Abstract
Proximal hamstring tendon avulsions are a relatively rare type of hamstring injury associated with persistent morbidity, including pain, weakness, and functional limitations. Open or endoscopic surgical repair is the standard treatment for complete tendon avulsions or partial tears that remain symptomatic despite conservative management in relatively young, healthy, and active patients. However, complications known to occur include retearing of the hamstring, infection, nerve injury, inability to return to work or sport, subjective persistent weakness, and subjective persistent pain. In the case of persistent pain where the repair is partially retorn, a careful history, physical examination, and scrutiny of radiologic studies can help guide management. We describe a technique for using revision endoscopy and augmentation with a bovine bioinductive patch in a case of chronic persistently painful partial retear after a proximal hamstring repair.
Technique Video
This video demonstrates a technique for endoscopic implantation of a bioinductive patch for symptomatic chronic partial retearing of the proximal hamstring after failed repair. This is a case of chronic partial retearing of the right proximal hamstring, 2 years out from the index repair. T2 coronal imaging shows signal within the repair and the axial view shows a prominent suture anchor laterally with retearing of the semimembranosus. The patient was indicated for revision endoscopy after not responding to nonoperative management. The patient was placed in the prone position after general anesthesia. The right lower extremity was prepped and draped in the usual sterile fashion. The previous direct posterior portal in the gluteal crease was used to introduce a 30° arthroscope into the space between the gluteus maximus and ischial tuberosity. Next, a superolateral working portal was established to perform an ischial bursectomy. The sciatic nerve was then identified and a thorough neurolysis was performed. The proximal hamstring repair site was debrided and visualized revealing a prominent suture anchor and partial retear superolaterally. The suture anchor was burred down using a 5.5 partially hooded round burr. The arthroscope was then switched to the superolateral portal, and a third portal was made approximately 3-4 fingerbreadths proximal to the direct posterior portal to help facilitate staple fixation of the implant. A medium bioinductive patch is then passed through the direct posterior portal with the assistance of a skid and centered over the proximal hamstring tendon origin. Staple fixation was performed until sufficiently stable to remove the inserter. Additional fixation was then performed until adequate stability was achieved.
Proximal hamstring tears or avulsions account for a mere 9% to 12% of all hamstring complex injuries.1 The proximal hamstring anatomy consists of the semimembranosus, semitendinosus, and biceps femoris long head tendons attaching to the posterior, superior, and lateral aspect of the ischial tuberosity. The semitendinosus and long head of the biceps femoris typically form a conjoined tendon with a slightly more medial attachment on the ischial tuberosity than the semimembranosus.2 Indications for repair include complete 3-tendon tear with or without significant retraction in a young active patient or partial tears that remain symptomatic despite extensive conservative treatment.3, 4, 5, 6
Outcomes of open proximal hamstring repair have been shown to be superior to nonoperative management.7, 8, 9, 10, 11, 12 However, the overall complication rate is 23.17%, and neurologic complications comprise with the greatest incidence.7 Endoscopic proximal hamstring repair has shown promise with equivalent clinical outcomes and fewer complications reported.9,12 A notorious minor complication is persistent pain with sitting, with rates as high as 41% to 48% reported in the literature.9,13 Kurowicki et al.12 reported their outcomes on endoscopic proximal hamstring repair and found an incidence of pain with sitting of 16%. A follow-up magnetic resonance imaging (MRI) outcome study by Chahal et al.14 showed that by a mean of 36.9 months after repair, all the patients in their series showed signs of healing on MRI, although 3 of 12 with signs of tendinopathy and mild atrophy. Chronic pain with often-subtle signs on MRI of partial retearing or significant tendinopathy can present a challenge to practitioners.
Nonoperative management should be exhausted in patients after repair where there is persistent pain, particularly with activities of daily living, and signs of partial retearing or tendinopathy. Surgical options traditionally include revision with debridement, sciatic neurolysis, or revision repair. However, there is a promising technology to promote vascularization and growth. The REGENETEN (Smith & Nephew, Memphis, TN) bovine bioinductive implant acts as a highly porous collagen scaffold that allows vascular ingrowth, induces collagen formation, remodeling, and increased overall thickness of damaged tendon.15 This implant has been used in other applications, most convincingly and successfully in partial, medium, large, and massive rotator cuff tears with a reported 96% healing rate in the latter with no reported complications.15, 16, 17 It has also been described for use in the augmentation of a hip capsular reconstruction.18 Persistent pain and tendinopathy of the proximal hamstring can cause significant patient morbidity and dissatisfaction. In these cases, there may be a role for augmentation with a bovine bioinductive implant using the technique described in this article and summarized in Table 1. The clinical indications for this technique are outlined in Table 2, and the surgical pearls and pitfalls are described in Table 3.
