Abstract
Data on the efficacy and safety of interferon (IFN)-α for the treatment of essential thrombocythemia (ET) and polycythemia vera (PV) are inconsistent. We conducted a systematic review and meta-analysis and searched MEDLINE and EMBASE via Ovid, Scopus, COCHRANE registry of clinical trials (CENTRAL), and Web of Science from inception through 03/2019 for studies of pegylated IFN (peg-IFN) and non-pegylated IFN (non-peg-IFN) in PV and ET patients. Random-effects models were used to pool response rates for the primary outcome of overall response rate (ORR) defined as a composite of complete response, partial response, complete hematologic response (CHR) and partial hematologic response. Peg-IFN and non-peg-IFN were compared by meta-regression analyses.
44 studies with 1359 patients (730 ET, 629 PV) were included. ORR were 80.6% (95% confidence interval: 76.6–84.1%, CHR: 59.0% [51.5%−66.1%]) and 76.7% (67.4–84.0%; CHR: 48.5% [37.8–59.4%]) for ET and PV patients, respectively. In meta-regression analyses results did not differ significantly for non-peg-IFN vs. peg-IFN. Annualized rates of thromboembolic complications and treatment discontinuation due to adverse events were low at 1.2% and 8.8% for ET and 0.5% and 6.5% for PV patients, respectively. Both peg-IFN and non-peg-IFN can be effective and safe long-term treatments for ET and PV.
Keywords: ET, interferon, meta-analysis, MPN, PV
Introduction:
Essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF) comprise the heterogenous group of BCR-ABL1-negative myeloproliferative neoplasms (MPN) and manifest with a wide spectrum of clinical presentations ranging from asymptomatic to limiting constitutional symptoms as well as an increased risk of thromboembolic events and transformation to acute myeloid leukemia (AML).1–3
Treatment of ET and PV aims to reduce the risk of thromboembolic events using antiplatelet therapies such as low-dose acetylsalicylic acid (ASA) and cytoreduction with phlebotomy and hydroxyurea with both strategies having been shown to reduce the risk of thrombotic events.4, 5 Given the potential teratogenicity and the concerns about potential leukemogenic effects of hydroxyurea, interferon-α (IFN), is considered an alternative treatment for cytoreduction, mitigation of thrombosis risk and symptom management.1, 6–8 Despite its long track record since the 1980’s, the role of IFN in the treatment landscape of MPN continues to evolve. Recent studies have demonstrated that IFN treatment can lead to a reduction in the allele burden of driver mutations, which suggests a disease-modifying effect that is not seen with the purely symptomatic treatment with ASA and hydroxyurea.9–11 This disease-modifying potential has been attributed to the anti-proliferative, pro-apoptotic, and immunomodulatory effects of IFN on hematopoietic progenitor and immune cells in the bone marrow leading to the upregulation of tumor antigen presentation, restoration of immune surveillance and normal hematopoiesis.12, 13
Given the extensive but heterogenous literature, we conducted a systematic review and meta-analysis of the use of IFN in the treatment of ET and PV, two common types of MPN, to synthesize the efficacy and adverse event profile and to better define the role of these agents in the treatment landscape of this group of diseases.
Methods:
Search strategy:
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines14. MEDLINE and EMBASE via Ovid, COCHRANE registry of clinical trials (CENTRAL), Scopus, and Web of Science electronic databases were searched without language restriction from inception (inception dates: MEDLINE: 1946; EMBASE: 1974; CENTRAL: 1996; Scopus: 1970; Web of Science: 1900) through March 21st, 2019, using the following combination of free-text terms linked by Boolean operators: [“polycythemia” OR “polycythemia vera” OR “essential thrombocytosis” OR “essential thrombocythemia” OR “myelofibrosis” OR “myeloproliferative neoplasm” OR “MPN”] AND [“interferon” OR “IFN” OR “pegylated interferon” OR “peginterferon” or “alpha2b interferon” OR “alpha2 interferon” OR “alpha interferon”].
After removal of duplicates, two authors (JPB and MS) independently screened the titles and abstracts of all retrieved studies for eligibility. Based on this initial eligibility assessment studies were excluded if they were (I) clearly identified as review articles, commentaries or basic research articles, (II) reporting results from diseases other than ET, PV, or MF (e.g. chronic myeloid leukemia), or (III) case series with less than 5 patients. Subsequently, full texts of the potentially eligible studies were reviewed for eligibility. We excluded studies that 1) lacked information on the primary outcome of overall response rate (ORR), 2) listed IFN only among “other therapies” without separate reporting of outcome data, 3) were published only in abstract form, 4) were duplicate publications from the same patient cohort, 5) used IFN as part of combination therapy, 6) clinical trials without published results, and 7) studies without an available English full text. There was no disagreement among the two reviewers regarding the inclusion of any study. Studies on MF have been reported separately and were excluded from this analysis.15 The study selection process is illustrated in a flow diagram (Figure 1).
Figure 1: Flow chart showing study selection as per the MOOSE guidelines.
Figure 1 illustrates the search strategy and stepwise process of study selection used in this meta-analysis. MEDLINE and EMBASE via Ovid, the COCHRANE registry of clinical trials (CENTRAL), Scopus and the Web of Science electronic databases were searched without language restriction from inception through March 21st, 2019, using the following combination of free-text terms linked by Boolean operators: [“polycythemia” OR “polycythemia vera” OR “essential thrombocytosis” OR “essential thrombocythemia” OR “myelofibrosis” OR “myeloproliferative neoplasm” OR “MPN”] AND [“interferon” OR “IFN” OR “pegylated interferon” OR “peginterferon” or “alpha2b interferon” OR “alpha2 interferon” OR “alpha interferon”]. After removal of duplicates, two authors (JPB and MS) independently screened the titles and abstracts of all retrieved studies for eligibility. Studies were excluded if they were (I) review articles, commentaries or basic research articles, (II) reporting results from diseases other than ET, PV, or MF (e.g. chronic myeloid leukemia), or (III) case series with less than 5 patients. Subsequently, full texts of the potentially eligible studies were reviewed for eligibility. We excluded studies that 1) lacked information on the primary outcome of overall response rates, 2) IFN listed only among “other therapies” without separate reporting of outcome data, 3) studies published only in abstract form, 4) duplicate publications from the same patient cohort, 5) IFN given as part of combination therapy, 6) clinical trials without published results, and 7) without an available English full text. Studies on myelofibrosis have been reported separately and were excluded from this meta-analysis.
Quality assessment:
Two investigators (JPB and MS) extracted data using a standardized data-extraction form. A Downs and Black checklist was used independently by two authors (JPB and MS) to assess study quality as published previously.16, 17 The Downs and Black checklist is a validated tool for quality assessment for both randomized and non-randomized studies in systematic reviews. In its original version it contains 27 items assessing study quality in terms of reporting for a maximum score of 28 points.16
Definition of response and endpoints:
Primary outcome was the ORR defined as a composite of complete response (CR), partial response (PR), complete hematologic response (CHR) and partial hematologic response (PHR). Given the range of publication dates among the included studies, definitions used by the individual publications varied slightly but were primarily based on the normalization/improvement of peripheral blood counts and resolution of MPN-associated symptoms. For this meta-analysis definitions of CHR and PHR as reported by the original studies were used (Table 1). Key secondary outcomes included the rate of CHR and freedom of phlebotomy. Safety endpoints were the rate of treatment discontinuation and of thromboembolic complications.
Table 1:
Treatment characteristics, outcomes and adverse effects of studies of essential thrombocythemia
| Author (ref) | Year | Treatment and treatment schedule | N (patients) | Outcomes | Outcome definition | Adverse effects (AE) |
|---|---|---|---|---|---|---|
| Abegg-Werter et al.19 | 1990 | rIFN-α−2c 5×106 IU 3–7 days per week based on response and tolerability | 6 | ORR: 100% (CHR: 83%; PHR: 17%) | CHR: platelet count <350×109/L PHR: platelet count 350–600×109/L |
100% with adverse events, no grading provided |
| Alvarado et al.20 | 2003 | Peg-IFN-α−2b 1.5–6.0 μg/kg once weekly based on response and tolerability | 11 | ORR: 100% (CHR: 100%, PHR: 0%) | CHR: platelet count <400×109/L, no thromboembolic events PHR: platelet count 400–600×109/L, no thromboembolic events |
0 thromboembolic events, otherwise not reported |
| Bentley et al.21 | 1999 | IFN-α−2a 3–9×106 IU daily; maintenance treatement with IFN-α−2a 3×106 IU t.i.w. | 34 | ORR: 79% (CHR: 50%; PHR: 28%) | CHR: platelet count <400×109/L PHR: platelet count 400–600×109/L |
No grade ≥3 adverse events; 28% discontinuation rate due to adverse effects |
| Berte et al.47 | 1996 | IFN-α−2a or IFN-α−2b 3×106 IU t.i.w. | 12 | ORR: 83% (CHR: 42%; PHR: 42%) | CHR: platelet count <400×109/L, no splenomegaly PHR: platelet count 400–600×109/L; >50% reduction in splenomegaly |
No grading provided, 1 treatment discontinuation due to AE |
| Cervantes et al.22 | 1991 | IFN-α−2b 3–5×106 IU daily; maintenance treatement with IFN-α−2b 3×106 IU 2–4 days per week | 13 | ORR: 100% (CHR: 100%, PHR: 0%) | CHR: platelet count <400×109/L PHR: not defined |
11 out of 13 patients with flu-like symptoms, no other AE reported |
| Giles et al.23 | 1990 | rIFN-α−2a or IFN-α−2b 3–6×106 IU daily; maintenance treatement with IFN-α−2b 3×106 IU t.i.w | 22 | ORR: 86% (CR and PR not defined) | Not defined | All patients with AE, no grading provided, 3 patients disconitnued treatment due to AE |
| Giralt et al.24 | 1991 | IFN-α−2b 3×106 IU daily; dose reduction based on tolerability; maintenance treatment with IFN-α−2b 3–5×106 IU twice weekly | 13 | ORR: 69% (CR: 15%, PR: 54%) | CR: platelet count <450×109/L with resolution of splenomegaly and symptoms PR: reduction in platelet count (not defined) with spleen size (>50%) and absence of symptoms |
