Abstract
Concepts to ameliorate the continued mismatch between demand for liver allografts and supply include the acceptance of allografts that meet extended donor criteria (ECD). ECD grafts are generally associated with an increased rate of complications such as early allograft dysfunction (EAD). The costs of liver transplantation for the health care system with respect to specific risk factors remain unclear and are subject to change. We analyzed 317 liver transplant recipients from 2013 to 2018 for outcome after liver transplantation and hospital costs in a German transplant center. In our study period, 1‐year survival after transplantation was 80.1% (95% confidence interval: 75.8%‐84.6%) and median hospital stay was 33 days (interquartile rage: 24), with mean hospital costs of €115,924 (SD €113,347). There was a positive correlation between costs and laboratory Model for End‐Stage Liver Disease score (rs = 0.48, P < 0.001), and the development of EAD increased hospital costs by €26,229. ECD grafts were not associated with a higher risk of EAD in our cohort. When adjusting for recipient‐associated risk factors such as laboratory Model for End‐Stage Liver Disease score, recipient age, and split liver transplantation with propensity score matching, only EAD and cold ischemia increased total costs. Conclusion: Our data show that EAD leads to significantly higher hospital costs for liver transplantation, which are primarily attributed to recipient health status. Strategies to reduce the incidence of EAD are needed to control costs in liver transplantation.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate Aminotransferase
- BMI
body mass index
- CI
confidence interval
- CIT
cold ischemia time
- EAD
early allograft dysfunction
- ECD
extended criteria donor
- ICU
intensive care unit
- IQR
interquartile range
- labMELD
laboratory MELD score
- LOS
length of hospital stay
- MELD
Model for End‐Stage Liver Disease
Liver transplantation remains the treatment of choice for end‐stage liver disease and can additionally be a life‐saving procedure in patients with hepatocellular malignancies. The continued mismatch of organ supply and demand may pose a problem for the continued application of this procedure.( 1 , 2 ) Optimizing wait‐list mortality remains a priority,( 3 , 4 ) as alternative concepts to ameliorate these discrepancies include, but are not limited to, the expansion of living‐donor liver transplantation, donation after cardiac death, and the expansion of the donor pool to grafts from donors that meet extended criteria.( 5 , 6 )
Extended criteria donors (ECDs) fulfill certain parameters, such as higher age, elevated biochemical laboratory parameters before transplantation, drug abuse, or steatosis hepatis. The transplantation of such ECD grafts has been associated with an increase in primary nonfunction, early allograft dysfunction (EAD), and higher rates of retransplantation.( 7 , 8 , 9 , 10 ) The expected temporal trends for higher age and steatosis hepatis will most likely lead to an increasingly higher proportion of ECD grafts in the donor population in the near future.( 11 , 12 ) Liver transplantation tends to be very resource‐demanding, requiring highly specialized staff from different disciplines and on‐call complication management if needed. Higher use of ECD grafts will therefore likely incur increased rates of complications. As costs play an essential role in resource management in health care plays, the effect of complications such as EAD on the individual health care departments are of special interest. EAD is not only associated with graft and patient survival after transplantation, but also later complications such as chronic kidney injury, potentially incurring higher costs through the necessity of dialysis.( 7 , 13 ) Previously, studies identified the donor risk index (DRI), graft failure, length of hospital stay (LOS), as well as the recipient Model for End‐Stage Liver Disease (MELD) status as cost factors for liver transplantation.( 14 , 15 , 16 )
The aim of this study was to investigate the association of EAD with the costs of the initial hospital stay of liver transplantation. To identify potential contributors to higher cost, we analyzed the effect of donor risk factors such as steatosis hepatis, liver enzymes, and cold ischemia times, as well as recipient condition before transplant, and studied their interaction with EAD and costs of liver transplantation.( 17 )
Materials and Methods
Study Design and Participants
All patients receiving a liver transplantation over a 6‐year period between 2013 and 2018 at the Department of Surgery, Charité Campus Mitte | Campus Virchow‐Klinikum, Charité–Universitätsmedizin Berlin, Germany, were included in the study. Multi‐organ recipients were not included. Patient data were extracted from the individual electronic health record and anonymized; data on organ donors were retrieved from Eurotransplant. All patients were monitored at an intensive care unit (ICU) after transplantation. Follow‐up after transplantation was managed at our in‐house outpatient clinic; data were equally retrieved. The study was approved by the institutional ethics board (EA1/369/16).
Risk Stratification and Cost Data
Donor data were classified into extended criteria risk factors as proposed by Eurotransplant.( 17 , 18 ) These are donor age >65 years, donor body mass index (BMI) > 30 kg/m2, ICU stay > 7 days, sodium > 165 mmol/L, macrovesicular steatosis > 40%, aspartate aminotransferase (AST) > 90 U/L, alanine aminotransferase (ALT) > 105 U/L, and bilirubin > 51.3 µmol/l. The DRI was calculated as reported by Feng et al.; no donors in this study sample were of donations after cardiac death.( 19 ) EAD was defined by peak ALT and AST values in the first 7 days after transplantation >2,000 U/L, in addition to bilirubin (≥170 µmol/L) and international normalized ratio at day 7 (≥ 1.6), as defined by Olthoff et al.( 20 ) Cost data were requested from the medical controlling office and are structured according to the German Institute for the Hospital Remuneration System; they form the basis of payment according to the German diagnosis‐related group (DRG) system. The current reimbursement for liver transplantation in Germany is based on preset DRG bundled costs. Quality control is performed through an independent party (Institute for Quality and Transparency in the Healthcare System) without direct financial consequences for each individual case. Costs were calculated from admission date to discharge date. Follow‐up outpatient costs were not included. Data are categorized into the responsible department delivering therapy and respectively into personnel costs, medication, medical supply, and infrastructure costs for billing health insurances in Germany. These include the ICU, intermediate care unit, and normal ward of the following departments: nephrology/dialysis, anesthesiology, endoscopy, radiology, surgery, as well as the laboratory. In cases of retransplantation during the same hospital stay, the index transplantation was used for donor and recipient criteria, with costs summed for the entire hospital stay.