Table 1.
Surgical Steps in Endoscopic Bioinductive Patch Augmentation for Proximal Hamstring
|
|
|
|
|
|
|
Table 2.
Indications and Contraindications for Bioinductive Implant Augmentation for Painful Chronic Retearing or Tendinopathy After Repair
| Indications | Contraindications |
|---|---|
|
|
|
|
|
|
|
|
Table 3.
Surgical Pearls, Pitfalls, Risks, and Limitations
| Pearls |
|---|
| A thorough diagnostic endoscopy will reveal what else can be done to remove pain |
| generators. |
| Appropriate sciatic neurolysis is recommended. |
| Ensure the direct posterior portal will provide appropriate trajectory for overlying the |
| graft on the proximal hamstring origin. |
| Accessory portal 3-4 fingerbreadths proximal to direct posterior portal will aid in staple |
| fixation. |
| A skid may facilitate graft passage. |
| A minimum of 6 staples are required for graft stability. |
| Pitfalls |
| Poor patient selection (differential includes lumbar radiculopathy, ischiofemoral |
| impingement, deep gluteal syndrome) can lead to suboptimal results. |
| Inadequate accessory portal placement can lead to staple misfire. |
| Risks and limitations |
| The sciatic nerve is at risk and should be identified, neurolysis performed, and protected throughout the entire procedure. |
| This procedure is indicated for patients who have exhausted a thorough trial of nonoperative management and should not be first line treatment for symptomatic partial proximal hamstring tears |
Surgical Technique (With Video Illustration)
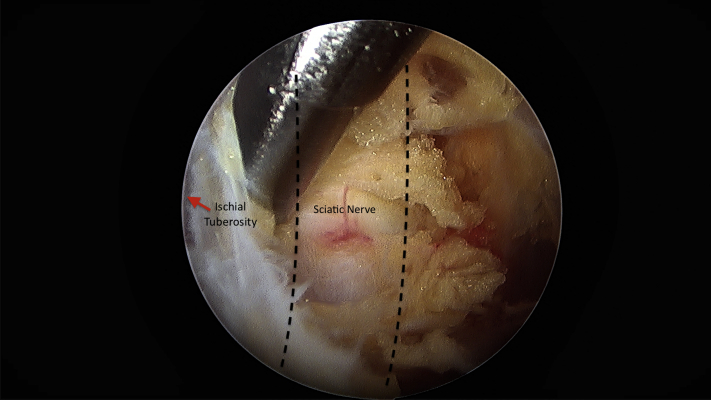
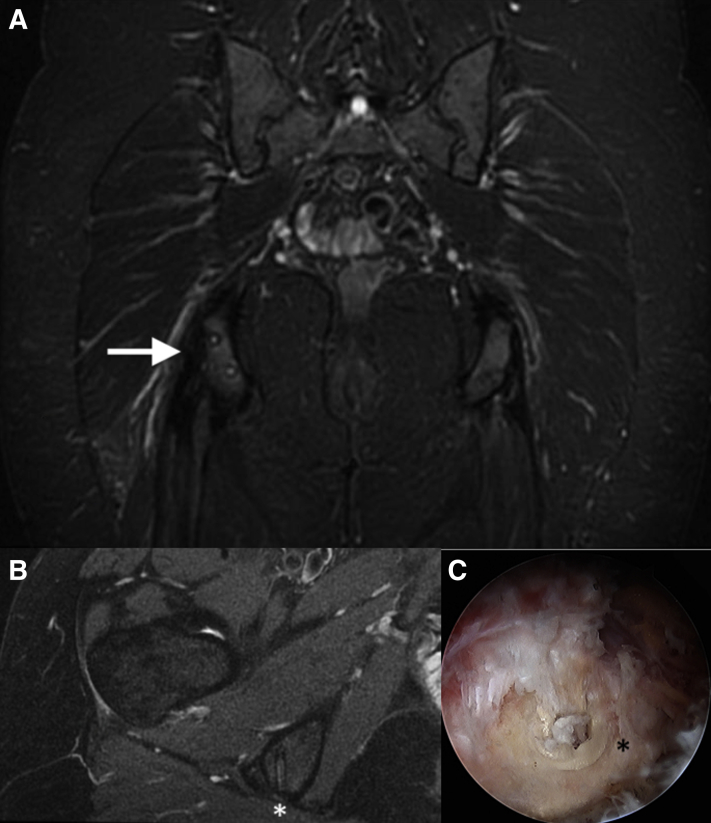
The patient is placed in the prone position under general anesthesia. The previous direct posterior portal in the gluteal crease is used, centered over the ischial tuberosity. A 30° arthroscope is introduced into the space between the gluteus maximus and the ischial tuberosity. Next, a