning the accumulation of all their genetic material (i.e., DNA and RNA). Both organs are characterized by a low microbial diversity at the phylum level but high diversity at the species level [6]. The microbiome provides a multitude of benefits to the host, such as shaping the immune system, protecting against pathogens, breaking down metabolites, and maintaining a healthy barrier [3].
The immuno-modulating potential of the microbiome on distant organ sites is an expanding research field. Especially the influence of the gut microbiome on distant organs, such as the lung, brain, and skin, have created the following areas of research: gut–lung axis, gut–brain axis, and gut–skin axis [7]. The innate and adaptive immune systems alter the microbial composition; however, the local microbiome can also modulate the immune system. The underlying mechanisms of how the gut microbiome alters the skin’s immune system, and vice versa, are currently being investigated. Several skin pathologies pose as gut comorbidities. Several studies have demonstrated the bidirectional link between gut dysbiosis and skin homeostasis imbalances, with a particular role of gut microbiota dysbiosis in the pathophysiology of multiple inflammatory diseases [8,9,10].
A summary of recent findings in the skin and gut microbiome in multiple skin disorders is given in this review, highlighting some potential mechanisms underlying the gut–skin axis.
2. Skin Versus Gut Barrier
The gut and skin barrier share surprisingly many features. The gut and skin are highly analogous to each other in purpose and functionality. Both organs are highly innervated and vascularized, as they are both essential for immune and neuroendocrine function [11]. The gut–skin axis results from this resemblance [11]. The inner surface of the gut and the outer surface of the skin are both covered by epithelial cells (ECs) which have direct contact with the exogenous environment [12]. This way, the immune system is continuously primed to distinguish between harmful and beneficial compounds. Immune cell priming starts early on in life and forms the basis of tolerance, a crucial concept hypothesized to be flawed in several autoimmune disorders [13]. ECs maintain an important link between the internal body and the external environment. They act as a first line of defense, preventing the entry of microorganisms [12]. Keratin, which is present in the stratified squamous epithelium of the skin, presents a formidable physical barrier to most microorganisms [14]. In addition, this compound makes the skin resistant to weak acids and bases, bacterial enzymes, and toxins [15]. Mucosae provide similar mechanical barriers, as it comprises a glycoprotein layer on top of the epithelium in which commensal bacteria reside [16,17]. The epithelial membranes produce protective chemicals that eliminate microorganisms [18]. The skin acidity (pH of 5.4 to 5.9) creates an inhospitable environment for potential pathogens and inhibits bacterial growth [19]. Sebum produced by the sebaceous glands acts as a seal for hair follicles and contains several antimicrobial molecules as well as specific nutritional lipids for beneficial microorganisms [20,21]. Meanwhile, in the digestive system, saliva and lacrimal fluid contain lysozyme, followed by the stomach mucosae that secrete strong acid and protein-digesting enzymes [22]. In addition, mucus traps microorganisms that enter the digestive and respiratory tract [23].
The second line of defense are the antimicrobial peptides (AMPs), phagocytes, and innate lymphoid cells (ILCs) [24]. These two first lines of defense form the innate immune system [23]. AMPs produced by keratinocytes, such as cathelicidin and psorasin, provide an effective barrier function to the skin [25,26]. The serine protease Kallikrein 5 (KLK5) cleaves cathelicidin into active peptides, such as LL-37 [27]. Compared to the skin, the composition of the intestinal epithelial barrier varies throughout the gastrointestinal tract. The proximal part of the gastrointestinal tract, the mouth and esophagus, is analogous to the skin, covered by multiple layers of squamous epithelium, which is cleansed by mucus from salivary and other glands [28]. The remaining part of the digestive tract includes a single layer of active cells, e.g., goblet cells (mucus secretion), enteroendocrine cells (hormone secretion), enterocytes or colonocytes (absorption), etc. [29,30]. The intestinal epithelium constitutes a single layer of enterocytes or colonocytes, and its barrier integrity is protected by the immune system. The absorptive functionality of the enterocytes in the small intestine ensues a discontinuous layer of mucus with fewer goblet cells [31]. Paneth cells are enriched in the crypts of the small intestine that secrete AMPs, which integrate in the complex mucus layer [32].
Microbial-associated molecular patterns (MAMPs) are sampled through antigen uptake by membranous (M) cells and goblet cells to dendritic cells (DCs), together with direct transepithelial luminal DCs. Microbial signals are sensed by RORt innate lymphoid Cclls (group 3 ILCs) that produce interleukin-17 (IL-17) and IL-22 [33]. The latter acts directly on the intestinal epithelial cells (IECs) and activates damage repair mechanisms, AMPs, and mucin genes [34]. Plasma cells in Peyer’s patches, which are stimulated by DCs, produce IgA in the lamina propria in a T cell-independent manner [35,36]. The large intestine on the other hand contains a thick, continuous mucus layer to compartmentalize the microbiota, with IgA and AMPs having a secondary role [17]. The control of immunological processes within mucosal tissues is dependent on the interaction between ECs and DCs, as both cell types are involved in the sensing and sampling of antigens [12]. In the skin and intestine, pathogens are sampled via M cell-independent mechanisms [37]. The only DCs that are found within the epidermis are the Langerhans cells (LCs) [12].
Other similarities between the gut and skin tissues is the high cellular turnover rate, which inhibits adherence and infection by the colonizing microbiome [38,39]. The skin and the gut are the two major niches that host prokaryotic and eukaryotic symbiotic microorganisms [40,41]. However, the resident microbiota are frequently involved and play a crucial role in the pathogenesis of several diseases [42]. Both tissues are very responsive to stress and anxiety, as they face similar challenges. Remarkably, diseases such as inflammatory bowel Disease (IBD) and psoriasis comprise an epithelial barrier dysfunction and an increased epithelial cellular turnover rate. The increased permeability of the epidermal skin and intestinal barrier is due to the augmented interaction of allergens and pathogens with inflammatory receptors of immune cells. Both diseases have an analogous immune response and involve phagocytic, dendritic, and natural killer cells along with a range of cytokines and AMPs that induce a T cell response [43]. In addition, both diseases are characterized by dysbiosis in the microbiome composition that covers the respective interface linings [43].
The gut microbiome is the largest endocrine organ, producing at least 30 hormone-like compounds, e.g., short chain fatty acids (SCFAs); secondary bile acids; cortisol; and neurotransmitters such as gamma-aminobutyric acid (GABA), serotonin, dopamin, and tryptophan. Certain members of the gut microbiome respond to hormones secreted by the host [44]. The hormone-like pleiotropic compounds that are produced by the gut microbiome are released into the bloodstream and can act at distant organs and systems, such as the skin [44]. Numerous studies provided evidence for a profound bidirectional link between gastrointestinal health and skin homeostasis through modification of the immune system [45,46,47]. Modulation of the immune system occurs primarily through the gut microbiota. However, commensal skin microbiota are evenly essential for maintenance of the skin immune homeostasis [48]. Both the intestine and the skin host diverse bacterial, fungal, and viral species that maintain symbiosis with the human habitat. Disrupting this balance might lead to an impaired barrier function. Skin homeostasis recovery after disturbance or stress through gut microbiota enacts on both innate and adaptive immunity.
3. Skin and Gut Microbiome Involvements
The skin is the largest and most external barrier of the body with the outer environment. It is richly perfused with immune cells and heavily colonized by microbial cells, which in turn train the immune cells and determine the well-being of the host [49]. The skin microbiome has gained significant attention in recent years in dermatology, skin disorders, and its connection and influence on the immune system. Many skin conditions are associated with an imbalance in the skin microbiome (Table 1). More and more studies have shown enriched pathogens and microbiota that are associated with skin conditions, some of which are obvious and others more surprising. It is nonetheless difficult to determine whether the altered skin microbiome is a cause or consequence of the skin disorder.
Table 1.
Skin microbiota associated with nine common skin disorders.
| Disease | Associated Skin Microbiota | Additional Remarks | Reference |
|---|---|---|---|
| 1. Acne vulgaris | Particular C. acnes strains | Administered probiotic bacteria could play a protective role. | [71,72,73,74,75,76,77,78,79] |
| 2. Atopic Dermatitis | Decreased bacterial diversity. Increased abundance of S. aureus. | Herpes simplex virus and coxsackie virus can infect AD * skin. | [55,80,81,82,83] |
| 3. Psoriasis | Higher abundance of Staphylococcus and Streptococcus. | Anti-psoriasis treatments lead to skin microbial changes. | [84,85,86,87,88] |
| 4. Hidradenitis suppurativa | Saccharomyces cerevisiae(yeast), Prevotella, and Porphyromonas (bacteria) | Anaerobic species in lesions. | [89,90] |
| 5. Rosacea | Demodex folliculorum(mites) | C. acnes decreased and Snodgrassella alvi increased. Geobacillus and Gordonia. | [91,92] |
| 6. Dandruff and Seborrheic dermatitis | Malassezia spp. (yeast) | Potential bacterial imbalance. | [93,94,95,96] |
| 7. Alopecia areata | Limited data. Possible imbalance C. acnes/ S. epidermidis. | Potential role of cytomegalovirus and/or Alternaria fungi. | [97,98,99] |
| 8. Skin cancer | Merkel bell Polyomavirus, Fusobacterium, and Trueperella, S. aureus. | Increase in certain strains of S. aureus in combination with a decrease in skin commensals can be associated to SCC * or BCC *, and that in MCPyV * can be associated to MCC *. | [100,101,102,103] |
| 9. Wound healing | S. aureus and biofilm-forming bacteria. | Lactobacilli and fermented products can be beneficial. | [104,105] |
* Abbreviations: Atopic dermatitis (AD), basal cell carcinoma (BCC), hidradenitis suppurativa (HS), Merkel cell carcinoma (MCC), Merkel cell Polyomavirus (MCPyV), and squamous cell carcinoma (SCC).
The intestinal tract harbors a diverse collection of bacteria, fungi, and protozoa [50]. Many of these microorganisms are essential for metabolic and immune function, as they metabolize indigestible complex polysaccharides into essential nutrients such as vitamin K and B12, butyrate, and propionate [51,52]. The latter have a positive effect on the epithelial barrier integrity. Intestinal barrier integrity plays a crucial role in protecting microbiota from entering the systemic circulation and in avoiding inflammation in the gut. Diet can have a vital role in the maintenance of particular skin pathologies, when those food ingredients impair the intestinal barrier, which leads to gut bacteria entering the bloodstream.
Lifestyle factors such as diet and hygiene have a determining impact on the tolerance of the immune system to commensal microbiota, which in combination with genetic susceptibility, leads to microbial dysbiosis and disease. For instance, a Western diet has been associated with the development of numerous immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis, psoriasis, and atopic dermatitis (AD). Similarly, the hygiene hypothesis has been linked to the development of Th2-mediated diseases such as asthma and atopic dermatitis. The hygiene hypothesis implies that a reduced exposure to microbes through modern health practices can lead to increased inflammatory diseases in the urbanized society [53]. An overly hygienic lifestyle prevents microbial stimulation and can cause an atopic Th2-skewed response. People living in non-urbanized environments (indigenous people and farming environments) are usually not characterized by inflammatory diseases [54,55]. The mechanism of the Western diet, or high-fat diet (HFD) relies on the resulting intestinal dysbiosis, leading to an increase in the ratio of Firmicutes to Bacteroidetes. The mechanism for this phenomenon is outlined by Guo et al., who found that, in mice, HFD leads to a decreased release of AMPs in the small intestine, which is followed by changes in the composition of the gut microbiota and subsequent alterations in serological inflammatory cytokine levels [56].
Other nutritional components such as glycoalkaloids, alpha tomatine, and capsaicin, which are characteristic for the nightshade family legumes, have been associated with intestinal permeability [57]. In a similar manner, gluten can have an impact on skin health. Coeliac disease and gluten sensitivity have been linked with several skin conditions [58]. Dermatitis herpetiformis is a cutaneous manifestation of celiac disease, and patients can clear up skin rashes when shifting to a gluten-free diet for several months up to several years. The rashes generally return when patients resume gluten consumption [59]. Other allergic and autoimmune diseases, including psoriasis, have been associated with gluten intolerance [58]. Similarly, the strong association between atopic dermatitis and food allergy demonstrates the importance of food underlying the gut–skin axis [60].
However, the gut–skin axis not only is governed by diet but also acts bidirectionally. Skin exposure to ultraviolet B (UVB) and therefore indirectly to serum vitamin D levels increase the and diversity of the gut microbiome [61]. Bacteria from several families were enriched, and the serum vitamin D levels were correlated with the relative abundance of Lachnospira and Fusicatenibacter genera [61]. Moreover, food allergies may result from an impaired skin barrier: atopic dermatitis sensitizes to peanut allergy due to epicutaneous peanut protein exposure in household dust, leading ultimately to immunoglobulin E (IgE)-mediated mast cell expansion in the gut [62,63]. More specifically, the duodenum and oesophagus act as reservoirs for IgE+ B lineage cells [64].
Dysbiosis in the gastrointestinal system is quite often linked to inflammatory diseases (Table 2) [8,9,10]. Gastrointestinal disorders are associated with certain dermatoses, for instance, 7–11% of patients with IBD also suffer from psoriasis [65]. The connection between the skin and gut seems to be mediated by the host immune system. The interaction of the microorganisms and the host immune system is important to maintain the skin homeostasis. The gut–skin axis may be viewed as an integral part of the gut–brain–skin axis, elegantly described by Arck et al. and by Bowe and Logan [7,66]. Table 3 lists neurotransmitters that are produced by intestinal microbiota that might cross the intestinal barrier, enter the bloodstream, and instigate systemic effects (Figure 1) [67,68]. In addition, SCFAs, such as butyrate, acetate, and propionate, are fermentation products derived from undigested polysaccharides by intestinal bacteria (e.g., Bacteroides, Bifidobacterium, Cutibacterium, Eubacterium, Lactobacillus, and Prevotella) [69]. These SCFAs, especially butyrate, enhance epithelial barrier function and decrease the permeability of the intestinal barrier [70]. However, the SCFA quantity that enters the bloodstream is dependent on the individual fiber intake, the microbial fermentation rate, and the amount of colon absorption. All these compounds, which are derived from the gut, could all interact with skin receptors and could directly affect the skin or modify the skin’s commensal bacteria. Further research is needed to reveal if a clinical significant amount of SCFAs is reached in the bloodstream to affect the skin [11]. The studies from Table 3 support that the gut and skin interact with one another via the diet, microbial metabolites, the neuroendocrine pathways, and the central nervous system.
Table 2.
Gut microbiota associated with nine common skin disorders.
| Disease | Associated Gut Microbiota | Additional Remarks | Reference |
|---|---|---|---|
| 1. Acne vulgaris | Decrease in Firmicutes and increase in Bacteroides. | Distinct gut microbiome composition and decreased diversity. | [106] |
| 2. Atopic Dermatitis | Higher levels of Faecalibacterium prausnitzii, Clostridium, and Escherichia (in infants). Lower levels of Akkermansia, Bacteroidetes, and Bifidobacterium. | Probiotics consumption can prevent AD *. | [107,108,109,110,111,112,113,114] |
| 3. Psoriasis | Changes in -diversity. Gut microbiome changes in reaction to biologicals. | Increased risk of intestinal immune disorders. Diet and gut microbiome can have an impact on inflammation. | [115,116,117,118,119,120] |
| 4. Hidradenitis suppurativa | Unknown | Increased risk in developing CD * and UC *. | [121,122] |
| 5. Rosacea | Can be associated with SIBO *. Acidaminococcus and Megasphaera increase and Peptococcaceae and Methanobrevibacter decrease. | Can be associated with H. pylori infection. | [123,124,125] |
| 6. Dandruff and Seborrheic dermatitis | Unclear | Probiotic consumption can alleviate moderate to severe dandruff | [126] |
| 7. Alopecia areata | No major differences | FMT * in 2 patients showed restoration of hair growth | [127,128] |
| 8. Skin cancer | Not reported | Other cancers are associated with microbial dysbiosis | [129,130,131] |
| 9. Wound healing | Not reported | Not reported |
* Abbreviations: atopic dermatitis (AD), Crohn’s disease (CD), fecal microbiota transplant (FMT), hidradenitis suppurativa (HS), small intestinal bacterial overgrowth (SIBO), and ulcerative colitis (UC).
Table 3.
Molecules with potential a modulatory effect on skin and gut either directly or indirectly.
| Molecule | Documented/Possible effect in gut | Documented/Possible effect on skin | Reference |
|---|---|---|---|
| Bacterial metabolites | |||
| SCFAs * | Anti-inflammatory effects | Anti-inflammatory effects | [132] |
| Vitamin D | Suppress inflammation in IBD* | Not reported | [133] |
| Urocanic Acid | Suppress inflammation in IBD* | Not reported | [134] |
| GABA * | Neurotransmitter modulation | Itch restriction | [135,136] |
| Dopamine | Neurotransmitter modulation | Inhibition of hair growth | [135,137] |
| Serotonin | Neurotransmitter modulation | Melatonin modulation | [135,138] |
| Acetylcholine | Neurotransmitter modulation | Barrier function | [135,139] |
| Phenol and p-cresol | Biomarker of gut dysbiosis | Impaired epidermal barrier function | [140] |
| Dietary components | |||
| Catechins | Anti-inflammatory effects | Anti-inflammatory effects | [141] |
| Polyphenols | Anti-inflammatory effects | Anti-inflammatory effects | [142] |
| Lycopene | Selectively utilized by host microbiota | Protection against photodamage | [143,144] |
| Prolamin | Not reported | Protection against AD * | [145] |
| Phytomolecules | Not reported | Anti-ageing | [146] |
| Gluten | Coeliac disease | Skin Rashes | [58,59] |
* Abbreviations: Atopic dermatitis (AD), gamma-aminobutyric acid (GABA), inflammatory bowel disease (IBD), and short chain fatty acids (SCFAs).
