Abstract
Objective
Periodic Limb Movements of Sleep (PLMS) is a poorly understood comorbidity with close association to Obstructive Sleep Apnea (OSA). The mechanistic link between the two is unclear. Recent studies on the latter have uncovered low respiratory arousal threshold as an important non-anatomical cause of the disorder. This study sought to investigate whether periodic limb movements are associated with the low respiratory arousal threshold (ArTH) in OSA.
Methods
Retrospective data on 720 OSA patients (mean age = 47.0) who underwent Polysomnography (PSG) were collected. Using PLMS diagnostic criteria of PLMS index ≥ 15, patients were divided into the OSA-PLMS group (n=95) and the OSA-only group (n=625). Binary logistic regression analysis was used to examine the correlation between PLMS and the presence of low ArTH, classified using a predicted tool (developed by Edward et al) requiring meeting at least two of the three criteria: apnea-hypopnea index (AHI) < 30/h, nadir oxygen saturation (SaO2) > 82.5%, and fraction of hypopneas > 58.3%. The resulting model was validated in the external MrOS database.
Results
The patients in the OSA-PLMS group tend to be older, with a higher prevalence of hypertension, diabetes, and stroke. PLMS was associated with age, diabetes, oxygen desaturation index, and low respiratory arousal threshold (OR=8.78 (4.73–16.30), p<0.001). When validated against the MrOS database, low ArTH remained a significant predictor of PLMS with an odds ratio of 1.33 (1.08–1.64, p = 0.009).
Conclusion
This is the first study that demonstrated a strong correlation between PLMS and low respiratory arousal threshold. This suggests a possible mechanistic link between the physical manifestation of PLMS and the non-anatomical low arousal threshold phenotype in OSA.
Keywords: obstructive sleep apnea, periodic limb movements, low respiratory arousal threshold
Statement of Significance
This study examined the hitherto poorly understood association between OSA and PLMS and demonstrated for the first time a strong correlation between PLMS and a low respiratory threshold (a non-anatomical cause of OSA). This potentially poses the respiratory arousal threshold as the missing link between the respiratory phenomenon of OSA and the neurologically driven disorder of PLMS.
Clinically, this provides insights into why OSA patients may be more susceptible to PLMS and identified the low respiratory arousal threshold as a possible new therapeutic target in this group of patients, who are especially vulnerable to an elevated risk of cardiovascular diseases. As such, we call for more attention to this group of OSA patients with PLMS.
Introduction
Periodic limb movement in sleep (PLMS) is characterized as periodic episodes of stereotyped limb movements that occur involuntarily and repetitively during nocturnal rest,1 though patients are usually unaware of such movements and the arousals induced.2 Standard criteria define periodic limb movements as ≥15 leg movements per hour during sleep.1 The incidence of PLMS increases with age and is associated with several predisposing medical conditions, including narcolepsy, Parkinson’s disease, idiopathic REM sleep behavior disorder, and diabetes mellitus.3 Though there appears to be no clear link between PLMS and excessive daytime sleepiness,4,5 PLMS has been reported to be associated with poorer sleep quality,6 arousals7,8 and possibly sleep fragmentation4 and increased risks of cardiovascular diseases.9 PLM is also observed in 80% of patients with restless legs syndrome (RLS)10 and 24–48% of patients with Obstructive Sleep Apnea (OSA).11
A large body of literature has demonstrated the close clinical association between Obstructive Sleep Apnea (OSA) and Periodic Limb Movements (PLM).4–6,12 Yet, up till now, the pathogenesis and etiology of PLM and the mechanism of its close association with OSA remain unclear. In many cases, PLMS is noted only as an accompanying incidental finding in PSG conducted for OSA.5,11 One study has nevertheless shown that periodic limb movements in moderate to severe OSA patients worsens after CPAP treatment,13 and another demonstrated a reduced long-term adherence to CPAP treatment in OSA patients with PLMS.14 This can be explained by the fact that CPAP only tackles the anatomical causes of OSA, ie the upper airway obstruction, while failing to address the other non-anatomical factors. Indeed, these non-anatomical factors of OSA have recently become another focus of much academic interest,15–18 identifying patients with distinct phenotypes including (1) ineffective upper-airway dilator muscles; (2) unstable ventilatory control, ie high loop gain; (3) low respiratory arousal. Given the PLM’s propensity to lead to arousals,7,8 we hypothesized that such periodic limb movements may be associated with the low respiratory arousal phenotype seen in OSA. As such, we set out to examine retrospective data as a first step to explore the contributory factors leading to PLMS in OSA patients and the role of low arousal threshold.
