Abstract
Background
Chronic lung allograft dysfunction (CLAD), is a major hurdle for long-term lung allograft survival after lung transplant and roughly 50% of lung transplant recipients (LTxRs) develop CLAD within 5 years. The mechanisms of CLAD development remain unknown. Donor-specific immune responses to human leukocyte antigen (HLA) and lung self-antigens (SAgs) are vital to the pathogenesis of CLAD. Reduction in Club cell secretory protein (CCSP) has been reported in bronchoalveolar lavage (BAL) fluid samples from LTxRs with bronchiolitis obliterans syndrome (BOS).
Methods
CCSP levels in BAL fluid and development of antibodies to lung SAgs in plasma were determined by ELISA. Cytokines in BAL fluid were analyzed by 30-plex Luminex panel. Exosomes from BAL fluid or plasma were analyzed for SAgs, natural killer cells markers, and cytotoxic molecules.
Results
We demonstrate that LTxRs with BOS have lower CCSP levels up to 9 months before BOS diagnosis. LTxRs with antibodies to SAgs 1 year posttransplant also developed DSA (43%) and had lower CCSP. BOS with lower CCSP also induced Interleukin-8 and reduced vascular endothelial growth factor. Exosomes from BOS contained increased SAgs, natural killer cells markers, and cytotoxic molecules.
Conclusion
We conclude lower CCSP leads to inflammation, pro-inflammatory cytokine production, immune responses to HLA and SAgs, and induction of exosomes. For the first time, we demonstrate that CCSP loss results in exosome release from natural killer cells capable of stimulating innate and adaptive immunity posttransplant. This increases the risk of BOS, suggesting a role of natural killer cell exosomes in CLAD development.
Introduction
Club cells are nonciliated bronchiolar epithelial cells, mainly found on bronchioles, which contribute to host defense through the production of Club cell secreted protein (CCSP).1 CCSP, which is anti-inflammatory, is used as a biomarker for respiratory stress in athletes, in individuals with asthma, and in experimental models of acute and chronic lung injury. Kelly et al reported that lung transplant recipients (LTxRs) who develop bronchiolitis obliterans syndrome (BOS) show significant decreases in CCSP levels and Club cell numbers in bronchoalveolar lavage (BAL) fluid compared with stable LTxRs.2 According to the International Society for Heart and Lung Transplantation (ISHLT), in 2018 the 5-year survival of LTxRs was approximately 50%, which is much lower than 5-year survival for recipients of other solid organ transplants. A major complication that limits long-term graft survival after lung transplant (LTx) is chronic lung allograft dysfunction (CLAD), which includes restrictive allograft syndrome and BOS, and has been shown to be triggered by donor-specific alloimmune responses such as antibodies (Abs) to mismatched donor human leukocyte antigens (HLA).3, 4
Bronchiolitis obliterans syndrome is a fibroproliferative disease of unknown etiology, and is a major risk factor for morbidity and mortality after LTx. Our laboratory has demonstrated a strong correlation between development of donor-specific anti-HLA (DSA), Abs to lung self-antigens (SAgs), and development of BOS.5, 6 Furthermore, in our studies, DSA often preceded the development of Abs to SAgs and BOS.5 Although DSA can be transient, Abs to SAgs are often persistent and have been shown to be independent of DSA.6 Cell`s gene expression profiles analyzed in the BAL fluid of recipients with CLAD have demonstrated genes related to immune responses, including genes involved in recruitment, retention, activation, and proliferation of cytotoxic lymphocytes (CD8+ T-cells and natural killer [NK] cells).7 Recent studies have shown cytomegalovirus-related graft injury, which can lead to CLAD, is associated with an increased NKG2C NK cell population in BAL fluid.8 With their diverse receptors, NK cells have the potential to influence clinical outcomes after LTx. However, the mechanisms by which NK cells contribute to CLAD remain largely unknown. NK cell exosomes have been shown to carry cytotoxic proteins (ie, Fas ligand [FasL] and perforin) with antitumor activities.9 Our previous studies have demonstrated that exosomes isolated from LTxRs diagnosed with BOS contain donor HLA, lung SAgs, MHC-class II, costimulatory molecules, cell adhesion molecules, and various transcription factors.10–13 In addition, we were able to isolate circulating exosomes with lung SAgs in certain LTxRs months before they were diagnosed with acute rejection and BOS diagnosis, indicating that exosomes with lung SAgs may not only serve as biomarkers for rejection, but may in fact contribute to the pathogenesis of rejection. This is further supported by results showing that exosomes isolated from LTxRs with BOS are immunogenic and caused development of humoral and cellular immune responses to lung SAgs.10, 14 In this communication, we sought to determine the immune mechanisms by which a decline in CCSP contributes to induction of exosomes with NK cell markers, leading to production of pro-inflammatory cytokines, Abs to donor HLA, and lung SAgs, ultimately resulting in CLAD after human LTx.
Material and Methods
Patients
We performed a retrospective study and cross-section analysis. Informed consent and approval from the Institutional Review Boards for human research at Washington University/Barnes Hospital (St. Louis, MO, USA, IRB#: 201103312) and St. Joseph’s Hospital and Medical Center (Phoenix, AZ, USA, IRB: PHXB-16–0027-10–18) were obtained. Plasma and BAL fluid samples were collected from patients 2 to 3 years after undergoing LTx at either of the 2 aforementioned institutions. BOS was diagnosed according to ISHLT criteria.15–17 In total, 18 LTxRs with BOS and 34 stable LTxRs were included in this study. For serial study following LTx for 1year posttransplant, the number of patients included in the study were different time points: 3 month (n= 48), 6 month (n=39) and 1 year (n=25).
CCSP levels in BAL fluid
Club cell secretory proteins were analyzed using a human CCSP enzyme-linked immunoassay (ELISA) kit (BioVendor, Brno, Czech Republic). The ELISA technique was performed according to the manufacturer’s recommended protocol.
Cytokine analysis in BAL fluid
Analysis of the cytokines found in BAL fluid samples was performed using the Cytokine Human Magnetic 30-Plex Assay Panel (ThermoFisher Scientific, USA). Analysis was done according to the manufacturer’s recommended protocol and read using a Bio-Plex 200 array reader system (Bio-Rad, Hercules, California, USA).
