Abstract
Background and Aims:
Endoscopic full thickness resection (eFTR) is a field of increasing interest that offers a minimally invasive resection modality for lesions that are not amenable for resection by conventional methods. Full-thickness resection device (FTRD) is a new device that was developed for a single-step eFTR using an over-the scope-clip (OTSC). In this meta-analysis, we aim to assess the efficacy and safety of FTRD for eFTR of colorectal lesions.
Methods:
A Comprehensive literature review of different databases to identify studies reporting FTRD with outcomes of interest was performed. Studies with <10 cases were excluded. Rates of histologic complete resection (R0), technical success, and complications were extracted. Efficacy was assessed by using the technical and the R0 rates whereas safety was assessed by using the complications rates. Weighted pooled rates (WPR) and the 95% confidence interval (CI) were calculated depending on the heterogeneity (I2 statistics).
Results:
Nine studies including 551 patients with 555 lesions were included in this study. The WPR for overall R0 was 82.4% (95% CI: 79.0–85.5%),with moderate heterogeneity (I2 = 34.8%). The WPR rate for technical success was 89.25% (95% CI: 86.4–91.7%), with low heterogeneity (I2 = 23.7%). The WPR for total complications rate was 10.2% (7.8,12.8%) with no heterogeneity. The pooled rate for minor bleeding, major bleeding, postpolypectomy syndrome, and perforation were 3.2%, 0.97%, 2.2%, and 1.2%, respectively. Of 44 peri-appendicular lesions, the pooled rate for acute appendicitis was 19.7%.
Conclusion:
FTRD seems to be effective and safe for eFTR of difficult colorectal lesions. Large prospective studies comparing FTRD with conventional resection techniques are warranted.
Keywords: Endoscopic full-thickness resection, endoscopic full-thickness resection device, colorectal lesions
Introduction:
While conventional endoscopic resection methods such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are highly effective techniques for colorectal lesions resection, those techniques harbor high perforation and incomplete resection rates in cases of non-lifting adenomas such as recurrent/residual adenomas; adenomas in difficult locations such as peri-appendicular and peri-diverticular lesions, and in cases of subepithelial lesions due to the presence of submucosal fibrosis 1–4. Endoscopic full-thickness resection (eFTR) is an emerging field that offers a minimally invasive modality for resection of gastrointestinal (GI) lesions that are not amenable to conventional methods 5,6. Two eFTR techniques; exposed (free-hand eFTR) and non-exposed (Device-assisted eFTR), have been described in term of managing the GI wall defect that results from eFTR 7,8. In the exposed or free-hand technique, ESD full-thickness resection is done first followed by wall defect closure with clips, endoloop, and/or endoscopic suturing. This technique has been widely used for gastric submucosal lesions resection especially in Asian countries with good clinical outcomes 9–11. The non-exposed or device-assisted technique consists of securing the GI wall patency first with a clip followed by full-thickness resection 7,8. Over-the-scope clip (OTSC, Ovesco Endoscopy GmbH, Tübingen, Germany) is an example of clips that can be used either to secure the wall patency or to close the iatrogenic wall defect 7. Full thickness resection device (FTRD) is a device that was developed for one-step eFTR using a 14mm OTSC mounted over an elongated cap (21mm) as well as with an integrated snare system 12. Two FTRD systems have been manufactured; colonic FTRD and gastroduodenal FTRD 13. Colonic FTRD is commercially available, U.S. Food and Drug Administration (FDA) approved for lower GI lesions, as well as with an increasingly experience using it in clinical settings compared to gastroduodenal FTRD which is still not commercially available and with very limited clinical experience 13. In this meta-analysis study, our aim was to assess safety and efficacy of FTRD for colorectal lesions eFTR.
Methods
Study Selection, Data Extraction, and Quality Assessment
This meta-analysis study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines (PRISMA guideline) 14. A comprehensive literature search from the inception until April 2019 of MEDLINE, Cochrane library, and Scopus databases was done using the same search strategy; (endoscopic full thickness resection) And ((FTRD) OR (full thickness resection device) OR (over the scope) OR (OTS)). To increase the yield of our search strategy; references of the included studies as well as the last two issues of Gastrointestinal Endoscopy Journal were reviewed to identify any relevant study that was missed during the initial search strategy. Eligibility criteria were pre-determined by two authors (Y.F and M.M). Only studies in English reporting technical success, complete resection (R0), and complication rates of FTRD for colorectal lesions were included. As well as, only studies with 10 or more patients were included to reduce bias associated with case reports and small number of case studies. Animal and experimental studies, FTRD for upper GI lesions, and reviews and commentaries, were excluded. In addition to that, studies were excluded if their data were included in a more recent or a larger study which was already included in our study. All results were downloaded into EndNote X9 (Thompson ISI ResearchSoft, Philadelphia, Pennsylvania, USA). Any duplication was identified and removed. Two reviewers (Y.F and A.H) screened the titles and abstracts of the initially extracted studies. Both reviewers reviewed the full text of the potentially eligible studies. Any disagreement was resolved by consensus or by consulting a third author (M.M).
