Abstract
The ability of epigenetic markers to affect genome function has enabled transformative changes in drug discovery, especially in cancer and other emerging therapeutic areas. Concordant with the introduction of the term ‘epi-informatics’, the size of the epigenetically relevant chemical space has grown substantially and so did the number of applications of cheminformatic methods to epigenetics. Recent progress in epi-informatics has improved our understanding of the structure–epigenetic activity relationships and boosted the development of models predicting novel epigenetic agents. Herein, we review the advances in computational approaches to drug discovery of small molecules with epigenetic modulation profiles, summarize the current chemogenomics data available for epigenetics targets, and provide a perspective on the greater utility of biomedical knowledge mining as a means to advance the epigenetic drug discovery.
Keywords: chemical space, drug discovery, epigenetics, epi-drugs, epi-informatics, focused libraries, molecular modeling, polypharmacology, virtual screening, structure-activity relationships
Introduction
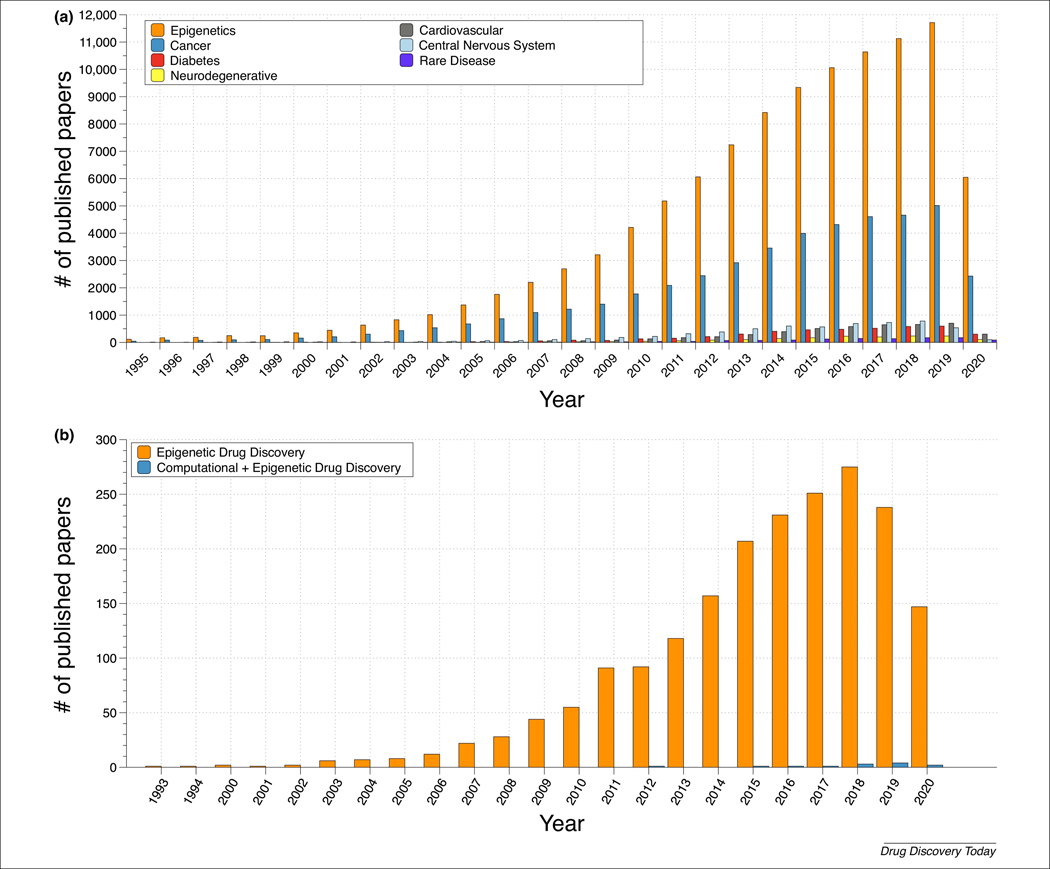
The term ‘epigenetics’ has its historical roots in the work of C. Waddington (1940s) and D.L. Nanney (1950s), where it was initially defined to denote a cellular memory, persistent homeostasis in the absence of an original perturbation, or an effect on cell fate not attributable to changes in DNA [1,2]. However, the term is now used with multiple meanings. For example, epigenetics has been used to describe the (i) heritable phenotype (cellular memory) without modification of DNA sequences [3]; (ii) ‘the structural adaptation of chromosomal regions to register, signal, or perpetuate altered activity states’ in the genome [4]; and (iii) the mechanism by which the environment conveys its influence to the cell, tissue, or organism [5]. Despite these ambiguous definitions, the growing importance of epigenetics in new therapeutics discovery and understanding disease etiology can be illustrated by the trends in the number of scientific publications on the subject. Figure 1a shows the number of papers published in the literature with the word ‘epigenetics’ and six co-occurring diseases per year; now, 25 years after the first six published reports on ‘epigenetics’ in 1994 [6], there are cumulatively >105 000 relevant publications. Although the most frequent co-occurring term is ‘cancer’ and most epidrugs are approved for cancer treatment, epigenetic modifications are not limited to malignant conditions. Instead, the data suggest that exceptionally deadly diseases were the first to be studied in the epigenetic field [6].
Figure 1.
Title. (a) The number of papers in PubMed with the keyword ‘Epigenetics’ alone or co-occurring with multiple diseases (cancer, diabetes, neurodegenerative, cardiovascular, central nervous system, and rare disease) by year. (b) The number of papers in PubMed with the keywords ‘epigenetics drug discovery’ and ‘epigenetics + computer-aided drug discovery / computational drug discovery’ by year.
