Abstract
The Bristow–Latarjet procedure has been one of the most recognized procedures for the treatment of recurrent shoulder dislocation with anterior glenoid bone loss, revision surgery after failed Bankart repair, contact and collision sport injuries, and patients with a high risk of recurrence. Open and arthroscopic approaches have recently shown similar outcomes by several authors. However, complications related to metal implants, despite being low, are still a matter of concern. We describe an all-arthroscopic Latarjet technique with a metal-free fixation method using 2 ultra-high-strength sutures, creating a cerclage construct through 2.4mm glenoid and coracoid tunnels with a final capsulolabral complex reconstruction.
Technique Video
We present the first all arthroscopic metal-free Latarjet cerclage technique. The patient is positioned in the beach chair position. We use a 70° arthroscopic scope through the posterior portal and a radiofrequency device through the anterosuperior portal to release the rotator interval, the coracoacromial ligament, the clavipectoral fascia and the inferior, lateral and anterior border of the conjoined tendon. The pectoralis minor tendon detachment is performed from the coracoid portal. The coracoid’s inferior border is flattened with a rasp or burr introduced through the anterosuperior portal. The coracoid’s length is measured. With the help of a 7 mm offset-specific guide inserted through the coracoid portal, 2 tunnels are performed with two 2.4-mm cannulated drills. An 8.2- to 5-mm cannula is inserted through the anteroinferior portal for suture shuttling. Two nitinol wires are subsequently passed through the cannulated drills. The nitinol wires are retrieved one after the other through the cannula and switched with 2 high-strength FiberLink sutures. The capsulolabral complex is detached from 1 to 6 o’clock through the anterosuperior portal. The hook component of the glenoid drilling guide is passed through the standard posterior portal and engaged on the anterior glenoid rim. Two tunnels are then drilled through the glenoid with the same 2.4-mm cannulated drills previously used for the coracoid drilling. A Wissinger rod is passed from the posterior portal through the subscapularis muscle to determine the level of the horizontal split on its lower portion. With a radiofrequency device, a horizontal split is performed. A specific retractor is inserted through the coracoid portal. A nitinol wire is passed through the inferior glenoid cannulated drill and retrieved across the subscapularis split through the cannula. The nitinol wire is replaced by a blue FiberLink, shuttling it through the glenoid, leaving its loop posteriorly. Two preconfigured FiberTape Cerclage sutures are transported, by pulling the blue inferior glenoid tunnel FiberLink and retrieved through the cannula. The inferior end of the blue distal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid process, from ventral to dorsal. The white proximal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid, from dorsal to ventral. A second nitinol is passed through the superior glenoid cannulated drill. The nitinol is replaced with a white FiberLink, which is used to transport the FiberTape through the superior glenoid tunnel from anterior to posterior. Looking through the anterosuperior portal, we use an arthroscopic grasp and chisel through the anterior portal to cut the coracoid at its base. The coracoid graft is transferred through the subscapularis split by pulling on the FiberTapes sutures symmetrically. The cerclage tapes are interconnected and manually, the knots are reduced against the posterior glenoid. With a tensioner, each suture is tensioned separately and an 80-N secure fixation is achieved. Finally, the system is locked with 3 alternating knots. Reconstruction of the capsulolabral complex on the native anterior glenoid rim is achieved leaving the coracoid in an extra-articular position.
In 1954, Michel Latarjet described his technique for the treatment of recurrent shoulder dislocation.1 The Latarjet procedure has shown excellent long-term functional outcomes, becoming one of the most widely used techniques for the treatment of recurrent shoulder instability.2, 3, 4, 5
With the technological evolution of arthroscopy, it has been possible to perform the treatment of the unstable shoulder from an arthroscopic approach. In 1980, the first arthroscopic Bankart lesion repair was reported by Johnson.6 Almost 30 years later, in 2007, Laurent Lafosse7 first described the arthroscopic Latarjet procedure. After that, several authors have been able to report similar results to open procedures.8,9
Despite their excellent outcomes, both open and arthroscopic Latarjet are not without complications.9, 10, 11, 12, 13 There is great concern about metal fixation because most complications are associated to metal implants.12,14, 15, 16 Multiple methods of fixation have been described for the arthroscopic Latarjet technique, such as 1 or 2 screws and metal buttons.17, 18, 19 We recently described an arthroscopically assisted Latarjet procedure without metal implants: The Latarjet Cerclage technique.20 Afterwards, we have been able to develop our metal-free fixation technique in a completely arthroscopic manner. In this Technical Note, we present our all-arthroscopic Latarjet cerclage technique, a completely arthroscopic metal-free fixation method using 2 ultra-high-strength sutures with an additional capsulolabral complex reconstruction. The advantages and disadvantages of this technique are described in Table 1.
Table 1.
Advantages and Disadvantages of the Technique
| Advantages |
|
|
|
|
|
|
|
| Disadvantages |
|
|
|
|
Surgical Technique (With Video Illustration)
The surgical technique is demonstrated in the supplementary video (Video 1).
Preoperative Assessment
All patients with recurrent anterior shoulder dislocations or subluxations events are studied with 3-dimensional computed tomography. In the sagittal plane, an “en face” view of the glenoid with humeral head suppression is used to assess anterior glenoid bone loss and the glenoid track. The best-fit circle technique is used for this purpose. Also, the Hill–Sachs (HS) lesion is measured in the axial plane and compared with the previously measured glenoid track to categorize HS lesion as an on-track (nonengaging HS lesion) or off-track (engaging HS lesion). This surgical technique is indicated for all recurrent anterior shoulder instability with more than 15% of glenoid bone loss, revision surgery after failed Bankart repair, contact and collision sports injuries, and patients with a high risk of recurrence.
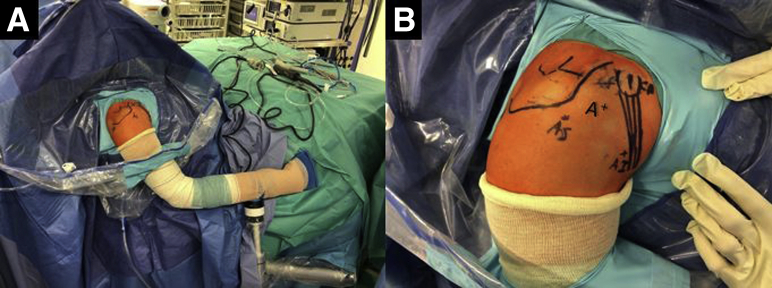
Patient Positioning and Portals
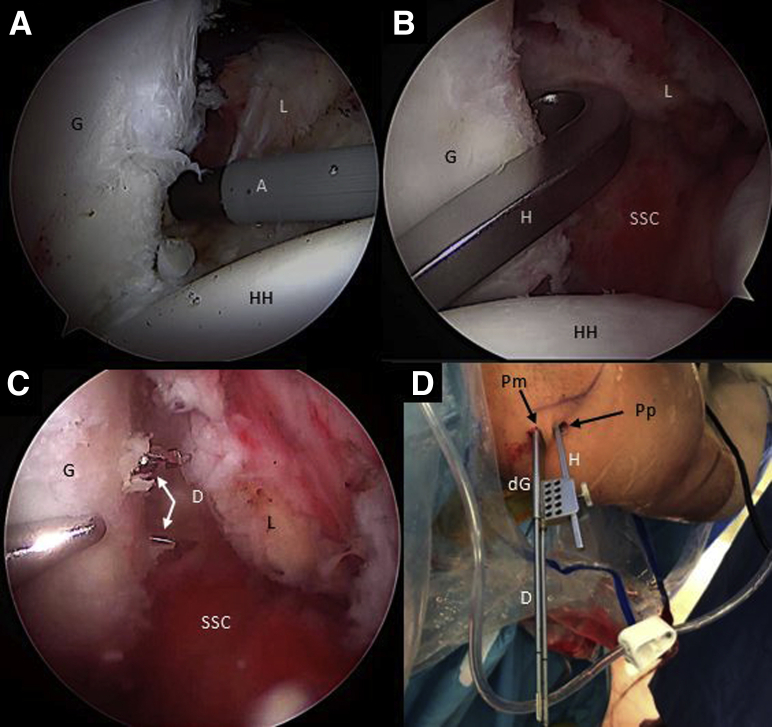
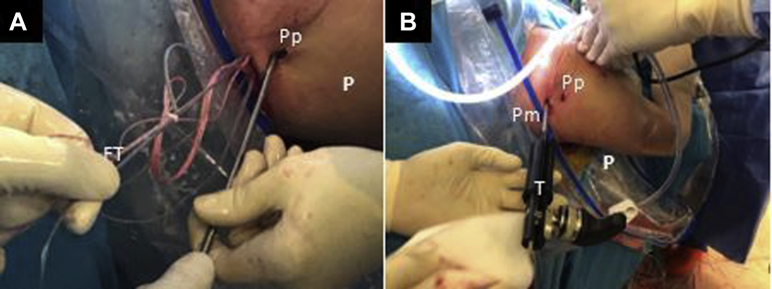
The patient, under general anesthesia, is positioned in the beach chair position (Fig 1A). The bony structures are marked on the skin. The following portals are also marked: a high standard posterior portal (P), an anterosuperior portal (AS) 1 cm distal to the anterolateral border of the acromion, the coracoid working portal 1 cm proximal and medial to the tip of the coracoid (CP), and an anteroinferior portal (AI), 5 cm distal to the coracoid process at the axillary pouch. An anterior standard rotator interval portal (A) can be created to have a good direction for the coracoid osteotomy (Fig 1B).