Two reviewers (Y.F and A.H) extracted the data of interest from the included studies independently using a standardized Excel sheet data. The extracted data include; Study authors, publication year, study design, patients demographics, lesions size and location, indications of the eFTR by FTRD, procedure time, complete resection, technical success, full-thickness resection rates, snare malfunction incidence, complication rates, OSTC fate, duration of hospital stay, and Follow-up period. After data extraction, data sheets from both the reviewers were compared. Any disagreement was resolved by consensus or by consulting a third author (M.M).
The quality of all of the included studies were assessed independently by two reviewers (Y.F and A.H) using the Newcastle-Ottawa Scale (NOS) 15. A score > 7 was considered high quality, 4–6 was considered moderate quality, and <4 was considered low quality. Disagreements between the two reviewers were resolved by consensus or by consulting a third investigator (M.M).
Definitions:
Difficult Adenomas: non-lifting recurrent, non-lifting residual, non-lifting primary, peri-appendicular, and peri-diverticular adenomas.
Complete resection (R0): Histologically tumor-free margins (Lateral and deep) resection
Technical success: reaching the lesion, then deploying the clip successfully, followed by macroscopically full resection using the integrated snare.
Full-thickness Resection (FTR) rate: Histologically confirmed full-thickness resection (mucosa, submucosa, and muscle layers).
Proximal Colon: Lesions that were in cecum, ascending colon, or transverse colon.
Distal Colon: Lesions that were in descending colon, sigmoid, or rectosigmoid areas.
Major bleeding: bleeding that required intervention or blood transfusion.
Minor bleeding: bleeding that did not require intervention or blood transfusion.
Statistical Analysis:
We evaluated safety and efficacy of eFTR of colorectal lesions using FTRD. Efficacy was assessed as technical and R0 rates whereas safety was assessed by post-procedural complication rates. Weighted pooled rate (WPR) with 95% confidence interval (CI) were calculated for the primary outcomes of interest; technical success, R0, and total complications rate. I2 statistics and Cochrane Q test were used to assess the presence of heterogeneity. A P value <0.1 resulting from Cochrane Q test was considered as an indication of the presence of heterogeneity. A significant heterogeneity was considered to be present if the I2 value was more than 50% 16. Depending on the heterogeneity, random or fixed effects model was chosen. If the heterogeneity was substantial (>50%), a random effect model was selected otherwise a fixed effects model was used. Only if it was reported, subgroup analysis was conducted to assess R0 rate according to the indications and site of lesions. For secondary outcomes; FTR rate, snare malfunction rate, and surgical intervention rate, only WPR was conducted. Publication bias was assessed using the funnel plots for technical success, R0 rates, and total complications rate. Quantifying the publication bias if present was not done given the small number of the included studies. The statistical analysis in this meta-analysis was performed using MetCalc by an expert statistician (M.S.M).
Results:
Study Characteristics and Quality Assessment
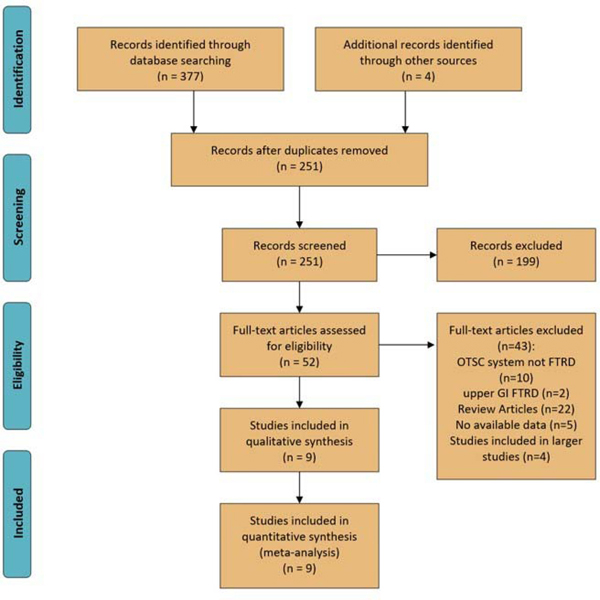
Figure 1 shows the study selection process and study characteristics. Three hundred and eighty-one studies were identified from the search strategy and from manual search, of which 130 were duplicates. Of the remaining 251 studies, 199 were excluded after screening titles and reviewing abstracts. Full-text review was performed on the remaining 52 studies. 9 cohort studies (6 retrospective and 3 prospective) were retained and included in this meta-analysis5,6,17–23. Two of the included studies were abstracts21,22. All the studies were conducted in Europe and were published between 2016–2019 except for one study that was conducted in the United States. Three studies were excluded for possible overlapping with larger multicenter studies12,24,25. A study by Kuellmer et al. evaluating FTRD only in early colorectal cancer resection was also excluded for probable data overlapping with larger included studies 26. eFTR using FTRD were planned to be done on 555 lesions in 551 patients. All nine included studies reported the primary outcomes of interest: complete resection (R0), technical success rate, and complication rates. Of these nine studies, six studies also reported subgroup analysis for R0 according to the indication 5,6,18–20,23and four reported the R0 according to the site of lesions 5,18,19,23.
