Abstract
Neutrophils are the most abundant white blood cell in the body and are key participants in the defense against fungal infections. Fungal infections occur often in patients with cirrhosis and are associated with increased 30‐day and 90‐day mortality. Previous studies have shown that specific neutrophil functions are abnormal in patients with cirrhosis, although the extent of neutrophil dysfunction is not well understood. We tested the ability of neutrophils from 21 hospitalized patients with cirrhosis and 23 healthy control patients to kill Candida albicans, a common fungal pathogen in patients with cirrhosis. Using an assay, we also measured the ability of neutrophils to coordinate multicellular, synchronized control of C. albicans hyphae through a process known as swarming. We found that neutrophils from patients with cirrhosis have significantly decreased fungicidal capacity compared with healthy control neutrophils (53% vs. 74%, P < 0.0001) and diminished ability to control hyphal growth normalized as a ratio to healthy control (0.22 vs. 0.65, P < 0.0001). Moreover, serum from patients with cirrhosis decreases the ability of healthy control neutrophils to kill C. albicans (from 60% to 41%, P < 0.003). Circulating concentration of the inflammatory cytokines tumor necrosis factor α, interleukin‐6, and interleukin‐8 were found to be significantly elevated in patients with cirrhosis compared to healthy controls. Following pretreatment with granulocyte‐colony stimulating factor and granulocyte‐macrophage colony‐stimulating factor, neutrophil function was restored to almost that of healthy controls. Conclusion: Our data establish profound neutrophil dysfunction against, and altered swarming to, C. albicans in patients with cirrhosis. This dysfunction can be partially reversed with cytokine augmentation ex vivo.
Neutrophils from patients with cirrhosis are defective in coordinated swarming response to the human fungal pathogen, Candida albicans.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANC
absolute neutrophil count
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- DM
diabetes mellitus
- EDTA
ethylene diamine tetraacetic acid
- EtOH
alcoholic
- FQ
fluoroquinolones
- G‐CSF
granulocyte‐colony stimulating factor
- GM‐CSF
granulocyte‐macrophage‐colony‐stimulating factor
- Hb
hemoglobin
- HCV
hepatitis C virus
- IL
interleukin
- MELD
Model for End‐Stage Liver Disease
- MOI
multiplicity of infection
- MOPS
3‐(N‐morpholino)propane sulfonic acid
- NAFLD
nonalcoholic fatty liver disease
- PPI
proton pump inhibitor
- ROS
reactive oxygen species
- RPMI
Roswell Park Memorial Institute 1640 medium
- SBP
spontaneous bacterial peritonitis
- TNF‐α
tumor necrosis factor α
- WBC
white blood cell
Neutrophils are the most abundant white blood cell in the body and are an important part of the innate immune system defense against bacterial and fungal infections. Patients with cirrhosis, especially in those with end‐stage liver disease and critical illness, are known to be at increased risk of fungal infections, which is associated with increased mortality.( 1 , 2 , 3 ) A diverse number of fungal species can infect patients with cirrhosis, including Candida species, Cryptococcus, and molds such as Aspergillus.( 2 , 3 , 4 , 5 ) Although prior studies have demonstrated neutrophil defects in response to bacterial and fungal pathogens,( 6 , 7 , 8 , 9 , 10 , 11 ) this study additionally highlights how both soluble extrinsic and cell‐intrinsic factors result in neutrophil defects with decreased ability to control C. albicans.
Neutrophils respond to infection through a multistep process that begins with migration to the site of infection. Recently, the process of neutrophil migration and accumulation at sites of infection was determined to involve neutrophil–neutrophil cooperation and positive‐feedback intercellular loops that accelerate neutrophil accumulation and enhance antifungal effectiveness.( 12 ) This process, termed “swarming,” is essential for restricting and killing pathogens.( 13 , 14 ) Neutrophils neutralize pathogens by phagocytosis, generation of reactive oxygen species (ROS), degranulation and release of antimicrobial proteins, and formation of neutrophil extracellular traps (NETs).( 15 , 16 ) Even at homeostasis, neutrophils are short‐lived cells with an estimated half‐life of 8‐18 hours.( 15 ) Despite their short life span, many variables can influence neutrophil function, including medications and cytokines. Beta blockers, for instance, can decrease oxidative burst.( 17 ) On the other hand, growth factors including granulocyte‐colony stimulating factor (G‐CSF) and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) have been shown to augment or “prime” neutrophil function, allowing for enhanced response to pathogens.( 18 , 19 )
In patients with cirrhosis, specific neutrophil functions including phagocytosis, generation of ROS, and intracellular killing are impaired when neutrophils are exposed to bacterial components or toxins.( 6 , 7 , 9 ) With the exception of rare studies,( 20 ) these functions have been found to be impaired when neutrophils from patients with cirrhosis interact with C. albicans.( 8 , 9 , 11 ) Although migration of neutrophils from patients with cirrhosis is defective, the coordinated process of swarming has not been previously studied.( 8 , 9 , 21 ) In addition, use of cytokine augmentation with GM‐CSF has been shown to improve neutrophil phagocytosis in patients with cirrhosis, but has not been studied in clinical trials.( 11 ) Similarly, G‐CSF has also been observed to improve neutrophil phagocytosis and generation of ROS.( 22 , 23 ) Clinical trials treating patients with compensated and decompensated cirrhosis with G‐CSF demonstrate contradictory results.( 23 , 24 , 25 , 26 , 27 )
We therefore postulated that neutrophils from patients with decompensated cirrhosis have a decreased ability to control the fungal pathogen C. albicans through loss of a coordinated swarming effort. We hypothesize this defect is due in part to both cell‐intrinsic and cell‐extrinsic defects, including serum cytokine levels. We further sought to define the effect of G‐CSF and GM‐CSF on the synchronized, swarming response of neutrophils, to determine whether this critical function is responsive to cytokine augmentation.
Materials and Methods
Materials
Nonidet P40 (NP40) was purchased from American Bioanalytical (Natick, MA). Culture medias used included liquid YPD (1% yeast extract, 2% peptone, 2% dextrose; Sigma‐Aldrich, St. Louis, MO), liquid MOPS‐RPMI (Roswell Park Memorial Institute 1640 medium containing 2% glucose and 0.165 M c3‐[N‐morpholino]propane sulfonic acid [MOPS], buffered at pH 7), and complete RPMI (RPMI with 2 mM l‐glutamine, 10% heat‐inactivated fetal bovine serum [FBS], and 1% penicillin‐streptomycin; Thermo Fisher Scientific, Waltham, MA).
Wild‐type C. albicans (SC5314) was purchased from the American Type Cell Culture (Manassas, VA). Yeast cultures were grown overnight in liquid YPD at 30°C with shaking, washed twice in phosphate‐buffered saline (PBS) after collection, counted with a Luna automated cell counter (Logos Biosystems, Annandale, VA), and resuspended in PBS at the desired inoculum.
