Abstract
Posterior glenohumeral instability is a relatively uncommon cause of shoulder instability. Recurrent posterior instability with static posterior humeral head subluxation is often associated with critical glenoid bone loss. Unlike anterior instability, the amount of bone loss for posterior instability that requires surgical reconstruction remains a topic of debate. Several techniques have been described to treat critical bony defects in patients with recurrent posterior shoulder instability with the use of both autografts and allografts depending on the amount of bone loss present. Open posterior glenoid bone block procedure is associated with increased risk of complications and morbidity to the patient. As such, all-arthroscopic techniques have emerged with the advantage of allowing for the diagnosis and treatment of concomitant glenohumeral pathology and minimizing soft-tissue dissection through the posterior deltoid and rotator cuff muscles. Reported short-term outcomes of arthroscopic posterior bone block stabilization are promising; however, it remains a technically challenging procedure due to intra-articular graft insertion and subsequent fixation congruent to the posterior glenoid articular margin. We describe an all-arthroscopic technique using a fresh distal tibia allograft fixation using 2 partially threaded screws in conjunction with an arthroscopic Latarjet fixation set for a patient with recurrent posterior shoulder instability and associated glenoid bone loss.
Technique Video
The right shoulder is shown, with the patient placed in an upright beach chair position with the operative shoulder positioned as far over as possible to have posterior incision (to deliver distal tibia graft) in line with glenoid. The shoulder is examined under general anesthesia to confirm range of motion and instability. The patient is draped and arm prepped in usual sterile fashion, and arm is placed in a pneumatic articulating limb-positioner. Anatomic landmarks and surgical sites are outlined. A 30° scope is placed in an anterior superior viewing portal via threaded cannula, and intra-articular instruments are accessed via standard posterior portal placement. Arthroscopic subscapularis repair, biceps tenodesis and anterior inferior labral repair was performed prior to glenoid bone grafting. Using a radiofrequency device and an arthroscopic burr, the remaining posterior capsule scar tissue and labrum are removed. The posterior glenoid and scapular neck are debrided at least 1cm (medial to lateral) for a flat surface to permit flush alignment with the distal tibia allograft. A vertical posterior capsulotomy is done with the radiofrequency device to allow passage of the graft. The posterior 3-cm mini-incision is made directly in line with the glenoid fossa, approximately 1 to 2 cm medial to posterior portal. Blunt dissection is performed to spread the posterior deltoid and soft tissue, to facilitate allograft passage. The flat surface with no scar tissue can also be manually confirmed. Using an arthroscopic measuring device, graft size can be estimated (usually 2-2.5 cm in length). On the back surgical table, the fresh distal tibia allograft is opened and bathed in BAN solution for 5 minutes. Using a micro-sagittal saw, 2 parallel flat cuts are made 1cm apart on the lateral aspect of the distal tibia. The graft dimensions were approximately 1 cm for width, 1 cm for depth, and 2.5 cm for length, which is based on the arthroscopic ruler measurement. The graft is then pulse lavaged for several minutes to help remove remaining debris and marrow components, which reduces potential allogenicity and immunogenicity. The DePuy Synthes Mitek arthroscopic Bristow-Latarjet set was used for this case. The distal tibia allograft is secured to the arthroscopic Latarjet set by first placing the coracoid guide flush to the graft, and then inserting 2 k-wires into alpha and beta holes perpendicular to the graft surface. Placing the holes centered in the graft avoids a graft fracture. The coracoid step drill is placed over the 2 k-wires, both holes drilled, and the tops of the holes tapped, followed by insertion of 2 Top Hats. Subsequently, the double coracoid cannula with two coracoid long 3.5-mm screws are inserted into the Top Hats to assist with controlling the graft during passage and fixation to the posterior glenoid rim. Under direct arthroscopic visualization via anterior superior viewing portal, the prepared graft is inserted into the shoulder joint through the posterior mini-incision with soft -tissue release and lined up flush to the posterior glenoid rim. A switching stick placed in a posterior portal can be used to lift posterior tissues to help with passage of the graft, as well as ensure articular congruence of the graft with the glenoid. When proper graft positioning is confirmed, two k-wires are placed into the long coracoid 3.5-mm screws via the alpha and beta holes. One of the long 3.5-mm coracoid screws is removed, and a 3.2-mm cannulated drill bit is used to drill the anterior glenoid neck over the k-wire with careful attention not to plunge and injure the neurovascular structures anteriorly. The drill hole is measured, and the first 4.5 mm partially threaded screw is inserted to compress the graft down to the glenoid, usually 32 to 34 mm in length. The k-wire can be removed, graft slightly rotated to optimize alignment if needed, and k-wire re-inserted, if there is any concern for inadvertent changes in position. The second 4.5-mm partially threaded screw is placed using the same technique as the first. The screws are tightened in an alternating manner under direct visualization to ensure full compression of graft to glenoid. The cannulas are removed and articular congruence confirmed using a switching stick in the posterior portal, feeling for a step-off. The final reconstruction is seen. All instrumentation was removed and 3-0 MONOCRYL was used for the deep dermal layer as well as epidermal layer in a running fashion. The surgical incisions were dressed with dry sterile dressings and the patient’s arm secured in a sling with bump.
Posterior glenohumeral instability is a relatively uncommon cause of shoulder instability, with posterior dislocations accounting for only 5% of all shoulder dislocations.1 Recurrent instability is often associated with glenoid bone loss.2 Unlike anterior instability, the amount of bone loss for posterior instability that requires surgical reconstruction with bone grafting remains a topic of debate.3
Several techniques have been described to treat bony defects in patients with recurrent posterior shoulder instability with the use of both autografts and allografts depending on the amount of bone loss present. Iliac crest autograft has been used as an extra-articular bone block with good outcomes with use of both open and arthroscopic techniques.4, 5, 6, 7, 8 Distal tibia allograft (DTA), as previously described by Provencher et al.9 for augmentation of anterior glenoid bone loss, has been advocated for fixation of posterior glenoid defects. Advantages of using the distal tibia include decreased morbidity associated with harvesting iliac crest autograft, as well as adding an additional articular cartilage surface to extend the glenoid with improved joint congruity.6,10,11 Other graft sources have also been explored, including the distal clavicle or scapular spine, which allow for graft harvest without a distant and separate surgical site.12,13
Previous reports regarding the use of an open posterior bone block in biomechanical cadaveric models have demonstrated inferior instability, which may lead to poor long-term outcomes.14,15 As such, all-arthroscopic techniques have emerged with the theoretical advantage of allowing for the diagnosis and treatment of concomitant shoulder pathology and minimizing surgical-site wounds/soft-tissue dissection through the deltoid and rotator cuff muscles. In this regard, an all-arthroscopic technique for posterior bone block was described by Boileau et al.,5 using suture anchors to assist graft delivery with extracapsular fixation. Reported short-term outcomes of arthroscopic posterior bone block stabilization are promising8; however, it remains a technically challenging approach with respect to intra-articular graft insertion and subsequent fixation congruent to the posterior glenoid articular margin. Various techniques have been described to facilitate graft delivery using different drill guides, Kirschner wires, and suture augmentation.4,5,7,10,16
We describe an all-arthroscopic technique for posterior glenoid reconstruction using a fresh DTA fixation, in conjunction with an arthroscopic Latarjet fixation set (Bristow-Latarjet Instability Shoulder System; DePuy Mitek, Inc., Raynham, MA), using 2 partially threaded screws in a patient with recurrent posterior shoulder instability, static posterior humeral head subluxation, and associated glenoid bone loss.
