Abstract
Hypoxylon, a large, cosmopolitan genus of Ascomycota is in the focus of our current poly-thetic taxonomic studies, and served as an excellent source for bioactive secondary metabolites at the same time. The present work concerns a survey of the Hypoxylon fuscum species complex based on specimens from Iran and Europe by morphological studies and high performance liquid chromatography coupled to mass spectrometry and diode array detection (HPLC-MS-DAD). Apart from known chemotaxonomic markers like binaphthalene tetrol (BNT) and daldinin F, two unprece-dented molecules were detected and subsequently isolated to purity by semi preparative HPLC. Their structures were established by nuclear-magnetic resonance (NMR) spectroscopy as 3′-malonyl-daldinin F (6) and pseudofuscochalasin A (4). The new daldinin derivative 6 showed weak cytotoxicity towards mammalian cells but bactericidal activity. The new cytochalasin 4 was compared to cytochalasin C in an actin disruption assay using fluorescence microscopy of human osteo-sarcoma U2OS cells, revealing comparable activity towards F-actin but being irreversible compared to cytochalasin C. Concurrently, a multilocus molecular phylogeny based on ribosomal and proteinogenic nucleotide sequences of Hypoxylon species resulted in a well-supported clade for H. fuscum and its allies. From a comparison of morphological, chemotaxonomic and phylogenetic evidence, we introduce the new species H. eurasiaticum and H. pseudofuscum.
Keywords: analytical chemistry, Ascomycota, bioactivity screening chemotaxonomy, molecular phylogenetics, polyphasic taxonomy, two new species
1. Introduction
The Hypoxylaceae are a family of the Xylariales (Sordariomycetes) that presently comprises 19 genera and approximately 400 taxa [1]. The family is characterized by the presence of stromatal pigments and a nodulisporium-like anamorph [2]. Most taxa in the family are saprobes, while some occur as endophytes and/or are associated with insect vectors [3]. The largest genus in the Hypoxylaceae is the type genus Hypoxylon with presently ca. 180 species. The generic concept of Hypoxylon was traditionally based on macromorphological features relating to the stromatal morphology until Ju and Rogers [4] restricted the genus to accommodate only those species that have a nodulisporium-like anamorph. Furthermore, they divided these anamorph stages into sub-types based on the complexity of the branching patterns of the conidiophores. Other genera whose species were traditionally placed in Hypoxylon (e.g., Nemania) are now accommodated in the Xylariaceae sensu stricto (see overview by Daranagama et al. [5]). Molecular studies have later validated this concept and also led to the segregation of further genera like Annulohypoxylon [6], Hypomontagnella [7], Jackrogersella, and Pyrenopolyporus [2] from the bulk of Hypoxylon. The current concept of the Hypoxylaceae is based on a multi gene genealogy but was recently even validated by a phylogenomic study based on 12 representatives using third generation genome sequencing techniques like Oxford nanopore and PACBIO [8]. However, both the multilocus phylogenetic tree and the phylogenomic reconstruction indicated that Hypoxylon is still polyphyletic and will need to be further segregated in the future. Until this can be accomplished, several complicated species complexes in the genus remain to be studied in-depth.
The Hypoxylon fuscum complex is one of them that needs additional comprehensive studies with polyphasic approaches. It is widely distributed in North temperate areas (Europe, North America) with occasional records from the tropics, the latter of which mostly came from higher altitudes [4]. The stromata are most frequently encountered on species of the Betulaceae but may grow on various other angiospermous host plants. A special case is Hypoxylon porphyreum, which is constantly associated with Quercus and was first reported by Granmo [9] from Norway, and later also found in France and (among the old herbarium specimens housed in New York Botanical Gardens) even in USA [10]. The latter study [10], which relied on HPLC profiling and morphological studies of several hundreds of herbarium specimens including many types, also confirmed that H. macrosporum, a species that is mostly known from Salix in the boreal-montane climates of the Northern hemisphere is related to H. fuscum. This was actually revealed by chemotaxonomic methodology in conjunction with morphological traits. In addition, the H. fuscum complex includes the (sub-)tropical H. anthochroum, the European H. fuscoides and various other potentially undescribed species that are thus far only known from old herbarium specimens [10,11].
The Hypoxylon fuscum complex is characterized by effused (on barkless wood) to effused-pulvinate (on bark), purple to red-brown stromata and olivaceous stromatal pigments in KOH. These major stromatal secondary metabolites of H. fuscum s. str. are binaphthalene tetrol (BNT; 1) and azaphilones like daldinins C, E and F (2, 3, 5) [12]. The latter compounds are also present in H. macrosporum but are actually lacking in H. porphyreum, which contains similar, yet unidentified azaphilones that could so far not be isolated due to their instability and the scarcity of the material [10]. The conidiogeneous states of these species as well as of the variants that are for now accommodated in H. fuscum have a characteristic virgariella-like branching pattern that was first described by Petrini [13] for various isolates derived from stromata growing on Alnus, Betula, Carpinus, and Corylus and it does not seem to be possible to use this feature to further segregate them based on anamorphic traits. However, Petrini et al. [14] also studied the ascospore size ranges of numerous specimens from different host plants and found striking correlations. These data were not taken into account by Ju and Rogers [4], who reported a very large ascospore size range (8–20 µm × 4–8 µm) for the specimens they treated as H. fuscum, but notably, their broad concept had even included H. porphyreum. In fact, Stadler and Fournier [15] and Stadler et al. [16] had already pointed out that specimens referable to H. fuscum ss. Ju and Rogers [4] had a deviating chemotype from the “typical” variant of the species, which frequently occurs on Corylus avellana in Europe. The study by Stadler et al. [10] included the holotype of Sphaeria fusca from the 18th century and revealed that this material has exactly the same metabolite profile as recently collected material, i.e., BNT (1) and daldinins C (2), E (3) and F (5) were still detected by HPLC in the ancient stromata as major components. For this reason, Wendt et al. [2] have selected an epitype from Corylus in Germany originating from the previous work by Triebel et al. [17] that was cultured. The ex-epitype culture CBS 113049 can be used in the future to further stabilize the taxonomy of this species complex.
Iran (especially the north of Iran) includes subtropical regions and houses numerous species of Xylariales. Only few surveys on their diversity have so far been conducted in the country [18,19,20,21], and even the Iranian species of Hypoxylon are still poorly known and need further examination.
In the present study we have examined several specimens belonging to the H. fuscum complex from Iran and Europe using the above described combination of morphology, chemotaxonomy and molecular phylogeny. During the course of this work we also have isolated some new molecules that can serve as chemotaxonomic markers and report their physicochemical characteristics and biological activities. The present paper is dedicated to reporting these findings.
2. Materials and Methods
2.1. Sample Sources
Samples were collected from Iran and Europe. Iranian samples were collected from Guilan and Mazandaran provinces (Northern Iran) during 2015–2017. European samples were collected during an excursion of the European mycological congress in the Białowieża national Park (Poland), in the vicinity of Braunschweig, Germany and near Engelhartstetten in Lower-Austria. Parts of corticated branches and trunks bearing Hypoxylaceae stromata were transferred to the laboratory. Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. Iranian specimens have been deposited in the fungarium of the Department of Plant Protection, Faculty of Agricultural Science, University of Guilan, Guilan, Iran (GUM). European specimens were deposited in the herbarium of the Staatl. Museum für Naturkunde, Karlsruhe, Germany (KR) and in the fungarium of the University of Vienna, Austria (WU). Living cultures were deposited in the culture collection MUCL (Louvain, Belgium) and DSMZ (Braunschweig, Germany). All fungal names used in this work and the corresponding taxonomy follow the recent overview on families of Sordariomycetes [1].
2.2. Morphological Characterisation
Morphological analyses of microscopic characters were carried out as described by Pourmoghaddam et al. [21]. Pigment colors were determined as described in the latter monograph, with colour codes following Rayner [22]. Macrophotographs were obtained with a Keyence VHX-6000 microscope. Light microscopy with Nomarski differential interference contrast (DIC) was done using a Zeiss Axio Imager.A1 compound microscope, equipped with a Zeiss Axiocam 506 colour digital camera. SEM of ascospores were recorded using a field-emission scanning electron microscope (FE-SEM Merlin, Zeiss, Germany), in a similar fashion as reported previously [23].
2.3. DNA Extraction, PCR and Sequencing
DNA extraction of fresh cultures and amplification of the ITS (nuc rDNA internal transcribed spacer region containing ITS1-5.8S-ITS2), LSU (5′ 1200 bp of the large subunit nuc 28S rDNA), rpb2 (partial second largest subunit of the DNA-directed RNA polymerase II), and tub2 (partial β-tubulin) loci were performed as described by [2]. DNA Sequences were generated by an in-house Sanger capillary sequencing solution on the HZI campus and processed with Geneious® 7.1.9 (http://www.geneious.com) [2].
2.4. Molecular Phylogenetic Analyses
The newly generated sequences were aligned with selected sequences from [2] and a combined matrix of the four loci (ITS, LSU, rpb2 and tub2) was produced for phylogenetic analyses, with four species (Biscogniauxia nummularia, Graphostroma platystomum, Xylaria arbuscula, and Xylaria hypoxylon) added as the outgroup. The GenBank accession numbers of sequences are listed in Table 1. Sequences were aligned with the server version of MAFFT (http://mafft.cbrc.jp/alignment/server/), checked and refined using BioEdit v7.2.6 [24,25].
Table 1.