Fig 1.
External view of the (A) preoperative setting with the patient placed in the beach-chair position with the right arm fixed on a Trimano Arthrex device and (B) drawing of bony structures and arthroscopic portals. (A, anterior portal; AS, anterosuperior portal; AI, anteroinferior portal; CP, coracoid portal.)
Step 1: Arthroscopic Diagnosis and Anterior Soft-Tissue Release
Initial arthroscopic diagnosis is performed through the standard P portal using a 70° scope, looking for concomitant intra-articular pathologies. Assessment of the engaging lesion between the humeral head and the anterior edge of the glenoid is done (Fig 2). Releasing of the rotator interval as well as the coracoacromial ligament, conoid and trapezoid ligaments, the clavipectoral fascia, the presubscapular tendon space, and inferior, lateral, anterior border of the conjoint tendon is performed using a shaver and a radiofrequency (RF) device through the AS portal (Fig 3 A-D).
Fig 2.
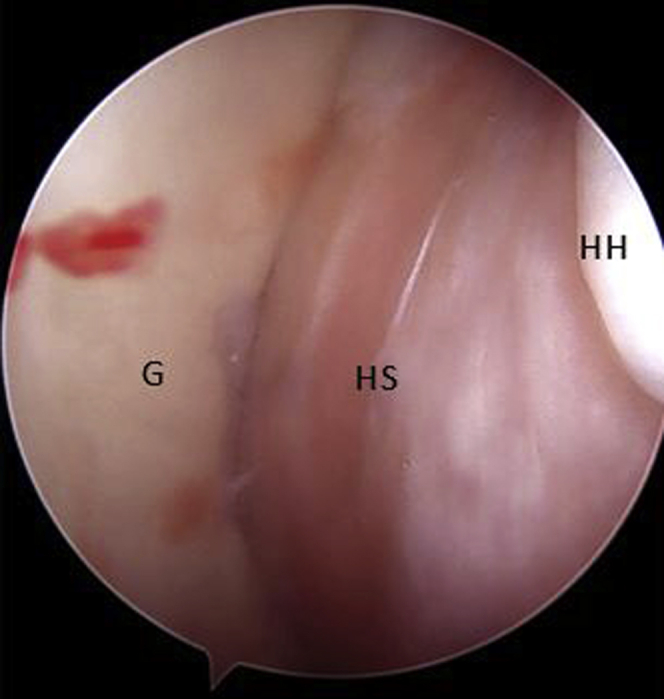
Arthroscopic view of a right shoulder through posterior portal with the patient placed in beach-chair position, showing an engaging lesion between the HS lesion and the anterior glenoid bone defect with the arm in abduction and external rotation. (G, glenoid surface; HH, humeral head; HS, Hill–Sachs lesion.)
Fig 3.
Right shoulder, Beach-chair position, Arthroscopic view, 30° scope. (A) The rotator interval is released looking through the posterior portal and working through the anterosuperior portal with an ablator. (B) The coracoacromial ligament is released through the anterosuperior portal with an ablator and looking through the posterior portal. (C) The superior and presubscapularis tendon space is released with an ablator looking through the anterosuperior portal and working through the anteroinferior portal. (D) The clavipectoral fascia is released with an ablator looking through the anterosuperior portal and working through the anteroinferior portal. (A, ablator; C, coracoid; CAL, coracoacromial ligament; CPF, clavipectoral fascia; CT, conjoint tendon; HH, humeral head; LHB, long head of the biceps tendon; RI, rotator interval; SSC, subscapularis tendon.)
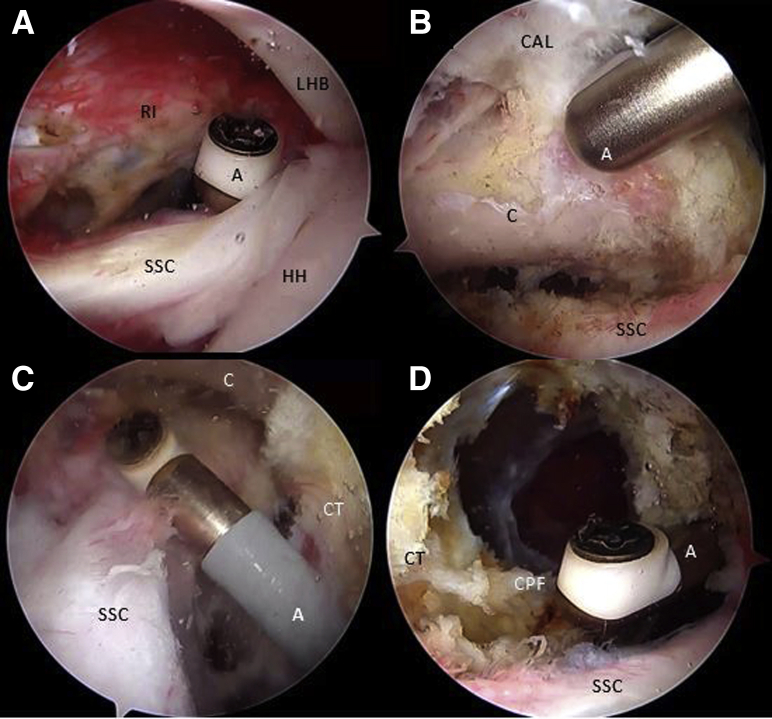
Step 2: Coracoid Preparation and Tunnel Drilling
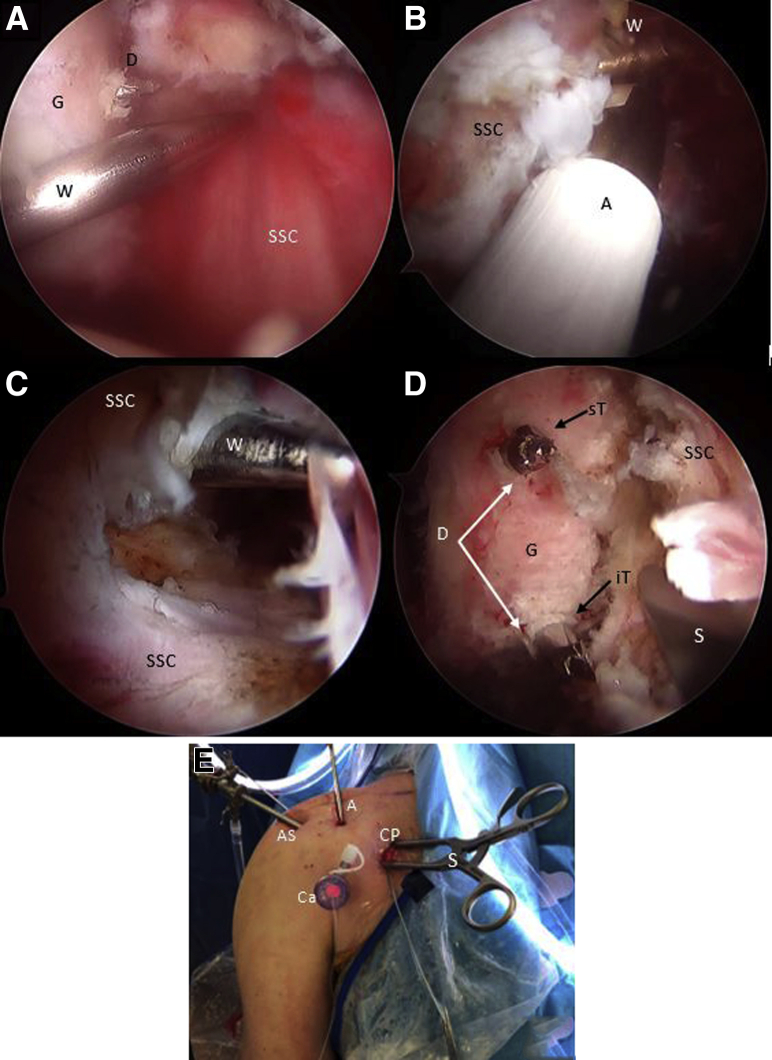
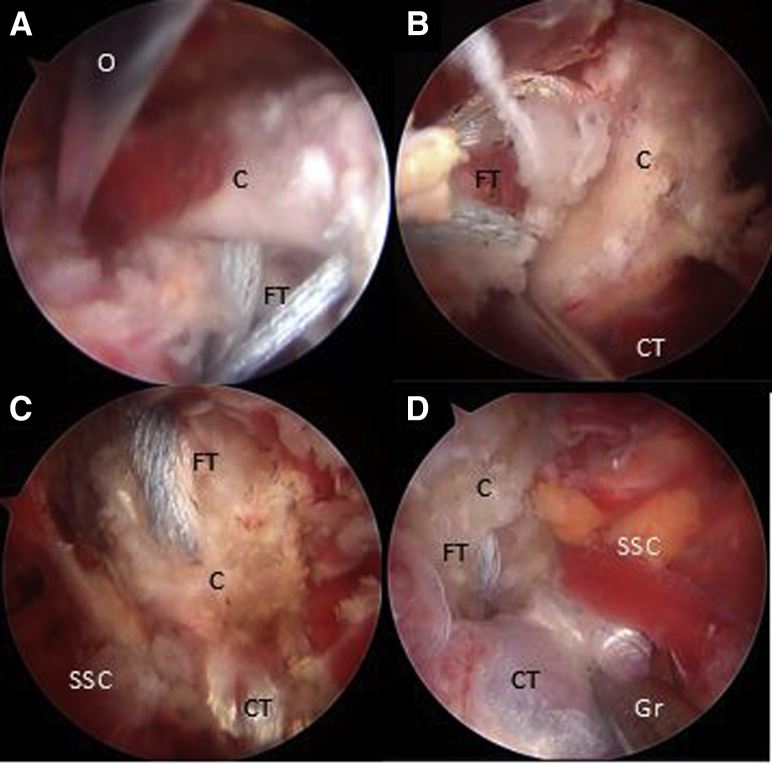
As one looks through the AS portal, the inferior border of the coracoid is flattened with an arthroscopic PowerRasp (Arthrex, Naples, FL) through the AI portal (Fig 4A). The length of the coracoid is measured with an arthroscopic measurement probe (Arthroscopic Measurement probe, 220 mm, 60°; Arthrex) and marked in the center to establish appropriate drilling guide positioning (Fig 4B). Using the RF wand through the CP, resection of the pectoralis minor tendon is performed (Fig 4C). We use an arthroscopic circular burr to flatten the undersurface of the coracoid, thereby obtaining a graft of approximately 25 mm in length. Using a proprietary drilling guide (Arthrex) inserted through the CP, placed at the mark previously made over the coracoid, two 2.4-mm cannulated drills are used to create 2 tunnels 10 mm apart from the superior to the inferior side of the coracoid (Fig 5 A-C). An 8.25-mm cannula is inserted through the AI portal for suture shuttling. Two nitinol wires are subsequently passed through the cannulated drills, the one in the distal coracoid tunnel with the loop placed superiorly and the other one with the loop placed inferiorly, after which the drills are removed (Fig 5 D and E). These nitinol wires are replaced with 2 high-strength FiberLink sutures (Arthrex) to avoid breakage of the nitinol wires when tractioning the cerclage sutures and are retrieved through the cannula at the AI portal. The distal coracoid tunnel is replaced with the FiberLink suture with the loop facing inferiorly, and the other proximal coracoid tunnel with the FiberLink suture with the loop facing superiorly. The FiberLink sutures must be colored differently to facilitate suture handling (we favor a blue suture through the distal tunnel and a white suture through the proximal tunnel). Both ends of the FiberLink sutures are then retrieved through the CP and stored safely (Fig 5 F and G).
Fig 4.
Right shoulder, beach-chair position, Arthroscopic view, 30° scope. (A) The coracoid is shaped on its lateral and inferior aspect looking through the anterior portal and working through the anterosuperior portal with a PowerRasp (Arthrex). (B) The length of the coracoid is measured with an Arthroscopic Probe (Arthrex) from the anterior portal and looking through the anterosuperior portal. (C) Looking through the anterosuperior portal, the pectoralis minor tendon is released from the medial aspect of the coracoid. (A, ablator; C, coracoid; CT, conjoint tendon; M, medial coracoid space; P, arthroscopic measurement probe; PW, PowerRasp; SSC, subscapularis tendon.)