Surgical Technique (With Video Illustration)
Patient Positioning
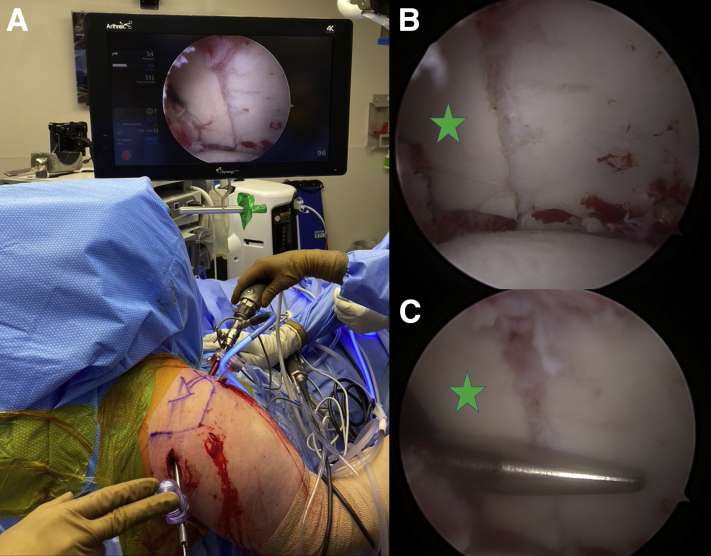
Please see Video 1 on the entire procedure. The patient is brought to the operating room, placed under general anesthesia, and situated in the upright beach-chair position. The right arm is placed in a pneumatic articulating limb-positioner, which affords increased mobility and the ability to rotate the arm providing ease of access to various anatomic locations about the shoulder (Fig 1 A and B). It is essential to have the patient’s shoulder move over as much as possible so that the posterior incision to deliver the distal tibia graft is in line with the glenoid fossa. Also, the anterior chest wall is prepped out as far medially as possible. Examination under anesthesia is critical to confirm both range of motion and posterior instability before the start of the operation.
Fig 1.
The patient is in the beach chair position with the right shoulder prepped out for the surgery and the arm is in the spider arm holder. (A) Viewing from the back shows the small incision in line with the glenoid used for the delivery of the distal tibia allograft. (B) Viewing from the front, you want to make sure to prepped out the anterior chest wall medially.
Diagnostic Scope and Addressing Other Intra-Articular Pathology
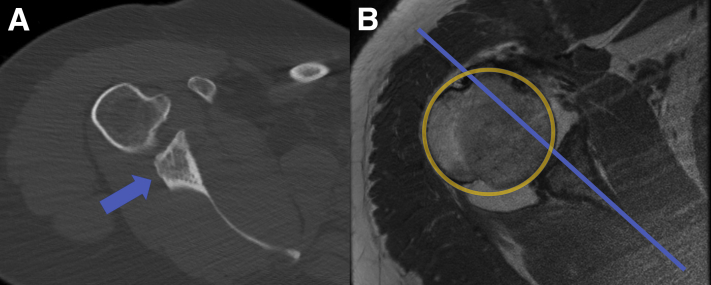
Intra-articular access to the shoulder is gained via standard posterior portal placement into the glenohumeral joint and viewing portal with 30° arthroscope. After comprehensive diagnostic arthroscopy confirms the posterior glenoid bone loss and other intra-articular pathology, standard anterior and anterior superior-lateral portals are made and threaded cannulas are inserted. The senior author (X.L.) prefers to address the intra-articular pathology first before the arthroscopic posterior glenoid procedure. In this case, the patient has a history of Ehlers−Danlos syndrome and had 2 previous surgeries at an outside hospital. The presenting symptom was multidirectional instability with large posterior glenoid bone loss on computed tomography (CT) scan (Fig 2A) and static posterior humeral head subluxation on magnetic resonance image (MRI) (Fig 2B) along with anterior labral and subscapularis tear. Arthroscopic subscapularis repair and revision anterior inferior labral repair with capsular shift along with arthroscopic biceps tenodesis was done before the arthroscopic posterior glenoid bone grafting.
Fig 2.
(A) Axial computed tomography view of the right shoulder shows major posterior glenoid bone loss (arrow) estimated at 25% to 30%. (B) Axial T1-weighted magnetic resonance imaging shows static posterior humeral head subluxation.
Preparation of the Posterior Glenoid
Following successful arthroscopic anterior inferior labral repair/capsular shift, biceps tenodesis and subscapularis fixation, attention was then guided to preparation of the posterior glenoid. The 30° arthroscope is placed into the anterior superior viewing portal via the threaded cannula (Fig 3A). A radiofrequency device is inserted through the posterior portal to prepare the posterior glenoid and to remove the remaining posterior capsule, labrum and scar tissue (Fig 3B). At least a 1-cm medial-to-lateral distance must be debrided on the posterior glenoid neck to allow the bone graft to sit flush to the surface. Attention is then drawn toward the posterior scapular neck to create a flat bleeding bony surface with use of an arthroscopic burr (Fig 3 C and D). Furthermore, a vertical capsulotomy is then performed here with the radiofrequency device to allow passage of the DTA. Subsequently, a small, 3-cm mini-incision is made posteriorly, just 1 to 2 cm medial to the original posterior portal to be in line with the glenoid fossa. Blunt dissection is carried out to spread the posterior deltoid and underlying soft tissue to allow for ease of graft passage. The contour of the surface of the posterior neck is then confirmed with manual palpation (Fig 4A). An arthroscopic measuring device (Arthrex. Naples, FL.) is used here to estimate the size of the posterior distal tibia bone graft (Fig 4B). The typical graft size will be 2 to 2.5 cm in length. Please see Table 1 on the advantages and disadvantages of this surgical technique.
Fig 3.
The patient is in the beach chair position with viewing via the anterior superior portal (30° scope), and the right shoulder is shown. (A) The glenoid fossa is seen with the star and the humeral head is labeled (H). (B) Arthroscopic radiofrequency device is inserted in the posterior portal to elevate and debridement the posterior glenoid neck to at least 1cm so that the graft can sit flush. (C) Arthroscopic burr is inserted via the posterior portal to debride the glenoid neck to the flat surface in preparation for the distal tibia allograft. (D) Preparation is done with a flush surface (arrow).
Fig 4.
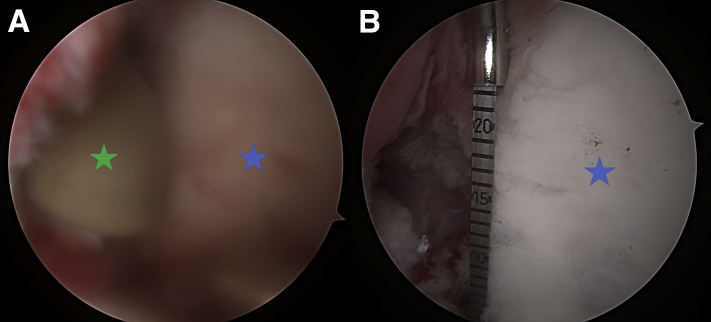
The patient is in the beach chair position with viewing via the anterior superior portal (30° scope), and the right shoulder is shown. Glenoid fossa is marked with a blue star. (A) A gloved finger (green star) is inserted via the posterior mini-incision to feel the posterior glenoid neck and confirm that it is flat with no scar tissue. (B) An arthroscopic measuring device (Arthrex) is used here to estimate the size of the graft, usually between 2 and 2.5 cm in length.
