Abstract
To gain regulatory approval for the clinical use of knee biologics and devices in humans, translational large-animal studies are typically required. Animal models that permit second-look arthroscopy are valuable because they allow for longitudinal assessment of the treated tissue without needing to sacrifice the animal. The minipig is an ideal preclinical animal model for the investigation of therapies for the knee, in part because arthroscopy can be performed in its stifle (knee) joint with the use of standard surgical equipment used in humans. The purpose of this Technical Note is to describe a reproducible technique for diagnostic arthroscopy of the minipig stifle (knee) joint.
Technique Video
Diagnostic arthroscopy of the right minipig stifle (knee) joint. An anterolateral portal is established, the arthroscope is introduced through the anterolateral portal and into the medial compartment, and an anteromedial portal is established under spinal needle localization. The medial compartment, intercondylar notch, lateral compartment, and distal trochlea can be visualized. Visualization of the lateral compartment can be enhanced by placing the arthroscope through the anteromedial portal. The proximal portion of the patellofemoral joint can be difficult to visualize through the anterior portals because of its elongated morphology and inability to fully extend the minipig knee. Visualization of the proximal patellofemoral joint can be performed through a separate superior portal if necessary.
Translational animal models are critical tools for investigating new therapies in preclinical studies before progression to human clinical trials. For treatments in the knee, the minipig stifle (knee) is an ideal animal model because of its docile nature, sufficient cartilage thickness,1 joint loading biomechanics that better simulate the adult human condition compared to other large animals,2,3 and feasibility of arthroscopy.4 Yucatan and Göttingen minipigs have been used for the study of anterior cruciate ligament (ACL) and articular cartilage repair strategies.5,6 Second-look knee arthroscopy is a valuable tool that allows for clear intra-articular visualization of the treated tissue without needing to sacrifice the animal, permitting longitudinal assessment of treatment strategies after surgery. The purpose of this Technical Note is to describe a reproducible technique for diagnostic arthroscopy of the minipig stifle (knee) joint.
Surgical Technique
Animal Positioning and Arthroscopy Set-Up
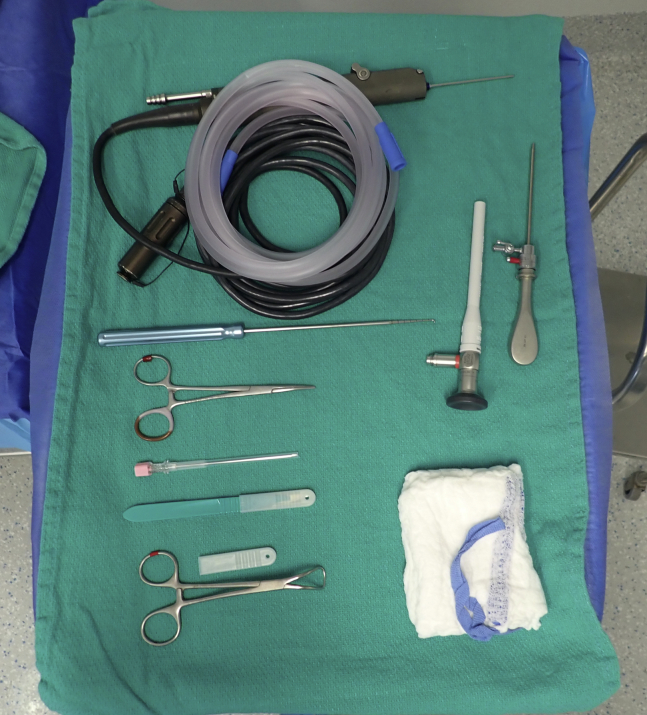
After induction of anesthesia, the minipig is placed supine on the operating room table. A padded bump is placed lateral to the operative leg to limit external rotation of the hip, allowing for more controlled range of motion of the knee. Alternatively, a lateral post may be used to assist with producing a valgus force to open the medial compartment. Standard arthroscopy instruments, including a 2.4-mm arthroscope with a 30° visual angle, 4-mm hook probe, and 2.0-mm arthroscopic shaver (Arthrex Sabre, SJ 2.0 mm × 7 cm), are used (Fig 1). Epinephrine is added to the saline solution bags at a standard dose of 0.33 mg/L. An arthroscopy pump system is used to maintain an intra-articular pressure of 40 mm Hg.