As one can see from Figure 1, epigenetics has implications for many different areas of research, as indicated by many extensive reviews on different topics, such as the waves of epigenetic drugs [4], the role of epigenetics in cancer [7], autism diagnosis [8], multiple sclerosis [9], depressive disorders [10], and rare diseases [11], to name a few. Although the general trends in drug discovery show that kinases and G-protein-coupled receptors to be the most investigated protein families [12], epigenetic research has predominantly focused on different classes of target, primarily histone deacetylases (HDACs), histone methyltransferases (HMTs), DNA methyltransferase (DNMT) 1, and other chromodomain and/or Tudor domain-containing proteins (TUD). Interest in the bromodomain and external terminal protein (BET) family has shed light on 42 bromodomain (BRD) targets [13], and further investigation of histone acetyltransferases (HATs) led to a distinctive class of lysine acetyltransferases (KATs). Since the report of the first adenine-specific DNMT in Escherichia coli [14], the first discovered epigenetic target, 515 human epigenetic targets (136 mappable onto epigenetic phylogenetic groups) have been described, along with respective chemogenomics data (Table S1 in the supplemental information online). Despite the immense growth of the field, several epigenetic proteins associated with important diseases have been largely understudied, such as protein arginine methyltransferases (PRMTs), histone methyl readers (HMRs), and DNMT3; limited funding allocated to the studies of such ‘dark’ targets could be a contributing factor [15]. Many compounds have been tested in histone demethylase (HDM) assays, some of them reaching preclinical and clinical stages, but none of them have been approved by the US Food and Drug Administration (FDA) as yet. Considering their potential impact on the field, more work needs to be done on dark targets to better understand their mechanisms and expand the number of available targets.
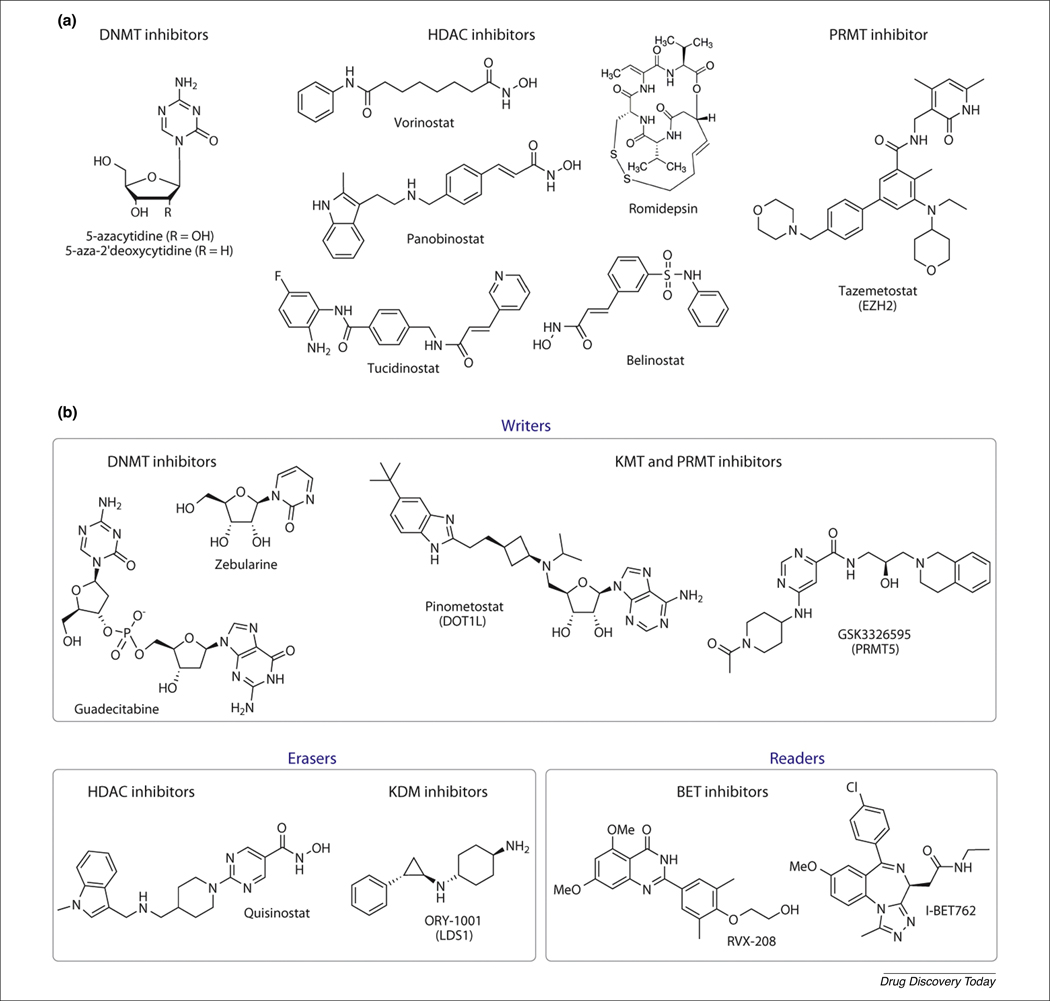
Epigenetics has a major role in the understanding of inheritance, development, and progression of diseases. Consequently, the discovery and development of epidrugs (or epigenetic drugs) have become a major research focus for multiple biotechnology companies [4]. As of August 2020, there were eight epigenetic drugs approved for clinical use [4,16]: two DNMT inhibitors (azacytidine and decitabine), five HDAC inhibitors (vorinostat, belinostat, panobinostat, romidepsin, and tucidinostat), and one HMT inhibitor (tazemetostat). Figure 2a and 2b show the chemical structures of representative FDA-approved drugs and compounds in clinical development as epidrugs, respectively [4]. An ongoing clinical trial is examining the FDA-approved DNMT inhibitor, azacitidine, in combination with a novel BET inhibitor, INCB057643, and a novel lysine dependent demethylase (LSD1) inhibitor, INCB059872, for solid metastatic tumors [17]. An extensive description of ongoing clinical trials related to epigenetic drugs has been reviewed recently [4]. Currently, most drugs focus on DNMT1 and HDACs (Figure 2a). The increased interest in epigenetic applications outside of cancer has not yet translated to a rise in viable drugs or drug candidates, despite the expansive therapeutic benefits of epigenetic modulation.