Patients and Data Collection
The study was performed following the 1975 Declaration of Helsinki and was approved by the institutional review board of the Massachusetts General Hospital (MGH). Twenty‐one patients with cirrhosis diagnosed by either liver biopsy or imaging admitted to the MGH and 23 healthy control patients presenting to a local primary care clinic were identified from September 2018 to April 2019. Written, informed consent was then obtained before sample and data collection. Sample collection consisted of obtaining one 10‐mL sample of peripheral blood in an ethylene diamine tetraacetic acid (EDTA)–coated tube at room temperature at the time of routine phlebotomy. Samples were collected and stored at room temperature for a maximum of 4 hours before processing. Data collected included age, race/ethnicity, etiology of cirrhosis, evidence of ascites, evidence of spontaneous bacterial peritonitis (SBP) by cell count or microbial culture, need for renal replacement therapy at least twice per week in the 2 weeks before sample collection, serum sodium, potassium, blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin levels, as well as complete blood cell counts including leukocyte differential. Such baseline lab values were collected either the day of, or up to 2 days before, consent. Model of End‐Stage Liver Disease (MELD) scores were calculated from the collected data. Medication use including midodrine, octreotide, beta blockers, proton pump inhibitors (PPIs), corticosteroids, vasopressors, antibiotics, and antifungals was recorded. Evidence of C. albicans colonization, defined by at least two cultures positive for C. albicans from two separate sites in the past 6 months, and evidence of other bacterial and fungal infections during admission was recorded.
Neutrophil Isolation
The buffy coat layer was isolated from each tube of whole blood through centrifugation. Plasma samples were collected and stored at −80°C for each patient. Neutrophils were isolated by negative selection from the buffy coat layer using the EasySep Direct Human Neutrophil Isolation Kit (STEMCELL Technologies, Cambridge, MA). In accordance with the manufacturer protocol, neutrophils underwent a series of incubations in the EasyEights EasySep Magnet (STEMCELL Technologies). The recovered neutrophils were washed and counted using an acridine orange/propidium iodide (AO/PI) viability dye on an automated cell counter (LUNA; Logos Biosystems, Annandale, VA). Purified neutrophils were resuspended in RPMI. To ensure purity, neutrophil preparations were also subjected to Wright‐Giemsa staining by staining 4 minutes in Wright, followed by 12 minutes in 20% Giemsa, then visualized with light microscopy. Final neutrophil purity and viability was measured at over 94%.
Killing Assays
Killing assays were performed in 96‐well clear‐bottom plates. A total of 5 × 104 human neutrophils were plated with C. albicans at a multiplicity of infection (MOI) of five in 100 μL of complete RPMI. The plates were incubated at 37°C and 5% CO2 for 2 hours. Following the initial incubation, mammalian cells were lysed with 4‐times NP40, and each well received an addition of optimized yeast growth media (MOPS‐RPMI) to supplement Candida growth. Finally, 10% PrestoBlue Cell Viability Reagent (Thermo Fisher Scientific) was added to each well. The plates were then incubated at 35°C and 5% CO2 until percent remaining live pathogen was measured at a predetermined endpoint in a plate reader (SpectraMax i3x; Molecular Devices, Sunnyvale, CA). Results were reported as the percent of pathogens killed by the neutrophils (“percent killing”).
To determine the effects of patient serum on neutrophil function, plasma samples were thawed and chelated with CaCl2 (20% m/v) at a 1:100 ratio to remove EDTA (as EDTA was observed to kill C. albicans independently) and convert plasma to serum. Healthy control neutrophils were incubated at 37°C and 5% CO2 with Candida at a MOI of 10 for 2 hours in RPMI containing 20% serum from healthy control and patients with cirrhosis. Mammalian cell lysis and percent remaining live pathogen was measured as described previously.
To determine the medication effects on neutrophil function, 5 × 104 human neutrophils were plated in 100 μL of complete RPMI media containing varying concentrations of drug and incubated at room temperature for 5 minutes. C. albicans was then added to wells at a MOI of 10. The plates were incubated at 37°C and 5% CO2. Following the initial incubation, cell viability was confirmed with AO/PI to ensure the drugs were not toxic to the neutrophils. Then, mammalian cells were lysed, and the percent remaining live pathogen was measured as described previously.
Swarming Assays
Neutrophil swarming was measured using arrays of clusters of C. albicans. Using a microarray printing platform (Picospotter PolyPico, Galaway, Ireland), arrays of spots using a solution of poly‐l‐lysine (Sigma‐Aldrich), Zetag, and fluorescein isothiocyanate (added to visualize the spots by microscopy) were printed onto ultraclean glass slides (Thermo Fisher Scientific). Slides were screened for accuracy and then dried for at least 2 hours before use. Sixteen‐well ProPlate wells (Grace Bio‐labs, Bend, OR) were attached to the glass slides with printed arrays. A total of 50 µL of a suspension of C. albicans inoculums in water was added to each well and incubated with rocking for 5‐10 minutes. Following incubation, wells were thoroughly washed with water to remove unbound yeast from the glass surface. Wells were screened to ensure appropriate patterning of targets onto the spots with minimal nonspecific binding before use. Swarming was observed using a Nikon Ti‐E microscope (Tokyo, Japan). Time‐lapse imaging was conducted using a ×10 Plan Fluor Ph1 DLL (numerical aperture [NA] = 0.3) lens, and endpoint images were taken with a ×2Plan Apo (NA = 0.10) lens. Swarming targets to be observed during the experiment were selected and saved using the multipoint function in NIS‐Elements before loading. A total of 5 × 105 neutrophils in Iscove’s modified Dulbecco’s medium with 20% FBS were then added to each well. All selected points were optimized for perfect focus before launching the experiment. For cells treated with cytokines, neutrophils were pre‐incubated for 30 minutes with G‐CSF at 300 ng/mL or GM‐CSF at 0.2 ng/mL before use. Hyphal escape analysis was performed by manually observing the time lapses to identify the time that C. albicans hyphae escaped the area of the neutrophil swarm. Fungal growth area analysis was done in ImageJ software by manually outlining the area covered by fungal growth after 16 hours of incubation.
Serum Cytokine Quantification
Patient plasma samples were assayed for serum cytokine levels using a Luminex assay (Biotechne, Minneapolis, MN). In brief, samples were thawed at 4°C before assay and kept on ice throughout the assay procedures. Manufacturers’ protocols were followed for all panels, with a general protocol as follows: All kit components were brought to room temperature. Reagents were prepared as per kit instructions. Assay plates (96‐well) were loaded with assay buffer, standards, samples, and beads and then covered and incubated on a plate shaker (500 rpm) overnight at 4°C. After primary incubation, plates were washed twice, and then detection antibody cocktail was added to all wells; the plates were covered and left to incubate at room temperature for 1 hour on a plate shaker. Streptavidin‐phycoerythrin fluorescent reporter was then added to all wells, and plates were covered and incubated for 30 minutes at room temperature on a plate shaker. Plates were then washed twice, beads were resuspended in sheath fluid, placed on a shaker for 5 minutes, and then read on Bio‐Plex 200 following the manufacturers’ specifications and using Bio‐Plex Manager software v6.0.