Statistical Analysis
Statistical analysis was performed using the software solutions R (version 4.0.0) and R Studio (version 1.25) for macOS (R Foundation for Statistical Computing, Vienna, Austria)( 21 ) Additional required packages for graph plotting and tabular analysis of statistics were ggplot2, ggpubr, ggstatsplot, scales, ggsci, gridExtra, BiocManager, DEGreport, mnormt, plyr, compareGroups, and arsenal. For descriptive statistics, variables were analyzed for normality and compared accordingly using one‐way analysis of variance, Kruskal–Wallis test, Student t test, or Wilcoxon rank‐sum test. Correlation was performed using Spearman’s rank correlation coefficient. Categorial variables were compared using the Pearson’s chi‐squared test.
To account for differences in allocation of extended and nonextended criteria of donor livers to recipients of differing overall health, propensity score matching was done using the R software packages Matching and MatchIt. The binominal outcome variable was the transplantation of an extended or nonextended criteria organ. Included in generation of the propensity score were recipient age, BMI, and laboratory MELD score (labMELD). A caliper of 0.05 was tolerated for matching. Of the initial data set of 349 patients, 120 remained in the matched data subset (i.e., 60 per subgroup). Overall, a two‐sided P value < 0.05 was considered significant. Data are reported as median and interquartile range (IQR) and mean and SD. The currency format is Euro (€), which translates approximately to US $1.15 in the third quarter of 2020. Yearly inflation in the study period was between 0.5% and 1.8% (2018); therefore, costs are not adjusted for inflation.( 22 )
Results
Clinical Outcome and Costs
In the 6‐year study period, a total of 364 liver transplantations were performed. Of these, 15 transplantations were not included in the analysis due to overlapping costs in two different cost‐calculation years; of the remaining 349 transplantations, 32 were retransplantations, and 4 patients received a third organ during the initial hospital stay. A total of 317 index cases were analyzed in this study. One‐year survival after transplantation was 80.1% (95% confidence interval [CI]: 75.8%‐84.6%). Median hospital stay was 33 days (IQR: 34), with a mean ICU stay of 10 days (IQR: 23). The mean total hospital costs associated with the transplantation procedure were €115,924 (SD €113,347); the median was €67,250 (IQR €106,070) (Fig. 1A). There were 10 (3.2%) outlier cases above €400,000 in the data set. Overall costs did not show any significant trend over the study period (P = 0.5), and most costs (52%) occurred during the ICU stay of the patients, while personnel costs dominated this subgroup (Fig. 1B). Average cost per hospital day was €2,120 (SD €2,382).
FIG. 1.
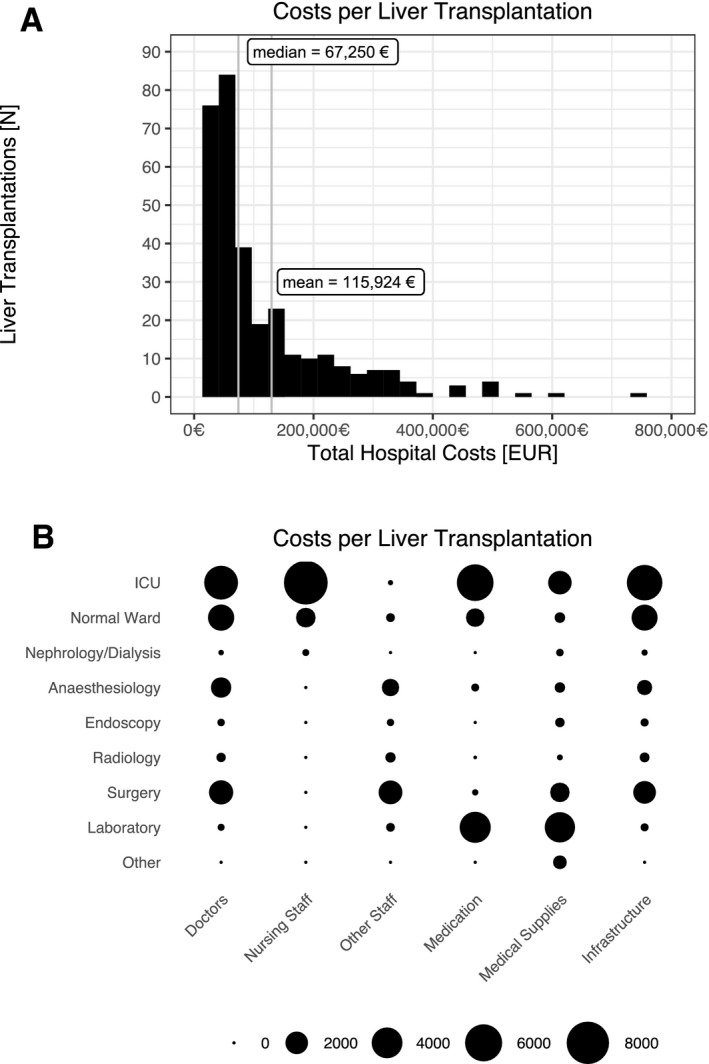
Costs per liver transplantation. (A) Histogram of total hospital‐associated costs (median and mean costs indicated). (B) Balloon plot of cost per liver transplantation distribution (larger dots equal higher costs). Currency format is EURO (€), which translates approximately to US $1.15 in the third quarter of 2020.
Liver transplant recipients were predominantly male (63%) with a median age of 55 years (IQR: 17). The median labMELD, as a surrogate parameter of overall health before transplantation, was 19 (IQR: 17) (Table 1).
TABLE 1.