12 patients with flu-like symptoms, 7 patients with leukopenia, and 3 patients with neurological, metabolic and hepatic AE |
| Gisslinger et al.48 | 1991 | IFN-α−2c 6–45×106 IU/week; dose adjustments based on efficacy and tolerability | 20 | ORR: 85% (CHR: 65%, PHR: 20%) | CHR: platelet count <440×109/L PHR: platelet count reduction >50% but still >440×109/L |
No grading provided, 4 treatment discontinuations due to AE |
| Kasparu et al.25 | 1992 | rIFN-α−2b 5×106 IU daily; maintenance treatement with IFN-α−2b every other day (unspecified dose) with subsequent taper | 14 | ORR: 100% (CHR: 86%, PHR: 0%) | Definition according to polycythemia vera study group86 CHR: platelet count <450×109/L Good PHR: platelet count 450–600×109/L Poor PHR: platelet count 600–1000×109/L |
All patients with AE, no grading provided, 1 thromboembolic event, 3 patients disconitnued due to AE |
| Langer et al.30 | 2005 | Peg-IFN-α−2b 25–150 μg once weekly; dose adjustments based on efficacy and tolerability | 36 | ORR: 75% (CHR: 67%, PHR: 8%) | CHR: platelet count <450×109/L PHR: platelet count 450–600×109/L |
10 patients discontinued due to AE |
| Lopes et al.31 | 1992 | IFN-α−2b 3×106 IU/m2 daily or 3–5×106 IU/m2 t.i.w.; 3×106 IU/m2 t.i.w. for maintenance |
7 | ORR: 100% (CR: 71%, PR: 29%) | CR: resolution of symptoms, splenomegaly, and normal platelet count PR: >50% reduction in baseline platelet count and clinical improvement |
5 patients with flu-like symptoms, 3 thromboembolic events |
| Gowin et al.26 | 2012 | Peg-IFN-α−2a starting dose 22.5–180 μg/week (median: 80 μg/week); maximum dose: 30–300 μg/week (median: 90 μg/week) | 46 | ORR: 78% (CR: 63%; PR: 15%) | 2009 ELN response criteria87 | Not reported separately |
| Gowin et al.27 | 2017 | Peg-IFN-α−2a starting dose 45–90 μg/week (median: 45 μg/week); maximum dose: 45–275 μg/week (median: 90 μg/week) | 20 | ORR: 65% (CR: 25%; PR: 20%) | 2013 ELN/IWG-MRT response criteria77 | 1 thromboembolic event; no other adverse effects reported separately |
| Huang et al.28 | 2014 | IFN-α−2b 3×106 IU t.i.w. for 2 years; 5×106 IU twice per week for maintenance | 123 | ORR: 73% (CHR: 29%; PHR: 45%) | CHR: normalization of platelet count and no thromboembolic events PHR: >50% reduction in platelet count but >400×109/L |
66 patients with any AE, 11 patients with ≥3 grade |
| Jabbour et al.29 | 2007 | Peg-IFN-α−2b 2–3 μg/kg/week; dose reduction for toxicity permitted | 13 | ORR: 69% (CHR: 54%; PHR: 15%) | CHR: platelet count <440×109/L PHR: >50% reduction of platelet count without achieving normal levels |
Not reported separately for ET patients |
| Lazzarino et al.32 | 1988 | IFN-α−2b 1–4 ×106 IU daily; dose adjustments based on efficacy and tolerability | 26 | ORR: 89% (CHR: 62%; PHR: 27%) | CHR: normalization of all blood counts and resolution of splenomegaly PHR: platelet count 400–600×109/L, WBC<20×109/L, reduction in spleen size by >50% |
Not reported separately for ET patients |
| Lindgren et al.33 | 2018 | Peg-IFN-α−2a 67.5–135 μg/week (median: 90 μg/week) Peg-IFN-α−2b 30–80 μg/week (median: 50 μg/week) IFN-α−2b 1.2–20×106 IU/week (median: 9×106 IU/week) |
43 | ORR: 100% (CHR: 70%; PHR: 30%) | CHR: normalization of all blood counts and resolution of symptoms PHR: platelet count <600×109/L or >50% reduction |
Not reported |
| Masarova et al.34 | 2017 | Peg-IFN-α−2a 90–450 μg/week | 40 | ORR: 80% (CHR: 73%; PHR: 3%) | Hematologic responses based on 2009 ELN response criteria87 | 100% with any adverse event; 4 patients with transformation to AML or myelofibrosis |
| Middelhoff et al.35 | 1992 | IFN-α (subtype not specified) induction with 4×106 IU/m2; maintenance with 3–6×106 IU every other day | 6 | ORR: 100% (CHR: 50%; PHR: 50%) | CHR: platelet count <400×109/L PHR: >50% decrease in platelet count |
Not reported |
| Pogliani et al.36 | 1995 | rIFN-α−2b 2–5×106 IU/m2 t.i.w.; dose adjustments based on response and tolerability | 25 | ORR: 80% (CHR: 56%; PHR: 24%) | CHR: platelet count <450 ×109/L PHR: platelet count <600 ×109/L |
9 treatment discontinuation due to AE |
| Radin et al.37 | 2003 | IFN-α2 2–5×106 IU/m2 daily; dose reductions based on adverse events | 17 | ORR: 88% (CR: 6%; PR: 82%) | CR: normalization of blood counts, normal blood smear, normal bone marrow, resolution of splenomegaly, and no thrombotic or hemorrhagic events PR: any one of (1) Hgb >10g/dL or (2) platelet count <800 ×109/L without thrombotic or hemorrhagic events or (3) spleen size reduction >50% |
96 adverse events in 17 patients, 11 patients with ≥3 grade |
| Rametta et al.38 | 1994 | IFN-α−2b 3×106 IU daily; maintenance treatment with IFN-α−2b 3×106 IU t.i.w. | 25 | ORR: 92% (CHR: 52%; PHR: 40%); 18% with reduction of splenomegaly | CHR: platelet count <400 ×109/L PHR: platelet count <600 ×109/L |
18 patients with flu-like symptoms, otherwise not reported; no treatment discontinuation due to AE |
| Saba et al.39 | 2005 | rIFN-α−2a 5×106/m2 IU daily; dose increase to 10×106/m2 IU daily permitted | 20 | ORR: 75% (CHR: 70%, CR: 30% ; PHR: 5%) | CHR: platelet count <440 ×109/L CR: CHR and normalization of bone marrow pathology PHR: >50% reduction in platelet count |
3 treatment discontinuation due to AE |
| Sacchi et al.40 | 1991 | IFN-α−2b 3×106 IU daily; reduced to IFN 3×106 IU 1–3x per week based on response | 35 | ORR: 89% (CHR: 46%; PHR: 43%) | CHR: platelet count <400 ×109/L PHR: platelet count <600 ×109/L |
100% of patients with flu-like symptoms, no grading provided; 3 treatment discontinuations due to AE |
| Sacchi et al.41 | 1998 | IFN-α n-1 3–6×106 IU daily | 11 | ORR: 100% (CHR: 91%; PHR: 9%); spleen size reduction in 2 out of 4 patients | CHR: platelet count <400 ×109/L PHR: platelet count <600 ×109/L |
9 patients with flu-like symptoms, otherwise not reported; no treatment discontinuation due to thyroiditis |
| Seewann et al.42 | 1991 | IFN-α−2b 3–5×106 IU daily; maintenance treatment for up to 2 years with IFN 3–5×106 IU t.i.w. | 19 | ORR: 68% (CHR: 68%; PHR: 0%) | CHR: platelet count <450 ×109/L PHR: >50% reduction in platelet count |
3 treatment discontinuation due to AE |
| Turri et al.43 | 1991 | IFN-α−2a 3×106 IU daily; maintenance treatement for responding patients with IFN-α2a 3×106 IU t.i.w. | 9 | ORR: 67% (CHR: 56%, PHR: 11%) | CHR: platelet count <450 ×109/L PHR: >50% reduction in platelet count |
Not reported |
| Verger et al.44 | 2015 | Peg-IFN-α 230–496 μg/month (mean dose) | 31 | ORR: 100% (CHR: 77%, PHR: 23%) | Hematologic responses based on 2009 ELN response criteria87 | 6 treatment discontinuation due to AE |
| Yataganas et al.45 | 1991 | IFN-α−2b 3–5×106 IU daily; maintenance treatement with IFN-α−2b 3×106 IU t.i.w. | 9 | ORR: 100% (CHR: 67%, PHR: 33%), 0/3 patients with improvement in splenomegaly | CHR: platelet count <400 ×109/L PHR: platelet count <600 ×109/L |
All patients with adverse events, no grading provided |
| Zhang et al.46 | 2014 | IFN-α−2b 300 U t.i.w.; maintenance treatment IFN-α−2b 300 U once or twice per week | 20 | ORR: 90% (CR: 40%, PR: 50%) | CR: normalization of blood counts and resolution of splenomegaly PR: improvement in blood counts by >50% |
4 thromboembolic events, otherwise not reported |
AE – adverse events; CHR – complete hematologic response; CR – complete remission; ELN – European Leukemia Network; IFN – Interferon; IU – international units; IWG-MRT - International Working Group-Myeloproliferative Neoplasms Research and Treatment; t.i.w. – three times a week, PHR – partial hematologic response; partial response – partial remission; rIFN – recombinant interferon; U - unit
Statistical analysis:
Random-effects models were used to pool ORR and rates of CR, PR, CHR, PHR, and thromboembolic events per patient year. All effect sizes underwent logarithmic transformation prior to pooling using an inverse variance weighting approach. Heterogeneity of studies was determined using Cochran Q and I2 indices and significant heterogeneity (defined as I2 > 60%) was further explored with sensitivity analyses.18 Planned subgroup analyses and univariate meta regression analyses were performed to statistically compare effect sizes of different studies based on the type of interferon administered (non-peg-IFN vs. peg-IFN) and duration of follow up (<24 months vs. ≥ 24 months). All analyses were performed with Comprehensive Meta-Analysis (CMA 2.2, Biostat).
Results:
Results of literature search:
Our search strategy identified 11,516 citations with 8,587 unique publications remaining after removal of duplicates. Based on title and abstract review, studies reporting results on diseases other than ET, PV, or MF, review articles, commentaries that did not report original data, basic research articles without clinical data, and case series with less than 5 patients were excluded, yielding a sample of 143 publications for full-text review. As outlined in the methods section, further exclusion criteria were applied to derive at the final sample of 44 studies included in the meta-analysis. Figure 1 outlines the study selection process.
Description of included studies:
Among the 44 studies, 30 studies (4 retrospective cohort studies, 13 prospective cohort studies, 1 phase I clinical trial, and 12 phase II clinical trials) reported outcomes on 730 patients with ET19–48 and 23 studies (5 retrospective cohort studies, 10 prospective cohort studies, 1 phase I clinical trial, 5 phase II clinical trials, and 2 phase III clinical trials) with a total of 629 patients with PV.9, 26–28, 33, 34, 37, 42, 43, 46, 49–60 Nine studies included patients with both ET and PV and were included in the disease-specific meta-analyses if they reported results separately for the different diseases.26–28, 33, 34, 37, 42, 43, 46
Disease-specific risk stratification using standardized tools such as the International Prognostic Score of Thrombosis in Essential Thrombocythemia (IPSET) were only reported by one study,27, 61 while four studies commented on risk assessment based on the patient’s age and history of prior thromboembolic events.30, 34, 49, 51 Information on prior treatments, prevalence of splenomegaly and baseline symptom burden were provided inconsistently by the original studies. Baseline characteristics of the patients included in the individual studies by disease entity are provided in Supplemental Tables 1 and 2.
Treatment characteristics:
Among the identified studies, patients were primarily treated with non-peg-IFN in 31 studies,11, 19, 21–25, 28, 31, 32, 35–43, 45–48, 50, 52, 53, 55–57, 59, 60 peg-IFN in 12 studies,9, 20, 26, 27, 29, 30, 33, 34, 44, 49, 54 and ropegylated-IFN in one study,51 respectively. Dosing and treatment schedules varied substantially between the individual studies with dose adjustments based on efficacy and adverse events being permitted (Tables 1 and 2).