Messenger RNA analysis in cell samples obtained from BAL fluid
Messenger RNA (mRNA) was quantified by reverse transcriptase polymerase chain reaction (RT-PCR). After separating total RNA from BAL cells, complementary DNA (cDNA) synthesis was performed using poly(A) selected RNA primed with oligo (dT),. PCR was then performed in another tube using primers specific for the gene of interest (Invitrogen). The thermal cycling parameters are described here. First, uracil-DNA glycosylase (UNG) was incubated for 2 minutes at 50°C. Second, polymerase activation was performed for 10 minutes at 95°C. Next, PCR was denatured for 15 seconds at 95°C, and annealed and extended for 1 minute at 60°C. 40 cycles of RT-PCR of target gene were performed using TaqMan Gene Expression Assays (ThermoFisher Scientific, Waltham, Massachusetts, USA). Ct values were determined using the AB StepOnePlus System (ThermoFisher Scientific) with StepOnePlus software version 2.3 (ThermoFisher Scientific). The change in expression was calculated using the 2-ΔΔCt method. We analyzed chemokine (C-X-C motif) ligand 13 (CXCL13), granzyme A (granzyme 1, cytotoxic T-lymphocyte-associated serine esterase 3) (GZMA), CD8a molecule (CD8A), killer cell lectin-like receptor subfamily K, member 1 (KLRK1), natural killer cell granule protein 7 (NKG7), cytotoxic T-lymphocyte-associated protein 4 (CTLA4),7 and oxidative stress marker metallothionein-1H (MT1H).
Detection of circulating Abs to lung SAgs (K-alpha 1 tubulin, Collagen V)
Enzyme-linked immunosorbent assay was performed to detect Abs to K-alpha 1 tubulin (Kα1T) and Collagen V (Col-V) in the plasma samples, as published previously.18 In brief, ELISA plates were coated overnight at 4°C with either recombinant K-α1T (1μg/ml) or Col-V (1μg/ml; Sigma-Aldrich, St. Louis, Missouri, USA) in phosphate-buffered saline and blocked for 2 hours with 1% bovine serum albumin. Plasma samples from LTxRs and plasma samples from normal patients were diluted 1:1000 for Col-V and 1:1250 for K-α1T and loaded. Color was developed using TMB substrate and Abs were detected using horseradish peroxidase conjugated anti-human IgG (1:10,000) and read at 450 nm. Samples were considered positive if the value was greater than the mean ± 2 standard deviations from normal serum samples from healthy controls (166ng/ml for K-α1T and 130ng/ml for Col-V). Ab concentrations were calculated using standard curves of known concentrations of anti-Kα1T (Santa Cruz Biotechnology, Dallas, Texas, USA) or anti-Col-V (Abcam, Cambridge, United Kingdom).
DSA determination
Abs to DSA and their specificity were detected in patient plasma by solid-phase assay (Luminex; One Lambda, Canoga Park, California, USA).
Exosome isolation and purity determination
Exosomes were isolated from BAL fluid or plasma using total exosome isolation (Invitrogen). Briefly, samples were added with reagent and centrifuged at 10,000G to obtain an exosome pellet. The pellet was dissolved in phosphate-buffered saline and centrifuged at 12,000G through a 0.22μm filter to obtain pure exosomes. Size distribution was verified, and exosomes were found to be <200nm (per MISEV guideline 201819) using NanoSight NS300 with NanoSight NTA software version 3.3 (Malvern Panalytical, Malvern, United Kingdom).
Characterization of exosomes
Exosomes were separated under reducing conditions using 4% to 12% gradient Bis Tris gel (Invitrogen) electrophoresis. The protein was transferred to a nitrocellulose membrane and blocked with Tris-buffered saline containing 5% skim milk protein for 1 hour. Exosomes isolated from stable LTxRs and from LTxRs with BOS were analyzed with Abs specific to target proteins, including Col-V (ab7046), CIITA (ab49132; Abcam), NF-κB (C22B4), cell-signaling technology, Kα1T (sc-12462-R), 20S proteasome (sc-166205), CD56 (sc-7326), NKG2D (sc-23869), perforin (sc-373943), FasL (sc-19681; Santa Cruz Biotechnology), per manufacturers’ protocol. The blots were then washed 3 times with Tris-buffered saline and incubated with horseradish peroxidase-conjugated goat anti-human IgG (1:10,000) (Jackson Immuno Research Laboratories, West Grove, Pennsylvania, USA) for 2 hours. The Western blot image was detected and quantitated by using Odyssey Fc with Image studio version 5.2 software (LI-COR Biotechnology, Lincoln, Nebraska, USA).
Cytotoxicity and immunofluorescence studies
Complement-dependent cytotoxicity (CDC) was determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Club cell line (H441 cell line, originated from human club cells (NCI-H441 [H441] (ATCC® HTB-174™)), rinsed twice with PBS, were seeded into a 96-well flat bottom plate in FBS free medium (1×105/well in 50 ul). Stable and BOS serum (1:10 dilution was incubated with club cells for 1 hr followed by rabbit complement 1:20 dilution) for 1 hr. The absorbances of the samples were measured at 595 nm and cytotoxicity was calculated based on UN-treated control cells.
For immunofluorescence studies, stable and BOS serum (1:10) dilution was incubated with club cell line H441 for 24 hrs at 4°C followed by incubation of FITC tagged anti-Human IgG and imaging was performed using Mantra quantitative pathology imaging system.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc, California, USA) was used to perform data analysis. CCSP levels, cytokine analysis in BAL fluid, and mRNA analysis in BAL fluid cells were analyzed by Mann-Whitney test. Optical density of exosomes by Western blot analysis was compared using Mann-Whitney or two-tailed student’s t-test. Statistical data in each cohort was expressed as mean ± standard deviation. P-values less than 0.05 were considered statistically significant in each comparative analysis. The fold changes were calculated after normalization of mean optical density of exosomes with CD-9.