Figure 1:
Study selection process using PRISMA flow diagram[14]
The quality of the included studies was assessed using the Newcastle-Ottawa score scale. All the included studies were moderate in the methodological quality.
Meta-Analysis Results:
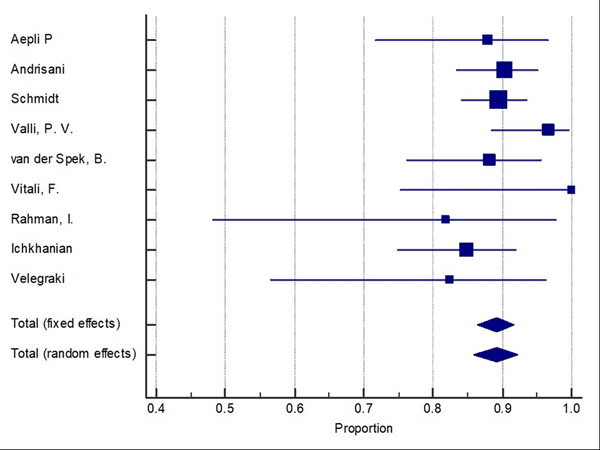
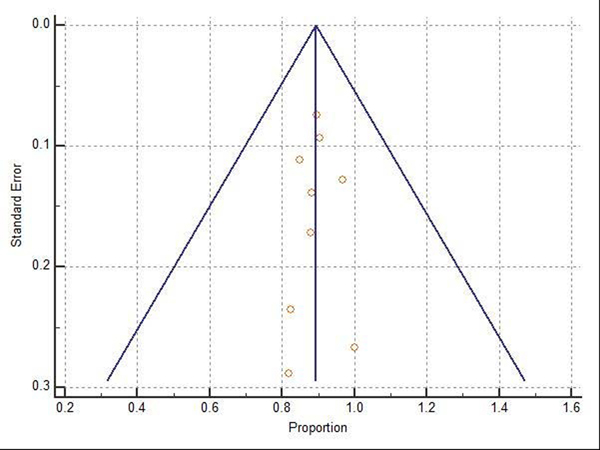
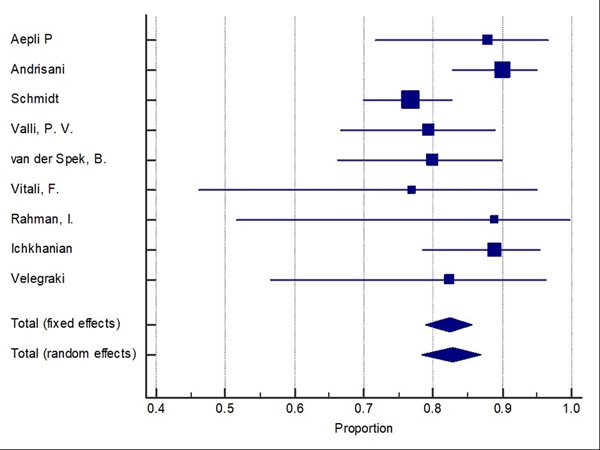
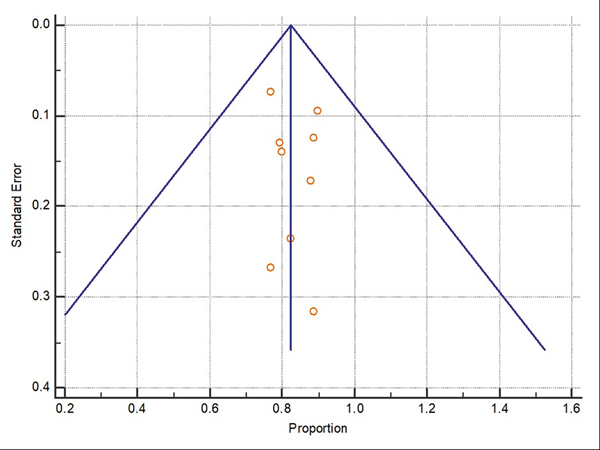
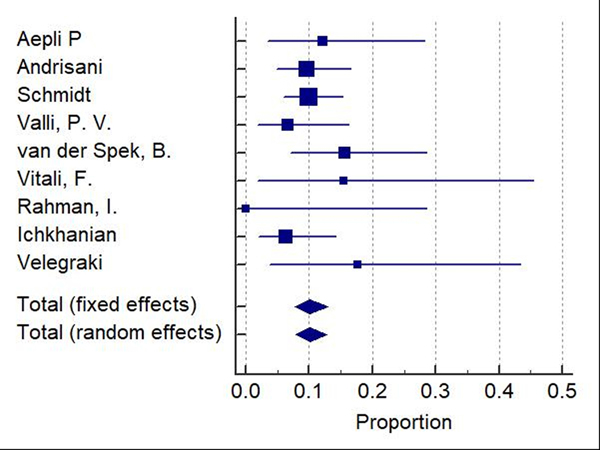
Difficult adenomas resection using FTRD was the most frequent indication (68.7%) followed by early carcinoma resection (17.5%), and then subepithelial lesions (10.7%). 47.3% of the lesions were in the proximal colon, 28.9% in the rectum, and 22.8% in the distal colon. Table 1, Table 2, and Table 3 show the indications, site of lesions, and the clinical outcomes. All the included studies reported the technical success rate, which was defined as reaching the lesion and successfully deploying the clip followed by macroscopically fully resecting the lesion with the integrated snare system. Failure to do any of these steps were considered as a technical failure. Pooled WPR for technical success of FTRD was 89.25% (95% CI: 86.4–91.7%), Cochran Q test P = 0.23, I2 = 23.7% (Figure 2). Funnel plot was fairly symmetrical (Figure 3). Technical failure due to snare malfunction were reported in 32 cases out of 420 cases (7.6%). The other main cause of the technical failure was non-reachable lesions as reported in 10 out of 420 cases. R0 rate which defined as histologically tumor-free margins of the resected lesion, was also reported in all included studies. Pooled WPR for R0 rate of FTRD was 82.4% (95% CI: 79.0– 85.5%), with moderate heterogeneity; Cochran Q test P = 0.14, I2 = 34.8% (Figure 4). Funnel plot was symmetrical (Figure 5). Subgroup analysis of the R0 rate according to the indications and site of lesions were conducted. Six studies reported the R0 rate according to the indication. From those six studies, 297 difficult adenomas were reported with R0 WPR of 82.7% (95% CI: 74.7–89.5%). Peri-appendicular and peri-diverticular lesions were considered as difficult adenomas. Six studies reported specifically the R0 for peri-appendicular lesions with WPR of 82.8%. The WPR for R0 rate for early carcinoma was 81.3% (95% CI: 70.3–89.6%). For subepithelial lesions, the WPR was 81.9% (95% CI: 68.8–91.2%). According to locations, the WPR for R0 rate for lesions located in proximal colon was 76.6% (95% CI: 68.9–83.3%). For lesions located in the distal colon, the WPR for R0 rate was 78.6% (95% CI: 65.8–88.4%). Finally, the WPR for R0 rate for rectal lesions was 78.5% (95% CI: 65.1–88.5%). From the nine included studies, six studies reported the FTR rate. The WPR for FTR was 88.6%. Out of the six studies, three studies reported FTR according to the lesion site. For lesions located in the proximal colon, the WPR for FTR was 77.6% whereas the WPR for lesions located in the distal colon and rectum were 72.8% and 71.9%, respectively.
Table 1.
Studies characteristics. eFTR: Endscopic full-thickness resection
| Study | Country | Study Design | Patient number | Age | Gender (M/F) | Number of lesions | Lesion Size before resection (mm) | Indications | Study quality Using NOS |