Table 2:
Treatment characteristics, outcomes and adverse effects of studies of polycythemia vera
| Author (ref) | Year | Treatment and treatment schedule | N (patients) | Outcomes | Outcome definition | Adverse events (AE) |
|---|---|---|---|---|---|---|
| Crisa et al.49 | 2017 | Peg-IFN-α−2a 90–135 μg/week | 30 | ORR: 87% (CR: 70%; PR: 17%) | 2009 ELN response criteria87 | 87% with any AE, 13% with ≥3 grade AE; 3% annual discontinuation rate due to AE |
| Foa et al.50 | 1998 | rIFN-α−2a 3×106 IU/m2 t.i.w.; dose adjustments based on efficacy and tolerability permitted | 38 | ORR: 66% (CHR: 38%; PHR: 28%) 11 out of 38 patients free of phlebotomy |
CHR: (<52% in men; <47% in females) without phlebotomy PHR: >50% reduction in phlebotomy requirement |
14 treatment discontinuations due to AE; 1 patient each with transformation to AML or myelofibrosis |
| Gisslinger et al.51 | 2015 | Ropeginterferon α−2b 50–540 mg every 2 weeks (mean dose 263 μg every 2 weeks) | 51 | ORR: 90% (CR: 47%; PR: 43%); 68% molecular response; 82% free of phlebotomy | 2009 ELN response criteria87 | 45 patients with any AE, no grading provided, 12% annual treatment discontinuation rate due to AE |
| Gowin et al.26 | 2012 | Peg-IFN-α−2a starting dose 22.5–180 μg/week (median: 80 μg/week); maximum dose: 30–300 μg/week (median: 90 μg/week) | 55 | ORR: 87% (CR: 54%; PR: 33%) | 2009 ELN response criteria87 | Not reported separately |
| Gowin et al.27 | 2017 | Peg-IFN-α−2a starting dose 45–90 μg/week (median: 45 μg/week); maximum dose: 45–275 μg/week (median: 90 μg/week) | 36 | ORR: 86% (CR: 8%; PR: 39%) | 2013 ELN/IWG-MRT response criteria77 | 1 thromboembolic event; no other AE reported separately |
| Heis et al.52 | 1999 | rIFN-α−2c 4–35×106 IU/week or lymphoblastoid IFN-α 9×106 IU/week | 21 | ORR: 33% (CHR: 10%; PHR: 24%) | CHR: Hct <45% without phlebotomy PHR: Hct >45% and >50% reduction in phlebotomy requirement |
40 AE in 32 patients, no grading provided; 20% annual treatment discontinuation rate due to AE |
| Huang et al.28 | 2014 | IFN-α−2b 3×106 IU t.i.w. for 2 years; 5×106 IU twice per week for maintenance | 64 | ORR: 70% (CR: 30%; PR: 41%) 53 out of 55 patients free of phlebotomy |
CR: normalization of CBC and spleen size, no thromboembolic events PR: >50% reduction in spleen size or phlebotomy requirement |
7 patients with ≥3 grade AE; 5.3% annual discontinuation rate due to AE |
| Jones et al.53 | 2006 | rIFN-α 2–4.25×106 IU ranging from daily to t.i.w. dosing | 7 | ORR: 100% (CHR: 86%; PHR: 14%) | CHR: Hct <45% (men) or <42% (women) and platelet count <600/mm3 without phlebotomy PHR: Hct <45% (men) or <42% (women) and platelet count 600–1000/mm3 without phlebotomy |
Not reported |
| Lindgren et al.33 | 2018 | Peg-IFN-α−2a 45–135 μg/week (median: 90 μg/week) Peg-IFN-α−2b 30–50 μg/week (median: 35 μg/week) IFN-α−2b 4.5–15×106 IU/week (median: 6.3×106 IU/week) |
43 | ORR: 64% (CHR: 60%; PHR: 4%) | CHR: Hct <45% without phlebotomoy with normal WBC and platelet count and resolution of symptoms PHR: Hct <45% or 3 other criteria |
Not reported |
| Kiladjian et al.9 | 2008 | Peg-IFN-α−2a 90–180 μg weekly | 37 | ORR: 100% (CHR: 95%; PHR: 5%); 72% with complete or partial molecular response | CHR: Hct <45% (men) and 42% (women), no phlebotomy, absence of splenomegaly, WBC (<10× 109/L) and platelet count (<400×109/L). PHR: Hct <45% (men) or 42% (women) but platelet count >400 ×109/L or persistent splenomegaly; >50% reduction of phlebotomy |
89% with any AE, 1 AE grade ≥3; 9 treatment discontinuations due to AE |
| Kiladjian et al.54 | 2018 | peg-IFN-α−2b 50–80 μg/day peg-IFN-α−2a 45–180 μg/day IFN-α−2a 3×106 IU/day IFN-α−2b 3×106 IU/day |
13 | ORR: 23% (CHR: 15%; PHR: 8%); no spleen response, no MPN-SAF symptom improvement | CHR: normalization of CBC withoutphlebotomy PHR: Hct <48% or 3% lower than baseline |
36 AE in 13 patients; 5 patients with AE grade ≥3; no treatment discontinuations due to AE |
| Kiladjian et al.54 | 2018 | peg-IFN-α−2b 25–50 μg/day peg-IFN-α−2a 45–135 μg/day IFN-α−2b 3–5×106 IU/day |
13 | ORR: 31% (CHR: 15%; PHR: 15%); no spleen response, no MPN-SAF symptom improvement | CHR: normalization of CBC without phlebotomy PHR: Hct <48% or 3% lower than baseline |
21 AE in 13 patients; 1 patient with AE grade ≥3; 1 treatment discontinuation due to AE |
| Kuriakose et al.60 | 2012 | rIFN-α−2b 0.5–3×106 IU t.i.w. peg-IFN-α−2a 15–225μg/week; dose adjustments based on efficacy and tolerance |
46 | ORR: 89% (CHR: 26%; PHR: 63%) | CHR: Hct <45% (men) or 42% (women), no phlebotomy, no splenomegaly, platelet count <600 ×109/L PHR: platelet count <600 ×109/L; >50% reduction of phlebotomy, persistent splenomegaly |
Not reported |
| Masarova et al.34 | 2017 | Peg-IFN-α−2a 90–450 μg/week | 43 | ORR: 84% (CHR: 77%; PHR: 7%) | Hematologic responsed based on 2009 ELN response criteria87 | 100% with any adverse event; 3 patients with transformation to AML or myelofibrosis |
| Ozturk et al.55 | 1997 | IFN-α−2b 3–10×106 IU t.i.w.; dose adjustments based on efficacy and tolerability | 7 | ORR: 100% (CHR: 86%; PHR: 14%) | CHR: Hct <45% without phlebotomy PHR: Hct 45–50% and >50% reduction in phlebotomy requirement |
100% with any AE, no grading provided, 1 treatment discontinuation due to AEs |
| Utke Rank et al.56 | 2016 | IFN-α−2b and peg-IFN-α−2b; dosing and schedule not specified | 9 | ORR: 100% (CHR: 67%; PR: 33%) | CHR: Hct <45% without phlebotomoy, normal WBC and platelet count, and resolution of symptoms and splenomegaly PR: Hct <45% or 3 other criteria |
Not reported |
| Radin et al.37 | 2003 | IFN-α2 2–5×106 IU/m2 daily; dose reductions based on adverse events | 12 | ORR: 42% (CR: 8%; PR: 33%); reduction in spleen size in 5 out of 7 patients, | CR: normalization of blood counts, normal blood smear, normal bone marrow, resolution of splenomegaly, no thrombotic or hemorrhagic events PR: any one of (1) Hb >10g/dL or (2) platelet count <800,000/mm3 without thrombotic or hemorrhagic events or (3) spleen size reduction >50% |
53 adverse events in 12 patients, 5 patients with ≥3 grade |
| Seewann et al.42 | 1991 | rIFN-α−2b 3–5×106 IU daily; maintenance treatement for up to 2 years with IFN 3–5×106 IU t.i.w. | 6 | ORR: 67% (CHR: 67%; PHR: 0%); 3 out of 3 patients free of phlebotomoy | CHR: platelet count <450,000/mm3 PHR: >50% reduction in platelet count |
Not reported |
| Stasi et al.57 | 1998 | Human leukocyte IFN-α 1.5–6×106 IU t.i.w. | 18 | ORR: 94% (CHR: 61%; PHR: 33%); 11 out of 18 patients free of phlebotomoy | CHR: Hct <45% withoutphlebotomies PHR: Hct 45–50% and >50% reduction in phlebotomy burden |
89% with flu-like symptoms; no treatment discontinuations due to AE |
| Stauffer Larsen et al.58 | 2009 | IFN-α−2b; dose and regimen not specified | 7 | ORR: 100% (CHR: 100%); all with major molecular response | CHR: normalization of blood counts without phlebotomies Major molecular response: JAK2 V617F VAF <0.2% |
Not reported |
| Taylor et al.59 | 1995 | rIFN-α−2a or rIFN-α−2b 3–8×106 IU t.i.w.; dose adjustments based efficacy and tolerability | 17 | ORR: 88% (CHR: 53%; PHR: 29%); 8 out of 9 patients with resolution of splenomegaly, freedom of phlebotomy not reported | CHR: Hct <45% without phlebotomies PHR: Hct 45–50% and >50% reduction in phlebotomy burden |
100% with flu-like symptoms; 6 treatment discontinuations due to AE |
| Turri et al.43 | 1991 | IFN-α−2a 3×106 IU daily; maintenance treatement for responding patients with IFN-α−2a 3×106 IU t.i.w. | 11 | ORR: 64% (CHR: 36%, PHR: 27%); 4 patients free of phlebotomy and reduction in spleen size | CHR: Hct <48% without need for phlebotomy PHR: reduction in phlebotomy requirements |
Not reported |
| Zhang et al.46 | 2014 | IFN-α−2b 300 U t.i.w.; maintenance treatment IFN-α−2b 300 U once or twice per week | 27 | ORR: 89% (CR: 67%, PR: 22%) | CR: normalization of blood counts, and resolution of splenomegaly PR: improvement in blood counts by >50% |
5 thromboembolic events, otherwise not reported |
AE – adverse events; AML – acute myeloid leukemia; CHR – complete hematologic response; CR – complete remission; ; ELN – European Leukemia Network; Hb – hemoglobin; Hct – Hematocrit; IFN - Interferon; IU - International units; IWG-MRT – International Working Group for Myeloproliferative Neoplasms Research and Treatment; MPN-SAF – myeloproliferative neoplasm symptom assessment form; ORR – overall response rate; PHR – partial hematologic response; PR – partial remission; rIFN – recombinant interferon; t.i.w. – three times per week; U – unit; VAF – variant allele frequency
Assessment of study quality:
Overall study quality was limited by the single-arm design employed by most studies. Notable exceptions were the studies by Crisa et al.49 and Kiladjian et al.54 that used hydroxyurea or ruxolitinib as an active comparator, respectively. The multi-arm studies by Crisa et al. and Kiladijian et al. scored 22 and 23 points, respectively, on the Downs and Black checklist.49, 54 Included single-arm studies achieved 12–19 points on the rating scale with the studies by Masarova et al., Saba et al., and Gisslinger et al. scoring highest.34, 39, 51 A detailed quality assessment for the individual studies and the subcategories of the Downs and Black checklist is provided in Supplemental Table 3.
Response to IFN in patients with ET:
The ORR was reported by all 30 studies and was 80.6% (95% CI 76.6–84.1%) for all studies combined (Figure 2 A). Heterogeneity among the various studies was low with a Cochran’s Q statistic of 29 (p=0.15) and an I2 statistic of 21.2%. ORR was 80.8% (95% CI 76.4–84.6%; I2=8.5%) for non-peg-IFN and 79.8% (95% CI 69.2–87.5%; I2=48.4%) for peg-IFN, which was not statistically significantly different in meta-regression analysis (p=0.46) (Figure 2 A).
Figure 2: Response to non-pegylated IFN (IFN) and pegylated IFN (peg-IFN) in ET.