Method
Participants
A retrospective cohort study was carried out with data from 793 OSA patients diagnosed at the Department of the Second Affiliated Hospital of Soochow University from January 2013 to July 2019. All patients were over 18 years old. Individuals taking medications or suffering from diseases that affect PLM such as restless legs, narcolepsy, multisystem atrophy, spinal cord injury, Parkinson’s disease were also excluded. Individuals with insomnia were also excluded. Only PSG readings with effective monitoring time ≥ 8 hours were included for analysis. The resultant cohort was then divided into the OSA-PLMS group (n=95) and the OSA-only group (n=625), using the PLMS diagnostic criteria of PLMS index (PLMI) ≥ 15 (See below for a detailed definition of PLMI). The participants in the study gave informed consent and the study protocol has been approved by the Research Ethics Committee of the Second Affiliated Hospital of Soochow University, Suzhou, China (JD-LK-2018-004-02).
Polysomnography
The participants underwent overnight, supervised, laboratory-based video polysomnography. The PSG recorded from 22:00 to 07:00 the next morning. Compumedics Grael multifunctional PSG monitoring system was used for all signal acquisition (Compumedics Company, Australia). All polysomnographic recordings included six electroencephalogram (EEG) channels (F3/A2, F4/A1, C3/A2, C4/A1, O1/A2, O2/A1), bilateral electrooculograms (EOGs), submental and bilateral anterior tibialis electromyograms (EMGs), electrocardiograms (ECGs), the nasal and oronasal airflow (by using nasal pressure monitor and thermistor), arterial oxygen saturation (via finger pulse oximetry), chest and abdominal movements (via inductance plethysmography).
Sleep Stage Analysis and PLM Scoring
Sleep staging and sleep-related respiratory analyses were conducted manually by a registered technician according to the AASM scoring criteria.19 The apnea-hypopnea index (AHI) was calculated as the mean number of apneas and hypopneas (with ≥3% desaturation or an arousal) per hour of sleep. The oxygen desaturation index was defined as the mean number of ≥3% desaturation events per hour of sleep.20 Based on the scoring of sleep stages and respiratory analyses, limb movements were scored manually by a registered technician blind to the study. According to the World Association of Sleep Medicine (WASM) 2016 standard,21 Candidate Leg Movements (CLM) for the evaluation of PLM were defined as unilateral limb movements (0.5~10 seconds) or bilateral limb movements (0.5~15 seconds, not more than 4 movements). LM events within 2.0s before or 10.25s after respiratory events were manually excluded. PLM was defined as a minimum of four consecutive CLM events with a 10 to 90 seconds interval. As the older AASM standard is still used in some studies, we have included a parallel set of results in Supplemental Material 1 (with very similar results, see Supplemental Material 1). The PLM Index (PLMI) was scored as the number of PLM per hour of sleep.22 In the scoring of arousals, during non-rapid eye movement sleep (NREM), an EEG arousal was defined as
An abrupt shift in EEG frequency, which may include theta waves, alpha waves, and/or frequencies greater than 16 Hz, but not ‘spindles’ of 3s or greater in duration.
An arousal was scored during rapid eye movement (REM) sleep when the required EEG changes were accompanied by a concurrent increase in electromyography amplitude. Other measures include total sleep time (TST), proportions of each sleep stage, minimum arterial oxygen saturation (minSaO₂), average arterial oxygen saturation (avgSaO₂), percentage of total sleep time at SaO₂ < 90% (TS90), sleep efficiency, sleep latency, REM latency and Arousal index.
