Abstract
New therapies offer hope for a cure to millions of persons living with hepatitis C virus (HCV) infection. HCV elimination is a global goal that will be difficult to achieve using the traditional paradigms of diagnosis and care. The current standard has evolved toward universal HCV screening and treatment, to achieve elimination goals. There are several steps between HCV diagnosis and cure with major barriers along the way. Innovative models of care can address barriers to better serve hardly reached populations and scale national efforts in the United States and abroad. Herein, we highlight innovative models of HCV care that aid in our progress toward HCV elimination.
Abbreviations
- AIDS
acquired immune deficiency syndrome
- CDC
Centers for Disease Control and Prevention
- DAA
direct acting antiviral
- DBS
dried blood spot
- ECHO
Extension for Community Healthcare Outcomes
- EHR
electronic health record
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injection drug use
- OAT
opioid agonist therapy
- OUD
opioid use disorder
- NYC
New York City
- POC
point of care
- PWID
persons who inject drugs
- SEP
syringe exchange program
- SVR
sustained virological response
- TCC
transitional care coordination
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Hepatitis C virus (HCV) infection is a major public health threat worldwide, with approximately 71 million people living with chronic infection.( 1 , 2 ) The approval of direct‐acting antivirals (DAAs) starting in 2014 revolutionized treatment and allows nearly all patients to be cured.( 3 ) The number of individuals initiating HCV treatment has increased from approximately 500,000 in 2014 to over 2 million in 2017.( 4 ) In 2016, the World Health Organization called for HCV to be eliminated as a global public health threat by 2030, setting a goal of reducing new infections by 90%, treating 80% of chronic infections, and reducing mortality by 65%.( 5 )
However, few countries are on track to reaching these HCV elimination targets. Globally, only 19% of chronically infected individuals report being aware of their infection, and 15.3% had been treated with DAAs by the end of 2017.( 1 ) In the United States, HCV remains the most common bloodborne infection, affecting 2 million people,( 2 ) and in 2016, more than half of individuals reported being unaware of their infection.( 6 ) HCV‐related mortality continues to rise, surpassing the combined total of 60 other nationally notifiable infectious conditions, including human immunodeficiency virus (HIV).( 2 , 7 , 8 , 9 ) The United States Preventive Services Task Force, the American Association for the Study of Liver Diseases (AASLD), the Infectious Diseases Society of America (IDSA), and Centers for Disease Control and Prevention (CDC) recently updated their guidelines to recommend universal HCV testing among adults.( 10 , 11 )
The 2020 standard of HCV care has evolved toward universal screening and treatment.( 12 ) However, there is currently a considerable drop‐off between each step of the HCV “cascade to cure,” from screening, diagnosis, evaluation, treatment, cure, prevention of reinfection, and care for cirrhosis (Fig. 1).( 13 ) Low rates of diagnosis result in even lower rates of treatment and ultimately cure. Innovation can help address major barriers in these steps to move us toward HCV elimination (Fig. 2). In this review, we focus on a combination of barriers at the system, provider, and patient level, with an emphasis on how system‐level and provider‐level enhancements are critical in overcoming what have traditionally been deemed patient‐level barriers. Since the interferon era, there has been focus on persons living with HCV in silos, some with blame and consequently stigma for their behaviors, when it is system‐level and provider‐level policies and practices that have presented as barriers that need to be addressed. Implementing interventions tailored toward “hardly reached” populations, the micro‐elimination approach, is a key strategy for achieving HCV elimination.( 14 , 15 ) They complement population‐level macro‐elimination programs. Herein, we highlight interventions that address the HCV cascade to cure in hardly reached populations, including (1) persons who inject drugs (PWIDs) and persons who are marginally housed; (2) correctional populations; and (3) women who are pregnant (Table 1). We hope readers can conceptualize members of these groups as being underserved by traditional engagement efforts, rather than as people with inherent qualities that make them challenging to engage and treat. We also discuss broader efforts to use innovation to eliminate HCV across health systems and countries. The interventions in this review specifically improve screening, case finding, linkage to care (broadly defined as strategies that lead to access to HCV care), treatment delivery and/or adherence, and cure.
Fig. 1.
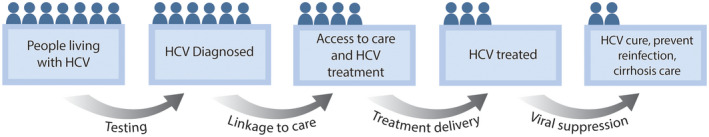
HCV cascade to cure. There are currently many steps, with considerable drop‐off between each step, along the HCV cascade to cure, from diagnosis, access to care, treatment, cure, prevention of reinfection, and care for cirrhosis. More effective strategies to overcome barriers around testing, linkage to care, treatment delivery, and viral suppression are needed to successfully move more people living with HCV from one step to the next to achieve the goal of HCV elimination. Printed with permission © Mount Sinai Health System.
Fig. 2.
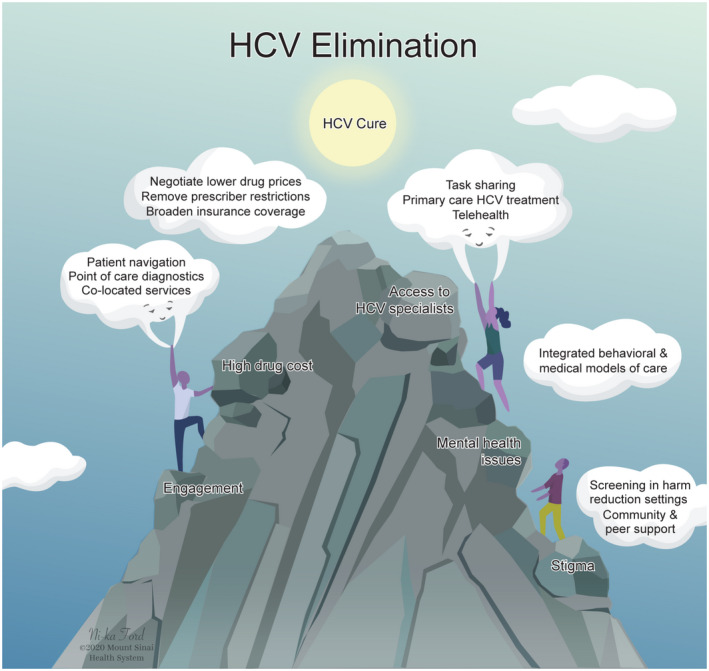
Barriers to HCV elimination can be overcome by innovation. System‐level innovations of HCV care can overcome gaps in care from testing, linkage to care, treatment, and cure. Barriers such as access to HCV specialists can be overcome by task sharing with a multidisciplinary team–based approach to care, removal of specialist restrictions by payers, coupled with task shifting to primary care and use of telehealth. Printed with permission © Mount Sinai Health System.
Table 1.
Innovation in HCV Screening, Linkage to Care, and Treatment
| Population or Setting | Key Elements |
|---|---|
| Global innovations in micro‐elimination | |
| PWIDs |
|
| Correctional facilities |
|
| Women who are pregnant |
|
| US macro‐elimination | |
| Community |
|
| Academic health systems |
|
| VHA |
|
| Global innovations in macro‐elimination | |
|
|
Innovations in Micro‐Elimination
PWIDs and Persons Who Are Marginally Housed
In developed countries, injection drug use (IDU) is the primary route of HCV transmission; 67% of PWIDs are estimated to be infected globally.( 16 , 17 , 18 , 19 , 20 ) IDU contributes to HCV transmission among homeless adults and people living with HIV/acquired immune deficiency syndrome (AIDS), two prominent hardly reached populations.( 21 , 22 ) Harm reduction interventions such opioid agonist therapy (OAT), encompassing methadone and buprenorphine, and syringe exchange programs (SEPs) can modestly reduce HCV transmission and provide a foundation for innovative models.( 23 , 24 , 25 , 26 ) OAT, essential treatment for opioid use disorder (OUD), can reduce HCV incidence and reinfection and facilitate treatment delivery and cure, along with decreasing opioid‐related morbidity and mortality.( 27 ) Major barriers to HCV cure in these populations are system‐level and provider‐level, including a lack of integrated/co‐located care models, low‐threshold patient‐centered care, and discrimination.