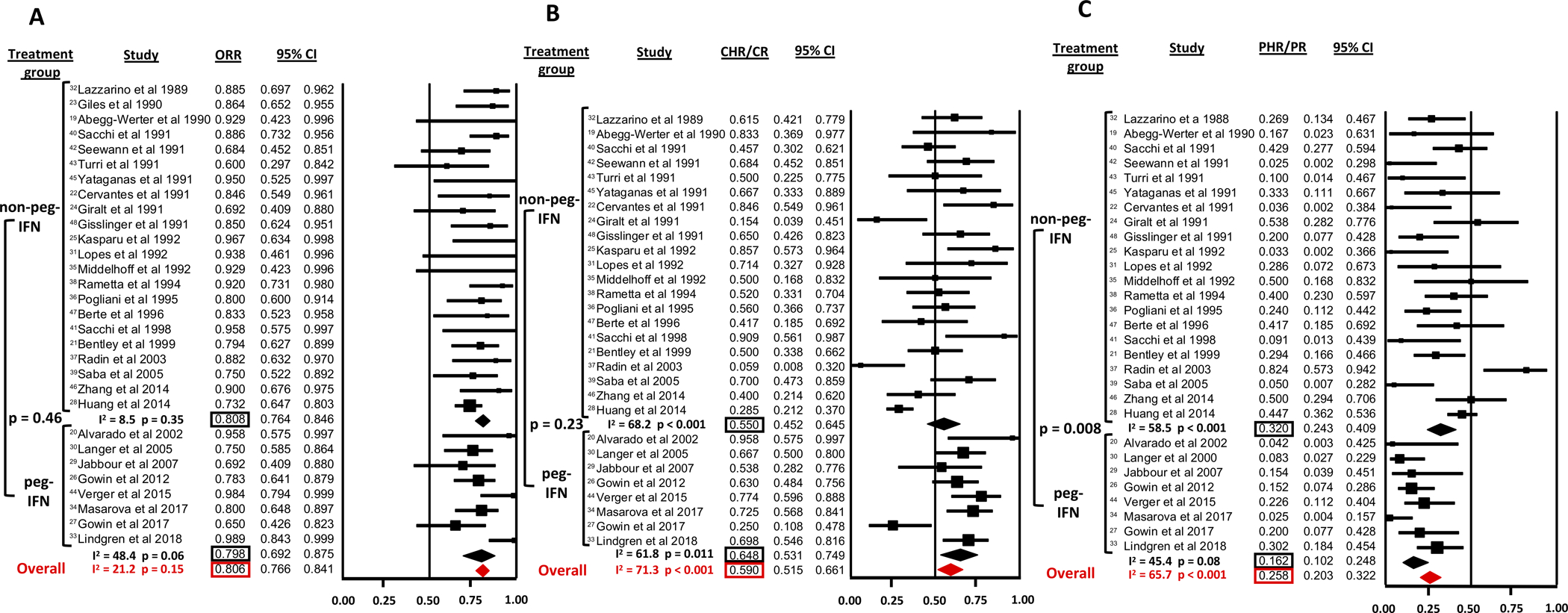
A: Overall response rate (ORR)
B: Complete hematologic response (CHR) rate
C: Partial hematologic response (PHR) rate
The CHR/CR and PHR/PR rates were reported by 29 studies (Figure 2 B and C). For all studies combined, the CHR/CR rate was 59.0% (95% CI 51.5–66.1%) with significant heterogeneity among the studies (Cochran’s Q=97.4 p<0.001; I2=71.3%). The CHR/CR rate was not statistically significantly different (p=0.23) between peg-IFN (64.8%, 95% CI 53.1–74.9%; I2=61.8%) and non-peg-IFN (55.0%, 95% CI 45.2–64.5%; I2=68.2%). The PHR/PR rate for all studies was 25.8% (95% CI 20.3–32.2%) with significant heterogeneity among studies (Cochran’s Q=81.6; p<0.001; I2=65.7%). Contrary to the CHR/CR rate, the PHR/PR rate was higher for non-peg-IFN (32.0%, 95% CI 24.3–40.9%; I2=58.5%) than for peg-IFN (16.2%, 95% CI 10.2–24.8%; I2=45.4%), which reached statistical significance in meta-regression analysis (p=0.008). Table 1 provides a detailed overview of response rates for individual studies.
Response to IFN in patients with PV:
The ORR was reported by all 23 studies (Figure 3 A). For all studies combined, the ORR was 76.7% (95% CI 67.4–84.0%). There was significant heterogeneity among the various studies with a Cochran’s Q statistic of 85.0 (p<0.001) and an I2 statistic of 74.1%. ORR was comparable between non-peg-IFN at 76.3% (95% CI 64.2–85.3%; I2=66.1%) and peg-IFN at 77.5% (95% CI 61.4–88.2%; I2=82.1%), which was not statistically significantly different in meta-regression analysis (p=0.95) (Figure 3 A).
Figure 3: Response to non-pegylated IFN (IFN) and pegylated IFN (peg-IFN) in PV.
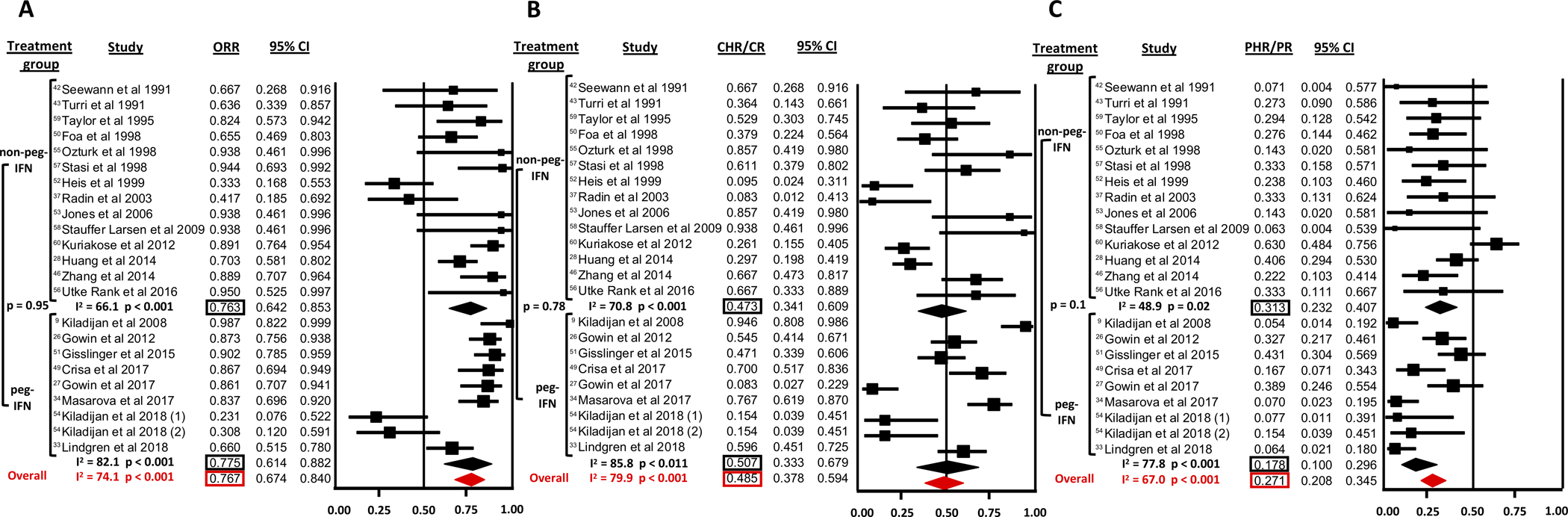
A: Overall response rate (ORR)
B: Complete hematologic response (CHR) rate
C: Partial hematologic response (PHR) rate
The CHR/CR and PHR/PR rates were reported by all 23 studies (Figure 3 B and C). For all studies combined, the CHR/CR rate was 48.5% (95% CI 37.8–59.4%) with significant heterogeneity among studies (Cochran’s Q=105.5; p<0.001; I2=79.9%). CHR/CR rate was not statistically significantly different (p=0.78) between non-peg-IFN (47.3%, 95% CI 34.1–60.9%; I2=70.8%) and peg-IFN (50.7%, 95% CI 33.3–67.9%; I2=85.8%). The PHR/PR rate for all studies was 27.1% (95% CI 20.8–34.5%) with significant heterogeneity among studies (Cochran’s Q=85.0; p<0.001; I2=67.0%). The PHR/PR rate was not statistically significantly different (p=0.10) between non-peg-IFN (31.3%, 95% CI 23.2–40.7%; I2=48.9%) and peg-IFN (17.8%, 95% CI 10.0–29.6%; I2=77.8%).
The percentage of patients, who achieved freedom from phlebotomy was reported by 11 studies (Supplemental Figure 1). The freedom from phlebotomy rate for all studies combined was 58.1% (95% CI 44.3–70.7%) with significant heterogeneity among studies (Cochran’s Q=41.2; p<0.001; I2=75.7%). The freedom from phlebotomy rate was not statistically significantly different (p=0.57) between non-peg-IFN (63.3%, 95% CI 41.9–80.4%; I2=77.0%) and peg-IFN (54.3%, 95% CI 36.6–71.1%; I2=78.1%). Table 2 provides a detailed overview of response rates for individual studies.
Response rate based on duration of follow up:
As response rates to IFN can increase over time,62 we conducted a subgroup and meta-regression analysis of the ORR based on the median duration of follow up. Studies which did not report the median duration of follow up were excluded from this analysis. For ET patients, we stratified studies by either ≤ 6 vs. >6 months of follow up (Supplemental Figure 2A), <24 vs. ≥24 months of follow up (Supplemental Figure 2B), ≤36 vs. >36 months of follow up (Supplemental Figure 2C). The ORR was not statistically significantly different in any of those comparisons and was in line with the ORR reported for all studies combined.
For PV patients, we stratified studies by either ≤12 vs. >12 months of follow up (Supplemental Figure 3A), <24 vs. ≥24 months of follow up (Supplemental Figure 3B), ≤36 vs. >36 months of follow up (Supplemental Figure 3C). The ORR was not statistically significantly different in any of those comparisons and was in line with the ORR reported for all studies combined. However, we did notice that response rates and especially CHR rates improved over time in several studies.9, 51
Response rate based on median patient age:
To assess whether the median patient age of study patients had an influence on the ORR for IFN-treated patients, we conducted a meta-regression analysis comparing studies with a median patient age of <60 years with studies in which the median patient age was ≥60 years of age (Supplemental Figure 4). ORR was similar in studies in ET patients (ORR: 79.7% [95% CI: 74.4–84.2%] for studies with median age <60 years vs 78.5% [95% CI: 41.9–94.8%]; p=0.70). However, among studies in PV patients the ORR in studies with a median patient age <60 years was statistically significantly higher than in studies with a median patient age ≥60 years (ORR: 79.0% [95% CI: 70.7–85.3%] for studies with median age <60 years vs 41.8% [95% CI: 22.6–63.8%]; p<0.001).
