Abstract
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) is the causative agent of coronavirus disease 2019 (COVID‐19). The presenting symptoms of this virus are variable, and there is an increasing body of literature on risk factors for mortality. The aim of this study was to evaluate the effect of initial aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and preexisting liver disease, including cirrhosis, in a cohort of patients admitted with COVID‐19 infection at a tertiary care hospital network in the Bronx, New York. We reviewed 3,352 patients who had a positive SARS‐CoV2 nasal swab, were over 18 years of age, and had an associated inpatient admission and discharge (or death) to the Montefiore Medical Center from February 28, 2020, to May 22, 2020. Of these, 39/86 (45%) patients died when the initial ALT was >5 times the upper limit of normal (ULN); 115/230 (50%) patients died when the initial AST was >3 times the ULN. The mortality of patients without preexisting liver disease was 26.6% compared to a mortality rate of 29.5% in patients with liver disease. Subgroup analysis showed a mortality of 36.1% in the patients with cirrhosis. Cirrhosis conferred a hazard ratio for mortality of 1.67 (95% confidence interval, 1.09, 2.55; P = 0.019). The baseline Model for End‐Stage Liver Disease score was not prognostic in the cirrhosis cohort. There was no statistical difference between mortality in patients with a history of compensated or decompensated cirrhosis. The most common cause of death in the cirrhosis cohort was respiratory failure. Conclusion: COVID‐19 hepatitis may lead to poor outcomes in patients who are hospitalized for the disease. Patients with cirrhosis are at a higher risk of COVID‐19‐related mortality.
Abbreviations
- ALD
alcohol‐related liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- COVID‐19
coronavirus disease 2019
- F
female
- FIB‐4
fibrosis‐4
- ICD‐10
International Classification of Diseases, Tenth Revision
- INR
international normalized ratio
- IQR
interquartile range
- LOS
length of stay
- M
male
- MELD
Model for End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- PT
prothrombin time
- PTT
partial thromboplastin time
- SARS‐CoV2
severe acute respiratory syndrome coronavirus 2
- ULN
upper limit of normal
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2), the causative agent of coronavirus disease 2019 (COVID‐19), is a novel beta‐coronavirus first reported in Wuhan, China, in late December 2019.( 1 ) It has led to a global pandemic with an estimated overall mortality between 1.4% and 5%.( 2 ) The pandemic had caused 1,461,249 deaths globally and over 50,000 deaths in the United States as of November 30, 2020.( 3 ) New York City, especially the Bronx and Queens boroughs, has been particularly impacted by this virus.( 4 ) COVID‐19 manifestations include fever, cough, and diarrhea.( 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 ) Patients with immune suppression and chronic disease, such as hypertension, diabetes, and obesity, are at a higher risk of mortality.( 2 , 8 , 9 , 10 ) Ethnicity and race have also emerged as risk factors for COVID‐19 death.( 11 ) Similar risk factors for mortality were observed with the 2002/2003 SARS‐CoV and 2012 Middle East respiratory syndrome coronavirus outbreaks.( 12 , 13 , 14 )
There have also been multiple studies published characterizing the possible direct hepatotoxic effect of SARS‐Cov2,( 15 , 16 , 17 , 18 , 19 , 20 , 21 ) as evidenced by an elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST). ALT and AST are elevated in 16.8%‐53% of patients with COVID‐19.( 2 , 15 , 22 ) Initial ALT levels have not been shown to correlate to inpatient mortality, although peak AST levels have been shown to correlate with COVID‐19‐related mortality.( 16 ) AST is thought to be elevated to a greater extent due to COVID‐19 proclivity to affect zone 3, as is seen with mitochondrial/ischemic injury, influenza infection, and cytokine storm.( 23 , 24 , 25 , 26 , 27 ) It has recently been shown that COVID‐19 infection is associated with higher mortality among patients with chronic liver disease when compared to patients without liver disease.( 1 , 28 , 29 , 30 , 31 , 32 ) The effect of COVID‐19 infection in patients admitted to the hospital with a previous history of liver disease still must be characterized. Furthermore, it is unknown whether the presence of cirrhosis or different etiologies of liver disease can explain differing survival among these patients. The aim of this study was to assess the prognostic ability of initial admission AST and ALT on inpatient mortality, which may be related to an acute SARS‐CoV2‐related mediated injury. We also sought to determine the impact of chronic liver disease, the presence of advanced liver disease, and the etiology of liver disease on mortality and hospital course among patients hospitalized with COVID‐19 infection in three large urban teaching hospitals of the Montefiore Health System located in the Bronx, New York.
