Abstract
Liver transplantation (LT) is definitive treatment for end‐stage liver disease. This study evaluated factors predicting successful evaluation in patients transferred for urgent inpatient LT evaluation. Eighty‐two patients with cirrhosis were transferred for urgent LT evaluation from January 2016 to December 2018. Alcohol‐associated liver disease was the common etiology of liver disease (42/82). Of these 82 patients, 35 (43%) were declined for LT, 27 (33%) were wait‐listed for LT, 5 (6%) improved, and 15 (18%) died. Psychosocial factors were the most common reasons for being declined for LT (49%). Predictors for listing and receiving LT on multivariate analysis included Hispanic race (odds ratio [OR], 1.89; P = 0.003), Asian race (OR, 1.52; P = 0.02), non‐Hispanic ethnicity (OR, 1.49; P = 0.04), hyponatremia (OR, 1.38; P = 0.04), serum albumin (OR, 1.13; P = 0.01), and Model for End‐Stage Liver Disease (MELD)‐Na (OR, 1.02; P = 0.003). Public insurance (i.e., Medicaid) was a predictor of not being listed for LT on multivariate analysis (OR, 0.77; P = 0.02). Excluding patients declined for psychosocial reasons, predictors of being declined for LT on multivariate analysis included Chronic Liver Failure Consortium (CLIF‐C) score >51.5 (OR, 1.26; P = 0.03), acute‐on‐chronic liver failure (ACLF) grade 3 (OR, 1.41; P = 0.01), hepatorenal syndrome (HRS) (OR, 1.38; P = 0.01), and respiratory failure (OR, 1.51; P = 0.01). Predictors of 3‐month mortality included CLIF‐C score >51.5 (hazard ratio [HR], 2.52; P = 0.04) and intensive care unit (HR, 8.25; P < 0.001). Conclusion: MELD‐Na, albumin, hyponatremia, ACLF grade 3, HRS, respiratory failure, public insurance, Hispanic race, Asian race, and non‐Hispanic ethnicity predicted liver transplant outcome. Lack of psychosocial support was a major reason for being declined for LT. The CLIF‐C score predicted being declined for LT and mortality.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- ALD
alcohol‐associated liver disease
- CI
confidence interval
- CLIF‐C
Chronic Liver Failure Consortium
- HCC
hepatocellular carcinoma
- HRS
hepatorenal syndrome
- ICU
intensive care unit
- IQR
interquartile ratio
- LT
liver transplantation
- MELD
Model for End‐stage Liver Disease
- OR
odds ratio
- SIPAT
Stanford Integrated Psychosocial Assessment for Transplant
Liver transplantation (LT) is the definitive treatment option for patients with acute liver failure, end‐stage liver disease, and selected primary liver malignancies. Patients with cirrhosis are typically evaluated for LT when there is evidence of decompensation and their biologic Model for End‐stage Liver Disease (MELD) score is ≥15 or if they develop hepatocellular carcinoma (HCC) that is within or can be downstaged to within the Milan criteria.( 1 , 2 ) While the evaluation process typically occurs in the outpatient setting, those with advanced liver disease, high MELD scores, acute decompensation, and acute liver failure are evaluated urgently during an inpatient admission.( 3 )
Many individuals with end‐stage liver disease are unaware of their disease until they develop an episode of acute decompensation or acute‐on‐chronic liver failure (ACLF). As these individuals are not yet engaged in hepatology care, transplant centers are often contacted to accept these patients as transfers for urgent LT evaluation and listing. Our aim was to evaluate factors that predict successful evaluation, listing, and transplantation in patients with decompensated cirrhosis who are transferred to our transplant center for urgent inpatient LT evaluation.
Patients and Methods
This was a single‐center retrospective cohort study at Stanford University Hospital. All transfers for patients 18 years or older who were referred for urgent inpatient LT evaluation from January 1, 2016, until December 31, 2018, with no prior contact with any transplant center were included. At our center, the transferring physician will request the transfer for urgent LT and the hepatologist on call will discuss the case with the referring physician to determine the appropriateness of the transfer and confirm with the transplant administrator that there is insurance coverage for transplant evaluation.