Early Allograft Dysfunction
| Early Allograft Dysfunction (n = 120) | No Early Allograft Dysfunction (n = 197) | Total (n = 317) | P Value | |
|---|---|---|---|---|
| Donor age (years) | 58 (23) | 55 (29) | 56 (26) | 0.79 |
| Donor BMI (kg/m2) | 26 (5) | 25 (5) | 26 (5) | 0.001 |
| Donor gender (female) | 63 (52.5%) | 101 (51.3%) | 164 (51.7%) | 0.92 |
| Donor ICU (days) | 3.5 (6) | 4 (5) | 4 (5) | 0.55 |
| Donor CPR | 13 (10.8%) | 14 (7.1%) | 27 (8.5%) | 0.34 |
| Donor sodium (mmol/L) | 147 (11) | 148 (10) | 148 (10) | 0.59 |
| Donor AST (U/L) | 57 (94) | 46 (85) | 50.4 (88.2) | 0.40 |
| Donor ALT (U/L) | 39.3 (59.2) | 31.5 (61.8) | 36 (62.4) | 0.09 |
| Donor GGT (U/L) | 58.5 (94) | 41.1 (82) | 51 (88) | 0.03 |
| Donor bilirubin (µmol/L) | 9.3 (11) | 8.4 (8.6) | 8.6 (9.7) | 0.17 |
| Donor quick (%) | 75 (24) | 79 (28) | 78 (27.2) | 0.13 |
| Donor INR | 1.2 (0.26) | 1.17 (0.27) | 1.17 (0.27) | 0.20 |
| Donor steatosis | 57 (47.5%) | 66 (33.5%) | 123 (38.8%) | 0.02 |
| Donor cause of death | 0.88 | |||
| Anoxia | 20 (16.7%) | 32 (16.2%) | 52 (16.4%) | |
| CVA | 65 (54.2%) | 112 (56.9%) | 177 (55.8%) | |
| Trauma | 19 (15.8%) | 25 (12.7%) | 44 (13.9%) | |
| Other | 16 (13.3%) | 28 (14.2%) | 44 (13.9%) | |
| Split transplantation | 4 (3.3%) | 12 (6.1%) | 16 (5.1%) | 0.41 |
| Recipient age (years) | 56 (13.2) | 54 (20) | 55 (17) | 0.06 |
| Recipient gender (female) | 43 (35.8%) | 74 (37.6%) | 117 (36.9%) | 0.85 |
| Recipient BMI (kg/m2)* | 26.7 (5.59) | 25.4 (5.14) | 25.9 (5.35) | 0.03 |
| Recipient labMELD | 17.5 (16.4) | 19 (17) | 19 (17) | 0.57 |
| Recipient CIT (minutes) | 603 (235) | 558 (147) | 571 (173) | 0.01 |
| Recipient ICU (days) | 13 (31) | 9 (20) | 10 (23) | 0.004 |
| Recipient hospital stay (days) | 35 (36) | 31 (32) | 33 (24) | 0.11 |
| ECD factors | 0.74 | |||
| None | 28 (23.3%) | 48 (24.4%) | 76 (24.0%) | |
| One | 41 (34.2%) | 79 (40.1%) | 120 (37.9%) | |
| Two | 13 (10.8%) | 16 (8.12%) | 29 (9.15%) | |
| Three | 34 (28.3%) | 49 (24.9%) | 83 (26.2%) | |
| Four | 4 (3.33%) | 5 (2.54%) | 9 (2.84%) | |
| ECD organ | 92 (76.7%) | 149 (75.6%) | 241 (76%) | 0.94 |
| Donor age > 65 years | 37 (30.8%) | 65 (33%) | 102 (32.2%) | 0.78 |
| BMI > 30 kg/m2 | 19 (15.8%) | 18 (9.1%) | 37 (11.7%) | 0.11 |
| Steatosis hepatis > 40 (%) | 9 (7.5%) | 8 (4.1%) | 17 (5.4%) | 0.29 |
| Donor AST > 90 U/L | 37 (30.8%) | 62 (31.5%) | 99 (31.2) | 1 |
| Donor ALT > 105 U/L | 24 (20%) | 38 (19.3%) | 62 (19.6%) | 0.99 |
| Donor sodium > 165 mmol/L | 5 (4.2%) | 6 (3%) | 11 (3.5%) | 0.83 |
| Donor bilirubin > 51.3 µmol/L | 2 (1.7%) | 4 (2%) | 6 (1.9%) | 1 |
| Donor ICU > 7 days | 31 (25.8%) | 45 (22.8%) | 76 (24%) | 0.64 |
| DRI | 2.45 (0.92) | 2.34 (0.83) | 2.36 (0.85) | 0.66 |
| Retransplantation | 21 (17.5%) | 7 (3.5%) | 28 (98.8%) | <0.001 |
Data are reported as median (IQR). Currency format is EURO (€), which translates approximately to US $1.15 in the third quarter of 2020.
Data are reported as mean (SD).
Abbreviations: CPR, cardiopulmonary resuscitation; CVA, cerebrovascular accident; GGT, gamma‐glutamyltransferase; INR, international normalized ratio.
Cost‐Attributing Factors
Analysis of total hospital costs after liver transplantation revealed ECD donors, donor age, donor BMI, EAD, retransplantation, split transplantation, recipient labMELD, and recipient ICU stay to be major attributing factors (Figs. 2 and 3). Recipients of ECD grafts indeed had a mean reduction in cost of €39,028 (P = 0.02). The same was true for livers from elderly donors (€24,521 less; P = 0.03) and donors with obesity (€16,094 less in overall hospital costs; P = 0.05). The remaining ECD factors had no direct effect. On the recipient side, split liver transplantation (P = 0.001) or early retransplantation (P < 0.001) was associated with an increase in cost of €33,158 and €143,034, respectively. There was a positive correlation between costs and recipient labMELD (rs = 0.45; P < 0.001) as well as costs and the length of ICU stay (rs = 0.8; P < 0.001).