Figure 2.
Title. (a) Chemical structures of epigenetic drugs approved for clinical use. (b) Chemical structures of selected compounds in clinical development as epidrugs. Epigenetic targets are grouped as writers, erasers, and readers. A comprehensive review of compounds in clinical development the reader was recently provided by Ganesan et al. [4]. For definitions of abbreviations, please see the main text.
In recent years, the substantial growth in epigenetics-related data has prompted the development of cheminformatics methods with application to this field. In 2015, the term ‘epi-informatics’ was introduced and conceptualized [6] to summarize advances in epigenetic drug and chemical probe discovery driven by computational methods. Since then, molecular modeling and cheminformatics approaches have made substantial contributions to the field (Figure 1b) [18–20]. Computational methods have helped to explore the mechanism of action of active compounds at the molecular level and guide lead optimization programs. Herein, we review recent advances in computational approaches to epigenetic drug discovery and summarize, to the best of our knowledge, all the publicly available chemogenomics data for epigenetics targets.
Chemogenomics data and databases
Over the past decade, several open-source databases have compiled targets and compounds with epigenetic profiles. However, most do not use the same criteria for target selection/classification or use a systematic drug target ontology [21]. Table 1 summarizes the chemogenomics databases related to epigenetics published thus far, including web links to each resource. Although all of them were accessible as of August 2020, none have been updated since their release. This fact highlights the need for tools that automate the analysis of the constantly increasing body of epigenetic data in the public domain.
Table 1.
Major public databases relevant to epigenetic drug discovery
| Database | Last update | Number of targets | Number of compounds | URLa | Refs |
|---|---|---|---|---|---|
| Human epigenetic enzyme and modulator database (HEMD) | 2012 | 269 | 4377 | http://mdl.shsmu.edu.cn/HEMD/ | [22] |
| EpiDBase | 2015 | Not available | 5784 | www.epidbase.org/ | [23] |
| EpiFactors | 2015 | 815 | – | http://epifactors.autosome.ru | [24] |
| Database of Epigenetic Modifiers (dbEM) | 2016 | 167 | – | http://crdd.osdd.net/raghava/dbem | [25] |
| Epigenomics chemical database | 2018 | 54 | 7820 | www.difacquim.com/d-databases/ | [18] |
Last accessed: 25 August 2020.
One of the first web-accessible databases was the Human Epigenetic Enzyme and Modulator Database (HEMD). This resource, published in 2012, includes 4377 small-molecule modulators and 269 epigenetic targets that are annotated with information on epigenetic mechanisms, catalytic processes, and related diseases [22]. EpiDBase is also a web-accessible database that was released in 2015 [23]. It contains 5784 different ligands annotated with experimental activity (IC50) against writers, erasers, and readers, as well as calculated properties.
EpiFactors [24], also published in 2015, is a manually curated database that provides information on epigenetic regulators, their complexes, targets, and products. The database contains 815 human epigenetic proteins and 69 protein complexes involved in epigenetic regulation. EpiFactors also provides the corresponding genes and their expression levels in 458 human primary cell samples, 255 different cancer cell lines, and 134 human post-mortem tissues.
The Database of Epigenetic Modifiers (dbEM) is a web-accessible database released in 2016 [25] that contains the genomic information on 167 epigenetic targets. This resource maintains the information of mutations, copy number variation, and gene expression in tumor samples, cancer cell lines, and healthy samples.
In 2018, a database of epigenetic small molecules inhibitors was released [18] integrated from other public access databases such as ChEMBL (www.ebi.ac.uk/chembl/) and PubChem (https://pubchem.ncbi.nlm.nih.gov/). The epigenomics database includes 7820 unique compounds, of which 3456 have information for more than one epigenetic target. The database has 16 102 compound–target associations, of which 15 887 have quantitative potency data associated with them. The database has associations with 60 epigenetic targets.
Current approaches, models, and best achievements
Epigenetic-relevant chemical space
According to the Chemical Space project, the total number of synthetically feasible organic molecules exceeds 166 billion compounds [26]. Through chemical clustering and visualization, cheminformatics has allowed the navigation of large databases to identify therapeutically relevant chemical spaces. In this context, the ‘epigenetic-relevant chemical space’ (ERCS) was the first attempt to comprise a list of HDACs, DNMTs, and BET inhibitors (2772 compounds in total) [27]. The design of selective inhibitors remains a challenge in epigenetic drug discovery. For instance, most HDAC inhibitors approved so far are non-isoform selective, even though it has been hypothesized that selective inhibitors might have fewer adverse effects [3,28]. A comprehensive characterization of the ERCS can uncover multitarget relationships as well as guide the design of selective inhibitors, which could significantly improve the effectiveness of drug therapies [29].