Data were fully anonymized after extraction. Data abstracted included demographic information (age, sex, race), laboratory data, MELD‐Na score, Chronic Liver Failure Consortium (CLIF‐C) ACLF score and ACLF grade at presentation, referral care level (medical/surgical floor, intensive care unit [ICU]), liver disease etiology, complication precipitating transfer, vasopressor requirement, respiratory failure, Stanford Integrated Psychosocial Assessment for Transplant (SIPAT) score (the tool used to assess psychosocial appropriateness for transplant at our center),( 4 ) insurance type (commercial, Medicare, Medicaid), and outcome after transfer (declined, listed, improved, died before presentation, received transplant). In those who were not deemed appropriate transplant candidates, medical (advanced malignancy, cardiopulmonary, neurologic, surgical/anatomic abnormalities, uncontrolled sepsis, deconditioning) and psychosocial (lack of sobriety, insurance, lack of adequate family social support, noncompliance with prior substance abuse recommendations, nonadherence with medical care, untreated other substance abuse) reasons for declining to list were also captured. ACLF identification with organ failures and CLIF‐C ACLF scores were calculated based on European Association for the Study of the Liver‐CLIF‐C score.( 5 , 6 ) Statistical analysis was conducted using RStudio version 1.1.463. The Shapiro‐Wilk test was used to test normality of continuous variables. The two‐sample t test was used to compare normally distributed continuous variables, and the Wilcoxon rank sum test was used to compare continuous variables that were not normally distributed. Categorical variables were compared using Fisher’s exact test. We analyzed factors to predict three outcomes, i.e., listing for liver transplant, receiving liver transplant, and declined for transplant, by creating multivariate models. Multivariate analysis models were built by performing backward elimination of variables and selecting the model with the highest adjusted R‐squared value and the most significant F statistics P value. Missing SIPAT scores were substituted with the median SIPAT score for the multivariate analysis model. Survival analysis was assessed with the Kaplan‐Meier method, with differences in survival probabilities measured by log‐rank testing. Predictors of 3‐month mortality were analyzed with multivariable Cox proportional hazards modeling and confirmed by competing risk survival analysis with the Fine‐Gary model. Three patients were lost at follow‐up, and they were excluded from mortality predictors analysis. Area under the receiver operating characteristic curve analysis was used for the threshold values. P < 0.05 was considered statistically significant. The Stanford University Institutional Review Board approved this study.
Results
From January 1, 2016, to December 31, 2018, 82 patients with cirrhosis were transferred for urgent LT evaluation. Alcohol‐associated liver disease (ALD) was the most common etiology (42/82) followed by nonalcoholic fatty liver disease (17/82), hepatitis C (12/82), and hepatitis B (7/82). The baseline characteristics of patients with ALD compared to patients with other liver disease etiologies are summarized in Table 1. The most common complications precipitating transfer were hepatic encephalopathy in 30 (37%), hepatorenal syndrome in 15 (18%), and jaundice in 14 (17%). Regarding outcomes, of the 82 individuals who were accepted for transfer, 35 (43%) were declined for LT, 27 (33%) were wait‐listed for LT, 5 (6%) improved, and 15 (18%) died (Fig. 1). Of the 27 who were wait‐listed, 21 (78%) underwent LT with 12 (57%) receiving LT during the index hospitalization and 9 (43%) subsequently receiving LT after hospital discharge. The baseline characteristics of patients who were listed for LT compared to those not listed for LT and differences between the two groups are summarized in Supporting Table S1A. Of the 35 who were declined for LT, 21 (60%) died within 3 months, 4 (11%) died within 1 year, 7 (20%) survived >1 year, and 3 (8%) were lost to follow‐up with unknown outcomes. Patients who were lost to follow‐up were censored for survival analysis.
TABLE 1.
Baseline Characteristics of Patients With ALD Compared to Patients With Other Liver Disease Etiologies
| Variable n (%) or Median (IQR) | ALD (42 Patients) | Others (40 Patients) | P Value* |
|---|---|---|---|
| Male sex | 27/42 (64%) | 14/40 (35%) | 0.01 |
| Female sex | 15/42 (36%) | 26/40 (65%) | 0.01 |
| Age (years) | 57.5 (51‐62) | 61 (56.75‐66.25) | 0.07 |
| Hispanic ethnicity | 13/42 (31%) | 9/40 (22%) | 0.46 |
| Non‐Hispanic ethnicity | 29/42 (69%) | 31/40 (77%) | 0.46 |
| White race | 20/42 (48%) | 18/40 (45%) | 0.83 |
| Asian race | 0/42 (0%) | 5/40 (12%) | 0.05 |
| Hispanic race | 13/42 (31%) | 9/40 (22%) | 0.46 |
| African American race | 2/42 (5%) | 3/40 (7%) | 0.67 |
| Public insurance | 11/42 (26%) | 16/40 (40%) | 0.24 |
| Medicare + commercial insurance | 31/42 (74%) | 24/40 (60%) | 0.24 |
| Received LT | 15/42 (36%) | 6/40 (15%) | 0.04 |
| Listed for LT | 16/42 (38%) | 11/40 (27%) | 0.35 |
| Declined for LT | 19/42 (45%) | 16/40 (40%) | 0.66 |
| ALP (U/L) | 136.5 (89.25‐193) | 145 (104.5‐203.25) | 0.41 |
| Albumin (g/dL) | 2.65 (1.93‐3.6) | 2.7 (2.05‐3.1) | 0.65 |
| ALT (U/L) | 34.5 (24.25‐54) | 52 (26.75‐168) | 0.02 |
| AST (U/L) | 67.5 (45‐100.75) | 101.5 (49.5‐305) | 0.05 |
| Creatinine (mg/dL) | 1.71 (0.84‐2.34) | 1.195 (0.75‐2.25) | 0.46 |
| INR | 2.45 (1.8‐3.05) | 1.95 (1.675‐2.6) | 0.18 |
| Sodium (mmol/L) | 132 (126‐134.75) | 135 (131.75‐140) | 0.005 |
| Total bilirubin (mg/dL) | 9.85 (5.275‐15.775) | 7.15 (3.3‐18.95) | 0.51 |
| MELD‐Na score | 33 (28.25‐36) | 28 (25.25‐33) | 0.06 |
| CLIF‐C score | 55 (49‐60.75) | 54.5 (51‐58) | 0.74 |
| SIPAT score | 34 (25‐44) | 23 (18‐30) | 0.0005 |
| ACLF grade 3 | 11/42 (26%) | 7/40 (17%) | 0.43 |
| Referred from floor | 39/42 (93%) | 35/40 (87%) | 0.48 |
| Referred from ICU | 3/42 (7%) | 5/40 (13%) | 0.48 |
| Vasopressor support | 2/42 (4.8%) | 4/40 (10%) | 0.42 |
| Respiratory failure | 1/42 (2.4%) | 6/40 (15%) | 0.05 |
| HRS | 12/42 (28.6%) | 3/40 (7.5%) | 0.02 |
| HE | 16/42 (38%) | 14/40 (35%) | 0.82 |
P < 0.05 is considered significant.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HE, hepatic encephalopathy; INR, international normalized ratio.
FIG. 1.
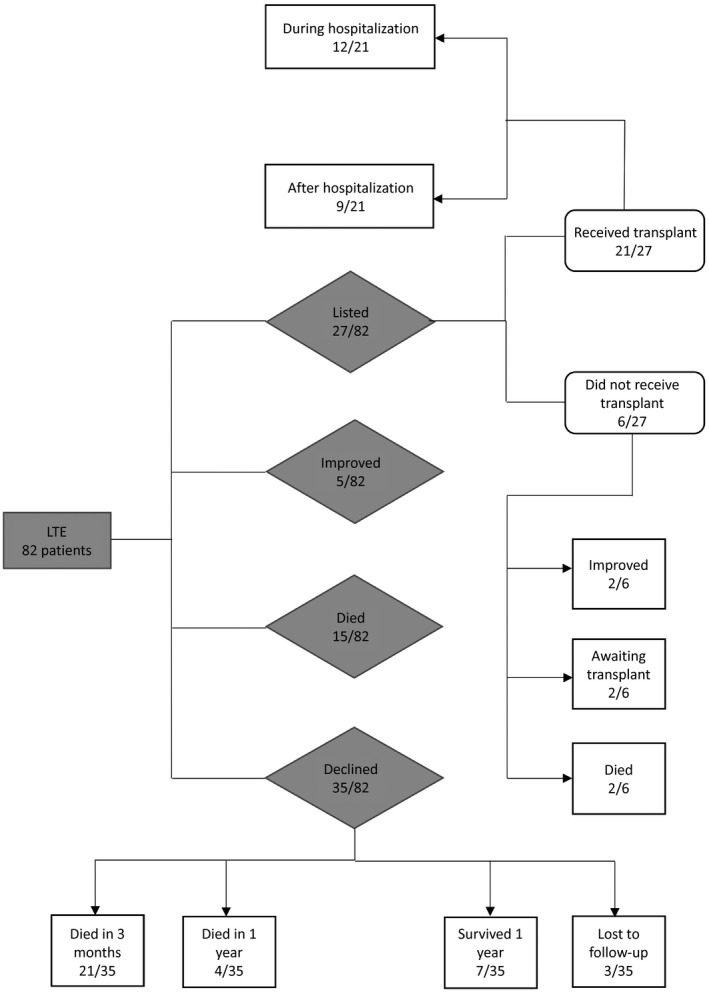
LT evaluation outcomes flow chart. Abbreviation: LTE, liver transplant evaluation.
The SIPAT score was obtained during transplant evaluation for 62 (76%) patients, with the majority (33/62 [53%]) of these patients assessed as minimally acceptable candidates (SIPAT score, 21‐39), 16/62 (26%) assessed as poor candidates (SIPAT score, >40), and 13/62 (21%) assessed as good candidates (SIPAT score, <20). Mean SIPAT score for patients who were listed for LT was 29 compared to mean SIPAT score of 35 for patients who were declined for LT. Detailed SIPAT analysis for all patients assessed for LT is summarized in Table 2. Univariate analysis demonstrated that the SIPAT score predicted being declined for LT (SIPAT score, 35.3 ± 14.9; P = 0.039).
TABLE 2.