Results
CCSP levels in BAL fluid were significantly reduced in LTxRs diagnosed with BOS
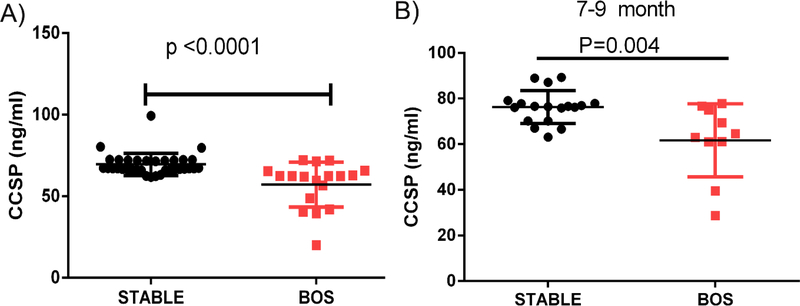
As has been reported previously,2 LTxRs diagnosed with BOS demonstrated significant decline in BAL fluid CCSP level (57.08±13.8ng/ml [n=18] vs 69.5±6.8 ng/ml [n=34], respectively; p<0.0001; Figure 1). We also found that a significant decline in CCSP levels in the BAL fluid of LTxRs with BOS of same study population used before and with samples available demonstrated 7 to 9 months before BOS can be clinically diagnosed (BOS LTxRs: 61.7±16.02ng/m [n=10] vs stable LTxRs: 76.2 ±7.2 ng/ml [n=18]; p<0.004; Figure 1B).
Figure 1:
(A) Lung transplant recipients with bronchiolitis obliterans syndrome (BOS) had low levels of club cell secretory protein (CCSP) in bronchoalveolar lavage fluid samples both at the time of BOS diagnosis and (B) 7 to 9 months before BOS diagnosis.
Decline in CCSP level and de novo development of Abs to DSA and lung SAgs in LTxRs diagnosed with BOS
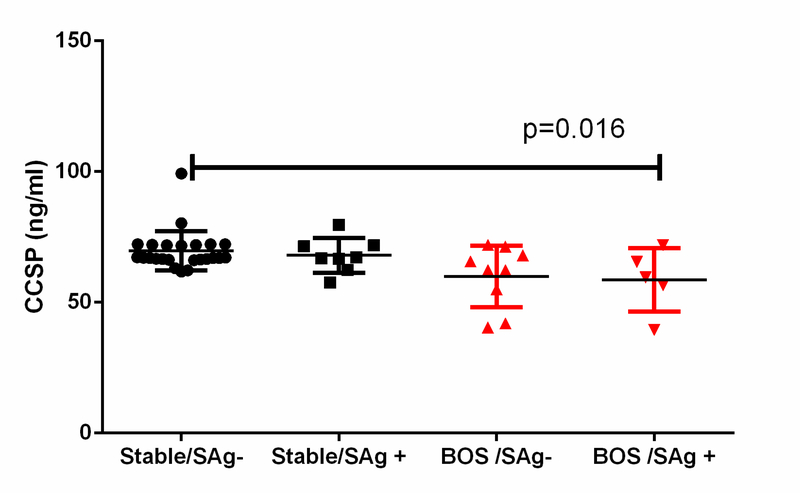
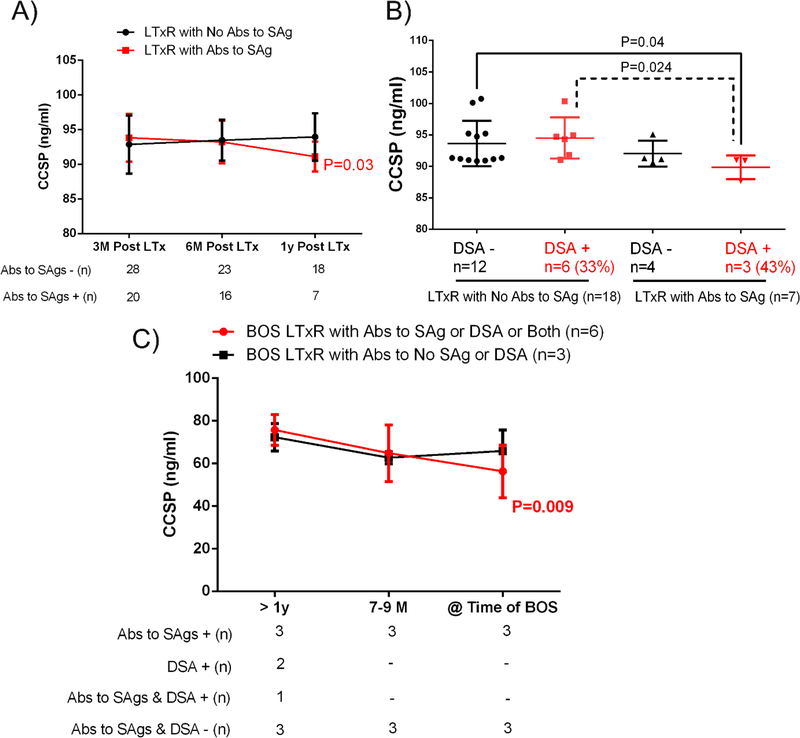
Lung transplant recipients who developed Abs to lung SAgs at the time they were diagnosed with BOS had lower levels of CCSPs in BAL fluid compared with stable LTxRs without Abs to lung SAgs (58.6±12.2ng/ml [n=5] vs 69.7±7.5ng/ml [n=24], respectively; P = 0.016). This suggests that CCSPs play a role in regulating immune responses against lung SAgs (Figure 2). In addition, LTxRs who developed de novo Abs to lung SAgs within 1 year of LTx also had lower levels of CCSPs (91.1±2.15 vs 94±3.4 ng/ml) than LTxRs without Abs de novo DSA to lung SAgs (P = 0.03; Figure 3A). LTxRs who developed both de novo DSA (43% of the study cohort) and Abs to lung SAgs within 1 year of LTx also had lower levels of CCSPs than LTxRs who did not develop Abs to SAgs with (P = 0.024) or without de novo DSA (P = 0.04; Figure 3B), suggesting that loss of CCSPs activates immune responses against donor antigens, leading to development of de novo DSA and lung SAgs. Interestingly, LTxRs who developed DSA or lung SAgs 1 year before diagnosis of BOS and whose DSA or abs to lung SAgs persisted had progressive decline in CCSP levels (P = 0.009). It is significant that LTxRs who did not develop DSA or abs to lung SAgs 1 year before diagnosis of BOS had no decline in CCSP levels (Figure 3C).