Fig 5.
Right shoulder, beach-chair position. (A) Beach-chair position, right shoulder, Anterosuperior extraarticular view of the drilling guide inserted through the coracoid portal. (B) Arthroscopic view, 70° scope, looking through the anterosuperior portal, drilling guide inserted through the coracoid portal and (C) 2 cannulated drills, can be observed. Note: In (A-C), black arrows show the distal coracoid tunnel and white arrows show the proximal coracoid tunnel. (D-F) Arthroscopic view, 70° scope, looking through the anterosuperior portal, a nitinol wire is retrieved from the distal coracoid tunnel through the cannula in the anteroinferior portal and then replaced with a with a blue FiberLink. (G) The procedure is repeated with the proximal coracoid tunnel, exchanging the nitinol wire with a different-colored FiberLink than in the distal tunnel. Then all FiberLink tails are retrieved through the coracoid portal. Note: white arrows show FiberLink strands that are passing through coracoid tunnels. Black arrows show FiberLink strands that are passing medial to the coracoid process. (C, coracoid process; Ca, cannula; CT, conjoint tendon; D, cannulated drill; dG, drilling guide; DT, distal coracoid tunnel; FL, FiberLink; Gr, grasper; N, nitinol wire.)
Step 3: Glenoid Exposure and Tunnel Drilling
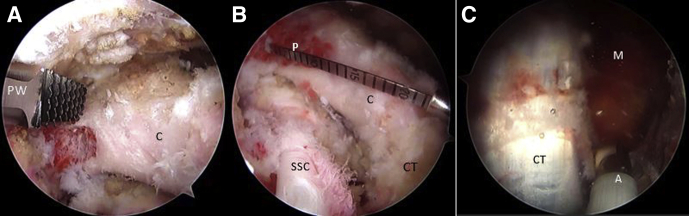
Looking through the AS portal, the capsulolabral complex is detached from 1 to 6 o’clock with a dissector working through the CP portal or the A portal (rotator interval), until the fibers of the subscapularis muscle are seen (Fig 6A). A polyester PDS suture is passed through the capsulolabral complex—including the middle glenohumeral ligament—using a percutaneous portal passing through the upper portion of the subscapularis tendon with the help of a SutureLasso (Arthrex). When traction is applied to this suture, it separates the anterior soft tissue from the anterior glenoid rim and improves visualization of the anterior glenoid surface. Next, the anterior glenoid defect is debrided with a shaver, a tissue dissector, and a curette. Exposing the bone surface of the anterior glenoid rim is necessary for the subsequent coupling of the coracoid to the anterior glenoid bone bed and reinsertion of the articular capsulolabral complex.
Fig 6.
Right shoulder, beach chair position, anterosuperior portal, arthroscopic view, 70° scope. (A) The anterior capsulolabral complex is released with an ablator through the coracoid portal. (B) The hook component of the drilling guide is inserted through the posterior portal and engaged in the anteroinferior glenoid neck. (C) Then, the hook is connected with the drilling guide outside the patient and pushed against the posterior glenoid neck through a separate posteromedial portal. Two cannulated drills are inserted through the glenoid drilling guide in the posteromedial portal to perform the glenoid tunnels. (D) Posterosuperior external view of the right shoulder in the beach chair position showing the drilling guide construct insertion through the posterior and the posteromedial portals. (A, ablator; D, cannulated drill; dG, drilling guide; G, glenoid; H, hook component of the drilling construct; HH, humeral head; L, capsulolabral complex; Pm, posteromedial portal; Pp, posterior portal; SSC, subscapularis muscle.)
The hook component of the Arthrex drilling guide is passed through the standard posterior portal and engaged on the anterior glenoid rim at the previous mark at the center of the bony defect (Fig 6B). The drilling guide is coupled to the hook component and inserted through a new posteromedial portal (parallel to the standard posterior portal) and pushed against the posterior glenoid bone. A scissor is used to dilate the infraspinatus muscle to place the drill guide directly against the posterior bony glenoid surface. Two tunnels are then drilled through the glenoid from posterior to anterior using the same 2.4-mm cannulated drills previously used for coracoid drilling (Fig 6 C and D). The drilling guides are then removed, leaving both cannulated sheaths inserted in the glenoid for subsequent sutures shuttling after performing the subscapular split. The distance from the glenoid surface to the tunnels (off set distance) and the most distal tunnel to the lowest margin of the glenoid can be measured to match the previously established coracoid dimensions.