Histopathologic and molecular responses in ET and PV patients treated with IFN:
Histopathologic response to IFN treatment with repeat bone marrow biopsies was assessed by only six and two studies for ET and PV patients, respectively.37–40, 45, 48, 56, 58 Among IFN-treated ET patients, 6–30% of patients experienced a normalization of baseline bone marrow abnormalities (mainly megakaryocyte hyperplasia and bone marrow fibrosis),37–40, 45 while up to 40% of patients with PV achieved normalization of bone marrow morphology in the two studies with follow up bone marrow assessment (Supplemental Table 4).56, 58
Baseline JAK2 V617F status was available for three and 13 studies of ET and PV patients, respectively, although not all studies reported the baseline JAK2 V617F allele burden.9, 28, 33, 34, 46, 49, 51, 53, 54, 56, 58, 60 Three additional studies also reported information on CALR status among ET patients.33, 34, 44 When reported, 48–100% of ET patients and 83–100% of PV patients were JAK2 V617F-positive, respectively.9, 28, 33, 34, 46, 51, 53, 56, 58 Only one study reported the median baseline JAK2 V617F allele burden for ET patients.34 The rate of complete (CMR; undetectable JAK2 V617F) and partial molecular response (PMR; ≥50% reduction in allele burden) was 9–27% and 17–33% in the two studies on ET that reported this information, respectively.34, 46 Verger et al. included only patients with CALR mutations with a median allele burden of 41% at baseline and reported a PMR in 42% of patients.44
Among studies of PV patients, the median baseline JAK2 V617F allele burden ranged from 40.5–70%.9, 34, 49, 51, 54, 56 Molecular response definitions varied among studies (Supplemental Table 5). Six studies reported rates of CMR and PMR, which was achieved in 0–33% and 15–57% of patients, respectively.9, 28, 34, 46, 51, 60 In the studies with serial molecular response assessments, JAK2 V617F allele burden tended to decrease over time with ongoing IFN treatment.9, 51 Due to the heterogeneity of outcome assessments and definitions, we were unable to conduct a meta-analysis on the molecular response rate or histopathologic changes in the bone marrow. A summary of those outcomes for individual studies is provided in Supplemental Table 4 and 5.
Rate of thromboembolic events in patients with ET and PV treated with IFN:
The rate of thromboembolic events was reported by 13 studies reporting outcomes for non-peg-IFN and peg-IFN treated ET patients (Figure 4 A).20, 21, 23, 25, 27, 30, 31, 35, 38, 39, 42, 46 The rate of thromboembolic complications was uniformly low at 1.2% per patient year (95% CI 0.3–2.2%; Cochran’s Q statistic: 10.5; p=0.57; I2 statistic: 0%) for ET patients and was not statistically significantly different (p=0.68) between non-peg-IFN (1.1%, I2=10.2%) and peg-IFN (1.5%, I2=0%).
Figure 4: Rate of thromboembolic events (per patient year).
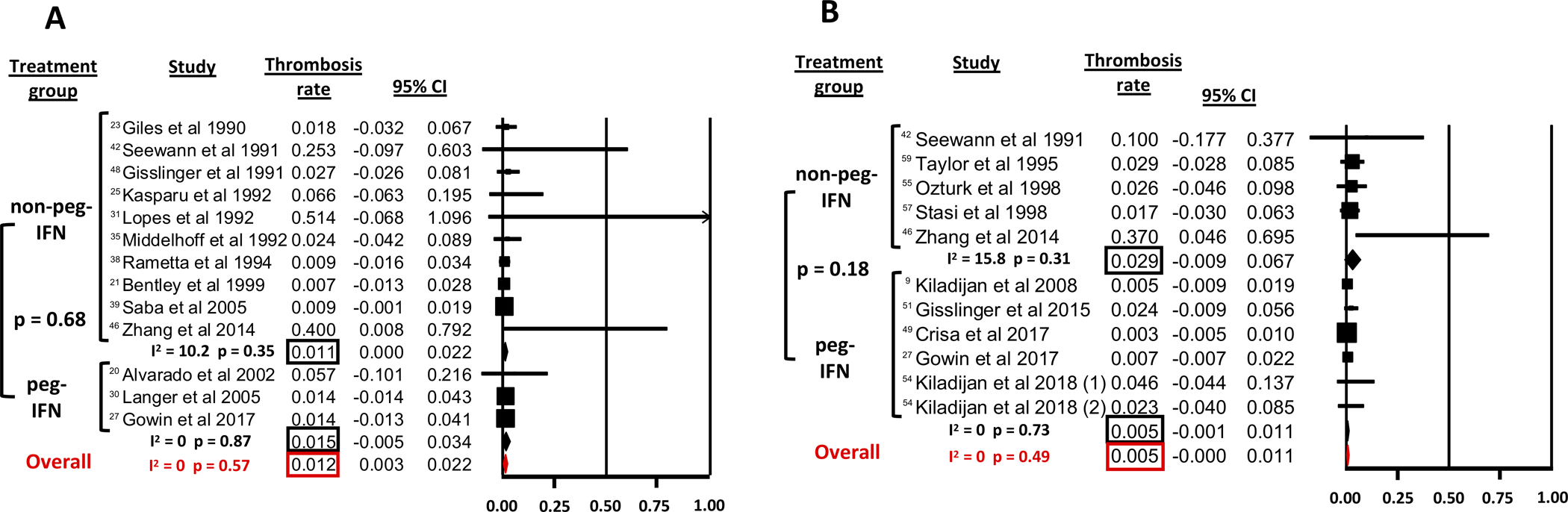
A: in ET
B: in PV
The rate of thromboembolic events was reported by 11 studies as outcomes for non-peg-IFN and peg-IFN treated PV patients (Figure 4 B).9, 27, 42, 46, 49, 51, 54, 55, 57, 59 For PV patients, the rate of thromboembolic complications was uniformly low at 0.5% per patient year (95% CI 0.0–1.1%; Cochran’s Q=9.36; p=0.49; I2=0%) and was not statistically significantly different (p=0.18) between peg-IFN (0.5% per patient year, I2=0%) and non-peg-IFN (2.9% per patient year, I2=15.8%).
As the risk for thromboembolic events increases with age and patients older than 60 years are classified as high-risk,61, 63 we conducted a subset analysis comparing the rates of thromboembolic events between studies with a median patient age <60 years and studies with a median patient age of ≥60 years (Supplemental Figure 5). There was a non-statistically significant trend towards a higher rate of thromboembolic events in studies with an older patient population on average for both ET and PV.
Discontinuation rate of IFN therapy in patients with ET and PV:
Studies that did not explicitly report the adverse event-related treatment discontinuation rate or the median duration of follow up were excluded from this analysis. Additionally, we excluded the study by Saba et al. as a median duration of follow up of 174 months would have led to an artificially low annualized discontinuation rate. For all studies combined, the discontinuation rate per patient year was 8.8% (95% CI 5.8–11.8%) for ET patients with significant heterogeneity among the studies (Cochran’s Q=53.9; p<0.001; I2= 68.5%) (Figure 5 A). The treatment discontinuation rate was higher for peg-IFN (10.9%, 95% CI 6.2–15.7%; I2=0%) compared to non-peg-IFN (7.5%, 95% CI 3.7–11.3%; I2=64.3%) but did not reach statistical significance in meta-regression analysis (p=0.23).
Figure 5: Discontinuation rate of non-pegylated IFN (IFN) and pegylated IFN (peg-IFN) therapy (for all patients while on study).
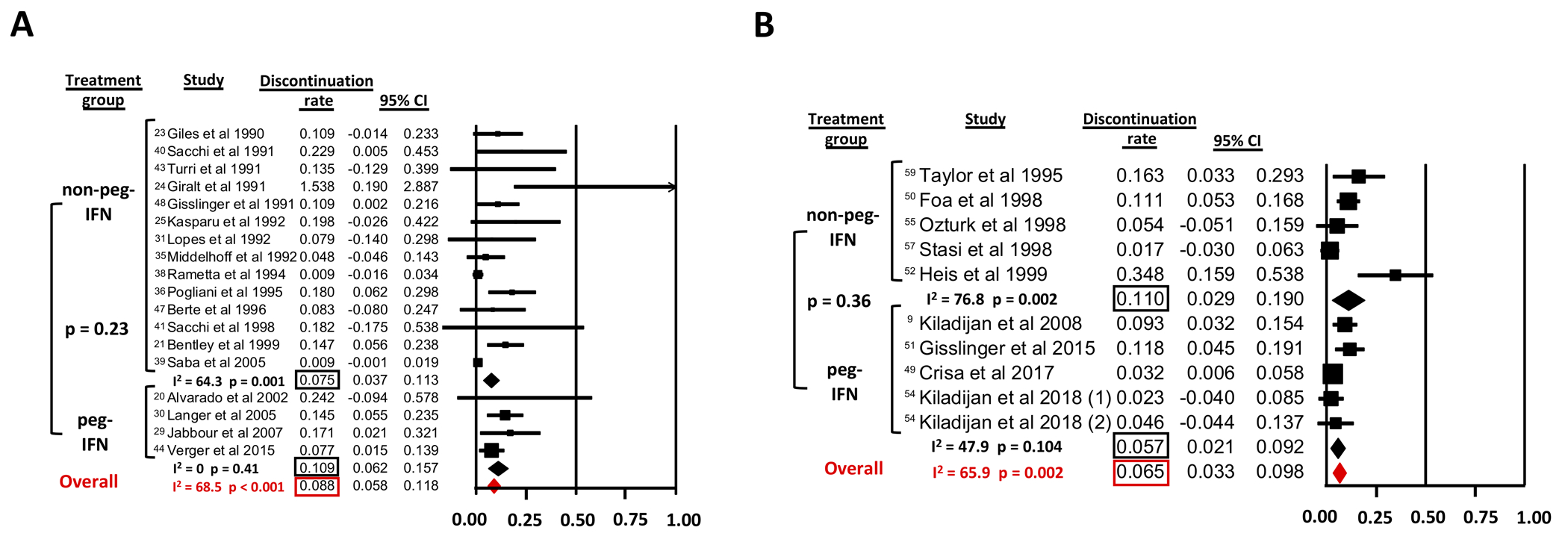
A: in ET
B: in PV
For studies examining IFN use in PV, the discontinuation rate per patient year for all studies was 6.5% (95% CI 3.3–9.8%) with significant heterogeneity among studies (Cochran’s Q=26.38; p=0.002; I2=65.9%) (Figure 5 B). In a meta-regression analysis treatment discontinuation rate per patient year was not statistically significantly different (p=0.36) between non-peg-IFN (11.0%, 95% CI 2.9–19.0%; I2=76.8%) and peg-IFN (5.7%, 95% CI 2.1–9.2%; I2=47.9%) (Figure 5 B).
Adverse events:
Given the long time-span covered by studies in this meta-analysis, reporting of adverse events was inconsistent among studies with 11 studies not providing any assessment of adverse events at all. Especially older studies did not use a standardized grading system for the severity of adverse events which precluded a formal meta-analysis. Qualitatively, flu-like symptoms were highly prevalent especially with non-peg-IFN formulations affecting almost all patients and constituting the most common cause for early treatment discontinuation despite supportive treatment with antipyretics. Serious and ≥grade 3 adverse events were rare although only 10 studies provided an objective grading. When reported, rates of ≥grade 3 adverse events ranged from 0% to 64.7%. We were unable to systematically assess whether these adverse events were felt to be treatment-related or secondary to the underlying disease. While this heterogeneity of outcome reporting precluded a formal meta-analysis, descriptive summaries of adverse events as reported by the original studies by disease entity can be found in Tables 1 and 2.
Sensitivity analysis:
Despite the various IFN formulations used, differences in outcome definitions, and the long interval of publication dates included in our meta-analysis, there was no significant heterogeneity regarding the ORR for IFN use in patients with ET (I2 = 21.2%, p=0.15). However, there was statistically significant heterogeneity for CHR/CR (I2 = 71.3%, p<0.001) and PHR/PR rates (I2 = 65.7%, p<0.001) among studies on ET. One reason for the heterogeneity between CHR and PHR rates could have been a variable response definition by individual studies with some studies requiring complete resolution of splenomegaly and ET-associated symptoms as well as a repeat bone marrow biopsy, while most other studies defined CHR using normalization of peripheral blood counts only. Therefore, we conducted a subgroup analysis of studies that reported CHR as defined by a normalization of blood counts (Supplemental Figure 6A). Studies which only reported CR rate but did not specify the CHR rate were excluded from this analysis. The CHR rate of all studies reporting a CHR rate (66.0%; I2=69.9%; p<0.001) appeared slightly higher than the combined CHR/CR rate of all studies included in the analysis (59.0%, I2=71.3%; p<0.001). However, the significant heterogeneity among studies did not change arguing against differences in response definition as a reason for heterogeneity among studies.