SIPAT Analysis Comparing Patients Declined for LT to Other Patients*
| SIPAT Domain | Declined for LT | Not Declined for LT | P Value † |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| A. Patient’s readiness level | |||
| I. Knowledge and understanding of medical illness process | 1.56 (0.79) | 1.59 (0.82) | 0.91 |
| II. Knowledge and understanding of the process of transplantation | 1.73 (0.69) | 1.74 (0.75) | 0.98 |
| III. Willingness/desire for treatment (transplant) | 1.22 (0.73) | 1.15 (0.78) | 0.75 |
| IV. Treatment compliance/adherence | 3.48 (2.02) | 2.56 (1.71) | 0.06 |
| V. Lifestyle factors (diet, exercise, fluid restrictions, habits according to organ) | 1.21 (0.85) | 1.20 (0.83) | 0.96 |
| B. Social support system | |||
| VI. Availability of social support system | 2.78 (1.78) | 1.74 (1.31) | 0.01 |
| VII. Functionality of social support system | 2.52 (1.83) | 1.69 (1.42) | 0.05 |
| VIII. Appropriateness of physical living space and environment | 0.65 (0.93) | 0.67 (0.62) | 0.94 |
| C. Psychological stability and psychopathology | |||
| IX. Presence of psychopathology (mood, anxiety, psychosis, others) | 3.12 (1.79) | 2.20 (1.82) | 0.06 |
| IXa. Assessment of depression | 1.52 (0.79) | 1.15 (0.93) | 0.12 |
| IXb. Assessment of anxiety | 1.13 (0.62) | 0.89 (0.88) | 0.27 |
| X. History of organic psychopathology or neurocognitive impairment | 0.56 (1.04) | 0.23 (0.48) | 0.09 |
| Xa. Assessment of current cognitive functioning | 0.13 (0.46) | 1.1 (0.38) | 0.79 |
| XI. Influence of personality traits versus disorder | 0 (0‐0.5) | 0 (0‐0) | 0.39 |
| XII. Effect of truthfulness versus deceptive behavior in presentation | 1.17 (1.87) | 1.20 (1.97) | 0.95 |
| XIII. Overall risk for psychopathology | 1.96 (1.15) | 1.67 (1.47) | 0.42 |
| D. Lifestyle and effect of substance use | |||
| XIV. Alcohol use/abuse/dependance | 4.09 (2.66) | 3.49 (1.99) | 0.32 |
| XV. Alcohol use/abuse/dependence: risk for recidivism | 2 (1.31) | 1.67 (1.06) | 0.28 |
| XVI. Substance use/abuse/dependance, including prescribed and illicit substances | 1 (1.24) | 0.87 (1.57) | 0.74 |
| XVII. Substance use/abuse/dependance, including prescribed and illicit substances: risk for recidivism | 0.56 (0.51) | 0.74 (0.71) | 0.29 |
| XVIII. Nicotine use/abuse/dependence | 1 (1.21) | 0.74 (1.31) | 0.45 |
Performed for the 62 patients in our cohort with a reported SIPAT score. Score interpretation per domain: I. 1, good understanding; 2, moderate understanding. II. 1, good understanding; 2, moderate understanding. III. 1, good; 2, moderate. IV. 2, good; 4, moderate. V. 1, patient is responsive to recommended changes; 2, patient is reluctant but compliant with recommended changes after much prompting and encouragement from support and transplant team. VI. 0, excellent; 2, good; 4, moderate. VII. 0, excellent; 2, good; 4, moderate. VIII. 0, excellent; 1, good. IX. 2, mild psychopathology; 4, moderate psychopathology. IXa. 1, mild; 2, moderate. IXb. 0, no; 1, mild; 2, moderate. X. 0, none; 1, mild organic psychopathology. Xa. 0, cognitive functioning with normal limits; 1, borderline; 2, impaired. XI. 0, none; 1, minimal. XII. 0, no evidence of deceptive behavior in history or present; 2, patient has not volunteered some negative information but truthfully answered on direct questioning. XIII. 1, minimal; 2, mild. XIV. 2, alcohol use without abuse; 4, moderate alcohol abuse. XV. 1, low risk; 2, moderate risk. XVI. 0, none; 1, history of minimal substance abuse. XVII. 0, none; 1, low risk. XVIII. 0, none; 1, past use.
P < 0.05 is considered significant.
Patients were declined as LT candidates for psychosocial reasons (49%), advanced HCC (14%), uncontrolled sepsis (14%), deconditioning (11%), neurologic (6%), cardiopulmonary (3%), and anatomic reasons (3%). Mean SIPAT score for patients declined for psychosocial reasons was 40 compared to a mean SIPAT score of 26 for patients declined for other reasons. Excluding patients with ALD, patients were declined as LT candidates for psychosocial reasons (31%), advanced HCC (19%), deconditioning (19%), and uncontrolled sepsis (12.5%), without difference in distribution comparable to those with ALD. Psychosocial reasons for being declined for LT included lack of adequate family/social support 8/17 (47%), untreated other substance abuse 3/17 (17.5%), lack of 6 months of alcohol sobriety 3/17 (17.5%), insurance 1/17 (6%), nonadherence with Alcoholics Anonymous meetings 1/17 (6%), and persistent nonadherence with medical care 1/17 (6%).