Step 4: Subscapular Split
Under direct vision with the scope in the anterosuperior portal (AS), a Wissinger rod is passed from the posterior portal through the subscapularis muscle to determine the horizontal split’s level (Fig 7A). The axillary nerve can be identified following the “three sisters” medially and subsequently protected. Through palpation of the posterior Wissinger rod through the subscapularis tendon and with the help of an RF wand introduce from the AI portal, a horizontal split is performed at the junction of the two-thirds proximal and one-third distal level of the subscapularis (Fig 7 B-D). The capsulolabral complex is protected with a Wissinger rod, just posterior to the subscapularis tendon, until the anterior glenoid border and drills are visualized. An additional Wissinger rod or a specific retractor can be inserted through the CP to aid in visualization and suture management (Fig 7E).
Fig 7.
Right shoulder, beach-chair position, arthroscopic view, 70° scope, anterosuperior portal. (A) The Wissinger is inserted through the posterior portal to determine the split level of the subscapularis tendon. (B-C) The Wissinger rod is identified across the anterior aspect of the subscapularis muscle and the split is performed parallel to the muscle fibers with an ablator. (D) Deep to the subscapularis split, the glenoid and the cannulated drills are identified. A specific spreader is inserted through the coracoid portal to facilitate anterior glenoid neck visualization and sutures shuttling. (E) Anterior external view of the right shoulder in the beach-chair position showing the spreader insertion through the coracoid portal and the cannula insertion in the anteroinferior portal for subsequent sutures shuttling. (A, ablator; AP, anterior portal; AS, anterosuperior portal; C, coracoid process; Ca, cannula; CP, coracoid portal; D, cannulated drill; G, glenoid; iT, inferior glenoid tunnel; S, self-retaining retractor; SSC, subscapularis muscle; sT, superior glenoid tunnel; W, Wissinger rod.)
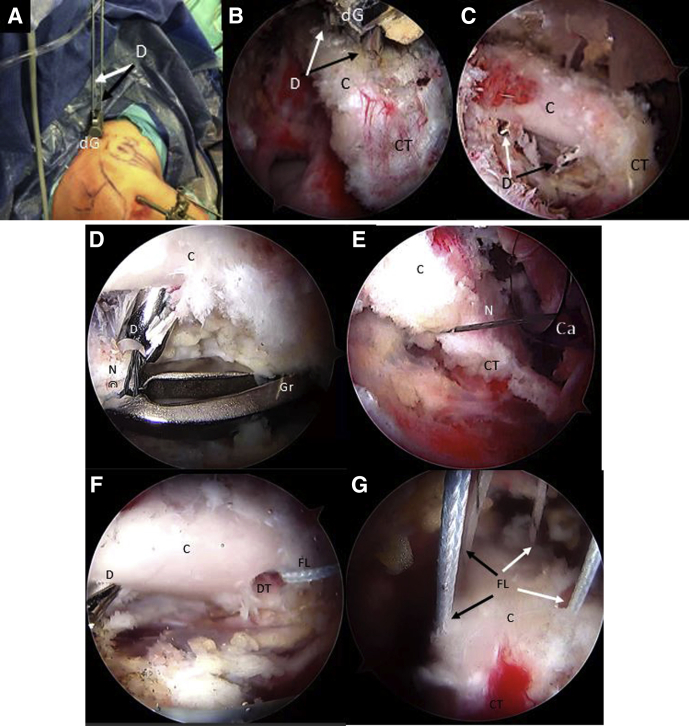
Step 5: Suture Shuttling
The sutures shuttling process is divided in 4 phases with an inbound journey (Phase 1 and 2) and an outbound journey (Phase 3 and 4).
Phase 1
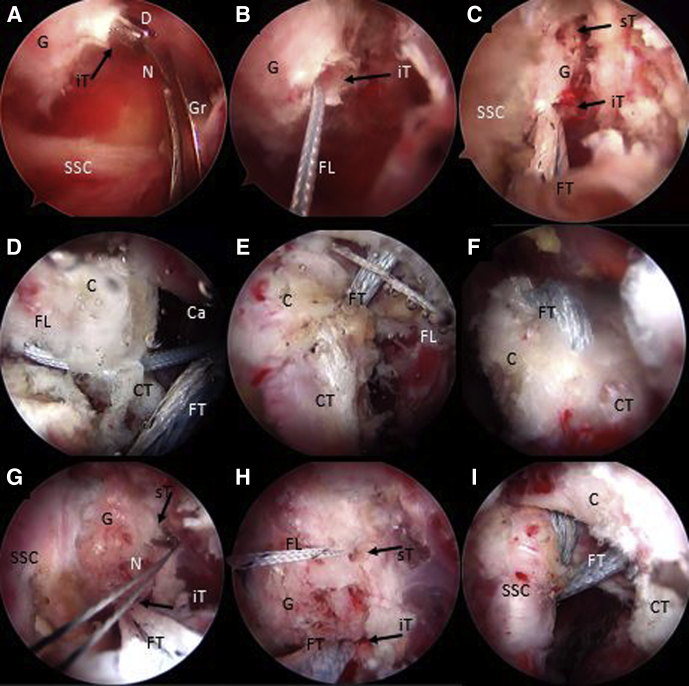
With the scope in the AS portal, the nitinol wire is retrieved through the subscapularis split and the cannula in the AI portal. It is replaced with a blue FiberLink, shuttled from posterior to anterior in the inferior glenoid tunnel, leaving the loop posteriorly (Fig 8 A and B). The FiberLinks sutures must be colored differently to facilitate suture management (blue FiberLink for the inferior tunnel, white FiberLink through the superior Tunnel). Two preconfigured FiberTape Cerclage sutures (FTC-s; Arthrex) are transported by pulling the blue FiberLink from posterior to anterior across the inferior glenoid tunnel passing through the subscapular split and retrieved through the canula at the AI portal (Fig 8C).
Fig 8.
Right shoulder, beach-chair position, arthroscopic view, 70° scope, anterosuperior portal. (A-C) With an arthroscopic grasper, a nitinol wire is retrieved from the inferior glenoid tunnel, through the subscapularis muscle split and the cannula in the anteroinferior portal. The nitinol is replaced with a FiberLink and then subsequently with the FiberTape from the posterior to the anterior side of the glenoid. (D-F) By pulling the blue distal coracoid FiberLink assembled with the FiberTape through the cannula outside the patient, these are shuttled from the inferior to the superior side of the coracoid (distal coracoid drill hole). In the same way, the white FiberLink is pulled through the cannula to shuttle the FiberTape from the superior to the inferior side of the coracoid process (proximal drill hole). (G-I) With an arthroscopic grasper, a nitinol wire is retrieved from the superior glenoid tunnel, through the subscapularis muscle split and the cannula in the anteroinferior portal. The nitinol is replaced with a FiberLink. Then the FiberLink, previously connected with the FiberTape outside the patient, is pulled from the cannula from the anterior side to the posterior side of the glenoid. (C, coracoid process; Ca, cannula; CT, conjoint tendon; D, cannulated drill; FL, FiberLink; FT, FiberTape; G, glenoid; Gr, arthroscopic grasper; iT, inferior glenoid tunnel; N, nitinol wire; SSC, subscapularis muscle; sT, superior glenoid tunnel.)
Phase 2
Afterward, the inferior looped end of the blue distal coracoid tunnel FiberLink is retrieved through the cannula and is used to shuttle the anterior limbs of the FTC-s from the inferior to the superior side of the coracoid and retrieved through coracoid portal CP (Fig 8D).
Phase 3
The inferior end of the white proximal coracoid tunnel FiberLink is retrieved through the cannula and used to shuttle incoming ends of the FTC-s from superior to inferior of the coracoid by pulling it through the canula at the AI portal (Fig 8 E and F). We must take into account not to cross the tapes during this step, leaving the proximal coracoid tapes freed to be transported to the superior glenoid tunnel in phase 4. We have to see how the FTC sutures are tensioned without slacks and in full contact with the coracoid between their tunnels.