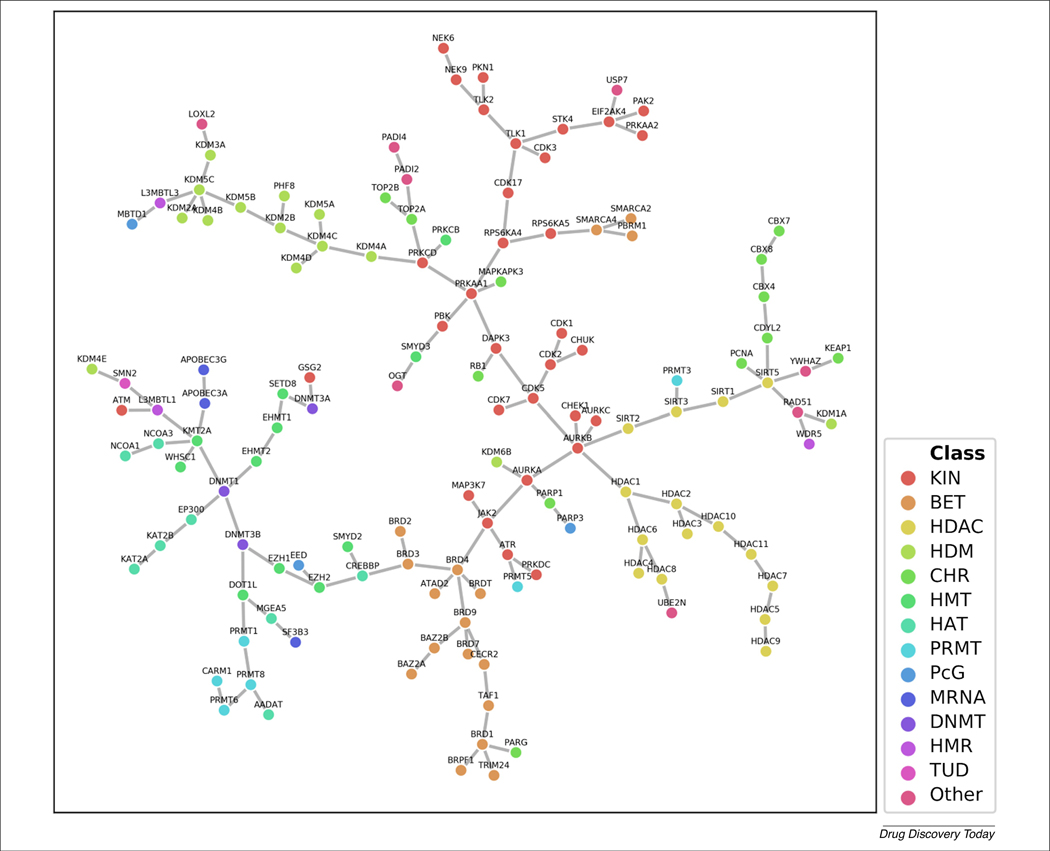
We surveyed the status of the compounds tested against the epigenetic targets and currently available in the public domain. Figure 3 visualizes the current ERCS using a Tree Manifold Approximation and Projection (TMAP) [30], generated with Statistical-Based Database Fingerprints (SB-DFPs) to represent target-associated compound datasets [31]. The SB-DFPs are novel condensed representations of compound data sets that have also been shown to capture these relationships between the compound data sets. This representation is based on statistical comparisons of molecular fingerprint bit proportions among any two data sets. These condensed representations are connected by a minimum spanning tree, as described in detail in the original publication [30]. The TMAP displays the relationships among compounds with biological data for a particular target as nodes that are connected to similar data sets of other targets through branches and subbranches. This map could also help to guide the design of multitarget epigenetic agents. In the map, several targets that are in the same or neighboring branches share the same role in histone modifications, such as kinases (KINs; writers), the BET family (readers), HDACs and HDMs (erasers), as well as chromatin remodelers (CHR). This observation is consistent with the known difficulty of designing a selective inhibitor within each class [4]. The map also shows a close relation between targets related to DNA methylation (i.e., DNMTs) to those involved in RNA modification (mRNAs) as well as different histone writers (HMTs, PRMTs, and HATs). Targets associated with other histone modifications tend to appear in the terminal regions of these branches, illustrating a weak relationship between different target classes. By contrast, Polycomb-group proteins (PcG) and other targets, such as the chromatin remodeler PARG2 and methyltransferases PRMT3 and PRMT5, do not appear to be closely related to targets with the same function, suggesting them as promising targets for the development of selective compounds.
Figure 3.
Visual representation of the current epigenetics-relevant chemical space. The visualization was generated with the recent Tree Manifold Approximation and Projection method and Statistical-Based Database Fingerprints as condensed representations of compounds associated to the epigenetic targets [31]. Individual nodes of the tree are epigenetic targets, colored by their class. The nodes are clustered according to the pairwise similarity of their condensed representations. The branches represent the connection between the chemical space of two different targets. Proximal data sets can contribute to the discovery of compounds acting on multiple targets. For definitions of abbreviations, please see the main text.
Structure-epigenetic activity relationship analyses
The increasing number of reports on compounds with experimental activity against one or more epigenetic targets has allowed the exploration of the structure–activity landscape for several epigenetic target datasets. Here, we review the respective models developed and published in recent years.
Quantitative structure-activity relationships (QSAR) modeling
QSAR modeling is a major computational approach to drug discovery [32] and, with the growth of chemogenomics datasets for epigenetic targets, its application to find bioactive compounds with epigenetics profile has grown. A recent review discussed 3D-QSAR studies performed with several different sets of HDAC inhibitors [33]. More recently, models were reported for 11 sets of inhibitors of HDAC using conformal prediction [34], showing that these models demonstrated high accuracy for both training and external test sets. The efficiencies for the predictions were >80% for most data sets and >90% for four data sets at different significance levels. Another paper expanded the discussion on HDAC-1 inhibitors, specifically, aminophenyl benzamide derivatives, and was able to produce an externally validated model with a high correlation (R2) and predictive (r2ext_ts) values of 0.96 and 0.79, respectively [35]. García-Sánchez et al. [36] reviewed more recent examples of QSAR models developed, again, mostly for HDACs and DNMTs. In an interesting effort to obtain novel DNMT1 inhibitors, the authors modified the structures of known inhibitors and used QSAR models to predict the activity of the novel compounds. They also identified electronegativity and the bond information content index as the most influential descriptors [37]. Unfortunately, other epigenetic targets have been minimally explored. BET inhibitors have been studied using QSAR modeling approaches; one study used such models to predict six putative multitarget BET bromodomain inhibitors [38]. Six reversible LSD1 inhibitors were proposed based on 3D-QSAR, molecular docking, and dynamics studies, but these predictions have not been supported by the experimental binding affinity measurements [39]. Similar approaches have been applied to lysine methyltransferase DOTL1 inhibitors, resulting in the computer-assisted design of two compounds that demonstrated inhibition at micromolar levels in confirmatory assays [40].