superolateral working portal is established approximately 3-4 fingerbreadths proximally and 2-3 fingerbreadths laterally. This portal is used to perform an ischial bursectomy. A 90° radiofrequency ablator is paramount to achieve and maintain hemostasis in this space. The sciatic nerve is then identified laterally between the ischial tuberosity and greater trochanter. The nerve is safely found just lateral to the ischial tuberosity, and a motorized shaver is used to clear bursa alternating with use as a blunt dissection tool to avoid injury to the sciatic nerve. A thorough neurolysis is then performed (Fig 1). The proximal hamstring repair is identified and probed under direct visualization. While the repair appears mostly intact, there is a partial retear superolaterally with an exposed prominent anchor identified both on preoperative MRI and intraoperatively (Fig 2). This is then burred down to eliminate any prominent hardware irritation.
Fig 1.
Ischial bursectomy is performed on a right hip viewing from the direct posterior portal and alternating a motorized shaver with a 90° radiofrequency ablator through a superolateral portal. The sciatic nerve is identified using either instrument as a blunt dissector and sciatic neurolysis is performed.
Fig 2.
(A) T2-weighted coronal image of a right hip showing intact repair with signal intensity suggestive of chronic retearing and associated tendinopathy (arrow), (B) T2-weighted axial image, and (C) arthroscopic image confirming prominent suture anchor (asterisk).
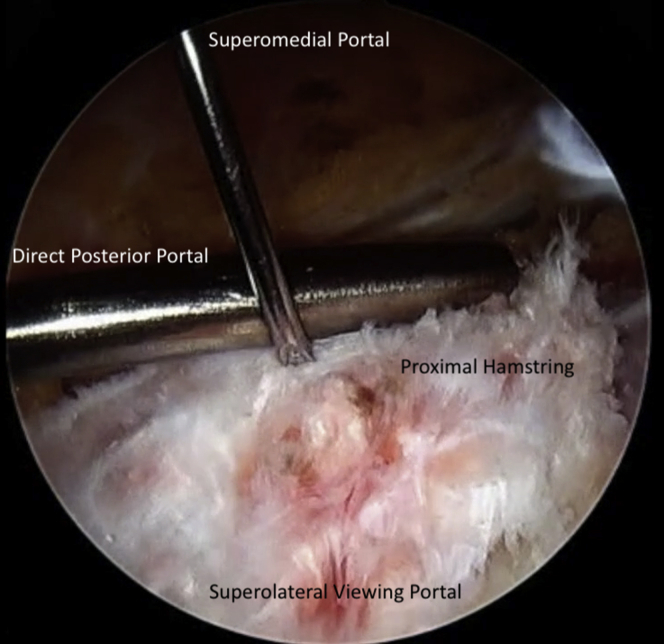
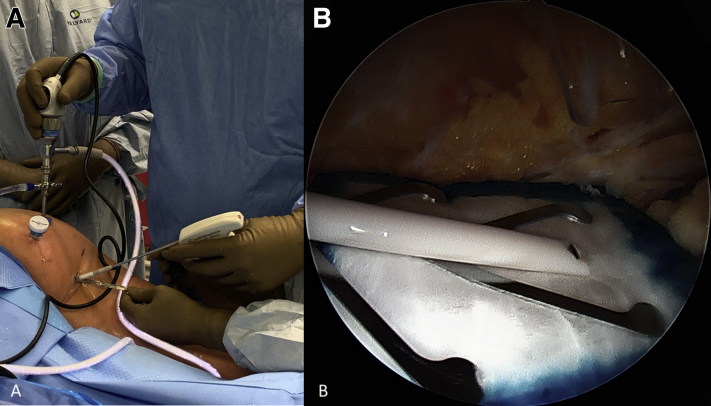
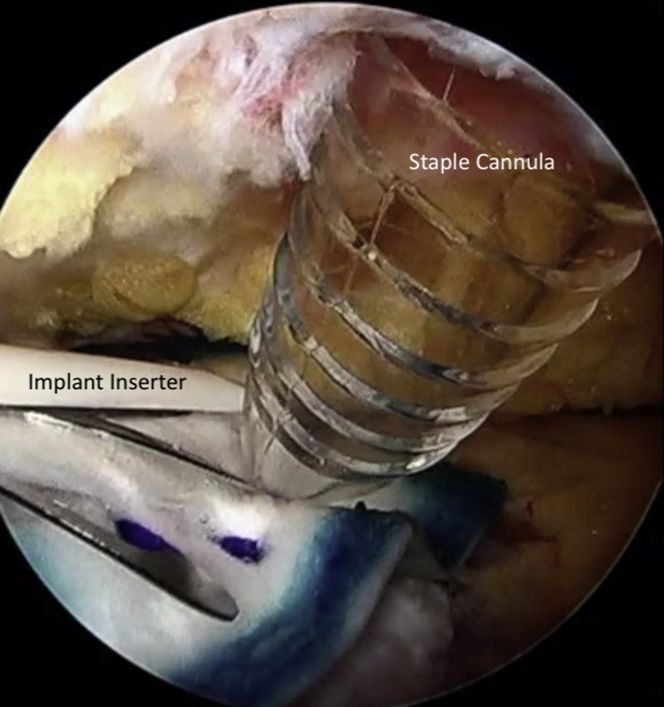
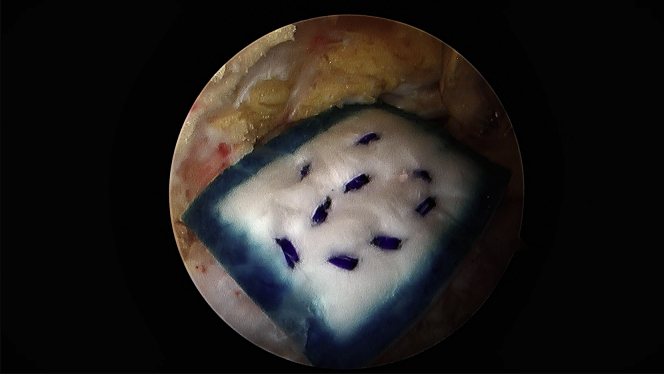