Table 1.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| Allograft with decreased donor-site morbidity as compared with iliac crest autograft harvest | Theoretic increase in allogenicity and immunogenicity |
| Larger graft size contributing to increased glenoid surface area with improved joint congruity for significant defects | Risk of allograft bone resorption and compromised bone healing |
| Fresh cartilage surface on the distal tibia allograft allows for restoration of the articular cartilage | Technically challenging procedure |
| Arthroscopic approach allows for the diagnosis and management of concomitant intraarticular pathology | If difficulties are encountered during the passage of the allograft, a conversion to an open approach may be needed |
| Arthroscopic approach minimizes surgical- site wounds/soft-tissue dissection through the deltoid and rotator cuff muscles | Drilling from a posterior to anterior direction is associated with a small risk of neurovascular injury |
| Arthroscopic approach allows for direct visualization of both articular congruency and graft compression for anatomic reduction | |
| Arthroscopic approach allows for quick postoperative recovery with decreased patient morbidity compared with the traditional open approach |
Distal Tibia Allograft Preparation
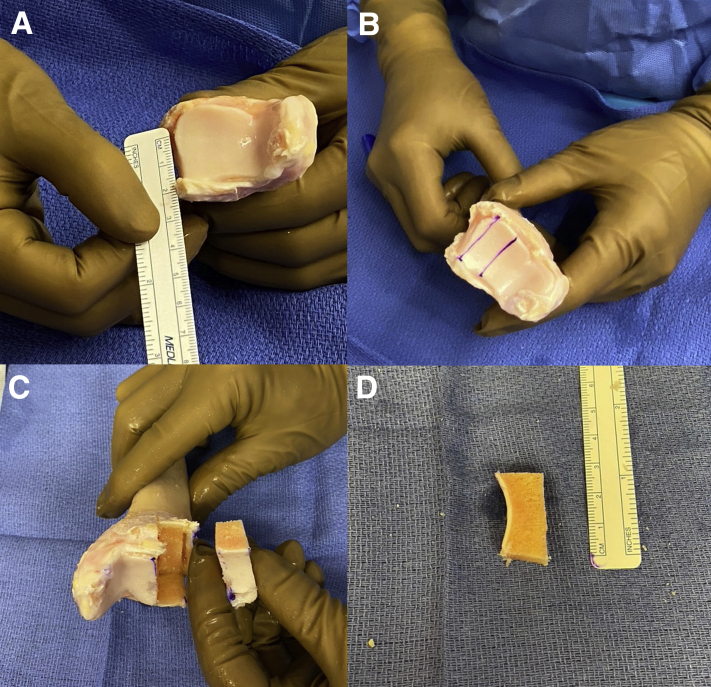
The fresh DTA is opened on the back surgical table and allowed to sit in BAN solution for 5 minutes. The graft is cut with a microsagittal saw to match the native posterior glenoid anatomy (Fig 5A). Two flat cuts are created on the lateral aspect of the distal tibia to fashion the graft about 1 cm in width and 1 cm in depth (Fig 5 B and C). The length is determined with the arthroscopic ruler, and for most cases, 2 to 2.5 cm is the appropriate length (Fig 5D) for the reconstruction. The graft is subsequently pulse-lavaged for several minutes to remove any remaining debris and marrow elements to reduce potential allo- and immunogenicity. At this point, an orthobiologic may be supplemented prior to graft insertion, however no orthobiologic substance was used in this case.
Fig 5.
(A) Fresh distal tibia allograft is seen here and a ruler is used to measure the length of the graft. (B) Two cuts are made parallel to each other about 1 cm apart to create the 2 flat surfaces. (C) Final graft is harvested from the distal tibia. (D) The graft is again measured to confirm the correct length, width, and height.
Graft Delivery
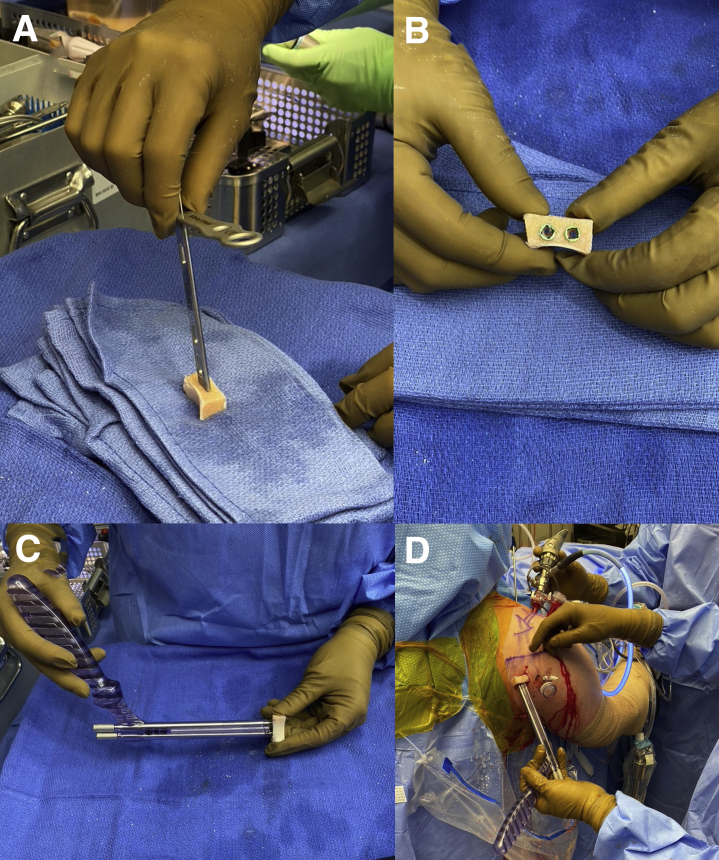
The DePuy Synthes Mitek arthroscopic Bristow-Latarjet set is used for this case. The prepared DTA is secured to the arthroscopic Latarjet set using first the coracoid guide placed flush to the graft with 2 k-wires inserted into the alpha and beta holes (Fig 6A). These holes should be centered in the graft and perpendicular to the graft surface to avoid fracture of the graft. Use the Coracoid step drill over the 2 k-wires, tap the top of the hole, and insert the 2 Top Hats (Fig 6B). Next, the double Coracoid cannula with the Coracoid long 3.5-mm screws are inserted into the Top Hats to assist with controlling the graft during passage and securing the graft to the posterior glenoid rim (Fig 6C). The posterior soft tissue is again completely released through the previously developed 3-cm posterior incision with a blunt instrument or manually with your finger tip. Under direct arthroscopic visualization through the anterior superior viewing portal, the prepared graft is inserted into the shoulder joint and lined up flush to the posterior glenoid rim (Fig 6D). A switching stick can be inserted from the posterior portal to the anterior chest wall and used anteriorly to confirm that the graft is flush to the glenoid rim and also can assist in lifting the posterior tissue up to further help with passage of the graft (Fig 7A). After care is taken to achieve articular congruence of the distal tibia graft under direct visualization and confirmation with the switching stick (Fig 7B), the graft is secured to the posterior glenoid neck via two 4.5-mm screw fixation. While holding the double Coracoid cannula and confirmation that the graft is flush with the glenoid fossa, 2 k-wires are placed into the long Coracoid 3.5-mm screws into the alpha and beta holes. One of the long 3.5-mm Coracoid screws is taken out and a 3.2-mm cannulated drill bit is used via the k-wire to drill to the anterior glenoid neck (Fig 7C). Attention is paid to not plunge the drill bit anteriorly, which may risk injuring the neurovascular bundle. The drill hole is measured and the first 4.5-mm partially threaded screw is inserted to compress the graft (Fig 7D). At this time, it is essential to check that the graft is still flush to the glenoid (Fig 8A). If any concern exists, the k-wire is taken out and the graft is slightly rotated to make it flush to the glenoid fossa and the k-wire is reinserted. The same technique is repeated for the second hole and another partially threaded 4.5-mm screw is inserted. Typically, the length of the screws will be between 32 and 34 mm. Alternate tightening of the 2 screws were done with direct compression visualized through the arthroscope to make sure the graft is fully compressed down. The final graft fixation is seen in Fig 8B. The cannulas is removed and a switching stick is again used to confirm congruence as defined by the absence of articular step-off (Fig 8C). All instrumentation is removed from the joint. A postoperative radiograph in the scapular Y view shows anatomic placement of the DTA on to the posterior glenoid (Fig 9). Please see Table 2 on the pearls and pitfalls of this surgical technique.