The traditional approach to HCV diagnosis requires a two‐step process. The first step involves venipuncture for screening with an antibody test, which if reactive, is followed by a second venipuncture for diagnostic confirmation of viremia, usually using a polymerase chain reaction–based assay to quantify the level of HCV RNA. This traditional two‐step process can take several days or weeks and lead to drop‐off, thereby decreasing the number of cases diagnosed. To address the gap between screening and diagnostic testing,( 13 ) where follow‐up is particularly challenging in PWID and homeless populations, novel diagnostics with point‐of‐care (POC) and rapid diagnostic tests have been developed (Table 2). Rapid POC‐HCV antibody tests can offer results in minutes, and reflex HCV‐RNA testing (a process in which a single venous sample that is found to have a reactive HCV antibody result can be tested for HCV RNA, bypassing a second venipuncture) has been more widely implemented in various settings. Additionally, HCV‐RNA diagnostic testing can be reflexed with genotype testing. Innovation in reflex RNA testing with dried blood spot (DBS) sampling and POC‐RNA testing with tests like Xpert (Xpert Solutions, Aliso Viejo, CA) have further facilitated screening and diagnosis. These have been successfully evaluated in studies of PWIDs in Australia and Spain.( 28 , 29 ) In the study from Spain, investigators found that POC and DBS were 98.4% and 93.7% sensitive for detecting HCV RNA with venous plasma samples as the gold‐standard comparison. Eighty percent of patients received their POC testing results on the same day, which was preferred by most participants.( 28 ) The feasibility of self‐sampling DBS at home is currently being evaluated.( 30 ) The novel approach of POC‐RNA testing as one‐step, rapid screening in high‐prevalence PWID populations coupled with rapid (same‐day) DAA initiation are underway in Australia (ClinicalTrials.gov, NCT03492112).
Table 2.
Innovation in HCV Diagnostics
| Test or Approach | Details and Process | Time to Result | Pros | Cons |
|---|---|---|---|---|
| Reflex HCV RNA | Serum (venipuncture), drawn with HCV antibody testing | Hours to days |
|
|
| DBS RNA | Fingerstick for whole blood, applied to a filter paper card, stable at room temperature, allow card to dry for up to 24 hours and send to lab | Days to week(s) |
|
|
| POC antibody | Blood/serum/oral | ~20 minutes |
|
|
| POC RNA | Drop of whole blood or serum, individual cartridges for blood collection, automated nucleic acid extraction and amplification | ~105 minutes | Reduce time to confirmed diagnosis |
|
Following diagnosis of HCV, there is a drop‐off particularly in PWID populations before they are linked to care, because off‐site referral to subspecialty care has not been effective in this population.( 31 )
Advancements in system‐level interventions to provide an “under one roof” approach can facilitate HCV‐related outcomes. Integration of telemedicine at SEPs has also broadened access to HCV treatment by bypassing off‐site referrals while enabling specialist involvement.( 32 ) Under‐one‐roof test and treat models in community settings facilitate linkage to care when integrated into or co‐located with drug, alcohol and psychiatric services, thereby improving access to HCV care. Strategies that reduce unnecessary obstacles to medication, such as urine drug testing, and promote access to stable housing can also improve medication adherence.( 33 , 34 ) These illustrate the impact of services that address social determinants of health, to improve long‐term outcomes in this population.( 35 ) Notable features of test‐and‐treat models include task sharing (i.e., team‐based approach), decentralization of services (i.e., moving testing and treatment out of tertiary or specialist practices to local‐level care), and a multidisciplinary approach. Two successful examples are Project ITTREAT (Integrated Community Test–Stage–TREAT), which is based at a drug and alcohol treatment center in the United Kingdom, and the Cool Aid Community Health Center in British Columbia.( 36 , 37 ) Both test‐and‐treat models incorporate task sharing with a nurse coordinator, who integrates all components of the cascade of care and provides services to address barriers caused by low income, insufficient housing, and mental health. In Project ITTREAT, care was directed by an experienced hepatitis nurse and clinics were designed in a “drop‐in” style where mental health, medication for OUD, social support, and SEPs were available. Nearly all 125 patients with a detectable HCV‐RNA test who were deemed eligible for treatment received and completed therapy (98%), with a sustained virological response (SVR) rate of almost 90%. The Cool Aid Community Health Center features a similar medical home and multidisciplinary team model that uses multiple strategies to improve access to care, including easier self‐referral, creating “Liver Days” to consolidate treatment and counseling, creating integrated treatment plans, and having an on‐site pharmacy team be available to administer medications and answer questions. By using a test‐and‐treat model, this clinic consistently achieved SVR rates of 72%‐88%, even during the pre‐DAA era. Other interventions that improve linkage to care include colocation, peer‐based HCV support groups, contingency management, and use of financial incentives.( 38 , 39 , 40 ) In PWID populations, a randomized controlled trial in Australia and New Zealand demonstrated that HCV treatment outcomes for PWIDs were superior in a primary care setting compared with hospital‐based specialty care, supporting the expansion of treatment to primary care providers.( 41 ) Models of HCV care in harm‐reduction programs have also leveraged telemedicine successfully in PWID populations.( 32 ) Colocation of treatment, primary‐based and community‐based care models, and peer‐based support have also been demonstrated to be effective in patients who are homeless and underhoused.( 42 , 43 , 44 , 45 ) Other facilitators include simplified treatment regimens, shortened treatment, elimination of response‐guided treatment, and availability of blood tests to estimate fibrosis rather than sending patients off site for imaging.
There is strong evidence of high cure rates and low rates of reinfection (1%‐5% per year) in PWIDs.( 46 , 47 , 48 , 49 ) Re‐infection, while low, is more common among individuals with ongoing IDU after DAA therapy.( 50 ) Engagement in OAT reduces re‐infection risk, and re‐infection should not be used as a reason to withhold therapy from people with ongoing IDU. Modeling studies suggest that HCV prevalence decreases in PWIDs even when treating 10 infections per 10,000, even if risks of re‐infection are higher and SVR lower.( 51 , 52 ) Additionally prompt retreatment and rapid scale‐up can reduce reinfection rates. Another method that may reduce HCV incidence is treatment of PWIDs through social networks.( 53 ) In one study, investigators modeled the effect of different treatment strategies on long‐term HCV prevalence in an Australian cohort of PWIDs. This transmission model found that a strategy of treatment of contacts within a patient’s social network (“treat your friends”) versus treatment of random patients leads to a drop in HCV prevalence over 10 years. For this reason, treatment as prevention warrants further study as an innovative model.
Correctional Populations
Seroprevalence of HCV is estimated to be 16.1% for US inmates, with 10.7% having a confirmed infection using RNA testing.( 2 ) Opportunities for initiating the HCV cascade differ between high‐turnover jails (mean stay of 25 days( 54 )) and longer‐term prisons (stays typically longer than 1 year), as well as access to OAT, which can assist with primary prevention.( 55 ) Jails may be primed for innovations in rapid screening and linkage to care, although jail‐release patterns suggest that a tailored approach is needed, given heterogeneity in length of stay.( 42 , 56 ) Conversely, the defined length of time in prison populations affords the opportunity to complete HCV treatment in a highly supervised environment.