|---|---|---|---|---|---|---|---|---|---|
| Aepli P et al., 2017 18 | Switzerland | Retrospective | 33 | 65.9 (mean) | (23/10) | 33 | 13.5 | Recurrent adenoma (18) Staging after resection of a malignant polyp (4), Primary non-lifting adenoma (2), Peri-appendicular adenomas (2), Primary eFTR of polyps suspected to be malignant (2), Non-lifting malignancy recurrence after eFTR (1), Incomplete resection of neuroendocrine tumor (1). | Moderate |
| Andrisani et al.,20196 | Italy | Retrospective | 110 | 68 (mean) | (61/49) | 114 | 17.8 | Residual/recurrent adenoma (39), Histologic R1 resection (26), Non-lifting sign adenoma (12), Para-diverticular and para appendicular adenoma (4), Submucosal lesion (10), suspected T1 carcinoma (16), Diagnostic resection of the colo-rectal wall (3). | Moderate |
| Schmidt et al., 20185 | Germany | Prospective | 181 | 65 (median) | (99/82) | 181 | 15.0 | Difficult adenoma (143) (Adenoma with negative lifting sign – 104/143, adenoma involving the appendiceal orifice 34/143 and adenoma involving diverticulum 5/143) T1 carcinoma (15), Subepithelial tumor (23) | Moderate |
| Valli et al., 201819 | Germany | Retrospective | 60 | 68 (mean) | N/A | 60 | N/A | Recurrent adenomas (22), primary nonlifting adenoma (2), eFTR in addition to piecemeal resection (10), para-diverticular (2), peri-appendicular (4), Submucosal lesions (5), early carcinoma (7), follow-up resection of a malignant polyp (6), eFTR over endoloop (2). | Moderate |
| Vitali et al., 201820 | Germany | Prospective | 12 | 64.3 (mean) | (7/5) | 13 | N/A | Primary non-lifting adenomas (6), Recurrent/residual adenomas (5), Para-diverticular adenoma (1), Subepithelial lesion (1). | Moderate |
| van der Spek et al., 201817 | Netherland | Retrospective | 48 | 67 (mean) | (30/18) | 51 | 12.2 | Non-lifting Adenoma (19) T1 carcinoma (28), Adenoma involving a diverticulum (2), Neuroendocrine tumor (2) | Moderate |
| Rahman et al., 201622 | United Kingdom | Prospective | 11 | 76 (Median) | N/A | 11 | N/A | Non-lifting adenomas (5), T1 polyps (4),and Subepithelial lesion (2). | Moderate |
| Ichkhanian et al., 201921 | USA | Retrospective | 79 | 65 (mean) | (48/31) | 79 | 15.3 | Difficult adenoma (48), Subepithelial lesion (10), Early carcinoma (17) | Moderate |
| Velegraki et al., 201923 | Greece | Retrospective | 17 | 59.7 (mean) | (10/7) | 17 | 12.7 | Recurrent/residual (5), Primary non-lifting adenomas (1), Peri-appendicular (2), T1 carcinoma (3), Subepithelial tumor (6) | Moderate |
Table 2:
Indications and site of lesions
| Indications | Locations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difficult Adenomas | Early Carcinoma | Subepithelial | Others | Proximal Colon | Distal Colon | Rectum | Others | ||||||||
| Study | Recurrent nonlifitng adenomas | Resiudal nonlifitng adenomas | Primary nonlifting adenomas | Peri-appendicular adenomas | Peri-diverticular adenomas | Cecum | Ascending Colon | Transverse | Descending | Sigmoid | |||||
| Aepli P et al., 201718 | 19 | 0 | 4 | 2 | 1 | 2 | 1 | 4 (staging) | 9 | 9 | 0 | 2 | 1 | 12 | N/A |
| Andrisani et al.,20186 | 39 | 26 | 12 | 2 | 2 | 16 | 10 | 3 (Diagnostic resection) | 7 | 11 | 17 | 6 | 10 | 59 | N/A |