Isolation and accession numbers of sequences used in the phylogenetic analyses. Type specimens are labeled with HT (holotype) ET (epitype) and PT (paratype). Isolates/sequences in bold were isolated/sequenced in present study. N/A: not available.
| Species | Strain Number | Origin | Status | GenBank Accession Number | References | |||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | TUB2 | |||||
| Annulohypoxylon annulatum | CBS 140775 | Texas | ET | KY610418 | KY610418 | KY624263 | KX376353 | [2,23] |
| Annulohypoxylon michelianum | CBS 119993 | Spain | KX376320 | KY610423 | KY624234 | KX271239 | [2,29] | |
| Annulohypoxylon moriforme | CBS 123579 | Martinique | KX376321 | KY610425 | KY624289 | KX271261 | [2,23] | |
| Annulohypoxylon nitens | MFLUCC 12–0823 | Thailand | KJ934991 | KJ934992 | KJ934994 | KJ934993 | [30] | |
| Annulohypoxylon stygium | MUCL 54601 | French Guiana | KY610409 | KY610475 | KY624292 | KX271263 | [2] | |
| Annulohypoxylon truncatum | CBS 140778 | Texas | ET | KY610419 | KY610419 | KY624277 | KX376352 | [2,23] |
| Biscogniauxia nummularia | MUCL 51395 | France | ET | KY610382 | KY610427 | KY624236 | KX271241 | [2] |
| Daldinia andina | CBS 114736 | Ecuador | HT | AM749918 | KY610430 | KY624239 | KC977259 | [2,29,31] |
| Daldinia bambusicola | CBS 122872 | Thailand | HT | KY610385 | KY610431 | KY624241 | AY951688 | [2,6] |
| Daldinia caldariorum | MUCL 49211 | France | AM749934 | KY610433 | KY624242 | KC977282 | [2,29,31] | |
| Daldinia childiae | CBS 122881 | France | HT | KU683757 | MH874773 | KU684290 | KU684129 | [32,33] |
| Daldinia concentrica | CBS 113277 | Germany | AY616683 | KY610434 | KY624243 | KC977274 | [2,17,29] | |
| Daldinia dennisii | CBS 114741 | Australia | HT | JX658477 | KY610435 | KY624244 | KC977262 | [2,29,34] |
| Daldinia eschscholtzii | MUCL 45435 | Benin | JX658484 | KY610437 | KY624246 | KC977266 | [2,29,34] | |
| Daldinia loculatoides | CBS 113279 | UK | ET | AF176982 | KY610438 | KY624247 | KX271246 | [2] |
| Daldinia macaronesica | CBS 113040 | Spain | PT | KY610398 | KY610477 | KY624294 | KX271266 | [2] |
| Daldinia petriniae | MUCL 49214 | Austria | ET | AM749937 | KY610439 | KY624248 | KC977261 | [2,29,31] |
| Daldinia placentiformis | MUCL 47603 | Mexico | AM749921 | KY610440 | KY624249 | KC977278 | [2,29,31] | |
| Daldinia pyrenaica | MUCL 53969 | France | KY610413 | KY610413 | KY624274 | KY624312 | [2] | |
| Daldinia steglichii | MUCL 43512 | Papua New Guinea | PT | KY610399 | KY610479 | KY624250 | KX271269 | [2] |
| Daldinia theissenii | CBS 113044 | Argentina | PT | KY610388 | KY610441 | KY624251 | KX271247 | [2] |
| Daldinia vernicosa | CBS 119316 | Germany | ET | KY610395 | KY610442 | KY624252 | KC977260 | [2,29] |
| Entonaema liquescens | ATCC 46302 | USA | KY610389 | KY610443 | KY624253 | KX271248 | [2] | |
| Graphostroma platystomum | CBS 270.87 | France | JX658535 | DQ836906 | KY624296 | HG934108 | [2,34,35,36] | |
| Hypomontagnella barbarensis | STMA 14081 | Argentina | HT | MK131720 | MK131718 | MK135891 | MK135893 | [7] |
| Hypomontagnella monticulosa | MUCL 54604 | French Guiana | ET | KY610404 | KY610487 | KY624305 | KX271273 | [2] |
| Hypomontagnella submonticulosa | CBS 115280 | France | KC968923 | KY610457 | KY624226 | KC977267 | [2,29] | |
| Hypoxylon carneum | MUCL 54177 | France | KY610400 | KY610480 | KY624297 | KX271270 | [2] | |
| Hypoxylon cercidicola | CBS 119009 | France | KC968908 | KY610444 | KY624254 | KC977263 | [2,29] | |
| Hypoxylon crocopeplum | CBS 119004 | France | KC968907 | KY610445 | KY624255 | KC977268 | [2,29] | |
| Hypoxylon eurasiaticum | MUCL 57720 | Iran | HT | MW367851 | not obtained | MW373852 | MW373861 | This study |
| Hypoxylon eurasiaticum | MUCL 57721 | Iran | MW367852 | not obtained | MW373853 | MW373862 | This study | |
| Hypoxylon eurasiaticum | MUCL 57722 | Iran | MW367853 | not obtained | MW373854 | MW373863 | This study | |
| Hypoxylon eurasiaticum | MUCL 57723 | Iran | MW367854 | not obtained | MW373855 | MW373864 | This study | |
| Hypoxylon eurasiaticum | DSM 112037 | Poland | MW367855 | not obtained | MW373856 | MW373865 | This study | |
| Hypoxylon fendleri | MUCL 54792 | French Guiana | KF234421 | KY610481 | KY624298 | KF300547 | [2,29] | |
| Hypoxylon fragiforme | MUCL 51264 | Germany | ET | KC477229 | KM186295 | KM186296 | KX271282 | [2,30,37] |
| Hypoxylon fuscum sensu stricto | CBS 113049 | France | ET | KY610401 | KY610482 | KY624299 | KX271271 | [2] |
| Hypoxylon fuscum s. str. | DSM 112039 | Austria | MW367856 | MW367847 | MW373857 | MW373866 | This study | |
| Hypoxylon griseobrunneum | CBS 331.73 | India | HT | KY610402 | KY610483 | KY624300 | KC977303 | [2,29] |
| Hypoxylon guilanense | MUCL 57726 | Iran | HT | MT214997 | MT214992 | MT212235 | MT212239 | [21] |
| Hypoxylon haematostroma | MUCL 53301 | Martinique | ET | KC968911 | KY610484 | KY624301 | KC977291 | [2,29] |
| Hypoxylon howeanum | MUCL 47599 | Germany | AM749928 | KY610448 | KY624258 | KC977277 | [2,29,31] | |
| Hypoxylon hypomiltum | MUCL 51845 | Guadeloupe | KY610403 | KY610449 | KY624302 | KX271249 | [2] | |
| Hypoxylon invadens | MUCL 51475 | France | HT | MT809133 | MT809132 | MT813037 | MT813038 | [38] |
| Hypoxylon investiens | CBS 118183 | Malaysia | KC968925 | KY610450 | KY624259 | KC977270 | [2,29] | |
| Hypoxylon lateripigmentum | MUCL 53304 | Martinique | HT | KC968933 | KY610486 | KY624304 | KC977290 | [2,29] |
| Hypoxylon lenormandii | CBS 119003 | Ecuador | KC968943 | KY610452 | KY624261 | KC977273 | [2,29] | |
| Hypoxylon ochraceum | MUCL 54625 | Martinique | ET | KC968937 | N/A | KY624271 | KC977300 | [2,29] |
| Hypoxylon lienhwacheense | MFLUCC 14-1231 | Thailand | KU604558 | MK287550 | MK287563 | KU159522 | [39,40] | |
| Hypoxylon musceum | MUCL 53765 | Guadeloupe | KC968926 | KY610488 | KY624306 | KC977280 | [2,29] | |
| Hypoxylon olivaceopigmentum | DSM 107924 | USA | HT | MK287530 | MK287542 | MK287555 | MK287568 | [39] |
| Hypoxylon papillatum | ATCC 58729 | USA | HT | KC968919 | KY610454 | KY624223 | KC977258 | [2,29] |
| Hypoxylon perforatum | CBS 115281 | France | KY610391 | KY610455 | KY624224 | KX271250 | [2] | |
| Hypoxylon petriniae | CBS 114746 | France | HT | KY610405 | KY610491 | KY624279 | KX271274 | [2,23] |
| Hypoxylon pilgerianum | STMA 13455 | Martinique | KY610412 | KY610412 | KY624308 | KY624315 | [2] | |
| Hypoxylon porphyreum | CBS 119022 | France | KC968921 | KY610456 | KY624225 | KC977264 | [2,29] | |
| Hypoxylon pseudofuscum | DSM 112038 | Germany | HT | MW367857 | MW367848 | MW373858 | MW373867 | This study |
| Hypoxylon pseudofuscum | DSM 112035 | Germany | MW367858 | MW367849 | MW373859 | MW373868 | This study | |
| Hypoxylon pseudofuscum | DSM 112036 | Germany | MW367859 | MW367850 | MW373860 | MW373869 | This study | |
| Hypoxylon pulicicidum | CBS 122622 | Martinique | HT | JX183075 | KY610492 | KY624280 | JX183072 | [2,41] |
| Hypoxylon rickii | MUCL 53309 | Martinique | ET | KC968932 | KY610416 | KY624281 | KC977288 | [2,29] |
| Hypoxylon rubiginosum | MUCL 52887 | Germany | ET | KC477232 | KY610469 | KY624266 | KY624311 | [2,29] |
| Hypoxylon samuelsii | MUCL 51843 | Guadeloupe | ET | KC968916 | KY610466 | KY624269 | KC977286 | [2,29] |
| Hypoxylon texense | DSM 107933 | USA | HT | MK287536 | MK287548 | MK287561 | MK287574 | [39] |
| Hypoxylon ticinense | CBS 115271 | France | JQ009317 | KY610471 | KY624272 | AY951757 | [2,6] | |
| Hypoxylon trugodes | MUCL 54794 | Sri Lanka | ET | KF234422 | KY610493 | KY624282 | KF300548 | [2,29] |
| Hypoxylon vogesiacum | CBS 115273 | France | KC968920 | KY610417 | KY624283 | KX271275 | [2,23,29] | |
| Jackrogersella cohaerens | CBS 119126 | Germany | KY610396 | KY610497 | KY624270 | KY624314 | [2] | |
| Jackrogersella minutella | CBS 119015 | Portugal | KY610381 | KY610424 | KY624235 | KX271240 | [2,23] | |
| Jackrogersella multiformis | CBS 119016 | Germany | ET | KC477234 | KY610473 | KY624290 | KX271262 | [2,23,29] |
| Pyrenopolyporus hunteri | MUCL 52673 | Ivory Coast | ET | KY610421 | KY610472 | KY624309 | KU159530 | [2,23] |
| Pyrenopolyporus laminosus | MUCL 53305 | Martinique | HT | KC968934 | KY610485 | KY624303 | KC977292 | [2,29] |
| Pyrenopolyporus nicaraguensis | CBS 117739 | Burkina_Faso | AM749922 | KY610489 | KY624307 | KC977272 | [2,29,31] | |
| Rhopalostroma angolense | CBS 126414 | Ivory Coast | KY610420 | KY610459 | KY624228 | KX271277 | [2] | |
| Ruwenzoria pseudoannulata | MUCL 51394 | D. R. Congo | HT | KY610406 | KY610494 | KY624286 | KX271278 | [2] |
| Thamnomyces dendroidea | CBS 123578 | French Guiana | HT | FN428831 | KY610467 | KY624232 | KY624313 | [2,42] |
| Xylaria arbuscula | CBS 126415 | Germany | KY610394 | KY610463 | KY624287 | KX271257 | [2,43] | |
| Xylaria hypoxylon | CBS 122620 | Sweden | ET | KY610407 | KY610495 | KY624231 | KX271279 | [2,44] |
Maximum Parsimony (MP) analyses were performed with PAUP v4.0a165 [26]. All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to MINBRLEN. MP analysis of the combined multilocus matrix was done using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analyses with 1000 replicates were performed in the same way but using 10 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate.
Maximum Likelihood (ML) analyses were performed with RAxML [27] as implemented in raxmlGUI 1.3 [28], using the ML + rapid bootstrap setting and the GTRGAMMA substitution model with 1000 bootstrap replicates. The matrix was partitioned for the different gene regions. Bootstrap values ≤70% are considered low, between 70 and 90% intermediate and ≥90% high in the discussion of the data that follows further below.