FIG. 2.
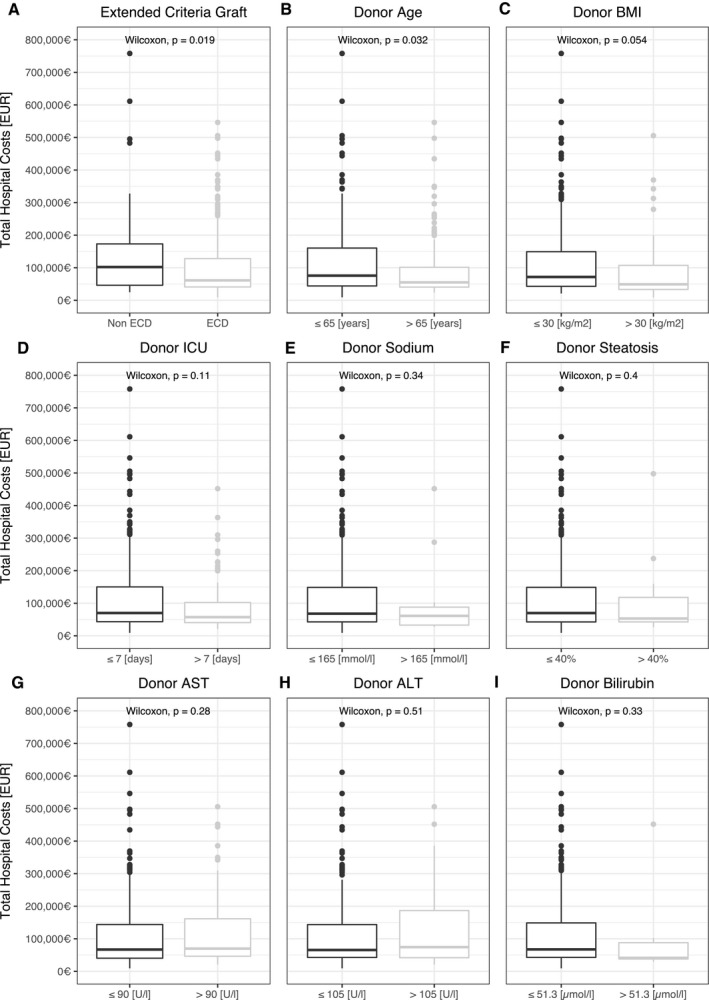
Donor criteria and total hospital costs. (A‐I) Donor risk factors and association with total hospital cost. Currency format is EURO (€), which translates approximately to US $1.15 in the third quarter of 2020.
FIG. 3.
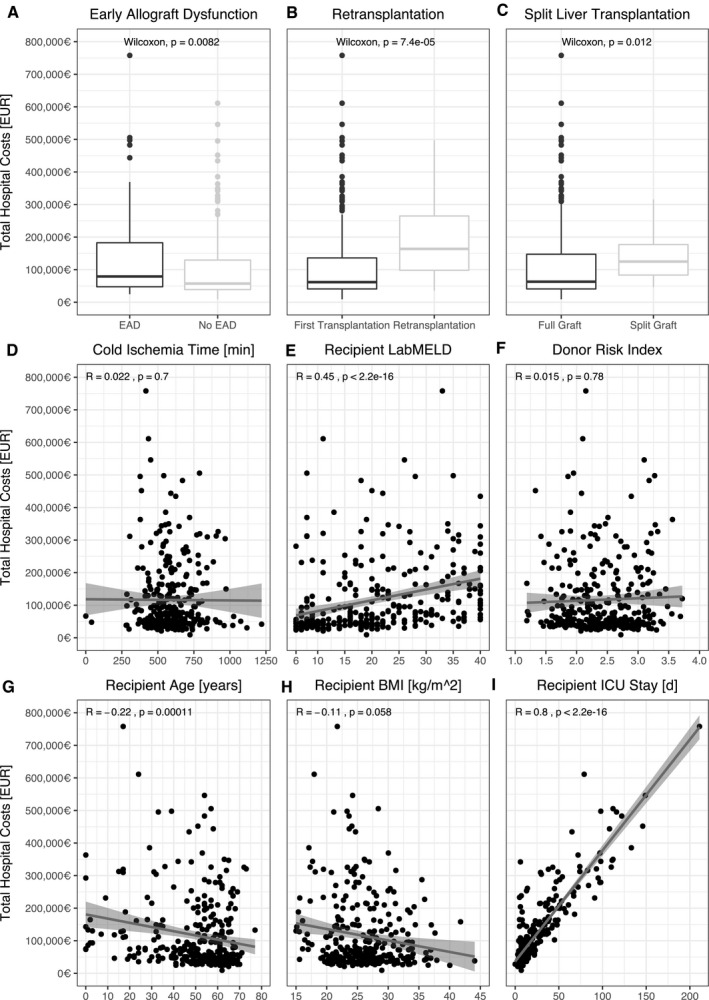
Recipient criteria and total hospital costs. (A‐I) Recipient risk factors and association with total hospital cost. Currency format is EURO (€), which translates approximately to US $1.15 in the third quarter of 2020.
Early Allograft Dysfunction
During the study period, EAD occurred in 120 cases (38%) and was associated with a reduced 1‐year survival rate of 70% (95% CI: 62.3%‐78.7%) compared with 86.3% (95% CI: 81.6%‐91.2%) in patients without graft EAD (P < 0.001) (Table 1). Retransplantation after EAD was three times more frequent and occurred in 21 cases (18%). Development of EAD was associated with higher donor or recipient BMI (P = 0.001 and P = 0.03, respectively) and higher donor gamma‐glutamyl‐transferase levels (P = 0.03). Additionally, reported donor steatosis was an influence on the development of EAD (P = 0.02). Donor cause of death did not differ in between grafts that developed EAD and those that did not. There was no direct association between extended donor criteria and EAD, even when accounting for the number of ECD factors met. The DRI did not differ (P = 0.66) and was above 2.3 in either group. Recipient labMELD was not associated with development of EAD (P = 0.57); however, CIT was approximately 45 minutes longer in the patient group, which developed EAD (P = 0.01).