| Schmidt et al., 20185 | 53 | 19 | 32 | 34 | 5 | 15 | 23 | N/A | 55 | 35 | 22 | 8 | 30 | 30 | N/A |
| Valli et al., 201819 | 22 | 10 | 2 | 4 | 2 | 13 | 5 | 2 (Over endoloop) | 9 | 15 | 1 | 16 | N/A | 14 | 5 (stomach) |
| Vitali et al., 201820 | 2 | 3 | 6 | N/A | 1 | N/A | 1 | N/A | 2 | 4 | 0 | 1 | 0 | 6 | N/A |
| Van der Spek et al., 201817 | 3 | 12 | 4 | N/A | 2 | 28 | 2 | N/A | 1 | 8 | 2 | 6 | 16 | 18 | N/A |
| Velegraki et al., 201923 | 0 | 5 | 1 | 2 | 0 | 3 | 6 | N/A | 3 | 0 | 1 | 0 | 11 | 2 | N/A |
| Rahman et al., 201622 | 5 | 4 | 2 | N/A | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | N/A | ||||
| Ichkhanian et al., 201921 | 48 | 17 | 10 | N/A | 46 | 17 | 16 | N/A | |||||||
| Total | 384 (68.7%) | 98 (17.5%) | 60 (10.7%) | 257 (47.3%) | 124 (22.8%) | 157 (28.9%) | 5 (1%) | ||||||||
Table 3:
Studies clinical outcomes. eFTR: Endoscopic full-thickness resection, N/A: Not Applicable.
| Study | Procedure Time (Minutes) | Complete Resection (R0) Rate | Technical Success Rate | eFTR Resection Rate | Mean Diameter of Resected Specimen (mm) | Complications | Surgery secondary to complication | Technical failure | OTSC Fate | Hospital Stay (Days) | Follow up | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aepli P et al., 201718 | 63 (mean) | 29/33 (87.9%) | 29/33 (87.9%) | 25/31 (80.6%) | 27.0 | Minor bleeding (2), Major bleeding (1), Perforation (1) | N/A | Not reachable lesions (1), Snare malfunction (3) | Not reported | 3.1 (mean) | Based on histologic type of the resected tissue | Not reported |
| Andrisani et al.,20186 | 45 (mean) | 99/110 (90%) | 103/114 (90.3%) | 100/110 (90.9%) | 20.0 | Traumatic wall injury (4), Stenosis after the deployment of the clip (1), Appendicitis (1), Perforation (1), Postpolypectomy syndrome (1), Tenesmus and perineal pain (2), Major bleeding (1) | 2 | Snare malfunction (12), Not reachable lesions (4) | Spontaneously fallen off (100), In place (10), Removed Endoscopically (2) | 91/110 (82.7%) stayed one day, 19/110 (17.3%) as outpatient | 3 months in all pts | Residual/recurrent disease in only 7 pts on 3-month follow-up |
| Schmidt et al., 20185 | 50 (median) | 139/181 (76.8%) | 162/181 (89.5%) | 162/181 (89.5%) | Not reported | Minor bleeding (4),Appendicitis (3), Postpolypectomy (3), Recurrent Abdominal pain (1), Perforation (6), Enterocolonic fistula (1) | 4 | Snare malfunction (13) | Spontaneously fallen off (106/154), In place (48), Enodscopically removed (10) | 4 (median) | 3 months 154/181 | 19/154 |
| Valli et al., 201819 | 60 (Median) | 46/58 (79.3%) | 58/60 (96.7%) | 51/58 (87.9%) | 24.0 | Minor bleeding (2), Appendicitis (1), Incomplete OTSC deployment (1). | N/A | Not reachable lesions (2), Snare malfunction (4) | Spont. Fallen off (26), In place (4), Removed endoscopically (3), unknown (24) | Not reported | Mean 16 months | No recurrence reported. |
| Vitali et al., 201820 | 68 (mean) | 10/13 (76.9%) | 13/13 (100%) | Not reported | 17.0 | Postpolypectomy (2) | N/A | Snare malfunction (1) | N/A | 2.5 (mean) | 8.7±7.2 months | 3/11 |
| Van der Spek et al., 201817 | Not reported | 40/50 (80%) | 45/51 (88.2%) | 43/50 (86%) | 21.0 | Minor bleeding (4), Major bleeding (1), Perforation (1), Postprocedural cardiac event (1), urinary retention (1) | N/A | N/A | N/A | N/A | 42/48 130 days (+/−11 days) | 5/42 |