Attention is turned to the proximal hamstring and a third portal is made in line with the viewing portal, approximately 3-4 fingerbreadths proximal to facilitate staple fixation of the bioinductive patch. This allows for a perpendicular trajectory for staple placement (Fig 3). A medium bioinductive patch (20 mm × 24 mm) is then passed through the direct posterior portal with the assistance of a skid and centered over the proximal hamstring tendon origin (Fig 4). A minimum of 3 to 4 staples are placed before the patch inserter can be removed safely. The cannula for staple passage can be used to stabilize the implant while the inserter is gently backed out (Fig 5). Staple fixation is performed until satisfactory stability, typically no fewer than 6 bioabsorbable staples (Fig 6). Video 1 demonstrates this technique.
Fig 3.
An 18-gauge spinal needle is used to establish a superomedial portal approximately 3-4 fingerbreadths proximal to the direct posterior portal. This will be used for staple fixation of the bioinductive implant.
Fig 4.
(A) A skid is used to facilitate patch passage and (B) it is centered over the proximal hamstring.
Fig 5.
After a minimum of 3-4 staples are placed, the inserter can be safely removed by using the cannula for staple fixation as a stabilizer.
Fig 6.
Completed construct, viewing from the posterolateral portal showing appropriate bioinductive patch placement and adequate staple fixation.
Rehabilitation and Recovery
Postoperative care for this technique resembles our protocol for primary endoscopic proximal hamstring repair. Weight-bearing is protected with crutches for 4 to 6 weeks, followed by unrestricted weight-bearing, stretching, and closed chain exercises for the next 6 weeks. There is no limitation placed on range of motion and by 3 months gradual strengthening is permitted. Progressive return to usual exercise activities was allowed after 3 months, and the patient continued physical therapy until approximately 4.5 months after surgery. Her pain has now improved, and she has been able to resume ultimate frisbee.