Statistics
Data are presented as mean ± SD. Groups were compared using the Mann‐Whitney U test. For comparison of more than two groups, parametric or nonparametric one‐way analysis of variance was used, where appropriate. Correlations were evaluated with the Spearman’s correlation coefficient (two‐tailed P value). A P value less than or equal to 0.05 was considered statistically significant. GraphPad Prism8 (GraphPad Software Inc., San Diego, CA) software was used.
Results
Patient Baseline Demographics and Clinical Parameters
After obtaining informed consent, 21 hospitalized patients with cirrhosis and 23 healthy control patients were recruited from November 2018 to April 2019. Demographic, clinical, and laboratory information was extracted from the electronic medical record (Tables 1 and 2). In the cirrhosis group, the major etiology of cirrhosis was alcohol‐related (57%, n = 12), followed by nonalcoholic fatty liver disease (NAFLD; 29%, n = 6), hepatitis C virus (HCV; 14%, n = 3), and other (19%, n = 4). Three of those patients had multifactorial cirrhosis and therefore were included in all applicable groups. Fifteen patients with cirrhosis had acute on chronic liver failure, while 6 had acute decompensated cirrhosis without end organ failure. The average MELD score was 28. Fifteen of 23 (71%) had evidence of ascites on admission, and 4 of these patients had evidence of SBP by either cell count or microbial growth from culture. Four of 23 (19%) patients required inpatient renal replacement therapy at least twice per week in the 2 weeks before sample collection. Four of 23 (19%) were in the medical intensive care unit and on vasopressors at the time of sample collection. Most patients with cirrhosis were treated with lactulose (71%, n = 15), rifaximin (76%, n = 16), and PPIs (71%, n = 15). Relatively few patients were being treated with beta blockers (14%, n = 3) or diuretics, including furosemide (19%, n = 4) and spironolactone (29%, n = 6). Patients with cirrhosis had significantly different serum sodium, BUN, creatinine, AST, total bilirubin, albumin, hemoglobin (Hb), and platelet levels compared with healthy controls (Table 2). There was no statistically significant difference in the white blood cell (WBC) count or ALT, and patients with cirrhosis paradoxically had higher absolute neutrophil counts (ANCs) than healthy controls (Table 2).
TABLE 1.
Demographic and Clinical Characteristics of 23 Healthy Controls and 21 Patients With Cirrhosis
| Healthy Controls (n = 23) | Patients With Cirrhosis (n = 21) | |
|---|---|---|
| Age (years) | 49.3 ± 15.2 | 56.8 ± 10.7 |
| Ethnicity, n (%) | ||
| White | 20 (87%) | 20 (95%) |
| Black | 1 (4%) | 0 (0%) |
| Other | 2 (9%) | 1 (5%) |
| Etiology, n (%) | ||
| Alcohol | — | 12 (57%) |
| NAFLD | — | 6 (29%) |
| HCV | — | 3 (14%) |
| Other | — | 4 (19%) |
| Clinical characteristics | ||
| AD | — | 6 (29%) |
| ACLF | — | 15 (71%) |
| Ascites | — | 15 (71%) |
| SBP | — | 4 (19%) |
| DM | — | 5 (24%) |
| Renal replacement therapy | — | 4 (19%) |
| Candida colonization | — | 4 (19%) |
| Medications, n (%) | ||
| Beta‐blockers | 2 (9%) | 3 (14%) |
| Furosemide | 0 | 4 (19%) |
| Octreotide | 0 | 5 (24%) |
| PPI | 0 | 15 (71%) |
| Lactulose | 0 | 15 (71%) |
| Rifaximin | 0 | 16 (76%) |
Data are presented as mean ± SD or number (percentage) as appropriate.
Abbreviations: ACLF, acute on chronic liver failure; AD, acute decompensated cirrhosis.
TABLE 2.
Biochemical and Complete Blood Count Parameters of Healthy Controls and Patients With Cirrhosis
| Healthy Controls | Patients With Cirrhosis | P Value | |||
|---|---|---|---|---|---|
| n | Mean ± SD, range | n | Mean ± SD, range | ||
| Biochemistry/liver injury | |||||
| Sodium (mmol/L) | 17 | 140.2 ± 2.01, 136‐143 | 21 | 136.43 ± 4.47, 125‐149 | 0.0002 |
| BUN (mg/dL) | 17 | 15.35 ± 4.26, 9‐26 | 21 | 32.76 ± 24.97, 7‐111 | 0.0006 |
| Creatinine (mg/dL) | 17 | 0.93 ± 0.2, 0.48‐1.30 | 21 | 1.65 ± 0.87, 0.58‐4.00 | 0.002 |
| ALT (U/L) | 10 | 22.60 ± 12.44, 12‐50 | 21 | 35.57 ± 27.49, 9‐111 | 0.2539 |
| AST (U/L) | 9 | 23.78 ± 4.41, 17‐31 | 21 | 74.33 ± 47.72, 24‐165 | <0.0001 |
| ALP (U/L) | 9 | 63.44 ± 33.17, 4‐101 | 21 | 150.3 ± 52.47, 83‐268 | <0.0001 |
| Total bilirubin (mg/dL) | 9 | 0.62 ± 0.34, 0.3‐1.3 | 21 | 11.80 ± 11.55, 0.5‐37.9 | <0.0001 |
| Albumin (g/dL) | 9 | 4.32 ± 0.38, 3.4‐4.7 | 21 | 3.11 ± 0.58, 1.8‐4.2 | <0.0001 |
| INR | 0 | — | 21 | 2.05 ± 0.72, 1.1‐4.2 | — |
| MELD | — | — | 21 | 27.86 ± 9.23, 13‐45 | — |
| Complete blood cell counts | |||||
| WBC count (103/μL) | 14 | 6.57 ± 1.73, 3.7‐10.04 | 21 | 8.32 ± 4.29, 2.84‐18.44 | NS |
| ANC (103/μL) | 7 | 3.43 ± 1.07, 2.19‐5.32 | 14 | 6.24 ± 3.46, 1.9‐14.59 | 0.0379 |
| Hb (g/dL) | 14 | 14.24 ± 1.28, 12.1‐17 | 21 | 8.58 ± 1.43, 7‐12.2 | <0.0001 |
| Platelets (103/μL) | 14 | 274.6 ± 68.06, 166‐390 | 21 | 85.52 ± 82.03, 15‐311 | <0.0001 |
Data are presented as mean ± SD as appropriate. P values represent Mann‐Whitney U test results.
Abbreviations: INR, international normalized ratio; NS, no significance.