| Rahman et al., 201622 | 40 (Median) | 8/9 (88.9%) | 9/11 (81.8%) | 9/9 (100%) | 22.0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ichkhanian et al., 201921 | 63 (mean) | 56/63 (88.9%) | 67/79 (84.8%) | N/A | N/A | Appendicitis (1), Perforation (1), Minor bleeding (3) | 2 | Snare malfunction (3), clip closure (1),, Not reachable lesion (1) | N/A | 0.4 | N/A | N/A |
| Velegraki el al., 201923 | 36.9 (mean) | 14/17 (82.3%) | 14/17 (82.3%) | N/A | N/A | Minor bleeding (1), Appendicitis (1), Recurrent abdominal pain of unknown cause (1) | 1 | Not reachable lesion (2), Macroscopically incomplete resection (1) | N/A | 1–3 days | 6/17 3-month follow up | No recurrence both macro and microscopically |
Figure 2:
Forest plot for technical success of Full thickness resection device (FTRD). Size of the square is proportional to the precision of the study-specific effect estimates, and the bars indicate the corresponding 95% (Confidence Interval) CIs. The diamond is placed on the summary correlation coefficient of the observational studies, and the width indicates the corresponding 95% CI.
Figure 3:
Funnel plot for technical success
Figure 4:
Forest plot for complete resection (R0) of Full thickness resection device (FTRD). Size of the square is proportional to the precision of the study-specific effect estimates, and the bars indicate the corresponding 95% (Confidence Interval) CIs. The diamond is placed on the summary correlation coefficient of the observational studies, and the width indicates the corresponding 95% CI.
Figure 5:
Funnel plot for complete resection (R0) of Full thickness resection device (FTRD).
Complications:
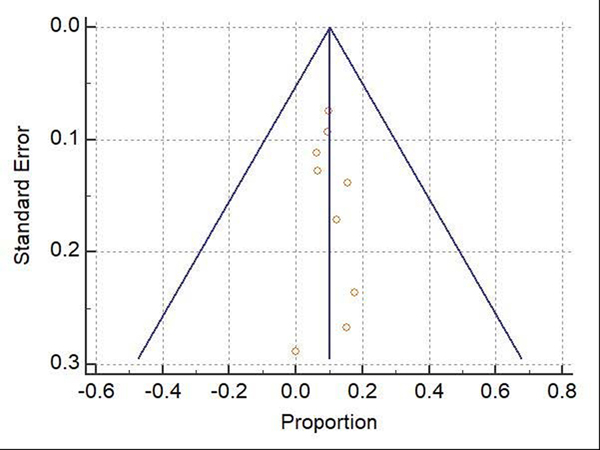
Table 4 summarizes the complication rates. The WPR for total complication rate was 10.2% (95% CI: 7.8–12.8%), Cochran Q test P = 0.51, I2 = 0% (Figure 6). Funnel plot for total complication rate was fairly symmetrical (Figure 7). The most common complication reported in the included studies was minor bleeding (3.2%). The WPR for major bleeding, postpolypectomy syndrome, perforation, and traumatic bowel wall injury, were 0.97%, 2.2%, 1.2%, and 0.78%, respectively. Of 44 patients with peri-appendicular lesions, the pooled rate of appendicitis was 19.7%. Following eFTR, 38 patients underwent surgery for any reason with WPR of 6.7%. For complications-related surgery, the WPR was 2.2%.
Table 4:
Complication rates. WPR: Weighted pooled rate
| Complications | WPR |
|---|---|
| Total | 10.2% |
| Minor bleeding | 3.2% |
| Major bleeding | 0.97% |
| Perforation | 1.2% |
| Postpolypectomy syndrome | 2.2% |
| Traumatic injury | 0.78% |
| Acute appendicitis: Rate out of total lesions Rate out of peri-appendicular lesions | 1.7% 19.7% |
| Surgery for any reason | 6.7% |
| Surgery secondary to complications | 2.2% |
Figure 6:
Forest plot for total complications rate. Size of the square is proportional to the precision of the study-specific effect estimates, and the bars indicate the corresponding 95% (Confidence Interval) CIs. The diamond is placed on the summary correlation coefficient of the observational studies, and the width indicates the corresponding 95% CI.