2.5. HPLC Profiling
Stromata from Hypoxylon specimens were extracted using acetone as described previously [23] and subsequently analysed by high-performance liquid chromatography coupled to a diode array and electrospray mass spectrometric detection system (HPLC/DAD-ESIMS) with instrument settings as described recently [21]. Resulting UV/Vis and mass spectrometric data were compared with internal databases, comprising standards of known Hypoxylaceae and literature data [10,11,12].
2.6. Extraction and Isolation of Compounds 4, 5, and 6
For a chemical study, 337 mg of stromata derived from the specimen 987 GUM was extracted five times with acetone as stated previously by sonication and subsequent filtration [23] with a yield of 85.8 mg extract. The crude extract was subjected to a Strata X-33 µm reversed-phase (RP) column to remove fatty acids and debris. The constituents of the pre-cleaned extract (47.6 mg) were further separated using a C18-Gemini 10 µm; 250 × 21 mm column in a PLC 2250 preparative HPLC system (Gilson, Middleton, WI, USA) with gradient settings as follows: isocratic conditions (equilibration) for 6.25 min, 60% H2O, 40% acetonitrile (ACN), 10 mL/min; gradient from 40% ACN to 55%, 45 min, 20 mL/min; gradient from 55% ACN to 100% ACN for 5 min, 20 mL/min; isocratic conditions for 10 min at 100% ACN, 20 mL/min. Fractions were collected for every 10 mL of solvent volume. Fractions at Retention time (RT) = 19−20.5 (I) and 35−37.5 min (II) were collected and dried in vacuo.
In a second attempt, 209 mg of stromata were extracted as stated previously to give rise to 64 mg of crude extract from strain 987 GUM and pre-cleaned using a Strata-X 33 µm column to remove fatty acids, debris and to achieve a pre-fractionation by using different solvent mixtures. A solvent mix of 80% H2O and 20% ACN resulted in a 23.87 mg fraction (III). Stromatal extracts of specimens 217H and 303H (134 mg and 199 mg dry mass, resulting in 39 mg and 27 mg crude extract in total) were pooled and fractionated following a similar strategy, however, with a solvent mixture of 59.5% H2O, 39.5% ACN with 1% formic acid (FA), resulting in a 48.7 mg fraction (IV).
Fraction I was purified by NP-TLC (pre-coated TLC plates Silgur-25, Macherey-Nagel, Düren, Germany) with 100 mL mobile phase comprised of 80% dichloromethane (DCM) and 20% acetone as solvent. TLC material was scraped and adsorbed compound eluted by acetone + 0.1% formic acid, which resulted in 0.3 mg of pure compound (4).
Fraction II was purified by NP-HPLC (Gilson, Middleton, WI, USA; GX-271 liquid handler, two pumps; 305 and 306, DAD wavelengths set to 210, 254 and 391 nm) with the mobile phase comprised of 50% isopropanol (A) and 50% of a solvent mixture containing 60% EtAc, 20% benzene, 20% n-heptane and 1% formic acid (B) to 100% B in 50 min. Fractions from RT = 9.3−10.7 min were combined to give rise to 2.1 mg of compound 5. An Orbit 100 5µm diol column (MZ-Analysentechnik GmbH, Mainz, Germany) was used as stationary phase.
Fraction III was purified by NP-TLC with 100 mL mobile phase comprised of 40% EtAc, 30% benzene, 30% n-heptane and 1% FA and subsequent elution similar to fraction I, which lead to 0.46 mg of pure compound (5).
Fraction IV was purified by NP-TLC with 100 mL mobile phase comprised of 4% EtAc, 30% benzene, 30% n-heptane and 1% FA and subsequent elution similar to fraction I, which lead to 3.27 mg of pure compound (6).
2.7. Spectral Data
2.7.1. Pseudofuscochalasin A (4) Figures S1–S6
Colorless oil. [α]25D = +166 (c 0.3, AcN); 1H NMR (700 MHz, CHCl3-d): δ ppm 7.33 (t, J = 7.4 Hz, 3′–H/5′–H), 7.25 (d, J = 7.4 Hz, 4′–H), 7.17 (d, J = 7.4 Hz, 2′–H/6′–H), 6.88 (d, J = 15.5 Hz, 20–H), 6.45 (d, J = 15.5 Hz, 19–H), 5.93 (ddd, J = 15.6, 10.2, 0.9 Hz, 13–H), 5.61 (br s, 2–NH), 5.20 (ddd, J = 15.6, 10.9, 5.0 Hz, 14–H), 4.10 (br s, 7–OH), 4.05 (d, J = 9.8 Hz, 7–H), 3.55 (br s, 4–H), 3.41 (td, J = 7.6, 1.3 Hz, 3–Ha), 2.73 (dqd, J = 10.9, 6.8, 1.4 Hz, 16–H), 2.603 (m, 10–H2), 2.598 (m, 15–Ha), 2.082 (m, 8–H), 2.078 (m, 15–Hb), 1.67 (br s, 12–H3), 1.64 (s, 23–H3), 1.44 (s, 11–H3), 1.24 (d, J = 6.8 Hz, 22–H3), 13C NMR (175 MHz, CHCl3-d): δ ppm 209.7 (C, C–17), 197.6 (C, C–21), 173.6 (C, C–1), 145.0 (CH, C–19), 137.1 (C, C–1′), 135.0 (CH, C–14), 133.6 (CH, C–20), 131.8 (C, C–6), 130.2 (CH, C–13), 129.2 (2 × CH, C–2′/C–6′), 128.8 (2 × CH, C–3′/C–5′), 127.0 (CH, C–4′), 126.2 (C, C–5), 78.8 (C, C–18), 69.3 (CH, C–7), 63.2 (C, C–9), 59.0 (CH, C–3), 53.6 (CH, C–8), 47.3 (CH, C–4), 43.0 (CH, C–16), 42.9 (CH2, C–10), 38.3 (CH2, C–15), 23.5 (CH3, C–23), 19.8 (CH3, C–22), 17.2 (CH3, C–11), 14.1 (CH3, C–12), ESIMS m/z 464.29 ([M + H]+, 462.34 ([M − H]-, HRESIMS m/z 464.2430 ([M + H]+, calcd for C28H34NO5 464.2431), 486.2250 ([M + Na]+, calcd for C28H33NO5Na 486.2251).
2.7.2. Daldinin F (5) Figures S7–S11
1H NMR (500 MHz, acetone-d6) δ ppm 7.73 (d, J = 1.4 Hz, 1–H), 7.30 (d, J = 15.6 Hz, 3′′–H), 6.15 (d, J = 1.4 Hz, 5–H), 6.09 (br q, J = 7.0 Hz, 5′′–H), 5.86 (s, 4–H), 5.77 (d, J = 15.6 Hz, 2′′–H), 4.57 (d, J = 6.0 Hz, 3′–OH), 4.01 (m, 3′–H), 3.92 (dqd, J = 11.5, 6.2, 2.4 Hz, 6′–H), 2.15 (m, 4–Ha), 2.05 (m, 2–H3), 1.88 (m, 4–Hb), 1.812 (m, 5′–Ha), 1.811 (d, J = 7.0 Hz, 6′’–H3), 1.77 (br s, 7′′–H3), 1.52 (m, 5′–Hb), 1.45 (s, 7-Me), 1.10 (d, J = 6.2 Hz, 7′–H), 13C NMR (125 MHz, acetone-d6): δ ppm 194.5 (C, C–6), 192.5 (C, C–8), 169.8 (C, C–1′), 166.2 (C, C–1′’), 155.8 (CH, C–1), 151.8 (CH, C–3′′), 144.0 (C, C–4a), 138.7 (CH, C–5′’), 134.7 (C, C–4′’), 122.8 (CH, C–5), 115.0 (CH, C–2′’), 111.7 (C, C–8a), 103.9 (C, C–3), 86.0 (C, C–7), 69.8 (CH, C–6′), 67.2 (CH, C–4), 62.8 (CH, C–3′), 26.9 (CH2, C–4′), 26.6 (CH2, C–5′), 22.8 (CH3, 7Me), 21.7 (CH3, C–7′), 20.0 (CH3, C–2′), 14.7 (CH3, C–6′′), 11.8 (CH3, C–7′’); HRESIMS m/z 461.1806 ([M + H]+, calcd for C24H29O9 461.1806), 483.1625 ([M + Na]+, calcd for C24H28O9Na 483.1625).
2.7.3. 3′-Malonyl-daldinin F (6) Figures S12–S17
Yellow oil. [α]25D = +18.0 (c 1.0, AcN). 1H NMR (700 MHz, acetone-d6) δ ppm 7.76 (d, J = 1.4 Hz, 1–H), 7.31 (d, J = 15.6 Hz, 3′′–H), 6.13 (d, J = 1.4 Hz, 5–H), 6.09 (br q, J = 7.1 Hz, 5′′–H), 5.84 (br s, 4–H), 5.73 (d, J = 15.6 Hz, 2′′–H), 5.11 (t, J = 2.8 Hz, 3′–H), 4.03 (dqd, J = 10.8, 6.2, 2.6 Hz, 6′–H), 3.48 (d, J = 15.9 Hz, 3′–′′–Ha), 3.37 (d, J = 15.9 Hz, 3′–′′–Hb), 2.19 (m, 4–Ha), 2.05 (m, 2–H3), 2.02 (m, 4–Hb), 1.81 (d, J = 7.0 Hz, 6′′–H3), 1.76 (br s, 7′′–H3), 1.68 (m, 5′–Ha), 1.64 (m, 5′–Hb), 1.46 (s, 7-Me), 1.14 (d, J = 6.2 Hz, 7′–H), 13C NMR (125 MHz, acetone-d6): δ ppm 194.3 (C, C–6), 192.2 (C, C–8), 169.9 (C, C–1′), 167.9 (C, C–3′′′), 166.2 (C, C–1′′′), 166.1 (C, C–1′′), 155.1 (CH, C–1), 152.5 (CH, C–3′′), 143.0 (C, C–4a), 139.1 (CH, C–5′′), 134.7 (C, C–4′′), 123.0 (CH, C–5), 114.2 (CH, C–2′′), 111.8 (C, C–8a), 102.0 (C, C–3), 86.0 (C, C–7), 70.1 (CH, C–6′), 66.6 (CH, C–4), 65.7 (CH, C–3′), 41.8 (CH2, C–2′′′), 26.9 (CH2, C–5′), 24.0 (CH2, C–4′), 22.6 (CH3, 7Me), 21.5 (CH3, C–7′), 20.0 (CH3, C–2′), 14.7 (CH3, C–6′′), 11.8 (CH3, C–7′′); HRESIMS m/z 547.1811 ([M + H]+, calcd for C27H31O12 547.1810), 569.1629 ([M + Na]+, calcd for C27H30O12Na 569.1629).