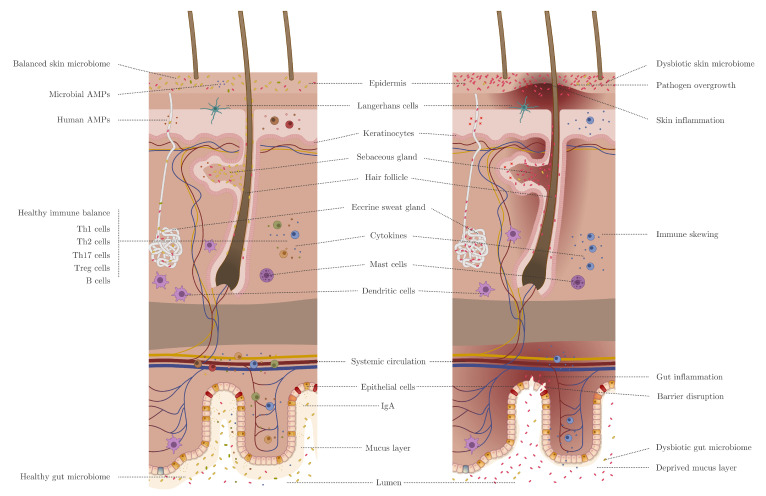
Figure 1.
Inflammatory and microbial influences between the gut and skin for a healthy state (left) and a dysbiotic state (right): The intestinal and epidermal barriers are connected through the systemic circulation (blood and lymph) and are visualized here together in a simplistic manner. The dysbiotic state is characterized by an impaired gut barrier (imbalance in gut microbiome, reduced mucus layer, reduced IgA secretion, barrier disruption, intestinal permeation into the bloodstream, and gut inflammation) and an impaired skin barrier (imbalance in skin microbiome, reduced human and microbial antimicrobial peptides (AMP) production, skin rashes/thickening/lesions, and skin inflammation). Gut and skin dysbiosis are connected through an immune imbalance (Th2 skewing in this example), whereas crosstalk can be bidirectional.
Here, we provide an overview of nine common skin disorders and their respective pathophysiology, and the body of knowledge regarding their skin microbiome alterations, imbalanced gut microbiome, and/or relation to a specific diet.
4. Acne Vulgaris
4.1. Acne Vulgaris Pathophysiology
Acne vulgaris is a multifactorial disease with the main drivers being the skin microbiome composition, the hormonal and immunological state of the host, sebum production, diet, FoxO1 deficiency, hormonal disorders, and deregulation of insulin-like growth factor. Acne vulgaris is the most common skin disorder in the Western world; it can affect 79% or 95% of the Western adolescent population [147]. It is surprisingly absent in hunter–gatherer communities and communities that live a traditional, non-Western lifestyle [147]. The immunology contribution is shaped by the innate immunity, adaptive immunity, and the T helper 17 (Th17) pathway [148,149]. However, the clinical relevance of the Th17 pathway in this disease still has to be evaluated as CD4+ IL-17-producing T cells were also found next to non-inflamed sebaceous glands [150,151]. A very different axis in the development of acne vulgaris is the insulin levels and insulin-like growth factor (IGF) concentrations. Some studies reported that IGF deficiency can protect from acne vulgaris [152]. A more complicated interplay with diet and other drivers in acne vulgaris is likely [153,154]. Currently, the main therapeutic approach includes antibacterial compounds, retinoids, or comedolytic actives. The diversity in mode of actions make it obvious that the underlying disease has many contributors [155].
4.2. Acne Vulgaris Skin Microbiome
Acne vulgaris is a widespread skin disease that usually affects sebaceous skin areas. The pathology and associated skin microbiome dysbiosis has been linked with certain strains of Cutibacterium acnes. The suggested factors range from sebum induction, direct immune system stimulation, diversity of the C. acnes population, porphyrin production, mobile genetics elements, and associated CRISPR/CAS loci to the production of SCFAs [156,157,158,159,160,161,162]. Many reviews on this topic are published, with the most recent being by Brüggemann [163]. Despite many years of research, the precise interdependence and choreography of pathogenic events in acne remain unclear. The main challenge is to distinguish between health- and disease-associated C. acnes strains. Multiple observational studies have been published on this subject and came to slightly varying conclusions. However, most of these studies overlap in the finding that Clade II strains are health-associated, while there is dispute on the functional reasoning behind this [164]. Some studies suggest that Clade IA strains are disease-associated [72]. However, this clade is also the most widespread in healthy skin. This leads to the search for pathogenicity-defining biomarkers in the subpopulation of Clade IA. Multiple biomarkers are suggested, such as mobile genetic elements, linoleic acid isomerase activity, porphyrin production, or cell adhesion. The search for an efficient biomarker that distinguishes acne-associated strains in Clade IA from health-associated strains is of particular interest as these strains have an evolutionary advantage over strains from Clade II, as demonstrated by their wide distribution. Additionally, Clade IA strains produce high levels of the antioxidant RoxP, which might be highly beneficial in protecting the host during the normal aging process [165]. One aspect that is often undervalued is the interplay of different C. acnes strains. A recent hypothesis stated that, instead of individual strains, the C. acnes strain diversity is a distinguishing driver of health and disease [166]. A limited number of studies have tested the potential use of probiotics (Lactobacillus, Bifidobacterium) to counteract the adverse effects of antibiotic treatments and as an alternative treatment for acne vulgaris [117]. These theories are promising and are currently further investigated [167].
4.3. Gut Microbiome and Diet Implications in Acne Vulgaris
Already many decades ago, a connection between the gastrointestinal part and acne vulgaris was suggested and followed by a first study in 1961 using oral Lactobacillus supplements [66]. Later, this was substantiated by a trial showing a strong association of diet and acne vulgaris [106]. Finally, in 2018, a study showed that acne vulgaris patients actually have a distinct gut microbiome composition [168]. Acne patients have decreased diversity of the gut microbiota with lower abundance of Firmicutes and increased levels of Bacteroides. Generally, Clostridium, Clostridiales, Lachnospiraceae, and Ruminococcaceae were depleted in the acne cohort. While a distinct difference between the acne cohort and the healthy controls was observed in this study, no correlation or distinctive biomarker was found correlating with acne severity. Multiple studies have been performed looking at the oral supplementation of probiotics in acne vulgaris [117,169,170]. The available results look promising, but a high heterogeneity in the provided products as well as shortcomings in the study design do not yet allow a final verdict on the efficacy of oral probiotic treatments in acne vulgaris. HFD contains a large quantity of saturated fats and a high glycemic load, which is strongly correlated with acne vulgaris [60,147]. The hypothetical cause is a disturbed nutrient signaling with an uncontrolled stimulation of sterol regulatory element-binding protein 1 (SREBP-1) and an increased synthesis of fatty acids and triglycerides in the sebum, which stimulates the growth of C. acnes [171]. While the gastrointestinal microbiome is only one of many factors contributing to acne, it has an undeniable impact on the skin condition in acne vulgaris. Until now, the exact mechanism is unclear but it is tempting to speculate a general influence of the gut microbiome on the immune system.
5. Atopic Dermatitis (AD)
5.1. AD Pathophysiology
Atopic dermatitis is the most common inflammatory skin disease (7% of adults and 15% of children). It is an inflammatory skin disorder characterized by barrier dysfunction, chronic inflammation, and microbial dysbiosis on skin [172]. AD is also influenced by host genetics and environment [173]. The inflammation is driven by a Th2 cytokine pathway, with the cytokines IL-4 and IL-13 playing an important role [174]. These play a central role in type 2 inflammation, not only for atopic dermatitis but also for several other allergic diseases. The IL-4 and IL-13 cytokines are involved in skin barrier disruption, decreased skin lipid metabolism, and inhibited antimicrobial peptide synthesis [175]. These conditions promote the growth and pathogenesis of Staphylococcus aureus [176]. The understanding of this disease has improved a lot in recent years. There is nonetheless still a large unmet need for long-term disease control.
Up to 30% of Caucasians have a mutation of the filaggrin gene, which codes for a crucial protein regulating the epidermal homeostasis [177]. Filaggrin is affected in both lesional and non-lesional skin.
Mild atopic dermatitis can be treated with skin moisturizers, topical corticosteroids, antihistamines, immunosuppressants, and phototherapy [178]. Twenty to thirty percent of patients have moderate-to-severe atopic dermatitis. For these patients, many biologics are in development or have been developed targeting the Th2 axis, such as IL-4, IL-13, IL-31, OX40, IL23p19, IL-5RA, and janus kinase (JAK)-inhibitors (which target several key AD cytokines) [179,180]. So far, only dupilumab has been shown to improve moderate-to-severe atopic dermatitis and subsequently approved [181].
5.2. Skin Microbiome in AD
Lesional skin in AD is commonly characterized by a low bacterial diversity [182]. The relative abundance of Staphylococcus aureus and Staphylococcus epidermidis is increased, while a decrease in Cutibacterium, Corynebacterium, Streptococcus, Acinetobacter, Prevotella, and Malassezia is found [80,83]. Specifically, S. aureus has long been associated with the skin pathology [183]. S. aureus has been found in higher relative and absolute abundances on lesional skin compared to nonlesional skin [184]. S. aureus was found more abundantly on AD skin compared to healthy controls [80,81]. The relative abundance of S. aureus is correlated with disease severity [82]. Colonization by S. aureus may play a critical role in perpetuating skin inflammation through the development of Th2 cells induced by peptidoglycan [185]. These are common cell components of S. aureus strains. The barrier disruption as well as reduced levels of ceramide cause the peptidoglycan to penetrate into the skin [185]. The peptidoglycan of S. aureus can induce human cathelicidin LL-37 and vascular endothelial growth factor (VEGF) expression in keratinocytes, with VEGF production being amplified by subsequent IL-13 overproduction [186]. S. aureus and its enterotoxins as such trigger inflammation through direct infection of the keratinocytes [187]. Due to the impaired barrier, AD lesions are also susceptible to viral infections, although the occurence is rather rare. The most common is infection with herpes simplex virus (called eczema herpeticum) [188]. Another recently described case is infection with coxsackie virus (called eczema coxsackium) [189]. Atopic dermatitis is usually treated with topical agents, including moisturizers, corticosteroids, calcineurin inhibitors, or antimicrobials [190]. The mode of action is restoring the skin barrier, reducing inflammation, and reducing the bacterial load. Bacteriotherapy has also been tested in the case of atopic dermatitis, through application of coagulase-negative Staphylococcus spp. (for instance, S. epidermidis and S. hominis). In a mouse model, S. hominis provided selected protection against S. aureus by secreting lantibiotics and showed potential in producing AMPs [191].
5.3. Gut Microbiome and Diet Implications in AD
Studies have shown a gut dysbiosis association in patients with atopic dermatitis. The gut microbiome of AD patients was enriched in Faecalibacterium prausnitzii, had more genes encoding for release of molecules that can damage the gut epithelium, and had lower levels of butyrate and propionate, which possess anti-inflammatory properties [107]. Higher levels of Clostridium and Escherichia were found in the gut of atopic infants compared to healthy controls [108,109,110,111]. Clostridium and Escherichia coli in the intestine can contribute to an inflammatory state [110]. On the other hand, lower levels of Akkermansia, Bacteroidetes, and Bifidobacterium were found in AD patients, compared to healthy controls [112,113]. Butyrate-producing bacteria (f.i. Coprococcus) were more abundant in healthy infants or infants with mild AD, compared to infants with severe AD [192]. A likely effective therapeutic option for AD involves the consumption of probiotics, for which a considerable number of studies have been published [193]. In most of the studies Lactobacillus and Bifidobacterium have been tested [194]. Studies have been conducted in children and adults and during pregnancy, for which often contrasting efficacy results have been obtained [195]. Evidence from a meta-analysis supports the use of probiotics for the treatment of AD in infants; however, the benefit likely results from primary prevention of atopic dermatitis, as also concluded by the World Allergy Organization [114,196,197]. The prophylactic effect of probiotics is likely due to its mediating role on the host immune system. Probiotics can interact with dendritic cells, can balance Th1/Th2 immunity, and can enhance Treg activity, as described in in vitro and in animal models [198,199]. These studies show the impact of the gut microbiome (dysbiosis) on Th2-type immune response to allergens in the skin [200]. Diet has been implicated in atopic dermatitis and Th2-driven inflammations. A reduced consumption of fruit, vegetables, and -3 fatty acids and increased consumption of -6 fatty acids have been linked to atopic dermatitis [201,202]. Epidemiologic studies have demonstrated associations of atopic dermatitis (and asthma) with margarine, fish, -6 polyunsaturated fatty acid (PUFA), and -3 PUFA [202]. Further research is nonetheless required to prove the conclusive effect of dietary manipulations on the reduction in atopic disease (and asthma), as previous studies have failed to do so [202,203].
6. Psoriasis
6.1. Psoriasis Pathophysiology
Psoriasis is an immune-mediated inflammatory disease (IMID) and one of the most prevalent chronic skin diseases (0.1–12%) in the world [204]. It is characterized by red, scaly, and thickened skin lesions that can occur at any site of the body [205]. It is a multifactorial disease with an intimate interplay between genetic susceptibility, lifestyle, and environment [205,206,207,208,209]. Numerous comorbidities are reported, suggesting psoriasis to be a systemic disease rather than just a skin disease [210,211]. Similarly, stress has been reported to be an important trigger and crucial exacerbating factor [212,213]. It is primarily considered a Th17 disease with a major role for IL-23/IL-17-mediated inflammation, where tumor necrosis factor (TNF) enhances the inflammatory feedback loop [214]. Consequently, moderate-to-severe psoriasis is treated with therapeutic antibodies, termed biologics, that target these cytokines, including TNF, IL-17, IL-23, and IL-12/23. These drugs have revolutionized the therapeutic landscape in related IMIDs as well, such as hidradenitis suppurativa (HS), rheumatoid arthritis, and IBD (Crohn’s disease (CD) and ulcerative colitis (UC)). Interestingly, psoriasis is also characterized by a type I interferon (IFN) signature in lesional skin, including upregulated expression of IFN-stimulated response element (IFN-ISRE) genes [215,216,217]. Since psoriasis has no clear cause, it is not considered a classic autoimmune disease. Nonetheless, the AMP LL-37 is found complexed with DNA in increased levels in lesional skin and is targeted by autoantibodies in the arthritis subform of psoriasis [218]. However, the presence of specific cytokine profiles in certain diseases associated with specific antimicrobial responses begs for the role of the microbiome in a disease such as psoriasis, which for instance includes an antiviral response (i.e., type I IFN).
6.2. Skin Microbiome in Psoriasis
The psoriatic skin microbiome has been described in several studies [84,85,86,87], and is mainly characterized by a relative higher abundance of Staphylococcus and Streptococcus species. The various studies often show different outcomes: some studies report a decreased microbial diversity, whereas others describe an increase in diversity. Yerushalmi et al. performed a systematic review on microbial studies in psoriatic disease and reported a general decrease in -diversity: a higher and lower relative abundance of Firmicutes and Actinobacteria, respectively, in comparison to healthy controls [88]. At the genus level, the results are less consistent according to the systematic review: Corynebacterium, Staphylococcus, and Streptococcus are reportedly more present in lesional skin, whereas a decrease in Cutibacterium is observed [88]. This discrepancy supposedly stems from the variety in study design (e.g., lesional skin versus nonlesional skin versus healthy controls, body sites, and medication) and analytic methodology [219]. In psoriasis, a lower abundance of S. epidermidis and C. acnes may enable increased colonization of S. aureus [87]. Indeed, S. aureus colonization has been found to stimulate Th17 polarization in mice, suggesting that S. aureus triggers IL-17-mediated skin inflammation [87].
A clinical subform of psoriasis, called guttate psoriasis, is usually triggered by a streptococcal throat infection and generally evolves into the vulgaris (plaque) form. People with psoriasis vulgaris also report exacerbation of the disease severity following tonsillitis. Psoriasis associated with tonsillitis may be controlled by tonsillectomy [220,221]. HLA-C*06:02, a well-known psoriasis-associated single nucleotide polymorphism (SNP), has been found to be associated with chronic and recurrent streptococcal tonsillitis [222].
The effect of anti-psoriasis treatments on the skin microbiome has been investigated as well. Psoriasis patiens treated with narrowband UVB light therapy displayed reduced presence of Firmicutes, Staphylococcus, Finegoldia, Anaerococcus, Peptoniphilus, Gardnerella, Prevotella, and Clostridium spp. in lesions posttreatment [223]. Conventional and biological systemic treatments (e.g., cyclosporin A, retinoic acids, fumarates, methotrexate, adalimumab, and ustekinumab) resulted in a change in the Actinobacteria to Firmicutes ratio, with biologics having the greatest effect [224].
Characterization of the viral microbiome in psoriasis has not been studied as extensively as its bacterial counterpart. Interestingly, the presence of a type I IFN signature in psoriasis suggests an antiviral response. Triggers through viral infections have been described, albeit evidence remains limited [225,226].
6.3. Gut Microbiome and Diet Implications in Psoriasis
People with psoriasis have an increased risk to develop intestinal immune disorders, such as IBD, UC, and celiac disease [118,119,227]. The exact mechanism is not entirely understood, and though many pro-inflammatory cytokines play similar roles in IMIDs, the responses to treatments may differ entirely: the IL-17 blockade is beneficial in psoriasis but rather harmful in IBD [228,229]. Integrity issues in psoriasis are found not only in the skin but also at the intestinal level. Structural aberration in the form of decreased surface in the jejunum was reported in psoriasis patients compared to healthy controls [230]. Other aberrations have been reported as well, including intestinal infiltration of lymphocytes. Lactose intolerance is significantly more present in psoriasis and is even associated with psoriatic severity [227]. Loss of intestinal integrity has been reported in psoriasis based on a 51Cr-labeled ethylenediaminetetraacetic acid (EDTA) absorption test [231] and more recently through increased levels of barrier-related proteins such as claudin-3 and intestinal fatty acid binding protein (I-FABP) in serum [232]. Higher levels of fecal calprotectin were found, were correlated to disease severity [233], and were especially increased when joint inflammation was involved [233]. Studies have shown the presence of ribosomal DNA in the peripheral blood of psoriasis patients, including DNA from Streptococcus and Staphylococcus spp. [234,235]. In mice, intestinal inflammation drove imiquimod-induced psoriasis-like skin inflammation [116].