For PV studies, there was significant heterogeneity among the ORR and CHR/CR rates. In a subgroup analysis of studies which defined CHR by normalization of blood counts (Supplemental Figure 6B), the CHR rate of all studies reporting a CHR rate (44.6%; I2=76.4%; p<0.001) was similar to the combined CHR/CR rate of all studies included in the analysis (48.5%, I2=79.9%, p<0.001) and there was a decrease in study heterogeneity observed arguing against differences in response definition as a reason for heterogeneity among studies.
Conversely, the lower ORR reported by Kiladjian et al. is not explained by different response criteria as this study did not require a repeat bone marrow biopsy to define CR. While data on prior treatment were not reported by all studies, 100% of patients in the study by Kiladjian et al. were pretreated with hydroxyurea and the included patient population was older than on average (mean median age: 54 years [all studies] vs 61 years and 70 years, in the study arms by Kiladjian et al., respectively).54
Discussion:
To our knowledge, this is the first published systematic review and meta-analysis on the use of IFN for the treatment of ET and PV. We included 44 studies with 1359 patients (730 ET, 629 PV) treated with various formulations of IFN over a period of more than three decades. With a pooled ORR of 80.6% (95%CI: 76.6–84.1%) and 76.7% (95%CI: 67.4–84.0%) for patients with ET and PV, respectively, IFN appears to be an effective treatment option. There was no statistically significant difference in terms of ORR for non-peg-IFN compared to peg-IFN in ET and PV patients and between shorter (<24 months) and longer (≥24 months) durations of follow up. Importantly, the thrombosis rate was uniformly low for studies examining IFN therapy for PV and ET and appeared similar or lower compared to historic controls of patients treated with cytoreduction.4, 64, 65 As thromboembolic and cardiovascular events account for a substantial proportion of deaths associated with ET and PV, reducing the risk for such complications especially in high-risk patients (e.g. age ≥60 or prior thrombotic events) is essential and cytoreduction with hydroxyurea and/or phlebotomy in combination with ASA is frequently used.63 In PV patients, strict hematocrit (Hct) control (i.e. Hct <45%) with phlebotomy and/or hydroxyurea has been shown to significantly reduce the risk for cardiovascular death and major thrombosis compared to a more lenient target.4 However, even in the landmark CYTO-PV trial 2.7% of patients in the group with a Hct target of <45% suffered a major thrombotic event or cardiovascular mortality after a median of 31 months of follow-up.4 In our analysis the annualized rate of thrombotic events was only 0.5% and 1.2% among PV and ET patients, respectively, without significant differences between peg-IFN and non-peg-IFN or heterogeneity across studies. This finding is in line with data from the recent PROUD-PV and CONTINUATION-PV studies that compared ropeginterferon alfa-2b to standard of care and reported similar rates of major thromboembolic and cardiovascular events between the two arms (major thromboembolic event: 3% vs. 3%; major cardiovascular events: 10% vs 6%).62 These data suggest that IFN may be an acceptable alternative to phlebotomy and/or hydroxyurea as it has comparable efficacy with regard to thrombotic complications. In the subset of ET and PV patients with splanchnic vein thrombosis, Mascerenhas et al. recently showed in a prospective phase II study of 20 ET and PV patients with prior splanchnic vein thrombosis treated with peg-IFN alfa-2a that 15% and 55% of patients achieved a CR and PR, respectively, after 12 months of therapy and none of the patients developed a recurrent splanchnic vein thrombosis.66 However, it is important to note that 18 out of 20 patients received either ASA, anticoagulation (coumadin or low molecular weight heparin), or a combination of both, as well.66 Therefore, the effect of IFN on thrombosis risk and its role in combination with ASA or anticoagulation warrants further studies.
Despite its effectiveness, a wider uptake of IFN in routine clinical practice has been hampered by its associated side effects and the absence of an oral formulation. In our meta-analysis the annual treatment discontinuation rate was 8.8% and 6.5% in ET and PV patients, respectively. The annualized treatment discontinuation rate for peg-IFN and non-peg-IFN was similar for both ET and PV patients although pegylated formulations are believed to have better tolerability and the weekly administration schedule is easier to adhere to for patients. Potential explanations include the comparatively short duration of follow up for studies of peg-IFN compared to non-peg IFN, which leads to early treatment discontinuations carrying a relatively greater weight in the studies using peg-IFN. Additionally, studies on peg-IFN included less patients compared to non-peg IFN, which supports the need for larger studies with longer follow up to better evaluate the safety profile of peg-IFN.
Reporting of adverse events and their severity grading was inconsistent among studies but mainly included flu-like symptoms, malaise, and fever with lower rates of liver toxicity, neurotoxicity, and psychiatric complications. While most adverse events, were reportedly manageable with antipyretics and dose adjustments, the frequency of dosing (up to daily for older formulations) can make even low-grade adverse events difficult to tolerate for patients and lead to treatment discontinuation. This is especially relevant as alternative therapies such as hydroxyurea, phlebotomy, or anagrelide seem to have less side effects and are effective in reducing the risk of thromboembolic complications.4, 5, 67–70 As there are currently no large randomized trial data showing superiority of IFN over either anagrelide or hydroxyurea available, both the National Comprehensive Cancer Network (NCCN) and European Leukemia Net (ELN) recommend hydroxyurea over IFN for cytoreduction in ET patients.1, 63 However, recommendations differ for PV patients with NCCN recommending hydroxyurea in addition to ASA and phlebotomy for high-risk patients (age ≥60 years and/or prior thrombosis) with IFN as an alternative mainly for younger or pregnant patients.1 Conversely, ELN recommendations suggest IFN and hydroxyurea as equally effective choices.63 While the combination of phlebotomy, ASA and cytoreductive therapy with hydroxyurea or IFN is the standard of care for higher-risk PV patients,1, 63 we were unable to specifically assess the effect of combinations of IFN with phlebotomy or hydroxyurea due to heterogeneity of practice patterns among studies. However, freedom of phlebotomy was achieved in 58.1% of PV patients in our meta-analysis and reductions in phlebotomy requirements with IFN treatment can constitute an important improvement in patients’ quality of life. Similarly, in recent clinical trials of IFN in PV patients such as PROUD-PV, phlebotomy was permitted in addition to IFN or hydroxyurea to achieve a Hct of <45%.62 Dedicated studies evaluating the combination of IFN, hydroxyurea, and phlebotomy are needed to fully assess the efficacy and safety of this approach.
The role of IFN in the treatment landscape of ET and PV will likely continue to evolve. Following our data cut-off date, the randomized phase III PROUD-PV (ropeginterferon α−2b vs hydroxyurea) and its continuation study CONTINUATION-PV (ropeginterferon α−2b vs best available treatment [BAT]; 97% hydroxyurea) trials that enrolled 257 early stage PV patients were published.62 While PROUD-PV failed to demonstrate non-inferiority of ropeginterferon α−2b compared to hydroxyurea at 12 months for the composite primary outcome of CHR and spleen size normalization, response rates to ropeginterferon α−2b improved over time and both the composite outcome of CHR and symptom improvement as well as CHR alone were superior to hydroxyurea after 36 months in CONTINUATION-PV.62 The response rates seen in those trials are overall in line with the findings of our meta-analysis and lend additional support to the validity of our results. Notably, ropeginterferon α−2b was well tolerated and led to molecular responses in 66% of cases, which further supports its disease-modifying potential.62 Based on these studies ropeginterferon α−2b was approved by the European Medicines Agency as monotherapy in adults for the treatment of PV without symptomatic splenomegaly in February 2019. The role of IFN will be further defined with publication of the final results of the randomized, open label phase III MPN-RC112 trial that compares hydroxyurea to peg-IFN-α−2a as initial treatment of high-risk ET and PV patients (NCT01259856). Preliminary results showed similar ORR for hydroxyurea and IFN after 12 months of treatment.71 However, in an intention-to-treat analysis at 24 months among 106 patients IFN appeared to be more effective than hydroxyurea but to be associated with more grade 3/4 adverse events and no clear advantage in quality of life.71, 72
In a meta-regression analysis of studies on PV, we found that IFN was more effective in studies with a median patient age <60 years compared to studies with a median patient age ≥60 years. Extrapolating these results to the individual patient level is limited as we only compared studies stratified by median patient age and cross-study heterogeneity remains a potential confounder. While the association of advanced age and increased risk of thrombotic events is well-established, age has not been consistently identified as a prognostic factor for hematologic responses to IFN.63, 73 However, given its disease-modifying potential, safety in pregnancy, and concerns about development of resistance and leukemogenic potential of hydroxyurea, IFN might be an attractive option for younger and lower-risk patients with PV.63, 73, 74 The randomized phase II LOW-PV trial (NCT03003325) is currently evaluating whether the addition of pegylated proline-IFN-α−2b to ASA and phlebotomy could enhance Hct control in low risk PV patients (age <60 years, no prior thrombotic events).
Several studies have shown that IFN can lead to a reduction of the JAK2 V617F variant allele frequency (VAF), which suggests a disease modifying effect that is unusual with hydroxyurea.9, 10, 75, 76 Molecular responses, which are usually defined as ≥50% reduction in allele burden in patients with >20% VAF at baseline (PMR) or undetectable (CMR),77 were only inconsistently defined and reported in the studies included in this meta-analysis which precluded a formal assessment of this endpoint but molecular response rates of up to 90% have been described.9, 11, 27 In our systematic review six studies reported rates of CMR and PMR in 0–33% and 15–57% of patients among IFN-treated PV patients, respectively, with six additional studies reporting a reduction in allele burden.9, 28, 34, 46, 49, 51, 53, 54, 56, 58, 60 Furthermore, molecular responses with CMR rates of up to 26.7% were also reported in ET patients, which supports the disease-modifying potential of IFN.34, 46 Additionally, the prolonged response duration even after treatment discontinuation demonstrated in several studies suggests a potential elimination of the underlying clonal population and even cure in some patients but further studies with extended follow-up are necessary.11, 58 However, it is important to note that prolonged treatment with IFN is often required to achieve a CMR, which is an important aspect to address when counseling patients.34, 51, 62
Since the prognosis of ET and PV patients can vary widely, appropriate treatment selection, patient counseling, and clinical trial eligibility criteria continue to evolve with the wider availability of molecular testing.63, 78, 79 Efforts to standardize the reporting of outcomes and patient selection for clinical trials based on relevant risk factors have been launched by the ELN and International Working Group – Myeloproliferative Neoplasms Research and Treatment (IWG-MRT).78, 79 While recent trials of ruxolitinib used standardized assessments of symptom burden using Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and spleen size reduction,54, 80 those information were not available in the majority of studies included in this manuscript which precluded a meta-analysis of those outcomes. Although an increasing body of evidence suggests that the addition of molecular testing can aid in the risk stratification of ET and PV patients and that molecular responses could be an important clinical trial endpoint, additional studies with longer follow-up evaluating more nuanced questions such as the impact of VAF and the impact of co-mutations are needed.34, 51, 63, 81 Furthermore, a potential impact of other factors such as white blood cell count on thrombosis risk could not be evaluated in our study but has been shown previously.82, 83 However, data are controversial with a recent publication suggesting an association of persistent leukocytosis with risk of disease progression to MF, myelodysplastic syndrome, or AML but not thrombosis incidence in PV patients.84
While our systematic review and meta-analysis yielded robust results despite the various IFN formulations and treatment schedules and settings, several limitations exist. First, we had to rely on outcome definitions provided by the authors of the original studies. However, in our sensitivity analysis of studies reporting only CHR based on peripheral blood counts, heterogeneity persisted, which argues against a systematic effect of outcome definitions on our results. Second, data on adverse events were inconsistently reported and the absence of a standardized grading system did not permit a meta-analysis of this endpoint. However, the annual treatment discontinuation rate of 6.5% in PV and 8.8% in ET patients, respectively, are in line with the original studies.51, 85 Third, genetic data both for molecular response assessment and for the identification of predictive biomarkers were not available for the majority of studies given that a significant proportion of studies was conducted before the routine use of molecular testing. Finally, as the nomenclature has been evolving over time we cannot exclude that studies using alternative terms such as “myeloproliferative syndrome” instead of MPN might have been missed. However, our search strategy retrieved over 11,500 citations and 44 studies were included in the final meta-analysis what makes it unlikely that an alternative, even more extensive search strategy would have changed the conclusions of the study as a whole.