Development of EAD increased total hospital costs by a mean of €26,229 and a median of €21,845 (P = 0.008), which resulted in a cost per day increase by €656. In further subgroup analysis of respective department costs, the largest increase in costs was doctor and nursing staff in the ICU, while ward cost through different categories was significantly lower (Supporting Table S1). Additionally, imaging and subsequent accumulated costs in the Radiology department, as well as costs in the Surgical department (i.e., the operating room) added to the overall cost increase of EAD. Further major factors were medications and medical supplies costs: both with €5,851 (P = 0.02) and €4,934(P = 0.03) increase per case of EAD, respectively.
Propensity Score Matching for Extended Donor Criteria
In univariate analysis, relevant recipient‐associated factors such as recipient labMELD, recipient age, and BMI affected the development of EAD and therefore the overall cost after liver transplantation. These factors differed in patients receiving ECD or non‐ECD liver grafts (Table 2). When adjusting for recipient‐associated risk factors such as labMELD, recipient age, split liver transplantation, or recipient BMI with propensity score matching, the effects were no longer significant (Table 3). In the propensity score–matched subgroup, EAD was equally distributed (P = 0.83) for ECD and non‐ECD graft, and the development of EAD was only associated with prolonged CIT (665 SD [166 minutes] vs. 562 SD [119 minutes]; P = 0.002). The DRI was slightly higher at 2.45 (IQR: 0.9) and did not differ between ECD groups.
TABLE 2.
Extended Criteria Grafts
| Extended Criteria Organ (n = 241) | Nonextended Criteria Organ (n = 76) | Total (n = 317) | P Value | |
|---|---|---|---|---|
| Split transplantation | 7 (2.9%) | 9 (11.8%) | 16 (5.1%) | 0.004 |
| Recipient age (years) | 55 (14) | 52 (30.2) | 55 (17) | 0.08 |
| Recipient gender (female) | 89 (36.9%) | 28 (36.8%) | 117 (36.9) | 1.00 |
| Recipient BMI (kg/m2)* | 26.5 (5.32) | 24 (5.04) | 25.9 (5.35) | <0.001 |
| Recipient labMELD | 18 (16) | 20 (17.5) | 19 (17) | 0.14 |
| Recipient CIT (minutes) | 575 (166) | 558 (182) | 571 (173) | 0.80 |
| Recipient ICU (days) | 9 (17) | 15 (25.75) | 10 (23) | 0.04 |
| Recipient hospital stay (days) | 33 (28) | 35.5 (45.7) | 33 (34) | 0.30 |
| DRI | 2.49 (0.89) | 2.17 (0.62) | 2.36 (0.85) | 0.006 |
| Retransplantation | 21 (8.7%) | 7 (9.2%) | 28 (8.8%) | 1.0 |
| Early allograft dysfunction | 92 (38.2%) | 28 (36.8%) | 120 (37.9%) | 0.94 |
Data are reported as median (IQR).
Data are reported as mean (SD).
TABLE 3.
Extended Criteria Grafts after Propensity Score Matching
| Extended Criteria Organ (n = 49) | Nonextended Criteria Organ (n = 49) | Total (n = 98) | P Value | |
|---|---|---|---|---|
| Split transplantation | 2 (4.1%) | 5 (10.2%) | 7 (7.1%) | 0.44 |
| Recipient age (years) | 51 (19) | 59 (27) | 54 (23) | 0.17 |
| Recipient gender (female) | 16 (32.7%) | 15 (30.6%) | 31 (31.6%) | 1.00 |
| Recipient BMI (kg/m2) | 25 (5.2) | 25 (5.5) | 25 (5.1) | 0.68 |
| Recipient labMELD | 21 (11) | 20 (15) | 20.5 (13) | 0.35 |
| Recipient CIT (minutes)* | 592 (135) | 609 (157) | 600 (146) | 0.56 |
| Recipient ICU (days) | 9 (21) | 15 (36) | 12 (27.5) | 0.09 |
| Recipient hospital stay (days) | 37 (27) | 37 (49) | 37 (40.3) | 0.96 |
| DRI | 2.45 (0.9) | 2.22 (0.68) | 2.34 (0.82) | 0.17 |
| Retransplantation | 6 (12.2%) | 3 (6.1%) | 9 (9.2%) | 0.48 |
| Early allograft dysfunction | 17 (34.7%) | 19 (38.8%) | 36 (36.7%) | 0.83 |
| Propensity score* | 0.26 (0.09) | 0.26 (0.09) | 0.26 (0.01) | 0.88 |
Data are reported as median (IQR).
Data are reported as mean (SD). Included in the generation of the propensity score were recipient age, BMI, and labMELD.
When analyzing the effect of ECD criteria on costs associated with liver transplantation in the matched subgroup, only donor age over 65 years reduced overall costs (P = 0.047) (Fig. 4). The presence of one factor or more than one ECD factor (P = 0.8) was neither associated with an increase nor decrease of total hospital costs.
FIG. 4.