Neutrophil Killing of C. albicans
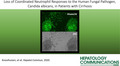
Neutrophil killing of C. albicans ex vivo is significantly decreased in patients with cirrhosis compared with healthy controls (53 ± 14% vs. 74 ± 11%, P < 0.0001) (Fig. 1A). Interestingly, neutrophil fungicidal activity did not correlate with higher MELD scores (r = 0.32, P = 0.23) (Fig. 1B). Candida killing capacity was not associated with ALT, ALP, or albumin (Fig. 1B). In addition, neutrophil function was not significantly correlated with any hematologic parameter (Fig. 1C). Neutrophil function was not significantly different in patients among various etiologies of cirrhosis, with neutrophil killing of C. albicans of 54%, 45%, 57%, and 61% in patients with alcohol, NAFLD, HCV, and other etiologies of cirrhosis, respectively (Fig. 1D). Given the importance of diabetes to neutrophil function, we examined the correlation of diabetes in the cirrhosis cohort. The presence of diabetes mellitus (DM) in patients with cirrhosis was not significantly related to Candida killing capacity (DM, 57.60 ± 18.30%; no DM, 50.13 ± 12.94%; P = 0.28). We also investigated the potential impact of antimicrobials on immune function. Patients with cirrhosis receiving fluoroquinolones (FQ) had a small, but significant decrease in fungicidal activity when compared with those patients not receiving FQ (FQ, 43.33 ± 9.56%; no FQ, 55.33 ± 14.61%; P < 0.05). Removing patients with FQ use did not alter the significant difference in Candida killing capacity between healthy controls versus patients with cirrhosis (percent killing of patients with cirrhosis not receiving FQ = 55.33 ± 14.61% vs. healthy controls = 71.88 ± 10.63%; P < 0.005). These results suggest an intrinsic neutrophil defect contributes to decreased C. albicans killing in patients with cirrhosis.
FIG. 1.
Neutrophils from patients with cirrhosis have a diminished ability kill C. albicans. (A) Neutrophil percent killing of C. albicans is significantly decreased in patients with cirrhosis (n = 21) compared to healthy controls (n = 14). Neutrophil percent killing of C. albicans is not correlated with biochemical measures of liver function, including MELD scores (B), nor WBC count, ANC, Hb, or platelet count (C). (D) Neutrophil percent killing of C. albicans does not differ in patients with alcoholic (EtOH), NAFLD, HCV, or other etiologies of cirrhosis. ****P < 0.0001 (Mann‐Whitney U test). Abbreviation: EtOH, ethanol.
Serum Effect on Healthy‐Control Neutrophil Fungicidal Capacity
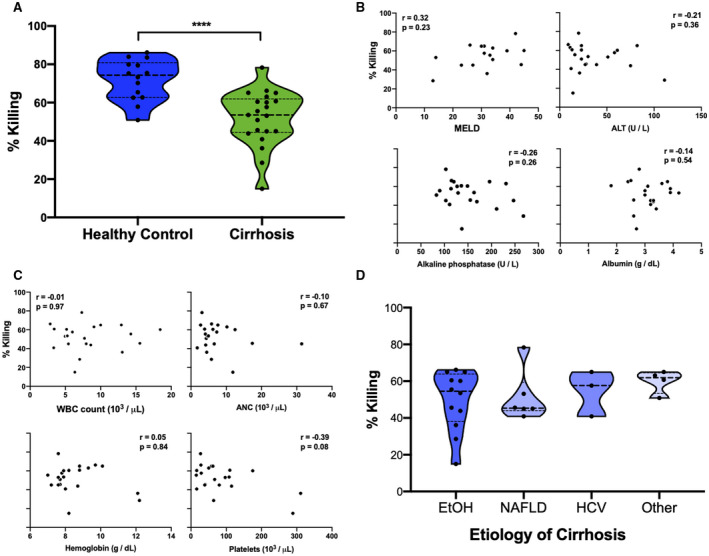
Incubation of healthy control neutrophils with serum from patients with cirrhosis, but not with serum from healthy control patients, significantly decreased fungicidal activity (cirrhosis serum, 41 ± 19%; healthy control serum, 60 ± 12%; P < 0.003) (Fig. 2A). The neutrophil killing defect was not significantly related to MELD score nor serum ALT values (Fig. 2B). The defect was also not significantly related to the etiology of cirrhosis or hematologic parameters (data not shown). Interestingly, neutrophil killing was negatively correlated with albumin (r = −0.25, P = 0.28) and ALP levels (r = −0.35, P = 0.13) (Fig. 2B) in serum from patients with cirrhosis, although they did not reach statistical significance. Of note, patients with higher serum albumin levels had not received more albumin infusions before sample collection, although MELD scores were higher. These data suggest that there is an additional extrinsic serum factor that affects neutrophil function. We therefore tested the effect of medications commonly used to manage cirrhosis on healthy‐control neutrophil function, and additionally measured circulating cytokine levels.
FIG. 2.
Serum from patients with cirrhosis impairs fungicidal capacity of healthy neutrophils. (A) Incubation of healthy neutrophils with serum from patients with cirrhosis (n = 20) significantly decreased neutrophil percent killing of C. albicans compared with incubation with control serum (n = 23). (B) The decrease in neutrophil killing capacity was not associated with calculated end‐stage liver disease (MELD) scores, ALT levels, ALP, or albumin in serum from patients with cirrhosis. **P < 0.002 (Mann‐Whitney U test).