Figure 2:
Lung transplant recipients with bronchiolitis obliterans syndrome (BOS) (n=5) with antibodies to self-antigens (SAgs) Collagen V and K-alpha 1 tubulin had low levels of club cell secretory protein (CCSP) in bronchoalveolar lavage fluid samples compared to stable (n=24).
Figure 3:
(A) Lung transplant recipients (LTxRs) with low levels of club cell secretory protein (CCSP) developed antibodies (Abs) to lung self-antigens (SAgs) within 1 year of transplant, compared to LTxRs without Abs. (B) LTxRs with low CCSP levels developed de novo donor-specific anti-HLA (DSA), along with Abs to lung SAgs within 1 year of LTx. (C) Abs to SAgs (Collagen V and K-alpha 1 tubulin), if persistent, lower CCSP levels in bronchoalveolar lavage fluid samples.
Increased levels of Interleukin-8 and reduced levels of vascular endothelial growth factor in BAL fluid samples from LTxRs with BOS
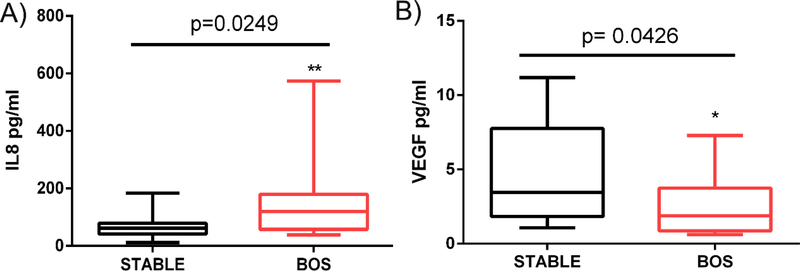
We analyzed the composition of the BAL fluid cytokines in LTxRs with BOS and stable LTxRs using the Cytokine Human Magnetic 30-Plex Assay Panel (ThermoFisher Scientific). LTxRs with BOS (n=18) had lower levels of CCSPs, which was associated with significantly increased levels of Interleukin-8 (IL-8) compared with stable LTxRs (n=18) (156.5±143.4 vs 70.73±46.6 pg/ml, respectively; P = 0.025). The LTxRs with BOS also had reduced levels of vascular endothelial growth factor (VEGF) compared with stable LTxRs (2.65±2.1 vs 4.5±3.3pg/ml, respectively; P = 0.043; Figure 4). Therefore, loss of CCSP may contribute to increased production of IL-8, suggesting that CCSPs play an important role in regulating the pro-inflammatory cascade.
Figure 4:
(A) Interleukin-8 (IL8) was upregulated and (B) vascular endothelial growth factor (VEGF) was downregulated in bronchoalveolar lavage fluid samples from lung transplant recipients with bronchiolitis obliterans syndrome (BOS; n=18).
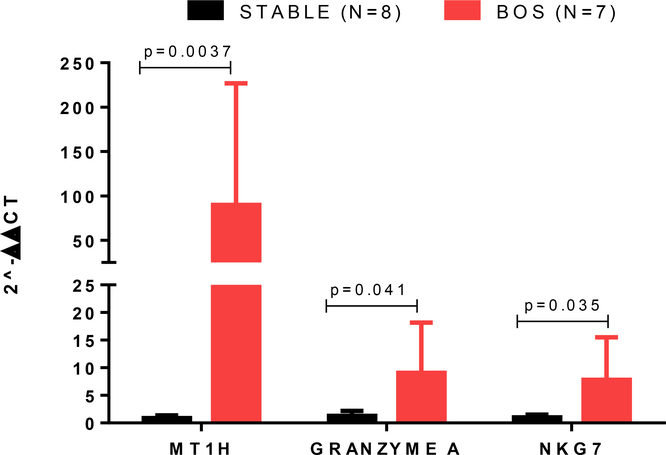
Cells isolated from BAL fluid of LTxRs with BOS have oxidative stress marker MT1H, GRANZYME, and NK-cell-related NKG7 genes
Quantitative polymerase chain reaction (qPCR) analysis of cells in BAL fluid samples of LTxRs with BOS and stable LTxRs demonstrated upregulated mRNA levels of CXCL13 (21.4 fold)(), CD8A(4.1-fold), KLRK1(1.5-fold), and CTLA4(5.2-fold) in LTxRs with BOS, but these values were not statistically significant (Supplementary Figure 1). However, oxidative stress marker MT1H (P = 0.0037), GRANZYME A (P = 0.041), and NK-cell-related NKG7 (P = 0.035) mRNA levels were significantly increased in LTxRs with BOS compared to stable LTxRs (Figure 5). Upregulation of MT1H and NKG7 in BAL fluid of LTxRs with BOS indicates that loss of CCSP may lead to activation of oxidative stress and NK cells, which may play a role in the pathogenesis of BOS.
Figure 5:
MT1H, GRANZYME A, and NKG7 were upregulated in bronchoalveolar lavage fluid samples from lung transplant recipients with bronchiolitis obliterans syndrome.