Fig 6.
(A) The Coracoid guide placed flush to the graft with 2 k-wires inserted into the alpha and beta holes. The Coracoid step drill is used and then each hole is also tapped. (B) Two Top Hats are placed into the graft. (C) The Coracoid double cannula is used with the long 3.5-mm Coracoid screws over the Top Hats to secure the distal tibia allograft. (D) The patient is in the beach chair position with viewing via the anterior superior portal (30° scope), and the right shoulder is shown. The graft is passed from the posterior mini-incision into the shoulder joint and to the back of the glenoid rim.
Fig 7.
The patient is in the beach chair position with viewing via the anterior superior portal (30° scope), and the right shoulder is shown. (A) A switching stick (arrow) can be inserted from the posterior portal to the anterior chest wall and used anteriorly to confirm that the graft is flush to the glenoid rim and also can assist in lifting the posterior tissue up to further help with passage of the graft. (B) Care is taken to achieve articular congruence of the distal tibia graft (green star) to the glenoid fossa (blue star) under direct visualization and confirmation with the switching stick. (C) One of the long 3.5-mm Coracoid screws is taken out and a 3.2-mm cannulated drill bit is used via the k-wire to drill to the anterior glenoid neck. (D) The drill hole is measured and the first 4.5-mm partially threaded screw is inserted to compress the graft down.
Fig 8.
The patient is in the beach chair position with viewing via the anterior superior portal (30° scope), and the right shoulder is shown. (A) Viewing anteriorly, it is essential to check that the graft is flush to the native glenoid fossa. (B) A switching stick is inserted posteriorly to make sure that the graft is flush and the final distal tibia allograft reconstruction is seen here in (C).
Fig 9.
Right shoulder postoperative radiograph in the scapular Y view shows anatomic all-arthroscopic reconstruction of the posterior glenoid bone defect with the distal tibia allograft. The yellow circle shows the original glenoid fossa and the 2 arrows points to the distal tibia allograft.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Beach chair with use of articulating pneumatic arm-holder allows for maneuverability of the shoulder | Failure to sufficiently remove posterior labrum and capsule will inhibit graft insertion and fixation |
| The shoulder must be prepped out very medially to allow the posterior incision and passage of the switching stick anteriorly to the chest wall | |
| The posterior mini-incision for the allograft must be made 1 to 2 cm medial to the posterior portal to allow the graft to sit flush on the glenoid neck | |
| Meticulous graft preparation is vital to allow for the success of each additional step | Undersized graft may contribute to inappropriate surface and articular congruence |
| Typical graft size is 2.0 to 2.5 cm in length, 1cm in width and depth | |
| Using the Top Hats and the double Coracoid cannula will allow excellent fixation of the graft and facilitate passage into the joint | |
| Careful preparation of posterior glenoid neck to a flat surface and at least 1 cm over medially from the glenoid fossa to encourage maximum osseous integration and stability of the graft fixation | Failure to address concomitant intra-articular pathology may negatively impact outcomes |
| Arthroscopic posterior vertical capsulotomy is created with a radiofrequency device to allow passage of the graft | |
| Use of blunt instrument or manually with one fingertip to open the posterior soft tissue and increase ease of intraarticular graft passage across the soft-tissues | |
| With a switching stick anteriorly, lift up the posterior opening to allow better passage of the graft | |
| Assure desired articular congruence with the switching stick in the front prior to screw fixation via anterior superior viewing with 30 degrees scope | |
| When drilling posterior to anterior, do NOT plunge the drill bit to avoid neurovascular damage | |
| When the graft is flush to the glenoid surface, place 2 k-wires via the double Coracoid cannula to hold the graft in position | |
| Do not over tighten the first 4.5-mm partially threaded screw to allow rotation of the graft in case it is not flush to the glenoid surface. | |
| When tightening the two 4.5-mm screws, alternate tightening to ensure maximal compression of the graft to the glenoid neck |
Closure
The deep dermal layer is closed with an interrupted 3-0 MONOCRYL as well as a running 3-0 MONOCRYL for epidermal closure. A dry sterile dressing is applied and a sling with bump is utilized to secure the operative extremity. A step-by-step guide is listed in Table 3.
Table 3.