A number of studies investigated the gut microbiome of psoriasis patients, which showed differences in -diversity (Table 2). Two studies reported lower relative abundance of Bacteroidetes and higher Firmicutes in psoriasis patients compared to healthy controls [115]. The gut microbiome has also been investigated in response to anti-psoriasis treatment: secukinumab, an IL-17 inhibitor, had a greater impact on the gut microbiome in comparison to ustekinumab, an IL-12/23-p40 inhibitor. In detail, the relative abundance of Proteobacteria, Pseudomonadaceae, Enterobacteriaceae, and Pseudomonadales increased in response to secukinumab, whereas Bacteroidetes and Firmicutes declined [120]. These findings suggest a link between psoriasis and the intestinal health.
Lifestyle has major implications in psoriasis: smoking and alcohol have been associated with the exacerbation of skin lesions and even suboptimal responses to treatments, whereas obesity is an independent risk factor for the development of psoriasis [236]. A healthy weight is associated with beneficial effects: HFDs are associated with the exacerbation of psoriasis, whereas weight reduction had a positive outcome on psoriasis severity [237,238,239]. A response to treatment may also be susceptible to dietary intake, as a very low-calorie ketogenic diet has been shown to improve response in patients with a psoriasis relapse [237,240]. Intermittent fasting according to the Ramadan regimen has also been found to positively impact moderate-to-severe psoriasis [241]. Indeed, the intestinal dysbiosis in patients with psoriasis has been the target for probiotics [242]. A mixture of strains was tested in psoriasis and found to be beneficial, up to 6 months after intervention, with fewer relapses in the group treated with the probiotic mixture [243]. Recently, an oral derivative of a single strain of Prevotella histicola was tested for psoriasis. In the murine imiquimod model, it was found to be effective, which was confirmed in a phase 1b trial in humans, yet the results remain to be published [122,244].
7. Hidradenitis Suppurativa (HS)
7.1. HS Pathophysiology
Another chronic cutaneous IMID is hidradenitis suppurativa, with a global prevalence of 0.3% [245]. HS is characterized by occlusion of the apocrine glands, though it is also commonly known as acne inversa, as it occurs in the inverse areas such as the axillae, inframammary regions, groin, and genital and perianal regions. It typically involves recurring, draining, and inflamed lesions that are painful and disfiguring. The lesions consist of chronic subcutaneous sinus tracts, cutaneous fistulae, or dermal-cutaneous scars. Though its etiology remains incompletely understood, research reports a dysregulation of inflammatory cytokines and occlusion of the follicles. TNF is considered a key cytokine, orchestrating the inflammatory loop with VEGF, IL-8, and IL-1, whilst skin biopsies show an increased ratio of Th17 cells compared to Tregs [246,247]. The use of adalimumab, an anti-TNF antagonist, was found to be effective in the treatment of HS and to normalize the Th17/Treg population. It is a multifactorial disease, where lifestyle plays a significant role, including exacerbating effects of smoking and obesity. It remains incompletely understood what the exact underlying mechanism is, and therapeutic interventions, additionally to anti-TNF, include laser-assisted hair removal and antibiotics, yet no cure exists.
7.2. Skin Microbiome in HS
HS lesions present with bacterial infections, which are clinically considered secondary. However, the lesions exhibit a distinct cutaneous microbiome in lesional and nonlesional HS skin in comparison to healthy controls. More specifically, anaerobic species were found in lesions, e.g., Prevotella and Porphyromonas, whereas aerobic commensals were reduced. Interestingly, the HS-microbiome may have clinical relevance as Fusobacterium and Parvimonas spp. were found to correlate to disease severity [89]. In addition, Saccharomyces cerevisiae yeast and one of its wall components, mannans, may also play a role in HS: anti-Saccharomyces cerevisiae antibodies (ASCAs) have been found in HS-serum and to be specifically present in comparison to psoriasis vulgaris and healthy controls, underlining its importance as a biomarker [90]. Assan et al. even reported a significant elevation in the severe cases (Hurley III stage), suggestive for a prognostic marker for disease severity [90].
7.3. Gut Microbiome and Diet Implications in HS
The main link with the gut is based on the increased risk in developing CD and UC: based on a systematic review, usually a two-fold odds ratio was found [248]. Moreover, both diseases respond to anti-TNF treatment, suggesting similar inflammatory pathomechanisms. Especially the presence of perianal fistulae in CD is interesting, and molecular research of HS and CD fistulae has found that CD161+ T lymphocytes are enriched in these lesions, which can differentiate into pathogenic Th17 cells [249]. Interestingly, the presence of ASCAs in HS can be linked to several intestinal disorders such as Crohn’s and celiac disease, where ASCAs are prevalent as well. Positivity for ASCA suggests a systemic response to the oligomannosidic epitopes of yeast. Such observations imply that systemic tolerance to microbial antigens may be exhibited in tissue-specific manifestations, including cutaneous (HS) and intestinal (Crohn’s and celiac disease) [250]. In HS, several lifestyle factors such as smoking, alcohol, and obesity are considered exacerbating factors. Especially cessation of smoking has been associated with significant improvement in HS lesions [251,252].
Based on the intimate link with Crohn’s disease, dietary interventions have been suggested as clinical interventions for HS. However more importantly, obesity is also a known independent risk factor for HS, and a weight loss of at least 15% has been associated with diminished disease severity [253]. Imbalance in the gut microbiome has been associated with diets high in fat, including an increment in Firmicutes and a reduction in Bacteroidetes. How the bacterial HS-associated gut microbiome responds to low fat diets remains to be elucidated. Avoidance of food containing or made with the yeast S. cerevisiae has resulted in promising long-term results in HS, including a reduction in inflammation and amelioration of clinical response to (surgical) treatments [254]. Though other “avoidance” diets are suggested for the treatment of HS, randomized controlled trials are lacking to provide solid evidence for HS [255].
8. Rosacea
8.1. Rosacea Pathophysiology
Rosacea is a chronic inflammatory dermatosis characterized by various skin lesions predominantly on the face including erythema, papulopustules, telangiectasia, and/or ophthalmic involvement that affects up to 15% of the Caucasion population with fair sun-sensitive skin (skin phototypes I and II) [256,257,258]. Although the pathophysiology of rosacea remains unclear, neurovascular dysregulation, impaired immunity, external factors, and genetic inheritance are suggested to play important roles in the disease progression [92]. Rosacea patients retain a dysregulated innate immune system that causes an abnormal inflammatory cytokine release and an AMP response. Cathelicidin expression is significantly increased in the epidermis of rosacea affected skin compared to normal skin [259,260]. In granular or cornified layers of normal skin, cathelicidin nis early absent whereas its expression is greatly induced by wounding or infection [261]. LL-37 is the most common ly found cathelicidin peptide in rosacea patients [260]. Toll-like receptor 2 (TLR2) levels are increased in rosacea patients and stimulate KLK5 [262]. In addition, particular cathelicidin types stimulate and control leukocyte chemotaxis, vasodilation, angiogenesis, and the expression of extracellular matrix proteins [263,264,265]. Neurogenic inflammation might also play an important role in the pathogenesis of rosacea. Various rosacea triggers, including heat and dietary factors, might activate and upregulate the transient receptor potential (TRP) ion channels of vanilloid type (TRPV), which are expressed by sensory nerves as well as by keratinocytes [266]. The TRP channels might be targets for rosacea patients, as they play a role in inflammation, pain perception, and vasoregulation [262,266].
8.2. Skin Microbiome in Rosacea
The skin of rosacea patients regularly contains an overgrowth of commensal skin microorganisms. Higher concentrations of Demodex folliculorum were detected, which usually inhabit the sebaceous glands. The D. folliculorum mite density has been reported to attain up to 10.8/cm in rosacea patients in comparison to 0.7/cm in controls [91]. TLR2 is activated by cell-membrane components of the Demodex mite, which triggers KLK5 activity [267]. The use of permethrin against D. folliculorum reduced the mite abundance; however, the skin lesions did not recover [268]. For this reason, researchers proposed bacteria as a causative agent for the inflammatory responses in rosacea, and the role of Bacillus oleronius and Staphylococcus epidermidis have been investigated [262,267,269,270]. The study of Woo et al. analyzed the influence of oral antibiotics on the composition and diversity of the skin microbiome in rosacea patients. Staphylococcus epidermidis, a skin commensal, is the predominant species, followed by C. acnes. Rosacea severity increased with age and the relative abundance of C. acnes decreased, whereas the relative abundance of Snodgrassella alvi increased. Geobacillus and Gordonia were significantly associated with rosacea severity [271]. The use of topical metronidazole (1% cream) did not alter the skin microbiota composition [272]. Zaidi et al. described that oral doxycycline (100 mg for 6 weeks) did not affect the -diversity but demonstrated an increase in the relative abundance of Weissella confusa [273]. In contrast, Woo et al. reported a decrease in W. confusa [271]. Further studies are thus needed to assess the effect of oral antibiotics on the skin microbiome composition.
8.3. Gut Microbiome and Diet Implications in Rosacea
A link between gut microbial dysbiosis and rosacea has been hypothesised, as there is an increased risk of gastrointestinal disorders in rosacea patients [274]. Especially Helicobacter pylori infection (HPI) has been associated with the disease [123]. The prevalence of small intestinal bacterial overgrowth (SIBO) is increased in rosacea patients. The elimination of SIBO resulted in a significant reduction in cutaneous lesions [124]. A population-based cohort study with 50,000 Danish rosacea patients could identify a higher prevalence of celiac disease, CD, UC, HPI, SIBO, and irritable bowel syndrome (IBS) among the rosacea subjects compared to the control subjects [274,275]. However, comprehensive studies are missing that describe the role of gut dysbiosis in rosacea. A recent Korean study found a link between several enteral microbiota and rosacea in a group of 12 female subjects with rosacea [125]. The abundance of enteral microbiota was similar between patients with rosacea and rosacea-free controls yet differed in composition. A higher abundance of Acidaminococcus and Megasphaera and a lower abundance of Peptococcaceae and Methanobrevibacter were reported [125]. The study of Chen et al. demonstrated a reduction in the fecal microbial richness in rosacea patients as well as a distinct fecal microbial community. The altered microbial composition might be due to sulfur metabolism, cobalamin, and carbohydrate transport [276]. The microbiome might be a critical therapeutic target. An important note is that there is a lot of interindividual variability in the human intestinal microbiome composition. Several reasons may account for this variability, including genetics, environmental exposure, hygiene, geography, ethnicity, etc. [277].
Rosacea exacerbations are frequently linked to dietary factors that can mainly be categorised into heat-related, alcohol-related, capsaicin-related, and cinnamaldehyde-related [278]. There are multiple proposed mechanisms of action (MOA); one of them is through the activation of TRP channels [279]. A second MOAPlease define if appropriate. is through the gut–skin connection [274].
9. Dandruff and Seborrheic Dermatitis
9.1. Dandruff and Seborrheic Dermatitis Pathophysiology
Dandruff is a skin condition that mainly affects the scalp, resulting in skin flaking and pruritus. It occurs in 30–50% of the world’s population, with males generally more affected than females [280,281]. Severe forms of dandruff include inflammation of the skin and are known as seborrheic dermatitis. Seborrheic dermatitis is a chronic and inflammatory dermatosis with recurrent character, and its pathophysiology is very similar to that of dandruff [282]. The exact causes of these conditions remain unknown. However, several factors have been implicated in the progression of the skin conditions, including sebum levels, immune response, stress, environmental and hormonal changes, and individual sensitivity [283]. Moreover, seborrheic dermatitis has been linked and can be caused by an inflammatory immune response to Malassezia spp. [284]. It is usually treated with anti-dandruff shampoos, containing antibacterial and antifungal agents.
9.2. Skin Microbiome in Dandruff and Seborrheic Dermatitis
Dandruff and seborrheic dermatitis are generally associated with a fungal component. Malassezia spp. are lipophilic and dominant fungi colonizing the human scalp and are the most abundant yeast species of the skin mycobiome [280]. Malassezia restricta, Malassezia furfur, and Malassezia globosa are the most abundant species of the Malassezia genus. An inflammatory reaction to excess Malassezia spp. growth on skin has been associated with seborrheic dermatitis [93]. Malassezia spp. are thought to cause an overproduction of oleic acid, which disturbs the stratum corneum cells and evokes an inflammatory response on the scalp [93]. This results in irritating free fatty acids and other metabolites, which can lead to more sebaceous secretions on the scalp, which in turn leads to an inflammatory response that results in skin changes [285]. Dandruff and seborrheic dermatitis occur solely on skin areas with high levels of sebum [93]. Preferencial sites are sebaceous gland-rich areas such as the face, ears, scalp, and upper trunk. Patients with oily skin are prone to developing seborrheic dermatitis [94]. Analysis of the M. globosa genome showed an absence of fatty acid synthase, while many lipase and phospholipase genes/enzymes were present and active on human scalp [93]. This explains the nature of this yeast to heavily rely on external (sebaceous) fatty acids to survive. A bacterial impact was also suggested, with an imbalance in Cutibacterium and Staphylococcus species [95,96].
9.3. Gut Microbiome and Diet Implications in Dandruff and Seborrheic Dermatitis
The link between gut dysbiosis and dandruff/seborrheic dermatitis has been controversial. Some deviations have been detected in the intestinal mucosa of patients with seborrheic dermatitis [286]. A clinical study on probiotics consumption (Lactobacillus paracasei strain) found significant improvements in severity and symptoms of moderate to severe dandruff compared to a placebo treatment [126]. However, the influence of the gut microbiome composition on seborrheic dermatitis and dandruff remains to be elucidated.
Diet has been reported as having an important contribution in the production of sebum. Dietary lipids, glucose intake, and acetate have been indicated as influencing for sebaceous gland activity [287]. Sugar consumption is often higher in patients with seborrheic dermatitis, compared to healthy control groups [288]. Caloric restrictions have been linked to reduced sebum production [289]. Increased levels of vitamin A in the blood has also been linked to a decreased sebum production [290]. Dandruff patients are often advised to avoid sugar, animal fats, and greasy food products and instead consume more vegetables, water-based fruits, seeds, fish, biotin, and vitamin B, although no conclusive evidence has been found for such recommendations [291].
10. Alopecia
10.1. Alopecia Pathophysiology
Alopecia areata is a skin condition with a prevalence of 2% and is clinically characterized by small areas of hair loss on the scalp and/or all over the body [292]. The pathophysiology is still unclear but there is some strong evidence that autoimmune reactions cause inflammations at the site of the hair follicle. Research indicates that different cells of the innate and adaptive immune system are correlated to alopecia areata. Th cells, cytotoxic T cells, natural killer cells, and DCs are present at the hair follicle during the anagen (growth) phase of the hair. The autoimmune responses of these cells cause the production of cytokines such as IFN- and TNF-, which leads to collapse of the hair follicle [293]. The factors that cause this immune response remain unknown. However, there is some evidence that genetic disposition, several environmental factors, and even maybe the skin microbiome can have some influences on the disease as well [294].
10.2. Skin Microbiome in Alopecia
The scalp microbiome mainly consists of Corynebacteriaceae, Propionibacteriaceae, and Staphylococcaceae [99]. A small fraction of the scalp microbiome also consists of fungi, with Malassezia restricta being the most important one. These microorganisms have a symbiotic relationship on healthy scalp, while dysbiosis can cause pathological conditions. A higher abundance of pathogenic taxa in the hair follicle can lead to infections and can contribute to a pro-inflammatory state on the scalp [295]. Analysis of the scalp microbiome of patients with alopecia areata demonstrated an increase in C. acnes in combination with a decrease in S. epidermidis [99]. A disbalance in Cutibacterium/Staphylococcus spp. can potentially play a role in alopecia areata [99]. An increase in cytomegalovirus and Alternaria fungi in alopecia areata has also been postulated [97,98]. Skin microbiome data on this scalp condition remains nonetheless scarce.
10.3. Gut Microbiome and Diet Implications in Alopecia
An association between gut dysbiosis and alopecia areata has been considered. Genes that are related to alopecia areata may also affect gut colonization with microorganisms that induce a Th1 response, which leads to the production of IFN-, as IFN- signals through a JAK/signal transducer and activator of transcription (STAT) signal pathway [293]. Induction of this pathway can cause abnormal growth of hair follicle cells and can even progress into hair loss. Furthermore, dysbiosis of the gut microbiome provokes other diseases through manipulation of the T cell activity near and distant to the site of induction [296]. A case report revealed hair growth in two patients with alopecia areata who were treated with a fecal microbiota transplant (FMT) [128]. This also supports the hypothesis of the potential role of the gut microbiome in the pathophysiology of alopecia areata. Some gut bacterial differences were identified in alopecia areata patients, without major differences [127]. Based on the limited studies found in literature, a clear association between gut dysbiosis and alopecia areata has not yet been determined.
A nutrient deficiency can impact hair growth and structure. Metal deficiencies, such as iron and zinc, can cause hair loss. Low serum ferritin and zinc are more prevalent in patients with alopecia areata. Vitamin deficiencies can also result in hair loss. Niacin and biotin deficiency are proven to cause alopecia areata. Vitamin D takes part in hair follicle cycling and vitamin A activates hair follicle stem cells. [297]. These deficiencies are linked to hair loss and/or alopecia areata; however, limited information is available on the effect of supplement intake and its association with hair loss and alopecia areata [297]. Furthermore, some diet alterations might also benefit the hair growth of alopecia areata patients [298]. A gluten-free diet stimulated the hair growth of patients suffering from celiac disease [298]. People who followed a soya-based eastern diet have a decreased risk for alopecia areata, less than 1% instead of a global +/−2% risk [293]. The Mediterranean diet, rich in raw vegetables and fresh herbs, or a high protein diet is a potential treatment for alopecia [298]. However, the effect of a food limitation as a treatment for alopecia areata needs to be further explored.