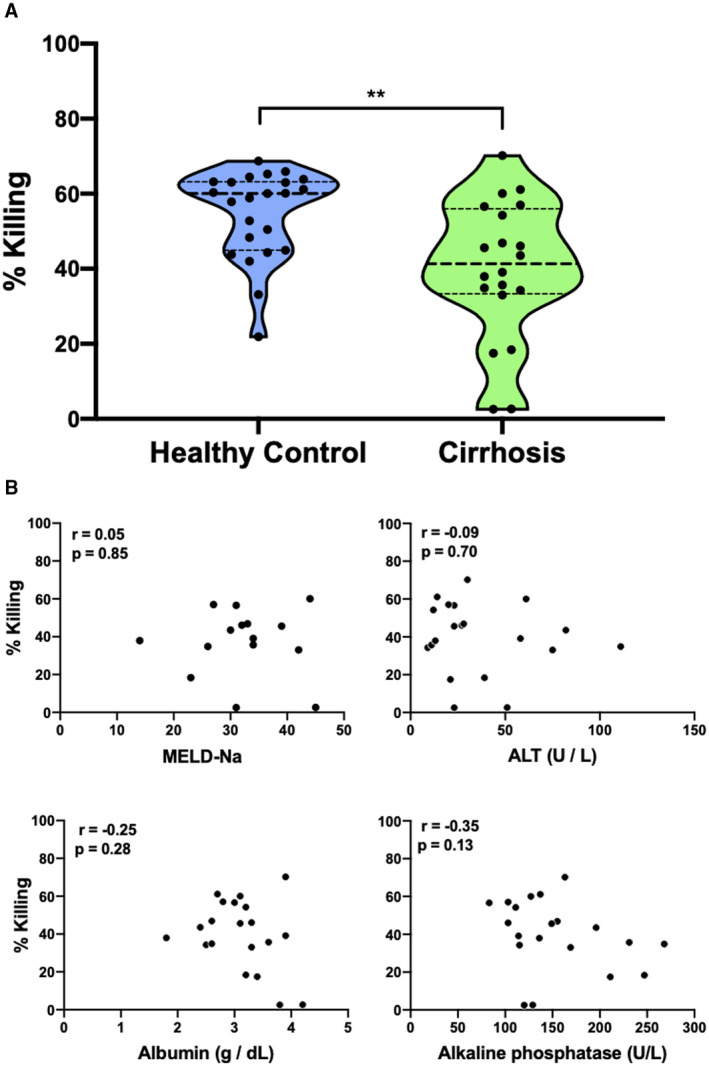
Medication Effect On Neutrophil Fungicidal Capacity
Healthy neutrophils were incubated with concentrations of nadolol, propranolol, octreotide, furosemide, and pantoprazole, likely to be used in routine care of patients with cirrhosis. Cephalosporin antibiotics were not tested due to their lack of documented effect on neutrophil function.( 28 ) Drug dose ranges were based on prior pharmacokinetic studies.( 26 , 29 , 30 , 31 ) The fungicidal capacity of neutrophils from patients with cirrhosis treated with spironolactone, furosemide, beta blockers, octreotide, or pantoprazole on the day of sample collection did not show significantly altered Candida killing by healthy neutrophils (Supporting Fig. S1). Together, these data indicate that these specific medications do not influence neutrophil function (Fig. 3).
FIG. 3.
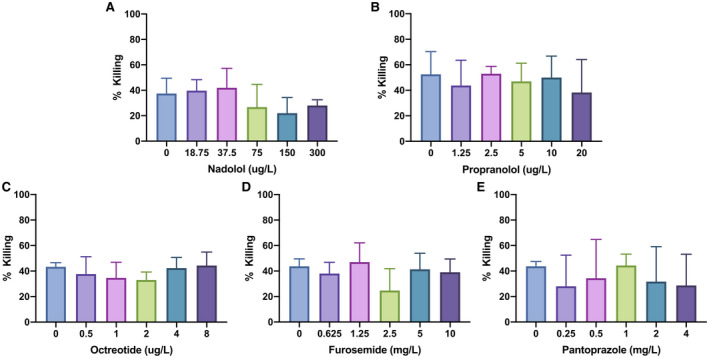
Medications commonly used to manage cirrhosis do not affect neutrophil killing of C. albicans. Incubation of healthy neutrophils with nadolol (A), propranolol (B), octreotide (C), furosemide (D), and pantoprazole (E) at concentrations likely to be found in human serum based on prior pharmacokinetic studies did not significantly alter C. albicans killing capacity.
Plasma Cytokine Levels
Plasma concentration of the cytokines interleukin (IL)‐6, IL‐8, and tumor necrosis factor α (TNF‐α) were measured from plasma of 23 healthy‐control patients and 16 patients with cirrhosis. Patients with cirrhosis were found to have significantly higher plasma levels (2‐times to 35‐times concentration compared with healthy‐control serum) of IL‐6, IL‐8, and TNF‐α (Table 3). To determine whether the presence of SBP contributed to the elevation of inflammatory cytokines, patients with cirrhosis were separated into cohorts: those with proven SBP and those without. Patients with cirrhosis with SBP did not have significantly different plasma levels of IL‐6 (SBP, 97.54 ± 39.22 pg/mL; no SBP, 86.67 ± 161 pg/mL; P = 0.07), IL‐8 (SBP, 23.37 ± 23.90 pg/mL; no SBP, 24.13 ± 32.83 pg/mL; P = 0.65), IL‐15 (SBP, 3.63 ± 2.98 pg/mL; no SBP, 2.75 ± 3.36 pg/mL; P = 0.45), or TNF‐a (SBP, 5.96 ± 1.85 pg/mL; no SBP, 5.59 ± 2.55 pg/mL; P = 0.77) when compared to patients with cirrhosis without SBP, suggesting that while cirrhosis results in elevated cytokines, there was no further elevation accountable to SBP alone.
TABLE 3.
Biochemical Characteristics of Healthy Controls and Patients With Cirrhosis
| Controls (n = 23) | Patients With Cirrhosis (n = 16) | P Value | |
|---|---|---|---|
| IL‐6 (pg/mL) | 2.02 ± 4.55 | 88.71 ± 145.53 | 0.0001 |
| IL‐8 (pg/mL) | 0.67 ± 1.74 | 23.99 ± 31.01 | <0.0001 |
| IL‐15 (pg/mL) | 2.55 ± 12.03 | 2.92 ± 3.25 | NS |
| TNF‐α (pg/mL) | 2.22 ± 5.88 | 5.66 ± 2.41 | <0.001 |
Data are presented as mean ± SD. P values represent Mann‐Whitney U test results.
Abbreviation: NS, no significance.
Neutrophil Swarming and C. albicans Escape
Six representative patients with cirrhosis with an average MELD of 32 were randomly selected for swarming studies in the setting of fixed Candida spots (Supporting Table S1). The etiology of cirrhosis was alcohol in 4 of 6 (67%); 4 of 6 (67%) had evidence of ascites on admission; and 2 of 6 (33%) had SBP at the time of sample collection. Additionally, most were taking lactulose (67%, n = 4) and rifaximin (83%, n = 5). None were taking beta blockers; half were taking spironolactone (50%); and two were on furosemide (33%) (Supporting Table S1).
Patients with cirrhosis had relatively normal neutrophil accumulation during swarming responses to C. albicans, as observed under fluorescent light microscopy (Fig. 4A). However, the swarms were severely defective in their ability to control fungal growth, as demonstrated by early C. albicans hyphae escape from neutrophil swarms from patients with cirrhosis compared to healthy controls (Fig. 4B). The total area covered by growing Candida was significantly larger after 16 hours of incubation (Fig. 4C), suggesting an intrinsic defect in neutrophil‐to‐neutrophil cooperative communication and organization from patients with cirrhosis.
FIG. 4.
