Abstract
Background
Skeletal involvement of Cryptococcus neoformans is infrequent and usually associated with disseminated cryptococcosis or underlying predisposing conditions. We present an atypical case of osteoarticular cryptococcosis in an immunocompetent patient.
Case Presentation
We herein report a case of bone and soft tissue cryptococcal infection in a 42-year-old male from Pakistan with well-controlled diabetes without other associated immunodeficiencies treated with antifungal therapy without surgical debridement. Furthermore, the patient developed toxidermia due to fluconazole use, so a fluconazole desensitization was performed. Therapeutic management also included the performance of therapeutic drug monitoring of fluconazole plasma concentrations.
Conclusion
To our knowledge, this is the first case of osteoarticular cryptococcosis treated with this treatment regimen. This strategy may be of interest to try to reduce hospital stay and associated complications.
Keywords: Cryptococcus neoformans, osteoarticular cryptococcosis, immunocompetent, liposomal amphotericin B, fluconazole desensitization
Case Report
A 42-year-old male from Pakistan was admitted to hospital in July 2018 complaining of chronic lumbar pain. The patient, who had been living in Barcelona since 2003, was recently diagnosed with type 2 diabetes mellitus, established with two hemoglobin A1C tests of over 6.5%, and was well controlled on diet. He had no other medical conditions. He worked in public works water and sewer maintenance and claimed to have had no prior contact with animals. The patient reported a 6-month history of mechanical lumbar pain, without any previous trauma. The only symptoms reported during this time were 5 kg weight loss and 24-hr fever. After five months of pain, nodular lesions in the right gluteal region were noticed and antibiotic treatment was initiated by his general practitioner (amoxicillin-clavulanate 1g/200mg TID for 10 days and thereafter ciprofloxacin 500 mg BID for 10 days). Despite antibiotic treatment, pain and inflammatory signs increased and spontaneous drainage of the gluteal abscess occurred. A sample was taken for microbiological cultures and Cryptococcus neoformans was isolated. The patient was hospitalized for further study. Physical examination at admission revealed two indurated areas without inflammatory signs in the right gluteus and right lumbar area. The rest of the exploration was unremarkable. Laboratory tests showed renal and liver function values within the normal range (total bilirubin 0.36 mg/dl, GOT 31 UI/L, GPT 29 UI/L, FA 67 UI/L), hemoglobin 15.3 g/dl, white cell count 6940 cells/µL (44.2% neutrophils, 44.5% lymphocyte). The plasma cryptococcal antigen latex agglutination test (CLAT) was positive (1:100), suggesting a disseminated infection. A lumbar puncture was performed with normal results, including a negative CLAT. Human immunodeficiency virus (HIV), primary immunodeficiencies and a lymphoproliferative disorder were ruled out. CD4 and CD8 counts were in the normal range. Except for diabetes, no other predisposing factors were found. Thoraco-abdominal computed tomography ruled out lung involvement and revealed a gluteal abscess and a 3 cm lytic lesion on the right iliac crest (Figure 1). An ultrasound-guided fine-needle aspiration biopsy of the iliac crest was performed. No further surgical debridement was performed. The sample was inoculated on Polyvitex and Sabouraud agar plates (Biomèrieux®) and incubated at 37°C. Growth was observed after 72h incubation and the strain was identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS, BrukerDaltonics®) as C. neoformans. Antifungal susceptibility testing was performed using the Sensititre YeastOne® method, showing susceptibility to fluconazole [minimum inhibitory concentration (MIC) of 2 mg/L] and amphotericin B.
Figure 1.
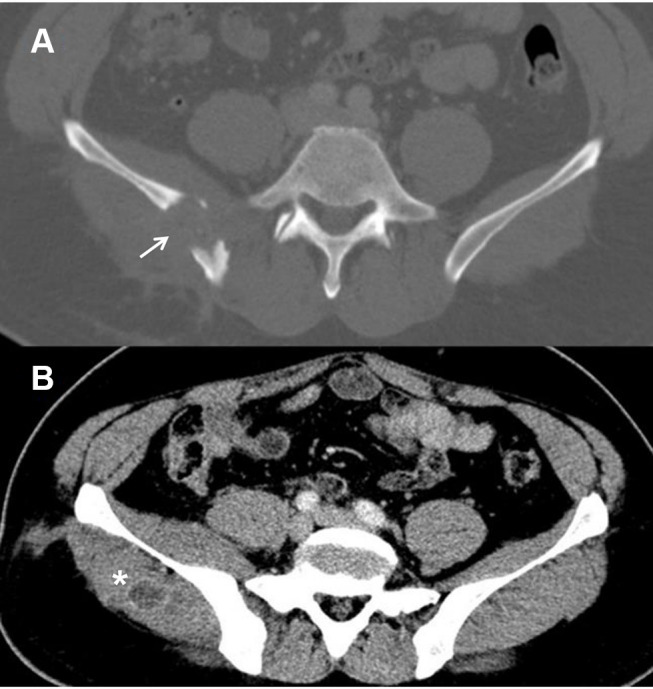
Transverse computed tomography image of the pelvis.