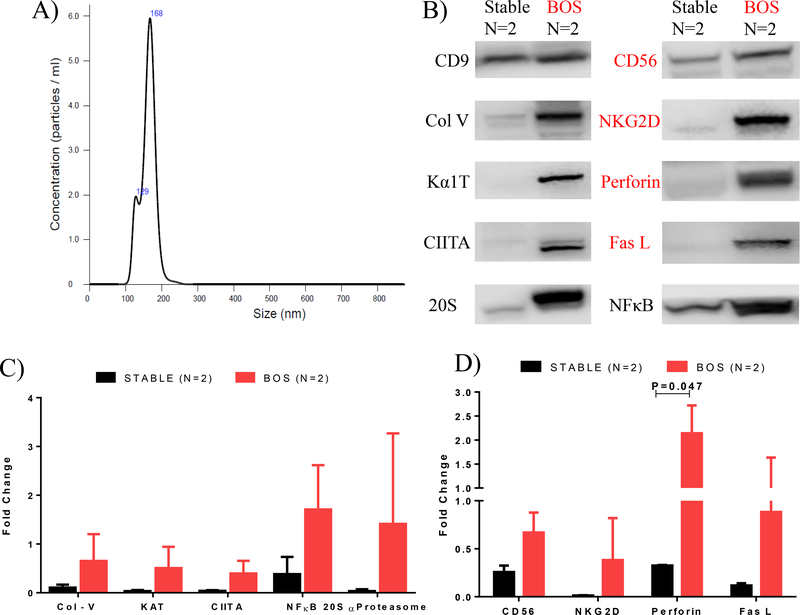
Induction of circulating exosomes in BAL fluid with lung SAgs, transcription factors, and 20S proteasome
Exosomes were isolated from BAL fluid and plasma samples of 10 stable of LTxRs (rejection-free) and 10 LTxRs with BOS. Exosome sizes were <200nm (Figure 6A). Because the amount of exosomes that can be isolated from BAL fluid were limited, the isolated exosomes from 5 patients in each group were pooled and Western blotting was performed using Abs specific to Col-V, Kα1T, CIITA, NFκB, and 20S proteasome (Supplemental Figure 2A). As reported earlier, we found exosomes in LTxRs with BOS that had Col-V, Kα1T, CIITA, NFκB and 20S proteasome.10, 14 Exosomes derived from BAL fluid of LTxRs with BOS (compared with BAL fluid from stable LTxRs) had elevated levels of lung SAgs Col-V (0.65±0.55 vs 0.1±0.06; 6.09-fold), Kα1T (0.51±0.4vs 0.03±0.02; 14.75-fold), CIITA (0.4±0.26 vs 0.04±0.01; 9.72 fold), NF-κB (1.71±0.91 vs 0.38±0.35; 4.45-fold), and 20S proteasome (1.41±1.85 vs 0.05±0.07; 23.9-fold; Figure 6B&C).
Figure 6:
(A) Exosome size, verified by Nanosight, was less than 200nm. (B) Exosomes from bronchoalveolar lavage fluid samples from lung transplant recipients (LTxRs) with bronchiolitis obliterans syndrome (BOS) contained increased levels of self-antigens (CIITA, 20S proteasome, NFκB, CD56, NKG2D, FasL and perforin). (C) Densitometry analysis of self-antigens (CIITA, 20S proteasome, NFκB). (D) Representative densitometry analysis of natural killer (NK) cells related and cytotoxic molecules in LTxRs with BOS (n=2) vs stable LTxRs (n=2), demonstrating increased levels of CD56, NKG2D, FasL, and perforin.
NK cells (CD56, NKG2D) and cytotoxic molecules (perforin, FasL) are significantly increased in the exosomes of LTxRs with BOS
Exosomes that were isolated in BAL fluid samples from LTxRs diagnosed with BOS showed higher levels (compared to stable LTxRs) of the NK-cell-associated molecules CD56 (0.67±0.2 vs 0.26±0.7; 2.6-fold) and NKG2D (0.38±0.4 vs 0.01±0.003; 30.3-fold; Figure 6D). The exosomes isolated from LTxRs diagnosed with BOS also demonstrated the presence of cytotoxic molecules (perforin, FasL; Figure 6B). FasL levels were 0.88±0.75 in LTxRs with BOS and 0.11±0.02 in stable LTxRs (7.5-fold higher in LTxRs with BOS). Perforin levels were 6.6-fold higher in LTxRs with BOS compared with stable LTxRs (2.14±0.58 vs 0.32±.006; P = 0.047; Figure 6D).
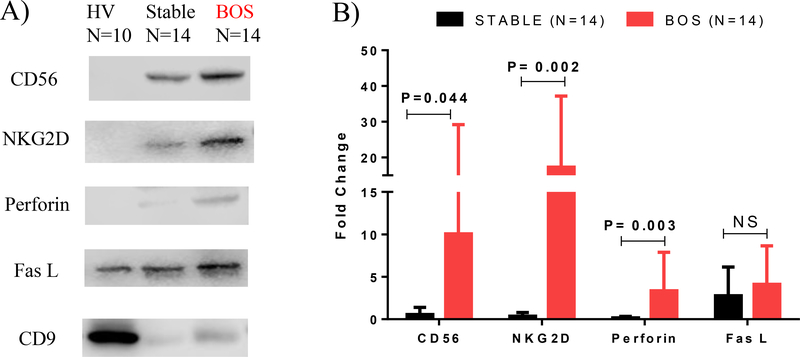
Exosomes isolated from plasma samples of LTxRs with BOS also contained NK cells associated (CD56, NKG2D) as noted in BAL but not in stable and healthy volunteers (Figure 7A & Supplemental Figure 2B). Exosomes from LTxRs diagnosed with BOS had higher level of NK cells associated (CD56 (10.1±19.2 vs 0.55±0.861; 18.4-fold P = 0.044, NKG2D (17.29±19.9 vs 0.37±0.44; 47.29-fold (P = 0.002)) and cytotoxic molecules (Perforin (3.35±4.53 vs 0.17± 0.16; 19.37-fold P = 0.003), FasL (4.1±4.53 vs 2.76±3.39; 1.5-fold)) as compared to stable (Figure 7B).
Figure 7:
(A) Exosomes isolated from plasma samples of healthy volunteers (HV), stable lunt transplant recipients (LTxRs), and LTxRs with bronchiolitis obliterans syndrome (BOS) demonstrate increased levels of CD56, NKG2D, FasL, and perforin in LTxRs with BOS. (B) Densitometry analysis of natural killer (NK) cells related and cytotoxic molecules optical density in LTxRs with BOS (n=14) compared with stable LTxRs (n=14).
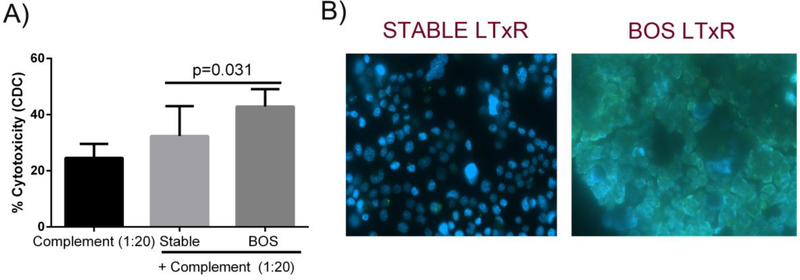
Cytotoxic activity and IgG Abs binding by plasma of BOS LTxRs against H441 club cell line
The potential of sera from BOS LTxRs to induce CDC of club cells was assessed in a MTT assay. Incubation of club cells with sera of BOS LTxRs resulted in significant lysis of 42.9±6.2% (P = 0.031) of club cells compared with stable LTxRs plasma (32.4±10.6%). Incubation of club cells with sera of BOS LTxRs also demonstrated increased binding of Abs present in BOS sera as compared to sera from stable LTxRS (Figure 8B). This binding is mediated by IgG Abs present in BOS sera.