Materials and Methods
We reviewed the records of 3,352 patients who had a positive SARS‐CoV2 nasal swab (Cepheid Xpert Xpress SARS‐CoV‐2 real‐time polymerase chain reaction assay), were over 18 years of age, and had an associated inpatient admission and discharge (or death) to the Montefiore Medical Center (Moses, Einstein/Weiler, and Wakefield Divisions) from February 28, 2020, to May 22, 2020. Data were compiled directly from Montefiore’s Enterprise Data Warehouse (EDW). Data collection and analysis were approved by the Montefiore Medical Center Institutional Review Board.
Laboratory values were indexed as the first within 36 hours after triage or admission or up to 8 hours before admission. Fibrosis‐4 (FIB‐4), AST‐to‐platelet ratio index, and Model for End‐Stage Liver Disease (MELD) scores were calculated using their standard formulas (see Supporting Materials). All calculations were performed based on laboratory data obtained before the index COVID‐19 hospital admission. Transient elastography data were obtained between July 11, 2016, and February 17, 2020. The final MELD score was calculated using the closest laboratory parameters within 36 hours of discharge or death. A total of 216 current inpatients were excluded from the analysis because their outcomes were not known (see Supporting Materials).
International Classification of Diseases, Tenth Revision, (ICD‐10) codes were used to establish the presence of liver disease, which was then further categorized by etiology into alcohol‐related liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), viral hepatitis, and other/mixed etiologies (including cholestatic liver disease, autoimmune hepatitis, hepatocellular carcinoma, and acute on chronic liver failure; see Supporting Materials). ICD‐10 codes were also used to further classify the cirrhosis cohort into patients with a history of compensated or decompensated liver disease.
The ICD‐10 code time frame considered was from December 2015 up to a 3‐day exclusion period before admission to prevent codes during hospitalization from the analysis. The presence of hepatitis B DNA, hepatitis C RNA, urine ethyl glucuronide, and transient elastography (FibroScan; in kPa) results were queried to validate the ICD‐10 liver disease subclassifications. Several patients were found to fit in more than one category. Preference in categorization was given when laboratory results aligned with the ICD‐10 code (see Supporting Materials).
Patients with advanced hepatic fibrosis and cirrhosis were identified by a calculated FIB‐4 of >3.25 and/or FibroScan transient elastography results of >12.5 kPa. The outcomes of patients with cirrhosis were compared to those without cirrhosis in a subgroup analysis.
COVID‐19 treatment data were not available at the time of data collection for the entire cohort.
Statistical Methods
Demographic and baseline group differences were assessed using chi‐square tests and two‐sample Student t tests, and the Mann‐Whitney U test was used for non‐normally distributed data. The relationship between length of stay, morbidity, liver disease, clinical risk factors, and biomarkers was modeled using cox proportional hazards. The number of days since admission was set as the underlying time metric. Proportionality between the predictors and the hazard was validated through an evaluation of Schoenfeld residuals that found P > 0.05 and thus confirmed proportionality. A sensitivity analysis was carried out that assessed patterns in missing data and study‐result sensitivity to statistical technique and covariate selection (see Supporting Materials). Analyses were performed using R version 3.6.2. P < 0.05 was considered statistically significant.
Results
Demographics
A total of 457 patients (13.6%) of those admitted for COVID‐19 were found to have underlying liver disease. The mean age of the overall cohort was 64.7 years (SD, 16.6). The overall cohort consisted of 1,718 women (51.3%) and 1,634 men (48.7%). The mean body mass index (BMI) was 29.6 (SD, 8.3). Of the total cohort, 630 patients (18.8%) required intubation and 904 patients (27.0%) died. Baseline vital signs and laboratory values are presented in Table 1. The mean age of the control (no liver disease) cohort was 64.87 years (SD, 17.04), while the mean age in the liver disease cohort was 64.23 years (SD, 13.84). The control cohort consisted of 1,492 women and 1,408 men (51.4%/48.6%). There were more Latino‐identified patients in the liver disease group (Table 2). The BMI was not significantly different between the two groups.
Table 1.