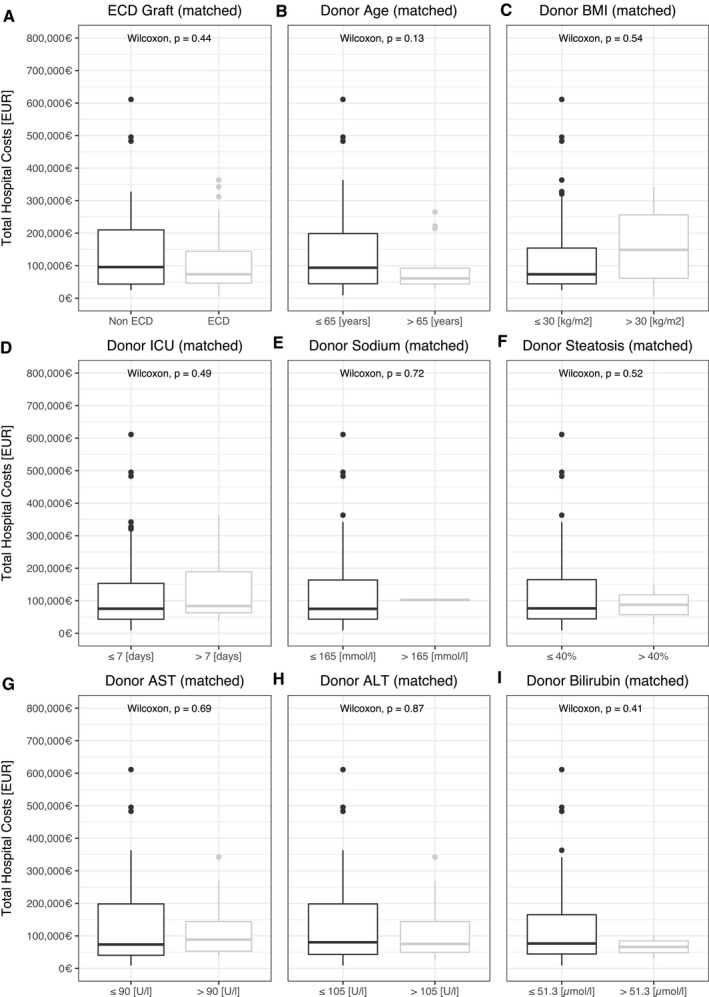
Donor criteria and total hospital costs after propensity score matching. (A‐I) Donor risk factors and association with total hospital cost after propensity score matching for recipient risk factors. Currency format is EURO (€), which translates approximately to US $1.15 in the third quarter of 2020.
Discussion
The continued mismatch between liver allograft demand and supply has, among other strategies, led to broader acceptance of organs that meet extended donor criteria. As transplantation medicine is especially resource‐demanding, we studied how these acceptance policies have affected costs after liver transplantation.
We show in this single‐center retrospective study of 317 cases that most costs can be attributed to the recipient health status before liver transplantation. By careful selection of ECD organs, costs of liver transplantation do not increase, even when matching for recipient‐associated risk factors. However, the occurrence of EAD had disastrous effects on patient survival, retransplantation rate, and therefore overall costs. Developing EAD is generally associated with ECD or high‐risk factors such as prolonged CIT, despite there being no direct effect in our study sample.( 7 , 13 , 23 ) We assumed a selection bias and possibly limited applicability of ECD criteria, such as higher donor age, for this case. Previous cost‐modeling approaches have, however, found similar results.( 24 ) Nonetheless, it is clear that the examined donors were overall of high risk, with a median DRI of 2.36 and 228 cases (72%) with a DRI ≥ 2.( 10 , 14 , 19 ) Additionally, in our patient group, there was a larger number of patients with a high labMELD ≥ 35 (n = 45, 14%), which is an accepted, albeit not perfect, predictor of mortality after liver transplantation, LOS, and disease burden.( 25 , 26 , 27 )
Our propensity score matching tried to address this problem. It is common practice for organs through rescue allocation (i.e., mostly ECD organs) to be transplanted in patients with considerably good remnant liver function (i.e., patients with a hepatocellular carcinoma and low labMELD score). When negating these effects through matching, the total cost of transplantation was still almost double in recipients of non‐ECD organs. However, accepting organs for transplantation is always an individual decision, reflected by the surgeon/hepatologist in charge and equally depends on the current health of the recipient, logistical factors in procurement and donor quality. At least from a financial standpoint, our results would suggest that with careful selection, ECD organs are not only feasible for transplantation in times of organ scarcity, but do not affect the total cost of a hospital stay. Zhang et al. proposed a more aggressive use of ECD grafts after analyzing over 75,000 donors from the United Network for Organ Sharing database.( 28 ) However, we would like to highlight that the development of EAD was associated with donor quality and prolonged CIT. In our own center, we could show that macrovesicular steatosis hepatis, especially in cases with long CIT, leads to EAD in up to 70% of cases.( 29 ) The trend of increasing percentage of ECD donors required to be accepted for transplantation therefore comes with a literal price. If the graft does develop an early dysfunction, the associated cost and complications are immense.
Previous publications have highlighted that the DRI is an attributing factor to LOS and that donors with a DRI > 2.5 can lead to increased costs of up to US $50,000.( 14 ) Previous studies from Germany have shown rising costs of liver transplantation since the introduction of labMELD‐based allocation as well as increasing costs with increasing MELD score.( 30 , 31 ) The effect of labMELD on costs after liver transplantation has additionally been shown in a cohort of 403 patients in Switzerland, with double the overall costs in a high MELD group.( 16 ) Interestingly, health care costs during the waiting time for an allograft appear to be about €9,500 in Germany; patients with a lower MELD score had especially higher costs, due to a longer waiting time.( 31 ) Despite there being no time trend in the costs during our limited study period of 6 years, the mean difference in comparison with data from our own center over a decade ago, accounting for inflation, was €54,186.( 15 ) This coincides with the introduction of the MELD‐based allocation by Eurotransplant in December 2006, and confirms that MELD base allocation has significantly affected costs of liver transplantation.
Allocation policies using labMELD will likely not change in the foreseeable future, and patients with high MELD scores will continue to incur high costs on the health care system if these patients cannot be transplanted earlier. Manageable or preventable effects on graft function and associated costs, however, can be addressed. Our data show that prolonged CIT was most relevant for EAD in our recipient risk factor–matched subgroup. CIT has been linked directly to LOS and prolonged LOS after liver transplantation.( 32 ) When considering costs in liver transplantation, priority should be in preventing EAD and consequent retransplantation of patients. With careful matching of donors and recipients, this can, in part, be avoided. Nevertheless, we show in our propensity score–matched subgroup that CIT remains of utmost relevance to the developments of EAD. Consequently, we propose focusing on methods to reduce CIT and therefore potentially limit the disastrous effect that EAD has on patient and graft survival and, to an extent, the resource‐limited liver transplantation field in general.