The Federal Bureau of Prisons currently recommends “opt‐out” HCV testing for all inmates, which is informed decline of testing rather than informed consent( 43 ); however, only 13 state prison systems currently offer routine HCV screening,( 44 ) and just 4% of jails reported routine screening in a 2012 survey.( 45 ) Several factors contribute to low screening rates. By US Supreme Court case law, prisons and jails must act on diagnosed diseases.( ) Given the difficulty in linking prisoners to care and the high price of DAAs, correctional systems have a disincentive to detect HCV infections, despite new evidence demonstrating the cost effectiveness of a test‐all, treat‐all linkage to care at release approach in this population.( 57 , 58 ) Some jails have implemented opt‐out HCV screening. Dallas County Jail transitioned from targeted and by‐request screening to opt‐out testing in 2015. Testing for both HIV and HCV antibodies was offered during the intake process and every phlebotomy, increasing HCV testing from 12.9% to 80.5% within a year.( 59 ) Implementation of reflex HCV‐RNA testing with antibody screening further streamlined diagnosis.( 60 )
A comprehensive approach can couple screening with linkage to care. Drawing on a nationally recognized linkage‐to‐care program called transitional care coordination (TCC),( 61 ) which provides medical case management, counseling, and comprehensive discharge planning for incarcerated people living with HIV/AIDS, Akiyama et al. developed a similar program for inmates with reported HCV mono‐infection at three New York City (NYC) jails and one hospital correctional health ward.( 62 ) Patients were contacted by a coordinator during or after incarceration and followed for 180 days. The coordinator scheduled appointments, made reminder calls, accompanied patients to appointments, and offered free public transit passes. Of 84 inmates, 31% attended an HCV appointment, 18% completed treatment, and 8% achieved SVR. Strengthening and adapting the TCC strategy, such as with peer navigation, may improve effectiveness.( 63 )
There have also been increased models of patients being treated on site in correctional facilities.( 64 , 65 ) Telemedicine has emerged as an promising model of HCV treatment in prisons, obviating challenges associated with transporting prisoners to off‐site clinics.( 66 ) Efficacy of telemedicine in HCV treatment was demonstrated beginning during the pre‐DAA era through Project ECHO (Extension for Community Healthcare Outcomes; discussed subsequently) and when the Virginia Department of Corrections partnered with an academic medical center to treat 59 inmates. Patients achieved comparable or better rates of SVR than published literature at the time.( 67 ) A similar study conducted in Australia during the pre‐DAA era found high SVR rates when HCV treatment was comanaged by nurses and medical staff.( 68 ) A telemedicine‐based DAA treatment program in a large Spanish prison recently achieved prison HCV elimination (n = 131) over 3 years, further demonstrating the promise of this approach in correctional populations.( 69 ) Project ECHO has partnered specialists from the University of New Mexico with providers working in the New Mexico Corrections Department since 2003, to offer treatment on site, and has continued to have success during the DAA era.( 70 )
Women Who Are Pregnant
In the United States, the number of HCV cases in women of reproductive age (WORA) doubled between 2006 and 2014.( 71 ) In 2014, the prevalence of HCV in the US pregnant population was estimated to be 0.73%, suggesting that 29,000 HCV‐infected women gave birth.( 71 ) This population has unique opportunities and challenges. Pregnancy and the postpartum period may be the only time women in the United States have access to health insurance, presenting an opportunity to screen and treat.
Routine screening of women who are pregnant for HCV infection offers significant advantages over risk‐based screening, which is poorly implemented by clinicians and inadequately sensitive.( 72 , 73 , 74 , 75 , 76 , 77 ) In 2018, the AASLD and IDSA updated guidelines, based on expert opinion, recommending universal HCV screening with each pregnancy,( 78 ) with the CDC following suit in 2020, except in settings where HCV prevalence is less than 0.1%.( 79 ) Routine HCV antepartum screening is under review by the American College of Obstetricians and Gynecologists.( 80 ) In the DAA era, universal screening appears to be cost‐effective.( 81 , 82 )
HCV infection in pregnancy in the United States is a marker for potential IDU in 2020. Women who are pregnant may not want to be screened for reasons that include stigma, involvement of state agencies with risk of women losing their children, and criminalization of substance use in pregnancy. The critical intervention is health care and support for the woman living with OUD, with treatment to prevent and/or reduce IDU‐related morbidity or mortality, and to ameliorate drug‐related harms to a developing fetus. OAT is indicated for women who are pregnant and is a great medical priority.
In general, linkage to HCV care is low( 76 , 77 ) in women who are pregnant, and treatment‐completion( 83 ) rates postpartum are even lower. Retrospective case finding and linkage to care using public health records may improve postpartum cure rates. Using Tennessee’s National Electronic Disease Surveillance System Base System to identify recent, probable, or confirmed HCV cases in WORA, researchers found that 30% of women were pregnant at time of diagnosis of HCV exposure, and 28% of women with contact information responded by telephone to a mailed inquiry, resulting in nearly 300 women receiving posttest counseling with referral to the local health department for confirmatory HCV testing or to a patient navigator for HCV care.( 84 )
Other than preventing long‐term complications of advanced liver disease, one major goal of HCV treatment is reducing the risk of perinatal transmission. Rates of infection seen in partners of pregnant women in one Swedish cohort study also suggest a broader benefit to knowing one’s status and considering treatment.( 85 ) Currently, treatment of HCV with DAAs is only recommended after breastfeeding is completed. The postpartum period can include loss of health insurance, and mothers may be lost to follow‐up. HCV treatment during pregnancy may increase uptake and adherence; however, more evidence is needed to establish safety. A phase 1 study with ledipasvir/sofosbuvir therapy in pregnancy was recently completed. The preliminary results indicate that all 8 women achieved SVR and delivered at term with no safety concerns identified to date.( 86 , 87 ) A similar phase 1 study of sofosbuvir/velpatasvir treatment in women who are pregnant will start enrolling soon.( 88 ) A researcher in India treated 15 pregnant women with ledipasvir/sofosbuvir, with no identified safety concerns and 100% SVR.( )
Innovations in Macro‐elimination in the United States
The US Health and Human Services has developed a National Viral Hepatitis Action Plan and is tracking local progress.( 89 , 90 ) Several states (13 at the time of this publication) and cities have developed strategies to implement HCV‐elimination programs by building innovative coalitions of community advocates, social service providers, researchers, legal experts, and government representatives.( 91 ) In this section we will highlight a number of the innovations in the United States. While not exhaustive, the aim is to highlight innovations on the community level, within academic health centers and through the national Veterans Health Administration (VHA) system.
US Community‐Based Programs
Novel studies have explored HCV screening and linkage‐to‐care programs in community and nonclinical settings, to help reach hardly reached populations. Many HCV infections in the United States cluster geographically, with higher prevalence in specific regions with significant socioeconomic, racial/ethnic, and educational disparities seen in both rural and urban areas.( 92 ) Community‐based HCV screening and linkage‐to‐care interventions that are geographically focused may help identify people living with HCV, similar to HIV,( 93 , 94 ) and help overcome barriers to care.
The Project ECHO model was initially developed to provide access to HCV care in rural areas with severe shortages of specialty providers and in prisons. Core innovative features include task sharing, integrating primary care with specialty and behavioral health, and telemedicine to expand HCV provider capacity. In this model, primary care providers were mentored by a multidisciplinary team of experts at the University of New Mexico via telemedicine during weekly clinics, where community providers presented cases. Collaboration was built under the principles of (1) longitudinal co‐management of patients with specialists, (2) shared case‐management decision making with other providers in the network, and (3) short didactic presentations. Community providers who were able to manage 20 patients in a year and complete the necessary training were deemed ready to treat patients independently. The ECHO model was piloted in a prospective study,( 70 ) conducted in the pre‐DAA era between 2004 and 2008. The study compared the standard of care of in‐person HCV treatment with treatment by primary care providers at 21 ECHO telemedicine visits in rural areas and prisons. Of the 407 patients with HCV enrolled and treated, similar SVR rates were reported between the two groups (57.5% in‐person vs. 58.2% telemedicine; P = 0.89). The model demonstrated improvement in community provider knowledge, self‐efficacy, and satisfaction.( 95 )
Community‐based strategies that couple targeted outreach with patient navigation appear promising to enhance HCV screening and linkage‐to‐care efforts. Check Hep C was a public health department, community‐based patient navigation program that began in the pre‐DAA era; it aimed to scale up HCV screening and linkage to care in partnership with federally qualified health centers and SEPs in NYC. As part of a year‐long pilot (2012‐2013), Check Hep C funded several organizations to provide HCV testing and related services. Sites were located in low‐income neighborhoods with high rates of HCV infection and included programs that served PWID populations. Interventions included (1) rapid POC‐HCV antibody screening, (2) immediate on‐site phlebotomy for confirmatory RNA testing, (3) linkage to care via patient navigators, and (4) telemedicine. HCV screening was conducted in a diverse, at‐risk cohort of 4,751 persons (49% Hispanic and 40% Black non‐Hispanics, 41% reporting a prior incarceration, 25% with prior IDU, and 15% homeless). Check Hep C identified 11% (n = 512) with confirmed infection, 85% of whom attended at least one follow‐up appointment. These innovative models show how targeted outreach, decentralized testing, and community patient navigation can increase HCV screening and linkage to care. Improved outcomes with expanding access care coordination has also been shown. A care coordination model, Project INSPIRE (2014‐2017), integrated primary care with behavioral health services in NYC to assess the effect on HCV cure rates in the DAA era. Care coordinators worked with patients with HCV (n = 2,775) to schedule appointments, provide health promotion and appointment reminders, coordinate medication insurance approval, and connect patients to mental health and substance use care. Participants enrolled had higher rates of HCV treatment initiation (72% vs. 36%) and cure (65% vs. 47%) than patients not enrolled in Project INSPIRE.( 96 ) This model increased HCV provider capacity via telemedicine for primary care providers( 97 ) and was found to cost very few resources (i.e., only 30 patients needed to be treated at each site to break even with budgetary expenses, and the cost of the intervention totaled to less than $100 per month).( 98 , 99 ) Increased funding to reimburse supportive services, ability to bill and reimburse for outpatient HCV care in community‐based settings, elimination of DAA restrictions, and innovative payer models could help lead to wider implementation of HCV care.