Activity landscape modeling
Activity landscapes can be defined as representations that compare compound similarity and activity relationships [41]. One of the main goals of this approach is to identify activity cliffs that can help lead optimization efforts [42] as well as data sets with a smooth (continuous) SAR that would be more likely to yield predictive models. Similarly, one can identify epigenetic datasets with a ‘rough’ (i.e., discontinuous) SAR landscape, for which the development of predictive models is expected to be difficult. Based on this concept, the SEAR of various epigenetic target data sets has been analyzed, including DNMTs [43,44], BRDs [36], HDACS [45], and lysine methyltransferases (G9a or EHMT2) [46]. To characterize the epigenetic activity landscape, a SAR analysis of 52 compounds tested against different epigenetic targets was used [47], which showed DNMT3B and DOT1L to have the highest percentage of activity cliffs (i.e., the respective data sets had more discontinuous SAR). The study also revealed that HDACs were the targets with the most continuous SAR, making them most suitable to carry out hit-to-lead optimization. This result is also consistent with the generation of a large number of predictive QSAR models. Finally, this large-scale epigenetic activity landscape study highlighted SMARCA2 and HAT as the epigenetic targets more prone to scaffold hopping (i.e., the search for compounds with different scaffolds but similar activity).
Epigenetic Target Profiler
Recently, a free online service was developed to predict the potential activity of small molecules against epigenetic targets. Briefly, the Epigenetic Target Profiler is a user-friendly web application that uses binary classification models relying on machine-learning algorithms (support vector machines and artificial neural networks) to predict the most probable epigenetic targets for a small molecule. Classification models were built on the available bioactivity data for 35 epigenetic regulators from ChEMBL26; the development of a new version using the latest release of ChEMBL is in progress. Figure S1 in the supplemental information online shows the graphical user interface of the webserver.
Reshaping the discovery and development of epidrug candidates with current and emerging technologies in cheminformatics
Molecular modeling and cheminformatics have made notable contributions to drug discovery [48]. Several important drugs have been developed with computational methods, including imatinib (a kinase inhibitor used to treat certain types of cancer), dorzolamide (a carboanydrase II inhibitor used to treat high pressure inside the eye, including glaucoma), enfuvirtide (a first-in-class antiretroviral drug used in combination therapy for the treatment of HIV-1), oseltamivir (a neuraminidase inhibitor used to treat flu), and many others [49,50]. The increasing chemogenomic body of data in the field of epigenetics has facilitated the application of several well-established computational methodologies. One example is the development of the Epigenetic Target Profiler discussed in the previous section. However, the application of computational approaches in epigenetic drug discovery is still recent and, so far, no epi-drugs have been discovered with computational strategies. Therefore, the development and application of new epi-informatics methodologies should continue to be an actively growing area of research.
Over the past few years, several groups have used multiple computational approaches to epigenetics research [18–20]. A recent review [51] summarized the advantages and limitations of several structure-based approaches in multitarget drug design, including examples from epigenetics. For example, Kuang et al. [52] reported on a comprehensive modeling study of several BRD isoforms with molecular dynamics simulations; the authors exploited the non-negligible ligand-binding kinetics features of these proteins, enhancing the understanding of the binding site of the BRD family. Although these techniques are promising, some limitations associated with molecular simulations [53] need to be overcome to enable their broader application to epigenetic research. For instance, an improvement in parametrization protocols for both the simulation of drug-like molecules [54] and protein–protein interfaces [55] is necessary.
Computational approaches can be used to find hidden allosteric binding sites [56] and protein–protein interaction hotspots [57] for epigenetic targets. Computational approaches can also help improve both the pharmacodynamics and pharmacokinetics of compounds in the hit-to-lead stage. Recently, Letfus et al. [58] used quantum/molecular mechanics (QM/MM) approaches to improve toxoflavin-based inhibitors of the human histone lysine demethylase enzymes of the subfamily KDM4C (a molecular target related to prostate and breast cancer). These studies led to compounds with activity in the low nM range in biochemical assays and mM activity in cell-based assays that also had enhanced pharmacokinetics properties in vitro. QM/MM methods were used because conventional force fields used in docking programs cannot model most metalloenzymes and metal–ligand interactions properly.
Epigenetic drug discovery can mostly benefit from cheminformatics to identify and prioritize hits for experimental validation. Recently, Tao et al. [59] reported the identification of a novel small molecule with in vitro enzymatic inhibition at a submicromolar level for PRMT5, an anticancer therapeutic target, using molecular docking and pharmacophore-based virtual screening. In another study, Song et al. [60] used a combination of pharmacophore searches, molecular docking, and molecular dynamic simulations and found compounds with selective activity against the proliferation of cancer cells.
We expect that QSAR modeling will continue to be an important methodology to accelerate the discovery of epidrugs. This approach has been substantially enriched over the past few years with modern machine learning and artificial intelligence algorithms [32]. Although some studies have reported the development of QSAR models and the identification of virtual hits for epigenetic targets [37,38], these hits were not experimentally tested. However, compounds have been identified computationally that were later validated experimentally [61–66], confirming how this approach could help identifying promising bioactive compounds for epigenetic targets. We have summarized available libraries that have been designed for epigenetics targeted using computational approaches, and a comprehensive list of these libraries is available in Table S2 in the supplemental information online. Meanwhile, structural diversity and scaffold content of such libraries, as well as their in silico pharmacokinetic and toxicological profiles, still need to be estimated [67].
Molecular docking and molecular dynamics simulations as well as artificial intelligence methods have gained attention because of increased computational capabilities and efficient scaling with the advent of graphics processing units. For instance, Lyu et al. [68] recently ran molecular docking on a 170-million compound library leading to the experimental discovery of a new scaffold of phenolate inhibitors of AmpC. After optimization, one of the compounds showed inhibitory activity of 77 nM. The authors also identified 30 compounds with submicromolar activity for the D4 dopamine receptor. Gentile et al. [69] developed Deep Docking, a hybrid platform built on deep learning QSAR models trained on docking scores, which afforded rapid accurate prediction of docking scores for billions of molecular structures. Several recent applications of deep learning and generative neural network models have been described in the literature. For instance, a deep reinforcement learning algorithm, termed ReLEASE (Reinforcement Learning for Structural Evolution) was developed that can help to design chemical libraries with a bias toward desired inhibitory activity [70]. By using a similar approach, Zhavoronkov et al. [71] reported the discovery of a potent candidate for DDR1, a kinase target implicated in fibrosis and other diseases, noting that the entire project was accomplished in 21 days. Although this work received criticism because the new molecule was similar to an approved drug [72], the new compound was de facto not available in any open database. The application of these advanced and accelerated computational approaches to epigenetic targets is pending.