Among the 42 patients with ALD, 16 (38%) were wait‐listed and 15/16 (94%) received LT compared to six transplants performed among 11 wait‐listed candidates with other etiologies. Significantly more patients with ALD received LT compared to other liver disease etiologies (odds ratio [OR], 3.1; 95% confidence interval [CI], 0.97‐11.14; P = 0.04). Significant predictors for listing for LT on multivariate analysis included Hispanic race, non‐Hispanic ethnicity, and hyponatremia, whereas having public insurance (i.e., Medicaid) was a predictor of not being listed for LT (Table 3A). Predictors of receiving LT on multivariate analysis included Asian race, Hispanic race, non‐Hispanic ethnicity, hyponatremia, serum albumin, and MELD‐Na score; however, having public insurance predicted not receiving LT on multivariate analysis (Table 3B).
TABLE 3A.
Multivariate Analysis Model for Predictors for Listing for LT (Model F Statistics P = 0.008)
| Factor | Listed for LT | Not Listed for LT | OR (95% CI) | P Value* |
|---|---|---|---|---|
| n (%), Median (IQR) | n (%), Median (IQR) | |||
| Asian race | 3/27 (11%) | 2/55 (3.6%) | 1.41 (0.93‐2.16) | 0.1 |
| African American race | 0/27 (0%) | 5/55 (9%) | 0.72 (0.47‐1.11) | 0.13 |
| Hispanic race | 10/27 (37%) | 12/55 (22%) | 1.89 (1.25‐2.87) | 0.003 |
| Non‐Hispanic ethnicity | 17/27 (63%) | 36/55 (65%) | 1.49 (1.01‐2.19) | 0.04 |
| Public insurance | 5/27 (18%) | 22/55 (40%) | 0.77 (0.62‐0.95) | 0.02 |
| Age in years | 60 (55‐62) | 61 (55‐64) | 1.006 (0.99‐1.02) | 0.33 |
| Vasopressors | 2/27 (7.4%) | 4/55 (7.3%) | 1.25 (0.83‐1.89) | 0.27 |
| Hyponatremia | 5/27 (18%) | 5/55 (9%) | 1.38 (1.008‐1.89) | 0.04 |
| MELD‐Na score | 33 (26‐36) | 30 (26.5‐35) | 1.009 (0.99‐1.02) | 0.19 |
P < 0.05 is considered significant.
TABLE 3B.
Multivariate Analysis Model for Predictors of Receiving LT (Model F Statistics P = 0.0002)
| Variable | Received LT | Did Not Receive LT | OR (95% CI) | P Value* |
|---|---|---|---|---|
| n (%), Median (IQR) | n (%), Median (IQR) | |||
| Asian race | 3/21 (14%) | 2/61 (3.2%) | 1.52 (1.06‐2.19) | 0.02 |
| Hispanic race | 6/21 (28%) | 16/61 (26%) | 1.65 (1.15‐2.37) | 0.007 |
| Ethnicity, non‐Hispanic | 15/21 (71%) | 38/61 (62%) | 1.52 (1.08‐2.12) | 0.02 |
| Public insurance | 4/21 (19%) | 17/61 (28%) | 0.82 (0.68‐0.98) | 0.03 |
| Vasopressors support | 2/21 (10%) | 19/61 (31%) | 1.25 (0.87‐1.78) | 0.22 |
| Hyponatremia | 5/21 (24%) | 16/61 (26%) | 1.53 (1.16‐2.01) | 0.003 |
| MELD‐Na score | 35 (30‐37) | 30 (26‐34) | 1.02 (1.002‐1.03) | 0.02 |
| Albumin (mg/dL) | 3.0 (2.5‐3.9) | 2.6 (1.9‐3.1) | 1.13 (1.02‐1.24) | 0.01 |
P < 0.05 is considered significant.
Predictors for being declined for LT on multivariate analysis included African American race, CLIF‐C score, and MELD‐Na score (Table 3C). To predict a precise clinical model for being declined for LT, we excluded patients declined for LT for psychosocial reasons as patients were declined for psychosocial reasons regardless of their clinical condition.
TABLE 3C.
Multivariate Analysis Model for Predictors of Being Declined for LT for Entire Cohort (Model F Statistics P = 0.0002)
| Variable n (%) or Median (IQR) | Declined for LT | Not Declined for LT | OR (95% CI) | P Value* |
|---|---|---|---|---|
| 35 Patients | 47 Patients | |||
| SIPAT | 29.5 (28‐35.5) | 29.5 (23‐34.5) | 1.007 (0.99‐1.01) | 0.07 |
| African American race | 5/35 (14%) | 0/47 (0%) | 1.74 (1.15‐2.63) | 0.009 |
| ACLF grade 3 | 11/35 (31%) | 7/47 (15%) | 1.29 (0.99‐1.66) | 0.05 |
| CLIF‐C score | 57 (52‐62) | 54 (47.5‐58) | 1.01 (1.001‐1.03) | 0.04 |
| MELD‐Na score | 29 (26.5‐34) | 33 (26‐36) | 0.98 (0.97‐0.99) | 0.02 |
P < 0.05 is considered significant.
