Abstract
Objective
To compare Pneumocystis jirovecii pneumonia (PJP) risk between patients with autoimmune rheumatic diseases (ARD) and the general population
Methods
We identified patients with ARD recorded in the National Health Insurance Research Database of Taiwan from 2002 to 2015 and randomly selected a comparison cohort from the general population matched for age and sex. We analyzed PJP risk stratified by sex, age, comorbidities, and medications using Cox proportional hazard model.
Results
We enrolled 103,117 patients with ARD. PJP risk significantly increased in patients with any ARD and with each individual ARD like rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjogren’s syndrome (SjS), polymyositis and dermatomyositis (PM/DM), systemic sclerosis (SSc), and systemic vasculitis. Patients with PM/DM showed prominent risk with incidence rate of 12.47/100,000 patient year (95% confidence interval (CI), 32.16–86.70). In a time-dependent Cox proportional hazard model with comorbidities and medications as covariates, PM/DM, SSc, SLE, and SjS significantly increased adjusted hazard ratios (aHR) of 5.40, 5.12, 4.09, and 3.64, respectively (95% CI of 2.82–10.35, 2.16–12.13, 2.41–6.95, and 2.06–6.42, respectively). AHR after adjusting for male sex, cancer, human immunodeficiency virus infection (HIV), and interstitial lung disease also significantly increased. Use of daily oral steroid dose of >10 mg conferred the highest risk followed by mycophenolate. Use of injected steroids, cyclophosphamide, biological agents, methotrexate, and cyclosporine conferred a significantly higher risk.
Conclusion
Underlying ARD significantly predisposes patients to PJP, with PM/DM posing the highest threat. In addition to underlying disease, comorbidities and concomitant immunosuppressants are major risks. The strongest risk is recent daily steroid dose of >10 mg. Mycophenolate seems to be a more prominent risk factor than cyclophosphamide.
|
Key Points • Autoimmune rheumatic diseases (ARD) significantly increased the overall risk of PJP, and so did each individual ARD. • Use of steroids, mycophenolate, cyclophosphamide, biological agents, methotrexate, and cyclosporine all significantly increased risk of PJP. • Male, elderly, malignancy, HIV, and interstitial lung disease are also related to increased risk of PJP. • Underlying ARD, comorbidities, and use of immunosuppressant should all be considered in determining the overall risk of PJP. |
Keywords: Autoimmune rheumatic diseases, Cyclophosphamide, Mycophenolate, Pneumocystis jirovecii pneumonia, Steroid
Introduction
Pneumocystis jirovecii is an opportunistic organism that can cause high mortality among immunosuppressed patients including those with human immunodeficiency virus infection (HIV), malignancy, and underlying autoimmune rheumatic diseases (ARD) [1, 2]. In recent years, due to the progress of organ transplantation, tumor chemotherapy, and ARD treatment, the number of non-HIV patients infected by Pneumocystis jirovecii has gradually increased [3]. Patients with underlying ARD may be exposed to immunosuppressive drugs, corticosteroid, biological agents, and cyclophosphamide, rendering them more vulnerable to Pneumocystis jirovecii pneumonia (PJP) [4, 5]. Mortality rates among non-HIV patients diagnosed with PJP are high (estimated to be between 39.4 and 59.1%), making this an important population to target for prophylaxis [6]. Trimethoprim/sulfamethoxazole prophylaxis in patients with ARD can significantly reduce incidence of PJP [5, 7]. Therefore, identifying patients with ARD and a high risk of PJP is crucial for adequate initiation of Pneumocystis jirovecii prophylaxis, which may improve patient outcomes as has proven effective for HIV patients [8].
In previous reports of ARD, the most common underlying condition for development of PJP is Wegener’s granulomatosis (8–12%), followed by polyarteritis nodosa (PAN) (6.5%), polymyositis and dermatomyositis (PM/DM) (2.7%), systemic lupus erythematosus (SLE) (2%), and rheumatoid arthritis (RA) (0.1–0.3%) [9]. However, data on the prevalence of PJP in ARD is relatively limited. Several comorbidities other than HIV, malignancy, or ARD may also increase risks of PJP, such as renal transplantation [10, 11], end-stage renal disease (ESRD) [11], or interstitial lung disease (ILD) [12], which adds to the complexity of risk evaluation and management.