Conclusion:
This is the first published systematic review and meta-analysis of IFN in PV and ET covering 44 studies with 1359 patients and over three decades of clinical experience with various formulations and treatment schedules of this agent. IFN is a potent treatment option in both treatment-naïve and refractory patients with ORR of 80.6% (CHR: 59.0%) and 76.7% (CHR: 48.5%) in ET and PV patients, respectively, with comparable efficacy seen with pegylated and non-pegylated formulations. Adverse events remain a major limitation to treatment with IFN, but the high ORR and the durable molecular remission seen in a subset of patients suggest IFN to be a reasonable therapeutic option for patients with ET or PV.
Supplementary Material
Acknowledgments:
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359 and Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
N.A.P. consulted for and received honoraria from Alexion, Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene, Bristol-Myers Squib and CTI biopharma. N.A.P. received research funding (all to the institution) from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Jazz Pharmaceuticals, Pfizer, Astex Pharmaceuticals, CTI biopharma, Celgene, Genentech, AI Therapeutics, Samus Therapeutics, Arog Pharmaceuticals and Kartos Therapeutics. M.S.T. has received research funding from Abbvie, Cellerant, Orsenix, ADC Therapeutics, and Biosight. R.K.R has received consulting fees from: Constellation, Incyte, Celgene, Promedior, CTI, Jazz Pharmaceuticals, Blueprint, Stemline, and research funding from Incyte, Constellation, Stemline. M.S.T. has received honoraria for M.S.T. has received research funding from Abbvie, Cellerant, Orsenix, ADC Therapeutics, and Biosight. M.S.T. has received honoraria for advisory board membership from Abbvie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, and Jazz Pharma. M.S.T. received patents and royalties from UpToDate. A.M.Z. received research funding (institutional) from Celgene, Acceleron, Abbvie, Novartis, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics. A.M.Z had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Ionis, Epizyme, Amgen, Thyme, Janssen, and Takeda. A.M.Z received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this manuscript. All other authors report no relevant disclosures/competing interests.
References:
- 1.Network NCC. NCCN Guidelines Version 3.2019: Myeloproliferative neoplasms. 2019. [cited 2019 10/11/2019]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
- 2.Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood 2017; 130(23): 2475–2483. [DOI] [PubMed] [Google Scholar]
- 3.Szuber N, Mudireddy M, Nicolosi M, Penna D, Vallapureddy RR, Lasho TL, et al. 3023 Mayo Clinic Patients With Myeloproliferative Neoplasms: Risk-Stratified Comparison of Survival and Outcomes Data Among Disease Subgroups. Mayo Clin Proc 2019. April; 94(4): 599–610. [DOI] [PubMed] [Google Scholar]
- 4.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013. January 3; 368(1): 22–33. [DOI] [PubMed] [Google Scholar]
- 5.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005. July 7; 353(1): 33–45. [DOI] [PubMed] [Google Scholar]
- 6.Sterkers Y, Preudhomme C, Lai JL, Demory JL, Caulier MT, Wattel E, et al. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: high proportion of cases with 17p deletion. Blood 1998. January 15; 91(2): 616–622. [PubMed] [Google Scholar]
- 7.Nand S, Stock W, Godwin J, Fisher SG. Leukemogenic risk of hydroxyurea therapy in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Am J Hematol 1996. May; 52(1): 42–46. [DOI] [PubMed] [Google Scholar]
- 8.Kiladjian JJ, Rain JD, Bernard JF, Briere J, Chomienne C, Fenaux P. Long-term incidence of hematological evolution in three French prospective studies of hydroxyurea and pipobroman in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost 2006. June; 32(4 Pt 2): 417–421. [DOI] [PubMed] [Google Scholar]
- 9.Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008. 15 October; 112(8): 3065–3072. [DOI] [PubMed] [Google Scholar]
- 10.Quintas-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood 2013. August 8; 122(6): 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stauffer Larsen T, Iversen KF, Hansen E, Mathiasen AB, Marcher C, Frederiksen M, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res 2013. September; 37(9): 1041–1045. [DOI] [PubMed] [Google Scholar]
- 12.Kiladjian J-J, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011; 117(18): 4706–4715. [DOI] [PubMed] [Google Scholar]
- 13.Masarova L, Bose P, Verstovsek S. The Rationale for Immunotherapy in Myeloproliferative Neoplasms. Curr Hematol Malig Rep 2019 2019/August/01; 14(4): 310–327. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000. April 19; 283(15): 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Bewersdorf JP, Giri S, Wang R, Podoltsev N, Williams RT, Rampal RK, et al. Interferon therapy in myelofibrosis - a systematic review and meta-analysis. Clinical Lymphoma Myeloma and Leukemia 2020 2020/May/28/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998. June; 52(6): 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl M, Bewersdorf JP, Giri S, Wang R, Zeidan AM. Use of Immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis. Haematologica 2019. April 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaboration TC. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.02011. 2011.
- 19.Abegg-Werter MJBP, Raemaekers JMM, De Pauw BE, Haanen C recombinant interferon-alpha, but not interferon-gamma is effective therapie for essential thrombocythemia. Blut 1990; 60(1): 37–40. [DOI] [PubMed] [Google Scholar]
- 20.Alvarado Y, Cortes J, Verstovsek S, Thomas D, Faderl S, Estrov Z, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemotherapy and Pharmacology 2003; 51(1): 81–86. [DOI] [PubMed] [Google Scholar]
- 21.Bentley M, Taylor K, Grigg A, Kronenberg H, Gibson J, Bunce I, et al. Long-term interferon-alpha 2A does not induce sustained hematologic remission in younger patients with essential thrombocythemia. Leukemia & Lymphoma 1999. December; 36(1–2): 123–128. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes F, Salgado C, Feliu E, Montserrat E, Rozman C. Interferon alpha-2b for essential thrombocythaemia: Results in 13 previously untreated patients. Leukemia and Lymphoma 1991; 4(5–6): 351–354. [DOI] [PubMed] [Google Scholar]
- 23.Giles FJ, Anderson CC, Grant IR, Hoffbrand AV, Mehta AB, Machin SJ, et al. Recombinant alpha 2a interferon - An effective maintenance agent in essential thrombocythaemia. Leukemia and Lymphoma 1990; 3(2): 103–107. [DOI] [PubMed] [Google Scholar]
- 24.Giralt M, Rubio D, Cortes MT, Miguel JS, Steegmann JL, Serena J, et al. Alpha interferon in the management of essential thrombocythaemia. European Journal of Cancer 1991; 27(SUPPL. 4): S72–S74. [DOI] [PubMed] [Google Scholar]
- 25.Kasparu H, Bernhart M, Krieger O, Lutz D. Remission may continue after termination of rIFNalpha-2b treatment for essential thrombocythemia. European Journal of Haematology 1992; 48(1): 33–36. [DOI] [PubMed] [Google Scholar]
- 26.Gowin K, Thapaliy P, Samuelson J, Harrison C, Radia D, Andreasson B, et al. Experience with pegylated interferon alpha −2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica 2012; 97(10): 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gowin K, Jain T, Kosiorek H, Tibes R, Camoriano J, Palmer J, et al. Pegylated interferon alpha - 2a is clinically effective and tolerable in myeloproliferative neoplasm patients treated off clinical trial. Leukemia Research 2017. March; 54: 73–77. [DOI] [PubMed] [Google Scholar]
- 28.Huang BT, Zeng QC, Zhao WH, Li BS, Chen RL. Interferon alpha-2b gains high sustained response therapy for advanced essential thrombocythemia and polycythemia vera with JAK2V617F positive mutation. Leukemia Research 2014. 01 October; 38(10): 1177–1183. [DOI] [PubMed] [Google Scholar]
- 29.Jabbour E, Kantarjian H, Cortes J, Thomas D, Garcia-Manero G, Ferrajoli A, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: Final result of a phase 2 study. Cancer 2007; 110(9): 2012–2018. [DOI] [PubMed] [Google Scholar]
- 30.Langer C, Lengfelder E, Thiele J, Kvasnicka HM, Pahl HL, Beneke H, et al. Pegylated interferon for the treatment of high risk essential thrombocythemia: results of a phase II study. Haematologica 2005; 90(10): 1333–1338. [PubMed] [Google Scholar]
- 31.Lopes E, Ribeiro MM, Silva MJ, Gandra M, Principe F, Granato C. Essential thrombocythemia - clinical features, therapy and follow-up of 12 cases. Leukemia 1992; 6(SUPPL. 3): 138S–140S. [PubMed] [Google Scholar]
- 32.Lazzarino M, Vitale A, Morra E, Gagliardi A, Bernasconi P, Torromeo C, et al. Interferon alpha-2b as treatment for Philadelphia-negative chronic myeloproliferative disorders with excessive thrombocytosis. British Journal of Haematology 1989; 72(2): 173–177. [DOI] [PubMed] [Google Scholar]
- 33.Lindgren M, Samuelsson J, Nilsson L, Knutsen H, Ghanima W, Westin J, et al. Genetic variation in IL28B (IFNL3) and response to interferon-alpha treatment in myeloproliferative neoplasms. European journal of haematology 2018; 100(5): 419–425. [DOI] [PubMed] [Google Scholar]
- 34.Masarova L, Patel KP, Newberry KJ, Cortes J, Borthakur G, Konopleva M, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial. The Lancet Haematology 2017; 4(4): e165–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middelhoff G, Boll I. A long-term clinical trial of interferon alpha-therapy in essential thrombocythemia. Annals of Hematology 1992. May; 64(5): 207–209. [DOI] [PubMed] [Google Scholar]
- 36.Pogliani EM, Rossini F, Miccolis I, Ferrario A, Perego D, Casaroli I, et al. Alpha interferon as initial treatment of essential thrombocythemia. Analysis after two years of follow-up. Tumori 1995; 81(4): 245–248. [DOI] [PubMed] [Google Scholar]
- 37.Radin AI, Kim HT, Grant BW, Bennett JM, Kirkwood JM, Stewart JA, et al. Phase II study of alpha2 interferon in the treatment of the chronic myeloproliferative disorders (E5487): A trial of the Eastern Cooperative Oncology Group. Cancer 2003; 98(1): 100–109. [DOI] [PubMed] [Google Scholar]
- 38.Rametta V, Ferrara F, Marottoli V, Matera C, Mettivier V, Cimino R. Recombinant interferon alpha-2b as treatment of essential thrombocythaemia. Acta Haematologica 1994; 91(3): 126–129. [DOI] [PubMed] [Google Scholar]
- 39.Saba R, Jabbour E, Giles F, Cortes J, Talpaz M, O’Brien S, et al. Interferon alpha therapy for patients with essential thrombocythemia: final results of a phase II study initiated in 1986. Cancer 2005; 103(12): 2551–2557. [DOI] [PubMed] [Google Scholar]