11. Skin Cancer
11.1. Skin Cancer Pathophysiology
Skin cancer is a common malignancy and can be divided into two categories: invasive melanoma, in which melanocytes divide uncontrollably, and non-melanoma skin cancers (NMSCs). The latter covers tumors with keratinocytic origin such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [299]. There is a wide variety of risk factors that may lead to melanoma and NMSCs, which includes constitutional predisposition, immunosuppressive status, and exposure to environmental risk factors such as ultraviolet radiation [300]. In addition, actinic keratosis and Bowen’s disease may also result in SCC [301]. During the last decennia, immunology involving cutaneous components was better understood by the immunosurveillance mechanisms and the immunoediting framework. The immunogenicity of tumor cells changes by an altered expression of (tumor-associated) antigens, such as reduced MHC-1 expression, resulting in the development of malignancy [302].
11.2. Skin Microbiome in Skin Cancer
The impact of viruses and UV radiation on skin cancer has already been extensively examined. Recently, a lower incidence of skin cancer was discovered in germ-free rats. As a result, it is hypothesized that dysbiotic skin microbiota can result in the development of several skin cancers. However, it remains unclear whether tumor cells or microbial dysbiosis trigger progression [303]. A number of studies have explored the link between several skin cancers and dysbiosis of the bacterial skin microbiome in inflammatory diseases involving Th17, such as psoriasis and acne [103]. Moreover, SCC and actinic keratosis have recently also been associated with an increase in certain strains of S. aureus in combination with a decrease in skin commensals [100]. Cheng et al. associates the latter with the development of BCC [101]. Additionally, melanoma samples showed increased levels of Fusobacterium and Trueperella genera according to a recent study by Mrázek et al. [102]. Furthermore, an increase in Merkel cell polyomavirus (MCPyV), a virus thought to be a persistent resident of the skin, can lead to Merkel cell carcinoma (MCC) [103]. On the other hand, specific S. epidermidis strains were shown to selectively inhibit proliferation of tumor cell lines as it protects the progression of UVB-induced skin papillomas in preclinical models [304]. S. epidermidis produces 6-N-hydroxyaminopurin, which interferes in Streptococcus synthesizing DNA polymerase without interfering primary keratinocyte growth [303]. Protective reactive oxygen species (ROS) are reduced in actinic keratosis and BCCs [165,305]. This shows that skin commensals, such as C. acnes and S. epidermidis, can protect the host from UV-induced DNA damage. Furthermore, treatments with topical probiotics are suggested to reduce the risk of skin cancer due to increased immune surveillance and reduced chronic inflammation. In fact, topical probiotics may alter the tumor microenvironment by changing the immune responses, which may lead to therapeutic effects [103]. However, more research is still needed to fully understand the role of skin microbiota in skin cancer.
11.3. Gut Microbiome and Diet Implications in Skin Cancer
Cancer patients are also frequently subjected to dysbiotic gut microbiota because of therapies affecting the composition and immunity of these microbiota [306]. Although the link between this dysbiosis and skin cancer in particular remains unclear, the link with cancer in general has already been investigated to a limited extent. For example, colorectal cancer (CRC) is associated with an increase in Bacteroides fragilis in murine models. Additionally, an altered gut microbiome leads to an increased risk to develop CRC [129]. Moreover, Guo et al. discovered the link between Helicobacter pylori and an increased risk of pancreatic cancer [130]. This may be a consequence of a disturbed gut microbiome, which has been described in Helicobacter-associated diseases [131]. However, more research is needed to investigate the correlation between gut dysbiosis and skin cancer. Finally, it is uncertain whether tumor development is secondary to bacterial dysbiosis.
12. Wound Healing
12.1. Wound Pathophysiology
Cutaneous wound healing is a very complex and organized process and consists of overlapping phases of acute healing [307]. Multiple cell types, primarily epidermal keratinocytes, neutrophils, and macrophages, are involved and interact with residing commensal microbiota. The latter one may colonize wounds and may stimulate wound healing by promoting the innate immune system. Subsequently, keratinocytes expand and migrate, fibroblasts migrate and amass the extracellular matrix (ECM), and angiogenesis ensues during the proliferation phase. In the remodelling phase, the ECM restores, scar formation appears, and the epidermal skin barrier recovers [307]. When one of these phases is hampered, the epithelial barrier will not heal properly and a wound becomes chronic. Impaired wound healing is a major challenge for the health care system, as it affects roughly 1% to 2 % of the population in developed countries [308,309]. The prevalence of chronic wounds is higher in older people with underlying pathologies including diabetes mellitus, vascular disease, and obesity [310]. The cellular programs restoring the skin barrier do not function properly in chronic wounds [307,311,312]. Impaired wound healing is characterized by accelerated keratinocyte proliferation, impaired migration, and fibrosis. In addition, several processes such as angiogenesis, ECM remodelling, and induction of stem cells are hindered. Chronic wounds were also continuously inflamed, as was demonstrated by several studies [307,313,314,315,316,317]. However, the underlying cellular and molecular mechanisms of impaired wound healing are not yet fully understood. Especially the role of skin microbiome in impaired wound healing and the application of antimicrobial products are still questioned [312].
12.2. Wound Skin Microbiome
Wounds provide an ideal opportunity for microbiota to obtain access to underlying tissues and to meet the ideal conditions to colonize and grow [318,319]. Commensal microbiota are thought to be beneficial for the wound healing process. They are essential for regulating the skin innate immune system, as they stimulate the production of antimicrobial molecules that provide protection against intracellular pathogens [318,320,321]. Keratinocytes are effective killers of intracellular bacteria in a perforin-2 (P-2)-dependent manner [322]. Skin commensal bacteria, such as S. epidermidis, are able to regulate the gamma delta () T cells and to induce the P-2 expression. Intracellular S. aureus were destroyed by skin cells as a result of the increased P-2 expression, which was induced by S. epidermidis [323]. In addition, certain S. epidermidis strains produce trace amines that accelerate wound healing in mice [324]. Disruption of the normal skin microbiota may contribute to impaired wound closure and the chronic wound pathology. Wound microbiome studies reveal that 21 bacterial families account for the majority of microbiota that colonize chronic wounds [325,326]. Methicillin-resistant S. aureus (MRSA) is one of the most common pathogens to colonize wounds [104]. Similarly, biofilm forming bacteria have been associated with delayed wound healing [326]. Probiotics, like Lactobacillus as well as fermented products have been tested to counteract the detrimental effects of wound colonizing microbes [104,105].
12.3. Gut Microbiome and Diet Implications in Wound Healing
Alterations in the commensal skin microbiome may contribute to the formation of chronic wounds. Recent research in animal models suggests that probiotics may hinder and cure non-healing wounds. Kefir extracts in topical gels have improved the epithelialization and the collagen generation in burn injuries in rats compared to controls that were treated with silver sulfadiazine [327]. The administration of oral probiotics to ultraviolet injured mice modulated the quantity of immune cells in the skin as well as the IL-10 levels, illustrating the immunomodulating potential of probiotics in skin tissues [46]. Supplementation of lactic acid bacteria in drinking water stimulated the healing process in mice compared to controls. In addition, the probiotic strain Lactobacillus reuteri improved wound healing by stimulating oxytocin, which induced the CD4 + Foxp3 + CD25 + Treg lymphocytes that convey the wound healing capacity [328]. These data support the notion that Tregs have the potential to modulate the immune system beyond the gut.
13. Conclusions
The skin diseases as discussed in this manuscript result from a complex interaction between genetic susceptibility, lifestyle, and the immune system. More specifically, the latter is in constant orchestration with the nervous and endocrine systems. These interactions allow microbiota to play a key role, especially in organs such as the skin and gut that are richly perfused with immunoregulators and microbiota. Additionally, observations such as the prevention of AD through probiotics and the increased prevalence of intestinal comorbidities in chronic skin diseases suggest that skin diseases can be linked to the gastrointestinal system. The main hypothesis relies on the gut health, which is directed by dietary factors, mediated through the intestinal microbiome and the immune system, leading to systemic effects, including skin health. The integrity of the intestinal barrier plays a key role, which if compromised, leads to a “leaky gut”—an impaired intestinal barrier. However, its existence remains heavily debated.
This intestinal microbial dysbiosis poses an interesting field of investigation and applications. Pre- and probiotics aimed at the intestinal microbiome may be used for targeting skin health [45]. Probiotic-fed mice with Lactobacillus reuteri demonstrated a shinier and thicker fur mediated through IL-10 and, upon the addition of purified Foxp3+ T cells, also improved the integumentary system [329]. In a placebo controlled human study, healthy volunteers obtained a lower transepidermal water loss and lower skin sensitivity when consuming probiotics compared to the placebo group [330]. Interestingly, consumption of probiotics or live bacteria that are beneficial for the gastrointestinal system has potential to prevent and manage various skin diseases, such as acne vulgaris, atopic dermatitis, and psoriasis [331,332,333,334,335,336]. Similar beneficial health effects have been determined by consuming prebiotics and synbiotics [337,338]. However, specific diets such as caloric restriction and low fat diets have also been associated with improved intestinal epithelial barrier or with cutaneous improvements, including acne vulgaris, AD, psoriasis, wound healing, skin cancer, and even skin aging [339,340].
The attractiveness of targeting the gut microbiome through oral delivery seems inversely correlated with the complexity of targeting the gut–skin axis: modulation of the intestinal microbiome may lead to systemic effects, including the skin and other organs. Properly designed clinical trials are needed to assess the effectiveness of microbiome-targeted treatments. Recent advances in short-read and long-read sequencing technologies permit detailed understanding in gut and skin microbial mediators. These technologies should be accompanied by biomarker analyses (f.i. IgA, calprotectin, and immune measurements) to identify the interplay between the microbiome and the gut barrier integrity.
Though the microbiome also includes viral microbiota, little evidence is available on how viruses impact skin diseases and the intestinal health. The upcoming whole genome sequencing should facilitate our knowledge on the role of viruses in the gut–skin axis.
Skin health outcomes need to be defined in order to determine the impact. Skin tape stripping, which has recently been introduced for protein and mRNA quantification, will enable noninvasive sampling, which is less burdensome for the study subjects. However, one should take into account that skin tape stripping only reveals limited information in comparison to skin biopsies.
Lastly, the study protocols need to take this complexity into account: the quality of recording dietary habits is crucial and relies on the chosen method, such as patient-reported outcome measures (e.g., a food frequency questionnaire versus digital apps for calorie counting and food databases). Moreover, the moment of sampling should be carefully considered: study subjects may have fasted at the moment of sampling by skipping breakfasts versus those who did not. Moreover, the impact of the circadian rhythm needs to be incorporated into future clinical trials. The current literature lacks studies reporting on the impact of the circadian rhythm of nutrition uptake. The study of Parkar et al. reviewed current evidence that demonstrated the effect of altered sleep and eating patterns that may disrupt the host circadian system and that affect the gut microbiome. They concluded that a distortion of microbiome rhythms might at least partly be responsible for an increased risk of obesity and metabolic syndrome linked with insufficient sleep and circadian misalignment [341]. In addition to our own circadian rhythm, the microbiota has also been described to exhibit an internal clock regulated through microbial metabolites [342,343,344]. Presumably, certain nutritional components are better metabolized at certain time points during the day. Indeed, high caloric intake during evening hours has been associated with weight gain, whereas the same caloric intake during morning hours results in weight maintenance [345].
In conclusion, the gut–skin axis, with a central role for our microbiota, poses an exciting field of research, with promising therapeutic and cosmetic applications. Dissecting the interactions between the microbiome and the hosting tissues will lead to a better understanding of health and disease and will create novel opportunities. The need for well-designed trials is primordial and will require multidisciplinary teams to work together, reflecting the cooperation between our own bodies and microbiota.
Acknowledgments
B.D.P., L.G., M.D., and A.M. were supported by Ghent University. C.C. was supported by Research Foundation Flanders. B.P. was supported by S-Biomedic.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Atopic Dermatitis |
| AMP | Antimicrobial Peptide |
| ASCA | Anti-Saccharomyces cerevisiae Antibody |
| BCC | Basal Cell Carcinoma |
| CD | Crohn’s Disease |
| CRC | Colorectal Cancer |
| DC | Dendritic Cell |
| EC | Epithelial Cell |
| EDTA | Ethylenediaminetetraacetic Acid |
| FMT | Fecal Microbiota Transplant |
| GABA | Gamma-Aminobutyric Acid |
| Gamma Delta | |
| GI | Gastrointestinal |
| HFD | High-Fat Diet |
| HPI | Helicobacter pylori Infection |
| HS | Hidradenitis Suppurativa |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| IEC | Intestinal Epithelial Cell |
| I-FABP | Intestinal Fatty Acid Binding Protein |
| IFN | Interferon |
| IFN-ISRE | IFN-Stimulated Response Element |
| IgE | Immunoglobulin E |
| IGF | Insulin-like Growth Factor |
| IL | Interleukin |
| ILC | Innate Lymphoid Cell |
| IMID | Immune-Mediated Inflammatory Disease |
| JAK | Janus Kinase |
| KLK5 | Kallikrein 5 |
| LC | Langerhans Cell |
| LL-37 | Cathelicidin AMP |
| M | Membranous |
| MAMP | Microbial-Associated Molecular Pattern |
| MCPyV | Merkel Cell Polyomavirus |
| MCC | Merkel Cell Carcinoma |
| MOA | Mechanism of Action |
| MRSA | Methicillin-resistant S. aureus |
| NMSC | Non-Melanoma Skin Cancer |
| P-2 | Perforin-2 |
| PUFA | Polyunsaturated Fatty Acid |
| ROS | Reactive Oxygen Species |
| SCC | Squamous Cell Carcinoma |
| SCFA | Short Chain Fatty Acid |
| SIBO | Small Intestinal Bacterial Overgrowth |
| SREBP-1 | Sterol Regulatory Element-Binding Protein 1 |
| STAT | Signal Transducer and Activator of Transcription protein |
| TLR2 | Toll-like Receptor 2 |
| Th | Helper T cell |
| Treg | Regulatory T cell |
| TRP | Transient Receptor Potential |
| TRPV | Transient Receptor Potential Ion Channels of Vanilloid Type |
| UC | Ulcerative Colitis |
| UVB | Ultraviolet B |
| VEGF | Vascularendothelial Growth Factor |
Author Contributions
C.C. conceived the idea for the review article. All authors contributed to the conceptualisation, literature study, and the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. B.P is employee and stockholder of S-Biomedic. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gallo R.L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 2017;137:1213–1214. doi: 10.1016/j.jid.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helander H.F., Fändriks L. Surface area of the digestive tract–revisited. Scand. J. Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 3.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams R. Benefit and mischief from commensal bacteria. J. Clin. Pathol. 1973;26:811. doi: 10.1136/jcp.26.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 6.Grice E.A., Segre J.A. The skin microbiome. Nat. Rev. Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arck P., Handjiski B., Hagen E., Pincus M., Bruenahl C., Bienenstock J., Paus R. Is there a ‘gut–brain–skin axis’? Exp. Dermatol. 2010;19:401–405. doi: 10.1111/j.1600-0625.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 8.Shah K.R., Boland C.R., Patel M., Thrash B., Menter A. Cutaneous manifestations of gastrointestinal disease: Part I. J. Am. Acad. Dermatol. 2013;68:189.e1–189.e21. doi: 10.1016/j.jaad.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Thrash B., Patel M., Shah K.R., Boland C.R., Menter A. Cutaneous manifestations of gastrointestinal disease: Part II. J. Am. Acad. Dermatol. 2013;68:211.e1–211.e33. doi: 10.1016/j.jaad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Gloster H.M., Gebauer L.E., Mistur R.L. Absolute Dermatology Review. Springer; Berlin/Heidelberg, Germany: 2016. Cutaneous manifestations of gastrointestinal disease; pp. 171–179. [Google Scholar]
- 11.O’Neill C.A., Monteleone G., McLaughlin J.T., Paus R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays. 2016;38:1167–1176. doi: 10.1002/bies.201600008. [DOI] [PubMed] [Google Scholar]
- 12.Shaykhiev R., Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J. Leukoc. Biol. 2007;82:1–15. doi: 10.1189/jlb.0207096. [DOI] [PubMed] [Google Scholar]
- 13.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 14.Madison K.C. Barrier function of the skin:“la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 15.Lange L., Huang Y., Busk P.K. Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016;100:2083–2096. doi: 10.1007/s00253-015-7262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelaseyed T., Bergström J.H., Gustafsson J.K., Ermund A., Birchenough G.M., Schütte A., van der Post S., Svensson F., Rodríguez-Piñeiro A.M., Nyström E.E., et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janeway C.A., Jr., Travers P., Walport M., Shlomchik M.J. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science; New York, NY, USA: 2001. The front line of host defense. [Google Scholar]
- 19.Schmid-Wendtner M.H., Korting H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 20.Nakatsuji T., Kao M.C., Zhang L., Zouboulis C.C., Gallo R.L., Huang C.M. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating β-defensin-2 expression. J. Investig. Dermatol. 2010;130:985–994. doi: 10.1038/jid.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlhoff M., Zouboulis C.C., Schneider M.R. Expression of dermcidin in sebocytes supports a role for sebum in the constitutive innate defense of human skin. J. Dermatol. Sci. 2016;81:124–126. doi: 10.1016/j.jdermsci.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Patricia J.J., Dhamoon A.S. Physiology, Digestion. [(accessed on 2 November 2020)];2019 Available online: https://europepmc.org/books/nbk544242.