In addition to the patient’s underlying diseases, high-dose steroid use and immunosuppressants were found to increase the risk of PJP in patients with ARD [10, 13]. Patients with ARD receiving an initial steroid dose of ≥60 mg/day prednisolone equivalent were also found to have a higher risk for PJP [5]. Significant risk factors for PJP included higher age, male sex, underlying lung disease, use of corticosteroids, use of methotrexate, and use of biologics (anti-TNF alpha, anti-IL-6 receptor antibody, or anti-T-cell costimulatory pathway agents) in patients with RA [8, 14]. Anti-CD20 and cyclophosphamide use were also related to increased risk of PJP in patients with SLE or malignancy [15, 16].
To summarize, the host’s underlying disease and comorbidity, which directs different combination of immunosuppressant use including different dosage and duration, contribute greatly to the variability and complexity of PJP in clinical practice. Due to the rare occurrence but high mortality of PJP infection in patients with ARD, it is crucial to determine the risk of PJP infection individually when considering the initiation of prophylaxis. This study aims to determine (1) the risk of PJP with underlying ARD as a whole or individual ARD, (2) comorbidities that pose additional risks, and (3) effects of immunosuppressants, steroid, biologics, and cyclophosphamide on the risk of PJP. Using nationwide data on PJP in patients with ARD, we aimed to better understand this condition under complex clinical settings.
Materials and methods
Data resources
The National Health Insurance (NHI) program in Taiwan was instituted in 1995 to provide comprehensive health care for nearly all Taiwanese citizens and has a coverage rate of 99%. For research purposes, the bureau of NHI in Taiwan released the National Health Insurance Research Database (NHIRD), which provides detailed claim data for outpatient clinics, admission, and drug prescriptions of patients enrolled in the NHI. For this study, we collected data from the NHIRD from January 1, 2002, to December 31, 2015. Since the data sets used in this study were comprised of de-identified secondary data for research purposes, patient consent was not required for this study. This study was approved by the Taipei Medical University institutional review board (approval numbers N201509007 and N201908055). The diagnostic codes used in this data set were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Study design and cohort definition
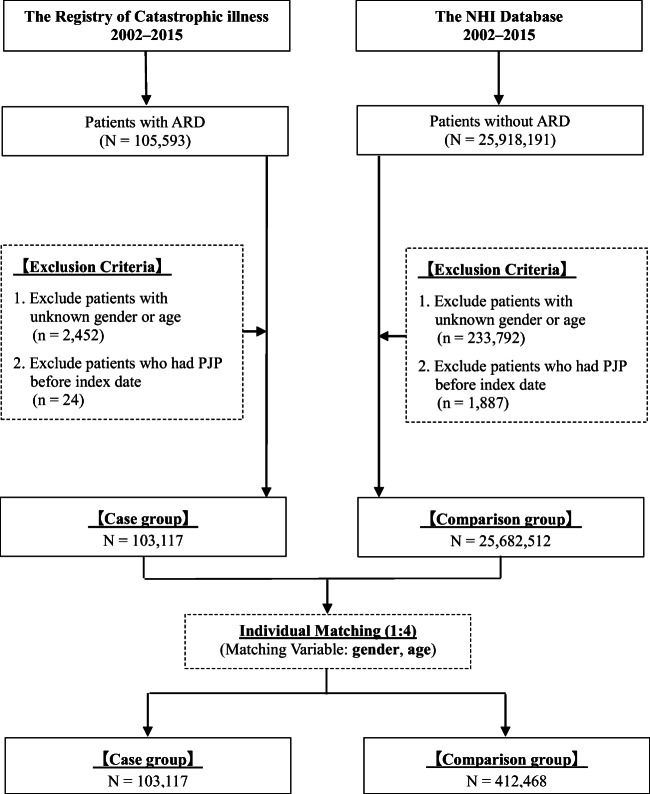
We conducted a retrospective matched cohort study using the NHIRD of Taiwan to investigate the risk of PJP of patients with and without ARD (Fig. 1). The NHIRD established a registry system for catastrophic illnesses. In Taiwan, after a rheumatologist makes a diagnosis based on a patient’s clinical features and laboratory and image results, patients who precisely meet the classification criteria for each ARD are eligible for catastrophic illness registration and are reviewed by a committee. The related classification criteria are as follows: American College of Rheumatology (ACR) revised criteria (1997) for SLE (ICD-9-CM: 710.0) [17], American Rheumatism Association revised criteria (1987) for RA (ICD-9-CM: 714.0) [18], American-European Consensus Group revised criteria (2002) for Sjogren’s syndrome (SjS, ICD-9-CM: 710.2) [19], ACR criteria for systemic sclerosis (SSc, ICD-9-CM: 710.1) [20], Bohan and Peter criteria (1975) for polymyositis and dermatomyositis (PM/DM; ICD-9-CM: 710.4 and 710.3) [21, 22], PAN (ICD-9-CM: 446.0), hypersensitivity angiitis (ICD-9-CM: 446.2) [23], Buerger’s disease (ICD-9-CM: 443.1) [24], Wegener’s granulomatosis (ICD-9-CM: 446.4) [25], and giant cell arteritis (ICD-9-CM: 446.5) [26].