- 40.Sacchi S, Tabilio A, Leoni P, Riccardi A, Vecchi A, Messora C, et al. Interferon alpha-2b in the long-term treatment of essential thrombocythemia. Annals of Hematology 1991; 63(4): 206–209. [DOI] [PubMed] [Google Scholar]
- 41.Sacchi S, Gugliotta L, Papineschi F, Liberati AM, Rupoli S, Delfini C, et al. Alfa-interferon in the treatment of essential thrombocythemia: clinical results and evaluation of its biological effects on the hematopoietic neoplastic clone. Leukemia 1998. March; 12(3): 289–294. [DOI] [PubMed] [Google Scholar]
- 42.Seewann HL, Zikulnig R, Gallhofer G, Schmid C. Treatment of thrombocytosis in chronic myeloproliferative disorders with interferon alfa-2b. European Journal of Cancer 1991; 27(SUPPL. 4): S58–S63. [DOI] [PubMed] [Google Scholar]
- 43.Turri D, Mitra ME, Di Trapani R, Lipari MG, Perricone R, Cajozzo A. Alpha-interferon in polycythemia vera and essential thrombocythemia. Haematologica 1991. Jan-Feb; 76(1): 75–77. [PubMed] [Google Scholar]
- 44.Verger E, Cassinat B, Chauveau A, Dosquet C, Giraudier S, Schlageter M-H, et al. Clinical and molecular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. Blood 2015; 126(24): 2585–2591. [DOI] [PubMed] [Google Scholar]
- 45.Yataganas X, Meletis J, Plata E, Viniou N, Deligiannis F, Tsekoura C, et al. Alpha interferon treatment of essential thrombocythaemia and other myeloproliferative disorders with excessive thrombocytosis. European Journal of Cancer 1991; 27(SUPPL. 4): S69–S71. [DOI] [PubMed] [Google Scholar]
- 46.Zhang ZR, Duan YC. Interferon Apha 2b for Treating Patients with JAK2V617F Positive Polycythemia Vera and Essential Thrombocytosis. Asian Pacific Journal of Cancer Prevention 2014; 15(4): 1681–1684. [DOI] [PubMed] [Google Scholar]
- 47.Berte R, Vallisa D, Ferrari B, Civardi G, Sbolli G, Cavanna L. Low-dose interferon alpha treatment in essential thrombocythemia. European Journal of Haematology 1996. Jan-Feb; 56(1–2): 104–105. [PubMed] [Google Scholar]
- 48.Gisslinger H, Chott A, Scheithauer W, Gilly B, Linkesch W, Ludwig H. Interferon in essential thrombocythaemia. British Journal of Haematology 1991. October; 79 Suppl 1: 42–47. [DOI] [PubMed] [Google Scholar]
- 49.Crisa E, Cerrano M, Beggiato E, Benevolo G, Lanzarone G, Manzini PM, et al. Can pegylated interferon improve the outcome of polycythemia vera patients? Journal of hematology & oncology 2017; 10(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foa P, Massaro P, Caldiera S, LaTargia ML, Iurlo A, Clerici C, et al. Long-term therapeutic efficacy and toxicity of recombinant interferon- alpha 2a in polycythaemia vera. European Journal of Haematology 1998; 60(5): 273–277. [DOI] [PubMed] [Google Scholar]
- 51.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 2015; 126(15): 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heis N, Rintelen C, Gisslinger B, Knobl P, Lechner K, Gisslinger H. The effect of interferon alpha on myeloproliferation and vascular complications in polycythemia vera. European Journal of Haematology 1999. January; 62(1): 27–31. [DOI] [PubMed] [Google Scholar]
- 53.Jones AV, Silver RT, Waghorn K, Curtis C, Kreil S, Zoi K, et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood 2006; 107(8): 3339–3341. [DOI] [PubMed] [Google Scholar]
- 54.Kiladjian JJ, Guglielmelli P, Griesshammer M, Saydam G, Masszi T, Durrant S, et al. Efficacy and safety of ruxolitinib after and versus interferon use in the RESPONSE studies. Annals of Hematology 2018. April; 97(4): 617–627. [DOI] [PubMed] [Google Scholar]
- 55.Öztürk A, Günay A, Üskent N. Therapeutic efficacy of recombinant interferon-alpha in polycythaemia vera. Acta Haematologica 1998; 99(2): 89–91. [DOI] [PubMed] [Google Scholar]
- 56.Utke Rank C, Weis Bjerrum O, Larsen TS, Kjaer L, De Stricker K, Riley CH, et al. Minimal residual disease after long-term interferon-alpha2 treatment: A report on hematological, molecular and histomorphological response patterns in 10 patients with essential thrombocythemia and polycythemia vera. Leukemia and Lymphoma 2016. 01 February; 57(2): 348–354. [DOI] [PubMed] [Google Scholar]
- 57.Stasi R, Venditti A, Del Poeta G, Conforti M, Brunetti M, Bussa S, et al. Role of human leukocyte interferon-alpha in the treatment of patients with polycythemia vera. American Journal of the Medical Sciences 1998; 315(4): 237–241. [DOI] [PubMed] [Google Scholar]
- 58.Larsen TS, Moller MB, de Stricker K, Norgaard P, Samuelsson J, Marcher C, et al. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology (Amsterdam, Netherlands) 2009; 14(6): 331–334. [DOI] [PubMed] [Google Scholar]
- 59.Taylor PC, Dolan G, Ng JP, Paul B, Collin R, Reilly JT. Efficacy of recombinant interferon-alpha (rIFN-alpha) in polycythaemia vera: A study of 17 patients and an analysis of published data. British Journal of Haematology 1996; 92(1): 55–59. [DOI] [PubMed] [Google Scholar]
- 60.Kuriakose E, Vandris K, Wang YL, Chow W, Jones AV, Christos P, et al. Decrease in JAK2 V617F allele burden is not a prerequisite to clinical response in patients with polycythemia vera. Haematologica 2012. April; 97(4): 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization–essential thrombocythemia (IPSET-thrombosis). Blood 2012; 120(26): 5128–5133. [DOI] [PubMed] [Google Scholar]
- 62.Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol 2020. March; 7(3): e196–e208. [DOI] [PubMed] [Google Scholar]
- 63.Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 2018; 32(5): 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005. April 1; 23(10): 2224–2232. [DOI] [PubMed] [Google Scholar]
- 65.Cortelazzo S, Viero P, Finazzi G, D’Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol 1990; 8(3): 556–562. [DOI] [PubMed] [Google Scholar]
- 66.Mascarenhas J, Kosiorek H, Prchal J, Yacoub A, Berenzon D, Baer MR, et al. A prospective evaluation of pegylated interferon alfa-2a therapy in patients with polycythemia vera and essential thrombocythemia with a prior splanchnic vein thrombosis. Leukemia 2019; 33(12): 2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito T, Hashimoto Y, Tanaka Y, Nakaya A, Fujita S, Satake A, et al. Efficacy and safety of anagrelide as a first-line drug in cytoreductive treatment-naïve essential thrombocythemia patients in a real-world setting. Eur J Haematol 2019; 103(2): 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gisslinger H, Gotic M, Holowiecki J, Penka M, Thiele J, Kvasnicka HM, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013. March 7; 121(10): 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spivak JL. Myeloproliferative Neoplasms. N Engl J Med 2017; 376(22): 2168–2181. [DOI] [PubMed] [Google Scholar]
- 70.Ferrari A, Carobbio A, Masciulli A, Ghirardi A, Finazzi G, De Stefano V, et al. Clinical outcomes under hydroxyurea treatment in polycythemia vera: a systematic review and meta-analysis. Haematologica 2019; 104(12): 2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A, et al. Results of the Myeloproliferative Neoplasms - Research Consortium (MPN-RC) 112 Randomized Trial of Pegylated Interferon Alfa-2a (PEG) Versus Hydroxyurea (HU) Therapy for the Treatment of High Risk Polycythemia Vera (PV) and High Risk Essential Thrombocythemia (ET). Blood 2018; 132(Supplement 1): 577–577.29954751 [Google Scholar]
- 72.Mesa RA, Kosiorek HE, Mascarenhas J, Prchal JT, Rambaldi A, Berenzon D, et al. Impact on MPN Symptoms and Quality of Life of Front Line Pegylated Interferon Alpha-2a Vs. Hydroxyurea in High Risk Polycythemia Vera and Essential Thrombocythemia: Results of Myeloproliferative Disorders Research Consortium (MPD-RC) 112 Global Phase III Trial. Blood 2018; 132(Supplement 1): 3032–3032. [Google Scholar]
- 73.Hatalova A, Schwarz J, Gotic M, Penka M, Hrubisko M, Kusec R, et al. Recommendations for the diagnosis and treatment of patients with polycythaemia vera. Eur J Haematol 2018; 101(5): 654–664. [DOI] [PubMed] [Google Scholar]
- 74.Beauverd Y, Radia D, Cargo C, Knapper S, Drummond M, Pillai A, et al. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: outcome and safety. A case series. Haematologica 2016; 101(5): e182–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antonioli E, Carobbio A, Pieri L, Pancrazzi A, Guglielmelli P, Delaini F, et al. Hydroxyurea does not appreciably reduce JAK2 V617F allele burden in patients with polycythemia vera or essential thrombocythemia. Haematologica 2010. August; 95(8): 1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Them NC, Bagienski K, Berg T, Gisslinger B, Schalling M, Chen D, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015. April; 90(4): 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barosi G, Mesa R, Finazzi G, Harrison C, Kiladjian J-J, Lengfelder E, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013; 121(23): 4778–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and Disease Progression in Essential Thrombocythemia Are Significantly Influenced by Accurate Morphologic Diagnosis: An International Study. J Clin Oncol 2011; 29(23): 3179–3184. [DOI] [PubMed] [Google Scholar]
- 79.Barosi G, Tefferi A, Besses C, Birgegard G, Cervantes F, Finazzi G, et al. Clinical end points for drug treatment trials in BCR-ABL1-negative classic myeloproliferative neoplasms: consensus statements from European LeukemiaNET (ELN) and Internation Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT). Leukemia 2015. January; 29(1): 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015. January 29; 372(5): 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guglielmelli P, Pietra D, Pane F, Pancrazzi A, Cazzola M, Vannucchi AM, et al. Recommendations for molecular testing in classical Ph1-neg myeloproliferative disorders–A consensus project of the Italian Society of Hematology. Leuk Res 2017 2017/July/01/; 58: 63–72. [DOI] [PubMed] [Google Scholar]
- 82.Barbui T, Masciulli A, Marfisi MR, Tognoni G, Finazzi G, Rambaldi A, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 2015. July 23; 126(4): 560–561. [DOI] [PubMed] [Google Scholar]
- 83.Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood 2009. July 23; 114(4): 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronner L, Podoltsev N, Gotlib J, Heaney ML, Kuykendall AT, O’Connell C, et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood 2020. May 7; 135(19): 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 2019. October 31; 134(18): 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy S, Iland H, Rosenthal D, Laszlo J. Essential thrombocythemia: an interim report from the Polycythemia Vera Study Group. Semin Hematol 1986. Jul; 23(3): 177–182. [PubMed] [Google Scholar]
- 87.Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood 2009. May 14; 113(20): 4829–4833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