- 23.Brown E.M., Sadarangani M., Finlay B.B. The role of the immune system in governing host-microbe interactions in the intestine. Nat. Immunol. 2013;14:660–667. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]
- 24.Spits H., Cupedo T. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 25.Braff M.H., Zaiou M., Fierer J., Nizet V., Gallo R.L. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gläser R., Harder J., Lange H., Bartels J., Christophers E., Schröder J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki K., Schauber J., Coda A., Lin H., Dorschner R.A., Schechter N.M., Bonnart C., Descargues P., Hovnanian A., Gallo R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 28.Johansson M.E., Sjövall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013;10:352. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng H., Leblond C. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am. J. Anat. 1974;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 30.Podolsky D.K., Lynch-Devaney K., Stow J.L., Oates P., Murgue B., DeBeaumont M., Sands B.E., Mahida Y.R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J. Biol. Chem. 1993;268:6694–6702. doi: 10.1016/S0021-9258(18)53305-6. [DOI] [PubMed] [Google Scholar]
- 31.Johansson M.E., Larsson J.M.H., Hansson G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu J., Heller J.J., Guo X., Zong-ming E.C., Fish K., Fu Y.X., Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnenberg G.F., Monticelli L.A., Elloso M.M., Fouser L.A., Artis D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niess J.H., Brand S., Gu X., Landsman L., Jung S., McCormick B.A., Vyas J.M., Boes M., Ploegh H.L., Fox J.G., et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 36.Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 37.Neutra M.R., Pringault E., Kraehenbuhl J.P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 38.Barker N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh E.A., Chai C.M., Benito O., Neese R.A., Hellerstein M.K. Dynamics of keratinocytes in vivo using 2H2O labeling: A sensitive marker of epidermal proliferation state. J. Investig. Dermatol. 2004;123:530–536. doi: 10.1111/j.0022-202X.2004.23303.x. [DOI] [PubMed] [Google Scholar]
- 40.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 41.Capone K.A., Dowd S.E., Stamatas G.N., Nikolovski J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011;131:2026–2032. doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzutsev A., Goldszmid R.S., Viaud S., Zitvogel L., Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur. J. Immunol. 2015;45:17–31. doi: 10.1002/eji.201444972. [DOI] [PubMed] [Google Scholar]
- 43.Vlachos C., Gaitanis G., Katsanos K.H., Christodoulou D.K., Tsianos E., Bassukas I.D. Psoriasis and inflammatory bowel disease: Links and risks. Psoriasis. 2016;6:73. doi: 10.2147/PTT.S85194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapat L., Chemin K., Dubois B., Bourdet-Sicard R., Kaiserlian D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur. J. Immunol. 2004;34:2520–2528. doi: 10.1002/eji.200425139. [DOI] [PubMed] [Google Scholar]
- 46.Guéniche A., Benyacoub J., Buetler T.M., Smola H., Blum S. Supplementation with oral probiotic bacteria maintains cutaneous immune homeostasis after UV exposure. Eur. J. Dermatol. 2006;16:511–517. [PubMed] [Google Scholar]
- 47.Benyacoub J., Bosco N., Blanchard C., Demont A., Philippe D., Castiel-Higounenc I., Guéniche A. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef. Microbes. 2014;5:129–136. doi: 10.3920/BM2013.0014. [DOI] [PubMed] [Google Scholar]
- 48.Belkaid Y., Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016;16:353–366. doi: 10.1038/nri.2016.48. [DOI] [PubMed] [Google Scholar]
- 49.Johnson L.R., Christensen J., Jackson M.J. Physiology of the Gastrointestinal Tract. 2nd ed. Raven; New York, NY, USA: 1987. pp. 665–693. [Google Scholar]
- 50.Ipci K., Altıntoprak N., Muluk N.B., Senturk M., Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Eur. Arch. Oto-Rhino. 2017;274:617–626. doi: 10.1007/s00405-016-4058-6. [DOI] [PubMed] [Google Scholar]
- 51.LeBlanc J.G., Milani C., De Giori G.S., Sesma F., Van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Yazdanbakhsh M., Kremsner P.G., Van Ree R. Allergy, parasites, and hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 54.Mccall L.I., Callewaert C., Zhu Q., Song S.J., Bouslimani A., Minich J.J., Ernst M., Ruiz-Calderon J.F., Cavallin H., Pereira H.S., et al. Home chemical and microbial transitions across urbanization. Nat. Microbiol. 2020;5:108–115. doi: 10.1038/s41564-019-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callewaert C., Helffer K.R., Lebaron P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol. 2020;21:4–11. doi: 10.1007/s40257-020-00551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X., Li J., Tang R., Zhang G., Zeng H., Wood R.J., Liu Z. High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediat. Inflamm. 2017;2017:9474896. doi: 10.1155/2017/9474896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gee J., Wortley G., Johnson I., Price K., Rutten A., Houben G., Penninks A. Effects of saponins and glycoalkaloids on the permeability and viability of mammalian intestinal cells and on the integrity of tissue preparations in vitro. Toxicol. Vitr. 1996;10:117–128. doi: 10.1016/0887-2333(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 58.Humbert P., Pelletier F., Dreno B., Puzenat E., Aubin F. Gluten intolerance and skin diseases. Eur. J. Dermatol. 2006;16:4–11. [PubMed] [Google Scholar]
- 59.Fry L., Riches D., Seah P., Hoffbrand A. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet. 1973;301:288–291. doi: 10.1016/S0140-6736(73)91539-0. [DOI] [PubMed] [Google Scholar]
- 60.Grossi E., Cazzaniga S., Crotti S., Naldi L., Di Landro A., Ingordo V., Cusano F., Atzori L., Tripodi Cutrì F., Musumeci M., et al. The constellation of dietary factors in adolescent acne: A semantic connectivity map approach. J. Eur. Acad. Dermatol. Venereol. 2016;30:96–100. doi: 10.1111/jdv.12878. [DOI] [PubMed] [Google Scholar]
- 61.Bosman E.S., Albert A.Y., Lui H., DUTZ J.P., Vallance B.A. Skin exposure to Narrow Band Ultraviolet (UV) B light modulates the human intestinal microbiome. Front. Microbiol. 2019;10:2410. doi: 10.3389/fmicb.2019.02410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brough H.A., Liu A.H., Sicherer S., Makinson K., Douiri A., Brown S.J., Stephens A.C., McLean W.I., Turcanu V., Wood R.A., et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 2015;135:164–170. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartnikas L.M., Gurish M.F., Burton O.T., Leisten S., Janssen E., Oettgen H.C., Beaupré J., Lewis C.N., Austen K.F., Schulte S., et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013;131:451–460. doi: 10.1016/j.jaci.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoh R.A., Joshi S.A., Lee J.Y., Martin B.A., Varma S., Kwok S., Nielsen S.C., Nejad P., Haraguchi E., Dixit P.S., et al. Origins and clonal convergence of gastrointestinal IgE+ B cells in human peanut allergy. Sci. Immunol. 2020;5:eaay4209. doi: 10.1126/sciimmunol.aay4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang B.L., Chandra S., Shih D.Q. Skin manifestations of inflammatory bowel disease. Front. Physiol. 2012;3:13. doi: 10.3389/fphys.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowe W.P., Logan A.C. Acne vulgaris, probiotics and the gut-brain-skin axis-back to the future? Gut Pathog. 2011;3:1–11. doi: 10.1186/1757-4749-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyte M. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2014. Microbial endocrinology and the microbiota-gut-brain axis; pp. 3–24. [DOI] [PubMed] [Google Scholar]
- 68.Rea K., Dinan T.G., Cryan J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cummings J.H., Macfarlane G.T. Role of intestinal bacteria in nutrient metabolism. Clin. Nutr. 1997;16:3–11. doi: 10.1016/S0261-5614(97)80252-X. [DOI] [PubMed] [Google Scholar]
- 70.Mariadason J., Catto-Smith A., Gibson P. Modulation of distal colonic epithelial barrier function by dietary fibre in normal rats. Gut. 1999;44:394–399. doi: 10.1136/gut.44.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lomholt H.B., Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS ONE. 2010;5:e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lomholt H., Scholz C., Brüggemann H., Tettelin H., Kilian M. A comparative study of Cutibacterium (Propionibacterium) acnes clones from acne patients and healthy controls. Anaerobe. 2017;47:57–63. doi: 10.1016/j.anaerobe.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 73.McDowell A., Gao A., Barnard E., Fink C., Murray P.I., Dowson C.G., Nagy I., Lambert P.A., Patrick S. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology. 2011;157:1990–2003. doi: 10.1099/mic.0.049676-0. [DOI] [PubMed] [Google Scholar]
- 74.Paugam C., Corvec S., Saint-Jean M., Le Moigne M., Khammari A., Boisrobert A., Nguyen J., Gaultier A., Dréno B. Propionibacterium acnes phylotypes and acne severity: An observational prospective study. J. Eur. Acad. Dermatol. Venereol. 2017;31:e398–e399. doi: 10.1111/jdv.14206. [DOI] [PubMed] [Google Scholar]
- 75.Fitz-Gibbon S., Tomida S., Chiu B.H., Nguyen L., Du C., Liu M., Elashoff D., Erfe M.C., Loncaric A., Kim J., et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013;133:2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karoglan A., Paetzold B., De Lima J.P., Brüggemann H., Tüting T., Schanze D., Güell M., Gollnick H. Safety and efficacy of topically applied selected cutibacterium acnes strains over five weeks in patients with acne vulgaris: An open-label, pilot study. Acta Derm. Venereol. 2019;99:1253–1257. doi: 10.2340/00015555-3323. [DOI] [PubMed] [Google Scholar]
- 77.Johnson T., Kang D., Barnard E., Li H. Strain-level differences in porphyrin production and regulation in Propionibacterium acnes elucidate disease associations. Msphere. 2016;1 doi: 10.1128/mSphere.00023-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barnard E., Shi B., Kang D., Craft N., Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lheure C., Grange P.A., Ollagnier G., Morand P., Désiré N., Sayon S., Corvec S., Raingeaud J., Marcelin A.G., Calvez V., et al. TLR-2 recognizes Propionibacterium acnes CAMP factor 1 from highly inflammatory strains. PLoS ONE. 2016;11:e0167237. doi: 10.1371/journal.pone.0167237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong H.H., Oh J., Deming C., Conlan S., Grice E.A., Beatson M.A., Nomicos E., Polley E.C., Komarow H.D., Murray P.R., et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi B., Bangayan N.J., Curd E., Taylor P.A., Gallo R.L., Leung D.Y., Li H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016;138:1233–1236. doi: 10.1016/j.jaci.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh J., Freeman A.F., Park M., Sokolic R., Candotti F., Holland S.M., Segre J.A., Kong H.H., NISC Comparative Sequencing Program The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 2013;23:2103–2114. doi: 10.1101/gr.159467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chng K.R., Tay A.S.L., Li C., Ng A.H.Q., Wang J., Suri B.K., Matta S.A., McGovern N., Janela B., Wong X.F.C.C., et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016;1:1–10. doi: 10.1038/nmicrobiol.2016.106. [DOI] [PubMed] [Google Scholar]
- 84.Alekseyenko A.V., Perez-Perez G.I., De Souza A., Strober B., Gao Z., Bihan M., Li K., Methé B.A., Blaser M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1:31. doi: 10.1186/2049-2618-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Statnikov A., Alekseyenko A.V., Li Z., Henaff M., Perez-Perez G.I., Blaser M.J., Aliferis C.F. Microbiomic signatures of psoriasis: Feasibility and methodology comparison. Sci. Rep. 2013;3:2620. doi: 10.1038/srep02620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takemoto A., Cho O., Morohoshi Y., Sugita T., Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J. Dermatol. 2015;42:166–170. doi: 10.1111/1346-8138.12739. [DOI] [PubMed] [Google Scholar]
- 87.Chang H.W., Yan D., Singh R., Liu J., Lu X., Ucmak D., Lee K., Afifi L., Fadrosh D., Leech J., et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6:154. doi: 10.1186/s40168-018-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yerushalmi M., Elalouf O., Anderson M., Chandran V. The skin microbiome in psoriatic disease: A systematic review and critical appraisal. J. Transl. Autoimmun. 2019;2:100009. doi: 10.1016/j.jtauto.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guet-Revillet H., Jais J.P., Ungeheuer M.N., Coignard-Biehler H., Duchatelet S., Delage M., Lam T., Hovnanian A., Lortholary O., Nassif X., et al. The microbiological landscape of anaerobic infections in hidradenitis suppurativa: A prospective metagenomic study. Clin. Infect. Dis. 2017;65:282–291. doi: 10.1093/cid/cix285. [DOI] [PubMed] [Google Scholar]
- 90.Assan F., Gottlieb J., Tubach F., Lebbah S., Guigue N., Hickman G., Pape E., Madrange M., Delaporte E., Sendid B., et al. Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J. Allergy Clin. Immunol. 2020;146:452–455. doi: 10.1016/j.jaci.2020.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Forton F., Seys B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993;128:650–659. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 92.Woo Y.R., Lim J.H., Cho D.H., Park H.J. Rosacea: Molecular mechanisms and management of a chronic cutaneous inflammatory condition. Int. J. Mol. Sci. 2016;17:1562. doi: 10.3390/ijms17091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dawson T.L., Jr. Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J. Investig. Dermatol. Symp. Proc. 2007;12:15–19. doi: 10.1038/sj.jidsymp.5650049. [DOI] [PubMed] [Google Scholar]
- 94.MacKee G.M., Lewis G.M., WTTA of Martha. Spence J., WTTA of Mary. Hopper E. Dandruff and seborrhea: I. flora of “normal” and diseased scalps. J. Investig. Dermatol. 1938;1:131–139. doi: 10.1038/jid.1938.14. [DOI] [Google Scholar]
- 95.Xu Z., Wang Z., Yuan C., Liu X., Yang F., Wang T., Wang J., Manabe K., Qin O., Wang X., et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clavaud C., Jourdain R., Bar-Hen A., Tichit M., Bouchier C., Pouradier F., El Rawadi C., Guillot J., Ménard-Szczebara F., Breton L., et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS ONE. 2013;8:e58203. doi: 10.1371/annotation/bcff4a59-10b7-442a-8181-12fa69209e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skinner R.B., Jr., Light W.H., Leonardi C., Bale G.F., Rosenberg E.W. A molecular approach to alopecia areata. J. Investig. Dermatol. 1995;104:3S. doi: 10.1038/jid.1995.27. [DOI] [PubMed] [Google Scholar]
- 98.Rudnicka L., Lukomska M. Alternaria scalp infection in a patient with alopecia areata. Coexistence or causative relationship? J. Dermatol. Case Rep. 2012;6:120. doi: 10.3315/jdcr.2012.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinto D., Sorbellini E., Marzani B., Rucco M., Giuliani G., Rinaldi F. Scalp bacterial shift in Alopecia areata. PLoS ONE. 2019;14:e0215206. doi: 10.1371/journal.pone.0215206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood D.L., Lachner N., Tan J.M., Tang S., Angel N., Laino A., Linedale R., Lê Cao K.A., Morrison M., Frazer I.H., et al. A natural history of actinic keratosis and cutaneous squamous cell carcinoma microbiomes. MBio. 2018;9 doi: 10.1128/mBio.01432-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng J., Zens M.S., Duell E., Perry A.E., Chapman M.S., Karagas M.R. History of allergy and atopic dermatitis in relation to squamous cell and basal cell carcinoma of the skin. Cancer Epidemiol. Prev. Biomarkers. 2015;24:749–754. doi: 10.1158/1055-9965.EPI-14-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mrázek J., Mekadim C., Kučerová P., Švejstil R., Salmonová H., Vlasáková J., Tarasová R., Čížková J., Červinková M. Melanoma-related changes in skin microbiome. Folia Microbiol. 2019;64:435–442. doi: 10.1007/s12223-018-00670-3. [DOI] [PubMed] [Google Scholar]