After excluding patients declined for LT for psychosocial reasons, we calculated a threshold CLIF‐C ACLF score of 51.5 (area under the curve, 70%) associated with being declined for LT with an OR of 5.3 (95% CI, 1.1‐52; P < 0.05) on univariate analysis. After excluding patients declined for LT for psychosocial reasons, predictors of being declined for LT on multivariate analysis included CLIF‐C score >51.5, ACLF grade 3, hepatorenal syndrome (HRS), respiratory failure, and MELD‐Na score; Hispanic race and non‐Hispanic ethnicity were predictors of not being declined for LT (Table 3D). As having public insurance was a predictor of not being listed for LT and not receiving LT on multivariate analysis, we explored baseline characteristics with no differences noted in demographics, presence of ALD, or disease severity (Supporting Table S1B).
TABLE 3D.
Multivariate Analysis Model for Predictors of Being Declined for LT Listing Excluding Patients Declined for Psychosocial Reasons (Model F Statistics P = 0.0001)
| Factor | Declined for LT | Not Declined for LT | OR (95% CI) | P Value* |
|---|---|---|---|---|
| n (%), Median (IQR) | n (%), Median (IQR) | |||
| African American race | 1/18 (5.5%) | 3/47 (6%) | 1.32 (0.89‐1.96) | 0.17 |
| Hispanic race | 5/18 (28%) | 12/47 (25.5%) | 0.45 (0.25‐0.79) | 0.007 |
| Non‐Hispanic race | 11/18 (61%) | 35/47 (74%) | 0.38 (0.22‐0.66) | 0.0008 |
| ACLF grade 3 | 6/18 (33%) | 7/47 (15%) | 1.41 (1.07‐1.85) | 0.01 |
| CLIF‐C score >51.5 | 16/18 (89%) | 28/47 (59%) | 1.26 (1.02‐1.56) | 0.03 |
| HRS | 4/18 (22%) | 10/47 (21%) | 1.38 (1.07‐1.77) | 0.01 |
| Respiratory failure | 3/18 (17%) | 4/47 (8%) | 1.51 (1.09‐2.07) | 0.01 |
| Hyponatremia | 3/18 (17%) | 6/47 (13%) | 1.31 (0.99‐1.73) | 0.05 |
| MELD‐Na score | 28 (23‐32) | 33 (26‐36) | 0.96 (0.94‐0.98) | 0.0001 |
P < 0.05 is considered significant.
Among patients referred from ICU level of care, 2/6 (33%) were given comfort measures only in the first 24 hours and died, 2/6 (33%) were declined for psychosocial reasons, 1/6 (17%) was declined for uncontrolled sepsis, and 1/6 (17%) was declined for neurologic deterioration. Although our center created a protocol to consider LT in those with ALD and limited sobriety in 2017, transplant rate by year did not differ during the study period.
Regarding transplant outcomes, of those who received LT, 20 (95%) survived at least 1 year and 1 (5%) died in the first year. Survival analysis by the Kaplan‐Meier method demonstrated a statistically significant difference in survival probability in patients who received LT compared to patients who did not undergo LT (P = 0.0001) (Supporting Fig. S1A). CLIF‐C ACLF score and ACLF grade were used to predict 3‐month mortality, 1‐year survival, and being declined for LT. Predictors of 3‐month mortality when analyzed with multivariable Cox proportional hazards modeling with censoring patients who received LT and excluding the 3 patients who were lost to follow‐up included CLIF‐C score >51.5 (hazard ratio [HR], 2.52; 95% CI, 1.06‐6.02; P = 0.04) and ICU level of care (HR, 8.25; 95% CI, 2.78‐24.51; P < 0.001). ACLF grade 0 (HR, 0.21; 95% CI, 0.07‐0.6; P = 0.004) and ACLF grade 2 (HR, 0.29; 95% CI, 0.1‐0.86; P = 0.02) were both predictors of 3‐month survival (Fig. 2). This was confirmed by competing risk survival analysis with the Fine‐Gary model with censoring patients who received LT. When analyzing ACLF grades, ACLF grade 3 was associated with higher 3‐month mortality and ACLF grade 0 was associated with higher 1‐year survival (Supporting Fig. S1B). One‐year survival analysis showed that ACLF grade 0 had significantly better survival and ACLF grade 3 had worse survival compared to other ACLF grades (P = 0.033) (Supporting Fig. S1C). There was no significant difference in 3‐month mortality and 1‐year survival for patients with ALD compared to patients with other liver disease etiologies. For those receiving a transplant, the average length of stay after LT was 25 days. Among patients receiving LT, 2 patients were on renal replacement therapy (RRT) before LT and 1 did not require RRT after LT.