Figure 8:
(A) Cytotoxicity assay demonstrated increased toxicity of club cell line incubated with plasma of BOS compared with stable LTxRs. (B) Immunofluorescence studies demonstrated BOS LTxRs plasma had increased level of IgG Abs bound to club cells compared to stable LtxRs.
Discussion
In this study, human LTxRs who developed BOS demonstrated significant decline in CCSP levels in BAL fluid samples compared with samples obtained from stable LTxRs (Figure 1A). Furthermore, LTxRs with BOS demonstrated significant decline in BAL CCSP levels 7 to 9 months before clinical diagnosis of BOS (Figure 1B), suggesting that CCSPs play an important role in regulating the pro-inflammatory cascade. CCSPs have been reported to have an anti-inflammatory function and have been shown to be reduced in—different pulmonary diseases, infections, injury, BOS, history of cigarette smoking, and others—that can lead to inflammatory responses.20–22
De novo development of DSA has been shown to increase the risk of CLAD development.23 However, DSA can also lead to immune responses to tissue-associated SAgs.5 Ischemia–reperfusion injury during LTx can result in exposure of otherwise-masked SAgs that are released in BAL fluid, leading to the development of immune responses to the SAg Col-V.24 Our previous studies have demonstrated that Abs to Col-V or Kα1T can increase the risk of BOS/chronic rejection after LTx.15, 25 To determine the role of declining CCSP levels and development of Abs to HLA (DSA) and lung SAgs, we analyzed de novo development of Abs to lung SAgs, DSA, or both in stable LTxRs and LTxRs with BOS. Our results demonstrated that loss of CCSP in the BAL fluid of LTxRs with BOS resulted in development of Abs to lung SAgs, whereas stable LTxRs did not develop these Abs to SAgs. We also noted LTxRs who did not have lower levels of CCSP and did not develop Abs to lung SAg developed BOS (Figure 2). However, LTxRs with lower levels of CCSPs also showed de novo development of DSA within 1 year of LTx (Figure 3B), Therefore, we conclude that loss of CCSP in the BAL fluid of LTxRs correlate with development of BOS as well as development of Abs to lung SAgs and DSA, suggesting that the loss of CCSPs can lead to development of allo- and autoimmunity. This further demonstrates the vital role of CCSP in regulating immune responses against donor antigens.
We also assessed LTxRs with BOS who developed Abs to HLA or abs to lung SAgs 1 year before BOS diagnosis and when these Abs to HLA or Abs to lung SAgs persisted, they coincided with a decline in CCSPs (Figure 3C). This suggests that development of DSA or Abs to lung SAgs can also lead to decline in CCSP. Our previous studies, which used a novel transgenic mouse model with lung-specific expression of MHC under a CCSP promoter, have shown that anti-MHC can induce damage to club cells and decrease in CCSP expression, resulting in the development of autoimmunity and obliterative airway disease, a correlate of BOS.26 Decline in CCSP alone or in combination with development of DSA and Abs to lung SAg can result in the development of BOS following human LTx.
Bronchoalveolar lavage fluid samples from patients with BOS with low levels of CCSP showed higher levels of pro-inflammatory cytokine IL-8 and lower levels of VEGF compared to stable LTxRs (Figure 4). This confirms the findings of other reports that showed that LTxRs who developed BOS had elevated levels of IL-8, resulting in neutrophil influx and chemotactic activity.27, 28 Another report showed that a decline in the concentrations of VEGF in BAL fluid occurs 6 months before BOS develops.29 VEGF can promote endothelial cell survival, lung angiogenesis, and alveolar development even in the setting of hyperoxia-induced lung injury;30 therefore, a decline in VEGF can increase the risk of BOS.
Our previous studies have demonstrated that exosomes with lung SAgs can be detected in LTxRs diagnosed with acute rejection and BOS.14, 31 Levels of lung-associated SAgs, Col-V and Kα1T; MHC class II molecules; costimulatory molecules CD40, CD80, and CD86; and transcription factors class II MHC trans-activator, NF-kB, hypoxia-inducible factor 1-α, IL-1R– associated kinase 1, MyD88, and 20S proteasome were increased in exosomes from LTxRs with BOS, but not in stable LTxRs. Furthermore, exosomes derived from LTxRs with BOS demonstrated immunogenic potential in a mouse model after immunization, resulting in the development of humoral and cellular immune response to lung SAgs.10, 14 In this communication, we demonstrate that exosomes from LTxRs with BOS also contained NK cell markers (Figure 6B). NK cell exosomes with perforin, CD56, and high levels of NKp46 and NKG2D molecules have been shown to be involved in both antiviral and antitumor immune surveillance.9 In our study we demonstrated the existence of NK cells (CD56, NKG2D) and cytotoxic molecules (perforin, FasL) in exosomes from LTxRs with BOS—both in cells isolated from BAL fluid samples (Figure 6B&D) and from plasma samples (Figure 7A&B). A recent study reported that NK-92 cells derived exosome-containing NK associated proteins (perforin and FasL), can be cytotoxic to melanoma cells.32 Therefore, we postulate that NK-cell-derived exosomes in LTxRs with BOS indicate NK cell activation, which can be cytotoxic to the lung parenchyma either directly or by Ab-dependent cellular cytotoxicity mechanisms. It is also possible that NK-cell-derived exosomes may interact with recipient’s cells, cross-dressing either by membrane fusion or receptor-to-its ligands MICA/B (humans) or rae-1 (mice), thereby inducing cytotoxic effects that damage lung tissue.33 Sera from BOS LTxRs resulted in CDC of club cells and also had increased level of IgG Abs bound to club cells suggesting that there are Abs specific to antigens present in the club cells (Figure 8). However, the antigen expressed on the club cell line remains to be identified.