Step-by-Step Surgical Technique Guide
| 1 | Place a 30° arthroscope in anterior superior portal for viewing. Address all intra-articular pathology first prior to proceeding with the all-arthroscopic posterior glenoid reconstruction. |
| 2 | Prepare posterior glenoid with radiofrequency ablator or device to debride the scar tissue, capsule and any remining tissue off the glenoid neck at least 1cm medial to the glenoid surface. A vertical capsulotomy is created to allow passage of the graft. |
| 3 | Arthroscopic burr to create flat bleeding bony surface on posterior glenoid neck |
| 4 | Make a 3-cm posterior skin incision just medial (1-2 cm) to the original posterior portal. |
| 5 | Blunt dissection to spread the posterior deltoid and underlying soft-tissue with either blunt instrument or manually with your finger tip. Make sure enough tissue is cleared to allow the graft to be flush to the posterior glenoid neck. |
| 6 | Insert a switching stick from posterior portal or from the mini-incision flush to the glenoid to the anterior chest wall. Use switching stick from anterior to make sure the graft is flush and also used the stick to lift up the posterior capsule to help passage of the graft. |
| 7 | Use arthroscopic measuring device to size the length of the glenoid defect. Typically the length is between 2 and 2.5cm in size. |
| 8 | Fresh distal tibia allograft opened on the back surgical table and soak in BAN solution for 5 minutes. |
| 9 | Cut allograft to size with microsagittal saw to create 2 flush surfaces about 1cm in width and 1 cm in depth. Pulse-lavage allograft for several minutes and orthobiologics can be added if desired. |
| 10 | Secure allograft to Mitek arthroscopic Latarjet set using 2 pins followed by tap and top hat on both sides |
| 11 | The Coracoid guide placed flush to the graft with 2 k-wires inserted into the alpha and beta holes. The Coracoid step drill is used and then each hole is also tapped. Two Top Hats are placed into the graft. |
| 12 | The Coracoid double cannula is used with the long 3.5-mm Coracoid screws over the Top Hats to secure the distal tibia allograft. |
| 13 | The graft is passed from the posterior mini-incision into the shoulder joint and to the back of the glenoid rim. |
| 14 | Arthroscopic confirmation of articular congruence of the allograft and native glenoid surface via a switching stick from the front. |
| 15 | Two k-wires are inserted into the cannulated long 3.5mm screws via the double Coracoid cannula holder to provisionally secure the graft to the glenoid neck. |
| 16 | One of the long 3.5mm Coracoid screws is taken out and a 3.2-mm cannulated drill bit is used via the k-wire to drill to the anterior glenoid neck. |
| 17 | The drill hole is measured and the first 4.5-mm partially threaded screw is inserted to compress the graft down. Same step is repeated and secure the allograft to the glenoid via two screws placed parallel to the articular surface. |
| 18 | Alternative tightening of each screw is important to maximally compress the allograft down to the posterior glenoid neck. |
| 19 | Remove cannulas and insert switching stick to check articular congruence. |
| 20 | Once confirmed, remove all instrumentation from the glenohumeral joint. |
| 21 | Incisions closed with absorbable sutures and the arm is placed in a sling and abduction brace. |
Rehabilitation
The patient is placed in a sling and abduction pillow for 6 weeks. Physical therapy is started 4 weeks after surgery. Passive range of motion is followed by active assisted range of motion for 4 to 6 weeks. Strengthening is started around 8 to 10 weeks after surgery. Return to sports or full activities without limitations usually takes 6 to 9 months depending on the sports and the occupation.
Discussion
Posterior glenohumeral instability can present in the setting of an acute traumatic posterior shoulder instability event17 or insidious onset following a series of repetitive stress.2 Both static and dynamic stabilizers work harmoniously to maintain congruence of the humeral head and glenoid cavity throughout activities of daily living. Posterior stabilizers, such as the posterior labrum, posterior capsule, and posterior band of the inferior glenohumeral ligament, function to increase the depth of the glenohumeral articulation, thus discouraging posterior shoulder translation. The intra-articular negative-pressure environment created by the osseous glenoid morphology and circumferential glenoid labrum is further reinforced by additional static and dynamic stabilizers, including the rotator interval and rotator cuff musculature, respectively. In the case of posterior and multidirectional instability, however, the glenohumeral joint experiences increased volume associated with soft tissue or capsule compromise with decreased stability. Lesser degrees of energy are thus required to disrupt the posterior capsular tissue and cause an instability event leading to recurrent instability over time.18
Several authors have proposed theories to explain shoulder instability in an effort to conceptually direct surgical planning. For example, Warren et al.19 introduced the “circle concept,” or the theory that for a shoulder to dislocate posteriorly, there must be concomitant soft-tissue damage anteriorly to the capsuloligamentous structures and rotator interval or vice versa. As such, some authors stress surgical fixation should include both repair of the anterior and posterior soft-tissue structures. While this has been debated over time, it alludes to the complexity of this stabilization network. Static constraint provided by the humeral head and glenoid fossa are critical when considering shoulder stability, specifically with respect to glenoid retroversion, hypoplasia20 and posterior bone loss. Increased retroversion leads to increased baseline external rotation and subsequent decreased internal rotation,21 which may increase the risk of posterior subluxation of the humeral head on the glenoid. In their review of the MRI findings of 143 patients who underwent stabilization for shoulder instability with ≤25% glenoid bone loss and no Hill−Sachs lesion, Gottschalk et al.22 found that increased retroversion was associated with greater likelihood of suffering a posterior dislocation event. Furthermore, in a prospective review of 714 young athletes over a 4-year period, Owens et al.23 demonstrated that posterior shoulder instability was associated with increased glenoid retroversion, increased external rotation strength in adduction and at 45° of abduction, and increased internal rotation strength in adduction.
While there is no true threshold in the literature at which bony augmentation for severe posterior glenoid bone loss is necessary, extrapolating from published studies on anterior shoulder instability, a range of 10% to 20% is cited.18 In addition, bony augmentation should be considered in patients with static posterior humeral head subluxation in the setting of posterior glenoid bone loss. Furthermore, it is difficult to use any single posterior glenoid bone loss number as a cut off for bony augmentation as this does not consider potential humeral head bone loss in the form of a reverse Hill−Sachs lesion or in patients with connective tissue disease and compromised soft tissue. Further, concomitant intra-articular and extra-articular pathologies, such as superior labrum anterior to posterior lesions, labral articular disruptions, or rotator cuff pathologies influence surgical management. An all-arthroscopic or open approach can be pursued; however, achieving adequate capsulolabral visualization can be challenging with the open approach.24,25
For patients with severe posterior glenoid bone loss with recurrent instability and static posterior humeral head subluxation with or without soft-tissue damage, several open reconstructive techniques have historically been described based on different preoperative or radiographic factors, in the form of glenoid osteotomies,26 proximal humerus rotational osteotomies, and bone block augmentation using acromial27 or iliac crest bone grafting.28,29 More recently, however, alternative open or all-arthroscopic approaches have been described using a fresh DTA.9,10 In a biomechanical evaluation of 8 fresh-frozen human cadaveric shoulders reconstructed using an iliac crest bone graft or fresh DTA, Frank et al.11 demonstrated similar contact pressure, peak force, and contact area between the 2 treatment arms, supporting the use of a fresh DTA as an alternative to iliac crest bone graft. DTA also confers the potential advantage of limiting donor-site morbidity, providing more structural support in that it is composed of dense weight-bearing cortical and metaphyseal bone,9 and restoring native joint congruity with fresh cartilage surface. To that end, Decker et al.30 found that CT measurement of the radius of curvatures of the glenoid, distal tibia and humeral head were reliable and reproducible in cadaveric studies. Obtaining CT measurements before the reconstruction can be useful to match the radius curvature of the glenoid fossa with the DTA. Other studies have compared DTA with more traditional reconstructive methods. Nacca et al.13 biomechanically tested 10 cadaveric shoulders subjected to glenoid reconstruction via a DTA or scapular spinal autograft. There was no significant difference in peak force and lateral displacement after reconstruction in both treatment arms compared to the intact glenoid, suggesting that both approaches can effectively restore glenohumeral instability in select settings. However, in comparison with a DTA, scapular spinal autograft lacks articular cartilage, which limits native joint restoration of articular cartilage. In addition, Provencher et al.31 reported 92% of patients with failed Latarjet procedure revised with a fresh DTA achieved complete union at the glenoid and DTA surface at final follow-up with CT imaging.