Future strategies such as bioengineering of autologous liver grafts have previously been proposed as potential methods to broaden the donor pool.( 33 ) Other technologies such as ex vivo machine perfusion appear, at this time, to be more feasible and readily applicable as a potential evaluation and reconditioning tool.( 34 , 35 ) Studies for renal transplantation have shown hypothermic perfusion to be superior to static cold storage in outcome and costs.( 36 , 37 ) We are not of aware of any such comparisons for liver transplantation at time of writing. However, EAD, which is also defined by a peak AST/ALT in the first 7 days, could be reduced by as much as 50% in first clinical trials of normothermic ex vivo machine perfusion (NEVLP).( 38 ) Controlled oxygenated rewarming before transplantation yielded equal results,( 39 ) and a combination of hypothermic and normothermic machine perfusion showed a reduction in ischemia reperfusion injury.( 40 ) Furthermore, there is current research on potential manipulation of the lipid metabolism (i.e. defatting of grafts) through NEVLP.( 41 ) Ex vivo machine perfusion could therefore be a valuable tool for careful organ selection and optimization before transplantation. However, at this time, no specific approach has been unilaterally agreed upon concerning perfusion temperature, stage in allocation procedure, or perfusate substance.( 42 ) Considering the potential benefit of reducing EAD and therefore improving cost effectiveness does seem promising. Moreover, the additional costs of liver machine perfusion might be worth the potential for cost reduction by lowering the incidence of EAD.
Our data are limited by the single‐center, retrospective study design as well as the relatively high proportion of high DRI grafts transplanted, long median LOS, and lower 1‐year survival rate compared with most transplant centers in North America.( 43 ) Additionally, absolute costs described here may not reflect costs in other countries outside the European Union, where labor costs could be lower or higher. Nonetheless, the relative effects we show are, in our opinion, of utmost relevance for the transplant community, especially in low donation countries. Furthermore, our detailed cost structure for each liver transplantation enables a detailed comparison of individual cost‐driving factors and adds valuable insight for liver transplantation.
In conclusion, we show that EAD leads to significantly higher hospital costs for liver transplantation. While ECD criteria do not directly affect the total hospital costs of liver transplantation, costs can be attributed primarily to recipient health status as measured in the labMELD, and CIT as the most important donor risk factor. Because the MELD‐based allocation system will not change in the foreseeable future, we propose, not only from a financial standpoint, to focus on reduction of EAD and CIT, potentially by using liver‐preservation technologies such as ex vivo machine perfusion.
Supporting information
Table S1
Acknowledgment
Open access funding enabled and organized by ProjektDEAL.
Supported by the Department of Surgery, Campus Charité Mitte | Campus Virchow‐Klinikum, corporate member of Freie Universität Berlin, Humboldt‐ Universität zu Berlin and Berlin Institute of Health.
Potential conflict of interest: Nothing to report.
References
- 1. Orman ES, Mayorga ME, Wheeler SB, Townsley RM, Toro‐Diaz HH, Hayashi PH, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl 2015;21:1040‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toro‐Díaz H, Mayorga ME, Barritt AS, Orman ES, Wheeler SB. Predicting liver transplant capacity using discrete event simulation. Med Decis Making 2015;35:784‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishaque T, Massie AB, Bowring MG, Haugen CE, Ruck JM, Halpern SE, et al. Liver transplantation and waitlist mortality for HCC and non‐HCC candidates following the 2015 HCC exception policy change. Am J Transplant 2019;19:564‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haugen CE, McAdams‐DeMarco M, Holscher CM, Ying H, Gurakar AO, Garonzik‐Wang J, et al. Multicenter study of age, frailty, and waitlist mortality among liver transplant candidates. Ann Surg 2020;271:1132‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson KR, Motter JD, Haugen CE, Holscher C, Long JJ, Massie AB, et al. Temporal trends in utilization and outcomes of steatotic donor livers in the United States. Am J Transplant 2020;20:855‐863. [DOI] [PubMed] [Google Scholar]
- 6. Vodkin I, Kuo A. Extended criteria donors in liver transplantation. Clin Liver Dis 2017;21:289‐301. [DOI] [PubMed] [Google Scholar]
- 7. Wadei HM, Lee DD, Croome KP, Mai ML, Golan E, Brotman R, et al. Early allograft dysfunction after liver transplantation is associated with short‐ and long‐term kidney function impairment. Am J Transplant 2016;16:850‐859. [DOI] [PubMed] [Google Scholar]
- 8. Pratschke S, Bender A, Boesch F, Andrassy J, van Rosmalen M, Samuel U, et al. Association between donor age and risk of graft failure after liver transplantation: an analysis of the Eurotransplant database. Transpl Int 2019;32:270‐279. [DOI] [PubMed] [Google Scholar]
- 9. Moosburner S, Ritschl PV, Wiering L, Gassner J, Ollinger R, Pratschke J, et al. High donor age for liver transplantation: tackling organ scarcity in Germany. Chirurg 2019;90:744‐751. [DOI] [PubMed] [Google Scholar]
- 10. Flores A, Asrani SK. The donor risk index: a decade of experience. Liver Transpl 2017;23:1216‐1225. [DOI] [PubMed] [Google Scholar]
- 11. GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019;70:745‐758. [DOI] [PubMed] [Google Scholar]
- 13. Lee DD, Croome KP, Shalev JA, Musto KR, Sharma M, Keaveny AP, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol 2016;15:53‐60. [DOI] [PubMed] [Google Scholar]