Fig. 1.
Study design. NHI, national health insurance; ARD, autoimmune rheumatic diseases; PJP, Pneumocystis jirovecii pneumonia
Patients with their first new catastrophic illness registration of each ARD between 2002 and 2015 were enrolled, including those with RA, SLE, SjS, PM/DM, SSc, and systemic vasculitis (SV). The SV group comprised patients with PAN, hypersensitivity angiitis, Wegener’s granulomatosis, giant cell arteritis, and Buerger’s disease. The primary endpoint was incidence of PJP infection defined as pneumocystosis (ICD-9-CM: 136.3) in the discharge diagnosis. Patients with unknown sex or age or with PJP prior to enrollment were excluded. Patients with overlapping ARDs were excluded. Each patient with ARD was matched with four non-ARD subjects for age and sex based on the same exclusion criteria. All patients were followed until the development of primary endpoint, end of the study period, or discontinuance of insurance.
Statistical analysis
For continuous variables, we employed Student’s t tests to evaluate the differences between the case group and the comparison group, and for categorical variables in our baseline analysis, we evaluated differences between the two groups using Pearson’s chi-squared tests. An incidence rate ratio (IRR) was constructed to compare the incidence between the groups, and the confidence interval (CI) of IRR was calculated using normal approximation. The cumulative incidence rate and Gray’s test for equality of cumulative incidence functions were employed to compare the risk of PJP infection between the two cohorts.
A Cox proportional hazard model was applied to evaluate the factors associated with PJP infection. Factors analyzed in the model included age, sex, type of ARD, comorbidities, and medications leading to immunosuppression status. Comorbidities within 1 year before the index date that may lead to immunosuppression were identified using the corresponding ICD-9-CM, including cancer, HIV, renal transplant, ESRD, ILD, congestive heart failure, diabetes mellitus, hypertension, cirrhosis, and stroke. Medications used during the study period were analyzed as time-dependent covariates, such as hydroxychloroquine, methotrexate, mycophenolate, azathioprine, cyclosporine, cyclophosphamide, steroid, and biologics. Biologics consisted of etanercept, adalimumab, golimumab, rituximab, tocilizumab, and abatacept. The follow-up period of each patient was retrospectively divided into successive 120-day blocks; the prescription status of each medication in each 120-day block and its association with the occurrence of the event at the end of each time block were analyzed. Oral steroid use was analyzed according to the average daily dose in the time block, with a cutoff point of 5 and 10 mg prednisolone equivalent. All factors with p < 0.1 in the univariate analysis were selected for Cox multivariate forward stepwise analysis. The results of all statistical tests were considered significant if p < 0.05.
Results
Demographic data
There were 103,117 patients with ARD and 412,468 matched controls enrolled in this study (Table 1). The majority of patients in both cohorts were female (81.58%) and the mean age was 50.4 years in both groups. The ARD group had significantly increased comorbidities and medication use. The detailed use of each medication is shown in Supplementary Material Table 1.
Table 1.