Notes: (A) Bone window: Osteolytic lesion with cortical bone destruction involving the right iliac bone (white arrow). (B) Soft tissue window: bulking of the right iliac and gluteus medius muscle with an abscess in the latter (*).
Since the patient refused to remain in hospital due to family and work responsibilities, a treatment regimen was planned based on a single-dose of liposomal amphotericin B (L-AmB) (10 mg/kg, total dose 870 mg), infused over 3h, followed by outpatient therapy with oral fluconazole (800 mg/day). However, after the second dose of fluconazole, the patient developed a pruritic erythematous maculopapular rash. Given the lack of alternatives due to his personal circumstances and based on previous data in patients without HIV,1 a rapid oral desensitization protocol was performed (Table 1). Desensitization was conducted over 8 hours without complications, and the patient was discharged afterwards. He received 800 mg fluconazole daily for 14 days, followed by 400mg daily for one year. The treatment was well tolerated with no adverse events. The pain resolved and plasma CLAT was undetectable at the end of treatment. During follow-up, on-site monitoring of fluconazole blood concentrations was conducted. While patient was on 800 mg, the area under the curve (AUC)/MIC was 420 (objective >389). With the later 400 mg dosage, a further 3 samples were obtained, yielding AUC/MICs of 144, 180 and 156 (goal >100). At the end of follow-up, a mild alteration in liver function was observed (total bilirubin 0.24 mg/dl, AST 41 UI/L, ALT 45 UI/L, GGT 62 UI/L and FA 67 UI/L), which was attributable to moderate liver steatosis confirmed by abdominal echography. After 20 months of follow-up, the patient continued asymptomatic without signs of recurrence.
Table 1.
Protocol of Fluconazole Desensitization
| Step | Fluconazole Dilution (mg/mL) | Volume Administered (mL) | Fluconazole Dose (mg) | Cumulative Dose |
|---|---|---|---|---|
| 1 | 0.002 | 10 | 0.02 | 0.02 |
| 2 | 0.005 | 10 | 0.05 | 0.07 |
| 3 | 0.01 | 10 | 0.1 | 0.17 |
| 4 | 0.02 | 10 | 0.2 | 0.37 |
| 5 | 0.04 | 10 | 0.4 | 0.77 |
| 6 | 0.08 | 10 | 0.8 | 1.57 |
| 7 | 0.16 | 10 | 1.6 | 3.17 |
| 8 | 2.0 | 5 | 4 | 7.17 |
| 9 | 2.0 | 3.13 | 6.25 | 13.42 |
| 10 | 2.0 | 6.25 | 12.5 | 25.92 |
| 11 | 2.0 | 12.5 | 25 | 50.92 |
| 12 | 2.0 | 25 | 50 | 100.92 |
| 13 | 2.0 | 50 | 100 | 200.92 |
| 14 | – | – | 200 | 400.92 |
| 15 | – | – | 400 | 800.92 |
| 16 | – | – | 800 | 1600.92 |
Note: A time interval of 30 minutes between doses was established.