Fig 1.
Arthroscopy setup and instrumentation for diagnostic arthroscopy of the minipig stifle (knee).
Diagnostic Technique
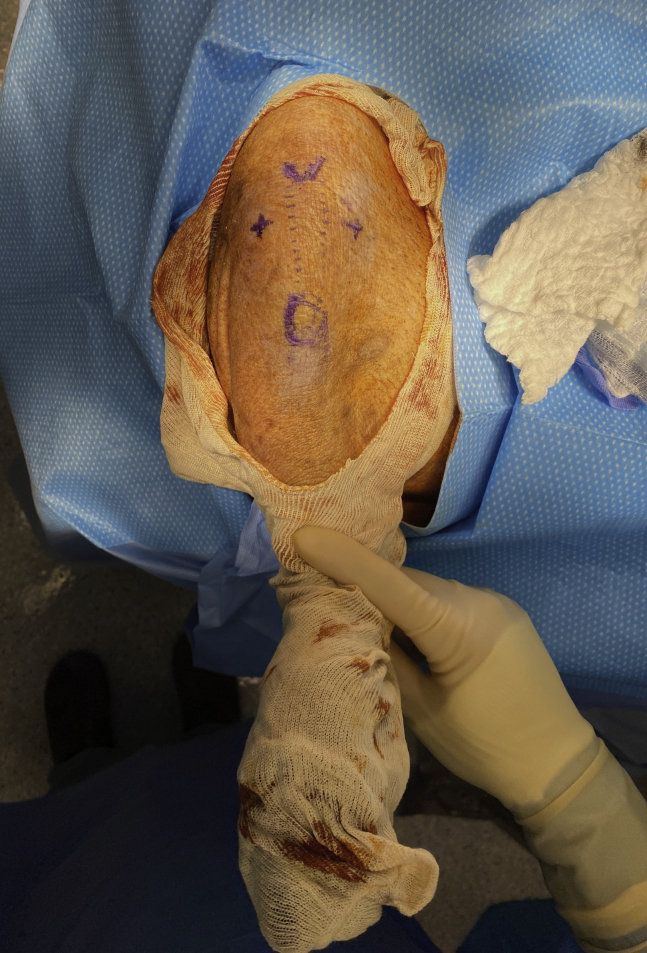
The joint line is palpated, and with the knee positioned at approximately 90° to 100° of flexion, the anterolateral and anteromedial portals are outlined with a surgical marker. Compared to the human knee, the patella of the minipig knee is typically positioned more proximally (equivalent to patella alta), and therefore the anterior portals should be made distal to the level of the inferior pole of the patella in order to gain access to the infrapatellar compartment (Fig 2). It is also important to note that minipig knees have a physiological passive range of motion from 42° to 144° of flexion.7 Pertinent anatomic differences between the minipig and human knee for arthroscopy should be recognized to successfully navigate the joint (Table 1 and Fig 3). The anterolateral portal is established with an 11-blade scalpel, and the arthroscope is placed intra-articularly and directed posterior and medial to the infrapatellar fat pad. The joint is then insufflated. The anteromedial portal can then be made under direct visualization with spinal needle localization and established with the 11-blade scalpel. A hemostat may be used to slightly enlarge the portals to facilitate easy passage of arthroscopic instruments.
Fig 2.
Right minipig knee with marked anterolateral and anteromedial portals (indicated by +) for arthroscopy. The inferior pole of the patella and tibial tubercle are also marked.
Table 1.
Pertinent differences between minipig and human anatomy for knee arthroscopy
| Difference in minipig knee compared to human knee |
| Smaller size |
| More proximal positioning of the patella (patella alta) |
| Physiologic passive range of motion from 42-144° of flexion |
| EDL is intra-articular |
| Elongated patellofemoral joint |
| Adjustment in arthroscopic surgical technique |
| Use of 2.4-mm arthroscope, instead of 4.0-mm arthroscope |
| Placement of anterior portals distal to the level of the inferior pole of the patella when the knee is at 90° of flexion |
| Arthroscope should be first introduced into the infrapatellar compartments |
| Need to retract EDL to fully visualize the lateral compartment |
| Through the anterior portals, only the middle and distal trochlea can be visualized. An accessory superolateral or superomedial portal is needed to access the proximal patellofemoral joint |
EDL, Extensor digitorum longus.