Baseline characteristics of case and sex- and age-matched comparison groups
| Variable | Case | Comparison | p value | ||
|---|---|---|---|---|---|
| Patients with ARD (N = 103117) | Patients without ARD (N = 412468) | ||||
| n | % | n | % | ||
| Gender | |||||
| Male | 18999 | 18.42% | 75996 | 18.42% | 1.0000 |
| Age | |||||
| Mean (SD) | 50.45 (16.77) | 50.44 (16.78) | 0.7849 | ||
| Comorbidities | |||||
| Cancer | 8628 | 8.37% | 33164 | 8.04% | 0.0006 |
| HIV | 57 | 0.06% | 148 | 0.04% | 0.0052 |
| Renal transplant | 206 | 0.20% | 170 | 0.04% | <0.0001 |
| End-stage renal disease | 2266 | 2.20% | 4927 | 1.19% | <0.0001 |
| Interstitial lung disease | 2071 | 2.01% | 375 | 0.09% | <0.0001 |
| Congestive heart failure | 1742 | 1.69% | 3948 | 0.96% | <0.0001 |
| Diabetes mellitus | 8599 | 8.34% | 39549 | 9.57% | <0.0001 |
| Hypertension | 6747 | 6.54% | 31163 | 7.56% | <0.0001 |
| Cirrhosis | 1016 | 0.99% | 2632 | 0.64% | <0.0001 |
| Stroke | 4169 | 4.04% | 14639 | 3.55% | <0.0001 |
| PJP | |||||
| 181 | 0.18% | 61 | 0.01% | <0.0001 | |
ARD, autoimmune rheumatic diseases; HIV, human immunodeficiency virus
Increased risk of PJP in patients with ARD
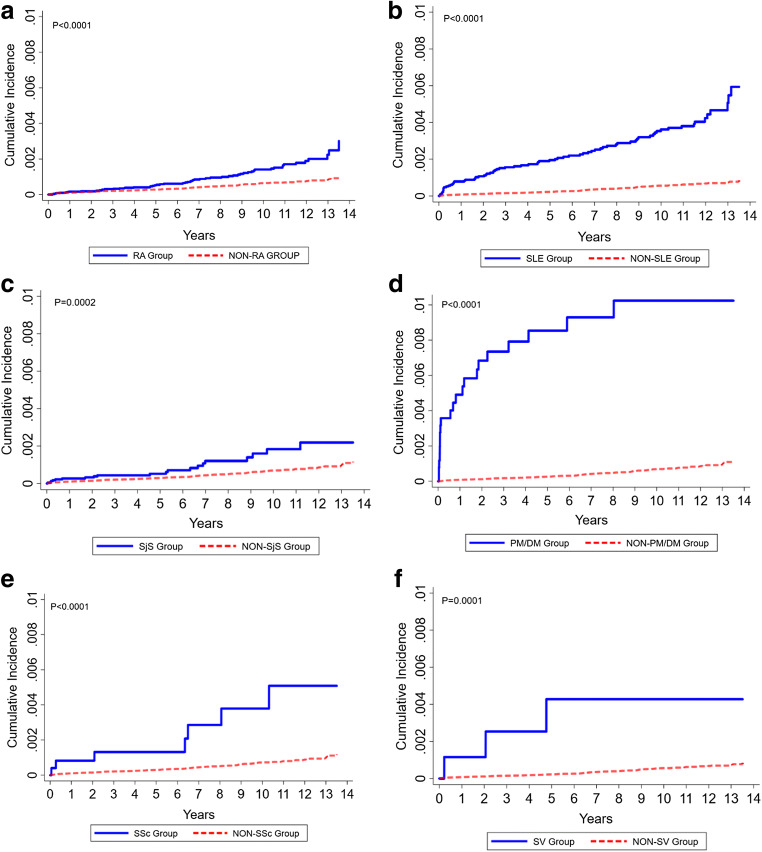
The ARD group had a significantly higher incidence of PJP development than the comparison group during the observation period (0.18% vs. 0.01%, p < 0.0001) (Table 1). The incidence rates (IR) and IRR of PJP during the follow-up period revealed a significantly higher risk in patients with each individual ARD (Table 2). The highest risk was observed in patients with PM/DM (IR 12.47, IRR 52.81, 95% CI 32.16–86.70), followed by SV (IR 4.87, IRR 20.61, 95% CI 6.46–65.70), SSc (IR 4.10, IRR 17.35, 95% CI 7.94–37.93), SLE (IR 3.79, IRR 16.03, 95% CI 11.42–22.50), SjS (IR 1.58, IRR 6.68, 95% CI 4.03–11.07), and RA (IR 1.46, IRR 6.19, 95% CI 4.31–8.89). The event rate of PJP in individual ARD can be found in Supplementary Material Table 2. The incidence rate analyses for individual ARD and overall ARD also revealed a significantly higher risk of PJP infection (Fig. 2, Supplementary Material Figure 1).
Table 2.