US Academic Health Systems
Several academic research groups in the United States have implemented programs to increase HCV screening and linkage to care, evolving in accordance with changes in CDC guidelines and gaining momentum with the introduction of DAAs in 2014. In 2012, the CDC issued guidelines recommending HCV antibody testing for persons born between1945 and 1965 (the baby boomer cohort). At the time, HCV prevalence was five‐fold higher in this cohort than in other segments of the population. Eight years later, baby boomers still harbor most HCV infections, and therefore remain an important population.
In the 2‐year period from November 2013 to November 2015, during the transition from interferon‐based treatment to interferon‐free DAA regimens, researchers in NYC tested the impact of a multifaceted intervention on rates of HCV screening among baby boomers in two primary care practices.( 100 ) The results demonstrated a beneficial effect of educational interventions and data feedback to providers, and to a lesser extent, patient navigation. Automated medical record alerts were relatively ineffective. During the study period, screening rates increased from 55% to 75% (P < 0.01), with far greater improvements among physician trainees than among the faculty. Among 84 HCV RNA–positive patients identified during the study, 60 completed an appointment with a hepatologist and 32 initiated HCV treatment.
Researchers at the University of Michigan tested the impact of an electronic health record (EHR) best practices alert on rates of HCV screening and linkage to care among baby boomers at 13 clinics.( 100 ) The alert was linked to educational materials, an order set, and streamlined access to specialty care; it was a soft alert that was repeated at 10‐month intervals if initially declined. Compared with practices during the 6‐month period before the alert, during the first year afterward, screening of eligible patients increased from 7.6% to 72% (P < 0.001), and the number of newly diagnosed HCV RNA–positive patients increased from 23 to 53, of whom 31 had received a DAA prescription and 20 had begun treatment at the time of publication.
These two studies establish the effectiveness of interventions that simultaneously increase HCV awareness and streamline access to treatment. However, a significant percentage of the HCV RNA–positive patients did not receive treatment, raising the possibility that screening is creating an ever‐increasing population of untreated persons with positive HCV‐RNA test results in their medical record. This was confirmed by a study carried out in an emergency department in the Bronx, New York.( 101 ) Building on these findings, researchers in a large health system in NYC developed a computer algorithm to find patients with positive HCV‐RNA tests and identified nearly 11,000 patients. Their records are currently being reviewed, and treatment candidates are being offered linkage to care.( 102 )
US VHA
The VHA is the largest integrated health system in the United States. Serving more than 9 million veterans at 1,255 health care facilities, the VHA is the single largest provider of HCV care in the United States.( 103 ) VHA system‐level and provider‐level barriers impeded progress with HCV treatment for years before DAAs were introduced. Barriers to treatment included need to prove service relatedness for HCV diagnosis, the volume of patients in need of treatment with high cost of DAAs when introduced, and restrictions around alcohol and substance use as well as prescribers (only allowing certain specialists, who are not readily available, to prescribe a cure). At the advent of the DAA era in 2014, only 12,000 veterans had been cured of HCV, and it is unclear how many died due to a lack of treatment. Mental health and substance abuse disorders are common among HCV‐infected veterans and were deemed as barriers to treatment candidacy by providers.( 104 ) From 2015 to 2017, US Congress appropriated over $3 billion for HCV macro‐elimination efforts. The VHA ramped up HCV treatment, and during the period of 2015 to 2017, approximately 2,000 veterans per week were treated.( 105 ) By March 2019, 116,000 veterans had initiated DAA therapy with SVR rates of over 90%. The multipronged initiative included (1) centralized leadership and team‐based implementation structures, (2) innovative use of EHR databases, (3) alternative treatment models, and (4) negotiating lower drug prices, which are described in the coming sections.
In 2001, the VHA established the National Viral Hepatitis Program, which has published policies for diagnosis and management of HCV and developed resources for clinicians and patients.( 106 ) Following DAA approval, the program established Hepatitis C Innovations Teams and provided funding for administrators, organization, and system redesign efforts. The program addressed gaps in the HCV cascade of care using process improvement methods. Teams identified annual objectives regarding HCV screening and treatment, and created facility‐specific solutions. They shared best practices with monthly and quarterly regional meetings and an annual national meeting, as well as through a central portal of information. The strategies of (1) revising professional roles and (2) preparing champions were among the most successful in improving HCV uptake within the VHA.( 107 )
Since the late 1990s, the VHA has maintained an EHR, and since 2006 began to store data in the corporate data warehouse.( 108 ) For HCV care, the VHA created a national database (i.e., an HCV clinical dashboard with patient‐level data) that included data on location of care, HCV antibody and viral load results, and measures of liver disease.( 109 ) This allowed local teams to scale up HCV screening as well as identify and track veterans through the cascade of HCV care. Tools, including EHR reminders and note templates, were implemented to support these efforts.
The VHA has also successfully implemented multiple novel treatment models. Task sharing with nonspecialists, such as advanced practice practitioners (APPs), pharmacists, mental health providers, and primary care physicians, has been critical in a number of practice settings. At some VHA facilities, HCV treatment was led almost entirely by nonspecialists.( 110 ) Among the several successful models, VHA San Diego used a mental health APP to provide case management and coordinate multidisciplinary care, resulting in more patients (32%) starting DAAs when receiving integrated care, compared to just 16% who did not receive integrated care.( 111 ) Expansion of telehealth programs, including HCV management by specialty and midlevel providers using telehealth for remote locations, and expansion of the Veterans Affairs (VA) ECHO program, which trained primary care physicians, was also important. In one study of the VA‐ECHO program, HCV antiviral medication dispensation by primary care providers occurred in 21% of patients with VA‐ECHO, compared with just 2.5% among patients who did not receive the program.( 112 )
Finally, one component of improving HCV‐elimination efforts in the VHA was negotiating lower drug prices for DAAs.( 113 ) For the VHA, drug manufacturers are required to provide a 24% discount off the drug’s average price or charge the VHA the lowest price offered in the private sector to nonfederal buyers. This increased access to medications and capacity for treating patients.
Innovations in Macro‐elimination Outside the United States
As of 2018, fewer than 10 high‐income countries are on track to eliminate HCV by 2030. Modeling studies suggest 30 high‐income countries are not expected to eliminate HCV before 2050.( 114 ) Barriers have plagued high‐income countries, which may be counterintuitive to the notion that increased resources can lead to more progress toward elimination. Countries such as the United States have fragmented health systems, multiple competing payers, predominantly specialist‐based HCV care delivery, lack of transparency in drug pricing coupled with a strong pharmaceutical lobby to maintain high drug prices, and lack of research funding( 115 ) (Fig. 3)—all of which can impede innovation. Most of the growth in annual HCV treatment globally has been in middle‐income countries.( 4 ) Despite these challenges, some nations are notable for their progress toward achieving HCV elimination.( 1 , 116 )
Fig. 3.