- 103.Sherwani M.A., Tufail S., Muzaffar A.F., Yusuf N. The skin microbiome and immune system: Potential target for chemoprevention? Photodermatol. Photoimmunol. Photomed. 2018;34:25–34. doi: 10.1111/phpp.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sikorska H., Smoragiewicz W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents. 2013;42:475–481. doi: 10.1016/j.ijantimicag.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Guo H., Zheng Y., Wang B., Li Z. A note on an improved self-healing group key distribution scheme. Sensors. 2015;15:25033–25038. doi: 10.3390/s151025033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith R.N., Mann N.J., Braue A., Mäkeläinen H., Varigos G.A. A low-glycemic-load diet improves symptoms in acne vulgaris patients: A randomized controlled trial. Am. J. Clin. Nutr. 2007;86:107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 107.Song H., Yoo Y., Hwang J., Na Y.C., Kim H.S. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016;137:852–860. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 108.Kalliomäki M., Kirjavainen P., Eerola E., Kero P., Salminen S., Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 109.Penders J., Thijs C., van den Brandt P.A., Kummeling I., Snijders B., Stelma F., Adams H., van Ree R., Stobberingh E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee E., Lee S.Y., Kang M.J., Kim K., Won S., Kim B.J., Choi K.Y., Kim B.S., Cho H.J., Kim Y., et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann. Allergy Asthma Immunol. 2016;117:91–92. doi: 10.1016/j.anai.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 111.Kirjavainen P., Arvola T., Salminen S., Isolauri E. Aberrant composition of gut microbiota of allergic infants: A target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watanabe S., Narisawa Y., Arase S., Okamatsu H., Ikenaga T., Tajiri Y., Kumemura M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003;111:587–591. doi: 10.1067/mai.2003.105. [DOI] [PubMed] [Google Scholar]
- 113.Fujimura K.E., Sitarik A.R., Havstad S., Lin D.L., Levan S., Fadrosh D., Panzer A.R., LaMere B., Rackaityte E., Lukacs N.W., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fiocchi A., Pawankar R., Cuello-Garcia C., Ahn K., Al-Hammadi S., Agarwal A., Beyer K., Burks W., Canonica G.W., Ebisawa M., et al. World allergy organization-McMaster university guidelines for allergic disease prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015;8:1–13. doi: 10.1186/s40413-015-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sikora M., Stec A., Chrabaszcz M., Knot A., Waskiel-Burnat A., Rakowska A., Olszewska M., Rudnicka L. Gut microbiome in psoriasis: An updated review. Pathogens. 2020;9:463. doi: 10.3390/pathogens9060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grine L., Steeland S., Van Ryckeghem S., Ballegeer M., Lienenklaus S., Weiss S., Sanders N.N., Vandenbroucke R.E., Libert C. Topical imiquimod yields systemic effects due to unintended oral uptake. Sci. Rep. 2016;6:20134. doi: 10.1038/srep20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung G.W., Tse J.E., Guiha I., Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J. Cutan. Med. Surg. 2013;17:114–122. doi: 10.2310/7750.2012.12026. [DOI] [PubMed] [Google Scholar]
- 118.Wu J.J., Nguyen T.U., Poon K.Y.T., Herrinton L.J. The association of psoriasis with autoimmune diseases. J. Am. Acad. Dermatol. 2012;67:924–930. doi: 10.1016/j.jaad.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 119.Pietrzak D., Pietrzak A., Krasowska D., Borzęcki A., Franciszkiewicz-Pietrzak K., Polkowska-Pruszyńska B., Baranowska M., Reich K. Digestive system in psoriasis: An update. Arch. Dermatol. Res. 2017;309:679–693. doi: 10.1007/s00403-017-1775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yeh N.L., Hsu C.Y., Tsai T.F., Chiu H.Y. Gut microbiome in psoriasis is perturbed differently during secukinumab and ustekinumab therapy and associated with response to treatment. Clin. Drug Investig. 2019;39:1195–1203. doi: 10.1007/s40261-019-00849-7. [DOI] [PubMed] [Google Scholar]
- 121.Wark K.J., Cains G.D. Dermatology and Therapy. Vol. 11. Springer; Cham, Switzerland: 2020. The Microbiome in Hidradenitis Suppurativa: A Review; pp. 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brooks M. Gut microbe curbs systemic inflammation in psoriasis; Proceedings of the 29th European Academy of Dermatology and Venereology Congress (EADV); Vienna, Austria. 29–31 October 2020. [Google Scholar]
- 123.Rebora A., Drago F., Parodi A. May Helicohacter pylori be important for dermatologists. Dermatology. 1995;191:6–8. doi: 10.1159/000246470. [DOI] [PubMed] [Google Scholar]
- 124.Parodi A., Paolino S., Greco A., Drago F., Mansi C., Rebora A., Parodi A., Savarino V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008;6:759–764. doi: 10.1016/j.cgh.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 125.Nam J.H., Yun Y., Kim H.S., Kim H.N., Jung H.J., Chang Y., Ryu S., Shin H., Kim H.L., Kim W.S. Rosacea and its association with enteral microbiota in Korean females. Exp. Dermatol. 2018;27:37–42. doi: 10.1111/exd.13398. [DOI] [PubMed] [Google Scholar]
- 126.Reygagne P., Bastien P., Couavoux M., Philippe D., Renouf M., Castiel-Higounenc I., Gueniche A. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef. Microbes. 2017;8:671–680. doi: 10.3920/BM2016.0144. [DOI] [PubMed] [Google Scholar]
- 127.Moreno-Arrones O., Serrano-Villar S., Perez-Brocal V., Saceda-Corralo D., Morales-Raya C., Rodrigues-Barata R., Moya A., Jaen-Olasolo P., Vano-Galvan S. Analysis of the gut microbiota in alopecia areata: Identification of bacterial biomarkers. J. Eur. Acad. Dermatol. Venereol. 2020;34:400–405. doi: 10.1111/jdv.15885. [DOI] [PubMed] [Google Scholar]
- 128.Rebello D., Wang E., Yen E., Lio P.A., Kelly C.R. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep. J. 2017;4:e107. doi: 10.14309/crj.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen J., Domingue J.C., Sears C.L. Seminars in Immunology. Volume 32. Elsevier; Amsterdam, The Netherlands: 2017. Microbiota dysbiosis in select human cancers: Evidence of association and causality; pp. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo Y., Liu W., Wu J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016;12:229. doi: 10.4103/0973-1482.200744. [DOI] [PubMed] [Google Scholar]
- 131.Pichon M., Burucoa C. Impact of the gastro-intestinal bacterial microbiome on Helicobacter-associated diseases. Healthcare. 2019;7:34. doi: 10.3390/healthcare7010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Torki M., Gholamrezaei A., Mirbagher L., Danesh M., Kheiri S., Emami M.H. Vitamin D deficiency associated with disease activity in patients with inflammatory bowel diseases. Dig. Dis. Sci. 2015;60:3085–3091. doi: 10.1007/s10620-015-3727-4. [DOI] [PubMed] [Google Scholar]
- 134.Kammeyer A., Peters C.P., Meijer S.L., te Velde A.A. Anti-inflammatory effects of urocanic acid derivatives in models ex vivo and in vivo of inflammatory bowel disease. ISRN Inflamm. 2012;2012:898153. doi: 10.5402/2012/898153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akiyama T., Carstens M.I., Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS ONE. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Langan E., Lisztes E., Bíró T., Funk W., Kloepper J., Griffiths C., Paus R. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br. J. Dermatol. 2013;168:520–525. doi: 10.1111/bjd.12113. [DOI] [PubMed] [Google Scholar]
- 138.Lee H., Park M., Kim S., Park Choo H., Lee A., Lee C. Serotonin induces melanogenesis via serotonin receptor 2A. Br. J. Dermatol. 2011;165:1344–1348. doi: 10.1111/j.1365-2133.2011.10490.x. [DOI] [PubMed] [Google Scholar]
- 139.Yokoyama S., Hiramoto K., Koyama M., Ooi K. Impairment of skin barrier function via cholinergic signal transduction in a dextran sulphate sodium-induced colitis mouse model. Exp. Dermatol. 2015;24:779–784. doi: 10.1111/exd.12775. [DOI] [PubMed] [Google Scholar]
- 140.Miyazaki K., Masuoka N., Kano M., Iizuka R. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes. 2014;5:121–128. doi: 10.3920/BM2012.0066. [DOI] [PubMed] [Google Scholar]
- 141.Rhodes L.E., Darby G., Massey K.A., Clarke K.A., Dew T.P., Farrar M.D., Bennett S., Watson R.E., Williamson G., Nicolaou A. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br. J. Nutr. 2013;110:891–900. doi: 10.1017/S0007114512006071. [DOI] [PubMed] [Google Scholar]
- 142.Giampieri F., Alvarez-Suarez J.M., Mazzoni L., Forbes-Hernandez T.Y., Gasparrini M., Gonzàlez-Paramàs A.M., Santos-Buelga C., Quiles J.L., Bompadre S., Mezzetti B., et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules. 2014;19:7798–7816. doi: 10.3390/molecules19067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 144.Rizwan M., Rodriguez-Blanco I., Harbottle A., Birch-Machin M., Watson R., Rhodes L. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011;164:154–162. doi: 10.1111/j.1365-2133.2010.10057.x. [DOI] [PubMed] [Google Scholar]
- 145.Yoon H.J., Jang M.S., Kim H.W., Song D.U., Nam K.I., Bae C.S., Kim S.J., Lee S.R., Ku C.S., Jang D.I., et al. Protective effect of diet supplemented with rice prolamin extract against DNCB-induced atopic dermatitis in BALB/c mice. BMC Complement. Altern. Med. 2015;15:1–7. doi: 10.1186/s12906-015-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tundis R., Loizzo M., Bonesi M., Menichini F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015;22:1515–1538. doi: 10.2174/0929867322666150227151809. [DOI] [PubMed] [Google Scholar]
- 147.Cordain L., Lindeberg S., Hurtado M., Hill K., Eaton S.B., Brand-Miller J. Acne vulgaris: A disease of Western civilization. Arch. Dermatol. 2002;138:1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 148.Zouboulis C.C., Jourdan E., Picardo M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. JEADV. 2014;28:527–532. doi: 10.1111/jdv.12298. [DOI] [PubMed] [Google Scholar]
- 149.Agak G.W., Qin M., Nobe J., Kim M.H., Krutzik S.R., Tristan G.R., Elashoff D., Garbán H.J., Kim J. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J. Investig. Dermatol. 2014;134:366–373. doi: 10.1038/jid.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thiboutot D.M., Layton A.M., Eady E.A. IL-17: A key player in the P. acnes inflammatory cascade? J. Investig. Dermatol. 2014;134:307–310. doi: 10.1038/jid.2013.400. [DOI] [PubMed] [Google Scholar]
- 151.Mattii M., Lovászi M., Garzorz N., Atenhan A., Quaranta M., Lauffer F., Konstantinow A., Küpper M., Zouboulis C., Kemeny L., et al. Sebocytes contribute to skin inflammation by promoting the differentiation of T helper 17 cells. Br. J. Dermatol. 2018;178:722–730. doi: 10.1111/bjd.15879. [DOI] [PubMed] [Google Scholar]
- 152.Ben-Amitai D., Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J. Eur. Acad. Dermatol. Venereol. 2011;25:950–954. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 153.Melnik B.C., Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp. Dermatol. 2009;18:833–841. doi: 10.1111/j.1600-0625.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 154.Çerman A.A., Aktaş E., Altunay İ.K., Arıcı J.E., Tulunay A., Ozturk F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016;75:155–162. doi: 10.1016/j.jaad.2016.02.1220. [DOI] [PubMed] [Google Scholar]
- 155.Nast A., Dréno B., Bettoli V., Bukvic Mokos Z., Degitz K., Dressler C., Finlay A.Y., Haedersdal M., Lambert J., Layton A., et al. European evidence-based (S3) guideline for the treatment of acne–update 2016–short version. J. Eur. Acad. Dermatol. Venereol. 2016;30:1261–1268. doi: 10.1111/jdv.13776. [DOI] [PubMed] [Google Scholar]
- 156.Iinuma K., Sato T., Akimoto N., Noguchi N., Sasatsu M., Nishijima S., Kurokawa I., Ito A. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J. Investig. Dermatol. 2009;129:2113–2119. doi: 10.1038/jid.2009.46. [DOI] [PubMed] [Google Scholar]
- 157.Yu Y., Champer J., Agak G.W., Kao S., Modlin R.L., Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J. Investig. Dermatol. 2016;136:2221–2228. doi: 10.1016/j.jid.2016.06.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dagnelie M.A., Corvec S., Saint-Jean M., Bourdès V., Nguyen J.M., Khammari A., Dréno B. Decrease in diversity of Propionibacterium acnes phylotypes in patients with severe acne on the back. Acta Derm. Venereol. 2018;98:262–267. doi: 10.2340/00015555-2847. [DOI] [PubMed] [Google Scholar]
- 159.Borelli C., Merk K., Schaller M., Jacob K., Vogeser M., Weindl G., Berger U., Plewig G. In vivo porphyrin production by P. acnes in untreated acne patients and its modulation by acne treatment. Acta Derm. Venereol. 2006;86:316–319. doi: 10.2340/00015555-0088. [DOI] [PubMed] [Google Scholar]
- 160.Kasimatis G., Fitz-Gibbon S., Tomida S., Wong M., Li H. Analysis of complete genomes of Propionibacterium acnes reveals a novel plasmid and increased pseudogenes in an acne associated strain. BioMed. Res. Int. 2013;2013 doi: 10.1155/2013/918320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brüggemann H., Lomholt H.B., Tettelin H., Kilian M. CRISPR/cas loci of type II Propionibacterium acnes confer immunity against acquisition of mobile elements present in type I P. acnes. PLoS ONE. 2012;7:e34171. doi: 10.1371/journal.pone.0034171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sanford J.A., O’Neill A.M., Zouboulis C.C., Gallo R.L. Short-chain fatty acids from Cutibacterium acnes activate both a canonical and epigenetic inflammatory response in human sebocytes. J. Immunol. 2019;202:1767–1776. doi: 10.4049/jimmunol.1800893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brüggemann H. Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology. Springer; Cham, Switzerland: 2020. Skin: Cutibacterium (formerly Propionibacterium) acnes and Acne Vulgaris; pp. 225–243. [Google Scholar]
- 164.O’Neill A.M., Gallo R.L. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6:177. doi: 10.1186/s40168-018-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Andersson T., Bergdahl G.E., Saleh K., Magnúsdóttir H., Stødkilde K., Andersen C.B.F., Lundqvist K., Jensen A., Brüggemann H., Lood R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-40471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dréno B., Pécastaings S., Corvec S., Veraldi S., Khammari A., Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018;32:5–14. doi: 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 167.Callewaert C., Knödlseder N., Karoglan A., Güell M., Paetzold B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021;19:624–631. doi: 10.1016/j.csbj.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deng Y., Wang H., Zhou J., Mou Y., Wang G., Xiong X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm. Venereol. 2018;98:783–790. doi: 10.2340/00015555-2968. [DOI] [PubMed] [Google Scholar]
- 169.Fabbrocini G., Bertona M., Picazo O., Pareja-Galeano H., Monfrecola G., Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes. 2016;7:625–630. doi: 10.3920/BM2016.0089. [DOI] [PubMed] [Google Scholar]
- 170.Kim J., Ko Y., Park Y.K., Kim N.I., Ha W.K., Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26:902–909. doi: 10.1016/j.nut.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 171.Melnik B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmetic Investig. dermatol. 2015;8:371–388. doi: 10.2147/CCID.S69135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bieber T. Mechanisms of disease. N. Engl. J. Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 173.Williams H.C. Epidemiology of atopic dermatitis. Clin. Exp. Dermatol. 2000;25:522–529. doi: 10.1046/j.1365-2230.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 174.Brunner P.M., Guttman-Yassky E., Leung D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017;139:S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leung D.Y., Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014;134:769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cho S.H., Strickland I., Tomkinson A., Fehringer A.P., Gelfand E.W., Leung D.Y. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J. Investig. Dermatol. 2001;116:658–663. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 177.Morar N., Cookson W.O., Harper J.I., Moffatt M.F. Filaggrin mutations in children with severe atopic dermatitis. J. Investig. Dermatol. 2007;127:1667–1672. doi: 10.1038/sj.jid.5700739. [DOI] [PubMed] [Google Scholar]
- 178.Tanei R. Drugs & Aging. Springer; Cham, Switzerland: 2020. Atopic Dermatitis in Older Adults: A Review of Treatment Options; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paller A.S., Kabashima K., Bieber T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017;140:633–643. doi: 10.1016/j.jaci.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 180.Guttman-Yassky E., Pavel A.B., Zhou L., Estrada Y.D., Zhang N., Xu H., Peng X., Wen H.C., Govas P., Gudi G., et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;144:482–493. doi: 10.1016/j.jaci.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 181.Shirley M. Dupilumab: First global approval. Drugs. 2017;77:1115–1121. doi: 10.1007/s40265-017-0768-3. [DOI] [PubMed] [Google Scholar]
- 182.Bjerre R., Bandier J., Skov L., Engstrand L., Johansen J. The role of the skin microbiome in atopic dermatitis: A systematic review. Br. J. Dermatol. 2017;177:1272–1278. doi: 10.1111/bjd.15390. [DOI] [PubMed] [Google Scholar]
- 183.Leyden J.J., Marples R.R., Kligman A.M. Staphylococcus aureus in the lesions of atopic dermatitis. Br. J. Dermatol. 1974;90:525. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 184.Callewaert C., Nakatsuji T., Knight R., Kosciolek T., Vrbanac A., Kotol P., Ardeleanu M., Hultsch T., Guttman-Yassky E., Bissonnette R., et al. IL-4Rα blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J. Investig. Dermatol. 2020;140:191–202. doi: 10.1016/j.jid.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Matsui K., Nishikawa A. Peptidoglycan-induced T helper 2 immune response in mice involves interleukin-10 secretion from Langerhans cells. Microbiol. Immunol. 2013;57:130–138. doi: 10.1111/j.1348-0421.2012.12006.x. [DOI] [PubMed] [Google Scholar]