Definitions of Low Arousal Threshold
For the evaluation of the arousal threshold, we adopted a clinical screening tool developed by Edwards et al.23 In their study, epiglottic catheter data were collected from 146 participants who underwent overnight polysomnography to physically measure their ArTH (nadir epiglottic pressure before arousal). Comparing against this gold standard of epiglottic ArTH measurement, a clinical screening tool was developed to include three criteria: (1) AHI < 30/h; (2) minSaO₂ > 82.5% and (3) Fraction of hypopneas (FHypopnea) > 58.3% of the total number of respiratory events, allocating a score of one to each criterion. A total score of two or more categorizes the patient as having a low respiratory arousal threshold (defined as an epiglottic pressure on the breath before arousal greater than −15 cmH2O in the paper by Edwards et al). This tool achieved a sensitivity and a specificity of 80.4% and 88.0%, respectively, and has been adopted widely.24–28
Statistical Analysis
Statistical analyses were conducted using SPSS 22.0 statistical software.29 Firstly, we tested the Gaussian distribution of values using the Kolmogorov–Smirnov test. Non-normally distributed data are represented by the median interquartile range (IQR). The categorical variables were compared using the chi-square test, continuous correction chi-square test, or Fisher exact test. Mann–Whitney rank sum test was used for data with non-normal distribution or variable variances. A binary logistic regression analysis was conducted to include clinical and PSG parameters that showed significant cross-group differences (p<0.05). All independent variables with p < 0.2 in the univariable analysis were included in a final multivariable analysis. A probability value of p < 0.05 was considered statistically significant.
Validation of the Relationship Between PLMS and ArTH Using the MrOS Database
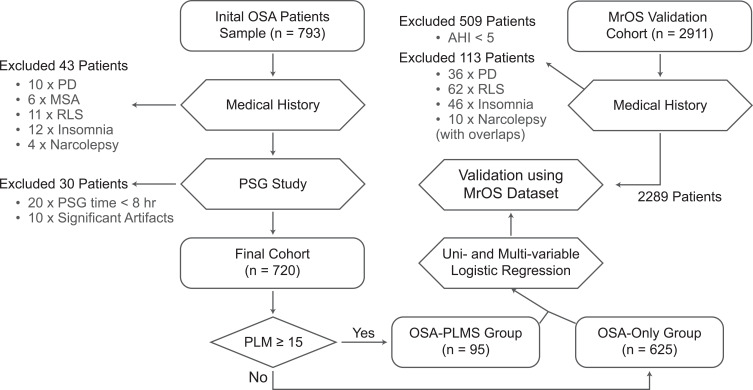
The relationship between low arousal thresholds and PLMS found in the sample was then validated in the MrOS database. The details of the MrOS study have been published elsewhere.30–33 Among the study population, 2911 community-dwelling men aged 65 years or older were chosen to undergo complete sleep monitoring. Using the same inclusion criteria as in our study, we excluded 509 patients with AHI < 5 and a further 113 patients with medical conditions potentially affecting PLMS, resulting in a cohort of 2289 subjects with complete PSG data in the validation analysis, where the average age was 76.4 years. (See Figure 1) Three binary logistic models with increasing numbers of variables were created, with the final model being the most comprehensive.
Figure 1.
Study flow diagram. In this study, 793 OSA patients with an AHI of ≥ 5 were enrolled. Among the 793 patients, 720 participants were eventually included in the study. The same inclusion and exclusion criteria were used for the MrOS patients.
Abbreviations: PD, Parkinson’s disease; MSA, multiple system atrophy; RLS, restless leg syndrome; PLMI, periodic leg movement index; PLMS, periodic leg movement in sleep; ArTH, arousal threshold.
Results
Among the data of 793 OSA patients initially sampled, we excluded 43 patients with conditions and/or drug therapies known or suspected to influence limb movements, 20 patients with effective PSG study time < 8 hours, and 10 further patients with significant artifacts in PSG recordings. The final study population consisted of 720 patients (see Figure 1), in which there were 107 females, 613 males, with a mean age of 47.0 years.
Using PLMI ≥ 15 as criteria, 625 patients were classified as OSA-only and 95 as OSA with PLMS. Patients in the OSA-PLMS group tend to be older (61 vs 42 years in OSA-only, p < 0.001). In terms of complications, the patients in the OSA-PLMS group exhibited higher prevalence of hypertension (52/95 vs 205/625 in OSA-only, p < 0.001), diabetes (19/95 vs 29/625, p < 0.001), Arrhythmia (12/95 vs 43/625, p = 0.049) and stroke (20/95 vs 47/625, p < 0.001). (See Table 1)
Table 1.