The potential of polypharmacological profiles allowed by epigenetic targets is an important consideration of direct relevance to epigenetic drug discovery. Very often, drugs hitting undesired targets are the primary source of toxicity, also known as ‘off-target’ effects [73]. Indeed, toxicity and lack of efficacy are the major causes of drug attrition [74]. However, recent studies highlighted the advantages of multitarget design (MTD), taking advantage of the ‘selective synergism’ [75]. Structure-based and machine-learning methods have been successfully applied in MTDs [76]. Epigenetic modulation is suitable for MTD because several targets are involved in the same pathway, which could lead to synergic results [18,77]. For the purposes of epigenetics drug discovery, promising MTD strategies should focus on designing compounds that inhibit different aspects (reading, writing, and erasing) of the same gene modification, such as (i) reader/writer (e.g., BET/HDAC inhibition) [78]; (ii) writer/writer of distinct changes (e.g., HDAC/DNMT) [79]; and (iii) writer/writer of the same modification (e.g., G9a/DNMT) [46,80]. Therefore, it is evident that epigenetic polypharmacology, or even the combination therapy of different epidrugs, offer a promising avenue for future epigenetic drug discovery [16,18].
Concluding remarks
Over the past 25 years, the number of scientific publications related to epigenetics has increased impressively from six papers in 1994 to >100 000 overall. Although cancer remains the primary therapeutic area associated with epigenetic drug discovery, other conditions, such as cardiovascular, neurodegenerative, central nervous system-related diseases, diabetes, and rare diseases, have been rapidly gaining interest in relation to epigenetics. In addition, we have seen a slight uptick in computational approaches used in this field. So far, epi-informatics has allowed the creation and maintenance of target-compound databases, exploring the increasing ERCS and SEARs, which eventually led to the development of the Epigenetic Target Profiler, a webserver to generate a predicted profile of potential inhibition of small molecules across a panel of epigenetic targets of pharmaceutical relevance.
Herein, we have highlighted recent advances in the use of cheminformatics in epigenetic drug discovery as well as discussed recent cutting-edge computer-aided drug design technologies that could be used to enable breakthrough discoveries in the field. Although no epigenetic drug has been discovered by computational approaches yet, the diversity of therapeutic areas associated with epigenetic targets, the growth of epigenetic chemical space, and the proliferation of robust computational approaches to drug discovery promise a substantial increase in the number of publications and the emergence of novel epigenetic drug candidates discovered by cheminformatics methods.
Supplementary Material
Highlight.
The interest in developing epigenetic drugs is substantially increasing.
We reviewed the recent epigenetics structure-activity relationships.
Epi-informatics has seen recent advances in databases and web servers.
Polypharmacology profiling is a must in the future of epigenetic drug discovery.
We briefly discuss the future directions of epi-informatics.
Acknowledgments
We thank Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico (grant 282785), and the National Institutes of Health, USA (grant R01GM114015). F.D.P-M. and N.S-C. are thankful to CONACyT for the PhD scholarships number, 660465/576637 and 335997, respectively.
Footnotes
Teaser: Epigenetic drug discovery is gaining growing attention both in industry and academia; ‘epi-informatics’ is an emerging concurrent area of computational research that helps accelerate this effort.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waddington CH (2012) The epigenotype, endeavor, 1942, vol. 1 (pg. 18–20) reprinted. Int. J. Epidemiol 41, 10–13 [DOI] [PubMed] [Google Scholar]
- 2.Greally JM (2018) A user’s guide to the ambiguous word ‘epigenetics’. Nat. Rev. Mol. Cell Biol 19, 207–208 [DOI] [PubMed] [Google Scholar]
- 3.Wu C and Morris JR (2001) Genes, genetics, and epigenetics: a correspondence. Science 293, 1103–1105 [DOI] [PubMed] [Google Scholar]
- 4.Ganesan A et al. (2019) The timeline of epigenetic drug discovery: from reality to dreams. Clin. Epigenet 11, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalli G and Heard E. (2019) Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 [DOI] [PubMed] [Google Scholar]
- 6.Dueñas-González A et al. (2016) Introduction of epigenetic targets in drug discovery and current status of epi-drugs and epi-probes. In Epi-informatics (Medina-Franco JL, ed.), pp. 1–20, Academic Press [Google Scholar]
- 7.Esteller M. (2008) Molecular origins of cancer: epigenetics in cancer. N. Engl. J. Med 358, 1148–1159 [DOI] [PubMed] [Google Scholar]
- 8.Waye MMY and Cheng HY (2018) Genetics and epigenetics of autism: a review. Psychiatry Clin. Neurosci 72, 228–244 [DOI] [PubMed] [Google Scholar]
- 9.Küçükali Cİ et al. (2015) Epigenetics of multiple sclerosis: an updated review. Neuro. Mol. Med 17, 83–96 [DOI] [PubMed] [Google Scholar]
- 10.Januar V et al. (2015) Epigenetics and depressive disorders: a review of current progress and future directions. Int. J. Epidemiol 44, 1364–1387 [DOI] [PubMed] [Google Scholar]
- 11.Brindisi M et al. (2020) Old but gold: tracking the new guise of histone deacetylase 6 (HDAC6) enzyme as a biomarker and therapeutic target in rare diseases. J. Med. Chem 63, 23–39 [DOI] [PubMed] [Google Scholar]
- 12.Zdrazil B et al. (2019) Moving targets: monitoring target trends in drug discovery by mapping targets, go terms, and diseases. bioRxiv 691550 [Google Scholar]
- 13.Fujisawa T and Filippakopoulos P. (2017) Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol 18, 246–262 [DOI] [PubMed] [Google Scholar]