Discussion
Proximal hamstring avulsions are uncommon yet debilitating injuries associated with pain, weakness, and functional limitations. Surgical repair is becoming the standard of care for patients who are active and high demand. While surgical repair has been demonstrated to outperform nonoperative management, it is important to keep in mind the well-documented complications. Of the possible complications, persistent pain with activities of daily living and sitting is relatively common. Chronicity of the tear has been shown to affect outcomes negatively in some studies, whereas others have shown repairing partial tears does not produce equivalent outcomes compared with acutely repaired complete tears.4,7,19, 20, 21 A recent case series by Kayani et al.22 showed excellent clinical results in 41 patients with chronic partial proximal hamstring avulsions, with all returning to preinjury activity levels by a mean of 22.2 months and no recurrent tears. There was high patient satisfaction, and improved functional outcomes were observed through the latest follow-up at 2 years.
The cause of postoperative pain can be multifactorial. A careful history and physical examination should pre-empt any treatment recommendation. In addition, MRI can help elucidate what pain generators may be present. The proximal hamstring origin is in close proximity to several neural structures, including the pudendal nerve superiorly, the posterior femoral cutaneous nerve superficially, and the sciatic nerve laterally.23 Any of these can be contributing to postoperative pain. Chahal et al.14 also showed that a significant portion of these repairs heal with residual signs of tendinopathy and atrophy. However, not all of these are symptomatic. In symptomatic cases, there may be a role for a bioinductive implant once nonoperative management has been exhausted. In this particular case, the patient underwent revision endoscopy 2 years after the index procedure. Patients should try a prolonged trial of nonoperative management before consideration for endoscopic bioinductive patch implantation.
The bovine bioinductive implant has shown promise for damaged tissue that needs augmentation to fully heal. It has been proven to induce the formation of new tendon consisting of well-organized collagen fibers in the direction of load transmission in a sheep model.15 In human trials, Bokor et al.15 showed 2 mm of increased rotator cuff tendon thickness at final follow-up after augmenting repair in medium-sized tears. Similarly, another study by the same authors showed a 2.2-mm increase in rotator cuff tendon thickness by 3 months even when a repair was not performed.24 Schlegel et al.16 further demonstrated that in partial-thickness rotator cuff tears, there was either no progression of tears or a reduction in defect size after 1 year. Thon et al.17 most recently published their results on repair augmentation in large and massive rotator cuff tears and found a 96% healing rate on ultrasound and MRI. We believe that these results indicate the induced tissue formation and increased thickness decreases the stress and strain at the tendon during dynamic activity. This technique should be reserved for those patients with persistent pain, functional limitation, chronic partial retearing, and tendinopathy in whom conservative management has been exhausted.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.B. reports personal fees from Stryker Endoscopy and Smith & Nephew and nonfinancial support from Vericel, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This video demonstrates a technique for endoscopic implantation of a bioinductive patch for symptomatic chronic partial retearing of the proximal hamstring after failed repair. This is a case of chronic partial retearing of the right proximal hamstring, 2 years out from the index repair. T2 coronal imaging shows signal within the repair and the axial view shows a prominent suture anchor laterally with retearing of the semimembranosus. The patient was indicated for revision endoscopy after not responding to nonoperative management. The patient was placed in the prone position after general anesthesia. The right lower extremity was prepped and draped in the usual sterile fashion. The previous direct posterior portal in the gluteal crease was used to introduce a 30° arthroscope into the space between the gluteus maximus and ischial tuberosity. Next, a superolateral working portal was established to perform an ischial bursectomy. The sciatic nerve was then identified and a thorough neurolysis was performed. The proximal hamstring repair site was debrided and visualized revealing a prominent suture anchor and partial retear superolaterally. The suture anchor was burred down using a 5.5 partially hooded round burr. The arthroscope was then switched to the superolateral portal, and a third portal was made approximately 3-4 fingerbreadths proximal to the direct posterior portal to help facilitate staple fixation of the implant. A medium bioinductive patch is then passed through the direct posterior portal with the assistance of a skid and centered over the proximal hamstring tendon origin. Staple fixation was performed until sufficiently stable to remove the inserter. Additional fixation was then performed until adequate stability was achieved.