Inpatient COVID‐Positive Cohort Demographic and Laboratory Data
| Number | ||
|---|---|---|
| Overall | 3,352 | |
| Sex (%) | F | 1,718 (51.3) |
| M | 1,634 (48.7) | |
| Race (%) | Asian | 83 (2.5) |
| Black | 1,304 (38.9) | |
| Not reported | 1,612 (48.1) | |
| White | 353 (10.5) | |
| Ethnicity (%) | Latino | 1,225 (36.5) |
| Not Latino | 1,805 (53.8) | |
| Not reported | 322 (9.6) | |
| Expired (%) | No | 2,448 (73.0) |
| Yes | 904 (27.0) | |
| Intubated (%) | No | 2,722 (81.2) |
| Yes | 630 (18.8) | |
| Liver disease group (%) | Control | 2,895 (86.4) |
| ALD | 19 (0.6) | |
| Mixed/other | 279 (8.3) | |
| NASH/NAFLD | 74 (2.2) | |
| Viral | 85 (2.5) | |
| Cirrhosis (%) | Cirrhosis | 83 (2.5) |
| No cirrhosis | 3,269 (97.5) | |
| Age, mean years (SD) | 64.79 (16.64) | |
| BMI, mean (SD) | 29.64 (8.36) | |
| LOS, mean days (SD) | 7.86 (7.52) | |
| Albumin g/dL, mean (SD) | 3.72 (0.55) | |
| Alkaline phosphatase U/L, median [IQR] | 79.00 [62.00, 106.00] | |
| ALT U/L, median [IQR] | 25.00 [16.00, 40.00] | |
| AST U/L, median [IQR] | 38.00 [26.00, 62.00] | |
| Total bilirubin mg/dL, mean (SD) | 0.58 (0.76) | |
| INR, median [IQR] | 1.10 [1.00, 1.20] | |
| PT seconds, median [IQR] | 14.30 [13.60, 15.60] | |
| PTT seconds, mean (SD) | 35.11 (13.47) | |
| Platelets k/µL, mean (SD) | 229.00 (106.32) | |
| Procalcitonin ng/mL, mean (SD) | 2.43 (7.65) | |
| Fibrinogen mg/dL, mean (SD) | 618.53 (195.04) |
Table 2.
Demographic and Laboratory Data by Clinical Group
| Control | Liver Disease | P Value | ||
|---|---|---|---|---|
| Number | 2,895 | 457 | ||
| Sex (%) | F | 1,488 (51.4) | 230 (50.3) | 0.707 |
| M | 1,407 (48.6) | 227 (49.7) | ||
| Race (%) | Asian | 76 (2.6) | 7 (1.5) | 0.033 |
| Black | 1,149 (39.7) | 155 (33.9) | ||
| Not reported | 1,367 (47.2) | 245 (53.6) | ||
| White | 303 (10.5) | 50 (10.9) | ||
| Ethnicity (%) | Latino | 1,016 (35.1) | 209 (45.7) | <0.001 |
| Not Latino | 1,593 (55.0) | 212 (46.4) | ||
| Not reported | 286 (9.9) | 36 (7.9) | ||
| Expired (%) | No | 2,126 (73.4) | 322 (70.5) | 0.202 |
| Yes | 769 (26.6) | 135 (29.5) | ||
| Intubated (%) | No | 2,373 (82.0) | 349 (76.4) | 0.005 |
| Yes | 522 (18.0) | 108 (23.6) | ||
| Age, mean years (SD) | 64.88 (17.04) | 64.23 (13.84) | 0.444 | |
| BMI, mean (SD) | 29.69 (8.51) | 29.30 (7.38) | 0.364 | |
| LOS, mean days (SD) | 7.80 (7.47) | 8.21 (7.81) | 0.272 | |
| Albumin g/dL, mean (SD) | 3.72 (0.54) | 3.68 (0.60) | 0.108 | |
| Alkaline phosphatase U/L, median [IQR] | 78.00 [62.00, 103.00] | 89.00 [66.00, 127.75] | <0.001 | |
| ALT U/L, median [IQR] | 25.00 [16.00, 40.00] | 26.00 [16.00, 41.00] | 0.27 | |
| AST U/L, median [IQR] | 38.00 [25.00, 61.00] | 40.00 [27.00, 69.00] | 0.047 | |
| Total bilirubin mg/dL, mean (SD) | 0.55 (0.57) | 0.81 (1.44) | <0.001 | |
| INR, median [IQR] | 1.10 [1.00, 1.20] | 1.10 [1.00, 1.30] | <0.001 | |
| PT seconds, median [IQR] | 14.20 [13.50, 15.40] | 14.80 [13.80, 16.90] | <0.001 | |
| PTT seconds, mean (SD) | 35.10 (13.86) | 35.14 (10.63) | 0.953 | |
| Platelets k/µL, mean (SD) | 231.40 (102.30) | 213.84 (127.96) | 0.001 | |
| Fibrinogen mg/dL, mean (SD) | 622.34 (192.48) | 592.64 (210.37) | 0.06 | |
| Procalcitonin ng/mL, mean (SD) | 2.49 (7.83) | 2.08 (6.37) | 0.48 |
Fisher’s exact test was used on all nominal variables due to the small sample size; one‐way test/t test for continuous analysis; Kruskal‐Wallis test for nonparametric analysis.