- 186.Ruíz-González V., Cancino-Diaz J.C., Rodríguez-Martínez S., Cancino-Diaz M.E. Keratinocytes treated with peptidoglycan from Staphylococcus aureus produce vascular endothelial growth factor, and its expression is amplified by the subsequent production of interleukin-13. Int. J. Dermatol. 2009;48:846–854. doi: 10.1111/j.1365-4632.2008.03924.x. [DOI] [PubMed] [Google Scholar]
- 187.Travers J.B. Toxic interaction between Th2 cytokines and Staphylococcus aureus in atopic dermatitis. J. Investig. Dermatol. 2014;134:2069–2071. doi: 10.1038/jid.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wollenberg A., Zoch C., Wetzel S., Plewig G., Przybilla B. Predisposing factors and clinical features of eczema herpeticum: A retrospective analysis of 100 cases. J. Am. Acad. Dermatol. 2003;49:198–205. doi: 10.1067/S0190-9622(03)00896-X. [DOI] [PubMed] [Google Scholar]
- 189.Mathes E.F., Oza V., Frieden I.J., Cordoro K.M., Yagi S., Howard R., Kristal L., Ginocchio C.C., Schaffer J., Maguiness S., et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:e149–e157. doi: 10.1542/peds.2012-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eichenfield L.F., Tom W.L., Berger T.G., Krol A., Paller A.S., Schwarzenberger K., Bergman J.N., Chamlin S.L., Cohen D.E., Cooper K.D., et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014;71:116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nakatsuji T., Chen T.H., Narala S., Chun K.A., Two A.M., Yun T., Shafiq F., Kotol P.F., Bouslimani A., Melnik A.V., et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017;9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nylund L., Nermes M., Isolauri E., Salminen S., De Vos W., Satokari R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241–244. doi: 10.1111/all.12549. [DOI] [PubMed] [Google Scholar]
- 193.Rather I.A., Bajpai V.K., Kumar S., Lim J., Paek W.K., Park Y.H. Probiotics and atopic dermatitis: An overview. Front. Microbiol. 2016;7:507. doi: 10.3389/fmicb.2016.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.West C.E., Dzidic M., Prescott S.L., Jenmalm M.C. Bugging allergy; role of pre-, pro-and synbiotics in allergy prevention. Allergol. Int. 2017;66:529–538. doi: 10.1016/j.alit.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 195.Piqué N., Berlanga M., Miñana-Galbis D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chang Y.S., Trivedi M.K., Jha A., Lin Y.F., Dimaano L., Garcia-Romero M.T. Synbiotics for prevention and treatment of atopic dermatitis: A meta-analysis of randomized clinical trials. JAMA Pediatr. 2016;170:236–242. doi: 10.1001/jamapediatrics.2015.3943. [DOI] [PubMed] [Google Scholar]
- 197.Zuccotti G., Meneghin F., Aceti A., Barone G., Callegari M.L., Di Mauro A., Fantini M., Gori D., Indrio F., Maggio L., et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 198.Iemoli E., Trabattoni D., Parisotto S., Borgonovo L., Toscano M., Rizzardini G., Clerici M., Ricci E., Fusi A., De Vecchi E., et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J. Clin. Gastroenterol. 2012;46:S33–S40. doi: 10.1097/MCG.0b013e31826a8468. [DOI] [PubMed] [Google Scholar]
- 199.Kim N.Y., Ji G.E. Effects of probiotics on the prevention of atopic dermatitis. Korean J. Pediatr. 2012;55:193. doi: 10.3345/kjp.2012.55.6.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim J.E., Kim H.S. Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019;8:444. doi: 10.3390/jcm8040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Black P., Sharpe S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 202.Devereux G., Seaton A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 203.Mabin D., Sykes A., David T. Controlled trial of a few foods diet in severe atopic dermatitis. Arch. Dis. Child. 1995;73:202–207. doi: 10.1136/adc.73.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Caputo V., Strafella C., Termine A., Dattola A., Mazzilli S., Lanna C., Cosio T., Campione E., Novelli G., Giardina E., et al. Overview of the molecular determinants contributing to the expression of Psoriasis and Psoriatic Arthritis phenotypes. J. Cell. Mol. Med. 2020;24:13554–13563. doi: 10.1111/jcmm.15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nestle F., Kaplan D., Barker J. Mechanisms of Disease: Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 206.Grine L., Lambert J. Psoriasis: Burning Down the Host. [(accessed on 15 November 2020)];2016 doi: 10.3109/09546634.2015.1117567. Available online: https://www.tandfonline.com/doi/pdf/10.3109/09546634.2015.1117567. [DOI] [PubMed]
- 207.Li Q., Chandran V., Tsoi L., O’Rielly D., Nair R.P., Gladman D., Elder J.T., Rahman P. Quantifying differences in heritability among psoriatic arthritis (PsA), cutaneous psoriasis (PsC) and psoriasis vulgaris (PsV) Sci. Rep. 2020;10:1–6. doi: 10.1038/s41598-020-61981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dand N., Mahil S.K., Capon F., Smith C.H., Simpson M.A., Barker J.N. Psoriasis and genetics. Acta Derm Venereol. 2020;100:adv00030. doi: 10.2340/00015555-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ovejero-Benito M.C., Muñoz-Aceituno E., Sabador D., Almoguera B., Prieto-Pérez R., Hakonarson H., Coto-Segura P., Carretero G., Reolid A., Llamas-Velasco M., et al. Genome-wide association analysis of psoriasis patients treated with anti-TNF drugs. Exp. Dermatol. 2020;29:1225–1232. doi: 10.1111/exd.14215. [DOI] [PubMed] [Google Scholar]
- 210.Grozdev I., Korman N., Tsankov N. Psoriasis as a systemic disease. Clin. Dermatol. 2014;32:343–350. doi: 10.1016/j.clindermatol.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 211.Takeshita J., Grewal S., Langan S.M., Mehta N.N., Ogdie A., Van Voorhees A.S., Gelfand J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017;76:393–403. doi: 10.1016/j.jaad.2016.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Remröd C., Sjöström K., Svensson Å. Subjective stress reactivity in psoriasis–a cross sectional study of associated psychological traits. BMC Dermatol. 2015;15:6. doi: 10.1186/s12895-015-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peters E.M. Stressed skin?–a molecular psychosomatic update on stress-causes and effects in dermatologic diseases. J. Der Dtsch. Dermatol. Ges. 2016;14:233–252. doi: 10.1111/ddg.12957. [DOI] [PubMed] [Google Scholar]
- 214.Grine L., Dejager L., Libert C., Vandenbroucke R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. doi: 10.1016/j.cytogfr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 215.Li Y., Song Y., Zhu L., Wang X., Yang B., Lu P., Chen Q., Bin L., Deng L. Interferon Kappa Is Up-Regulated in Psoriasis and It Up-Regulates Psoriasis-Associated Cytokines in vivo. Clin. Cosmet. Investig. Dermatol. 2019;12:865. doi: 10.2147/CCID.S218243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang L.j. Type1 interferons: Potential initiating factors linking skin wounds with psoriasis pathogenesis. Front. Immunol. 2019;10:1440. doi: 10.3389/fimmu.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Conrad C., Di Domizio J., Mylonas A., Belkhodja C., Demaria O., Navarini A.A., Lapointe A.K., French L.E., Vernez M., Gilliet M. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-017-02466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Frasca L., Palazzo R., Chimenti M.S., Alivernini S., Tolusso B., Bui L., Botti E., Giunta A., Bianchi L., Petricca L., et al. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: New biomarkers in PsA. Front. Immunol. 2018;9:1936. doi: 10.3389/fimmu.2018.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kong H.H., Andersson B., Clavel T., Common J.E., Jackson S.A., Olson N.D., Segre J.A., Traidl-Hoffmann C. Performing skin microbiome research: A method to the madness. J. Investig. Dermatol. 2017;137:561–568. doi: 10.1016/j.jid.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thorleifsdottir R.H., Sigurdardottir S.L., Sigurgeirsson B., Olafsson J.H., Sigurdsson M.I., Petersen H., Gudjonsson J.E., Johnston A., Valdimarsson H. Patient-reported outcomes and clinical response in patients with moderate-to-severe plaque psoriasis treated with tonsillectomy: A randomized controlled trial. Acta Derm. Venereol. 2017;97:340–345. doi: 10.2340/00015555-2562. [DOI] [PubMed] [Google Scholar]
- 221.Cohn J.E., Pfeiffer M., Vernose G. Complete resolution of guttate psoriasis after tonsillectomy. Ear, Nose Throat J. 2018;97:62–63. doi: 10.1177/014556131809700306. [DOI] [PubMed] [Google Scholar]
- 222.Haapasalo K., Koskinen L.L., Suvilehto J., Jousilahti P., Wolin A., Suomela S., Trembath R., Barker J., Vuopio J., Kere J., et al. The psoriasis risk allele HLA-C* 06: 02 shows evidence of association with chronic or recurrent streptococcal tonsillitis. Infect. Immun. 2018;86 doi: 10.1128/IAI.00304-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Assarsson M., Duvetorp A., Dienus O., Söderman J., Seifert O. Significant changes in the skin microbiome in patients with chronic plaque psoriasis after treatment with narrowband ultraviolet B. Acta Derm. Venereol. 2018;98:428–436. doi: 10.2340/00015555-2859. [DOI] [PubMed] [Google Scholar]
- 224.Langan E., Künstner A., Miodovnik M., Zillikens D., Thaçi D., Baines J.F., Ibrahim S., Solbach W., Knobloch J. Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. Br. J. Dermatol. 2019;181:1254–1264. doi: 10.1111/bjd.17989. [DOI] [PubMed] [Google Scholar]
- 225.Paniz Mondolfi A., Hernandez Perez M., Blohm G., Marquez M., Mogollon Mendoza A., Hernandez-Pereira C., Escalona M., Lodeiro Colatosti A., Rothe DeArocha J., Rodriguez Morales A. Generalized pustular psoriasis triggered by Zika virus infection. Clin. Exp. Dermatol. 2018;43:171–174. doi: 10.1111/ced.13294. [DOI] [PubMed] [Google Scholar]
- 226.Sbidian E., Madrange M., Viguier M., Salmona M., Duchatelet S., Hovnanian A., Smahi A., Le Goff J., Bachelez H. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br. J. Dermatol. 2019;181:1304–1306. doi: 10.1111/bjd.18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sanchez I.M., Jiang W., Yang E.J., Singh R.K., Beck K., Liu C., Afifi L., Liao W. Enteropathy in psoriasis: A systematic review of gastrointestinal disease epidemiology and subclinical inflammatory and functional gut alterations. Curr. Dermatol. Rep. 2018;7:59–74. doi: 10.1007/s13671-018-0213-1. [DOI] [Google Scholar]
- 228.Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D., Wehkamp J., Feagan B.G., Yao M.D., Karczewski M., et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fobelo Lozano M.J., Serrano Giménez R., Castro Fernández M. Emergence of inflammatory bowel disease during treatment with secukinumab. J. Crohn’s Colitis. 2018;12:1131–1133. doi: 10.1093/ecco-jcc/jjy063. [DOI] [PubMed] [Google Scholar]
- 230.Barry R., Salmon P., Read A., Warin R. Mucosal architecture of the small bowel in cases of psoriasis. Gut. 1971;12:873–877. doi: 10.1136/gut.12.11.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Humbert P., Bidet A., Treffel P., Drobacheff C., Agache P. Intestinal permeability in patients with psoriasis. J. Dermatol. Sci. 1991;2:324–326. doi: 10.1016/0923-1811(91)90057-5. [DOI] [PubMed] [Google Scholar]
- 232.Sikora M., Stec A., Chrabaszcz M., Waskiel-Burnat A., Zaremba M., Olszewska M., Rudnicka L. Intestinal fatty acid binding protein, a biomarker of intestinal barrier, is associated with severity of psoriasis. J. Clin. Med. 2019;8:1021. doi: 10.3390/jcm8071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Adarsh M., Dogra S., Vaiphei K., Vaishnavi C., Sinha S., Sharma A. Evaluation of subclinical gut inflammation using faecal calprotectin levels and colonic mucosal biopsy in patients with psoriasis and psoriatic arthritis. Br. J. Dermatol. 2019;181:401–402. doi: 10.1111/bjd.17745. [DOI] [PubMed] [Google Scholar]
- 234.Munz O.H., Sela S., Baker B.S., Griffiths C.E., Powles A.V., Fry L. Evidence for the presence of bacteria in the blood of psoriasis patients. Arch. Dermatol. Res. 2010;302:495–498. doi: 10.1007/s00403-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 235.Ramírez-Boscá A., Navarro-López V., Martínez-Andrés A., Such J., Francés R., de la Parte J.H., Asín-Llorca M. Identification of bacterial DNA in the peripheral blood of patients with active psoriasis. JAMA Dermatol. 2015;151:670–671. doi: 10.1001/jamadermatol.2014.5585. [DOI] [PubMed] [Google Scholar]
- 236.Kim M., Han K.D., Lee J.H. Bodyweight variability and the risk of psoriasis: A nationwide population-based cohort study. J. Eur. Acad. Dermatol. Venereol. 2020;34:1019–1025. doi: 10.1111/jdv.16099. [DOI] [PubMed] [Google Scholar]
- 237.Jensen P., Christensen R., Zachariae C., Geiker N.R., Schaadt B.K., Stender S., Hansen P.R., Astrup A., Skov L. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: A prospective observational follow-up study. Am. J. Clin. Nutr. 2016;104:259–265. doi: 10.3945/ajcn.115.125849. [DOI] [PubMed] [Google Scholar]
- 238.Nakamizo S., Honda T., Adachi A., Nagatake T., Kunisawa J., Kitoh A., Otsuka A., Dainichi T., Nomura T., Ginhoux F., et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-14292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Herbert D., Franz S., Popkova Y., Anderegg U., Schiller J., Schwede K., Lorz A., Simon J.C., Saalbach A. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: Saturated fatty acids as key players. J. Investig. Dermatol. 2018;138:1999–2009. doi: 10.1016/j.jid.2018.03.1522. [DOI] [PubMed] [Google Scholar]
- 240.Castaldo G., Galdo G., Aufiero F.R., Cereda E. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis. Obes. Res. Clin. Pract. 2016;10:348–352. doi: 10.1016/j.orcp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 241.Damiani G., Watad A., Bridgewood C., Pigatto P.D.M., Pacifico A., Malagoli P., Bragazzi N.L., Adawi M. The impact of ramadan fasting on the reduction of PASI score, in moderate-to-severe psoriatic patients: A real-life multicenter study. Nutrients. 2019;11:277. doi: 10.3390/nu11020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Codoñer F.M., Ramírez-Bosca A., Climent E., Carrión-Gutierrez M., Guerrero M., Pérez-Orquín J.M., De La Parte J.H., Genovés S., Ramón D., Navarro-López V., et al. Gut microbial composition in patients with psoriasis. Sci. Rep. 2018;8:1–7. doi: 10.1038/s41598-018-22125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Navarro-López V., Martínez-Andrés A., Ramírez-Boscá A., Ruzafa-Costas B., Núñez-Delegido E., Carrión-Gutiérrez M.A., Prieto-Merino D., Codoñer-Cortés F., Ramón-Vidal D., Genovés-Martínez S., et al. Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: A randomized controlled clinical trial. Acta Derm. Venereol. 2019;99:1078–1084. doi: 10.2340/00015555-3305. [DOI] [PubMed] [Google Scholar]
- 244.Itano A., Cormack T., Ramani K., Barth K., Wang I., Mukherjee A., Ponichtera H., McKenna C., Jahic M., Bodmer M. Orally-administered EDP1815, a single strain of Prevotella histicola, has potent systemic anti-inflammatory effects in Type 1, Type 2, and Type 3 inflammatory models; Proceedings of the 29th European Academy of Dermatology and Venereology Congress (EADV); Vienna, Austria. 29–31 October 2020. [Google Scholar]
- 245.Phan K., Charlton O., Smith S.D. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. Biomed. Dermatol. 2020;4:1–6. doi: 10.1186/s41702-019-0052-0. [DOI] [Google Scholar]
- 246.Jørgensen A.H.R., Thomsen S.F., Karmisholt K.E., Ring H.C. Clinical, microbiological, immunological and imaging characteristics of tunnels and fistulas in hidradenitis suppurativa and Crohn’s disease. Exp. Dermatol. 2020;29:118–123. doi: 10.1111/exd.14036. [DOI] [PubMed] [Google Scholar]
- 247.Moran B., Sweeney C.M., Hughes R., Malara A., Kirthi S., Tobin A.M., Kirby B., Fletcher J.M. Hidradenitis suppurativa is characterized by dysregulation of the Th17: Treg cell axis, which is corrected by anti-TNF therapy. J. Investig. Dermatol. 2017;137:2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 248.Chen W.T., Chi C.C. Association of hidradenitis suppurativa with inflammatory bowel disease: A systematic review and meta-analysis. JAMA Dermatol. 2019;155:1022–1027. doi: 10.1001/jamadermatol.2019.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Giudici F., Maggi L., Santi R., Cosmi L., Scaletti C., Annunziato F., Nesi G., Barra G., Bassotti G., De Palma R., et al. Perianal Crohn’s disease and hidradenitis suppurativa: A possible common immunological scenario. Clin. Mol. Allergy. 2015;13:12. doi: 10.1186/s12948-015-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Barta Z., Zöld É., Csípõ I., Zeher M. ASCAs in (auto-) Immune Small Bowel Diseases. [(accessed on 20 November 2020)];2020 Available online: https://gut.bmj.com/content/ascas-auto-immune-small-bowel-diseases.