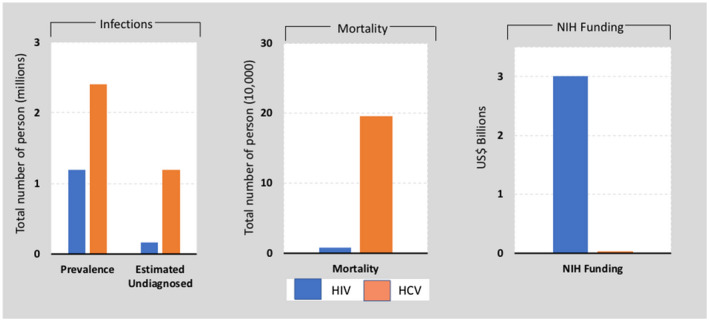
United States response to HIV and HCV epidemics.( 108 ) While the US estimated prevalence of HCV may vary by study, Saab et al. demonstrated how HCV is severely underfunded from research and care standpoints.( 108 ) This comparison shows the prevalence, undiagnosed infection, mortality, and National Institutes of Health research funding for HCV and HIV/AIDS in the United States. HCV is a more prevalent infection in the United States, with a substantial burden of undiagnosed cases and greater mortality compared with HIV.
A review of countries and their hepatitis elimination efforts highlights diverse approaches. Global comparisons show that HCV elimination progress is linked to strong leadership and centralized planning with multisectoral collaboration and innovative strategies for reducing barriers and increasing access for testing, care, and treatment.( 117 ) A report examining Australia, Pakistan, Malaysia, Fiji, Iceland, Egypt, Georgia, Scotland, Brazil, India, Qatar, Rwanda, and South Africa highlighted a wide spectrum of approaches to match the unique hepatitis burden, healthcare infrastructure, diagnostics and treatment markets, resources, and communities of each country.( 118 ) The decriminalization and medicalization of substance use disorders has served as a foundation of HCV elimination efforts in countries such as Australia, Portugal, and Iceland. Successful national models have embedded micro‐elimination efforts. For example, Australia has had increased uptake among PWID populations and HIV‐infected men who have sex with men.( 119 , 120 , 121 , 122 ) One example is the CEASE prospective cohort study in which people living with HIV/HCV coinfection were recruited (2014‐2017) across sites nationally (n = 402) and colocated HCV treatment in primary care clinics. This effort resulted in a decline of HCV viremic prevalence from 82% in 2014 to 8% in 2018 with low re‐infection rates.( 123 )
Effective national models have leveraged political action, contained costs, decentralized service delivery with removal of prescribing restrictions, and task shifting from specialists to primary care, and provided enhanced coverage for DAAs. Outside the United States, capping DAA prices has led to wider access to HCV treatment. Furthermore, high pricing in the United States has led to DAA rationing by state Medicaid programs. Prescribing restrictions such as the US Medicaid fibrosis restrictions should be eliminated. In March 2016, the Australian government made available unrestricted access to DAA therapy over 5 years.( 124 ) As of 2019, Australia has demonstrated rapid DAA uptake nationally with approximately 85,000 people treated. Additionally, with prescribing restrictions lifted and increased training of general practitioners, DAA prescriptions written by general practitioners in Australia increased from 9% to 37% in a year.( 125 ) The cost for rapid HCV testing and DAA treatment has fallen substantially in parts of the world. With one of the highest HCV prevalence rates globally, Egypt established a national response and by 2018 had treated 2 million people.( 126 ) The program in Egypt had novel components that included a public facing national campaign, leveraging a national voting registry, and establishing a web‐based registration system that increased access to treatment. Egypt aggressively negotiated DAA price, and locally produced generics also drove down the price.( 127 , 128 ) Notably, Iceland, considered a “closed system” with little immigration or emigration, is the first country anticipated to achieve HCV elimination through the Treatment as Prevention for Hepatitis C (the TraP Hep C) study. Launched in 2016, the TraP Hep C study is a nationwide elimination program that aims to( 2 ) (1) initiate HCV treatment for every person in need in Iceland within 3 years of commencement of the study( 2 , 7 , 8 ) and (2) reduce the domestic incidence of HCV in the population by 80% before the World Health Organization target of HCV elimination by the year 2030.( 2 ) Fifteen months after launching the TraP HepC, 557 individuals (~56%‐70% of the estimated total infected population) were evaluated. This cohesive, multipronged approach includes scaled‐up of prevention, testing, and early treatment of HCV in both hospital and community settings. It includes a multidisciplinary public health model of care and cooperation among government, health services, the penitentiary system, and community organizations.
Continued efforts to decentralize and destigmatize case finding efforts tailored to the needs of hardly reached populations are urgently required. Case finding efforts similar to what we describe in the United States with reaching patients already diagnosed with HCV and engaging them into care has been demonstrated in the Netherlands and other countries as well.( 129 ) Innovation across global settings will help inform which strategies can be realized in other settings to achieve global HCV elimination goals.
Keys to Future Success
Screening, case finding, linkage to care, and treatment in hardly reached populations needs continued study to overcome barriers in achieving HCV elimination. Groups such as the International Network on Hep C in Substance Users have helped create a body of work to guide how system‐level and provider‐level changes can effectively enhance our approach. As more and more people are cured, and with the rise of cases related to the opioid epidemic, additional system‐level interventions will be needed to aid case finding efforts. Now that we have demonstrated high efficacy of DAA therapy across various practice settings, we must work harder toward overcoming system‐level and provider‐level barriers with innovative models of care, working across defined roles to enhance collaboration, and increasing funding so that we can succeed toward the path of HCV elimination.
Disclosures: Mount Sinai receives research from Gilead Sciences to support Dr. Branch’s research. There are no other relevant financial disclosures.
Potential conflict of interest: Dr. Bhattacharya received grants from Gilead, AbbVie, and Regeneron. Dr. Branch received grants from Gilead. Dr. Wang received grants from Gilead.
REFERENCES
- 1. World Health Organization . Hepatitis C. https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐c. Published 2019. Accessed May 12, 2020. [Google Scholar]
- 2. Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013‐2016. Hepatology 2019;69:1020‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hutin YJBM, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc 2018;21(Suppl. 2):e25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razavi H. Global epidemiology of viral hepatitis. Gastroenterol Clin North Am 2020;49:179‐189. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 6. Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 2009;50:1750‐1755. [DOI] [PubMed] [Google Scholar]
- 7. Zhou K, Terrault NA. Gaps in viral hepatitis awareness in the United States in a population‐based study. Clin Gastroenterol Hepatol 2020;18:188‐195.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow‐up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 2012;55:1652‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003‐2013. Clin Infect Dis 2016;62:1287‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AASLD‐IDSA . HCV guidance: recommendations for testing, management, and treating hepatitis C. 2019. https://www.hcvguidelines.org/evaluate/testing‐and‐linkage. Accessed May 11, 2020. [Google Scholar]
- 11. USPSTF . Draft Recommendation Statement. Hepatitis C virus infection in adolescents and adults: screening. 2019. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft‐recommendation‐statement/hepatitis‐c‐screening‐citation2. Accessed May 10, 2020. [Google Scholar]