- 14.Kühnlein U et al. (1969) Host specificity of DNA produced by Escherichia coli. XI. In vitro modification of phage fd replicative form. Proc. Natl. Acad. Sci. U. S. A 63, 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oprea TI et al. (2018) Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discov 17, 317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lera AR and Ganesan A. (2020) Two-hit wonders: the expanding universe of multitargeting epigenetic agents. Curr. Opin. Chem. Biol 57, 135–154 [DOI] [PubMed] [Google Scholar]
- 17.Cor Incyte. (2016) Azacitidine Combined With Pembrolizumab and Epacadostat in Subjects With Advanced Solid Tumors (ECHO-206), US National Library of Medicine [Google Scholar]
- 18.Naveja JJ and Medina-Franco JL (2018) Insights from pharmacological similarity of epigenetic targets in epipolypharmacology. Drug Discov. Today 23, 141–150 [DOI] [PubMed] [Google Scholar]
- 19.Lim SJ et al. (2010) Computational epigenetics: the new scientific paradigm. Bioinformation 4, 331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W et al. (2018) Computer-aided drug design in epigenetics. Front. Chem 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y et al. (2017) Drug target ontology to classify and integrate drug discovery data. J. Biomed. Semantics 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z et al. (2012) HEMD: an integrated tool of human epigenetic enzymes and chemical modulators for therapeutics. PLoS ONE 7, e39917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loharch S et al. (2015) Epidbase: a manually curated database for small molecule modulators of epigenetic landscape. Database 2015, bav013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medvedeva YA et al. (2015) Epifactors: a comprehensive database of human epigenetic factors and complexes. Database 2015, bav067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh Nanda J et al. (2016) dbEM: a database of epigenetic modifiers curated from cancerous and normal genomes. Sci. Rep 6, 19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reymond J-L. (2015) The chemical space project. Acc. Chem. Res 48, 722–730 [DOI] [PubMed] [Google Scholar]
- 27.Prieto-Martinez FD et al. (2016) A chemical space odyssey of inhibitors of histone deacetylases and bromodomains. RSC Adv. 6, 56225–56239 [Google Scholar]
- 28.Chang P et al. (2018) Histone deacetylase inhibitors: Isoform selectivity improves survival in a hemorrhagic shock model. J. Trauma Acute Care Surg 84, 795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loharch S and Parkesh R. (2019) Epigenetic drug discovery: systematic assessment of chemical space. Fut. Med. Chem 11, 2803–2819 [DOI] [PubMed] [Google Scholar]
- 30.Probst D and Reymond J-L (2020) Visualization of very large high-dimensional data sets as minimum spanning trees. J. Cheminf 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Cruz N and Medina-Franco JL (2018) Statistical-based database fingerprint: chemical space dependent representation of compound databases. J. Cheminf 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muratov EN et al. (2020) QSAR without borders. Chem. Soc. Rev 49, 3525–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguayo-Ortiz R and Fernández-de Gortari E. (2016) Overview of computer-aided drug design for epigenetic targets. In Epi-informatics (MedinaFranco JL, ed.), pp. 21–52, Academic Press [Google Scholar]
- 34.Norinder U et al. (2019) Conformal prediction of HDAC inhibitors. SAR QSAR Environ. Res 30, 265–277 [DOI] [PubMed] [Google Scholar]
- 35.Sirous H et al. (2020) Computer-driven development of an in silico tool for finding selective histone deacetylase 1 inhibitors. Molecules 25, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Sánchez MO et al. (2017) Quantitative structure–epigenetic activity relationships. Adv. QSAR Model 24, 303–338 [Google Scholar]
- 37.Phanus-Umporn C et al. (2020) QSAR-driven rational design of novel DNA methyltransferase 1 inhibitors. EXCLI J. 19, 458–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speck-Planche A and Scotti MT (2019) BET bromodomain inhibitors: fragment-based in silico design using multi-target QSAR models. Mol. Diver 23, 555–572 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X et al. (2020) Molecular docking, 3D-QSAR, and molecular dynamics simulations of thieno[3,2-b]pyrrole derivatives against anticancer targets of KDM1A/LSD1. J. Biomol. Struct. Dyn Published online February 21, 2020. 10.1080/07391102.2020.1726819 [DOI] [PubMed] [Google Scholar]
- 40.Sabatino M et al. (2018) Disruptor of telomeric silencing 1-like (DOT1L): Disclosing a new class of non-nucleoside inhibitors by means of ligand-based and structure-based approaches. J. Comp. Aided Mol. Des 32, 435–458 [DOI] [PubMed] [Google Scholar]
- 41.Iqbal J et al. (2020) Activity landscape image analysis using convolutional neural networks. J. Cheminf 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H et al. (2019) Systematic identification of target set-dependent activity cliffs. Future Sci. OA 5, FSO363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naveja JJ and Medina-Franco JL (2015) Activity landscape of DNA methyltransferase inhibitors bridges chemoinformatics with epigenetic drug discovery. Expert Opin. Drug Discov 10, 1059–1070. [DOI] [PubMed] [Google Scholar]
- 44.Naveja JJ and Medina-Franco JL (2015) Activity landscape sweeping: into the mechanism of inhibition and optimization of dnmt1 inhibitors. RSC Adv. 5, 63882–63895 [Google Scholar]
- 45.Saldívar-González FI et al. (2017) Getting SMARt in drug discovery: chemoinformatics approaches for mining structure–multiple activity relationships. RSC Adv. 7, 632–641 [Google Scholar]
- 46.López-López E et al. (2020) Towards the understanding of the activity of G9a inhibitors: an activity landscape and molecular modeling approach. J. Comp. Aided Mol. Des 34, 659–669 [DOI] [PubMed] [Google Scholar]