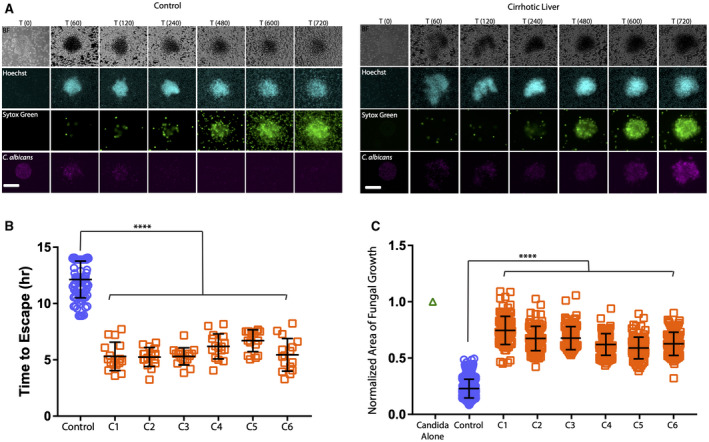
Ineffective control of C. albicans growth, despite swarming by neutrophils from patients with cirrhosis. Live C. albicans were patterned in clusters on poly‐l‐lysine/Zetag arrays. Human neutrophils were purified from peripheral blood from healthy donors or 6 different patients with cirrhosis and added to the C. albicans arrays to observe neutrophil swarming responses. (A) Time‐lapse images show the progression of neutrophil swarming to C. albicans patterns. Brightfield, 4´,6‐diamidino‐2‐phenylindole (Hoechst staining), fluorescein isothiocyanate (Sytox green) and Cy5 (C. albicans) channels are presented. (B) Time to escape of C. albicans hyphae from established neutrophil swarms from 6 patients with cirrhosis compared to control (n = 16 swarms per sample from patients with cirrhosis and n = 80 swarms across four healthy control samples). (C) Normalized area of fungal growth by C. albicans growth after 16 hours in the presence of neutrophils from patient with cirrhosis compared to control (n = 96 swarms per sample from patients with cirrhosis and n = 366 swarms across four healthy control samples). ****P ≤ 0.0001 (Kruskal‐Wallis). Error bars represent SD. Scale bar represents 100 µm.
Neutrophil G‐CSF and GM‐CSF Priming
Priming with the growth factors G‐CSF and GM‐CSF both significantly increased the time required for C. albicans hyphae to escape the swarm (Fig. 5A) and decreased the overall size of the C. albicans aggregate after 16 hours (Fig. 5B).
FIG. 5.
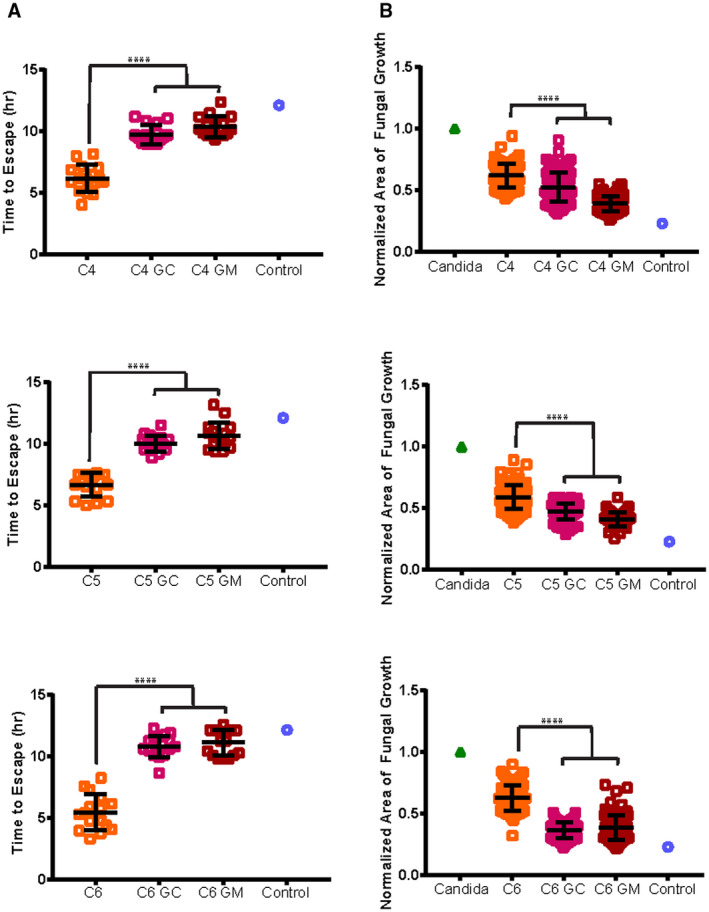
Restoration of coordinated neutrophil swarming response to C. albicans following priming with G‐CSF and GM‐CSF. Neutrophils from patients with cirrhosis were primed for 30 minutes with 0.2 ng/mL GM‐CSF, 300 ng/mL G‐CSF, or placebo before being added to C. albicans arrays to examine neutrophil swarming responses. (A) Time to escape of C. albicans hyphae from the swarm. (B) Normalized area of fungal growth by C. albicans by 16 hours comparing primed to untreated neutrophils from patients with cirrhosis: n = 16 swarms for each cirrhotic condition, for hyphal escape; and n = 96 swarms for each cirrhotic condition, for fungal growth, except for C5 GM‐CSF, which has 48 swarms. The average control value is shown as a reference in each graph. ****P ≤ 0.0001 (one‐way analysis of variance with Tukey’s post‐hoc test for (A), and Kruskal‐Wallis with Dunn’s posttest for (B). Error bars represent SD.
Discussion
Our study demonstrates that human neutrophil responses to the fungal pathogen C. albicans are significantly diminished in patients with cirrhosis compared to healthy controls. Clinically, our data suggest that neutrophil fungicidal capacity is not correlated with biochemical or hematologic parameters; however, we would need larger sample sizes for validation and statistical significance.
Neutrophil swarming is a newly recognized physiologic process involving a coordinated neutrophil cell‐to‐cell communication, which results in exponential recruitment to sites of inflammation and infection, and subsequent blockage of the growth and spread of pathogens.( 13 , 14 ) Very little work to date has examined neutrophil swarming function in patients with liver diseases. One study found that patients who suffered major trauma displayed defective swarming ability, recruiting less neutrophils and generating smaller swarms.( 32 ) Another study found that neutrophils from patients with chronic granulomatous disease form larger swarms, but their ability to counteract the growth of Candida clusters is reduced.( 12 ) Interestingly, in patients with cirrhosis, we found that swarms readily form and are comparable in size to healthy controls. This observation also stands in contrast with results from prior studies that have shown that neutrophils from patients with cirrhosis have a decreased ability to migrate to sites of infection.( 8 )
These differences may be explained by our ex vivo study experimental design that examines a two‐dimensional response to pathogens, whereas in vivo defects also include extravasation of neutrophils from the circulation toward sites of tissue damage and infection. Despite seemingly normal neutrophil recruitment to the swarms, there was still a significant inability of cirrhotic neutrophils to control Candida growth, shown both by faster hyphal escape from swarms and greater area of fungal growth. These data suggest that despite robust swarming recruitment, a fungicidal defect persists. This observation is in agreement with the results from the killing assay, which similarly point to an apparent intrinsic cell defect. There are multiple variables capable of explaining these findings, including immune cellular exhaustion related to high levels of inflammatory cytokines, as well as epigenetic changes, which are well known to influence the function state of similar myeloid cells such as monocytes.( 33 ) Changes to the gut microbiome, either as a result of drugs or cirrhosis, can also affect neutrophil function.( 34 ) Notably, the mechanisms responsible for Candida control tested in the swarming assay differ from that of the killing assay, as swarming represents the complex interaction of neutrophils with Candida clusters, while the latter assay involves polymorphonuclear neutrophil (PMN) interactions with planktonic, single yeast cells. Future studies are planned to determine the specific cell‐intrinsic factor responsible for this defective fungal control.