Upregulation of the NK-cell-related gene KLRK1 (encoded for NKG2D receptor ) and significant levels of NK cell NKG7 mRNA in BAL cells of LTxRs with BOS were observed in the current study (Figure 5), providing evidence for the role of NK cells in CLAD. NK cells can induce cytotoxicity both by direct cytotoxic mechanisms and Ab-dependent cytotoxicity. NK cells can cause rejection by triggering cytotoxic effects on allograft tissue recognized as “nonself” or “stressed,” via NKG2D receptor ligation to its ligands such as MICA/B (humans), or rae-1 (mice).34, 35 NK cells have been reported to contribute to both acute and chronic allograft rejection by their direct cytotoxic effect, which directly injures allogeneic donor cells and/or indirectly produces interferon-γ stimulated Th1 cell alloreactivity, leading to chronic inflammation via cytokines and sequestered alloantigen exposure, causing T cells activation.36
Other studies have shown hyperoxia exposure to CCSP −/− mice altered cytokine gene expression, with respect to oxidant injury via oxidative stress marker MT1H and/or altered regulation of the inflammatory response.37 We have demonstrated that LTxRs with BOS had elevated levels of oxidative stress marker MT1H compared to stable LTxRs (Figure 5). This finding signifies that loss of CCSP can also lead to oxidant-induced stress and thus increase inflammatory responses. Furthermore, qPCR analysis of BAL cells in LTxRs with BOS demonstrated upregulated mRNA levels of chemokine (CXCL13; Supplemental Figure 1). Recent studies from Halloran et al have demonstrated that allograft biopsies of kidney transplant recipients with DSA (complement activating) had interferon-γ induced transcripts of CXCL10, CXCL11, and CXCL13 in the endothelium due to Ab binding with the CD16a Fc receptor on NK cells.38 These authors have proposed that this can trigger interferon-γ response and NK cell-selective transcripts CD160 and chemokine (C Motif) Ligand 1 (XCL1) in biopsies from renal transplant recipients with Ab-mediated acute rejection.39 Recently, Koenig et al demonstrated that chronic vascular rejection of renal transplants caused by microvascular inflammation from Ab-mediated rejection likely results from innate immune effector NK cells damage of endothelial cells both Ab-dependent (Fc receptor activation in NK cells by DSA bound to endothelial cells) and independent. The authors attributed this to the phenomenon known as missing self (ie, reduced HLA class I molecule, a ligand for inhibition of NK cells in endothelial cells).40
There are some limitations to our study. Since this is a retrospective analysis of LTxRs, we were unable to perform studies that would show infiltration of NK cell population in the biopsies, due to unavailability. We did not find complete matching in the HLA phenotypes between donor and recipient in the study and therefore can’t infer the role of HLA class I on NK cells. Furthermore, only a small sample size was analyzed, and our study lacks serial samples, as BOS often occurs late in the posttransplant course. In addition, the quantity of exosomes isolated from BAL fluid was limited and, therefore, we were required to pool exosomes from different LTxRs for exosome characterization. However, consistent results were obtained from serial samples that were available for 1-year follow-up, before CLAD developed. In spite of these limitations, we were able to clearly demonstrate that loss of CCSP leads to allo- and autoimmunity, activates and induces NK cell exosomes containing NK cell marker (CD56, NKG2D) and cytotoxic molecule (perforin, FasL), thereby linking NK cells to activation of innate immunity along with adaptive immunity.
Conclusion
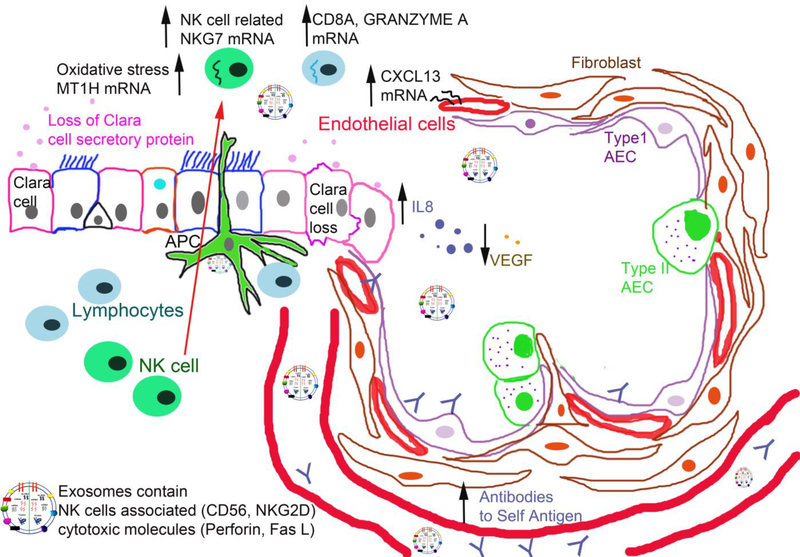
We demonstrated that CCSP levels in BAL fluid were significantly reduced in LTxRs with BOS. In fact, LTxRs with reduced levels of CCSP developed Abs to lung SAgs (Kα1T, Col-V) and DSA. IL-8 was upregulated and VEGF was downregulated in the BAL fluid samples obtained from LTxRs with BOS. Oxidative stress marker MT1H, GRANZYME A, and NK cell marker NKG7 were upregulated in BAL cells of LTxRs with BOS. Exosomes isolated from LTxRs with BOS contained not only lung SAgs, CIITA, 20S proteasome, and NFκβ, but also NK-cell-related proteins, including CD56, NKG2D, FasL, and perforin. This novel finding demonstrates an important role for NK cell activation, as it results in the release of NK cell exosomes during BOS after human LTx. We propose that loss of CCSP and NK cell activation, thereby inducing pro-inflammatory cytokines and oxidative stress and circulating exosomes with lung SAgs and NK-cell-related protein are observed in BOS. This induction in turn leads to de novo development of Abs to donor HLA and/or non-HLA lung SAgs, before ultimately manifesting into CLAD (Figure 9).
Figure 9:
Proposed role of club cell secretory proteins (CCSPs) regulating natural killer (NK) cells and NK-cell-derived exosomes. Decline of CCSPs and NK cell activation, thereby inducing pro-inflammatory cytokines and oxidative stress and circulating exosomes with lung SAgs and NK-cell-related protein. This induction in turn leads to de novo development of Abs to donor HLA and/or non-HLA lung SAgs, before ultimately manifesting into CLAD.