Incidence rate ratio of PJP in individual autoimmune rheumatic diseases
| N | PJP event | PY | IR | IRR | 95% CI | |
|---|---|---|---|---|---|---|
| Comparison | 412468 | 61 | 2583087 | 0.24 | Reference | |
| RA | 50341 | 56 | 383168.8 | 1.46 | 6.19 | (4.31–8.89) |
| SLE | 23716 | 74 | 195431.7 | 3.79 | 16.03 | (11.42–22.50) |
| SjS | 23048 | 20 | 126784.1 | 1.58 | 6.68 | (4.03–11.07) |
| PM/DM | 2589 | 21 | 16840.42 | 12.47 | 52.81 | (32.16–86.70) |
| SSc | 2499 | 7 | 17084.68 | 4.10 | 17.35 | (7.94–37.93) |
| SV | 924 | 3 | 6162.76 | 4.87 | 20.61 | (6.46–65.70) |
ARD, autoimmune rheumatic diseases; PJP, Pneumocystis jirovecii pneumonia; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SjS, primary Sjogren’s syndrome; PM/DM, polymyositis/dermatomyositis; SSc, systemic sclerosis; SV, systemic vasculitis; PY, patient year; IR, incidence rate of 100,000 patient year; IRR, incidence rate ratio; CI, confidence interval
Fig. 2.
Cumulative incidence plot of individual autoimmune rheumatic diseases. a Cumulative incidence plot of RA and non-RA group. b Cumulative incidence plot of SLE and non-SLE group. c Cumulative incidence plot of SjS and non-SjS group. d Cumulative incidence plot of PM/DM and non-PM/DM group. e Cumulative incidence plot of SSc and non-SSc group. f Cumulative incidence plot of SV and non-SV group. PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis; SjS, Sjogren’s syndrome; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; SV, systemic vasculitis
Risk factors for PJP in patients with ARD
To analyze risk factors associated with PJP infection, a multivariate Cox proportional hazard analysis was applied to all study participants (Table 3). Age, male sex, and comorbidities including malignancy, HIV, and ILD significantly increased the risk of PJP. After adjusting for sex, age, and comorbidities, several medications analyzed as time-dependent covariates in the model significantly related to PJP infection. The medication with the highest risk was daily oral steroid dose of >10 mg prednisolone equivalent (aHR 9.15, 95% CI 6.23–13.45), followed by (in order of risk) mycophenolate (aHR 7.14, 95% CI 4.21–12.13), injection steroid therapy (aHR 5.03, 95% CI 3.47–7.29), biological agents (aHR 3.53, 95% CI 2.08–5.98), cyclophosphamide (aHR 3.37, 95% CI 2.26–5.01), methotrexate (aHR 2.85, 95% CI 1.91–4.27), and cyclosporine (aHR 2.03, 95% CI 1.23–3.34). After adjusting for these factors, most of the individual ARD still conferred a significantly higher risk of PJP, with the highest risk in patients with PM/DM (aHR 5.40, 95% CI 2.82–10.35), followed by SSc (aHR 5.12, 95% CI 2.16–12.13), SLE (aHR 4.09, 95% CI 2.41–6.95), and SjS (aHR 3.64, 95% CI 2.06–6.42). After adjustment, RA and SV did not have a significantly increased PJP risk. The event rate of each medication for the individual ARD can be found in Supplementary Material Table 3.
Table 3.
Risk factors of PJP in patients with ARD
| aHR | 95% CI | |
|---|---|---|
| Types of ARD | ||
| Without ARD | Reference | |
| RA | 1.44 | (0.88–2.36) |
| SLE | 4.09*** | (2.41–6.95) |
| SjS | 3.64*** | (2.06–6.42) |
| PM/DM | 5.40*** | (2.82–10.35) |
| SSc | 5.12** | (2.16–12.13) |
| SV | 2.96 | (0.88–10.03) |
| Gender | ||
| Female | Reference | |
| Male | 1.77** | (1.31–2.39) |
| Age of diagnose ARD | ||
| Age | 1.02** | (1.00–1.03) |
| Comorbidities | ||
| Cancer | 3.74*** | (2.74–5.10) |
| HIV | 110.8*** | (47.69–257.5) |
| Renal transplant | 2.56 | (0.95–6.91) |
| End-stage renal disease | 2.04 | (0.95–4.41) |
| Interstitial lung disease | 2.41** | (1.39–4.18) |
| Congestive heart failure | 1.31 | (0.57–3.02) |
| Diabetes mellitus | 0.56 | (0.28–1.15) |
| Hypertension | 0.81 | (0.43–1.52) |
| Cirrhosis | 1.30 | (0.41–4.17) |
| Stroke | 1.43 | (0.73–2.80) |
| Medicine | ||
| Hydroxychloroquine | 0.73 | (0.52–1.02) |
| Methotrexate | 2.85*** | (1.91–4.27) |
| Mycophenolate | 7.14*** | (4.21–12.13) |
| Azathioprine | 1.33 | (0.84–2.10) |
| Cyclosporine | 2.03** | (1.23–3.34) |
| Cyclophosphamide | 3.37*** | (2.26–5.01) |
| Biological agents | 3.53*** | (2.08–5.98) |
| Steroid (injection) | 5.03*** | (3.47–7.29) |
| Steroid (oral) mg/day | ||
| <5 | Reference | |
| 5–10 | 1.89 | (0.98–3.64) |
| >10 | 9.15*** | (6.23–13.45) |
*p <0.05, **p <0.01, ***p <0.001
ARD, autoimmune rheumatic diseases; PJP, Pneumocystis jirovecii pneumonia; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SjS, primary Sjogren’s syndrome; PM/DM, polymyositis/dermatomyositis; SSc, systemic sclerosis; SV, systemic vasculitis; HIV, human immunodeficiency virus; aHR, adjusted hazard ratio; CI, confidence interval