Length of stay (LOS) was 7.80 days (SD, 7.47) for the nonliver disease cohort and 8.21 days (SD, 7.81) for the liver disease cohort (P = 0.269); 23.6% of patients with liver disease required intubation compared to 18.0% patients without liver disease (P = 0.005). The median ALT U/L (reference range <30 U/L) in the control group and liver disease group was 16.00 U/L (interquartile range [IQR], 12.00, 23.00) and 20.00 U/L (IQR, 13.00, 32.75), respectively (P < 0.001). The median AST (reference range <40 U/L) in the control group and liver disease group was 20.00 U/L (IQR, 20.00, 26.00) and 23.00 U/L (IQR, 20.00, 37.00), respectively (P < 0.001). Markers of inflammation (C‐reactive protein, ferritin, procalcitonin) were similar in both groups. A total of 26.6% of the control group died during the hospital admission compared to 29.5% of the liver disease group (P = 0.206). Baseline laboratory values and vital signs for both cohorts are presented in Table 2.
Categories of Liver Disease
The percentage of patients in different liver disease categories was 4.2% with ALD, 64.8% with mixed/other, 13.1% with NAFLD, and 17.9% with viral liver disease. The mean age, race, sex, and LOS were not different between the etiology subgroups. Patients with NAFLD had a mean BMI of 31.61 (SD, 6.62). Intubation was required for 21.1% of patients with ALD, 22.6% with mixed etiology, 29.7% with NASH/NAFLD, and 22.4% with viral hepatitis. A total of 83/457 (18.1%) patients with liver disease had cirrhosis or advanced hepatic fibrosis.
Outcomes of Patients With Specific Liver Diseases and Cirrhosis
Overall mortality in the cohort was 27.0%, and the mean LOS was 7.85 days (SD, 7.52). In patients without liver disease, mortality was 26.6%, proportion intubated was 18.0%, and mean LOS was 7.80 (SD, 7.47). However, liver disease of any type when compared to the control group was associated with a mortality of 29.5% (P = 0.202), intubation rate of 23.6% (P = 0.005), and LOS of 8.21 days (SD, 7.81; P = 0.0272). In a subgroup analysis of etiology of liver disease, ALD, NAFLD, mixed/other, and viral hepatitis did not confer a significant risk of mortality. Hazard ratios (HRs) were calculated for all relevant characteristics and controlled for comorbidities. There was no difference in risk of death in patients with all etiologies of liver disease; however, 30/83 (36.1%) patients with cirrhosis died (HR, 1.67; 95% confidence interval, 1.09, 2.55; P = 0.019) (Table 3; Fig. 1). After stratifying patients into a cirrhosis subgroup, there was no clear impact of baseline MELD on overall survival. There was a significant difference in initial MELD score versus final MELD (P < 0.001) for patients who died (Fig. 2). Patients with cirrhosis were further classified into those with a prior history of compensated (n = 67) or decompensated (n = 16) liver disease; 22/67 (32.8%) patients with compensated liver disease died, and 8/16 (50%) patients with decompensated liver disease died. There was no statistical significance between these two groups (P = 0.250), although this was limited by the small sample size. The most common cause of death in the cirrhosis population was respiratory failure. There were no documented cases of COVID‐related liver failure as the cause of death (see Supporting Materials).
Table 3.