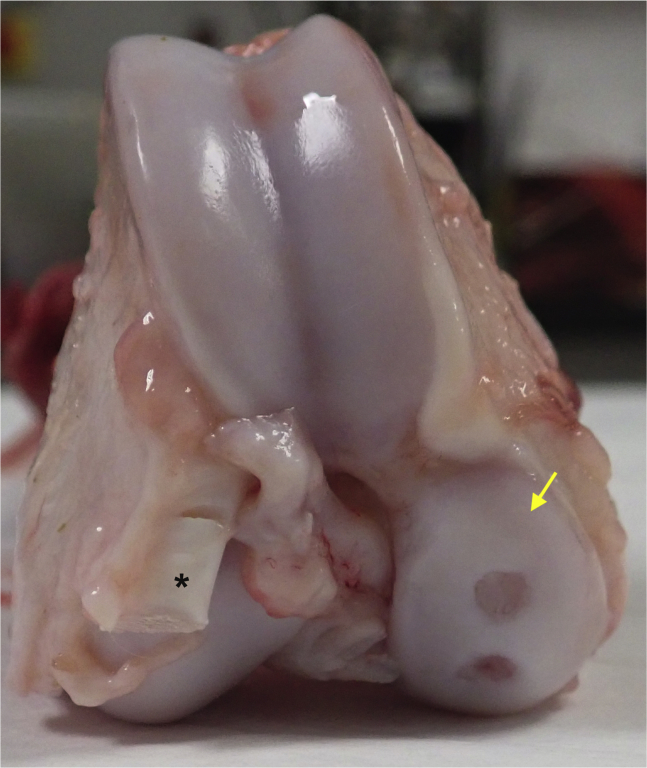
Fig 3.
Cadaveric specimen of a right minipig distal femur. Two chondral defects have been created on the medial femoral condyle (yellow arrow). Note the presence of the extensor digitorum longus (cut and marked by ∗) originating from the craniolateral aspect of the lateral femoral condyle and the relative length of the trochlea compared to that in humans.
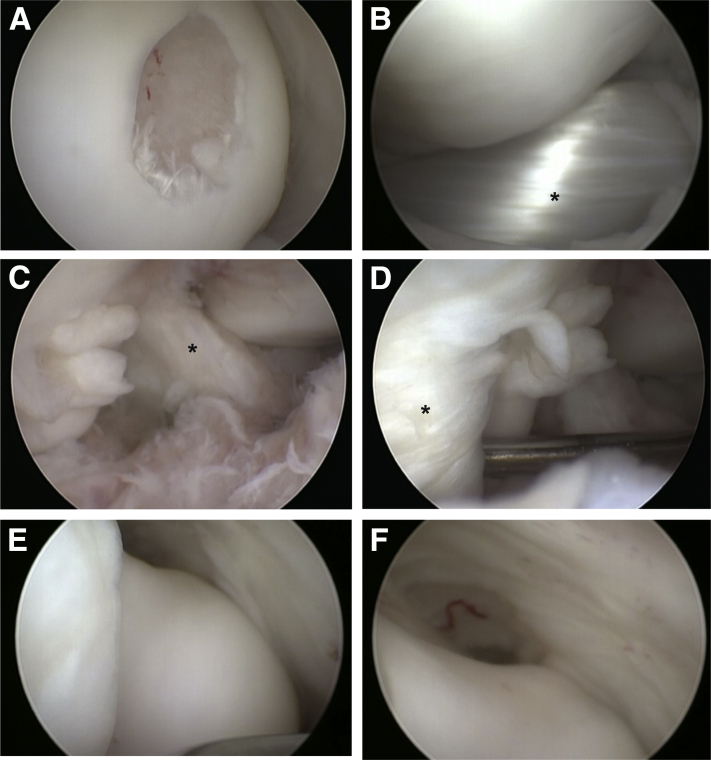
After irrigation of the joint, a standard arthroscopic examination can be performed (Video 1). The arthroscope can be directed to the medial compartment to visualize the medial femoral condyle and anterior horn and body of the medial meniscus (Fig. 4A and B). The knee can be extended to visualize the anterior aspect of the medial femoral condyle and flexed to visualize the posterior aspect of the medial femoral condyle. Visualization of the posterior horn of the medial meniscus can be difficult given the small size of the joint, even with a valgus force applied. An arthroscopic hook probe can be introduced through the anteromedial portal for manual palpation. The arthroscopic can then be turned to visualize the intercondylar notch (Fig. 4C). The ligamentum mucosum can be released at its origin from the anterior intercondylar notch with an arthroscopic shaver to facilitate retraction of the infrapatellar fat pad and visualization of the cruciate ligaments.