Discussion
Previous studies of PJP in autoimmune diseases are mostly case series and single-center experiences of one or a few ARD, with a relatively low incidence of PJP. To the best of our knowledge, this is the first study to attempt a broad, population-based perspective to better understand PJP in patients with ARD, especially in patients with SSc and SjS. Patients with ARD had a significantly higher risk of PJP. In our multivariate Cox proportional hazard analysis, patients with PM/DM had the highest risk, followed by those with SSc, SLE, and SjS. The factors associated with PJP included comorbidity-related secondary immunosuppression and immunosuppressant use such as steroid, mycophenolate, biologic agents, cyclophosphamide, methotrexate, and cyclosporine.
Patients with different ARD were at differing risks of PJP. In previous studies of PJP, the most common ARD were inflammatory myopathy, SLE, ANCA-associated vasculitis, and RA [3, 27, 28]. SSc and SjS have not been previously reported to be associated with higher risk of PJP infection, but in this study, patients with SSc or SjS had a significantly higher risk of PJP, and the risk in patients with SSc was even higher than that in patients with SLE. In addition, the ARD with the highest PJP risk was neither SLE nor SV; patients with PM/DM had the highest risk followed by SV and SSc. In our multivariate Cox proportional hazard analysis, most ARD still had a risk of PJP after adjusting for all related factors. This was not true for RA or SV, which means the increased risk of PJP in patients with RA and SV may come mostly from the immunosuppressants and related comorbidities such as ILD; therefore, the risk was no longer significant after adjusting for these factors. However, we believe that there were other reasons that lead to our failure to demonstrate the significant risk in patients with SV. The wide 95% CI implied that the patient year of SV was too low to have sufficient statistical power to show significance and only a trend toward higher risk remained in the model. The relatively lower patient year in the SV cohort might be due to the low prevalence and high mortality rate of this disease.
Among immunosuppressants, the risk of cyclophosphamide is well-documented and related prophylactic strategies were established in patients with hematological malignancies [29]. Of note, several medications had higher risk than cyclophosphamide in this study. The highest was daily oral steroid use over 10 mg prednisolone equivalent. Injected steroid therapy also had an increased risk. This result is consistent with previous reports emphasizing the risk of opportunistic infections such as Pneumocystis jirovecii in patients with high-dose steroid use, and this effect seems to be dose-dependent, regardless of underlying disease [5, 30, 31]. Cumulative steroid dose, duration of steroid use, and daily steroid dose all had prominent impacts on the risk of infection. It was demonstrated that current and recent doses of steroid have the greatest impact on infection risk, but the cumulative impact of doses taken in the past few years still affects risk in patients with RA [32].
Use of steroids, mycophenolate, biological agents, cyclophosphamide, methotrexate, and cyclosporine had significantly increased risks of PJP infection. It is noteworthy that mycophenolate is believed to be equally effective but better tolerated than cyclophosphamide, but in our model, the risk of PJP with mycophenolate was even higher than that with cyclophosphamide. There has been a single-center report of increased mortality of PJP in patients with ARD receiving mycophenolate [33]. Moreover, in Taiwan, biological agents endorsed by NHI were mostly used for treating RA. There have been reports of PJP infection in patients with RA under etanercept, adalimumab, golimumab, rituximab, tocilizumab, or abatacept treatment [8, 14, 34]. Our results also showed a significantly higher risk of PJP infection in patients using biological agents. Due to the paucity of evidence regarding primary prophylaxis of PJP in ARD, the initiation of prophylaxis in patients with ARD is still based on expert opinion with no clear recommendations [35]. The initiation and duration of trimethoprim/sulfamethoxazole prophylaxis should be considered in high risk patients in a patient-based manner [35]. This study provided data on the individual risk of each immunosuppressant, which may add insight to evaluating the overall risk of PJP under different clinical settings and assist decision-making on prophylaxis of this serious disease.