Demographic and Laboratory Data by Clinical Outcome
| Survived | Expired | P Value | ||
|---|---|---|---|---|
| Number | 53 | 30 | ||
| Sex (%) | F | 25 (47.2) | 15 (50.0) | 0.985 |
| M | 28 (52.8) | 15 (50.0) | ||
| Intubated (%) | No | 50 (94.3) | 11 (36.7) | <0.001 |
| Yes | 3 (5.7) | 19 (63.3) | ||
| Age, mean years (SD) | 66.30 (11.77) | 70.33 (11.35) | 0.133 | |
| BMI, mean (SD) | 27.89 (5.85) | 26.31 (5.61) | 0.24 | |
| LOS, mean days (SD) | 7.04 (6.03) | 6.13 (6.46) | 0.524 | |
| Albumin g/dL, mean (SD) | 3.52 (0.62) | 3.08 (0.78) | 0.007 | |
| Alkaline phosphatase U/L, median [IQR] | 133.00 [84.75, 169.25] | 133.00 [103.00, 190.00] | 0.522 | |
| ALT U/L, median [IQR] | 27.50 [19.00, 41.00] | 32.00 [16.00, 38.00] | 0.708 | |
| AST U/L, median [IQR] | 53.00 [36.00, 84.00] | 78.00 [50.25, 103.75] | 0.075 | |
| Total bilirubin mg/dL, mean (SD) | 1.21 (1.11) | 2.48 (4.58) | 0.059 | |
| INR, median [IQR] | 1.20 [1.10, 1.30] | 1.30 [1.20, 1.50] | 0.064 | |
| PT seconds, median [IQR] | 15.45 [14.12, 17.08] | 17.00 [15.45, 18.65] | 0.056 | |
| PTT seconds, mean (SD) | 35.60 (8.15) | 38.67 (10.70) | 0.203 | |
| Platelets k/µL, mean (SD) | 108.15 (66.61) | 145.80 (78.90) | 0.023 | |
| Procalcitonin ng/mL, mean (SD) | 1.17 (1.85) | 3.85 (6.90) | 0.093 | |
| Fibrinogen mg/dL, mean (SD) | 452.11 (175.11) | 423.44 (258.48) | 0.732 |
Fisher’s exact test was used on all nominal variables due to the small sample size; one‐way test/t test for continuous analysis; Kruskal‐Wallis test for nonparametric analysis.
FIG. 1.
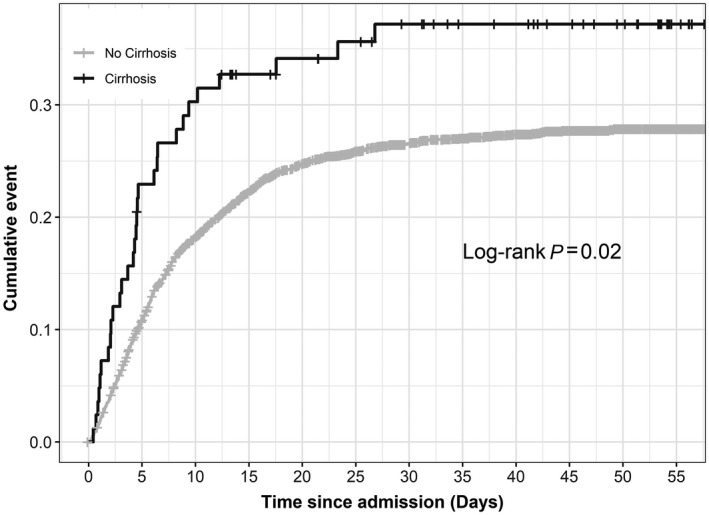
Cumulative mortality in COVID‐positive admissions by cirrhosis status. A total of 30/83 (36.1%) patients with cirrhosis died over a 60‐day interval. This was significantly different compared to the mortality of the remainder of the cohort (no cirrhosis); P = 02.
FIG. 2.
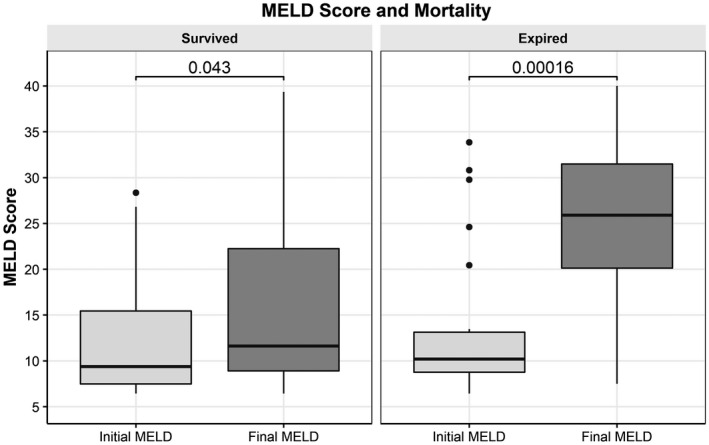
Mortality and MELD score at admission compared to MELD score at discharge or death in patients with cirrhosis. Unpaired Wilcoxon test was used for this analysis. MELD score was calculated on admission and at death or discharge. There was a significant difference between the calculated MELD scores in patients who died during the admission. Graphs show IQR (box), median (horizontal line), and outliers (whiskers).