Serum from patients with cirrhosis diminishes fungicidal capacity of neutrophils from healthy individuals, suggesting a soluble, neutrophil‐extrinsic component that can influence PMN function. We examined serum biochemical components and noted no significant correlations with impaired neutrophil function. Due to the relatively small group of patients with cirrhosis, additional sample size and power are required to clarify these correlations. Additionally, we showed that neutrophil fungicidal capacity is not affected by medications commonly used to manage cirrhosis (such as beta blockers, spironolactone, furosemide, octreotide, and PPIs) ex vivo. We also found that although few patients with decompensated cirrhosis were prescribed those medications, there was no significant difference in Candida killing capacity in patients, regardless of medication use. Controlling for the effect of antimicrobials on neutrophil killing within the cirrhotic cohort revealed no change in the relationship between healthy control and cirrhosis. Given that FQ can augment neutrophil function, we examined the patients treated with FQ to determine whether the drug influenced their neutrophil function. Six patients on ciprofloxacin exhibited a lower capacity to control C. albicans, although when sequestered from analysis there remained a statistical difference between cirrhotic and healthy‐control killing effect, thus confirming a cell‐intrinsic defect unrelated to the drug.
We next measured and compared cytokine levels in plasma from both patients with cirrhosis and healthy controls. Similar to prior publications, we found that levels of IL‐6, IL‐8, and TNF‐α were significantly higher in patients with cirrhosis compared to healthy controls.( 34 ) Neutrophil exhaustion is a term that describes decreased toxic granule release, ROS production, and decreased neutrophil extracellular trap formation in response to usual neutrophil‐activating signals, due to chronic excessive stimulation.( 6 , 10 , 35 ) It has been shown that patients with cirrhosis who harbor a genetic variant in the pro‐inflammatory cytokine TNF‐α result in decreased serum levels of TNF‐α and, interestingly, have overall reduced incidence of clinically severe bacterial infections.( 36 ) The finding of presumably improved neutrophil function in patients with low inflammatory cytokines together with our data suggests that altered neutrophil function in the setting of persistently high levels of inflammatory cytokines may result in neutrophil exhaustion and subsequent loss of proper fungal control in patients with cirrhosis.
Finally, we turned attention to cytokine augmentation in an attempt to restore the coordinated swarming function of neutrophils against Candida. It has been shown that G‐CSF administration is safe in patients with end‐stage liver disease( 36 ) and that it can restore neutrophil defects in acute liver failure.( 22 ) However, treatment of patients with decompensated cirrhosis following G‐CSF is controversial due to variant clinical outcomes.( 23 , 25 , 37 ) We attempted to restore neutrophil function by priming neutrophils ex vivo with G‐CSF and GM‐CSF. We observed that both G‐CSF and GM‐CSF directly restored the ability of neutrophils from patients with cirrhosis to control C. albicans growth, to levels comparable to healthy control neutrophils. This observation suggests that the signaling cascades of cytokines involved in inflammation (i.e., IL‐6, IL‐8, and TNF‐α) are distinct from those of the growth factors G‐CSF and GM‐CSF. It is known that, for instance, IL‐6 signals through Jak1/Tyk2, whereas G‐CSF and GM‐CSF both signal through Jak2.( 38 ) These distinct signaling pathways provide a plausible explanation for reduction of infectious diseases in prior studies using G‐CSF in patients with cirrhosis.( 22 , 37 ) Additional clinical investigations, including neutrophil function profiling assays, are warranted before using G‐CSF and GM‐CSF as prophylaxis against, or treatment for, fungal infections in patients with cirrhosis.( 39 )
Overall, we have shown that neutrophils from patients with cirrhosis have decreased fungicidal capacity. We confirm that neutrophils from patients with cirrhosis have a diminished ability to control planktonic C. albicans, while simultaneously reporting their lost capacity to form effective, coordinated swarms. This defect may be due to neutrophil exhaustion in the setting of persistently elevated circulating levels of inflammatory cytokines. We also have shown that neutrophil function can be partially restored after priming neutrophils with G‐CSF and GM‐CSF. Better understanding of the molecular mechanisms responsible for neutrophil dysfunction and potential use of cytokines or growth factors as therapeutics in patients with cirrhosis may help prevent invasive fungal infections and their associated high mortality.
Supporting information
Fig. S1
Table S1
Acknowledgment
The authors thank all of the patients who donated blood to participate in this study.
Supported by the National Institute of Allergy and Infectious Diseases (AI132638), Massachusetts General Hospital Research Scholars Program, Shriners Burns Hospitals, Shriners Hospitals for Children, and National Institute of General Medical Sciences (GM092804).
Potential conflict of interest: Dr. Chung received grants from AbbVie, Gilead, Merck, BMS, Boehringer, Janssen, Roche, Synlogic, and Kaleido. Dr. Mansour consults for Celularity, GenMark, Vericel, Day Zero, NED biosystems, and Globe Life House. He received grants from Thermo Fisher and Genentech. He owns stock in Pulsethera and Sorrento.
Contributor Information
Sally A.I. Knooihuizen, Email: mkmansour@mgh.harvard.edu.
Michael K. Mansour, Email: mkmansour@mgh.harvard.edu.