- 12. US Preventive Services Task Force ; Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for hepatitis C virus infection in adolescents and adults: US preventive services task force recommendation statement. JAMA 2020;323:970‐975. [DOI] [PubMed] [Google Scholar]
- 13. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta‐analysis. PLoS One 2014;9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lazarus JV, Safreed‐Harmon K, Thursz MR, Dillon J, El‐Sayed M, Elsharkawy A, et al. The micro‐elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis 2018;38:181‐192. [DOI] [PubMed] [Google Scholar]
- 15. Lazarus JV, Wiktor S, Colombo M, Thursz M. Micro‐elimination—a path to global elimination of hepatitis C. J Hepatol 2017;67:665‐666. [DOI] [PubMed] [Google Scholar]
- 16. Harris RJ, Ramsay M, Hope VD, Brant L, Hickman M, Foster GR, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012;22:187‐192. [DOI] [PubMed] [Google Scholar]
- 17. Alter MJ. Prevention of spread of hepatitis C. Hepatology 2002;36:s93‐s98. [DOI] [PubMed] [Google Scholar]
- 18. Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol 2003;26:171‐184. [DOI] [PubMed] [Google Scholar]
- 19. Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558‐567. [DOI] [PubMed] [Google Scholar]
- 21. Nyamathi AM, Dixon EL, Robbins W, Smith C, Wiley D, Leake B, et al. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med 2002;17:134‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta‐analysis. Hepatology 2017;66:13‐26. [DOI] [PubMed] [Google Scholar]
- 23. Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010;105:844‐859. [DOI] [PubMed] [Google Scholar]
- 24. Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction 2012;107:1984‐1995. [DOI] [PubMed] [Google Scholar]
- 25. Kwon JA, Iversen J, Maher L, Law MG, Wilson DP. The impact of needle and syringe programs on HIV and HCV transmissions in injecting drug users in Australia: a model‐based analysis. J Acquir Immune Defic Syndr 2009;51:462‐469. [DOI] [PubMed] [Google Scholar]
- 26. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013;57(Suppl. 2):S56‐S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Academies of Sciences, Engineering, and Medicine . Opportunities to improve opioid use disorder and infectious disease services: integrating responses to a dual epidemic. Washington, DC: National Academies Press; 2020. [PubMed] [Google Scholar]
- 28. Saludes V, Antuori A, Lazarus JV, Folch C, González‐Gómez S, González N, et al. Evaluation of the Xpert HCV VL Fingerstick point‐of‐care assay and dried blood spot HCV‐RNA testing as simplified diagnostic strategies among people who inject drugs in Catalonia, Spain. Int J Drug Policy 2020;80:102734. [DOI] [PubMed] [Google Scholar]
- 29. Williams B, Howell J, Doyle J, Thompson AJ, Draper B, Layton C, et al. Point‐of‐care hepatitis C testing from needle and syringe programs: an Australian feasibility study. Int J Drug Policy. 2019;72:91‐98. [DOI] [PubMed] [Google Scholar]
- 30. Prinsenberg T, Rebers S, Boyd A, Zuure F, Prins M, van der Valk M, et al. Dried blood spot self‐sampling at home is a feasible technique for hepatitis C RNA detection. PLoS One 2020;15:e0231385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falade‐Nwulia O, Irvin R, Merkow A, Sulkowski M, Niculescu A, Olsen Y, et al. Barriers and facilitators of hepatitis C treatment uptake among people who inject drugs enrolled in opioid treatment programs in Baltimore. J Subst Abuse Treat 2019;100:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Talal AH, Andrews P, McLeod A, Chen Y, Sylvester C, Markatou M, et al. Integrated, co‐located, telemedicine‐based treatment approaches for hepatitis C virus management in opioid use disorder patients on methadone. Clin Infect Dis 2019;69:323‐331. [DOI] [PubMed] [Google Scholar]
- 33. Ho CJ, Preston C, Fredericks K, Doorley SL, Kramer RJ, Kwan L, et al. A unique model for treating chronic hepatitis C in patients with psychiatric disorders, substance abuse, and/or housing instability. J Addict Med 2013;7:320‐324. [DOI] [PubMed] [Google Scholar]
- 34. Wolfe D, Luhmann N, Harris M, Momenghalibaf A, Albers E, Byrne J, et al. Human rights and access to hepatitis C treatment for people who inject drugs. Int J Drug Policy 2015;26:1072‐1080. [DOI] [PubMed] [Google Scholar]
- 35. Rourke SB, Sobota M, Tucker R, Bekele T, Gibson K, Greene S, et al. Social determinants of health associated with hepatitis C co‐infection among people living with HIV: results from the positive spaces. Healthy Places study. Open Med 2011;5:e120‐e131. [PMC free article] [PubMed] [Google Scholar]
- 36. O'Sullivan M, Jones AM, Gage H, Jordan J, MacPepple E, Williams H, et al. ITTREAT (Integrated Community Test ‐ Stage ‐ TREAT) hepatitis C service for people who use drugs: real‐world outcomes. Liver Int 2020;40:1021‐1031. [DOI] [PubMed] [Google Scholar]
- 37. Milne R, Price M, Wallace B, Drost A, Haigh‐Gidora I, Nezil FA, et al. From principles to practice: description of a novel equity‐based HCV primary care treatment model for PWID. Int J Drug Policy 2015;26:1020‐1027. [DOI] [PubMed] [Google Scholar]
- 38. Grebely J, Knight E, Genoway KA, Viljoen M, Khara M, Elliott D, et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. Eur J Gastroenterol Hepatol 2010;22:270‐277. [DOI] [PubMed] [Google Scholar]
- 39. Norton BL, Bachhuber MA, Singh R, Agyemang L, Arnsten JH, Cunningham CO, et al. Evaluation of contingency management as a strategy to improve HCV linkage to care and treatment in persons attending needle and syringe programs: a pilot study. Int J Drug Policy 2019;69:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roose RJ, Cockerham‐Colas L, Soloway I, Batchelder A, Litwin AH. Reducing barriers to hepatitis C treatment among drug users: an integrated hepatitis C peer education and support program. J Health Care Poor Underserved 2014;25:652‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364:2199‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention . Assessment of sexually transmitted diseases services in city and county jails–United States, 1997. MMWR Morb Mortal Wkly Rep 1998;47:429‐431. [PubMed] [Google Scholar]
- 43. Federal Bureau of Prisons . Evaluation and Management of chronic hepatitis C virus (HCV) infection. Clinical Guidance. Washington, DC: Federal Bureau of Prisons; 2018. [Google Scholar]
- 44. Spaulding AC, Chhatwal J, Thanthong‐Knight S, Zhan T, Ladd MA, Adee M,et al. Emory Center for the Health of Incarcerated Persons and MGH Institute for Technology Assessment. hepcorrections.org. Accessed May 30, 2020.
- 45. Beckwith CG, Kurth AE, Bazerman L, Solomon L, Patry E, Rich JD, et al. Survey of US correctional institutions for routine HCV testing. Am J Public Health 2015;105:68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta‐analysis. Clin Infect Dis. 2013;57(Suppl. 2):S80‐S89. [DOI] [PubMed] [Google Scholar]
- 47. Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019;170:594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cunningham EB, Applegate TL, Lloyd AR, Dore GJ, Grebely J. Mixed HCV infection and reinfection in people who inject drugs–impact on therapy. Nat Rev Gastroenterol Hepatol 2015;12:218‐230. [DOI] [PubMed] [Google Scholar]
- 49. Graf C, Mucke MM, Dultz G, Peiffer KH, Kubesch A, Ingiliz P, et al. Efficacy of direct‐acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta‐analysis. Clin Infect Dis 2020;70:2355‐2365. [DOI] [PubMed] [Google Scholar]
- 50. Akiyama MJ, Lipsey D, Heo M, Agyemang L, Norton BL, Hidalgo J, et al. Low hepatitis C reinfection following direct‐acting antiviral therapy among people who inject drugs on opioid agonist therapy. Clin Infect Dis 2020;70:2695‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol 2011;54:1137‐1144. [DOI] [PubMed] [Google Scholar]
- 52. Vickerman P, Martin N, Hickman M. Can hepatitis C virus treatment be used as a prevention strategy? Additional model projections for Australia and elsewhere. Drug Alcohol Depend 2011;113:83‐85;discussion 86‐87. [DOI] [PubMed] [Google Scholar]