2.8. Antimicrobial Activities and Cytotoxicity
Evaluated substances were solved in a concentration of 1 mg/mL in methanol and tested against a panel of microorganisms (Acinetobacter baumanii, Escherichia coli, Bacillus subtilis, Mycobacterium smegmatis, Staphylococcus aureus, Pseudomonas aeruginosa, Chromobacter violaceum) and fungi (Schizosaccharomyces pombe, Pichia anomala, Candida albicans, Mucor hiemalis) in a serial dilution assay [45]. Cytotoxicity was evaluated via a MTT-assay against two cell lines (L929 mouse fibroblasts, KB 3.1 endocervical carcinoma) as described previously [46].
2.9. Actin Disruption Assay
The actin disrupting potential of the newly described cytochalasin structurally related to cytochalasin C was investigated in the adherent mammalian osteosarcoma (U2OS, ATCC HTB-96) cell line by fluorescence microscopy as described [47]. Briefly, for the assay exponentially growing cells were seeded on fibronectin coated cover slips and allowed to spread overnight. On the next day, cells were treated with 5 and 1 µg/mL of the new compound dissolved in full medium for one hour. To probe reversibility, one set of treated cells was washed and incubated in fresh medium lacking compound for one hour prior to fixation. DMSO served as a negative control. Cells were washed and fixed with 4% para-formaldehyde for 20 min. Fixed cells were permeabilized using 0.1% Triton X-100 (Hercules, CA, USA) at room temperature for 1 min. Cytochalasin C is commercially available (Cayman chemical, MI, USA) and was used as a standard to for comparison with the novel cytochalasin due to its related structure. The actin cytoskeleton was stained with fluorescently-labelled phalloidin (ATTO-594, ATTO-Tec, Siegen, Germany) and the nucleus was stained with DAPI, contained in the mounting medium Pro-long Diamond Antifade (Invitrogen, Carlsbad, CA, USA). Pictures were recorded using an inverted microscope (Axio Vert 135 TV, Zeiss, Jena, Germany) equipped with a Coolsnap 4k camera (Photometrics, Tuscon, AZ, USA) operated by Metamorph software package (molecularDevices, San Jose, CA, USA) and processed by Image J (NIH, Bethesda, MD, USA).
3. Results
3.1. Molecular Phylogeny
Of the 4256 nucleotide characters of the combined matrix, 1637 are parsimony informative (310 of ITS, 162 of LSU, 490 of rpb2 and 675 of tub2). Figure 1 shows a phylogram of the best ML tree (lnL = −72,650.213864) obtained by RAxML. Maximum parsimony analyses revealed eight MP tree comprising 16,256 steps (data not shown). All major groups and deeper, highly supported nodes were consistent between the ML and MP analyses, but topologies of deeper unsupported nodes differed in the MP tree; as these differences are not relevant within the context of our new species, they are not further considered here. The phylogenies reveal a paraphyly of Hypoxylon, with the genera Annulohypoxylon, Daldinia, Entonaema, Jackrogersella, Hypomontagnella, Pyrenopolyporus, Rhopalostroma, Ruwenzoria, and Thamnomyces embedded within the former.
Figure 1.
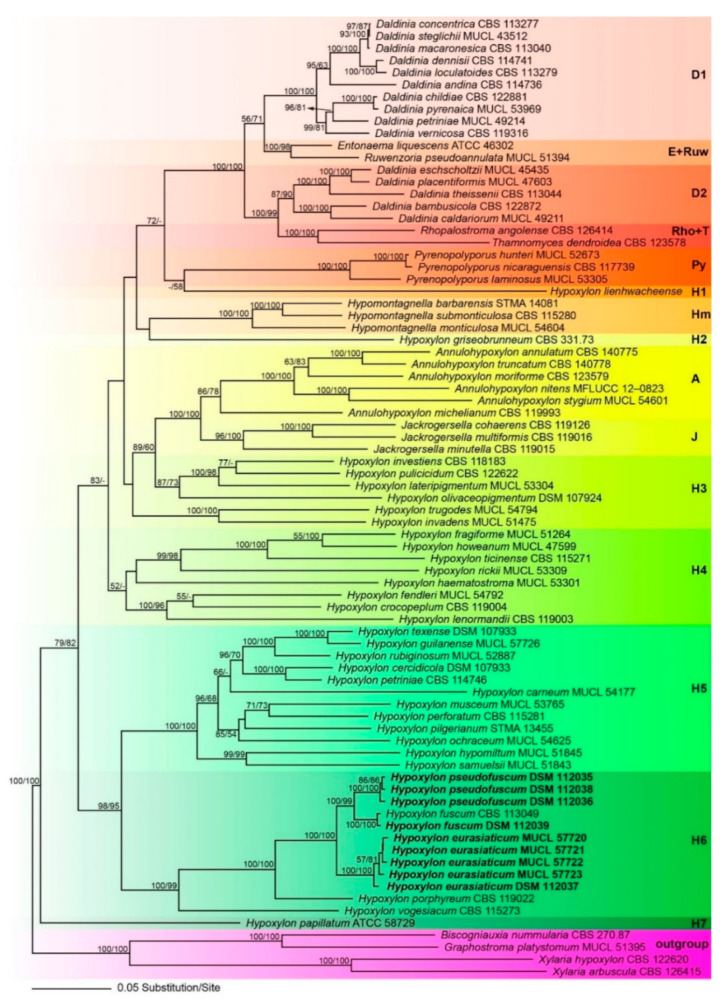
Phylogram of the best ML trees (lnL = −72650.213864) revealed by RAxML from an analysis of the combined ITS–LSU–rpb2–tub2 matrix of selected Xylariales. Strains in bold were sequenced in the current study. ML and MP bootstrap support above 50% are given at the first and second positions, respectively, above or below the branches. Different background colours have been applied to highlight the major clades.
All of the latter genera appeared monophyletic except for Daldinia (Figure 1). All of our new species described below are contained within the highly supported Hypoxylon clade H6. The new species (Hypoxylon eurasiaticum) is the sister group of H. pseudofuscum and H. fuscum sensu stricto (Figure 1). Sequences of H. pseudofuscum clustered together with Hypoxylon fuscum with 100% BS support. The sequences of the collection of Hypoxylon fuscum (DSM 112039) are almost identical to those of the ex-epitype culture and they clustered together with maximum support. The remaining clades are in accordance with previous results [2,7].
3.2. Taxonomic Part
Hypoxylon eurasiaticum Pourmoghaddam, Krisai-Greilhuber & Khodap., sp. nov.
MycoBank No: 838247, Figure 2 and Figure 3
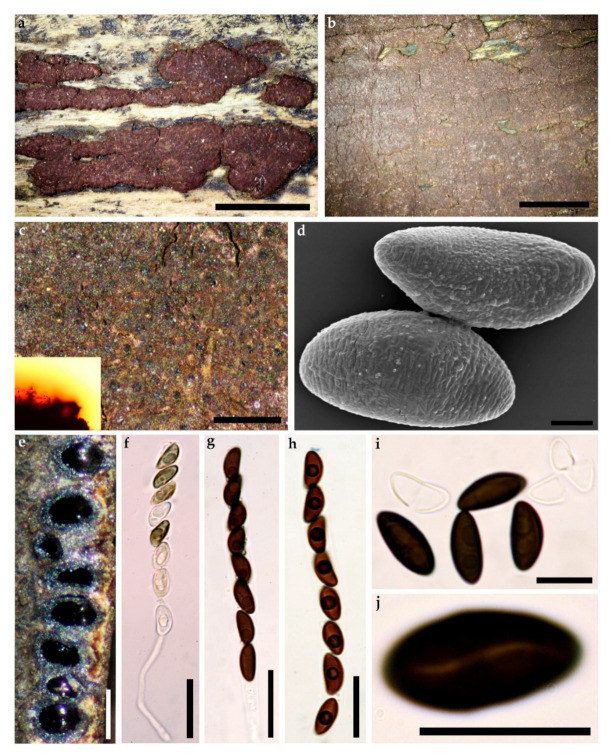
Figure 2.
Hypoxylon eurasiaticum (Holotype GUM 1597). (a) stromatal habit; (b,c) close-up view of stromatal surface, with stromatal pigments in 10% KOH; (d) ascospore under SEM; (e) stroma in section showing perithecia and ostioles; (f) immature ascus in water; (g) mature ascus in water; (h) ascus in Melzer’s reagent; (i) ascospores in 10% KOH with dehiscent perispore; (j) ascospore in water, with sigmoid germ-slit. Scales bars set as (a,b) 5 mm; (c) 0.5 mm; (d) 2 µm; (e) 0.5 mm; (f–h) 20 µm; (i,j) 10 µm.
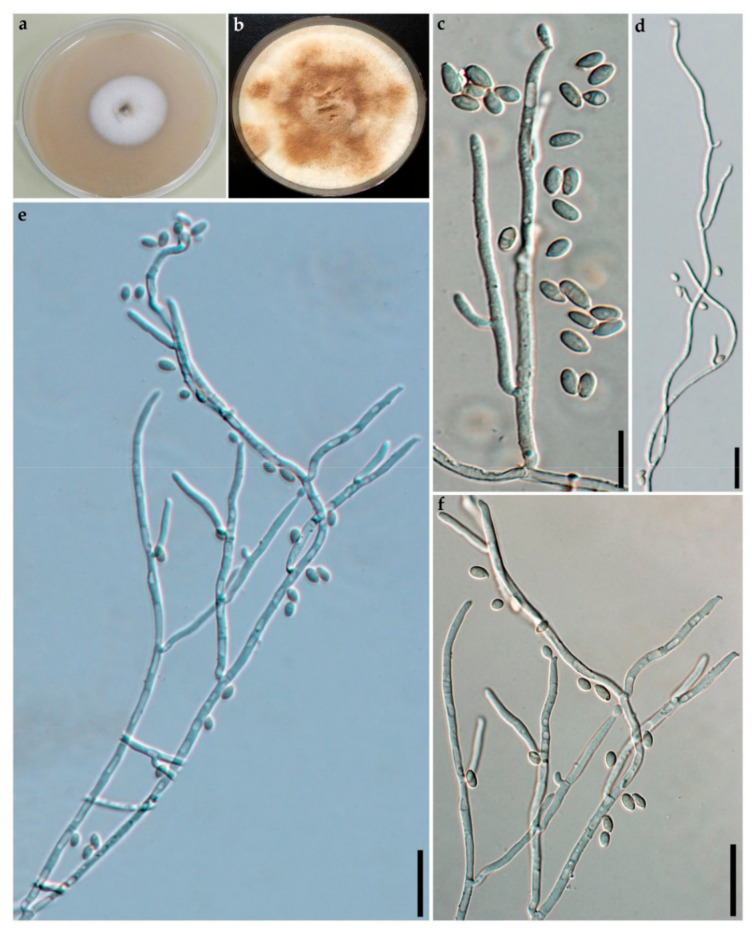
Figure 3.
Culture and anamorphic structures of Hypoxylon eurasiaticum (GUM 1597) on OA. (a,b) surface of colony after 1 and 8 weeks of incubation (respectively left to right); (c–f) general view of anamorph structure with virgariella-like branching patterns, conidiogenous cells, immature and mature conidia. Scales bars set as (c–f) 20 µm.