Phase 4
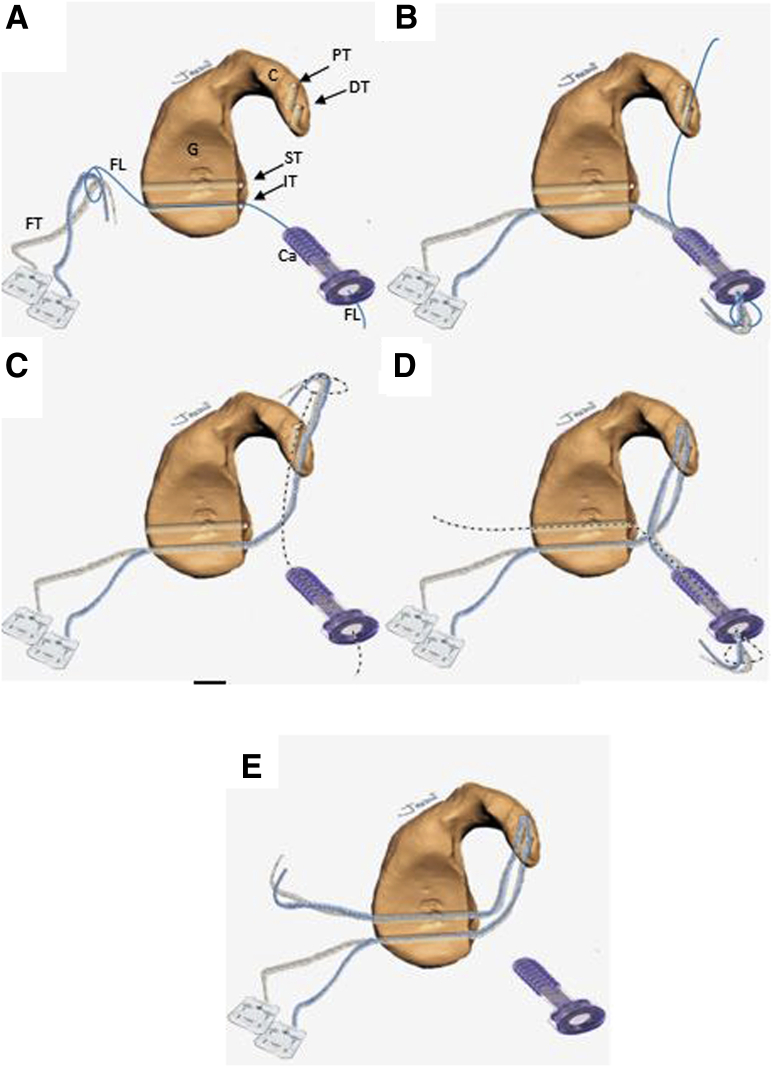
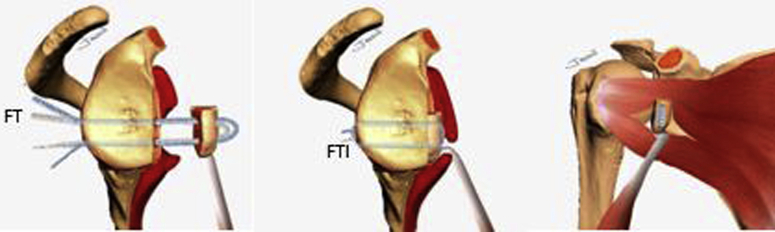
A second nitinol wire with the loop leading the way is passed from the posterior to the anterior side of the glenoid through the superior glenoid cannulated drill, which is then removed. The nitinol is then retrieved through the split in subscapularis muscle and the cannula at the AI portal (Fig 8G). The nitinol is replaced with a white FiberLink through the split of the subscapularis muscle from anterior to the posterior side of the glenoid with its loop left anteriorly (Fig 8H). This white FiberLink is used to transport the limbs of the anterior FTC-s from the cannula at the AI portal, through the subscapularis tendon split and the glenoid tunnel from anterior to posterior, completing the passage of the FTC-s around the glenoid and coracoid (Fig 8I). A schematic representation of FiberTapes suture shuttling is shown in Fig 9 A-E.
Fig 9.
Schematic representation of sutures shuttling technique through the glenoid and coracoid process sequentially from figure A to E. The inbound-outbound shuttling process is divided in 4 phases. The FiberTape must be equally tensioned at the same length in every shuttling phase. Inbound process: (A) Phase 1: From the posterior to the anterior side of the glenoid, the FiberTapes are transferred through the inferior glenoid tunnel, across the subscapularis muscle split and retrieved through the cannula at the anteroinferior portal. (B) Phase 2: The FiberTapes are transferer from the cannula through the distal coracoid tunnel from ventral to dorsal. Outbound process: (C) Phase 3: The FiberTapes are shuttled from the dorsal to the ventral side of the coracoid and retrieved through the cannula. (D) Phase 4: The FiberTapes are shuttled from the cannula through the subscapularis split and pulled from the anterior side to the posterior side of the glenoid. (E) Schematic representation of the final construct before performing the coracoid osteotomy. (C, coracoid; Ca, cannula; DT, distal coracoid tunnel; FT, FiberTape; FL, FiberLink; G, glenoid; IT, inferior glenoid tunnel; PT, proximal coracoid tunnel; ST, superior glenoid tunnel.)
Step 6. Cutting, Transportation, and Fixation of the Coracoid
As one looks through the AS portal and using a circular burr and a specific Arthrex chisel through the A portal, the coracoid is cut at its base (Fig 10A). The coracoid graft is transferred through the subscapularis split by pulling the FiberTape cerclage sutures from the posterior side of the glenoid, symmetrically, and simultaneously. It is important to pull on the sutures individually to avoid sutures slacks (Fig 10 B-D). The cerclage suture tapes are interconnected to each other using the preconfigured pre-tied knot in the Arthrex FiberTape cerclage sutures (Fig 11A). Manually, the knots are reduced and transferred to the posterior glenoid surface by pulling on each limb alternately and in a symmetrical manner. It is important to pull sequentially one by one on each end of the FTC-s separately to avoid the sutures blocking and interfering with the sliding of the knots.
Fig 10.
Right shoulder, beach-chair position, arthroscopic view, 70° scope, anterosuperior portal. An arthroscopic burr and chisel are used to osteotomized the coracoid, inserted through the anterior portal. (B-D) By Pulling the FiberTapes, the coracoid is transported through the subscapularis muscle split to the anterior glenoid neck. An arthroscopic grasper is used to facilitate the coracoid passage across the subscapularis split. (C, coracoid; CT, conjoint tendon; FT, FiberTape; Gr, grasper; O, chisel; SSC, subscapularis muscle.)
Fig 11.
Posterior extraarticular view of the right shoulder, beach-chair position. (A) The FiberTape Cerclage System is interconnected, and traction is performed subsequently one by one for each strand to fix the coracoid in the anterior glenoid neck. (B) A tensioner (Arthrex) is used to achieve a strong fixation of up to 100 N. (FT: FiberTape; P, posterior right shoulder; Pm, posteromedial portal; Pp, posterior portal; T, tensioner.).
The correct position and fixation of the coracoid process is checked under direct visualization. With a tensioner, each suture is tensioned separately up to 100 N (Fig 11B). The tensioner will help us to release the potential persistent slacks in the final construct. Finally, the system is locked with three alternating knots. The coracoid retractor is removed, and the subscapularis recovers its normal shape.
Step 7: Capsulolabral Complex Reconstruction
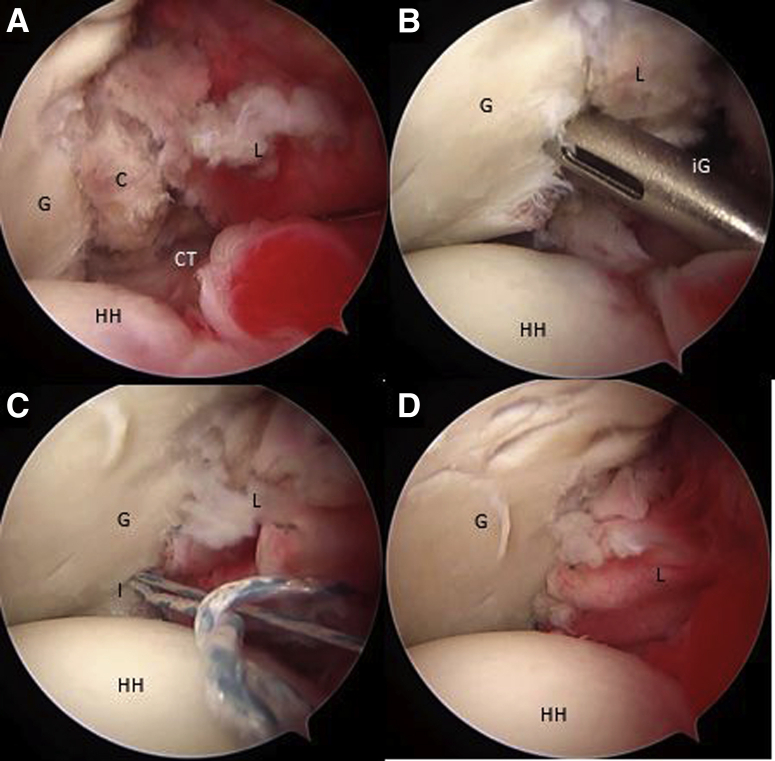
Reconstruction of the capsulolabral complex on the native anterior glenoid rim is achieved using 2 to 4 1.8-mm Knotless FiberTak anchors (Arthrex), leaving the coracoid in an extra-articular position (Fig 12 A-D). Schematic representation of final Latarjet cerclage construct is shown in Fig 13 A and B. The Tips and pitfalls of this technique are discussed in Table 2.