Figure 7:
Funnel plot for total complications rate
Discussion:
FTRD is a newly emerging over-the scope-clip device that is developed for single-step eFTR for colorectal lesions that are not amenable for resection by conventional methods. FTRD has been increasingly used for colorectal lesions resection with variations in its efficacy and safety profiles among the studies. Thereby, we aimed in this study to evaluate the cumulative efficacy and safety of this new device.
We found that FTRD had excellent efficacy for managing difficult colorectal lesions with technical success rate of 89.2% with low heterogeneity and R0 rate of 82.2% with moderate heterogeneity. In addition to its high technical success and R0 rates, FTRD has shown to have high FTR rate (88.6%). In two meta analyses investigating the clinical outcomes of ESD in colorectal lesions resection, the R0 rates were 80.3% and 82.9% with FTR of 91% in the two studies 27,28. These numbers seem to be similar to the numbers found in this study. However, certain points should be addressed. First, the study population is different in our study as FTRD was mainly used for non-lifting adenomas and adenomas in difficult locations. Given the presence of fibrosis and scar tissue, these non-lifting adenomas harbor high perforation and incomplete resection rates if they were resected by ESD even by expert hands 29,30. Second, in fact, the R0 rate for ESD in Western countries is significantly lower than in Eastern countries. In two meta-analyses, the R0 rates for ESD in Western countries were 74% and 71.3% compared to 89% and 85.6%, respectively 27,31. This difference between Western and Eastern R0 rates for ESD is most likely multifactorial as it could be due to long learning curve, long procedure times, differences in incidences of diseases, etc. On the other hand, in our study, all FTRD procedures were done in Western countries and the R0 rate was 82.2%. Third, further modification and development of the FTRD would increase the technical success rate thereby leading to increase in the R0 rate. As an example, in the current study, 7.6% of the technical failure was due to malfunction of the integrated snare. This technical failure would result in more cases with incomplete resection. According to Schmidt el al. 5, the integrated snare was modified by the company which could lead to an increase in the technical success and R0 rates in the future studies. Furthermore, evaluating the incorporation of the lesions prior to resection by using a novel ‘test-cap’ (prOVE CAP, Ovesco Endoscopy) could also increase the R0 rates. FTRD may not be attempted and another resection modality should be considered if the lesion cannot be fitted and pulled into this ‘test-cap’. Ultimately, given the different population, different ESD experience between Western and Eastern countries, and the room for further development and modification for FTRD, FTRD seems to be more effective than the conventional resection methods in selected patient population.
The most frequent indication for FTRD was difficult adenomas followed by early carcinoma resection, then by subepithelial lesions resection. Although not reported in all included studies, a subgroup analysis was conducted to evaluate the R0 rate for different indications and site of the lesion. The pooled R0 rate for difficult adenomas was 82.7%. For early carcinoma resection and subepithelial, the pooled R0 rates were 82.3% and 81.9%, respectively. The R0 rates for the different three indications were almost the same which indicate that FTRD has the same efficiency regardless of the indication. Similarly, R0 resection rates were almost similar for lesions located in the proximal colon, distal colon, or in the rectum (76.6%, 78.6%, and 78.5%, respectively). Therefore, indicating that FTRD is again efficient regardless of the site of the lesion in the colorectum.
In addition to its efficacy, we found that FTRD is safe with low complication and surgery requirements rates. The pooled total complication rate was 10.2% with no heterogeneity. Minor bleeding that was managed either endoscopically during the procedure or conservatively was the most common complication encountered (3.2%) followed by perforation (2.2%). The perforation rate for colorectal ESD has been reported in two different meta analyses to be near 5% 27,28. The perforation rate is higher in ESD as this technique becomes more difficult with extensive scar tissue and fibrosis which is expected to be seen in recurrent or residual adenomas. Usually, lesions involving or around the appendiceal orifices are managed surgically. From the included studies, 44 peri-appendicular lesions were resected using FTRD with pooled R0 of 82.8%. Despite its feasibility and efficacy to resect peri-appendicular lesions, closure of the appendiceal orifice during the procedure with the FTRD will increase the risk of acute appendicitis. In this meta-analysis, 19.7% of the peri-appendicular lesions that were resected using FTRD were complicated with acute appendicitis. In addition to the previous complications, the rates for other complications were low as well. The occurrence rates of major bleeding, postpolypectomy syndrome, traumatic injury, and other complications were 0.97%, 1.2%, 0.8%, and 1.9%, respectively. Finally, the pooled rate of surgical intervention regardless of the etiology was 6.7%. Nevertheless, surgical intervention secondary to post-FTRD complications was required only in 2.2% of cases. On the other hand, the rate of post-ESD surgical intervention was reported to be up to 9.9% 27,28. Again, extensive submucosal fibrosis and scar tissue could be the main cause of the high post-ESD surgical intervention rate.
One of the main limitations of this analysis is that all the included studies were non controlled cohort studies because no randomized clinical trials have been published. As well as, the variations in the endoscopists expertise could be a source of bias. In addition to these limitations, not all included studies stratified the R0 rates according to the site of the lesions or by the indications of the FTRD, which resulted in inadequately investigating the observed moderate heterogeneity for R0 (I2 =34.8%).