Etymology. Eurasiaticum, for its occurrence in both, Europe and Asia.
Holotype (designated here) Iran, Guilan Province, Shaft County, Babarekab forest, 37°00′26.88′′ N, 49°20′22.95′′ E, 289 m elev., on fallen branch of Quercus castaneifolia, 15 September 2016, leg. M.J. Pourmoghaddam (GUM 1597; ex-holotype culture preserved in metabolically inactive state, MUCL 57720).
Teleomorph. Stromata superficial, hemispherical, pulvinate to effused-pulvinate, up to 12 cm long × 0.2–2 cm wide, with inconspicuous to slightly conspicuous perithecial mounds, surface Vinaceaous (57) or Dark Vinaceaous (82), Brown Vinaceous (84); dull orange to orange-brown granules beneath the surface and dark dull granules between the perithecia, with Amber (47) to Honey (64), Isabelline (65), Olivaceous (48) or Hazel (88) KOH-extractable pigments. Perithecia obovoid to spherical, 0.15–0.4 mm high × 0.1 mm–0.3 mm wide. Ostioles umbilicate, inconspicuous. Asci with amyloid, discoid apical apparatus, 0.5–1.5 µm high × 2.5–3.5 µm wide, stipe up to 60 µm, and spore-bearing portion 70–90 × 7–10 µm. Ascospores smooth, unicellular, brown to dark brown, ellipsoid, inequilateral with narrowly rounded ends, 9–12.5 × 4–6 µm, with more sigmoid to less straight germ slit spore-length on convex side; perispore dehiscent in 10% KOH, conspicuous coil-like ornamentation in SEM; epispore smooth.
Cultures and anamorph. Colonies on OA covering a 9 cm Petri dish in 2 w, at first white, becoming Straw (46) from outwards, cottony; finally, attaining Umber (9) or Ochraceous (44). Conidiogenous structure branching virgariella-like as defined by Ju and Rogers [4], (Figure 3c–f). Conidiophores hyaline, smooth to finely roughened. Conidiogenous cells hyaline, smooth to finely roughened, 15–23 × 2–3 µm. Conidia hyaline, smooth to ellipsoid, 4–6 × 2–4 µm.
Secondary metabolites. BNT, daldinin F, 3′malonyl-daldinin F, unknown compounds related to naphthalene secondary metabolite family.
Other specimens examined. Iran, Guilan Province, Langaroud County, Liseroud forest, 37°7′44′′ N, 50°8′41′′ E, 28 m elev., on fallen branch of Quercus castaneifolia, 10 September 2017, leg. M.J. Pourmoghaddam (GUM 1598; culture MUCL 57721); Guilan Province, Siahkal County, Lonak Waterfall, 37°00′31.12′′ N, 49°51′52.69′′ E, 500 m elev., on fallen branch of Quercus castaneifolia, 10 Nov 2016, leg. M.J. Pourmoghaddam (GUM 1600; culture MUCL 57722); Guilan Province, Fouman County, Masouleh forest, 37°09′23.83′′ N, 48°59′58.12′′ E, 863 m elev., on fallen branch of Quercus castaneifolia, 23 July 2015, leg. M.J. Pourmoghaddam (GUM 988; culture MUCL 57723), Poland, Podlaskie Voivodeship, Białowieża National Park, on branch of cf. Betula, 20 September 2019, leg. C. Lambert (KR-M-0005886, culture DSM 112039).
Notes. This taxon differs in having smaller ascospores than the two other taxa in the Hypoxylon fuscum complex (see phylogenetic analyses (Figure 1). In addition, morphological and chemotaxonomic data (see Table 2 and Table 3) supported our hypothesis. The lack of malonyl-daldinin F in Hypoxylon fuscum provided an additional argument to regard Hypoxylon eurasiaticum as a separate taxon in this complicated species complex. The apparent host preference for Quercus castaneifolia in Iran is also striking, since the other records of similar Hypoxylon species from Quercus (H. porphyreum, as well as the type specimens of H. subchlorinum and H. commutatum subsp. holwayanum, whose status is still unclear) previously studied [10] showed yet different morphological characters and also deviating HPLC profiles. However, the material from Poland definitely was not collected from Quercus, hence further studies on the host range of the new taxon should be carried out in the future based on these data.
Table 2.
Diagnostic characters of the Hypoxylon fuscum complex.
| Taxon | Designation No/(Status) | Ascospores (µm) | Mean (µm) | Host | Known Distribution | KOH-Extractable Pigments | Secondary Metabolites |
|---|---|---|---|---|---|---|---|
| Hypoxylon fuscum sensu stricto | Ww3723/Epitype | 12.5–15.5 × 5–7 | 13.2 × 5.8 | Corylus avellana | Europe | Amber (47) to Honey (64) | 1,5 |
| Hypoxylon fuscum sensu stricto | WU 43621 | 13–15.8× 4.8–6 | 14.4 × 5.4 | Corylus avellana | Austria | Amber (47) to Honey (64) | 1,5 |
| Hypoxylon eurasiaticum | GUM 1597 (H) | 10–12.5 × 4.5–6 | 11.25 × 5.25 | Quercus castaneifolia | Iran | Amber (47) to Orange (7) | 1,5,6 |
| Hypoxylon eurasiaticum | KR-M-0005886 | 9–12 × 3.8–5 | 10.5 × 4.4 | cf. Betula | Poland | Isabelline (65), Olivaceous (47) or Hazel (88) | 1,5,6 |
| Hypoxylon pseudofuscum | KR-M-0005879 (H) | 12–16 × 4.8–7.3 | 14 × 6 | Alnus glutinosa | Germany | Isabelline (65), or Hazel (88) | 1,4,5,6 |
| Hypoxylon pseudofuscum | GUM 987 | 11–15 × 4.5–6.5 | 13 × 5.5 | Alnus sp. | Iran | Amber (47) to Oramge (7) | 1,4,5,6 |
| Hypoxylon pseudofuscum | KR-M-0005877/ KR-M-0005876 |
11–15 × 5.5–6.5 | 13 × 6 | Salix sp. | Germany | Isabelline (65), Olivaceous (47) or Hazel (88) | 1,4,5,6 |
Table 3.
Summary of chemotaxonomic results on selected specimens. 1: BNT; 4: Pseudofuscochalasin A; 5: Daldinin F 6: 3′malonyl-Daldinin F;. UC: Unidentified compound.
| Species | Specimen | Plant Host | Origin | 1 | 4 | 5 | 6 | UC 1 | UC 2 | UC 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxylon fuscum sensu stricto | WU 43621 | Corylus avellana | Austria | + | − | + | − | − | + | + |
| Hypoxylon eurasiaticum | GUM 1597 (H) | Quercus castaneifolia | Iran | + | − | + | + | − | + | + |
| Hypoxylon eurasiaticum | GUM 1598 | Quercus castaneifolia | Iran | + | − | + | + | − | + | + |
| Hypoxylon eurasiaticum | GUM 1600 | Quercus castaneifolia | Iran | + | − | + | + | − | + | + |
| Hypoxylon eurasiaticum | GUM 988 | Quercus castaneifolia | Iran | + | − | + | + | − | + | + |
| Hypoxylon eurasiaticum | KR-M-0005886 | cf. Betula | Poland | + | − | + | + | − | + | + |
| Hypoxylon pseudofuscum | KR-M-0005879 (H) | Alnus glutinosa | Germany | + | + | + | + | + | + | + |
| Hypoxylon pseudofuscum | GUM 987 | Alnus sp. | Iran | + | + | + | + | + | + | + |
| Hypoxylon pseudofuscum | KR-M-0005877 | Salix sp. | Germany | + | + | + | + | + | + | + |
| Hypoxylon pseudofuscum | KR-M-0005876 | Salix sp. | Germany | + | + | + | + | + | + | + |
Hypoxylon pseudofuscum Pourmoghaddam, Khodap. & Krisai-Greilhuber, sp. nov.
MycoBank No: 838248, Figure 4
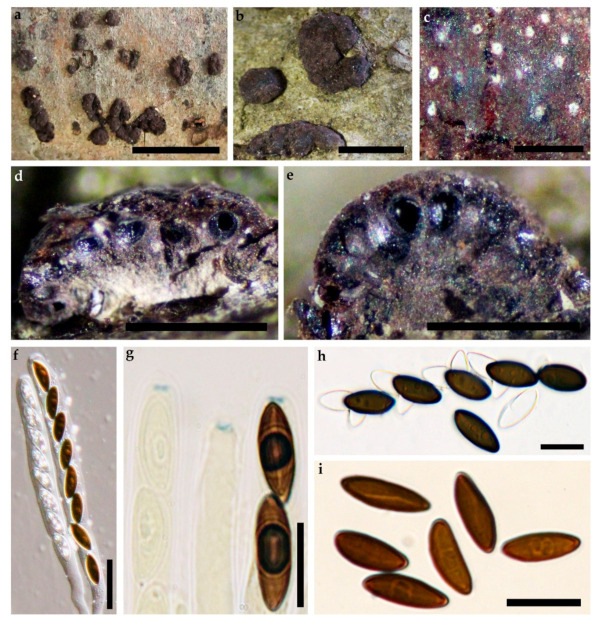
Figure 4.
Hypoxylon pseudofuscum (Holotype). (a,b) stromatal habit; (c) close-up view of stromatal surface; (d,e) stroma in section showing perithecia and ostioles; (f) mature and immature ascus in water; (g) mature and immature asci tips in Melzer’s reagent; (h) ascospores in 10% KOH with dehiscent perispore; (i) ascospores in water, with sigmoid germ-slit. Scales bars set as (a) 1 cm; (b) 2.5 mm; (c–e) 1mm; (f) 20 µm; (g–i) 10 µm.
Etymology. Pseudofuscum, referring to the morphological similarity to H. fuscum.
Holotype (designated here) Germany, Rhineland-Palatinate, Bad Dürkheim, Lake “Isenachweiher”, on fallen branch of Alnus glutinosa, 7 June 2020, leg. Barbara and M. Stadler (STMA 18264, KR-M-0005879), ex-type culture deposited in metabolically inactive state in DSM 112038.
Teleomorph. Stromata superficial, pulvinate to effused-pulvinate, up to 6 cm long × 1–3 cm wide, with inconspicuous to slightly conspicuous perithecial mounds, surface Rust (39), Brick (59), Vinaceaous (57) or Dark Vinaceaous (82), Brown Vinaceous (84); dull orange to orange-brown granules beneath the surface and dark dull granules between the perithecia, with Amber (47), Isabelline (65), Olivaceous (48) or Hazel (88) KOH-extractable pigments. Perithecia spherical to obovoid, 0.19–0.36 high × 0.12–0.28 mm wide. Ostioles umbilicate, inconspicuous. Asci with amyloid, discoid apical apparatus, 0.5–1.5 µm high× 2–3.5 µm wide, stipe up to 55 µm, and spore-bearing portion 65–85 × 6–10 µm. Ascospores smooth, unicellular, brown to dark brown, ellipsoid, inequilateral with narrowly rounded ends, 11–16 × 4.5–7.3 µm, with sigmoid to less frequently straight germ slit spore-length on convex side; perispore dehiscent in 10% KOH; epispore smooth.