Supplementary Material
Acknowledgements/Funding
This work was supported by Flinn Foundation, NIH R21 AI 123034 and HL 056643 (T.M). We thank Billie Glasscock and Clare Sonntag for assistance in preparing and submitting the manuscript.
Abbreviations
- Abs
antibodies
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- CCSP
Club cell secretory protein
- CDC
complement-dependent cytotoxicity
- cDNA
complementary DNA
- CLAD
chronic lung allograft dysfunction
- Col-V
Collagen V
- DSA
donor-specific anti-HLA
- ELISA
enzyme-linked immunoassay
- FasL
Fas ligand
- HLA
human leukocyte antigen
- IL-8
Interleukin 8
- ISHLT
International Society for Heart and Lung Transplantation
- Kα1T
K-alpha 1 tubulin
- LTx
lung transplant
- LTxR
lung transplant recipient
- mRNA
messenger RNA
- NK
natural killer
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- RT-PCR
reverse transcriptase polymerase chain reaction
- SAg
self-antigen
- UNG
uracil-DNA glycosylase
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am J Respir Crit Care Med. 1999;159:1585–1591. [DOI] [PubMed] [Google Scholar]
- 2.Kelly FL, Kennedy VE, Jain R, et al. Epithelial clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant. 2012;12:3076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hachem RR. Lung allograft rejection: diagnosis and management. Curr Opin Organ Transplant. 2009;14:477–482. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–195; discussion 96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachem RR, Tiriveedhi V, Patterson GA, et al. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigt SS, Wang X, Palchevsky V, et al. Gene expression profiling of bronchoalveolar lavage cells preceding a clinical diagnosis of chronic lung allograft dysfunction. PLoS One. 2017;12:e0169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese DR, Chong T, Wang A, et al. NKG2C Natural Killer Cells in Bronchoalveolar Lavage Are Associated With Cytomegalovirus Viremia and Poor Outcomes in Lung Allograft Recipients. Transplantation. 2019;103:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833–42. [DOI] [PubMed] [Google Scholar]
- 10.Gunasekaran M, Sharma M, Hachem R, et al. Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection. J Immunol. 2018;200:2535–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal S, Sharma M, Ranjithkumar R, et al. The role of exosomes in allograft immunity. Cell Immunol. 2018;331:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravichandran R, Bansal S, Rahman M, et al. The role of donor-derived exosomes in lung allograft rejection. Hum Immunol. 2019;80(8):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma M, Ravichandran R, Bansal S, et al. Tissue-associated self-antigens containing exosomes: Role in allograft rejection. Hum Immunol. 2018;79:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunasekaran M, Xu Z, Nayak DK, et al. Donor-derived exosomes with lung self-antigens in human lung allograft rejection. Am J Transpl. 2017;17(2):474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Translantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 16.Verleden GM, Raghu G, Meyer KC, et al. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. [DOI] [PubMed] [Google Scholar]
- 17.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:493–503. [DOI] [PubMed] [Google Scholar]
- 18.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Annals Thoracic Surgery. 2010;90(4):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nord M, Schubert K, Cassel TN, et al. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73:1264–1269. [DOI] [PubMed] [Google Scholar]
- 21.Wang SZ, Rosenberger CL, Bao YX, et al. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–60. [DOI] [PubMed] [Google Scholar]
- 22.Sunil VR, Vayas KN, Massa CB, et al. Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics. Toxicol Sci. 2013;133:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288–1294. [DOI] [PubMed] [Google Scholar]
- 24.Iwata T, Philipovskiy A, Fisher AJ, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiriveedhi V, Gautam B., Sarma N, et al. Pre-transplant antibodies to K-alpha-1 Tubulin and Collagen-V in lung transplantation: Clinical Correlations. J Heart and Lung Transplantation. 2013;32:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarma NJ, Tiriveedhi V, Mohanakumar T. Ligation of transgenic MHC class I molecule expressed only in the lungs by it’s specific antibodies induces epithelial injury, autoimmunity and obliterative airwar disease (OAD): A novel transgenic mouse model of OAD. J Heart and Lung Transplant. 2014;33:4S:S97. [Google Scholar]
- 27.Liu Z, Liao F, Scozzi D, et al. An obligatory role for club cells in preventing obliterative bronchiolitis in lung transplants. JCI Insight. 2019;4(9):e124732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy VE, Todd JL, Palmer SM. Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am J Transplant. 2013;13:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer KC, Cardoni AL, Xiang Z, et al. Vascular endothelial growth factor in human lung transplantation. Chest. 2001;119:137–143. [DOI] [PubMed] [Google Scholar]
- 30.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 31.Mohanakumar T, Sharma M, Bansal S, et al. A novel mechanism for immune regulation after human lung transplantation. J Thorac Cardiovasc Surg. 2019;157:2096–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Kalimuthu S, Gangadaran P, et al. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics. 2017;7:2732–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zingoni A, Molfetta R, Fionda C, et al. NKG2D and Its Ligands: “One for All, All for One”. Front Immunol. 2018;9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese DR, Lanier LL, Greenland JR. Natural killer cells in lung transplantation. Thorax. 2019;74:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alipoor SD, Mortaz E, Varahram M, et al. The Potential Biomarkers and Immunological Effects of Tumor-Derived Exosomes in Lung Cancer. Front Immunol. 2018;9:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benichou G, Yamada Y, Aoyama A, et al. Natural killer cells in rejection and tolerance of solid organ allografts. Curr Opin Organ Transplant. 2011;16:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mango GW, Johnston CJ, Reynolds SD, et al. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol. 1998;275:L348–56. [DOI] [PubMed] [Google Scholar]
- 38.Lefaucheur C, Viglietti D, Hidalgo LG, et al. Complement-Activating Anti-HLA Antibodies in Kidney Transplantation: Allograft Gene Expression Profiling and Response to Treatment. J Am Soc Nephrol. 2018;29:620–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkes MD, Halloran PF, Hidalgo LG. Evidence for CD16a-Mediated NK Cell Stimulation in Antibody-Mediated Kidney Transplant Rejection. Transplantation. 2017;101:e102–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenig A, Chen CC, Marcais A, et al. Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat Commun. 2019;10(1):5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.