Admission AST and ALT and Survival
Initial ALT and AST levels for all patients were investigated to determine mortality outcome. ALT was stratified into normal, 1‐5 times the upper limit of normal (ULN), and >5 times the ULN. AST was divided into normal, 1‐3 times the ULN, and >3 times the ULN. There was a statistically significant difference between both the initial ALT and AST level (P < 0.0001; Figs. 3 and 4).) Patients with an ALT >5 times the ULN at admission had 45% mortality at 60 days, and patients with AST >3 times the ULN had 50% 60‐day mortality. A total of 315/1,656 (19%) patients with a normal AST expired, and 482/1,935 (24.9%) patients with a normal ALT expired.
FIG. 3.
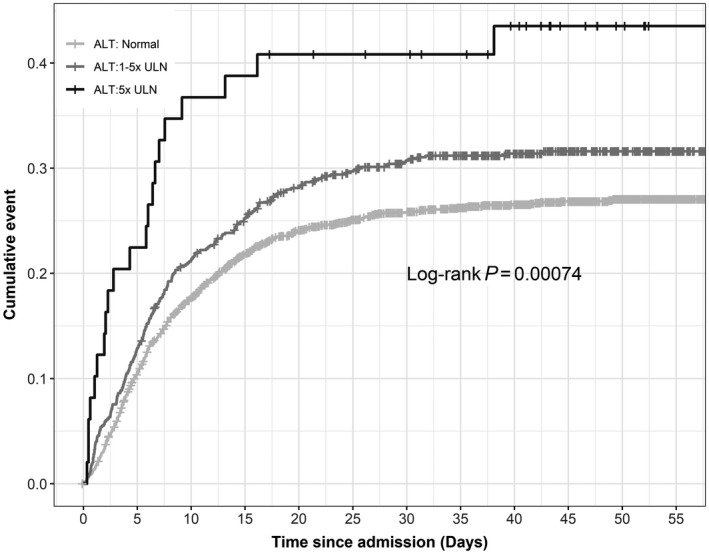
Cumulative mortality in patients positive for COVID by initial ALT result at admission. Baseline ALT values were subdivided into usual cut‐off points. A total of 39/86 (45%) patients died when the initial ALT was >5 times the ULN; P < 0.0001.
FIG. 4.
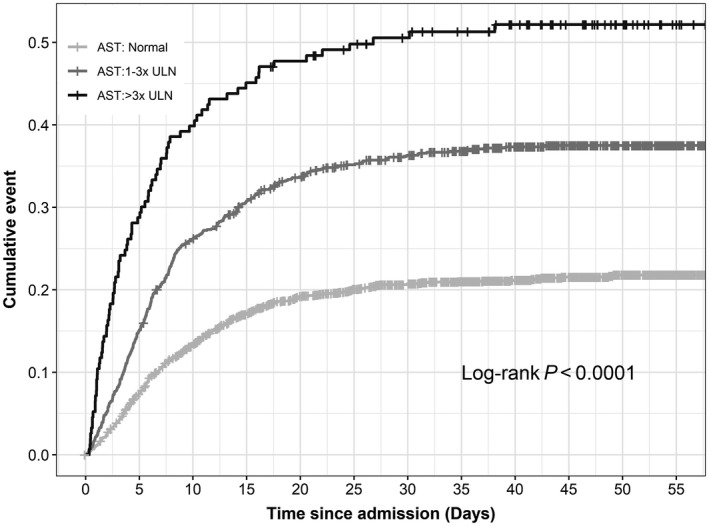
Cumulative mortality in patients positive for COVID by initial AST result at admission. Baseline AST values were subdivided into usual cut‐off points. A total of 115/230 (50%) patients died when the initial AST was >3 times the ULN; P < 0.0001.