Fig 4.
Viewing from the anterolateral portal of the right minipig knee, intra-articular arthroscopic visualization of the (A) medial femoral condyle with a surgically created 5-mm chondral defect, (B) medial meniscus (marked by ∗), (C) intercondylar notch and anterior cruciate ligament (marked by ∗), (D) lateral compartment with an arthroscopic probe behind the extensor digitorum longus and its synovial tendon sheath (marked by ∗), which is intra-articular in the minipig stifle, (E) distal trochlea, and (F) medial gutter.
Visualization of the lateral compartment can be obtained with the arthroscope through the anterolateral portal but may be enhanced by placing the arthroscope through the anteromedial portal with the 30° arthroscope. Unlike in humans, the extensor digitorum longus tendon is intra-articular and originates from the craniolateral aspect of the lateral femoral condyle (Figs 3 and 4D). It can obstruct visualization of the anterior lateral femoral condyle. A hook probe may be used to retract the tendon for better visualization of the lateral femoral condyle and meniscus.
Finally, the middle and distal trochlea can be visualized by placing the knee in maximum extension (Fig 4E and F). Because of the elongated anatomy of the minipig trochlea and inability to extend the knee to 0°, the proximal trochlea and patella are typically not well visualized through the anterior portals. Therefore a superolateral or superomedial portal can be made to access the proximal patellofemoral joint and suprapatellar pouch.
Postoperative Protocol
The minipig is recovered from anesthesia in a padded suspension sling (Fig 5) to maintain protected weightbearing of the operated limb, followed by transfer to the pen after the animal is capable of standing independently (typically 3-5 hours after extubation). Commercially available minipig slings are commonly used for temporary restraint of the animal to allow for veterinary exams and medical procedures, and other groups have used them to limit weightbearing of operated limbs after surgery.8 After transfer to the pen, the minipigs are allowed activity as desired.
Fig 5.
Mini-pig resting in padded sling to maintain non-weightbearing while recovering from anesthesia after surgery.
Discussion
The minipig is an ideal preclinical animal model to study knee conditions and therapies because of its docile nature, sufficient cartilage thickness (∼2-3 mm),1 joint loading biomechanics that better simulate the adult human condition compared to other large animals,2,3 and feasibility of arthroscopy of the stifle (knee) joint.4 The porcine knee is much larger than canine knee and compares favorably in size to goat and sheep knees,7 allowing for arthroscopy with standard surgical equipment used in humans. Many similarities exist between the human and minipig knee, and therefore arthroscopy skill sets are easily translatable between the two species. In fact, porcine knees have proven to be a valid model for arthroscopic surgical training and skill assessment.9
Minipigs are among one of several preferred large animal models for the study of knee ligament, cartilage, and meniscus treatments, and the ability to perform knee arthroscopy in these animals is valuable for testing the feasibility of arthroscopic methods and for second-look visualization of the treated tissue without needing to sacrifice the animal. For the Food and Drug Administration approval of new drugs, biologics, and devices, animal studies that assess endpoints such as safety, proof of concept for clinical efficacy, durability, and dose response, are typically required. To gain regulatory approval for clinical use of knee therapies in humans, translational large animal studies that are a minimum of 1 year in length are recommended. Second-look arthroscopic evaluations allow for longitudinal, interim assessments and are more cost-effective than magnetic resonance imaging. The minipig knee can be easily accessed with standard arthroscopy tools with good visualization of the medial and lateral compartments, intercondylar notch, and distal trochlea. Only the proximal trochlea and patella are difficult to visualize through the standard anterior portals. However, in many quadruped animals, an extended open medial or lateral parapatellar arthrotomy with subluxation of the patella often leads to iatrogenic patellar maltracking and instability postoperatively, even after meticulous repair of the retinaculum.10 Therefore this portion of the trochlea is typically not a region used for scientific investigation.