In conclusion, FTRD seems to be effective and safe in managing difficult colorectal lesions. However, the evidence presented in this study is derived from observational studies. Larger randomized controlled trials comparing FTRD with conventional resection methods are warranted.
ACKNOWLEDGMENT:
All authors gratefully thank professor Madhuri S. Mulekar, Ph.D for her help and input in the statistical analysis of this study.
Statistical analysis reported in this publication by Dr. Mulekar was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417. Dr. Mulekar has no financial or proprietary interest in the subject matter of this article.
Footnotes
COFLICT OF INTEREST:
All authors have no conflict of interest to disclose.
References:
- 1.Kuroki Y, Hoteya S, Mitani T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. Journal of gastroenterology and hepatology. 2010;25(11):1747–1753. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto H, Kitano M, Suetomi Y, et al. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound in medicine & biology. 2008;34(4):525–532. [DOI] [PubMed] [Google Scholar]
- 3.Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64(1):57–65. [DOI] [PubMed] [Google Scholar]
- 4.Hong SN, Byeon JS, Lee BI, et al. Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointestinal endoscopy. 2016;84(1):98–108. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67(7):1280–1289. [DOI] [PubMed] [Google Scholar]
- 6.Andrisani G, Soriani P, Manno M, et al. Colo-rectal endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD(®)): A multicenter Italian experience. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2019;51(3):375–381. [DOI] [PubMed] [Google Scholar]
- 7.Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2018;30 Suppl 1:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Mori H, Kobara H, Nishiyama N, Masaki T. Current status and future perspectives of endoscopic full-thickness resection. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2018;30 Suppl 1:25–31. [DOI] [PubMed] [Google Scholar]
- 9.Shi D, Li R, Chen W, et al. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surgical endoscopy. 2017;31(2):837–842. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Liu Z, Sun S, et al. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surgical endoscopy. 2015;29(11):3356–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surgical endoscopy. 2014;28(6):1978–1983. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47(8):719–725. [DOI] [PubMed] [Google Scholar]
- 13.Meier B, Schmidt A, Glaser N, et al. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surgical endoscopy. 2020;34(2):853–860. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 16.Deeks J. Analyzing data and undertaking meta-analyses In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions 5.0. 1. In: Oxford, UK: The Cochrane Collaboration; 2008. [Google Scholar]
- 17.van der Spek B, Haasnoot K, Meischl C, Heine D. Erratum: Endoscopic full-thickness resection in the colorectum: a single-center case series evaluating indication, efficacy and safety. Endoscopy international open. 2018;6(10):C4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aepli P, Criblez D, Baumeler S, Borovicka J, Frei R. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): Clinical experience from two tertiary referral centers in Switzerland. United European gastroenterology journal. 2018;6(3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valli PV, Mertens J, Bauerfeind P. Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD(®)). Surgical endoscopy. 2018;32(1):289–299. [DOI] [PubMed] [Google Scholar]
- 20.Vitali F, Naegel A, Siebler J, Neurath MF, Rath T. Endoscopic full-thickness resection with an over-the-scope clip device (FTRD) in the colorectum: results from a university tertiary referral center. Endoscopy international open. 2018;6(1):E98–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichkhanian Y, Vosoughi K, Sharaiha RZ, et al. 716 NON-EXPOSURE FULL-THICKNESS RESECTION OF COLONIC LESIONS IN THE US: THE FTRD EXPERIENCE. Gastrointestinal Endoscopy. 2019;89(6):AB108. [Google Scholar]
- 22.Rahman I, Boger P, Ishaq S, et al. OC-013 Endoscopic Full-Thickness Resection (eFTR) in the Colon with the FTRD System: The First UK Experience. In: BMJ Publishing Group; 2016. [Google Scholar]
- 23.Velegraki M, Trikola A, Vasiliadis K, et al. Endoscopic full-thickness resection of colorectal lesions with the full-thickness resection device: clinical experience from two referral centers in Greece. Annals of gastroenterology. 2019;32(5):482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrisani G, Pizzicannella M, Martino M, et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017;49(9):1009–1013. [DOI] [PubMed] [Google Scholar]
- 25.Richter-Schrag HJ, Walker C, Thimme R, Fischer A. [Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2016;87(4):316–325. [DOI] [PubMed] [Google Scholar]
- 26.Kuellmer A, Mueller J, Caca K, et al. Endoscopic full-thickness resection for early colorectal cancer. Gastrointestinal endoscopy. 2019;89(6):1180–1189.e1181. [DOI] [PubMed] [Google Scholar]
- 27.Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2017;86(1):74–86.e17. [DOI] [PubMed] [Google Scholar]
- 28.Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointestinal endoscopy. 2015;81(3):583–595. [DOI] [PubMed] [Google Scholar]
- 29.Hori K, Uraoka T, Harada K, et al. Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy. 2014;46(10):862–870. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointestinal endoscopy. 2010;72(6):1217–1225. [DOI] [PubMed] [Google Scholar]
- 31.Daoud DC, Suter N, Durand M, et al. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis. World journal of gastroenterology. 2018;24(23):2518–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]