Several basic characteristics were associated with PJP risk. Similar to a previous report in RA, older age and male sex were related to a higher risk of PJP [14]. Our study showed that underlying ARD, malignancy, HIV, and ILD contribute to PJP infection. ILD was associated with increased pulmonary colonization by Pneumocystis jirovecii, which may explain this increased risk [12].
Our study had several strengths including the long follow-up and the use of population-based, nationwide cohorts and the large study sample that may increase statistical precision. In addition, we validated ARD diagnosis by catastrophic illness registration. In the NHI system, patients with the catastrophic illness registration for ARD can be exempted from related medical expense. However, the verification requires fulfillment of the classification criteria of each ARD, as supported by medical records and examination reports. These features made the ARD diagnosis more reliable. Second, we analyzed immunosuppressants as time-dependent variables in a multivariate Cox-regression model, which could fit in with the temporal relationship between medication and the subsequent increased risk of PJP infection.
Our study had several limitations. First, because the NHI database does not include clinical data or culture results, we could not analyze information such as patients’ lymphocyte count, CD4 count, or serum LDH level, which might have an additional predictive value of PJP [6, 36]. However, the main goal of this study was to investigate the epidemiology of PJP in ARD, especially ARD that have been less studied in this context such as PM/DM, SSc, and SjS and the risk of individual medications. Second, different biologics were analyzed together despite the fact that different biologics might have different risks of PJP infection. We decided to simplify the analysis by combining different biologics as one variable because there were too many variables in the model given the low incidence of PJP events and the biologics were mainly used in patients with RA. We will focus on this issue in patients with RA in the future. The situation was similar in SV; we included different types of vasculitis as a single cohort due to relatively low case numbers of each type of vasculitis. However, we believe that the risk differed with different types of vasculitis.
This study revealed the increased risk and epidemiologic data of PJP infection in several ARD including ARD with few reports on, such as SSc and SjS. The risk was highest in patients with PM/DM followed by SV, SSc, SLE, SjS, and RA. This study also included risk factors for PJP infection such as male gender, older age, and comorbidities like cancer, HIV, or ILD, or immunosuppressants including steroid, mycophenolate, cyclophosphamide, biologics, methotrexate, and cyclosporine. These data provide fundamental insights for further studies on prophylactic antibiotics for PJP for these populations.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Hui-Ching Hsu and Yu-Sheng Chang contributed to study conception and design, article drafting, data interpretation, critical article revision for crucial intellectual content, and the final approval of the submitted version. Tsung-Yun Hou, Lung-Fang Chen, Li-Fang Hu, Tzu-Min Lin, Chi-Sheng Chiou, Kai-Len Tsai, Sheng-Hong Lin, Pei-I Kuo, Wei-Sheng Chen, and Yi-Chun Lin contributed to data analysis, critical article revision for crucial intellectual content, and the final approval of the submitted version. Jin-Hua Chen and Chi-Ching Chang were responsible for study conception and design, complete data analysis, critical article revision for crucial intellectual content, and correspondence regarding the final approval of the submitted version.
Compliance with ethical standards
The manuscript does not contain clinical studies or patient data.
Disclosures
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jin-Hua Chen and Chi-Ching Chang contributed equally to this work.