In summary, the minipig is an ideal preclinical animal model for the investigation of therapies for the knee, in part because its knee joint is large enough to permit arthroscopy with the use of standard surgical equipment. Because the human and minipig knees are very similar, an arthroscopy skill set for the human knee is easily translatable to the minipig knee.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Diagnostic arthroscopy of the right minipig stifle (knee) joint. An anterolateral portal is established, the arthroscope is introduced through the anterolateral portal and into the medial compartment, and an anteromedial portal is established under spinal needle localization. The medial compartment, intercondylar notch, lateral compartment, and distal trochlea can be visualized. Visualization of the lateral compartment can be enhanced by placing the arthroscope through the anteromedial portal. The proximal portion of the patellofemoral joint can be difficult to visualize through the anterior portals because of its elongated morphology and inability to fully extend the minipig knee. Visualization of the proximal patellofemoral joint can be performed through a separate superior portal if necessary.
References
- 1.Frisbie D.D., Cross M.W., McIlwraith C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142–146. [PubMed] [Google Scholar]
- 2.Jiang C.C., Chiang H., Liao C.J. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res. 2007;25:1277–1290. doi: 10.1002/jor.20442. [DOI] [PubMed] [Google Scholar]
- 3.Chu C.R., Szczodry M., Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelle S., Zantop T., Schanz S., Petersen W. Arthroscopic techniques for the fixation of a three-dimensional scaffold for autologous chondrocyte transplantation: structural properties in an in vitro model. Arthroscopy. 2007;23:1073–1078. doi: 10.1016/j.arthro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Christensen B.B., Foldager C.B., Olesen M.L. Experimental articular cartilage repair in the Gottingen minipig: the influence of multiple defects per knee. J Exp Orthop. 2015;2:13. doi: 10.1186/s40634-015-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray M.M., Fleming B.C. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proffen B.L., McElfresh M., Fleming B.C., Murray M.M. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–499. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A.C., Swindle M.M. Preparation of swine for the laboratory. ILAR J. 2006;47:358–363. doi: 10.1093/ilar.47.4.358. [DOI] [PubMed] [Google Scholar]
- 9.Martin R.K., Gillis D., Leiter J., Shantz J.S., MacDonald P. A Porcine Knee Model Is Valid for Use in the Evaluation of Arthroscopic Skills: A Pilot Study. Clin Orthop Relat Res. 2016;474:965–970. doi: 10.1007/s11999-015-4498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orth P., Madry H. A low morbidity surgical approach to the sheep femoral trochlea. BMC Musculoskelet Disord. 2013;14:5. doi: 10.1186/1471-2474-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic arthroscopy of the right minipig stifle (knee) joint. An anterolateral portal is established, the arthroscope is introduced through the anterolateral portal and into the medial compartment, and an anteromedial portal is established under spinal needle localization. The medial compartment, intercondylar notch, lateral compartment, and distal trochlea can be visualized. Visualization of the lateral compartment can be enhanced by placing the arthroscope through the anteromedial portal. The proximal portion of the patellofemoral joint can be difficult to visualize through the anterior portals because of its elongated morphology and inability to fully extend the minipig knee. Visualization of the proximal patellofemoral joint can be performed through a separate superior portal if necessary.
Diagnostic arthroscopy of the right minipig stifle (knee) joint. An anterolateral portal is established, the arthroscope is introduced through the anterolateral portal and into the medial compartment, and an anteromedial portal is established under spinal needle localization. The medial compartment, intercondylar notch, lateral compartment, and distal trochlea can be visualized. Visualization of the lateral compartment can be enhanced by placing the arthroscope through the anteromedial portal. The proximal portion of the patellofemoral joint can be difficult to visualize through the anterior portals because of its elongated morphology and inability to fully extend the minipig knee. Visualization of the proximal patellofemoral joint can be performed through a separate superior portal if necessary.