- 14. Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant 2007;7:990‐997. [DOI] [PubMed] [Google Scholar]
- 15. Lock J, Reinhold T, Bloch A, Malinowski M, Schmidt SC, Neuhaus P, et al. The cost of graft failure and other severe complications after liver transplantation—experience from a German Transplant Center. Ann Transplant 2010;15:11‐18. [PubMed] [Google Scholar]
- 16. Schlegel A, Linecker M, Kron P, Gyori G, De Oliveira ML, Mullhaupt B, et al. Risk assessment in high‐ and low‐MELD liver transplantation. Am J Transplant 2017;17:1050‐1063. [DOI] [PubMed] [Google Scholar]
- 17. Eurotransplant . Eurotransplant Manual. Leiden: Eurotransplant Foundation; 2019. [Google Scholar]
- 18. German Medical Association . Guideline § 16 Abs. 1 S. 1 No. 2 and 5 TPG for waitlist protocol and organ allocation for liver transplantation. Dtsch Arztebl International 2019;116:A‐1688. https://www.bundesaerztekammer.de/fileadmin/user_upload/downloads/pdf‐Ordner/RL/RiliOrgaWlOvLeberTx20190924.pdf. Accessed November 29, 2020. [Google Scholar]
- 19. Feng S, Goodrich NP, Bragg‐Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783‐790. [DOI] [PubMed] [Google Scholar]
- 20. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16:943‐949. [DOI] [PubMed] [Google Scholar]
- 21. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. http://www.r‐project.org/index.html. Accessed November 29, 2020. [Google Scholar]
- 22. Destatis . Consumer price index: Germany by years. 2020. https://www‐genesis.destatis.de/genesis/online?operation=previous&levelindex=2&step=2&titel=Ergebnis&levelid=1588535234733&acceptscookies=false. Accessed March 5, 2020.
- 23. Lee DD, Singh A, Burns JM, Perry DK, Nguyen JH, Taner CB. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl 2014;20:1447‐1453. [DOI] [PubMed] [Google Scholar]
- 24. Earl TM, Cooil B, Rubin JE, Chari RS. Cost prediction in liver transplantation using pretransplant donor and recipient characteristics. Transplantation 2008;86:238‐244. [DOI] [PubMed] [Google Scholar]
- 25. Roth JA, Chrobak C, Schadelin S, Hug BL. MELD score as a predictor of mortality, length of hospital stay, and disease burden: a single‐center retrospective study in 39,323 inpatients. Medicine (Baltimore) 2017;96:e7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864‐871. [DOI] [PubMed] [Google Scholar]
- 27. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 28. Zhang T, Dunson J, Kanwal F, Galvan NTN, Vierling JM, O'Mahony C, et al. Trends in outcomes for marginal allografts in liver transplant. JAMA Surg 2020;155:926‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moosburner S, Gassner JMGV, Nösser M, Pohl J, Wyrwal D, Claussen F, et al. Prevalence of steatosis hepatis in the eurotransplant region: impact on graft acceptance rates. HPB Surg 2018;2018:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruns H, Hillebrand N, Schneider T, Hinz U, Fischer L, Schmidt J, et al. LabMELD‐based organ allocation increases total costs of liver transplantation: a single‐center experience. Clin Transplant 2011;25:E558‐E565. [DOI] [PubMed] [Google Scholar]
- 31. Harries L, Schrem H, Stahmeyer JT, Krauth C, Amelung VE. High resource utilization in liver transplantation‐how strongly differ costs between the care sectors and what are the main cost drivers? A retrospective study. Transpl Int 2017;30:621‐637. [DOI] [PubMed] [Google Scholar]
- 32. Pan ET, Yoeli D, Galvan NTN, Kueht ML, Cotton RT, O'Mahony CA, et al. Cold ischemia time is an important risk factor for post‐liver transplant prolonged length of stay. Liver Transpl 2018;24:762‐768. [DOI] [PubMed] [Google Scholar]
- 33. Habka D, Mann D, Landes R, Soto‐Gutierrez A. Future economics of liver transplantation: a 20‐year cost modeling forecast and the prospect of bioengineering autologous liver grafts. PLoS One 2015;10:e0131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laing RW, Mergental H, Yap C, Kirkham A, Whilku M, Barton D, et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open‐label, non‐randomised, prospective, single‐arm trial. BMJ Open 2017;7:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghinolfi D, Tincani G, Rreka E, Roffi N, Coletti L, Balzano E, et al. Dual aortic and portal perfusion at procurement prevents ischaemic‐type biliary lesions in liver transplantation when using octogenarian donors: a retrospective cohort study. Transpl Int 2019;32:193‐205. [DOI] [PubMed] [Google Scholar]
- 36. Buchanan PM, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. Am J Transplant 2008;8:2391‐2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wight J, Chilcott J, Holmes M, Brewer N. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transplant 2003;17:293‐307. [DOI] [PubMed] [Google Scholar]
- 38. Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557:50‐56. [DOI] [PubMed] [Google Scholar]
- 39. Hoyer DP, Mathe Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation 2016;100:147‐152. [DOI] [PubMed] [Google Scholar]
- 40. Boteon YL, Laing RW, Schlegel A, Wallace L, Smith A, Attard J, et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl 2018;24:1699‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boteon YL, Attard J, Boteon APCS, Wallace L, Reynolds G, Hubscher S, et al. Manipulation of lipid metabolism during normothermic machine perfusion: effect of defatting therapies on donor liver functional recovery. Liver Transpl 2019;25:1007‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schlegel A, Muller X, Dutkowski P. Machine perfusion strategies in liver transplantation. Hepatobiliary Surg Nutr 2019;8:490‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bittermann T, Hubbard RA, Serper M, Lewis JD, Hohmann SF, VanWagner LB, et al. Healthcare utilization after liver transplantation is highly variable among both centers and recipients. Am J Transplant 2018;18:1197‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