References
- 1.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Teichtahl AJ, Morrisroe K, Ciciriello S, Jennens I, Tadros S, Wicks I. Pneumocystis jirovecci pneumonia in connective tissue diseases: comparison with other immunocompromised patients. Semin Arthritis Rheum. 2015;45(1):86–90. doi: 10.1016/j.semarthrit.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu AL, Traore K, Plekhanova I, Bouchrik M, Bossard C, Picot S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11–17. doi: 10.1016/j.ijid.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, Montagna P, Sulli A. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153–155. doi: 10.1016/j.autrev.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for Pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis. 2018;77(5):644–649. doi: 10.1136/annrheumdis-2017-211796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum. 2017;46(6):804–809. doi: 10.1016/j.semarthrit.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Sugimoto M. Pneumocystis jirovecii pneumonia in rheumatoid arthritis patients: risks and prophylaxis recommendations. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):29–40. doi: 10.4137/CCRPM.S23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Méd Mal Infect. 2014;44(5):185–198. doi: 10.1016/j.medmal.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Sugimoto M. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology (Oxford) 2012;51(12):2120–2130. doi: 10.1093/rheumatology/kes244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leth S, Jensen-Fangel S, Ostergaard L, Rostved AA, Jespersen B, Sogaard OS. Pneumocystis jirovecii pneumonia in patients with end-stage renal disease: a comparison with the general population. Scand J Infect Dis. 2014;46(10):704–711. doi: 10.3109/00365548.2014.936492. [DOI] [PubMed] [Google Scholar]
- 12.Vidal S, de la Horra C, Martin J, Montes-Cano MA, Rodriguez E, Respaldiza N, et al. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin Microbiol Infect. 2006;12(3):231–235. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZG, Liu XM, Wang Q, Chen NF, Tong SQ. A retrospective study of patients with systemic lupus erythematosus combined with Pneumocystis jiroveci pneumonia treated with caspofungin and trimethoprim/sulfamethoxazole. Medicine (Baltimore) 2019;98(23):e15997. doi: 10.1097/MD.0000000000015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto A, Suto S, Horie K, Fukuda H, Nogi S, Iwata K, Tsuno H, Ogihara H, Kawakami M, Komiya A, Furukawa H, Matsui T, Tohma S (2017) Incidence and risk factors for infections requiring hospitalization, including Pneumocystis pneumonia, in Japanese patients with rheumatoid arthritis. Int J Rheumatol 6730812. 10.1155/2017/6730812 [DOI] [PMC free article] [PubMed]
- 15.Tsai MJ, Chou CW, Lin FC, Chang SC. Pneumocystis jiroveci pneumonia in patients with systemic lupus erythematosus after rituximab therapy. Lupus. 2012;21:914–918. doi: 10.1177/0961203312436855. [DOI] [PubMed] [Google Scholar]
- 16.Cordonnier C, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71(9):2379–2385. doi: 10.1093/jac/dkw155. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FCES, Bloch DA, Mchane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 21.Bohan A, Peter JB. Polymyositis and dermatomyositis (First of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 22.Bohan A, Peter JB. Polymyositis and dermatomyositis (Second of two parts) N Engl J Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 24.Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. 2010;121(16):1858–1861. doi: 10.1161/CIRCULATIONAHA.110.942383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33(8):1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 26.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 27.Kronbichler A, Jayne DR, Mayer G. Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis. Eur J Clin Investig. 2015;45(3):346–368. doi: 10.1111/eci.12410. [DOI] [PubMed] [Google Scholar]
- 28.Mecoli CA, Saylor D, Gelber AC, Christopher-Stine L. Pneumocystis jiroveci pneumonia in rheumatic disease: a 20-year single-centre experience. Clin Exp Rheumatol. 2017;35:671–673. [PubMed] [Google Scholar]
- 29.Maertens J, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, Matos O, Bretagne S, Cordonnier C. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71(9):2397–2404. doi: 10.1093/jac/dkw157. [DOI] [PubMed] [Google Scholar]
- 30.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin N Am. 2016;42(1):157–176. doi: 10.1016/j.rdc.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winthrop KL, Baddley JW. Pneumocystis and glucocorticoid use: to prophylax or not to prophylax (and when?); that is the question. Ann Rheum Dis. 2018;77(5):631–633. doi: 10.1136/annrheumdis-2017-212588. [DOI] [PubMed] [Google Scholar]
- 32.Dixon WG, Abrahamowicz M, Beauchamp ME, Ray DW, Bernatsky S, Suissa S, Sylvestre MP. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis. 2012;71(7):1128–1133. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zheng Y. Pneumocystis jirovecii pneumonia in mycophenolate mofetil-treated patients with connective tissue disease: analysis of 17 cases. Rheumatol Int. 2014;34(12):1765–1771. doi: 10.1007/s00296-014-3073-4. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama M, Kaneko Y, Takeuchi T (2017) Comparison of the Clinical characteristics of Pneumocystis pneumonia between patients with rheumatoid arthritis being treated with biologics and those being treated without biologics. Biomed Res Int 3710652. 10.1155/2017/3710652 [DOI] [PMC free article] [PubMed]
- 35.Ghembaza A, Vautier M, Cacoub P, Pourcher V, Saadoun D. Risk factors and prevention of Pneumocystis jirovecii pneumonia in patients with autoimmune and inflammatory diseases. Chest. 2020;158(6):2323–2332. doi: 10.1016/j.chest.2020.05.558. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Huang XM, Fang WG, Zeng XJ. Pneumocystis carinii pneumonia in patients with connective tissue disease. J Clin Rheumatol. 2006;12(3):114–117. doi: 10.1097/01.rhu.0000221794.24431.36. [DOI] [PubMed] [Google Scholar]