FIG. 2.
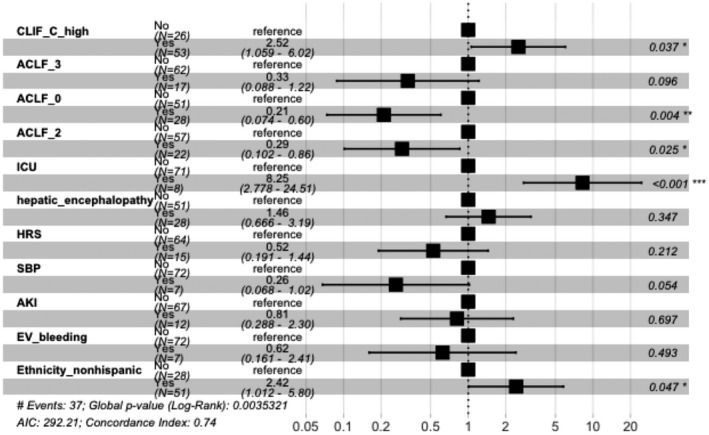
Predictors of 3‐month mortality analysis. CLIF_C_high is CLIF‐C ACLF score >51.5. There were 37 events resulting in global P (log‐rank) = 0.003532; Akaike information criterion, 292.21; concordance index, 0.74. *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: AKI, acute kidney injury; EV, esophageal varices; SBP, spontaneous bacterial peritonitis.
Discussion
Our study evaluated the outcomes of inpatient referrals for urgent LT evaluation in patients with cirrhosis as well as predictors of these outcomes. Among all causes of end‐stage liver disease, ALD was the most common indication for urgent LT evaluation, consistent with recent observations noting that ALD has replaced hepatitis C virus‐related cirrhosis as the leading indication for LT.( 2 , 7 , 8 , 9 , 10 ) The prevalence of 12‐month alcohol use, high‐risk drinking, and Diagnostic and Statistical Manual of Mental Disorders (fourth edition) alcohol use disorder are increasing throughout all sociodemographic subgroups in the United States, based on data from the National Epidemiologic Survey on Alcohol and Related Conditions (2001‐2013), and the recent corona virus disease 2019 pandemic may accelerate this rate of increase.( 11 , 12 ) In our cohort, all patients who received LT for ALD had evidence of cirrhosis on explant biopsy.
Among complications precipitating transfer for urgent LT, we found that refractory hepatic encephalopathy was the most common complication precipitating transfer and, while not part of the MELD‐Na score, is associated with higher mortality.( 13 ) Among complications precipitating transfer for urgent LT evaluation, hyponatremia was a predictor for listing for LT and for receiving LT, which may reflect the contribution of serum Na level to the traditional MELD score to improve prediction of short‐term mortality.( 14 ) The MELD‐Na score for patients with ALD who received LT was the highest with a median of 35. Higher MELD‐Na scores and higher serum albumin were both found to be predictors of receiving LT in our study. A prior report noted that lower albumin levels predicted higher wait‐list mortality in those awaiting transplant.( 15 ) HRS and respiratory failure were found to be predictors for being declined for LT.
CLIF‐C ACLF score >51.5 predicted LT decline in univariate and multivariate analysis after excluding patients declined for psychosocial reasons. Predictors of 3‐month mortality with censoring patients who received LT and excluding the 3 patients who were lost to follow‐up included CLIF‐C score >51.5 and ICU level of care. ACLF grade 0 and ACLF grade 2 were both predictors of 3‐month survival.
In addition, ACLF grade has been further classified based on number of organ failures, with ACLF grade 3 (defined as three or more organ failures) being the most severe category with 28‐ and 90‐day transplant‐free mortality rates of 76.7% and 79.1%, respectively.( 6 ) We found that those who presented with ACLF grade 3 had significantly lower 1‐year survival. A prior analysis of the United Network for Organ Sharing database noted that those with ACLF grade 3 at the time of listing were less likely to survive on the wait list regardless of MELD score.( 16 ) ACLF grade 3 was also found to be a predictor of being declined for LT in our study, again consistent with prior reports noting a greater number of organ failures is associated with higher wait‐list mortality in the setting of ACLF.( 16 ) Our population differs in that these individuals were not under care of a transplant hepatologist before transfer for LT evaluation and further supports that CLIF‐C ACLF scores can be used to predict outcome when considering accepting patients for transfer for urgent LT. In addition, while MELD score was predictive of receiving a transplant, MELD did not predict 3‐month mortality or 1‐year survival in our study; this was likely due to the sample size.