- 47.Naveja JJ et al. (2018) Computational methods for epigenetic drug discovery: a focus on activity landscape modeling. Adv. Protein Chem. Struct. Biol 113, 65–83 [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Mayorga K et al. (2020) The impact of chemoinformatics on drug discovery in the pharmaceutical industry. Expert Opin. Drug Discov 15, 293–306 [DOI] [PubMed] [Google Scholar]
- 49.Talele TT et al. (2010) Successful applications of computer aided drug discovery: moving drugs from concept to the clinic. Curr. Top. Med. Chem 10, 127–141 [DOI] [PubMed] [Google Scholar]
- 50.Leelananda SP and Lindert S. (2016) Computational methods in drug discovery. Beilstein J. Org. Chem 12, 2694–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sivakumar KC et al. (2020) Prospects of multitarget drug designing strategies by linking molecular docking and molecular dynamics to explore the protein–ligand recognition process. Drug Dev. Res 81, 685–699 [DOI] [PubMed] [Google Scholar]
- 52.Kuang M et al. (2015) Binding kinetics versus affinities in BRD4 inhibition. J. Chem. Inf. Model 55, 1926–1935 [DOI] [PubMed] [Google Scholar]
- 53.Chodera JD et al. (2011) Alchemical free energy methods for drug discovery: progress and challenges. Curr. Opin. Struct. Biol 21, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanette C et al. (2019) Toward learned chemical perception of force field typing rules. J. Chem. Theory. Comput 15, 402–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Best RB (2019) Atomistic force fields for proteins. Methods Mol. Biol 2022, 3–19 [DOI] [PubMed] [Google Scholar]
- 56.Lu S et al. (2018) Discovery of hidden allosteric sites as novel targets for allosteric drug design. Drug Discov. Today 23, 359–365 [DOI] [PubMed] [Google Scholar]
- 57.Yang CY and Wang S. (2010) Computational analysis of protein hotspots. ACS Med. Chem. Lett 1, 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Letfus V et al. (2020) Rational design, synthesis and biological profiling of new KDM4C inhibitors. Bioorg. Med. Chem 28, 115128 [DOI] [PubMed] [Google Scholar]
- 59.Tao H et al. (2019) Discovery of novel PRMT5 inhibitors by virtual screening and biological evaluations. Chem. Pharm. Bull 67, 382–388 [DOI] [PubMed] [Google Scholar]
- 60.Song Q et al. (2019) An improved protocol for the virtual screening discovery of novel histone deacetylase inhibitors. Chem. Pharm. Bull 67, 1076–1081 [DOI] [PubMed] [Google Scholar]
- 61.Alves V et al. (2020) QSAR modeling of SARS-CoV Mpro inhibitors identifies sufugolix, cenicriviroc, proglumetacin and other drugs as candidates for repurposing against SARS-CoV–2. Mol. Inf. Published online July 28, 2020. 10.1002/minf.202000113 [DOI] [PubMed] [Google Scholar]
- 62.Capuzzi SJ et al. (2018) Computer-aided discovery and characterization of novel ebola virus inhibitors. J. Med. Chem 61, 3582–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajjo R et al. (2010) Development, validation, and use of quantitative structure–activity relationship models of 5-hydroxytryptamine (2B) receptor ligands to identify novel receptor binders and putative valvulopathic compounds among common drugs. J. Med. Chem 53, 7573–7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gozalbes R et al. (2005) QSAR strategy and experimental validation for the development of a GPCR focused library. QSAR Comb. Sci 24, 508–516 [Google Scholar]
- 65.Lima MNN et al. (2018) QSAR-driven design and discovery of novel compounds with antiplasmodial and transmission blocking activities. Front. Pharmacol 9, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballante F et al. (2017) Structural insights of SmKDAC8 inhibitors: Targeting schistosoma epigenetics through a combined structure-based 3D QSAR, in vitro and synthesis strategy. Bioorg. Med. Chem 25, 2105–2132 [DOI] [PubMed] [Google Scholar]
- 67.Durán-Iturbide NA et al. (2020) In silico ADME/Tox profiling of natural products: a focus on BIOFACQUIM. ACS Omega 5, 16076–16084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyu J et al. (2019) Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gentile F et al. (2020) Deep docking: a deep learning platform for augmentation of structure based drug discovery. ACS Cent. Sci 6, 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popova M et al. (2018) Deep reinforcement learning for de novo drug design. Sci. Adv 4, eaap7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhavoronkov A et al. (2019) Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol 37, 1038–1040 [DOI] [PubMed] [Google Scholar]
- 72.Walters WP and Murcko M. (2020) Assessing the impact of generative ai on medicinal chemistry. Nat. Biotechnol 38, 143–145 [DOI] [PubMed] [Google Scholar]
- 73.Bantscheff M et al. (2009) Revealing promiscuous drug–target interactions by chemical proteomics. Drug Discov. Today 14, 1021–1029 [DOI] [PubMed] [Google Scholar]
- 74.Van Norman GA (2019) Phase II trials in drug development and adaptive trial design. JACC Basic Transl. Sci 4, 428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters JU (2013) Polypharmacology - foe or friend? J. Med. Chem 56, 8955–8971 [DOI] [PubMed] [Google Scholar]
- 76.Zhang W et al. (2017) Computational multitarget drug design. J Chem Inf Model 57, 403–412 [DOI] [PubMed] [Google Scholar]
- 77.Bechter O and Schöffski P. (2020) Make your best BET: the emerging role of BET inhibitor treatment in malignant tumors. Pharmacol. Ther 208, 107479 [DOI] [PubMed] [Google Scholar]
- 78.Atkinson SJ et al. (2014) The structure based design of dual HDAC/BET inhibitors as novel epigenetic probes. MedChemComm 5, 342–351 [Google Scholar]
- 79.Yuan Z et al. (2019) Development of a versatile DNMT and HDAC inhibitor C02S modulating multiple cancer hallmarks for breast cancer therapy. Bioorg. Chem 87, 200–208 [DOI] [PubMed] [Google Scholar]
- 80.San José-Enériz E et al. (2017) Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun 8, 15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.