- 53. Hellard M, Rolls DA, Sacks‐Davis R, Robins G, Pattison P, Higgs P, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology 2014;60:1861‐1870. [DOI] [PubMed] [Google Scholar]
- 54. Zhen Zeng PD. Jail inmates in 2018. bjs.gov. Bureau of Justice Statistics; 2020. [Google Scholar]
- 55. Rich JD, Beckwith CG, Macmadu A, Marshall BDL, Brinkley‐Rubinstein L, Amon JJ, et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet 2016;388:1103‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spaulding AC, Perez SD, Seals RM, Hallman MA, Kavasery R, Weiss PS. Diversity of release patterns for jail detainees: implications for public health interventions. Am J Public Health 2011;101(Suppl. 1):S347‐S352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spaulding AC, Chhatwal J, Adee MG, Lawrence RT, Beckwith CG, von Oehsen W. Funding hepatitis C treatment in correctional facilities by using a nominal pricing mechanism. J Correct Health Care 2019;25:15‐24. [DOI] [PubMed] [Google Scholar]
- 58. Assoumou SA, Tasillo A, Vellozzi C, Eftekhari Yazdi G, Wang J, Nolen S, et al. Cost‐effectiveness and budgetary impact of hepatitis C Virus testing, treatment, and linkage to care in us prisons. Clin Infect Dis 2020;70:1388‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de la Flor C, Porsa E, Nijhawan AE. Opt‐out HIV and hepatitis C testing at the dallas county jail: uptake, prevalence, and demographic characteristics of testers. Public Health Rep 2017;132:617‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abe CM, Aguwa M, Zhao M, Sullivan J, Porsa E, Nijhawan AE. Hepatitis C virus infection in the dallas county jail: implications for screening, prevention, and linkage to care. Public Health Rep 2019;134:626‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jordan AO, Cohen LR, Harriman G, Teixeira PA, Cruzado‐Quinones J, Venters H. Transitional care coordination in New York City jails: facilitating linkages to care for people with HIV returning home from Rikers Island. AIDS Behav 2013;17(Suppl. 2):S212‐S219. [DOI] [PubMed] [Google Scholar]
- 62. Akiyama MJ, Columbus D, MacDonald R, Jordan AO, Schwartz J, Litwin AH, et al. Linkage to hepatitis C care after incarceration in jail: a prospective, single arm clinical trial. BMC Infect Dis 2019;19:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cunningham WE, Weiss RE, Nakazono T, Malek MA, Shoptaw SJ, Ettner SL, et al. Effectiveness of a peer navigation intervention to sustain viral suppression among HIV‐positive men and transgender women released from jail: the LINK LA randomized clinical trial. JAMA Intern Med 2018;178:542‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sterling RK, Cherian R, Lewis S, Genther K, Driscoll C, Martin K, et al. Treatment of HCV in the department of corrections in the era of oral medications. J Correct Health Care 2018;24:127‐136. [DOI] [PubMed] [Google Scholar]
- 65. Chan J, Schwartz J, Kaba F, Bocour A, Akiyama MJ, Hobstetter L, et al. Outcomes of hepatitis C virus treatment in the New York City jail population: successes and challenges facing scale up of care. Open Forum Infectious Diseases 2020;7:ofaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Young JD, Badowski M. Telehealth: increasing access to high quality care by expanding the role of technology in correctional medicine. J Clin Med 2017;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sterling RK, Hofmann CM, Luketic VA, Sanyal AJ, Contos MJ, Mills AS, et al. Treatment of chronic hepatitis C virus in the virginia department of corrections: can compliance overcome racial differences to response? Am J Gastroenterol 2004;99:866‐872. [DOI] [PubMed] [Google Scholar]
- 68. Lloyd AR, Clegg J, Lange J, Stevenson A, Post JJ, Lloyd D, et al. Safety and effectiveness of a nurse‐led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis 2013;56:1078‐1084. [DOI] [PubMed] [Google Scholar]
- 69. Jimenez Galan G, Alia Alia C, Vegue Gonzalez M, García Berriguete RM, Fernández González F, Fernández Rodríguez CM, et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. Rev Esp Enferm Dig 2019;111:550‐555. [DOI] [PubMed] [Google Scholar]
- 70. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison‐based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep 2007;122(Suppl. 2):74‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive‐aged women and children in the United States, 2006 to 2014. Ann Intern Med 2017;166:775‐782. [DOI] [PubMed] [Google Scholar]
- 72. Ward C, Tudor‐Williams G, Cotzias T, Hargreaves S, Regan L, Foster GR. Prevalence of hepatitis C among pregnant women attending an inner London obstetric department: uptake and acceptability of named antenatal testing. Gut 2000;47:277‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lambert J, Jackson V, Coulter‐Smith S, Brennan M, Geary M, Kelleher TB, et al. Universal antenatal screening for hepatitis C. Ir Med J 2013;106:136‐139. [PubMed] [Google Scholar]
- 74. Diab‐Elschahawi M, Dosch V, Honsig C, Jatzko B, Segagni L, Assadian O, et al. Evaluation of a universal vs a targeted hepatitis C virus screening strategy among pregnant women at the Vienna University Hospital. Am J Infect Control 2013;41:459‐460. [DOI] [PubMed] [Google Scholar]
- 75. Boudova S, Mark K, El‐Kamary SS. Risk‐based hepatitis C screening in pregnancy is less reliable than universal screening: a retrospective chart review. Open Forum Infect Dis 2018;5:ofy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: a retrospective cohort study. Subst Abus 2016;37:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Berkley EM, Leslie KK, Arora S, Qualls C, Dunkelberg JC. Chronic hepatitis C in pregnancy. Obstet Gynecol 2008;112:304‐310. [DOI] [PubMed] [Google Scholar]
- 78. Jhaveri R, Broder T, Bhattacharya D, Peters MG, Kim AY, Jonas MM. Universal screening of pregnant women for hepatitis C: the time is now. Clin Infect Dis 2018;67:1493‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults ‐ United States, 2020. MMWR Recomm Rep 2020;69:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Screening for Hepatitis C Virus Infection . The American College of Obstetricians and Gynecologists. Practice Advisory Web Site. https://www.acog.org/clinical/clinical‐guidance/practice‐advisory/articles/2020/04/screening‐for‐hepatitis‐c‐virus‐infection. Published April 2020. Accessed January 6, 2020. [Google Scholar]
- 81. Chaillon A, Rand EB, Reau N, Martin NK. Cost‐effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis. 2019;69:1888‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rose MMJ, Evans J, Prince A, Espinosa C.Hepatitis C risk‐based vs. universal screening among pregnant women: implementation and cost‐effectiveness analysis. Presented at the Liver Meeting November, San Francisco, CA, 2018.
- 83. Mostafa A, Ebeid FSE, Khaled B, Ahmed RHM, El‐Sayed MH. Micro‐elimination of hepatitis C through testing of Egyptian pregnant women presenting at delivery: implications for screening policies. Trop Med Int Health 2020;25:850‐860. [DOI] [PubMed] [Google Scholar]
- 84. Oliver C, Black J, De Pont S, Sizemore L, Wester C. Pregnancy status, risk factors, and opportunities for referral to care among reproductive‐aged women with newly reported chronic hepatitis C virus infection in Tennessee. Public Health Rep 2020;135:90‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Millbourn C, Lybeck C, Psaros Einberg A, Nordin M, Lindh G, Hökeberg I, et al. Anti‐HCV prevalence and risk factor‐based screening for hepatitis C in pregnant women and their partners in Sweden. Infect Dis (Lond) 2020;52:776‐785. [DOI] [PubMed] [Google Scholar]
- 86. Chappell CA, Scarsi KK, Kirby BJ, Suri V, Gaggar A, Bogen DL,et al. Ledipasvir plus. sofosbuvir in pregnant women with hepatitis C virus infection: a phase 1 pharmacokinetic study. Lancet Microbe 2020;1:e200‐e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chappell CA.A phase 1 study of ledipasvir/sofosbuvir in pregnant women with hepatitis C virus. In: Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2019.