Cultures. Colonies on OA covering a 9 cm Petri dish in 2 weeks, at first white, cottony, becoming Pale Luteous (46) from outwards with concentric zones; finally, attaining Amber (47). Anamorph not observed.
Secondary metabolites. BNT, daldinin F, 3′malonyl daldinin F, pseudofuscochalasin A, one unknown compound with a conspicuous UV and two un-known compounds related to the naphthalene secondary metabolite family.
Specimens examined. Germany, Lower Saxony, Allerbüttel near Ilkerbruch, on Salix sp., 17 June 2020, leg. H. Andersson (KR-M-0005876; culture DSM 12036); Germany, Lower Saxony, Braunschweig, Viehmoor near Leiferde, on Salix sp., 17 June 2020, leg. H. Andersson (KR-M-0005877, culture DSM 112035 ); France, Pyrénées Atlantiques, Auterrive, Ile du Gave d’Oloron, on wood of Alnus glutinosa, 30 May 2004, leg. J. Fournier and M. Stadler (STMA 04048); Iran, Guilan Province, Talesh County, Gisoom forest, 37°39′41′′ N, 49°00′31′′ E, 11 m elev., on fallen branch of Alnus sp., 2 September 2015, leg. S. Raei (GUM 987).
Notes. This taxon is phylogenetically close to Hypoxylon fuscum (Figure 1). The phylogenetic analyses have good support to distinguish these two sister group. In addition, we found BNT, 3′-malonyl-daldinin F, and the new pseudofuscochalasin A as stromatal metabolites. It also differs from Hypoxylon eurasiaticum in having larger ascospores (see Table 2).
3.3. HPLC Profiling
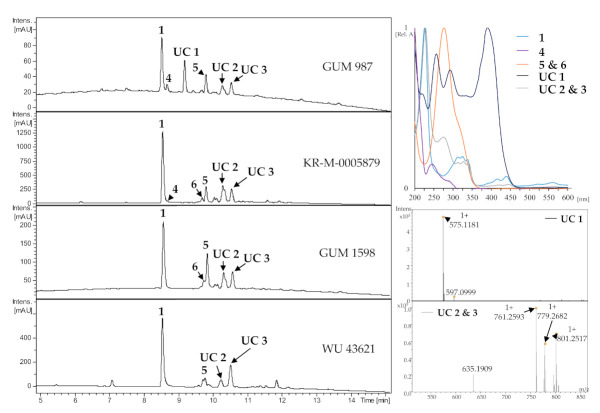
Among the studied specimens of the H. fuscum complex, five were derived from Iran (GUM 987, GUM 988, GUM 1597, GUM 1598, GUM 1600), one from Poland KR-M-0005886), three from Germany (KR-M-0005877, KR-M-0005876 and KR-M-0005879), and one from Austria (WU 43621). Host substrate, origin and identified compounds by comparison with an internal database are shown in Table 3 and briefly summarized further below.
The major constituents were identified as compounds 1 and 5 together with two unidentified compounds (UC 2 and UC 3, see supporting information for HRMS data) which share the typical crown-shape UV/vis pattern with BNT (1), pointing towards structural features shared with the naphthalene secondary metabolite family. Specimen growing on Alnus sp. were found to contain a member of the cytochalasin family and another unidentified compound with a conspicuous UV absorption pattern (UC 1). Compound UC 1 was only present in the specimens not derived from Corylus. These results corroborated the molecular phylogenetic and morphological data that we concurrently obtained.
3.4. Structure Elucidation (Figure 5 and Figure 6)
Figure 5.
Representative HPLC chromatograms (UV, 210 nm) of stromatal acetone extracts from H. fuscum (WU 43621), H. eurasiaticum (GUM 1598) and H. pseudofuscum (GUM 987 and KR-M-0005879/holotype). UV-Vis chromatograms from 200 to 600 nm and HRESIMS data of unknown compounds of designated peaks are given together with their normalized absorption. UC: Unknown compounds.
Figure 6.
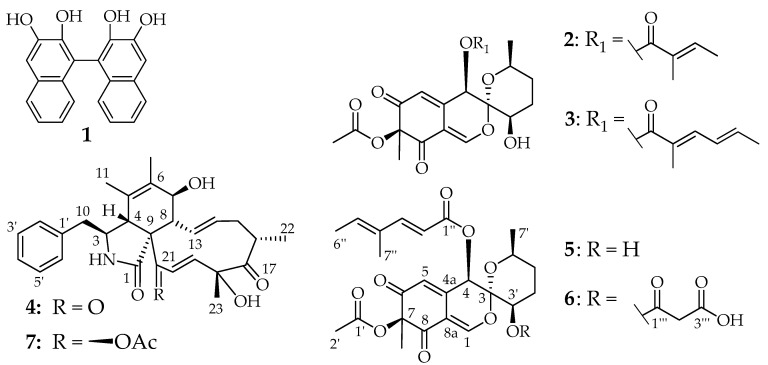
Chemical structures of secondary metabolites of the Hypoxylon fuscum complex: BNT (1), daldinin C (2), daldinin E (3), pseudofuscochalasin A (4), daldinin F (5), 3′-malonyl-daldinin F (6) and cytochalasin C (7).
The molecular formula of 4 was indicated as C28H33NO5 based on its [M + H]+ and [M + Na]+ peaks at m/z 464.2430 and 486.2250 in the HRESIMS spectrum, respectively, implying 13 degrees of unsaturation. 1H and HSQC NMR spectra revealed the presence of four methyls, two methylenes, and seven olefinic (two with dual intensity) as well as five aliphatic methines. In addition, the 13C spectrum specified two ketones, a carboxylic carbon, and five further carbons devoid of bound protons. HMBC correlations connected the 1H,1H COSY and TOCSY spin systems to a cytochalasin skeleton. The closest known structural relative of 4 is cytochalasin C (7), which formally constitutes its 21-deacetyl-didehydro derivative.
Compound 4 shares the stereochemistry with 7, which was confirmed by ROESY data. ROESY correlations between 13–H and 20–H on the α face as well as between 14–H and 19–H on the β face of the molecule supported the characteristic conformation described for the eleven-membered ring system, whereas the common 3S,4R,7S configuration can be assumed by analogous chemical shifts for the core structure as well as the biogenesis from l-Phe [48]. ROESY correlations between 23–H3 and 16–H as well as between 23–H3 and 19–H, located above the molecular main plain, endorse the upwards orientation of 23–H3 and thus an 18R configuration. We propose the trivial name pseudofuscochalasin for compound 4, whose systematic IUPAC name is (7S,13E,16S,18R,19E)-16,18-dimethyl-7,18-dihydroxy-10-phenyl [11]cytochalasa-5,13,19-triene 1,17,21-trione [13].
The molecular formulae of 5 and 6 were determined as C24H28O9 and C27H30O12 by their molecular ion clusters at m/z 461.1806 and 547.1811 in their HRESIMS spectra. The main metabolite 5 was identified as daldinin F by its 1H and 13C NMR data. Its derivative 6, which differed from 5 by the formal addition of a C3H2O3 fragment, exhibited very similar NMR data. Key difference in 1H and HSQC spectra were the presence of an additional methylene group and the low-field shift of oxymethine CH2–3′. HMBC correlations from 2′′′–H2 to C–1′’’ and C–3′′′ identified a malonyl moiety, which was connected to C–3′ due to the HMBC correlation of 3′–H to C–1′′′. Consequently, 6 was established as 3′-malonyl-daldinin F. The positive optical rotation of both 5 and 6 indicated a common 7S absolute configuration for the daldinins C–F [12,49,50].
3.5. Antibacterial and Cytotoxic Activities
The biological activities compounds 5 and 6 against microorganisms and mammalian cells were evaluated against a panel of bacteria (MIC) and cell lines (L929, fibroblasts; KB 3.1, endocervical adenocarcinoma cells). Neither compound showed activity in the selected concentration range, except for weak cytotoxicity of 6. (IC50 of 35 µg/mL against L929 murine fibroblasts and 17 µg/mL against Hela KB 3.1 cervix carcinoma cells), respectively.
3.6. Actin Disruption Assay (Figure 7)
Figure 7.

Overlay images of U2OS cells treated with compounds 4 ((a): 1 µg/mL; (b): 5 µg/mL; (g): 5 µg/mL, wash-out), 7 ((c): 1 µg/mL; (d): 5 µg/mL; (h): 5 µg/mL, wash-out) or vehicle control (DMSO, (e): 5 µl/mL; (f): wash-out control), PFA-fixed and stained for their actin cytoskeleton (phalloidin-ATTO594, red) and nuclei (DAPI, blue). Knot-like structures and aggregates of f-actin in treated cells demonstrate the disruptive effect of compounds 4 and 7 in comparison to the intact actin cytoskeleton depicted in the vehicle control (e). Note that the effect of 4 is not reversible, as a wash-out (f) with a recovery time of one hour did not lead to a regeneration of the f-actin network (compare (b,g)), while the effect conveyed by compound 7 was fully revertible (compare (d) and (h)). Scales bars set as 20 µm.
The new cytochalasin and its acetylated derivative were tested for its actin disrupting potential in a recently described actin disruption assay and a functional wash-out experiment with a one-hour of recovery time. With both, high and low concentrations, the typical consequences of an intoxication with cytochalasans arise as visible by f-actin aggregated knot-like structures (Figure 7b–d) as well as a reduction of stress-fiber-like structures in low-dose application of 4, where the degradation of the actin cytoskeleton was less pronounced (1 µg/mL; see Figure 7a). This reflects the well-known interference of cytochalasans with the fast-growing (barbed end) of f-actin filaments [51]. Interestingly, the effect of 4 is not functionally reversible as opposed to its acetylated derivative 7, pointing towards the loss of the enone function at C-21 as a key trait for the reversibility of the actin cytoskeleton degradative capabilities. It will be interesting to further embark on this pattern and verify this feat in differently organized cytochalasan core structures such as chaetoglobosins and other cytochalasans.