References
- 1. Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310‐1324. [DOI] [PubMed] [Google Scholar]
- 2. Theocharidou E, Agarwal B, Jeffrey G, Jalan R, Harrison D, Burroughs AK, et al. Early invasive fungal infections and colonization in patients with cirrhosis admitted to the intensive care unit. Clin Microbiol Infect 2016;22:189.e1‐189.e7. [DOI] [PubMed] [Google Scholar]
- 3. Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Biggins SW, et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am J Gastroenterol 2018;113:556‐563. [DOI] [PubMed] [Google Scholar]
- 4. Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis 2014;33:259‐264. [DOI] [PubMed] [Google Scholar]
- 5. Hassan EA, El‐Rehim ASA, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end‐stage liver disease: low frequency or low index of suspicion. Int J Infect Dis 2014;23:69‐74. [DOI] [PubMed] [Google Scholar]
- 6. Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 1986;6:252‐262. [DOI] [PubMed] [Google Scholar]
- 7. Taylor NJ, Manakkat Vijay GK, Abeles RD, Auzinger G, Bernal W, Ma Y, et al. The severity of circulating neutrophil dysfunction in patients with cirrhosis is associated with 90‐day and 1‐year mortality. Aliment Pharmacol Ther 2014;40:705‐715. [DOI] [PubMed] [Google Scholar]
- 8. Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis 2000;182:526‐533. [DOI] [PubMed] [Google Scholar]
- 9. Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol 2011;55:574‐581. [DOI] [PubMed] [Google Scholar]
- 10. Panasiuk A, Wysocka J, Maciorkowska E, Panasiuk B, Prokopowicz D, Zak J, et al. Phagocytic and oxidative burst activity of neutrophils in the end stage of liver cirrhosis. World J Gastroenterol 2005;11:7661‐7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia‐González M, Boixeda D, Herrero D, Burgaleta C. Effect of granulocyte‐macrophage colony‐stimulating factor on leukocyte function in cirrhosis. Gastroenterology 1993;105:527‐531. [DOI] [PubMed] [Google Scholar]
- 12. Hopke A, Allison S, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, et al. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun 2020;11:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kienle K, Lämmermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 2016;273:76‐93. [DOI] [PubMed] [Google Scholar]
- 14. Lämmermann T. In the eye of the neutrophil swarm‐navigation signals that bring neutrophils together in inflamed and infected tissues. J Leukoc Biol 2016;100:55‐63. [DOI] [PubMed] [Google Scholar]
- 15. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159‐175. [DOI] [PubMed] [Google Scholar]
- 16. Mócsai A. Diverse novel functions of neutrophils in immunity, infammation, and beyond. J Exp Med 2013;210:1289‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth E, Matos G, Guarnieri C, Papp B, Varga J. Influence of the beta‐blocker therapy on neutrophil superoxide generation and platelet aggregation in experimental myocardial ischemia and reflow. Acta Physiol Hung 1995;83:163‐170. [PubMed] [Google Scholar]
- 18. Balazovich KJ, Almeida HI, Boxer LA. Recombinant human G‐CSF and GM‐CSF prime human neutrophils for superoxide production through different signal transduction mechanisms. J Lab Clin Med 1991;118:576‐584. [PubMed] [Google Scholar]
- 19. Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: role of oxidants as priming agents. Antioxidants Redox Signal 2002;4:69‐83. [DOI] [PubMed] [Google Scholar]
- 20. Kirsch R, Woodburne VE, Shephard EG, Kirsch RE. Patients with stable uncomplicated cirrhosis have normal neutrophil function. J Gastroenterol Hepatol 2000;15:1298‐1306. [DOI] [PubMed] [Google Scholar]
- 21. Campbell AC, Dronfield MW, Toghill PJ, Reeves WG. Neutrophil function in chronic liver disease. Clin Exp Immunol 1981;45:81‐89. [PMC free article] [PubMed] [Google Scholar]
- 22. Rolando N, Clapperton M, Wade J, Wendon J. Administering granulocyte colony‐stimulating factor to acute liver failure patients corrects neutrophil defects. Eur J Gastroenterol Hepatol 2000;12:1323‐1328. [DOI] [PubMed] [Google Scholar]
- 23. Philips CA, Augustine P, Ahamed R, Rajesh S, George T, Valiathan GC, et al. Role of granulocyte colony‐stimulating factor therapy in cirrhosis, “inside any deep asking is the answering”. J Clin Transl Hepatol 2019;7:371‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, et al. Granulocyte‐colony stimulating factor therapy improves survival in patients with hepatitis B virus‐associated acute‐on‐chronic liver failure. World J Gastroenterol 2013;19:1104‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prajapati R, Arora A, Sharma P, Bansal N, Singla V, Kumar A. Granulocyte colony‐stimulating factor improves survival of patients with decompensated cirrhosis: a randomized‐controlled trial. Eur J Gastroenterol Hepatol 2017;29:448‐455. [DOI] [PubMed] [Google Scholar]
- 26. Taegtmeyer AB, Haschke M, Tchambaz L, Buylaert M, Tschöpl M, Beuers U, et al. A study of the relationship between serum bile acids and propranolol pharmacokinetics and pharmacodynamics in patients with liver cirrhosis and in healthy controls. PLoS One 2014;9:e97885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Philips CA, Augustine P, Rajesh S, Ahamed R, George T, Padsalgi G, et al. Granulocyte colony‐stimulating factor use in decompensated cirrhosis: lack of survival benefit. J Clin Exp HepatoI 2020;10:124‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pallister CJ, Warnock DW. Effect of antimicrobial and antineoplastic drugs alone and in combination on the phagocytic and candidacidal function of human polymorphonoclear leucocytes. J Antimicrob Chemother 1989;23:87‐94. [DOI] [PubMed] [Google Scholar]
- 29. Fukumoto K, Kobayashi T, Tachibana K, Kato R, Tanaka K, Komamura K, et al. Effect of amiodarone on the serum concentration/dose ratio of metoprolol in patients with cardiac arrhythmia. Drug Metab Pharmacokinet 2006;21:501‐505. [DOI] [PubMed] [Google Scholar]
- 30. Krukemyer J, Boudoulas H, Binkley P, Lima J. Comparison of single‐dose and steady‐state nadolol plasma concentrations. Pharm Res 1990;7:953‐956. [DOI] [PubMed] [Google Scholar]
- 31. Woltering EA, Salvo VA, O'Dorisio TM, Lyons J, Li G, Zhou Y, et al. Clinical value of monitoring plasma octreotide levels during chronic octreotide long‐acting repeatable therapy in carcinoid patients. Pancreas 2008;37:94‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reátegui E, Jalali F, Khankhel AH, Wong E, Cho H, Lee J, et al. Microscale arrays for the profiling of start and stop signals coordinating human‐neutrophil swarming. Nat Biomed Eng 2017;1:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quintin J, Saeed S, Martens J, Giamarellos‐Bourboulis E, Ifrim D, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012;12:223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stadlbauer V, Mookerjee RP, Hodges S, Wright GAK, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol 2008;48:945‐951. [DOI] [PubMed] [Google Scholar]
- 35. Hong CW. Current understanding in neutrophil differentiation and heterogeneity. Immune Netw 2017;17:298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senkerikova R, de Mare‐Bredemeijer E, Frankova S, Roelen D, Visseren T, Trunecka P, et al. Genetic variation in TNFA predicts protection from severe bacterial infections in patients with end‐stage liver disease awaiting liver transplantation. J Hepatol 2014;60:773‐781. [DOI] [PubMed] [Google Scholar]
- 37. Verma N, Kaur A, Sharma R, Bhalla A, Sharma N, De A, et al. Outcomes after multiple courses of granulocyte colony‐stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology 2018;68:1559‐1573. [DOI] [PubMed] [Google Scholar]
- 38. Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 2013;17:638‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexander NJ, Bozym DJ, Farmer JR, Parris P, Viens A, Atallah N, et al. Neutrophil functional profiling and cytokine augmentation for patients with multiple recurrent infections: a case study. J Allergy Clin Immunol Pract 2020. Aug 24:S2213‐2198(20)30840‐0. 10.1016/j.jaip.2020.08.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1
Table S1