Discussion
Since the start of the COVID‐19 pandemic in late December 2019, there has been a burgeoning body of literature defining the nature of COVID‐19‐related hepatic injury and comorbidities that are associated with COVID‐19 mortality. There has only been one U.S.‐based study analyzing the relationship between elevated AST and ALT in conjunction with COVID‐19 outcome.( 33 ) There have also been prior studies characterizing the effect of medical conditions on patients infected with SARS‐CoV2.( 7 , 8 , 10 ) Advanced age, cardiopulmonary disease, and metabolic syndrome are all associated with risk of death and morbidity in COVID‐19. The U.S. Centers for Disease Control and Prevention (CDC) has now characterized chronic liver disease as a high‐risk condition for COVID‐19‐related mortality.( 34 )
We initially investigated the prognostic ability of admission AST and ALT on overall COVID‐19‐related mortality. The higher the ALT/AST level was on admission, the more likely it was that mortality events would occur. A mortality of 45% and 50% was noted for the highest cutoff of ALT and AST, respectively. This observation was made for all patients in our cohort. These elevations of the liver biochemical profile may be related to the degree of inflammatory response precipitated by COVID‐19( 35 ) and may be considered an acute COVID‐19 hepatitis. Admission values of AST/ALT showed a significant difference between the control, liver disease, and cirrhosis groups using the Kruskal‐Wallis rank sum from a statistical sense (P < 0.001), but the values are so close that clinically the difference among the groups is minimal. There was a statistical difference in final AST between the control, liver disease, and cirrhosis groups. The rate/velocity of AST/ALT change was not analyzed.
A retrospective study in the United States with a mixed inpatient/outpatient cohort used ICD‐10 coding to determine liver disease. This study showed a liver disease‐related relative mortality risk (RR) of 2.8 and a cirrhosis RR of 4.6, indicating that liver disease is a risk factor for COVID mortality.( 28 ) Other studies from Italy and the United Kingdom also showed an increased risk of death for patients who were hospitalized with COVID‐19 with a history of preexisting liver disease and cirrhosis.( 31 , 32 ) These studies were limited by data collection from the electronic medical records, general characterization of liver disease, and lack of granular data, such as a definition of cirrhosis.
Our study evaluated the impact of underlying liver disease/severity of liver disease on COVID‐19 in a large cohort of hospitalized patients at a tertiary care center. Our hospital is in the Bronx, New York, which was one of the hardest hit communities in New York; the state has been recently considered the epicenter of the world COVID‐19 pandemic. Additionally, this is the only study to use well‐validated markers of fibrosis using a combination of transient elastography( 36 ) and FIB‐4 scores( 37 ) to determine the liver disease subgroup with cirrhosis. We were able to assess the MELD score and to determine hospital mortality in this patient population.
Our data suggest that preexisting liver disease may result in a small increase of COVID‐19‐associated mortality compared to those without liver disease (29.5% of patients with liver disease died compared to 26.6% of those without liver disease), but these results did not meet statistical significance. However, advanced liver disease (cirrhosis) had a significant negative impact on survival. The cirrhosis population died mainly of respiratory failure. The mechanistic basis for the observed increased mortality in patients with advanced liver disease is uncertain but could potentially be related to direct coronavirus effects on the liver parenchyma through the angiotensin‐converting enzyme 2 receptor,( 30 ) virus‐mediated systemic inflammation,( 21 ) and potentially the impact of impaired immune responses associated with advanced liver disease.( 38 ) There have been studies evaluating the effects of other Coronavirus species effects on the liver.( 39 ) Patients with preexisting liver disease, particularly viral hepatitis, were susceptible to liver chemistry elevations, although the reviewed cohorts were a heterogeneous population of patients.( 40 ) The MELD score is a verified prognostic score for patients with cirrhosis; however, the initial MELD score in our cohort did not show a statistically significant prognostic effect for the cutoff that was used. There was a significant difference between the initial and final MELD of the cohort, and perhaps the initial MELD score does not capture the acute inflammatory response that is at the center of COVID‐19‐related mortality. Patients who died from COVID‐19 had an elevated final MELD score as a measure of liver failure, which is a component of the multiorgan failure that is induced by SARS‐CoV2.
We further analyzed whether the etiology of liver disease had a differential impact on COVID‐19 infection. No specific category of liver disease (ALD, NAFLD, other, or viral hepatitis) appeared to have an impact on survival.
There are a few limitations to this study. We used ICD‐10 codes to define the liver group cohort. It is possible that patients were misclassified or inappropriately excluded from the liver disease cohort. To overcome this limitation, we performed an audit of our etiology by classification using hepatitis B and C viral‐load values in conjunction with urine alcohol screening tools to validate our patient selection. An additional limitation is that this analysis was performed using a large database (EDW) that demonstrated a higher baseline mortality rate in all patients with COVID‐19 compared to other studies. Lastly, our cohort analyzed data from inpatients alone. Emergency department visits and outpatient follow‐up were not performed.