4. Discussion
In this work, we characterized some specimens of the H. fuscum complex by a polyphasic approach using chemotaxonomic and multilocus sequencing data for the first time. This species complex had previously been recognized by the meticulous work of Petrini et al. [14], who had found correlations between the host plants from which the stromata were collected and the ascospore size range, but could not use the latter character to segregate species owing to the fact that the spore sizes were overlapping while the anamorphic structures from various mycelial cultures they made from specimens inhabiting different hosts were rather similar. The type specimen of Sphaeria fusca Pers., which is deposited in the fungarium of Leiden (L) represents the commonly encountered member of this species complex that is very frequently found on Corylus avellana. An epitype with matching characteristics was selected and sequenced as prerequisite to stabilize the taxonomy of the H. fuscum complex. While the broad concept of H. fuscum was upheld by Ju and Rogers [4], Mühlbauer et al. [52] provided first evidence on the existence of several chemotypes that could be useful for species segregation. Quang et al. [12] found that stromata of H. fuscum from Corylus contain daldinin C, which had previously been isolated from a Daldinia sp. and also described two congeners named daldinins E and F. Further HPLC profiling studies on H. fuscum specimen occurring on different hosts based on a large number of specimens revealed the existence of two different chemotypes [10,16]. Specimens from Corylus contains BNT, daldinal A, daldinins C, E and F, while a second chemotype mostly represented by specimens from Alnus and Salix were devoid of daldinal A and contained unidentified compounds. The identity of two of these metabolites was established in the current study as 3′ malonyl daldinin F (6) and the novel pseudofuscochalasin A (4). We have detected further yet unidentified compounds in the crude extracts but their isolation and structure elucidation was not possible, owing to the small amounts of stromatal material available.
The current work also provided a resolution of the H. fuscum species complex by molecular phylogeny for the first time. An earlier study based on ITS sequences using several vouchers of H. fuscum s. lat. showed no delimitation of H. fuscum across different host-species in a molecular phylogenetic inference [17]. However, the ITS sequences are of questionable utility in the Xylariales because of intraspecific and even intragenomic polymorphism on the one hand and high redundancies on the other hand [53,54]. Our current molecular phylogenetic analysis was congruent with published data, showing a paraphyly of Hypoxylon, which was not solved by the addition of the newly generated sequences. However, three clearly distinguished clades with high bootstrap support were formed by H. fuscum s. str. and its allies. Taking together morphological, chemical and phylogenetic data we introduce two new species. The previously reported specimens of the “Alnus/Salix chemotype” will have to be assigned to either H. eurasiaticum and H. pseudofuscum based on repetitive studies of their HPLC profiles (best using the standardized HPLC-MS system that was employed in the present study) and morphology. It remains possible that additional members of this species complex will be recognized as new taxa in the near future. This will definitely afford the availability of fresh material that can be cultured and subjected to a multi locus phylogeny.
The new chemotaxonomic marker metabolites were also subjected to a preliminary analysis of their biological effects, but due to the limited amounts available, no extensive activity spectra could be recorded. The difference in the activities of daldinin F, which was found inactive in our study, to previously reported data reported by Quang et al. [55], who had reported significant antibacterial effects for the compound, may be due to the use of different assay protocol. The new daldinin derivative 6 was also devoid of antimicrobial effects but showed only weak cytotoxicity against L929 murine fibroblasts and KB 3.1 cervix-cancer cells. The other new compound 4 belongs to the cytochalasins, which are well-known actin cytoskeleton disrupting agents with a huge structural diversity [56] and many different subtypes with varying biological effects have been reported. However, a clear-cut structure activity relationship is not yet available for this compound class and only few systematic studies were undertaken.
Recently we have established certain correlations between the chemical structures and the corresponding biological effects, such as the importance of an enone moiety for the general reversibility of the f-actin collapsing effect [47]. This was further corroborated by the comparison of four and seven in the present study. However, this adds only a small puzzle frame towards the many differently organized cytochalasin core structures, for which this pattern has to be verified.
5. Conclusions and Outlook
The present study has contributed further to the establishment of correlations between biological and chemical diversity of the fungal genus Hypoxylon and constitutes the first attempt to segregate a rather complicated species complex by using polyphasic taxonomy methodology and in particular the correlation of chemotaxonomy and a multi-locus genealogy has proven useful for this task. In the future additional specimens of the H. fuscum complex from other host plants and geographic areas should be included. It may also be of great interest to study the lichenicolous and endophytic strains that were assigned to “H. fuscum” (s. lat.) in the literature e.g., [57,58]. Unfortunately, the authors of these papers have apparently not deposited the cultures in public domain collections and they might not be accessible for phylogenetic studies. This is unfortunately the case for many other xylarialean endophytes that were reported to produce interesting secondary metabolites, and the reported taxonomy of the producer strains has frequently been inaccurate [59,60].
Acknowledgments
Mohammad Javad wants to express his appreciation to all colleagues in the labs of Marc Stadler (Braunschweig) and Irmgard Krisai-Greilhuber (Vienna) for their support, especially Kathrin Wittstein, Silke Reinecke, Esther Surges, Christel Kakoschke, Anke Skiba and to all colleagues in the University of Guilan. The authors want to express their gratitude to Manfred Rohde for recording the SEM pictures.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/7/2/131/s1, Figure S1: HRESIMS data of pseudofuscochalasin A (4); Figure S2: 1H NMR spectrum (500 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S3: 13C NMR spectrum (125 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S4: COSY NMR spectrum (500 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S5: ROESY NMR spectrum (500 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S6: HSQC NMR spectrum (500 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S7: HMBC NMR spectrum (500 MHz, chloroform-d) of pseudofuscochalasin A (4); Figure S8: HRESIMS data of daldinin F (5); Figure S9: 1H NMR spectrum (500 MHz, chloroform-d) of daldinin F (5); Figure S10: 13C NMR spectrum (125 MHz, chloroform-d) of daldinin F (5); Figure S11:. HRESIMS data of 3′-malonyl-daldinin F (6); Figure S12: 1H NMR spectrum (700 MHz, acetone-d6) of 3-malonyl daldinin F (6); Figure S13: 13C NMR spectrum (175 MHz, aceton-d6) of 3-malonyl daldinin F (6); Figure S14: COSY NMR spectrum (700 MHz, acetone-d6) of 3-malonyl daldinin F (6); Figure S15: ROESY NMR spectrum (700 MHz, aceton-d6) 3-malonyl daldinin F (6); Figure S16: HSQC NMR spectrum (700 MHz, acetone-d6) 3-malonyl daldinin F (6); Figure S17 HMBC NMR spectrum (700 MHz, acetone-d6) of 3-malonyl daldinin F (6).
Author Contributions
C.L.: Supervision, chemotaxonomic survey, compound isolation, sequencing, actin assay, writing; M.C.-S., M.J.P.: conceptualization, methodology, resources, software, investigation, writing—original draft preparation; S.A.K. writing—review and editing; I.K.-G. and H.V. writing—review and editing; F.S.: Structure elucidation, writing. M.S. and T.E.B.S. resources, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a grant from the Deputy of Research and Technology of the University of Guilan, Iran to M.J.P. In addition, C.L. is grateful for a stipend from the Life Science Foundation, Braunschweig, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyde K.D., Norphanphoun C., Maharachchikumbura S.S., Bhat D.J., Jones E.B., Bundhun D., Chen Y.J., Bao D.F., Boonmee S., Calabon M.S., et al. Refined families of Sordariomycetes. Mycosphere. 2020;11:305–1059. doi: 10.5943/mycosphere/11/1/7. [DOI] [Google Scholar]
- 2.Wendt L., Sir E.B., Kuhnert E., Heitkämper S., Lambert C., Hladki A.I., Romero A.I., Luangsa-ard J.J., Srikitikulchai P., Peršoh D. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018;17:115–154. doi: 10.1007/s11557-017-1311-3. [DOI] [Google Scholar]
- 3.Pažoutová S., Follert S., Bitzer J., Keck M., Surup F., Šrůtka P., Holuša J., Stadler M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013;60:107–123. doi: 10.1007/s13225-013-0238-5. [DOI] [Google Scholar]
- 4.Ju Y.M., Rogers J.D. A Revision of the Genus Hypoxylon. APS Press; St. Paul, MN, USA: 1996. p. 365. Mycologia Memoir n° 20. [Google Scholar]
- 5.Daranagama D.A., Hyde K.D., Sir E.B., Thambugala K.M., Tian Q., Samarakoon M.C., McKen-zie E.H.C., Jayasiri S.C., Tibpromma S., Bhat J.D., et al. Towards a natural classification and back-bone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018;88:1–165. doi: 10.1007/s13225-017-0388-y. [DOI] [Google Scholar]
- 6.Hsieh H.M., Ju Y.M., Rogers J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia. 2005;97:844–865. doi: 10.1080/15572536.2006.11832776. [DOI] [PubMed] [Google Scholar]
- 7.Lambert C., Wendt L., Hladki A.I., Romero A.I., Stadler M., Sir E.B. Hypomontagnella (Hypoxylaceae): A new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycol. Prog. 2019;18:187–201. doi: 10.1007/s11557-018-1452-z. [DOI] [Google Scholar]
- 8.Wibberg D., Stadler M., Lambert C., Bunk B., Spröer C., Rückert C., Kalinowski J., Cox R.J., Kuhnert E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2020 doi: 10.1007/s13225-020-00447-5. [DOI] [Google Scholar]
- 9.Granmo A. Ph.D. Thesis. Botanical Garden and Museum-University of Oslo; Oslo, Norway: 1999. Morphotaxonomy and Chorology of the Genus Hypoxylon (Xylariaceae) in Norway. [Google Scholar]
- 10.Stadler M., Fournier J., Granmo A., Beltrán-Tejera E. The “red Hypoxylons” of the temperate and subtropical Northern Hemisphere. N. Am. Fungi. 2008;3:73–125. doi: 10.2509/naf2008.003.0075. [DOI] [Google Scholar]
- 11.Fournier J., Köpcke B., Stadler M. New species of Hypoxylon from Western Europe and Ethiopia. Mycotaxon. 2010;113:209–235. doi: 10.5248/113.209. [DOI] [Google Scholar]