Regardless of the chosen bone graft, intra-articular placement and fixation can pose a technical challenge. Boileau et al.5 proposed an all-arthroscopic technique that uses 2 suture anchors for both bone block fixation and concomitant capsulolabral repair with no reported nonunion events. Others have advocated for the use of a double-barreled cannula to facilitate with graft positioning and fixation.7 To address the difficulties associated with intra-articular delivery and manipulation of the graft, Parada and Shaw16 recently proposed a technique that uses a dual-cannulated graft positioner (Arthrex) and 3-0 PROLENE suture to secure the graft to the positioner and allow for easy, intra-articular passage of the graft and instrument removal once secured in place. This low-cost approach allows for facilitated guidewire placement and subsequent suture removal before cannulated screw insertion.
The current described technique proposes an all-arthroscopic posterior glenoid reconstruction using a fresh DTA to restore bony anatomy and confer stability in a patient that failed several surgeries and presents with large posterior glenoid bone defect and static posterior humeral head subluxation. This technique offers facilitated all-arthroscopic graft passage using the DePuy Synthes Mitek Bristow-Latarjet arthroscopic kit so as to avoid the morbidity associated with the open posterior approach. Furthermore, an all-arthroscopic technique with direct visualization allows for anatomic reduction of the graft with the native glenoid fossa, which helps avoid the technical pitfalls associated with graft mispositioning and intra-articular manipulation due to lack of visualization associated with the open technique. Using the fresh DTA also avoids the morbidity of harvesting autograft, and the literature shows excellent bone incorporation with short- to mid-term follow-up.31 We present a step-by-step guide on how to accurately and reproducibly reconstruct the posterior glenoid bone defect with a fresh DTA. This all-arthroscopic technique will successfully address these complex recurrent posterior instability cases that are associated with large posterior glenoid bone defects.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: X.L. reports personal fees from FH Ortho and JOMI, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The right shoulder is shown, with the patient placed in an upright beach chair position with the operative shoulder positioned as far over as possible to have posterior incision (to deliver distal tibia graft) in line with glenoid. The shoulder is examined under general anesthesia to confirm range of motion and instability. The patient is draped and arm prepped in usual sterile fashion, and arm is placed in a pneumatic articulating limb-positioner. Anatomic landmarks and surgical sites are outlined. A 30° scope is placed in an anterior superior viewing portal via threaded cannula, and intra-articular instruments are accessed via standard posterior portal placement. Arthroscopic subscapularis repair, biceps tenodesis and anterior inferior labral repair was performed prior to glenoid bone grafting. Using a radiofrequency device and an arthroscopic burr, the remaining posterior capsule scar tissue and labrum are removed. The posterior glenoid and scapular neck are debrided at least 1cm (medial to lateral) for a flat surface to permit flush alignment with the distal tibia allograft. A vertical posterior capsulotomy is done with the radiofrequency device to allow passage of the graft. The posterior 3-cm mini-incision is made directly in line with the glenoid fossa, approximately 1 to 2 cm medial to posterior portal. Blunt dissection is performed to spread the posterior deltoid and soft tissue, to facilitate allograft passage. The flat surface with no scar tissue can also be manually confirmed. Using an arthroscopic measuring device, graft size can be estimated (usually 2-2.5 cm in length). On the back surgical table, the fresh distal tibia allograft is opened and bathed in BAN solution for 5 minutes. Using a micro-sagittal saw, 2 parallel flat cuts are made 1cm apart on the lateral aspect of the distal tibia. The graft dimensions were approximately 1 cm for width, 1 cm for depth, and 2.5 cm for length, which is based on the arthroscopic ruler measurement. The graft is then pulse lavaged for several minutes to help remove remaining debris and marrow components, which reduces potential allogenicity and immunogenicity. The DePuy Synthes Mitek arthroscopic Bristow-Latarjet set was used for this case. The distal tibia allograft is secured to the arthroscopic Latarjet set by first placing the coracoid guide flush to the graft, and then inserting 2 k-wires into alpha and beta holes perpendicular to the graft surface. Placing the holes centered in the graft avoids a graft fracture. The coracoid step drill is placed over the 2 k-wires, both holes drilled, and the tops of the holes tapped, followed by insertion of 2 Top Hats. Subsequently, the double coracoid cannula with two coracoid long 3.5-mm screws are inserted into the Top Hats to assist with controlling the graft during passage and fixation to the posterior glenoid rim. Under direct arthroscopic visualization via anterior superior viewing portal, the prepared graft is inserted into the shoulder joint through the posterior mini-incision with soft -tissue release and lined up flush to the posterior glenoid rim. A switching stick placed in a posterior portal can be used to lift posterior tissues to help with passage of the graft, as well as ensure articular congruence of the graft with the glenoid. When proper graft positioning is confirmed, two k-wires are placed into the long coracoid 3.5-mm screws via the alpha and beta holes. One of the long 3.5-mm coracoid screws is removed, and a 3.2-mm cannulated drill bit is used to drill the anterior glenoid neck over the k-wire with careful attention not to plunge and injure the neurovascular structures anteriorly. The drill hole is measured, and the first 4.5 mm partially threaded screw is inserted to compress the graft down to the glenoid, usually 32 to 34 mm in length. The k-wire can be removed, graft slightly rotated to optimize alignment if needed, and k-wire re-inserted, if there is any concern for inadvertent changes in position. The second 4.5-mm partially threaded screw is placed using the same technique as the first. The screws are tightened in an alternating manner under direct visualization to ensure full compression of graft to glenoid. The cannulas are removed and articular congruence confirmed using a switching stick in the posterior portal, feeling for a step-off. The final reconstruction is seen. All instrumentation was removed and 3-0 MONOCRYL was used for the deep dermal layer as well as epidermal layer in a running fashion. The surgical incisions were dressed with dry sterile dressings and the patient’s arm secured in a sling with bump.