Psychosocial reasons with lack of adequate social support emerged as the most common etiology for being declined to receive LT, even after excluding patients with ALD. Lack of adequate social support may explain, in part, why these individuals were not previously engaged with transplant centers to consider LT before acute decompensation. Further studies should address how to improve access to transplant in those with end‐stage liver disease before an episode of decompensation. In addition, having public insurance alone was a predictor of not being listed for LT and not receiving LT. This suggests disparities may exist in access to urgent transplantation in those with cirrhosis and acute decompensation. There were no significant differences in demographic data or presenting disease severity, as assessed by MELD, ACLF grade, or SIPAT score, and further studies are required to assess the underlying causes of these disparities. A previous report noted that those with public insurance and hepatocellular cancer were at increased risk for worse wait‐list outcomes compared to those with private insurance( 17 ) Hispanic race and non‐Hispanic ethnicity were both predictors of being listed for LT and receiving LT, and Asian race was also a predictor of receiving LT.
There are obvious limitations to our study. We reported single‐center data, although this allowed us to obtain granular data that are not captured by large databases, including all components of the CLIF‐C ACLF score. Criteria for accepting patients for urgent transplant evaluation vary from center to center as do insurance coverages, and these factors may introduce bias in our results. The sample size limits some analyses for outcome of predictors. Our center uses SIPAT scoring for psychosocial assessment, but this tool is not universally used by transplant centers. The expedited pathway for considering patients with <6 months sobriety for LT at our center was initiated in early 2017. We could not specifically determine whether patients before this time (2016) were not accepted in transfer who would have met the expedited pathway criteria for evaluation.
We conclude patients with cirrhosis with acute decompensation have an overall transplant rate of 26%, with ALD being the most common underlying liver disease. MELD score, albumin level, hyponatremia, ACLF grade 3, HRS, respiratory failure, public insurance, Hispanic race, Asian race, and non‐Hispanic ethnicity predicted liver transplant outcome. Lack of adequate psychosocial support was a major reason for being declined for LT. Finally, higher CLIF‐C ACLF score was associated with poor outcomes, with score >51.5 predicting LT decline and mortality, and ICU level of care also predicting mortality. These data, if validated, may inform clinicians how best to assess and prioritize patients with cirrhosis who are referred for urgent transplant evaluation.
Supporting information
Fig S1
Table S1a
Table S1b
Table S1c
Potential conflict of interest: Dr. Nguyen advises and received grants from Bristol‐Myers Squibb, Janssen, Lab for Advanced Medicine, Exact Sciences and Gilead. She advises Intercept, Roche, Dynavax, Alnylam, Novartis, Eisai, Bayer and Spring Bank. She received grants from NCI and B.K. Kee.
References
- 1. Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144‐1165. [DOI] [PubMed] [Google Scholar]
- 2. Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence‐based analysis of 15 years of experience. Liver Transpl 2011;17(Suppl. 2):S44‐S57. [DOI] [PubMed] [Google Scholar]
- 3. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al.; U.S. Acute Liver Failure Study Group . Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947‐954. [DOI] [PubMed] [Google Scholar]
- 4. Maldonado JR, Dubois HC, David EE, Sher Y, Lolak S, Dyal J, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre‐transplant candidates. Psychosomatics 2012;53:123‐132. [DOI] [PubMed] [Google Scholar]
- 5. Wu T, Sundaram V. Transplantation for acute‐on‐chronic liver failure. Clin Liver Dis (Hoboken) 2019;14:152‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chronic Liver Failure Consortium . Tools‐calculators: EASL‐CLIF Consortium Scores. https://www.clifresearch.com/ToolsCalculators.aspx. Published 2013. Accessed May 2020. [Google Scholar]
- 7. Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090‐1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, et al. Clinical impact of alcohol‐related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res 2015;39:2085‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kling CE, Perkins JD, Carithers RL, Donovan DM, Sibulesky L. Recent trends in liver transplantation for alcoholic liver disease in the United States. World J Hepatol 2017;9:1315‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2018;16:1356‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001‐2002 to 2012‐2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Da BL, Im GY, Schiano TD. COVID‐19 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology 2020. 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 13. Bajaj JS, O’Leary JG, Tandon P, Wong F, Garcia‐Tsao G, Kamath PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol 2017;15:565‐574.e4. [DOI] [PubMed] [Google Scholar]
- 14. Kim WR, Biggins SW, Kremers WK, Wisner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Eng J Med 2008;359:1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn J, Sundaram V, Ayoub WS, Frenette C, Wong RJ. Hypoalbuminemia is associated with significantly higher liver transplant waitlist mortality and lower probability of receiving liver transplant. J Clin Gastroenterol 2018;52:913‐917. [DOI] [PubMed] [Google Scholar]
- 16. Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, et al. Factors associated with survival of patients with severe acute‐on‐chronic liver failure before and after liver transplantation. Gastroenterology 2019;156:1381‐1391.e3. [DOI] [PubMed] [Google Scholar]
- 17. Gutin L, Yao F, Dodge JL, Grab J, Mehta N. Comparison of liver transplant wait‐list outcomes among patients with hepatocellular carcinoma with public vs private medical insurance. JAMA Netw Open 2019;2:e1910326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1a
Table S1b
Table S1c