Fig 12.
(A-D) Right shoulder, beach-chair position, arthroscopic view, 70° scope, anterosuperior portal. The capsulolabral complex is repaired and fixed in the native anterior glenoid rim with 3 knotless FiberTak 1.8 soft anchors (Arthrex) from the anterior portal. (C, coracoid; G, glenoid; L, capsulolabral complex; HH, humeral head; I, implant; iG, implant guide.)
Fig 13.
Schematic representation of the final Latarjet cerclage construct. The circle-like configuration of the fixation can be noted. FiberTape cerclage sutures (FT), FiberTape interconnected (FTI).
Table 2.
Tips and Pitfalls of the Technique
| Tips |
|
|
|
|
|
|
|
| Pitfalls |
|
|
|
|
|
Postoperative Care
During the postoperative period, the shoulder is immobilized with a sling in a neutral rotation for 3 weeks. Pendulum and passive assisted flexion exercises, as well as isometric strengthening exercises of the deltoid and the scapular stabilizing musculature, are prescribed. Mobility exercises of the elbow and hand are encouraged. External rotation less than 20° in adduction is permitted (elbow to the side of the body).
At 3 weeks postoperatively, the sling is removed, and active assisted mobilization exercises are initiated. Progressive stretches in external rotation are started at 4 to 6 weeks postoperatively to achieve a complete range of movement. Muscle-strengthening exercises are further increased at 6 weeks postoperatively. Return to sports activities is allowed at 4 months postoperatively. Radiographic postoperative controls are performed at 3 and 6 weeks of follow-up with neutral AP and Bernageau views of the shoulder. The position of the coracoid process is assessed with an early postoperative computed tomography scan. The limitations and risks of this technique are discussed in Table 3.
Table 3.
Risks and Limitations of This Technique
|
|
|
Discussion
The Latarjet procedure was first published more than 60 years ago and since its first description, many modifications have been described.21 However, it was indeed professor Gilles Walch who popularized it as the most effective treatment and standard of care for recurrent anterior shoulder instabilities with anterior glenoid bone defect, humeral head defect, or bipolar lesions, with several reported good long-term results.2,22, 23, 24 It has been performed typically through an open approach, although there is currently a trend to perform it arthroscopically.
Arthroscopic Latarjet was first described by L. Lafosse in 2007.7 Subsequently, similar outcomes were published between open and arthroscopic techniques.8,9,25 Boileau et al. presented the Bankart–Bristow–Latarjet procedure (2B3) in 201026 were the coracoid is fixed with only one screw and the capsulolabral complex is repaired at the end of the procedure following the “triple locking effect” described by Patte and Debeyre.27 Despite the excellent clinical results published in the literature, up to 30% complications have been reported in both open and arthroscopic approaches, with up to 7% reoperation rates.11,12,16 Nevertheless, several complications related to metal implants are still a matter of concern.11 Moreover, a recent study of American football players showed that up to 46% of reoperations were related to fixation with metallic screws, either due to symptomatic hardware or malpositioned screws.14 Some screw-related complications may involve screw avulsion, twisting, or breakage of the humeral head, which can lead to early degenerative changes.12,28
Athwal et al.29 reported on 7% of complications related to problems with screw fixation that could potentially be decreased with the implement of suture-fixation. Other reported screw-related complications include capsular and subscapular or infraspinatus muscle irritation from prominent screws or iatrogenic nerve injury involving the suprascapular, musculocutaneous or cubital nerves due to the angle of drilling from anterior to poserior.9,30, 31, 32 Furthermore, failure of screw fixation may result from fractures through one or both drill holes, overtightening of the screws at the coracoid bone block or even from screw breakage in bone graft resorption or pseudarthrosis.9,33,34 Recent research shows how the open technique is more accurate to achieve a better coracoid graft position of the screws than the arthroscopic technique.35 Other systems have been used for coracoid process fixation such as metallic buttress plates, bioabsorbable screws, and cortical buttons. However, metallic buttress plates have been found to cause soft-tissue irritation.36 In the same way, fixation with bioabsorbable screws are not recommended because of a high osteolysis rate, up to 67% in a recent study versus 33% of metallic screws.34 Recently, Boileau et al.19 introduced the fixation with suspensory cortical buttons as an alternative to avoid the complications related to screw fixation in this procedure. After, Valenti et al.,37 also published a fully arthroscopic technique using 2 cortical buttons. Indeed, it has been proposed that elastic fixation, leads to healing and remodeling that cannot be achieved by more rigid fixation methods.38 Cortical buttons, however, despite of good preliminary results, has been associated with higher rates of recurrent dislocation.39,40
In fact, the use of only one cortical button could facilitate the rotation around the button, which could lead to a compromised the graft-healing process.41 We recently described an arthroscopically assisted Latarjet procedure without metal implants: The Latarjet Cerclage technique,20 in this technique, we used 4 high-strength suture tapes through a 2.4-mm tunnels, with an outbound journey and inbound journey, configuring them in a circle, connecting both tunnels with the same tapes, achieving a strong fixation construct, mimicking a compression plate between the coracoid process and the glenoid. This configuration seems stronger than a parallel independent double fixation suspensory system and could improve bone graft healing. This technique uses smaller tunnels than any other fully arthroscopically Latarjet procedure already published.7,19,37,41 The usage of smaller diameter tunnels allows us to diminish both glenoid and coracoid bone loss and consequently reduce the risk of fracture. The specific designed metallic hook and the drilling guide make this technique reproducible and potentially decreases the risk of malpositioning of the tunnels, resulting in accurate placement of the coracoid graft. Afterwards, we have been able to develop our metal-free fixation technique, in a completely arthroscopic way, which we present in this Technical Note.
This new fixation is presented as an alternative to other methods of fixation for arthroscopic Latarjet Procedure. With this technique, we can eliminate screw-related complications, image scattering, and soft-tissue impingement, using a completely arthroscopic approach, taking advantage of all the benefits of arthroscopic surgery compared with open surgery such as smaller scars, less bleeding, reduced risk of infection, and faster rehabilitation.42
In addition, we avoid using the supra-mammary portal required for the ideal direction of the coracoid-fixing screws by using the FiberTape cerclage sutures and a posterior-to-anterior glenoid drilling technique. We accomplished an all-arthroscopic Latarjet technique and capsulolabral complex reconstruction fixed with an FTC-s Arthrex and 2 to 4 knotless soft anchors. We believe a strong stable fixation is obtained with this technique while interconnecting and tying the 2 FiberTape sutures of the Cerclage System and tensioning the fixation up to 100 N with a specific tensioning device.
In conclusion, we believe that this technique may be the next step in arthroscopic coracoid-fixation methods. It maintains the biomechanical benefits of the Latarjet procedure, using smaller tunnels and obtaining a strong fixation with excellent control of rotational stability, without the complications associated with the use of metal components.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: R.B. reports grants from Acumed and personal fees from Smith & Nephew, Exactech, and Conmed, outside the submitted work. A.-I.H. reports personal fees from Arthrex, outside the submitted work. In addition, A.-I.H. has a patent Bone Block Cerclage pending. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
We present the first all arthroscopic metal-free Latarjet cerclage technique. The patient is positioned in the beach chair position. We use a 70° arthroscopic scope through the posterior portal and a radiofrequency device through the anterosuperior portal to release the rotator interval, the coracoacromial ligament, the clavipectoral fascia and the inferior, lateral and anterior border of the conjoined tendon. The pectoralis minor tendon detachment is performed from the coracoid portal. The coracoid’s inferior border is flattened with a rasp or burr introduced through the anterosuperior portal. The coracoid’s length is measured. With the help of a 7 mm offset-specific guide inserted through the coracoid portal, 2 tunnels are performed with two 2.4-mm cannulated drills. An 8.2- to 5-mm cannula is inserted through the anteroinferior portal for suture shuttling. Two nitinol wires are subsequently passed through the cannulated drills. The nitinol wires are retrieved one after the other through the cannula and switched with 2 high-strength FiberLink sutures. The capsulolabral complex is detached from 1 to 6 o’clock through the anterosuperior portal. The hook component of the glenoid drilling guide is passed through the standard posterior portal and engaged on the anterior glenoid rim. Two tunnels are then drilled through the glenoid with the same 2.4-mm cannulated drills previously used for the coracoid drilling. A Wissinger rod is passed from the posterior portal through the subscapularis muscle to determine the level of the horizontal split on its lower portion. With a radiofrequency device, a horizontal split is performed. A specific retractor is inserted through the coracoid portal. A nitinol wire is passed through the inferior glenoid cannulated drill and retrieved across the subscapularis split through the cannula. The nitinol wire is replaced by a blue FiberLink, shuttling it through the glenoid, leaving its loop posteriorly. Two preconfigured FiberTape Cerclage sutures are transported, by pulling the blue inferior glenoid tunnel FiberLink and retrieved through the cannula. The inferior end of the blue distal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid process, from ventral to dorsal. The white proximal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid, from dorsal to ventral. A second nitinol is passed through the superior glenoid cannulated drill. The nitinol is replaced with a white FiberLink, which is used to transport the FiberTape through the superior glenoid tunnel from anterior to posterior. Looking through the anterosuperior portal, we use an arthroscopic grasp and chisel through the anterior portal to cut the coracoid at its base. The coracoid graft is transferred through the subscapularis split by pulling on the FiberTapes sutures symmetrically. The cerclage tapes are interconnected and manually, the knots are reduced against the posterior glenoid. With a tensioner, each suture is tensioned separately and an 80-N secure fixation is achieved. Finally, the system is locked with 3 alternating knots. Reconstruction of the capsulolabral complex on the native anterior glenoid rim is achieved leaving the coracoid in an extra-articular position.