- 88. Chappell CA. Phase 1 pharmacokinetic trial of sofosbuvir/velpatasvir in pregnant women with chronic hepatitis C virus infection (NCT04382404). University of Pittsburgh. [Google Scholar]
- 89. US Department of Health and Human Services NVHAP . https://clinicaltrials.gov/ct2/show/NCT04382404. [DOI] [PubMed]
- 90. Office of Infectious Disease and HIV/AIDS Policy . Mapping hepatitis elimination in action. https://www.hhs.gov/hepatitis/viral‐hepatitis‐action‐plan/index.html. Accessed June 29. [Google Scholar]
- 91. Gaudino A, Gay B, Garmon C, Selick M, Vreeland R, Burk K, et al. Localized US efforts to eliminate hepatitis C. Infect Dis Clin North Am 2018;32:293‐311. [DOI] [PubMed] [Google Scholar]
- 92. Bradley H, Hall EW, Rosenthal EM, Sullivan PS, Ryerson AB, Rosenberg ES. Hepatitis C virus prevalence in 50 U.S. states and D.C. by sex, birth cohort, and race: 2013‐2016. Hepatol Commun 2020;4:355‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nunn A, Yolken A, Cutler B, Trooskin S, Wilson P, Little S, et al. Geography should not be destiny: focusing HIV/AIDS implementation research and programs on microepidemics in US neighborhoods. Am J Public Health 2014;104:775‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Myers JE, Braunstein SL, Shepard CW, Cutler BH, Mantsios AR, Sweeney MM, et al. Assessing the impact of a community‐wide HIV testing scale‐up initiative in a major urban epidemic. J Acquir Immune Defic Syndr 2012;61:23‐31. [DOI] [PubMed] [Google Scholar]
- 95. Arora S, Kalishman S, Thornton K, Dion D, Murata G, Deming P, et al. Expanding access to hepatitis C virus treatment—Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology 2010;52:1124‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Deming R, Ford MM, Moore MS, Lim S, Perumalswami P, Weiss J, et al. Evaluation of a hepatitis C clinical care coordination programme's effect on treatment initiation and cure: a surveillance‐based propensity score matching approach. J Viral Hepat 2018;25:1236‐1243. [DOI] [PubMed] [Google Scholar]
- 97. Teixeira PA, Bresnahan MP, Laraque F, Litwin AH, Shukla SJ, Schwartz JM, et al. Telementoring of primary care providers delivering hepatitis C treatment in New York City: results from Project INSPIRE. Learn Health Syst 2018;2:e10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fluegge K, Bresnahan MP, Laraque F, Litwin AH, Perumalswami PV, Shukla SJ, et al. Evaluating reimbursement of integrated support services using chronic care management (CCM) codes for treatment of hepatitis C among Medicare beneficiaries. J Healthc Risk Manag 2019;39:31‐40. [DOI] [PubMed] [Google Scholar]
- 99. Behrends CN, Eggman AA, Gutkind S, Bresnahan MP, Fluegge K, Laraque F, et al. A cost reimbursement model for hepatitis C treatment care coordination. J Public Health Manag Pract 2019;25:253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Konerman MA, Thomson M, Gray K, Moore M, Choxi H, Seif E, et al. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology 2017;66:1805‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Torian LV, Felsen UR, Xia Q, Laraque F, Rude EJ, Rose H, et al. Undiagnosed HIV and HCV infection in a New York City Emergency Department, 2015. Am J Public Health 2018;108:652‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perumalswami PV, Wyatt B, Mageras A, Miller M, Dieterich DT, Dudley J, et al. An algorithm for digitally identifying hcv treatment candidates: use of digital case finding and care coordination to eliminate hcv across a large urban healthcare system. Hepatology 2019;70:387A. [Google Scholar]
- 103. Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology 2005;41:88‐96. [DOI] [PubMed] [Google Scholar]
- 104. Ho SB, Groessl E, Dollarhide A, Robinson S, Kravetz D, Dieperink E. Management of chronic hepatitis C in veterans: the potential of integrated care models. Am J Gastroenterol 2008;103:1810‐1823. [DOI] [PubMed] [Google Scholar]
- 105. U.S. Department of Veterans Affairs . VA on path to cure 100,000 Veterans of hepatitis C. Office of Public and Intergovernmental Affairs. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5219. [Google Scholar]
- 106. Belperio PS, Chartier M, Gonzalez RI, et al. Hepatitis C care in the department of veterans affairs: building a foundation for success. Infect Dis Clin North Am 2018;32:281‐292. [DOI] [PubMed] [Google Scholar]
- 107. Rogal SS, Yakovchenko V, Waltz TJ, Powell BJ, Kirchner JE, Proctor EK, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci 2017;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. U.S. General Services Administration . TTSDCCDWhcdgdc‐d‐w‐cAJ, 2020. https://www.data.va.gov/dataset/Corporate‐Data‐Warehouse‐CDW‐/ftpi‐epf7.
- 109. Lau M, Beste LA, Kryskalla J, Rongey C. Hepatitis C clinical dashboards: improving liver specialty care access and quality. Fed Pract. 2015;32(Suppl. 2):32S‐36S. [PMC free article] [PubMed] [Google Scholar]
- 110. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med 2017;167:499‐504. [DOI] [PubMed] [Google Scholar]
- 111. Ho SB, Brau N, Cheung R, Liu L, Sanchez C, Sklar M, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol 2015;13:2005‐2014.e3. [DOI] [PubMed] [Google Scholar]
- 112. Beste LA, Glorioso TJ, Ho PM, Au DH, Kirsh SR, Todd‐Stenberg J, et al. Telemedicine specialty support promotes hepatitis C treatment by primary care providers in the Department of Veterans Affairs. Am J Med. 2017;130:432‐438.e3. [DOI] [PubMed] [Google Scholar]
- 113. Blumenthal D, Squires D. Drug price control: how some government programs do it. Commonwealth Fund. May 10, 2016. https://www.commonwealthfund.org/blog/2016/drug‐price‐control‐how‐some‐government‐programs‐do‐it. Accessed June 20, 2020. [Google Scholar]
- 114. Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high‐income countries. Liver Int. 2020;40:522‐529. [DOI] [PubMed] [Google Scholar]
- 115. Saab S, Le L, Saggi S, Sundaram V, Tong MJ. Toward the elimination of hepatitis C in the United States. Hepatology 2018;67:2449‐2459. [DOI] [PubMed] [Google Scholar]
- 116. World Health Organization . Progress report on access to hepatitis C treatment. https://apps.who.int/iris/bitstream/handle/10665/260445/WHO‐CDS‐HIV‐18.4‐eng.pdf?sequence=1. Accessed June 20, 2020. [Google Scholar]
- 117. Schroeder SE, Pedrana A, Scott N, Wilson D, Kuschel C, Aufegger L, et al. Innovative strategies for the elimination of viral hepatitis at a national level: a country case series. Liver Int 2019;39:1818‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pedrana A, Howell J, Schröder S, Scott N, Wilson D, Kuschel C. Eliminating viral hepatitis: the investment case. Doha, Qatar: World Innovation Summit for Health; 2018. [Google Scholar]
- 119. Martinello M, Hajarizadeh B, Dore GJ. Hepatitis C elimination in Australia: progress and challenges. Med J Aust 2020;212:362‐363. [DOI] [PubMed] [Google Scholar]
- 120. Hajarizadeh B, Grebely J, Matthews GV, Martinello M, Dore GJ. Uptake of direct‐acting antiviral treatment for chronic hepatitis C in Australia. J Viral Hepat 2018;25:640‐648. [DOI] [PubMed] [Google Scholar]
- 121. Iversen J, Dore GJ, Catlett B, Cunningham P, Grebely J, Maher L. Association between rapid utilisation of direct hepatitis C antivirals and decline in the prevalence of viremia among people who inject drugs in Australia. J Hepatol 2019;70:33‐39. [DOI] [PubMed] [Google Scholar]
- 122. Iranpour N, Dore GJ, Martinello M, Matthews GV, Grebely J, Hajarizadeh B. Estimated uptake of hepatitis C direct‐acting antiviral treatment among individuals with HIV co‐infection in Australia: a retrospective cohort study. Sex Health 2020;17:223. [DOI] [PubMed] [Google Scholar]
- 123. Martinello M, Yee J, Bartlett SR, Read P, Baker D, Post JJ, et al. Moving towards hepatitis C micro‐elimination among people living with HIV in Australia: the CEASE study. Clin Infect Dis 2020;71:1502‐1510. [DOI] [PubMed] [Google Scholar]
- 124. Moon S, Erickson E. Universal medicine access through lump‐sum remuneration—Australia’s approach to hepatitis C. N Engl J Med 2019;380:607‐610. [DOI] [PubMed] [Google Scholar]
- 125. Doyle JS, Scott N, Sacks‐Davis R, Pedrana AE, Thompson AJ, Hellard ME. Treatment access is only the first step to hepatitis C elimination: experience of universal anti‐viral treatment access in Australia. Aliment Pharmacol Ther 2019;49:1223‐1229. [DOI] [PubMed] [Google Scholar]
- 126. Waked I, Esmat G, Elsharkawy A, El‐Serafy M, Abdel‐Razek W, Ghalab R, et al. Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med 2020;382:1166‐1174. [DOI] [PubMed] [Google Scholar]
- 127. Abdel‐Razek W, Hassany M, El‐Sayed MH, El‐Serafy M, Doss W, Esmat G, et al. Hepatitis C virus in Egypt: interim report from the world’s largest national program. Clin Liver Dis (Hoboken) 2019;14:203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cooke GS, Andrieux‐Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019;4:135‐184. [DOI] [PubMed] [Google Scholar]
- 129. Isfordink CJ, Brakenhoff SM, van Dijk M, van der Valk M, de Knegt RJ, Arends JE, et al. Hepatitis C elimination in the Netherlands (CELINE): study protocol for nationwide retrieval of lost to follow‐up patients with chronic hepatitis C. BMJ Open Gastroenterol. 2020;7:e000396. [DOI] [PMC free article] [PubMed] [Google Scholar]