References
- 1.Robinson C.M., Aderinto J. Recurrent posterior shoulder instability. J Bone Joint Surg Am. 2005;87-A:883–892. doi: 10.2106/JBJS.D.02906. [DOI] [PubMed] [Google Scholar]
- 2.Provencher M.T., LeClere L.E., King S. Posterior instability of the shoulder: Diagnosis and management. Am J Sports Med. 2011;39:874–886. doi: 10.1177/0363546510384232. [DOI] [PubMed] [Google Scholar]
- 3.Longo U.G., Rizzello G., Locher J. Bone loss in patients with posterior glenohumeral instability: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24:612–617. doi: 10.1007/s00167-014-3161-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith T., Goede F., Struck M., Wellmann M. Arthroscopic posterior shoulder stabilization with an iliac bone graft and capsular repair: A novel technique. Arthrosc Tech. 2012;1:e181–e185. doi: 10.1016/j.eats.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau P., Hardy M.B., McClelland W.B., Jr., Thélu C.E., Schwartz D.G. Arthroscopic posterior bone block procedure: A new technique using suture anchor fixation. Arthrosc Tech. 2013;2:e473–e477. doi: 10.1016/j.eats.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millett P.J., Schoenahl J.Y., Register B., Gaskill T.R., van Deurzen D.F., Martetschläger F. Reconstruction of posterior glenoid deficiency using distal tibial osteoarticular allograft. Knee Surg Sports Traumatol Arthrosc. 2013;21:445–449. doi: 10.1007/s00167-012-2254-5. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz D.G., Goebel S., Piper K., Kordasiewicz B., Boyle S., Lafosse L. Arthroscopic posterior bone block augmentation in posterior shoulder instability. J Shoulder Elbow Surg. 2013;22:1092–1101. doi: 10.1016/j.jse.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Wellmann M., Pastor M.F., Ettinger M., Koester K., Smith T. Arthroscopic posterior bone block stabilization-early results of an effective procedure for the recurrent posterior instability. Knee Surg Sports Traumatol Arthrosc. 2018;26:292–298. doi: 10.1007/s00167-017-4753-x. [DOI] [PubMed] [Google Scholar]
- 9.Provencher M.T., Ghodadra N., LeClere L., Solomon D.J., Romeo A.A. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy. 2009;25:446–452. doi: 10.1016/j.arthro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A.K., Chalmers P.N., Klosterman E., Harris J.D., Provencher M.T., Romeo A.A. Arthroscopic distal tibial allograft augmentation for posteriorshoulder instability with glenoid bone loss. Arthrosc Tech. 2013;2:405–411. doi: 10.1016/j.eats.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank R.M., Shin J., Saccomanno M.F. Comparison of glenohumeral contact pressures and contact areas after posterior glenoid reconstruction with an iliac crest bone graft or distal tibial osteochondral allograft. Am J Sports Med. 2014;42:2574–2582. doi: 10.1177/0363546514545860. [DOI] [PubMed] [Google Scholar]
- 12.Tokish J.M., Fitzpatrick K., Cook J.B., Mallon W.J. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3:e475–e481. doi: 10.1016/j.eats.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nacca C., Gil J.A., DeFroda S.F., Badida R., Owens B.D. Comparison of a distal tibial allograft and scapular spinal autograft for posterior shoulder instability with glenoid bone loss. Orthop J Sports Med. 2018;6(7) doi: 10.1177/2325967118786697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellmann M., Bobrowitsch E., Khan N. Biomechanical effectiveness of an arthroscopic posterior Bankart repair versus an open bone block procedure for posterior shoulder instability. Am J Sports Med. 2011;39:796–803. doi: 10.1177/0363546510389991. [DOI] [PubMed] [Google Scholar]
- 15.Meuffels D.E., Schuit H., van Biezen F.C., Reijman M., Verhaar J.A. The posterior bone block procedure in posterior shoulder instability: A long-term follow-up study. Ortho Traumatol Surg Res. 2009;95:100–107. doi: 10.1302/0301-620X.92B5.23529. [DOI] [PubMed] [Google Scholar]
- 16.Parada S.A., Shaw K.A. Graft transfer technique in arthroscopic posterior glenoid reconstruction with distal tibia allograft. Arthrosc Tech. 2017;6:1891–1895. doi: 10.1016/j.eats.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouleau D.M., Hebert-Davies J., Robinson C.M. Acute traumatic posterior shoulder dislocation. J Am Acad Orthop Surg. 2014;22:145–152. doi: 10.5435/JAAOS-22-03-145. [DOI] [PubMed] [Google Scholar]
- 18.Frank R.M., Romeo A.A., Provencher M.T. Posterior glenohumeral instability: Evidence-based treatment. J Am Acad Orthop Surg. 2017;25:610–623. doi: 10.5435/JAAOS-D-15-00631. [DOI] [PubMed] [Google Scholar]
- 19.Warren R.F., Kornblatt I.B., Marchand R. Static factors affecting posterior shoulder stability. Orthop Trans. 1989;8:89. [Google Scholar]
- 20.Inui H., Sugamoto K., Miyamoto T. Glenoid shape in atraumatic posterior instability of the shoulder. Clin Orthop Relat Res. 2002;403:87–92. doi: 10.1097/00003086-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kronberg M., Broström L.A., Söderlund V. Retroversion of the humeral head in the normal shoulder and its relationship to the normal range of motion. Clin Orthop Relat Res. 1990;253:113–117. [PubMed] [Google Scholar]
- 22.Gottschalk M.B., Ghasem A., Todd D., Daruwalla J., Xerogeanes J., Karas S. Does glenoid retroversion predict recurrence and contralateral instability? Arthroscopy. 2015;31:488–493. doi: 10.1016/j.arthro.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Owens B.D., Campbell S.E., Cameron K.L. Risk factors for posterior shoulder instability in young athletes. Am J Sports Med. 2013;41:2645–2649. doi: 10.1177/0363546513501508. [DOI] [PubMed] [Google Scholar]
- 24.Wolf E.M., Eakin C.L. Arthroscopic capsular plication for posterior shoulder instability. Arthroscopy. 1998;14:153–163. doi: 10.1016/s0749-8063(98)70034-9. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins R.J., Handa D.H. Posterior instability of the glenohumeral joint: A technique of repair. Am J Sports Med. 1996;24:275–278. doi: 10.1177/036354659602400305. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins R. Glenoid osteotomy for recurrent posterior subluxation of the shoulder: Assessment by computed axial tomography. J Shoulder Elbow Surg. 1996;5:393–400. doi: 10.1016/s1058-2746(96)80071-1. [DOI] [PubMed] [Google Scholar]
- 27.Arciero R.A., Augustus D. Posterior acromial bone block augmentation for the treatment of posterior glenoid bone loss associated with recurrent posterior shoulder instability. Tech Shoulder Elbow Surg. 2006;7:210–217. [Google Scholar]
- 28.Fronek J., Warren R.F., Bowen M. Posterior subluxation of the glenohumeral joint. J Bone Joint Surg Am. 1989;71:205–216. [PubMed] [Google Scholar]
- 29.Pollock R.G., LU Bigliani. Recurrent posterior shoulder instability: Diagnosis and treatment. Clin Orthop Relat Res. 1993;291:85–96. [PubMed] [Google Scholar]
- 30.Decker M.M., Strohmeyer G.C., Wood J.P. Distal tibai allograft for glenohumeral instability: Does radius of curvature match? J Shoulder Elbow Surg. 2016;25:1542–1548. doi: 10.1016/j.jse.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Provencher M.T., Peebles L.A., Aman Z.S. Management of the failed Latarjet procedure: Outcomes of revision surgery with fresh distal tibial allograft. Am J Sports Med. 2019;47:2795–2802. doi: 10.1177/0363546519871896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The right shoulder is shown, with the patient placed in an upright beach chair position with the operative shoulder positioned as far over as possible to have posterior incision (to deliver distal tibia graft) in line with glenoid. The shoulder is examined under general anesthesia to confirm range of motion and instability. The patient is draped and arm prepped in usual sterile fashion, and arm is placed in a pneumatic articulating limb-positioner. Anatomic landmarks and surgical sites are outlined. A 30° scope is placed in an anterior superior viewing portal via threaded cannula, and intra-articular instruments are accessed via standard posterior portal placement. Arthroscopic subscapularis repair, biceps tenodesis and anterior inferior labral repair was performed prior to glenoid bone grafting. Using a radiofrequency device and an arthroscopic burr, the remaining posterior capsule scar tissue and labrum are removed. The posterior glenoid and