References
- 1.Latarjet M. Treatment of recurrent dislocation of the shoulder. Lyon Chir. 1954;49:994–997. [PubMed] [Google Scholar]
- 2.Hurley E.T., Jamal M.S., Ali Z.S., Montgomery C., Pauzenberger L., Mullett H. Long-term outcomes of the Latarjet procedure for anterior shoulder instability: A systematic review of studies at 10-year follow-up. J Shoulder Elbow Surg. 2019;28:e33–e39. doi: 10.1016/j.jse.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Young A.A., Maia R., Berhouet J., Walch G. Open Latarjet procedure for management of bone loss in anterior instability of the glenohumeral joint. J Shoulder Elbow Surg. 2011;20:S61–S69. doi: 10.1016/j.jse.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Provencher M.T., Bhatia S., Ghodadra N.S. Recurrent shoulder instability: Current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg A. 2010;92(suppl 2):133–151. doi: 10.2106/JBJS.J.00906. [DOI] [PubMed] [Google Scholar]
- 5.Provencher M.T., Ferrari M.B., Sanchez G., Anavian J., Akamefula R., Lebus G.F. Current treatment options for glenohumeral instability and bone loss. JBJS Rev. 2017;5:1–11. doi: 10.2106/JBJS.RVW.16.00091. [DOI] [PubMed] [Google Scholar]
- 6.Donohue M.A., Mauntel T.C., Dickens J.F. Recurrent shoulder instability after primary Bankart repair. Sports Med Arthrosc. 2017;25:123–130. doi: 10.1097/JSA.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 7.Lafosse L., Lejeune E., Bouchard A., Kakuda C., Gobezie R., Kochhar T. The arthroscopic Latarjet procedure for the treatment of anterior shoulder instability. Arthroscopy. 2007;23:1242.e1–1242.e5. doi: 10.1016/j.arthro.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Malahias M.A., Fandridis E., Chytas D., Chronopulos E., Brilakis E., Antonogiannakis E. Arthroscopic versus open Latarjet: A step-by-step comprehensive and systematic review. Eur J Orthop Surg Traumatol. 2019;29:957–966. doi: 10.1007/s00590-019-02398-3. [DOI] [PubMed] [Google Scholar]
- 9.Hurley E.T., Lim Fat D., Farrington S.K., Mullett H. Open versus arthroscopic latarjet procedure for anterior shoulder instability: A systematic review and meta-analysis. Am J Sports Med. 2019;47:1248–1253. doi: 10.1177/0363546518759540. [DOI] [PubMed] [Google Scholar]
- 10.Bedeir Y.H., Schumaier A.P., Grawe B.M. The failed Latarjet procedure. JBJS Rev. 2018;6:e10. doi: 10.2106/JBJS.RVW.18.00002. [DOI] [PubMed] [Google Scholar]
- 11.Domos P., Lunini E., Walch G. Contraindications and complications of the Latarjet procedure. Shoulder Elbow. 2018;10:15–24. doi: 10.1177/1758573217728716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griesser M.J., Harris J.D., McCoy B.W. Complications and re-operations after Bristow-Latarjet shoulder stabilization: A systematic review. J Shoulder Elbow Surg. 2013;22:286–292. doi: 10.1016/j.jse.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Lädermann A., Lubbeke A., Stern R., Cunningham G., Bellotti V., Gazielly D.F. Risk factors for dislocation arthropathy after Latarjet procedure: A long-term study. Int Orthop. 2013;37:1093–1098. doi: 10.1007/s00264-013-1848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBus G.F., Chahla J., Sanchez G. The Latarjet procedure at the national football league scouting combine: An imaging and performance analysis. Orthop J Sport Med. 2017;5:1–6. doi: 10.1177/2325967117726045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magone K., Luckenbill D., Goswami T. Metal ions as inflammatory initiators of osteolysis. Arch Orthop Trauma Surg. 2015;135:683–695. doi: 10.1007/s00402-015-2196-8. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A., Delaney R., Petkin K., Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8:59–66. doi: 10.1007/s12178-015-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boileau P., Mercier N., Roussanne Y., Thélu C.É., Old J. Arthroscopic Bankart-Bristow-Latarjet procedure: The development and early results of a safe and reproducible technique. Arthroscopy. 2010;26:1434–1450. doi: 10.1016/j.arthro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Lafosse L., Boyle S. Arthroscopic Latarjet procedure. J Shoulder Elbow Surg. 2010;19(suppl 2):2–12. doi: 10.1016/j.jse.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Boileau P., Gendre P., Baba M. A guided surgical approach and novel fixation method for arthroscopic Latarjet. J Shoulder Elbow Surg. 2016;25:78–89. doi: 10.1016/j.jse.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Hachem A.I., Costa D.’O.G., Rondanelli S.R., Rius X., Barco R. Latarjet cerclage: The metal-free fixation. Arthrosc Tech. 2020;9:e1397–e1408. doi: 10.1016/j.eats.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemmex D., Cárdenas G., Ricks M., Woodmass J., Chelli M., Boileau P. Arthroscopic management of anterior glenoid bone loss. JBJS Rev. 2020;8 doi: 10.2106/JBJS.RVW.19.00049. [DOI] [PubMed] [Google Scholar]
- 22.Walch G., Boileau P. Latarjet-Bristow procedure for recurrent anterior instability. Tech Shoulder Elbow Surg. 2000;1:256–261. [Google Scholar]
- 23.Mizuno N., Denard P.J., Raiss P., Melis B., Walch G. Long-term results of the Latarjet procedure for anterior instability of the shoulder. J Shoulder Elbow Surg. 2014;23:1691–1699. doi: 10.1016/j.jse.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Yang J.S., Mazzocca A.D., Cote M.P., Edgar C.M., Arciero R.A. Recurrent anterior shoulder instability with combined bone loss. Am J Sports Med. 2015;44:922–932. doi: 10.1177/0363546515623929. [DOI] [PubMed] [Google Scholar]
- 25.Buckup J., Leuzinger C.S.D.S.J. Functional outcome and return to sports after the arthroscopic Latarjet procedure in young and physically active patients. Arch Orthop Trauma Surg. 2020;140:1487–1494. doi: 10.1007/s00402-020-03513-4. [DOI] [PubMed] [Google Scholar]
- 26.Boileau P., Mercier N., Old J. Arthroscopic bankart-bristow-latarjet (2B3) procedure: How to do it and tricks to make it easier and safe. Orthop Clin North Am. 2010;41:381–392. doi: 10.1016/j.ocl.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Patte D., Debeyre J. Recurrent dislocation of the shoulder. Encycl Med Chir Paris Tech Chir Orthop. 1980;(44-02):44265. [Google Scholar]
- 28.Butt U., Charalambous C.P. Complications associated with open coracoid transfer procedures for shoulder instability. J Shoulder Elbow Surg. 2012;21:1110–1119. doi: 10.1016/j.jse.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Athwal G.S., Meislin R., Getz C., Weinstein D., Favorito P. Short-term complications of the arthroscopic Latarjet procedure: A North American experience. Arthroscopy. 2016;32:1965–1970. doi: 10.1016/j.arthro.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Lädermann A., Denard P.J., Burkhart S.S. Injury of the suprascapular nerve during latarjet procedure: An anatomic study. Arthroscopy. 2012;28:316–321. doi: 10.1016/j.arthro.2011.08.307. [DOI] [PubMed] [Google Scholar]
- 31.Lafosse T., Amsallem L., Delgrande D., Gerometta A., Lafosse L. Arthroscopic screw removal after arthroscopic Latarjet procedure. Arthrosc Tech. 2017;6:e559–e566. doi: 10.1016/j.eats.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shishido H., Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: An anatomic study. J Shoulder Elbow Surg. 2001;10:372–376. doi: 10.1067/mse.2001.115988. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery S.R., Katthagen J.C., Mikula J.D. Anatomic and biomechanical comparison of the classic and congruent-arc techniques of the Latarjet procedure. Am J Sports Med. 2017;45:1252–1260. doi: 10.1177/0363546516685318. [DOI] [PubMed] [Google Scholar]
- 34.Weppe F., Magnussen R.A., Lustig S., Demey G., Neyret P., Servien E. A biomechanical evaluation of bicortical metal screw fixation versus absorbable interference screw fixation after coracoid transfer for anterior shoulder instability. Arthroscopy. 2011;27:1358–1363. doi: 10.1016/j.arthro.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 35.Minuesa-Asensio A., García-Esteo F., Mérida-Velasco J.R. Comparison of coracoid graft position and fixation in the open versus arthroscopic Latarjet techniques: A cadaveric study. Am J Sports Med. 2020;48:2105–2114. doi: 10.1177/0363546520930419. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhary D., Goyal A., Joshi D., Jain V., Mohindra M., Mehta N. Clinical and radiological outcome after mini-open Latarjet technique with fixation of coracoid with Arthrex wedge mini-plate. J Clin Orthop Trauma. 2016;7:23–29. doi: 10.1016/j.jcot.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valenti P., Maroun C., Wagner E., Werthel J.D. Arthroscopic Latarjet Procedure combined with Bankart repair: A technique using 2 cortical buttons and specific glenoid and coracoid guides. Arthrosc Tech. 2018;7:e313–e320. doi: 10.1016/j.eats.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J., Liu H., Lu W. Modified arthroscopic Latarjet procedure: Suture-Button fixation achieves excellent remodeling at 3-year follow-up. Am J Sports Med. 2020;48:39–47. doi: 10.1177/0363546519887959. [DOI] [PubMed] [Google Scholar]