- 12.Quang D.N., Hashimoto T., Tanaka M., Stadler M., Asakawa Y. Cyclic azaphilones daldinins E and F from the ascomycete fungus Hypoxylon fuscum (Xylariaceae) Phytochemistry. 2004;65:469–473. doi: 10.1016/j.phytochem.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Petrini L.E. Haupt-und Nebenfruchtformen europäischer Hypoxylon-Arten (Xylariaceae, Sphaeriales) und verwandter Pilze. Mycol. Helv. 1986;1:501–627. [Google Scholar]
- 14.Petrini L.E., Petrini O., Sieber T.N. Host specificity of Hypoxylon fuscum: A statistical approach to the problem. Sydowia. 1987;40:227–234. [Google Scholar]
- 15.Stadler M., Fournier J. Pigment chemistry, taxonomy and phylogeny of the Hypoxyloideae (Xylariaceae) Rev. Iberoam. Micol. 2006;23:160–170. doi: 10.1016/S1130-1406(06)70037-7. [DOI] [PubMed] [Google Scholar]
- 16.Stadler M., Fournier J., Quang D.N., Akulov A.Y. Metabolomic studies on the chemical ecology of the Xylariaceae (Ascomycota) Nat. Prod. Commun. 2007;2:287–304. doi: 10.1177/1934578X0700200311. [DOI] [Google Scholar]
- 17.Triebel D., Peršoh D., Wollweber H., Stadler M. Phylogenetic relationships among Daldinia, Entonaema and Hypoxylon as inferred from ITS nrDNA sequences. Nova Hedwig. 2005;80:25–43. doi: 10.1127/0029-5035/2005/0080-0025. [DOI] [Google Scholar]
- 18.Raei S., Khodaparast S.A., Abbasi M. Contribution to the knowledge of Hypoxylon and Annulohypoxylon in Guilan province (N Iran) Rostaniha. 2012;13:197–206. doi: 10.22092/BOTANY.2013.101336. [DOI] [Google Scholar]
- 19.Raei S., Khodaparast S.A., Abbasi M. More records of xylariaceous fungi from North of Iran. Rostaniha. 2014;15:110–121. doi: 10.22092/BOTANY.2014.101235. [DOI] [Google Scholar]
- 20.Pourmoghaddam M.J., Khodaparast S.A., Pedramfar H. The genus Daldinia in Guilan province (N Iran) Rostaniha. 2014;15:122–132. doi: 10.22092/BOTANY.2014.101236. [DOI] [Google Scholar]
- 21.Pourmoghaddam M.J., Lambert C., Surup F., Khodaparast S.A., Krisai-Greilhuber I., Voglmayr H., Stadler M. Discovery of a new species of the Hypoxylon rubiginosum complex from Iran and antagonistic activities of Hypoxylon spp. against the Ash Dieback pathogen, Hymenoscyphus fraxineus, in dual culture. MycoKeys. 2020;66:105–133. doi: 10.3897/mycokeys.66.50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayner R.W. A Mycological Colour Chart. British Mycological Society; Manchester, UK: 1970. Commonwealth Mycological Institute (Great Britain) [Google Scholar]
- 23.Kuhnert E., Sir E.B., Lambert C., Hyde K.D., Hladki A.I., Romero A.I., Rohde M., Stadler M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2017;85:1–43. doi: 10.1007/s13225-016-0377-6. [DOI] [Google Scholar]
- 24.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 25.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. In Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swofford D.L. PAUP* 4.0b10: Phylogenetic Analysis Using Parsimony (*and other Methods) Sinauer Associates; Sunderland, UK: 2002. [Google Scholar]
- 27.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 28.Silvestro D., Michalak I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012;12:335–337. doi: 10.1007/s13127-011-0056-0. [DOI] [Google Scholar]
- 29.Kuhnert E., Fournier J., Peršoh D., Luangsa-ard J.J.D., Stadler M. New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers. 2014;64:181–203. doi: 10.1007/s13225-013-0264-3. [DOI] [Google Scholar]
- 30.Daranagama D.A., Camporesi E., Tian Q., Liu X., Chamyuang S., Stadler M., Hyde K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015;73:203–238. doi: 10.1007/s13225-015-0329-6. [DOI] [Google Scholar]
- 31.Bitzer J., Laessoe T., Fournier J., Kummer V., Decock C., Tichy H.V., Piepenbring M., Persoh D., Stadler M. Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol. Res. 2008;112:251–270. doi: 10.1016/j.mycres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 32.U’Ren J.M., Miadlikowska J., Zimmerman N.B., Lutzoni F., Stajich J.E., Arnold A.E. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota) Mol. Phylogenetics Evol. 2016;98:210–232. doi: 10.1016/j.ympev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Vu D., Groenewald M., de Vries M., Gehrmann T., Stielow B., Eberhardt U., Al-Hatmi A., Groe-newald J.Z., Cardinali G., Houbraken J., et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadler M., Laessoe T., Fournier J., Decock C., Schmieschek B., Tichy H.V., Persoh D. A polyphasic taxonomy of Daldinia (Xylariaceae) Stud. Mycol. 2014;77:1–143. doi: 10.3114/sim0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N., Castlebury L.A., Miller A.N., Huhndorf S.M., Schoch C.L., Seifert K.A., Rossman A.Y., Rogers J.D., Kohlmeyer J., Volkmann-Kohlmeyer B., et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia. 2006;98:1076–1087. doi: 10.1080/15572536.2006.11832635. [DOI] [PubMed] [Google Scholar]
- 36.Koukol O., Kelnarová I., Černý K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For. Pathol. 2015;45:21–27. doi: 10.1111/efp.12129. [DOI] [Google Scholar]
- 37.Stadler M., Kuhnert E., Peršoh D., Fournier J. The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept. Mycology. 2013;4:5–21. doi: 10.1080/21501203.2013.782478. [DOI] [Google Scholar]
- 38.Becker K., Lambert C., Wieschhaus J., Stadler M. Phylogenetic assignment of the fungicolous Hypoxylon invadens (Ascomycota, Xylariales) and investigation of its secondary metabolites. Microorganisms. 2020;8:1397. doi: 10.3390/microorganisms8091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sir E.B., Becker K., Lambert C., Bills F.G., Kuhnert E. Observations on Texas hypoxylons, including two new Hypoxylon species and widespread environmental isolates of the H. croceum complex identified by a polyphasic approach. Mycologia. 2019;111:832–856. doi: 10.1080/00275514.2019.1637705. [DOI] [PubMed] [Google Scholar]
- 40.Li G.J., Hyde K.D., Zhao R.L., Hongsanan S., Abdel-Aziz F.A., Abdel-Wahab M.A., Alvarado P., Alves-Silva G., Ammirati J.F., Ariyawansa H.A. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;78:1–237. doi: 10.1007/s13225-016-0366-9. [DOI] [Google Scholar]
- 41.Bills G.F., Gonzalez-Menendez V., Platas G., Fournier J., Persoh D., Stadler M. Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a pantropical insecticide-producing endophyte. PLoS ONE. 2012:e46687. doi: 10.1371/journal.pone.0046687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stadler M., Fournier J., Læssøe T., Chlebicki A., Lechat C., Flessa F., Rambold G., Peršoh D. Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae) Mycoscience. 2010;51:189–207. doi: 10.1007/S10267-009-0028-9. [DOI] [Google Scholar]
- 43.Fournier J., Flessa F., Peršoh D., Stadler M. Three new Xylaria species from southwestern Europe. Mycol. Prog. 2011;10:33–52. doi: 10.1007/s11557-010-0671-8. [DOI] [Google Scholar]
- 44.Sir E.B., Kuhnert E., Lambert C., Hladki A.I., Romero A.I., Stadler M. New species and reports of Hypoxylon from Argentina recognized by a polyphasic approach. Mycol. Prog. 2016;15:42. doi: 10.1007/s11557-016-1182-z. [DOI] [Google Scholar]
- 45.Moussa A.Y., Lambert C., Stradal T.E., Ashrafi S., Maier W., Stadler M., Helaly S.E. New peptaibiotics and a cyclodepsipeptide from Ijuhya vitellina: Isolation, identification, cytotoxic and nematicidal activities. Antibiotics. 2020;9:132. doi: 10.3390/antibiotics9030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandargo B., Michehl M., Stadler M., Surup F. Antifungal sesquiterpenoids, rhodocoranes F-L from submerged cultures of the wrinkled peach mushroom, Rhodotus palmatus. J. Nat. Prod. 2020;83:720–724. doi: 10.1021/acs.jnatprod.9b00871. [DOI] [PubMed] [Google Scholar]
- 47.Kretz R., Wendt L., Wongkanoun S., Luangsa-ard J.J., Surup F., Helaly S.E., Noumeur S.R., Stadler M., Stradal T.E. The effect of cytochalasans on the actin cytoskeleton of eukaryotic cells and preliminary structure–activity relationships. Biomolecules. 2019;9:73. doi: 10.3390/biom9020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchanan M.S., Hashimoto T., Asakawa Y. Five 10-phenyl-[11]-cytochalasans from a Daldinia fungal species. Phytochemistry. 1995;40:135–140. doi: 10.1016/0031-9422(95)00201-H. [DOI] [Google Scholar]
- 49.Suzuki S., Hosoe T., Nozawa K., Yaguchi T., Udagawa S., Kawai K. Mitorubrin derivatives on ascomata of some Talaromyces species of ascomycetous fungi. J. Nat. Prod. 1999;62:1328–1329. doi: 10.1021/np990146f. [DOI] [PubMed] [Google Scholar]
- 50.Clark R.C., Lee S.Y., Boger D.L. Total synthesis of chlorofusin, its seven chromophore diastereomers, and key partial structures. J. Am. Chem. Soc. 2008;130:12355–12369. doi: 10.1021/ja8012819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yahara I., Harada F., Sekita S., Yoshihira K., Natori S. Correlation between effects of 24 different cytochalasins on cellular structures and cellular events and those on actin in vitro. J. Cell Biol. 1982;92:69–78. doi: 10.1083/jcb.92.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mühlbauer A., Triebel D., Persoh D., Wollweber H., Seip S., Stadler M. Macrocarpones, novel metabolites from stromata of Hypoxylon macrocarpum and new evidence on the chemotaxonomy of Hypoxylon species. Mycol. Prog. 2020;1:235–248. doi: 10.1007/s11557-006-0021-z. [DOI] [Google Scholar]
- 53.Hongsanan S., Xie N., Liu J.K., Dissanayake A., Ekanayaka A.H., Raspé O., Jayawardena R.S., Hyde K.D., Jeewon R., Purahong W., et al. Can we use environmental DNA as holotypes? Fungal Divers. 2018;92:1–30. doi: 10.1007/s13225-018-0404-x. [DOI] [Google Scholar]
- 54.Stadler M., Lambert C., Wibberg D., Kalinowski J., Cox R.J., Kolařík M., Kuhnert E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota) Mycol. Prog. 2020;19:235–245. doi: 10.1007/s11557-019-01552-9. [DOI] [Google Scholar]
- 55.Quang D.N., Hashimoto T., Radulovic N., Stadler M., Asakawa Y. Antimicrobial azaphilones from the xylariaceous inedible mushrooms. Int. J. Med. Mushrooms. 2005;7:452–455. doi: 10.1615/IntJMedMushr.v7.i3.880. [DOI] [Google Scholar]
- 56.Scherlach K., Boettger D., Remme N., Hertweck C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010;27:869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 57.Basnet B.B., Chen B., Suleimen Y.M., Ma K., Guo S., Bao L., Huang Y., Liu H. Cytotoxic secondary metabolites from the endolichenic fungus Hypoxylon fuscum. Planta Med. 2019;13:1088–1097. doi: 10.1055/a-0957-3567. [DOI] [PubMed] [Google Scholar]
- 58.Widmer T.L., McMahon M.B., Luster D.G. Plant pathogenic fungi are harbored as endophytes in Rhododendron spp. native to the Eastern USA. Fungal Ecol. 2020;47:100949. doi: 10.1016/j.funeco.2020.100949. [DOI] [Google Scholar]
- 59.Becker K., Stadler M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J. Antibiot. 2021;74:1–23. doi: 10.1038/s41429-020-00376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helaly S.E., Thongbai B., Stadler M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018;35:992–1014. doi: 10.1039/C8NP00010G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.