Discussion
C. neoformans is a yeast normally found in soil. It causes opportunistic infection in immunocompromised patients, typically those with acquired immune deficiency syndrome.2 Although localized pulmonary disease is the main primary infection, extrapulmonary disease is common; especially central nervous system (CNS) infection.3 Osteoarticular cryptococcosis as the sole manifestation of extrapulmonary disease is rare, with an incidence of 5% in patients with cryptococcal disease.3 Hematogenous dissemination after the primary pulmonary infection may explain bone affectation.3 All of these findings are even rarer in immunocompetent patients.4 In our case, diabetes mellitus was the only risk factor predisposing to cryptococcal infection. An exhaustive study ruled out other immunodeficiencies. Although diabetes mellitus is a known risk factor for fungal infection, our patient had non-severe diabetes, with glucose levels well controlled by diet. We, therefore, considered low-grade immunosuppression due to diabetes. The patient also denied exposure to other risk factors, such as avian guano. While exceptional, other cases of cryptococcal infection with osteoarticular involvement in seemingly healthy patients have been reported.3–8
Little is known about the optimal treatment of osteoarticular cryptococcosis. Most cases reported in the literature propose a wide variety of treatments, based on antifungal drug therapy and surgical debridement.3,5 In contrast, more evidence is available for the management of CNS infection. Recently, a Phase 2 non-inferiority clinical trial in HIV-associated cryptococcal meningitis showed that a single high-dose of L-AmB plus high-dose oral fluconazole was non-inferior to 14 days of 3 mg/kg/day L-AmB in clearing cerebrospinal fluid infection.9 In our case, although CNS infection was ruled out, high levels of cryptococcal antigen in blood suggested disseminated cryptococcal infection. Moreover, bone involvement requires high doses of antifungal agents due to its poor vasculature. Consequently, based on the latest IDSA guidelines10 and the evidence in CNS infection,11 we treated this case with induction therapy consisting of a single high-dose of L-AmB followed by maintenance treatment with oral fluconazole for twelve months. Remarkably, we report a successful desensitization therapy that may be useful in other patients with fluconazole allergy. Throughout the entire course of fluconazole treatment, the plasma levels were considered therapeutic, since they achieved the recommended AUC/MIC of ≥389 in induction therapy and AUC/MIC ≥100 during consolidation therapy.12
In conclusion, we present an atypical case of bone and soft tissue cryptococcal infection in an immunocompetent host, which was managed with antifungal treatment without surgical debridement. Induction therapy with a single high-dose of L-AmB followed by maintenance treatment with oral fluconazole appears to be effective. Fluconazole desensitization and therapeutic drug monitoring ensured the proper application of treatment for twelve months and suggests that results may be similarly effective when applied to other patients.
Funding Statement
The authors thank the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) which supports the research of Dr. Silvia Gómez-Zorrilla.
Abbreviations
L-AmB, liposomal amphotericin B; CNS, central nervous system; CLAT, cryptococcal antigen latex agglutination test; HIV, human immunodeficiency virus; MIC, minimum inhibitory concentration; AUC, area under the curve.
Data Sharing Statement
All data (image and table) used in this study are included in this published article. We do not have metadata sharing, thus, it is not applicable to this article.
Ethics Approval
No institutional approval was required to publish the case details.
Consent for Publication
The patient gave written informed consent to the publication of his history and photographs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Juan Pablo Horcajada reports being a speaker with honoraria advisory boards for MSD, Pfizer, and Menarini. The authors report no conflicts of interest in this work.
References
- 1.Randolph C, Kaplan C, Fraser B. Rapid desensitization to Fluconazole (Diflucan). Ann Allergy Asthma Immunol. 2008;100(6):616–617. doi: 10.1016/S1081-1206(10)60063-4 [DOI] [PubMed] [Google Scholar]
- 2.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20(3):507–544. [DOI] [PubMed] [Google Scholar]
- 3.Medaris LA, Ponce B, Hyde Z, et al. Cryptococcal osteomyelitis: a report of 5 cases and a review of the recent literature. Mycoses. 2016;59(6):334–342. [DOI] [PubMed] [Google Scholar]
- 4.Wood L, Miedzinski L. Skeletal cryptococcosis: case report and review of the literature. Can J Infect Dis. 1996;7:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou HX, Lu L, Chu T, et al. Skeletal cryptococcosis from 1977 to 2013. Front Microbiol. 2015;5:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn JH, Park C, Lee CW, Kim YC. Cryptococcal osteomyelitis of the first metatarsal head in an immunocompetent patient. J Am Pediatr Med Assoc. 2017;107(3):248–252. [DOI] [PubMed] [Google Scholar]
- 7.Sang J, Yang Y, Fan Y, et al. Isolated iliac cryptococcosis in an immunocompetent patient. PLoS Negl Trop Dis. 2018;12(3):e0006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Liang J, Shen J, Qiu G, Weng X. Thoracolumbar scoliosis due to cryptococcal osteomyelitis: a case report and review of the literature. Medicine. 2016;95(5):e2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvis JN, Leeme TB, Mooketsi M, et al. Short-course high-dose liposomal amphotericin b for human immunodeficiency virus–associated cryptococcal meningitis: a phase 2 randomized controlled trial. Clin Infect Dis. 2019;68(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378(11):1004–1017. [DOI] [PubMed] [Google Scholar]
- 12.Chesdachai S, Rajasingham R, Nicol MR, et al. Minimum inhibitory concentration distribution of fluconazole against cryptococcus species and the fluconazole exposure prediction model. Open Forum Infect Dis. 2019;6(10):ofz369. [DOI] [PMC free article] [PubMed] [Google Scholar]