References
- 1.Koulouris G., Connell D. Evaluation of the hamstring muscle complex following acute injury. Skeletal Radiol. 2003;32:582–589. doi: 10.1007/s00256-003-0674-5. [DOI] [PubMed] [Google Scholar]
- 2.Miller S.L., Gill J., Webb G.R. The proximal origin of the hamstrings and sorrounding anatomy encountered during repair: A cadaveric study. J Bone Joint Surg A. 2007;89:44–48. doi: 10.2106/JBJS.F.00094. [DOI] [PubMed] [Google Scholar]
- 3.Wood D.G., Packham I., Trikha S.P., Linklater J. Avulsion of the proximal hamstring origin. J Bone Joint Surg A. 2008;90:2365–2374. doi: 10.2106/JBJS.G.00685. [DOI] [PubMed] [Google Scholar]
- 4.Sarimo J., Lempainen L., Mattila K., Orava S. Complete proximal hamstring avulsions: A series of 41 patients with operative treatment. Am J Sports Med. 2008;36:1110–1115. doi: 10.1177/0363546508314427. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S., Bradley J. Acute proximal hamstring rupture. J Am Acad Orthop Surg. 2007;15:350–355. doi: 10.5435/00124635-200706000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Domb B.G., Linder D., Sharp K.G., Sadik A., Gerhardt M.B. Endoscopic repair of proximal hamstring avulsion. Arthrosc Tech. 2013;2:e35–e39. doi: 10.1016/j.eats.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodendorfer B.M., Curley A.J., Kotler J.A. Outcomes after operative and nonoperative treatment of proximal hamstring avulsions: A systematic review and meta-analysis. Am J Sports Med. 2018;46:2798–2808. doi: 10.1177/0363546517732526. [DOI] [PubMed] [Google Scholar]
- 8.Shambaugh B.C., Olsen J.R., Lacerte E., Kellum E., Miller S.L. A Comparison of nonoperative and operative treatment of complete proximal hamstring ruptures. Orthop J Sport Med. 2017;5:1–6. doi: 10.1177/2325967117738551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman E.N., Marshall N.E., Gerhardt M.B., Banffy M.B. Predictors of clinical outcomes after proximal hamstring repair. Orthop J Sport Med. 2019;7:1–6. doi: 10.1177/2325967118823712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakeney W.G., Zilko S.R., Edmonston S.J., Schupp N.E., Annear P.T. A prospective evaluation of proximal hamstring tendon avulsions: Improved functional outcomes following surgical repair. Knee Surg Sport Traumatol Arthrosc. 2017;25:1943–1950. doi: 10.1007/s00167-017-4475-0. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham P., Muller M., Wickiewicz T., Cavanaugh J., Rodeo S., Warren R. Functional outcome after repair of proximal hamstring avulsions. J Bone Joint Surg A. 2011;93:1819–1826. doi: 10.2106/JBJS.J.01372. [DOI] [PubMed] [Google Scholar]
- 12.Kurowicki J., Novack T.A., Simone E.S. Short-term outcomes following endoscopic proximal hamstring repair. Arthroscopy. 2020;36:1301–1307. doi: 10.1016/j.arthro.2019.11.126. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S.B., Rangavajjula A., Vyas D., Bradley J.P. Functional results and outcomes after repair of proximal hamstring avulsions. Am J Sports Med. 2012;40:2092–2098. doi: 10.1177/0363546512456012. [DOI] [PubMed] [Google Scholar]
- 14.Chahal J., Bush-Joseph C.A., Chow A. Clinical and magnetic resonance imaging outcomes after surgical repair of complete proximal hamstring ruptures: Does the tendon heal? Am J Sports Med. 2012;40:2325–2330. doi: 10.1177/0363546512453298. [DOI] [PubMed] [Google Scholar]
- 15.Bokor D.J., Sonnabend D., Deady L. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2015;5:144–150. doi: 10.11138/mltj/2015.5.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlegel T.F., Abrams J.S., Bushnell B.D., Brock J.L., Ho C.P. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018;27:242–251. doi: 10.1016/j.jse.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Thon S.G., O’Malley L., O’Brien M.J., Savoie F.H. Evaluation of healing rates and safety with a bioinductive collagen patch for large and massive rotator cuff tears: 2-year safety and clinical outcomes. Am J Sports Med. 2019;47:1901–1908. doi: 10.1177/0363546519850795. [DOI] [PubMed] [Google Scholar]
- 18.Larson C.M., Williams B.T., Bessa F. Revision hip capsular repair and augmentation with a bioinductive implant after a post-arthroscopy hip subluxation event. Arthrosc Tech. 2020;9:e453–e458. doi: 10.1016/j.eats.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnett A.J., Negus J.J., Barton T., Wood D.G. Reattachment of the proximal hamstring origin: outcome in patients with partial and complete tears. Knee Surgery, Sport Traumatol Arthrosc. 2015;23:2130–2135. doi: 10.1007/s00167-013-2817-0. [DOI] [PubMed] [Google Scholar]