In conclusion, this is the first study to stratify COVID‐19 outcomes in hospitalized patents by liver disease etiology and severity, with clear fibrosis cut‐off guidelines. This study also shows an AST/ALT prognostic benefit that implies the possibility of COVID‐19‐induced hepatitis. We also analyzed the use of MELD score in both prognosis and utility at the time of death. The study shows the effect of liver disease on the need for mechanical ventilation in COVID‐19. This study also shows that there is an increased mortality for patients with cirrhosis who are admitted with COVID‐19, and this observation should influence decisions for hospital admission and convince governmental agencies, such as the CDC, to include cirrhosis as a risk factor for COVID‐19 mortality.
Supporting information
Supplementary Material
Supported in part by the National Institutes of Health (grant P30 DK041296 to S.Z.F.).
Potential conflict of interest: Nothing to report.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. Erratum in: Lancet 2020; PMID:32007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. John Hopkins University and Medicine . Coronavirus Resource Center. COVID‐19 United States cases by county. https://coronavirus.jhu.edu/us‐map. Updated daily. Accessed November 2020.
- 4. New York State Department of Health . NYSDOH COVID‐19 Tracker. https://covid19tracker.health.ny.gov/views/NYS‐COVID19‐Tracker/NYSDOHCOVID‐19Tracker‐Map?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n. Updated daily. Accessed May 2020.
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. Erratum in: Lancet 2020; PMID:32171424 and PMID:32192581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sommer P, Lukovic E, Fagley E, Long D, Sobol J, Heller K, et al. Initial clinical impressions of the critical care of COVID‐19 patients in Seattle, New York City, and Chicago. Anesth Analg 2020;131:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052‐2059. Erratum in: JAMA 2020; PMID:32330939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al.; Washington State 2019‐nCoV Case Investigation Team . First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rentsch CT, Kidwai‐Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid‐19 by race and ethnicity: a national cohort study of 6 million United States veterans. medRxiv 2020; 10.1101/2020.05.12.20099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS‐CoV) infection ‐ clinicopathological and ultrastructural study. Histopathology 2018;72:516‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID‐19: abnormal liver function tests. J Hepatol 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology 2020;72:389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, et al. Liver biochemistries in hospitalized patients with COVID‐19. Hepatology 2020; 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 18. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol 2020;5:532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Judaibi B, Almaghrabi R, Alghamdi M, Al‐Hamoudi WK, AlQahtani M, Abaalkhail F, et al. Saudi association for the study of liver diseases and transplantation position statement on liver transplantation during the COVID‐19 pandemic. Saudi J Gastroenterol 2020;26:233‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol 2020;8:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O'Meara MJ, et al. A SARS‐CoV‐2‐human protein‐protein interaction. map reveals drug targets and potential drug‐repurposing. bioRxiv 2020; 10.1101/2020.03.22.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses 2012;6:e2‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell 2020;181:1036‐1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159:768‐771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology 2020;72:287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al.; The OpenSAFELY Collaborative . OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020; 10.1101/2020.05.06.20092999. [DOI] [Google Scholar]
- 32. Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol 2020;73:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, et al. Analysis of gastrointestinal and hepatic manifestations of SARS‐CoV‐2 infection in 892 patients in Queens, NY. Clin Gastroenterol Hepatol 2020;18:2378‐2379.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. CDC updates, expands list of people at risk of severe COVID‐19 illness [press release]. Atlanta, GA: Centers for Disease Control and Prevention; June 25, 2020. Available at: https://www.cdc.gov/media/releases/2020/p0625‐update‐expands‐covid‐19.html. Accessed [Google Scholar]
- 35. Schaefer EAK, Arvind A, Bloom PP, Chung RT. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis (Hoboken) 2020;15:175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castera L, Forns X, Alberti A. Non‐invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835‐847. [DOI] [PubMed] [Google Scholar]
- 37. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 38. Huang JF, Zheng KI, George J, Gao HN, Wei RN, Yan HD, et al. Fatal outcome in a liver transplant recipient with COVID‐19. Am J Transplant 2020;20:1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS‐associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 2004;39:302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang JT, Sheng WH, Fang CT, Chen YC, Wang JL, Yu CJ, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 2004;10:818‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