scapular neck are debrided at least 1cm (medial to lateral) for a flat surface to permit flush alignment with the distal tibia allograft. A vertical posterior capsulotomy is done with the radiofrequency device to allow passage of the graft. The posterior 3-cm mini-incision is made directly in line with the glenoid fossa, approximately 1 to 2 cm medial to posterior portal. Blunt dissection is performed to spread the posterior deltoid and soft tissue, to facilitate allograft passage. The flat surface with no scar tissue can also be manually confirmed. Using an arthroscopic measuring device, graft size can be estimated (usually 2-2.5 cm in length). On the back surgical table, the fresh distal tibia allograft is opened and bathed in BAN solution for 5 minutes. Using a micro-sagittal saw, 2 parallel flat cuts are made 1cm apart on the lateral aspect of the distal tibia. The graft dimensions were approximately 1 cm for width, 1 cm for depth, and 2.5 cm for length, which is based on the arthroscopic ruler measurement. The graft is then pulse lavaged for several minutes to help remove remaining debris and marrow components, which reduces potential allogenicity and immunogenicity. The DePuy Synthes Mitek arthroscopic Bristow-Latarjet set was used for this case. The distal tibia allograft is secured to the arthroscopic Latarjet set by first placing the coracoid guide flush to the graft, and then inserting 2 k-wires into alpha and beta holes perpendicular to the graft surface. Placing the holes centered in the graft avoids a graft fracture. The coracoid step drill is placed over the 2 k-wires, both holes drilled, and the tops of the holes tapped, followed by insertion of 2 Top Hats. Subsequently, the double coracoid cannula with two coracoid long 3.5-mm screws are inserted into the Top Hats to assist with controlling the graft during passage and fixation to the posterior glenoid rim. Under direct arthroscopic visualization via anterior superior viewing portal, the prepared graft is inserted into the shoulder joint through the posterior mini-incision with soft -tissue release and lined up flush to the posterior glenoid rim. A switching stick placed in a posterior portal can be used to lift posterior tissues to help with passage of the graft, as well as ensure articular congruence of the graft with the glenoid. When proper graft positioning is confirmed, two k-wires are placed into the long coracoid 3.5-mm screws via the alpha and beta holes. One of the long 3.5-mm coracoid screws is removed, and a 3.2-mm cannulated drill bit is used to drill the anterior glenoid neck over the k-wire with careful attention not to plunge and injure the neurovascular structures anteriorly. The drill hole is measured, and the first 4.5 mm partially threaded screw is inserted to compress the graft down to the glenoid, usually 32 to 34 mm in length. The k-wire can be removed, graft slightly rotated to optimize alignment if needed, and k-wire re-inserted, if there is any concern for inadvertent changes in position. The second 4.5-mm partially threaded screw is placed using the same technique as the first. The screws are tightened in an alternating manner under direct visualization to ensure full compression of graft to glenoid. The cannulas are removed and articular congruence confirmed using a switching stick in the posterior portal, feeling for a step-off. The final reconstruction is seen. All instrumentation was removed and 3-0 MONOCRYL was used for the deep dermal layer as well as epidermal layer in a running fashion. The surgical incisions were dressed with dry sterile dressings and the patient’s arm secured in a sling with bump.
The right shoulder is shown, with the patient placed in an upright beach chair position with the operative shoulder positioned as far over as possible to have posterior incision (to deliver distal tibia graft) in line with glenoid. The shoulder is examined under general anesthesia to confirm range of motion and instability. The patient is draped and arm prepped in usual sterile fashion, and arm is placed in a pneumatic articulating limb-positioner. Anatomic landmarks and surgical sites are outlined. A 30° scope is placed in an anterior superior viewing portal via threaded cannula, and intra-articular instruments are accessed via standard posterior portal placement. Arthroscopic subscapularis repair, biceps tenodesis and anterior inferior labral repair was performed prior to glenoid bone grafting. Using a radiofrequency device and an arthroscopic burr, the remaining posterior capsule scar tissue and labrum are removed. The posterior glenoid and scapular neck are debrided at least 1cm (medial to lateral) for a flat surface to permit flush alignment with the distal tibia allograft. A vertical posterior capsulotomy is done with the radiofrequency device to allow passage of the graft. The posterior 3-cm mini-incision is made directly in line with the glenoid fossa, approximately 1 to 2 cm medial to posterior portal. Blunt dissection is performed to spread the posterior deltoid and soft tissue, to facilitate allograft passage. The flat surface with no scar tissue can also be manually confirmed. Using an arthroscopic measuring device, graft size can be estimated (usually 2-2.5 cm in length). On the back surgical table, the fresh distal tibia allograft is opened and bathed in BAN solution for 5 minutes. Using a micro-sagittal saw, 2 parallel flat cuts are made 1cm apart on the lateral aspect of the distal tibia. The graft dimensions were approximately 1 cm for width, 1 cm for depth, and 2.5 cm for length, which is based on the arthroscopic ruler measurement. The graft is then pulse lavaged for several minutes to help remove remaining debris and marrow components, which reduces potential allogenicity and immunogenicity. The DePuy Synthes Mitek arthroscopic Bristow-Latarjet set was used for this case. The distal tibia allograft is secured to the arthroscopic Latarjet set by first placing the coracoid guide flush to the graft, and then inserting 2 k-wires into alpha and beta holes perpendicular to the graft surface. Placing the holes centered in the graft avoids a graft fracture. The coracoid step drill is placed over the 2 k-wires, both holes drilled, and the tops of the holes tapped, followed by insertion of 2 Top Hats. Subsequently, the double coracoid cannula with two coracoid long 3.5-mm screws are inserted into the Top Hats to assist with controlling the graft during passage and fixation to the posterior glenoid rim. Under direct arthroscopic visualization via anterior superior viewing portal, the prepared graft is inserted into the shoulder joint through the posterior mini-incision with soft -tissue release and lined up flush to the posterior glenoid rim. A switching stick placed in a posterior portal can be used to lift posterior tissues to help with passage of the graft, as well as ensure articular congruence of the graft with the glenoid. When proper graft positioning is confirmed, two k-wires are placed into the long coracoid 3.5-mm screws via the alpha and beta holes. One of the long 3.5-mm coracoid screws is removed, and a 3.2-mm cannulated drill bit is used to drill the anterior glenoid neck over the k-wire with careful attention not to plunge and injure the neurovascular structures anteriorly. The drill hole is measured, and the first 4.5 mm partially threaded screw is inserted to compress the graft down to the glenoid, usually 32 to 34 mm in length. The k-wire can be removed, graft slightly rotated to optimize alignment if needed, and k-wire re-inserted, if there is any concern for inadvertent changes in position. The second 4.5-mm partially threaded screw is placed using the same technique as the first. The screws are tightened in an alternating manner under direct visualization to ensure full compression of graft to glenoid. The cannulas are removed and articular congruence confirmed using a switching stick in the posterior portal, feeling for a step-off. The final reconstruction is seen. All instrumentation was removed and 3-0 MONOCRYL was used for the deep dermal layer as well as epidermal layer in a running fashion. The surgical incisions were dressed with dry sterile dressings and the patient’s arm secured in a sling with bump.