- 20.Harris J.D., Griesser M.J., Best T.M., Ellis T.J. Treatment of proximal hamstring ruptures a systematic review. Int J Sports Med. 2011;32:490–495. doi: 10.1055/s-0031-1273753. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Made A.D., Reurink G., Gouttebarge V., Tol J.L., Kerkhoffs G.M. Outcome after surgical repair of proximal hamstring avulsions. Am J Sports Med. 2015;43:2841–2851. doi: 10.1177/0363546514555327. [DOI] [PubMed] [Google Scholar]
- 22.Kayani B., Ayuob A., Begum F., Khan N., Haddad F.S. Surgical management of chronic incomplete proximal hamstring avulsion injuries. Am J Sports Med. 2020;48:1160–1167. doi: 10.1177/0363546520908819. [DOI] [PubMed] [Google Scholar]
- 23.Cvetanovich G.L., Saltzman B.M., Ukwuani G. Anatomy of the pudendal nerve and other neural structures around the proximal hamstring origin in males. Arthroscopy. 2018;34:2105–2110. doi: 10.1016/j.arthro.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Bokor D.J., Sonnabend D., Deady L. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2016;6:16–25. doi: 10.11138/mltj/2016.6.1.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates a technique for endoscopic implantation of a bioinductive patch for symptomatic chronic partial retearing of the proximal hamstring after failed repair. This is a case of chronic partial retearing of the right proximal hamstring, 2 years out from the index repair. T2 coronal imaging shows signal within the repair and the axial view shows a prominent suture anchor laterally with retearing of the semimembranosus. The patient was indicated for revision endoscopy after not responding to nonoperative management. The patient was placed in the prone position after general anesthesia. The right lower extremity was prepped and draped in the usual sterile fashion. The previous direct posterior portal in the gluteal crease was used to introduce a 30° arthroscope into the space between the gluteus maximus and ischial tuberosity. Next, a superolateral working portal was established to perform an ischial bursectomy. The sciatic nerve was then identified and a thorough neurolysis was performed. The proximal hamstring repair site was debrided and visualized revealing a prominent suture anchor and partial retear superolaterally. The suture anchor was burred down using a 5.5 partially hooded round burr. The arthroscope was then switched to the superolateral portal, and a third portal was made approximately 3-4 fingerbreadths proximal to the direct posterior portal to help facilitate staple fixation of the implant. A medium bioinductive patch is then passed through the direct posterior portal with the assistance of a skid and centered over the proximal hamstring tendon origin. Staple fixation was performed until sufficiently stable to remove the inserter. Additional fixation was then performed until adequate stability was achieved.
This video demonstrates a technique for endoscopic implantation of a bioinductive patch for symptomatic chronic partial retearing of the proximal hamstring after failed repair. This is a case of chronic partial retearing of the right proximal hamstring, 2 years out from the index repair. T2 coronal imaging shows signal within the repair and the axial view shows a prominent suture anchor laterally with retearing of the semimembranosus. The patient was indicated for revision endoscopy after not responding to nonoperative management. The patient was placed in the prone position after general anesthesia. The right lower extremity was prepped and draped in the usual sterile fashion. The previous direct posterior portal in the gluteal crease was used to introduce a 30° arthroscope into the space between the gluteus maximus and ischial tuberosity. Next, a superolateral working portal was established to perform an ischial bursectomy. The sciatic nerve was then identified and a thorough neurolysis was performed. The proximal hamstring repair site was debrided and visualized revealing a prominent suture anchor and partial retear superolaterally. The suture anchor was burred down using a 5.5 partially hooded round burr. The arthroscope was then switched to the superolateral portal, and a third portal was made approximately 3-4 fingerbreadths proximal to the direct posterior portal to help facilitate staple fixation of the implant. A medium bioinductive patch is then passed through the direct posterior portal with the assistance of a skid and centered over the proximal hamstring tendon origin. Staple fixation was performed until sufficiently stable to remove the inserter. Additional fixation was then performed until adequate stability was achieved.