- 39.Metais P., Clavert P., Barth J. Preliminary clinical outcomes of Latarjet-Patte coracoid transfer by arthroscopy vs. open surgery: Prospective multicentre study of 390 cases. Orthop Traumatol Surg Res. 2016;102:S271–S276. doi: 10.1016/j.otsr.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Hardy A., Sabatier V., Schoch B. Latarjet with cortical button fixation is associated with an increase of the risk of recurrent dislocation compared to screw fixation. Knee Surg Sport Traumatol Arthrosc. 2020;28:2354–2360. doi: 10.1007/s00167-019-05815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benedetto M., De, Galasso O. Arthroscopic Latarjet procedure: A technique using double round ENDOBUTTONs and specific glenoid and coracoid guides. Arthrosc Tech. 2020;9:e995–e1001. doi: 10.1016/j.eats.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah A.A., Butler R.B., Romanowski J., Goel D., Karadagli D., Warner J.J.P. Short-term complications of the Latarjet procedure. J Bone Joint Surg A. 2012;94:495–501. doi: 10.2106/JBJS.J.01830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We present the first all arthroscopic metal-free Latarjet cerclage technique. The patient is positioned in the beach chair position. We use a 70° arthroscopic scope through the posterior portal and a radiofrequency device through the anterosuperior portal to release the rotator interval, the coracoacromial ligament, the clavipectoral fascia and the inferior, lateral and anterior border of the conjoined tendon. The pectoralis minor tendon detachment is performed from the coracoid portal. The coracoid’s inferior border is flattened with a rasp or burr introduced through the anterosuperior portal. The coracoid’s length is measured. With the help of a 7 mm offset-specific guide inserted through the coracoid portal, 2 tunnels are performed with two 2.4-mm cannulated drills. An 8.2- to 5-mm cannula is inserted through the anteroinferior portal for suture shuttling. Two nitinol wires are subsequently passed through the cannulated drills. The nitinol wires are retrieved one after the other through the cannula and switched with 2 high-strength FiberLink sutures. The capsulolabral complex is detached from 1 to 6 o’clock through the anterosuperior portal. The hook component of the glenoid drilling guide is passed through the standard posterior portal and engaged on the anterior glenoid rim. Two tunnels are then drilled through the glenoid with the same 2.4-mm cannulated drills previously used for the coracoid drilling. A Wissinger rod is passed from the posterior portal through the subscapularis muscle to determine the level of the horizontal split on its lower portion. With a radiofrequency device, a horizontal split is performed. A specific retractor is inserted through the coracoid portal. A nitinol wire is passed through the inferior glenoid cannulated drill and retrieved across the subscapularis split through the cannula. The nitinol wire is replaced by a blue FiberLink, shuttling it through the glenoid, leaving its loop posteriorly. Two preconfigured FiberTape Cerclage sutures are transported, by pulling the blue inferior glenoid tunnel FiberLink and retrieved through the cannula. The inferior end of the blue distal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid process, from ventral to dorsal. The white proximal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid, from dorsal to ventral. A second nitinol is passed through the superior glenoid cannulated drill. The nitinol is replaced with a white FiberLink, which is used to transport the FiberTape through the superior glenoid tunnel from anterior to posterior. Looking through the anterosuperior portal, we use an arthroscopic grasp and chisel through the anterior portal to cut the coracoid at its base. The coracoid graft is transferred through the subscapularis split by pulling on the FiberTapes sutures symmetrically. The cerclage tapes are interconnected and manually, the knots are reduced against the posterior glenoid. With a tensioner, each suture is tensioned separately and an 80-N secure fixation is achieved. Finally, the system is locked with 3 alternating knots. Reconstruction of the capsulolabral complex on the native anterior glenoid rim is achieved leaving the coracoid in an extra-articular position.
We present the first all arthroscopic metal-free Latarjet cerclage technique. The patient is positioned in the beach chair position. We use a 70° arthroscopic scope through the posterior portal and a radiofrequency device through the anterosuperior portal to release the rotator interval, the coracoacromial ligament, the clavipectoral fascia and the inferior, lateral and anterior border of the conjoined tendon. The pectoralis minor tendon detachment is performed from the coracoid portal. The coracoid’s inferior border is flattened with a rasp or burr introduced through the anterosuperior portal. The coracoid’s length is measured. With the help of a 7 mm offset-specific guide inserted through the coracoid portal, 2 tunnels are performed with two 2.4-mm cannulated drills. An 8.2- to 5-mm cannula is inserted through the anteroinferior portal for suture shuttling. Two nitinol wires are subsequently passed through the cannulated drills. The nitinol wires are retrieved one after the other through the cannula and switched with 2 high-strength FiberLink sutures. The capsulolabral complex is detached from 1 to 6 o’clock through the anterosuperior portal. The hook component of the glenoid drilling guide is passed through the standard posterior portal and engaged on the anterior glenoid rim. Two tunnels are then drilled through the glenoid with the same 2.4-mm cannulated drills previously used for the coracoid drilling. A Wissinger rod is passed from the posterior portal through the subscapularis muscle to determine the level of the horizontal split on its lower portion. With a radiofrequency device, a horizontal split is performed. A specific retractor is inserted through the coracoid portal. A nitinol wire is passed through the inferior glenoid cannulated drill and retrieved across the subscapularis split through the cannula. The nitinol wire is replaced by a blue FiberLink, shuttling it through the glenoid, leaving its loop posteriorly. Two preconfigured FiberTape Cerclage sutures are transported, by pulling the blue inferior glenoid tunnel FiberLink and retrieved through the cannula. The inferior end of the blue distal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid process, from ventral to dorsal. The white proximal coracoid tunnel FiberLink is used to shuttle the FiberTapes through the coracoid, from dorsal to ventral. A second nitinol is passed through the superior glenoid cannulated drill. The nitinol is replaced with a white FiberLink, which is used to transport the FiberTape through the superior glenoid tunnel from anterior to posterior. Looking through the anterosuperior portal, we use an arthroscopic grasp and chisel through the anterior portal to cut the coracoid at its base. The coracoid graft is transferred through the subscapularis split by pulling on the FiberTapes sutures symmetrically. The cerclage tapes are interconnected and manually, the knots are reduced against the posterior glenoid. With a tensioner, each suture is tensioned separately and an 80-N secure fixation is achieved. Finally, the system is locked with 3 alternating knots. Reconstruction of the capsulolabral complex on the native anterior glenoid rim is achieved leaving the coracoid in an extra-articular position.