- 251.Denny G., Anadkat M.J. The effect of smoking and age on the response to first-line therapy of hidradenitis suppurativa: An institutional retrospective cohort study. J. Am. Acad. Dermatol. 2017;76:54–59. doi: 10.1016/j.jaad.2016.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.König A., Lehmann C., Rompel R., Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261–264. doi: 10.1159/000018126. [DOI] [PubMed] [Google Scholar]
- 253.Kromann C.B., Ibler K.S., Kristiansen V.B., Jemec G.B. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm. Venereol. 2014;94:553–557. doi: 10.2340/00015555-1800. [DOI] [PubMed] [Google Scholar]
- 254.Aboud C., Zamaria N., Cannistrà C. Treatment of hidradenitis suppurativa: Surgery and yeast (Saccharomyces cerevisiae)–exclusion diet. Results after 6 years. Surgery. 2020;167:1012–1015. doi: 10.1016/j.surg.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 255.Silfvast-Kaiser A., Youssef R., Paek S.Y. Diet in hidradenitis suppurativa: A review of published and lay literature. Int. J. Dermatol. 2019;58:1225–1230. doi: 10.1111/ijd.14465. [DOI] [PubMed] [Google Scholar]
- 256.Buechner S.A. Rosacea: An update. Dermatology. 2005;210:100–108. doi: 10.1159/000082564. [DOI] [PubMed] [Google Scholar]
- 257.Rainer B.M., Fischer A.H., Da Silva D.L.F., Kang S., Chien A.L. Rosacea is associated with chronic systemic diseases in a skin severity–dependent manner: Results of a case-control study. J. Am. Acad. Dermatol. 2015;73:604–608. doi: 10.1016/j.jaad.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 258.Tan J., Berg M. Rosacea: Current state of epidemiology. J. Am. Acad. Dermatol. 2013;69:S27–S35. doi: 10.1016/j.jaad.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 259.Duman N., Ersoy Evans S., Atakan N. Rosacea and cardiovascular risk factors: A case control study. J. Eur. Acad. Dermatol. Venereol. 2014;28:1165–1169. doi: 10.1111/jdv.12234. [DOI] [PubMed] [Google Scholar]
- 260.Yamasaki K., Di Nardo A., Bardan A., Murakami M., Ohtake T., Coda A., Dorschner R.A., Bonnart C., Descargues P., Hovnanian A., et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 261.Nizet V., Ohtake T., Lauth X., Trowbridge J., Rudisill J., Dorschner R.A., Pestonjamasp V., Piraino J., Huttner K., Gallo R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 262.Two A.M., Wu W., Gallo R.L., Hata T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015;72:749–758. doi: 10.1016/j.jaad.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 263.Gallo R.L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yang D., Chen Q., Schmidt A.P., Anderson G.M., Wang J.M., Wooters J., Oppenheim J.J., Chertov O. LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Koczulla R., Von Degenfeld G., Kupatt C., Krötz F., Zahler S., Gloe T., Issbrücker K., Unterberger P., Zaiou M., Lebherz C., et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sulk M., Seeliger S., Aubert J., Schwab V.D., Cevikbas F., Rivier M., Nowak P., Voegel J.J., Buddenkotte J., Steinhoff M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012;132:1253–1262. doi: 10.1038/jid.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ferrer L., Ravera I., Silbermayr K. Immunology and pathogenesis of canine demodicosis. Vet. Dermatol. 2014;25:427–e65. doi: 10.1111/vde.12136. [DOI] [PubMed] [Google Scholar]
- 268.Kocak M., Yagli S., Vahapoğlu G., Ekşioğlu M. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. Dermatology. 2002;205:265–270. doi: 10.1159/000065849. [DOI] [PubMed] [Google Scholar]
- 269.O’Reilly N., Menezes N., Kavanagh K. Positive correlation between serum immunoreactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosacea. Br. J. Dermatol. 2012;167:1032–1036. doi: 10.1111/j.1365-2133.2012.11114.x. [DOI] [PubMed] [Google Scholar]
- 270.Yamasaki K., Gallo R.L. The molecular pathology of rosacea. J. Dermatol. Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Woo Y.R., Lee S.H., Cho S.H., Lee J.D., Kim H.S. Characterization and Analysis of the Skin Microbiota in Rosacea: Impact of Systemic Antibiotics. J. Clin. Med. 2020;9:185. doi: 10.3390/jcm9010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Eriksson G., Nord C. Impact of topical metronidazole on the skin and colon microflora in patients with rosacea. Infection. 1987;15:8–10. doi: 10.1007/BF01646108. [DOI] [PubMed] [Google Scholar]
- 273.Zaidi A.K., Spaunhurst K., Sprockett D., Thomason Y., Mann M.W., Fu P., Ammons C., Gerstenblith M., Tuttle M.S., Popkin D.L. Characterization of the facial microbiome in twins discordant for rosacea. Exp. Dermatol. 2018;27:295–298. doi: 10.1111/exd.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Weiss E., Katta R. Diet and rosacea: The role of dietary change in the management of rosacea. Dermatol. Pract. Concept. 2017;7:31. doi: 10.5826/dpc.0704a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Egeberg A., Weinstock L., Thyssen E., Gislason G., Thyssen J. Rosacea and gastrointestinal disorders: A population-based cohort study. Br. J. Dermatol. 2017;176:100–106. doi: 10.1111/bjd.14930. [DOI] [PubMed] [Google Scholar]
- 276.Chen Y.J., Lee W.H., Ho H.J., Tseng C.H., Wu C.Y. An altered fecal microbial profiling in rosacea patients compared to matched controls. J. Formos. Med. Assoc. 2020;120:256–264. doi: 10.1016/j.jfma.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 277.Gupta V.K., Paul S., Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Scheman A., Rakowski E.M., Chou V., Chhatriwala A., Ross J., Jacob S.E. Balsam of Peru: Past and future. Dermatitis. 2013;24:153–160. doi: 10.1097/DER.0b013e31828afab2. [DOI] [PubMed] [Google Scholar]
- 279.Aubdool A.A., Brain S.D. Neurovascular aspects of skin neurogenic inflammation. J. Investig. Dermatol. Symp. Proc. 2011;15:33–39. doi: 10.1038/jidsymp.2011.8. [DOI] [PubMed] [Google Scholar]
- 280.Kamamoto C., Nishikaku A., Gompertz O., Melo A., Hassun K., Bagatin E. Cutaneous fungal microbiome: Malassezia yeasts in seborrheic dermatitis scalp in a randomized, comparative and therapeutic trial. Dermato-endocrinology. 2017;9:e1361573. doi: 10.1080/19381980.2017.1361573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tucker D., Masood S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2019. Seborrheic Dermatitis. [PubMed] [Google Scholar]
- 282.Borda L.J., Wikramanayake T.C. Seborrheic dermatitis and dandruff: A comprehensive review. J. Clin. Investig. Dermatol. 2015;3 doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mokos Z.B., Kralj M., Basta-Juzbasic A., Jukic I.L. Seborrheic dermatitis: An update. Acta Dermatovenerol Croat. 2012;20:98–104. [PubMed] [Google Scholar]
- 284.Rudramurthy S.M., Honnavar P., Chakrabarti A., Dogra S., Singh P., Handa S. Association of Malassezia species with psoriatic lesions. Mycoses. 2014;57:483–488. doi: 10.1111/myc.12186. [DOI] [PubMed] [Google Scholar]
- 285.DeAngelis Y.M., Gemmer C.M., Kaczvinsky J.R., Kenneally D.C., Schwartz J.R., Dawson T.L., Jr. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J. Investig. Dermatol. Symp. Proc. 2005;10:295–297. doi: 10.1111/j.1087-0024.2005.10119.x. [DOI] [PubMed] [Google Scholar]
- 286.Odintsova I., Dyudyun A. Features of the composition of microorganisms inhabiting the intestinal mucosa in patients with seborrheic dermatitis. Dermatovenerol. Cosmetol. Sexopathol. 2019:31–34. doi: 10.37321/dermatology.2019.1-2-05. [DOI] [Google Scholar]
- 287.Sakuma T.H., Maibach H.I. Oily skin: An overview. Skin Pharmacol. Physiol. 2012;25:227–235. doi: 10.1159/000338978. [DOI] [PubMed] [Google Scholar]
- 288.Bett D., Morland J., Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br. Med J. 1967;3:153. doi: 10.1136/bmj.3.5558.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pochi P.E., Downing D.T., Strauss J.S. Sebaceous gland response in man to prolonged total caloric deprivation. J. Investig. Dermatol. 1970;55:303–309. doi: 10.1111/1523-1747.ep12260136. [DOI] [PubMed] [Google Scholar]
- 290.Boelsma E., Van de Vijver L.P., Goldbohm R.A., Klöpping-Ketelaars I.A., Hendriks H.F., Roza L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am. J. Clin. Nutr. 2003;77:348–355. doi: 10.1093/ajcn/77.2.348. [DOI] [PubMed] [Google Scholar]
- 291.Tamer F. Relationship between diet and seborrheic dermatitis. Our Dermatol. Online. 2018;9:261–264. doi: 10.7241/ourd.20183.6. [DOI] [Google Scholar]
- 292.Lee H.H., Gwillim E., Patel K.R., Hua T., Rastogi S., Ibler E., Silverberg J.I. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020;82:675–682. doi: 10.1016/j.jaad.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 293.Simakou T., Butcher J.P., Reid S., Henriquez F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019;98:74–85. doi: 10.1016/j.jaut.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 294.Juhasz M., Chen S., Khosrovi-Eghbal A., Ekelem C., Landaverde Y., Baldi P., Mesinkovska N.A. Characterizing the Skin and Gut Microbiome of Alopecia Areata Patients. SKIN J. Cutan. Med. 2020;4:23–30. doi: 10.25251/skin.4.1.4. [DOI] [Google Scholar]
- 295.Polak-Witka K., Rudnicka L., Blume-Peytavi U., Vogt A. The role of the microbiome in scalp hair follicle biology and disease. Exp. Dermatol. 2020;29:286–294. doi: 10.1111/exd.13935. [DOI] [PubMed] [Google Scholar]
- 296.Migacz-Gruszka K., Branicki W., Obtulowicz A., Pirowska M., Gruszka K., Wojas-Pelc A. What’s new in the pathophysiology of alopecia areata? the possible contribution of skin and gut microbiome in the pathogenesis of alopecia–Big opportunities, big challenges, and novel perspectives. Int. J. Trichology. 2019;11:185. doi: 10.4103/ijt.ijt_76_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Guo E.L., Katta R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017;7:1. doi: 10.5826/dpc.0701a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pham C.T., Romero K., Almohanna H.M., Griggs J., Ahmed A., Tosti A. The Role of Diet as an Adjuvant Treatment in Scarring and Nonscarring Alopecia. Skin Appendage Disord. 2020;6:88–96. doi: 10.1159/000504786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Grosu-Bularda A., Lăzărescu L., Stoian A., Lascăr I. Immunology and skin cancer. Arch. Clin. Cases. 2018;5 doi: 10.22551/2018.20.0503.10137. [DOI] [Google Scholar]
- 300.Carr S., Smith C., Wernberg J. Epidemiology and risk factors of melanoma. Surg. Clin. 2020;100:1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 301.Marks R. An overview of skin cancers. Cancer. 1995;75:607–612. doi: 10.1002/1097-0142(19950115)75:2+<607::AID-CNCR2820751402>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 302.Rangwala S., Tsai K. Roles of the immune system in skin cancer. Br. J. Dermatol. 2011;165:953–965. doi: 10.1111/j.1365-2133.2011.10507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vergara D., Simeone P., Damato M., Maffia M., Lanuti P., Trerotola M. The cancer microbiota: EMT and inflammation as shared molecular mechanisms associated with plasticity and progression. J. Oncol. 2019;2019:1253727. doi: 10.1155/2019/1253727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nakatsuji T., Chen T.H., Butcher A.M., Trzoss L.L., Nam S.J., Shirakawa K.T., Zhou W., Oh J., Otto M., Fenical W., et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018;4:eaao4502. doi: 10.1126/sciadv.aao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Allhorn M., Arve S., Brüggemann H., Lood R. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci. Rep. 2016;6:36412. doi: 10.1038/srep36412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Frosali S., Pagliari D., Gambassi G., Landolfi R., Pandolfi F., Cianci R. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J. Immunol. Res. 2015;2015:489821. doi: 10.1155/2015/489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Heyer K., Herberger K., Protz K., Glaeske G., Augustin M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016;24:434–442. doi: 10.1111/wrr.12387. [DOI] [PubMed] [Google Scholar]
- 309.Guest J.F., Ayoub N., McIlwraith T., Uchegbu I., Gerrish A., Weidlich D., Vowden K., Vowden P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gould L., Abadir P., Brem H., Carter M., Conner-Kerr T., Davidson J., DiPietro L., Falanga V., Fife C., Gardner S., et al. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015;23:1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sawaya A.P., Stone R.C., Brooks S.R., Pastar I., Jozic I., Hasneen K., O’Neill K., Mehdizadeh S., Head C.R., Strbo N., et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-18276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tomic-Canic M., Burgess J.L., O’Neill K.E., Strbo N., Pastar I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020;21:36–43. doi: 10.1007/s40257-020-00536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ramirez H.A., Pastar I., Jozic I., Stojadinovic O., Stone R.C., Ojeh N., Gil J., Davis S.C., Kirsner R.S., Tomic-Canic M. Staphylococcus aureus triggers induction of miR-15B-5P to diminish DNA repair and deregulate inflammatory response in diabetic foot ulcers. J. Investig. Dermatol. 2018;138:1187–1196. doi: 10.1016/j.jid.2017.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Stone R.C., Stojadinovic O., Rosa A.M., Ramirez H.A., Badiavas E., Blumenberg M., Tomic-Canic M. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci. Transl. Med. 2017;9:eaaf8611. doi: 10.1126/scitranslmed.aaf8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Stone R.C., Stojadinovic O., Sawaya A.P., Glinos G.D., Lindley L.E., Pastar I., Badiavas E., Tomic-Canic M. A bioengineered living cell construct activates metallothionein/zinc/MMP8 and inhibits TGFβ to stimulate remodeling of fibrotic venous leg ulcers. Wound Repair Regen. 2020;28:164–176. doi: 10.1111/wrr.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pastar I., Wong L.L., Egger A.N., Tomic-Canic M. Descriptive vs mechanistic scientific approach to study wound healing and its inhibition: Is there a value of translational research involving human subjects? Exp. Dermatol. 2018;27:551–562. doi: 10.1111/exd.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Thom S.R., Hampton M., Troiano M.A., Mirza Z., Malay D.S., Shannon S., Jennato N.B., Donohue C.M., Hoffstad O., Woltereck D., et al. Measurements of CD34+/CD45-dim stem cells predict healing of diabetic neuropathic wounds. Diabetes. 2016;65:486–497. doi: 10.2337/db15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zeeuwen P.L., Boekhorst J., van den Bogaard E.H., de Koning H.D., van de Kerkhof P.M., Saulnier D.M., van Swam I.I., van Hijum S.A., Kleerebezem M., Schalkwijk J., et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012;13:1–18. doi: 10.1186/gb-2012-13-11-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Pastar I., Nusbaum A.G., Gil J., Patel S.B., Chen J., Valdes J., Stojadinovic O., Plano L.R., Tomic-Canic M., Davis S.C. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS ONE. 2013;8:e56846. doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Harrison O.J., Linehan J.L., Shih H.Y., Bouladoux N., Han S.J., Smelkinson M., Sen S.K., Byrd A.L., Enamorado M., Yao C., et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science. 2019;363:eaat6280. doi: 10.1126/science.aat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lai Y., Di Nardo A., Nakatsuji T., Leichtle A., Yang Y., Cogen A.L., Wu Z.R., Hooper L.V., Schmidt R.R., Von Aulock S., et al. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat. Med. 2009;15:1377. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.McCormack R.M., de Armas L.R., Shiratsuchi M., Fiorentino D.G., Olsson M.L., Lichtenheld M.G., Morales A., Lyapichev K., Gonzalez L.E., Strbo N., et al. Perforin-2 is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria. Elife. 2015;4:e06508. doi: 10.7554/eLife.06508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pastar I., O’Neill K., Padula L., Head C.R., Burgess J.L., Chen V., Garcia D., Stojadinovic O., Hower S., Plano G.V., et al. Staphylococcus epidermidis boosts innate immune response by activation of Gamma Delta T cells and induction of Perforin-2 in human skin. Front. Immunol. 2020;11:2253. doi: 10.3389/fimmu.2020.550946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Luqman A., Muttaqin M.Z., Yulaipi S., Ebner P., Matsuo M., Zabel S., Tribelli P.M., Nieselt K., Hidayati D., Götz F. Trace amines produced by skin bacteria accelerate wound healing in mice. Commun. Biol. 2020;3:1–10. doi: 10.1038/s42003-020-1000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Misic A.M., Gardner S.E., Grice E.A. The wound microbiome: Modern approaches to examining the role of microorganisms in impaired chronic wound healing. Adv. Wound Care. 2014;3:502–510. doi: 10.1089/wound.2012.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kalan L.R., Meisel J.S., Loesche M.A., Horwinski J., Soaita I., Chen X., Uberoi A., Gardner S.E., Grice E.A. Strain-and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25:641–655. doi: 10.1016/j.chom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Huseini H.F., Rahimzadeh G., Fazeli M.R., Mehrazma M., Salehi M. Evaluation of wound healing activities of kefir products. Burns. 2012;38:719–723. doi: 10.1016/j.burns.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 328.Poutahidis T., Kearney S.M., Levkovich T., Qi P., Varian B.J., Lakritz J.R., Ibrahim Y.M., Chatzigiagkos A., Alm E.J., Erdman S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Levkovich T., Poutahidis T., Smillie C., Varian B.J., Ibrahim Y.M., Lakritz J.R., Alm E.J., Erdman S.E. Probiotic bacteria induce a ‘glow of health’. PLoS ONE. 2013;8:e53867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gueniche A., Philippe D., Bastien P., Reuteler G., Blum S., Castiel-Higounenc I., Breton L., Benyacoub J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes. 2014;5:137–145. doi: 10.3920/BM2013.0001. [DOI] [PubMed] [Google Scholar]
- 331.Krutmann J. Pre-and probiotics for human skin. J. Dermatol. Sci. 2009;54:1–5. doi: 10.1016/j.jdermsci.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 332.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 333.Farris P.K. Are skincare products with probiotics worth the hype? DermatologyTimes. [(accessed on 20 November 2020)];2016 Available online: https://www.dermatologytimes.com/view/are-skincare-products-probiotics-worth-hype.
- 334.Grant M.C., Baker J.S. An overview of the effect of probiotics and exercise on mood and associated health conditions. Crit. Rev. Food Sci. Nutr. 2017;57:3887–3893. doi: 10.1080/10408398.2016.1189872. [DOI] [PubMed] [Google Scholar]
- 335.Sánchez B., Delgado S., Blanco-Míguez A., Lourenço A., Gueimonde M., Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 336.Sarao L.K., Arora M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017;57:344–371. doi: 10.1080/10408398.2014.887055. [DOI] [PubMed] [Google Scholar]
- 337.Muizzuddin N., Maher W., Sullivan M., Schnittger S., Mammone T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012;63:385–395. [PubMed] [Google Scholar]
- 338.Frei R., Akdis M., O’Mahony L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015;31:153–158. doi: 10.1097/MOG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 339.Longo V.D., Cortellino S. Fasting, dietary restriction, and immunosenescence. J. Allergy Clin. Immunol. 2020;146:1002–1004. doi: 10.1016/j.jaci.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 340.Bronsnick T., Murzaku E.C., Rao B.K. Diet in dermatology: Part I. Atopic dermatitis, acne, and nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2014;71:1039–e1. doi: 10.1016/j.jaad.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 341.Parkar S.G., Kalsbeek A., Cheeseman J.F. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 2019;7:41. doi: 10.3390/microorganisms7020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Voigt R., Forsyth C., Green S., Engen P., Keshavarzian A. International Review of Neurobiology. Volume 131. Elsevier; Amsterdam, The Netherlands: 2016. Circadian rhythm and the gut microbiome; pp. 193–205. [DOI] [PubMed] [Google Scholar]
- 343.Deaver J.A., Eum S.Y., Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front. Microbiol. 2018;9:737. doi: 10.3389/fmicb.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zeb F., Wu X., Chen L., Fatima S., Haq I.u., Chen A., Majeed F., Feng Q., Li M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020;123:1216–1226. doi: 10.1017/S0007114519003428. [DOI] [PubMed] [Google Scholar]
- 345.Jakubowicz D., Barnea M., Wainstein J., Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21:2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]