Subject Characteristics and Polysomnography Parameters in OSA-PLMS and OSA-Only Group
| Internal Cohort | MrOS Study Cohort | |||||
|---|---|---|---|---|---|---|
| OSA-Only (N=625) | OSA-PLMS (N=95) | p | OSA-Only (N=922) | OSA-PLMS (N=1367) | p | |
| Clinical Characteristics | ||||||
| Age | 42 (35–53) | 61 (53–70) | <0.001 | 75 (71–79) | 76 (72–81) | <0.001 |
| Gender (male) | 534 (85%) | 79 (83%) | 0.7 | All Male | ||
| Ethnicity (white) | All Chinese | 800 (87%) | 1275 (93%) | <0.001 | ||
| BMI | 26.2 (24.2–28.3) | 26.0 (23.9–28.3) | >0.9 | 27 (25–30) | 27 (25–30) | 0.8 |
| ESS | 8.0 (5.0–12.0) | 9.0 (5.0–12.5) | 0.6 | 6 (3–8) | 6 (3–8) | 0.6 |
| Hypertension | 205 (33%) | 52 (55%) | <0.001 | 451 (49%) | 711 (52%) | 0.2 |
| Diabetes | 29 (4.6%) | 19 (20%) | <0.001 | 109 (12%) | 201 (15%) | 0.056 |
| Arrhythmia | 43 (6.9%) | 12 (13%) | 0.049 | 84 (9.1%) | 153 (11%) | 0.13 |
| Stroke | 47 (7.5%) | 20 (21%) | <0.001 | 29 (3.1%) | 50 (3.7%) | 0.6 |
| CAD | 8 (1.3%) | 2 (2.1%) | 0.6 | 143 (16%) | 268 (20%) | 0.014 |
| Asthma | 8 (1.3%) | 1 (1.1%) | >0.9 | 58 (6.3%) | 113 (8.3%) | 0.093 |
| COPD | 5 (0.8%) | 1 (1.1%) | 0.6 | 54 (5.9%) | 61 (4.5%) | 0.2 |
| PSG Parameters | ||||||
| TST, min | 429 (373–472) | 394 (314–455) | <0.001 | 364 (323–401) | 359 (315–400) | 0.057 |
| Sleep Efficiency, % | 87 (77–93) | 78 (63–85) | <0.001 | 77 (69–84) | 78 (69–85) | >0.9 |
| Sleep Latency, min | 4 (2–11) | 6 (1–16) | 0.3 | 17 (8–29) | 15 (8–28) | 0.6 |
| NREM 1, % | 13 (8–20) | 21 (13–36) | <0.001 | 6.0 (4.0–9.0) | 6.0 (4.0–9.0) | 0.7 |
| NREM 2, % | 51 (45–58) | 44 (34–53) | <0.001 | 62 (56–68) | 64 (57–70) | <0.001 |
| NREM 3, % | 15 (10–20) | 14 (5–20) | 0.072 | 11 (4–17) | 9 (3–15) | <0.001 |
| REM, % | 19 (15–23) | 17 (12–23) | 0.11 | 20 (15–24) | 19 (14–23) | 0.056 |
| ODI, N/hr | 20 (13–27) | 15 (6–33) | 0.026 | 20 (12–33) | 19 (12–31) | 0.2 |
| AHI, N/hr | 25 (15–34) | 21 (10–39) | 0.2 | 18 (10–30) | 16 (10–28) | 0.089 |
| AvgSaO2, % | 95 (95–96) | 95 (94–96) | 0.081 | 93 (74–95) | 93 (74–95) | 0.6 |
| MinSaO2, % | 81 (77–84) | 84 (77–87) | <0.001 | 84 (80–87) | 85 (81–88) | 0.057 |
| TS90, % | 2.8 (1.1–5.2) | 1.4 (0.2–8.3) | 0.10 | 1 (0–5) | 1 (0–4) | 0.048 |
| Arousal Index, N/hr | 16 (10–24) | 17 (8–28) | 0.5 | 21 (16–29) | 23 (17–32) | <0.001 |
Note: Values were expressed as median (interquartile range in brackets) or count (percentage).
Abbreviations: PLMS, periodic limb movement syndrome; OSA, obstructive sleep apnea; BMI, body mass index; ESS, Epworth Sleepiness Scale; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; TST, total sleep time; NREM, non-rapid eye movement; REM, rapid eye movement; ODI, oxygen desaturation index; AHI, apnea-hypopnea index; avgSaO₂, mean arterial oxygen saturation; minSaO₂, lowest arterial oxygen saturation; TS90, percentage of total sleep time at oxygen saturation level < 90%.
Sleep Parameters
Patients in the OSA-PLMS group exhibited lower sleep efficiency (78% vs 87% in OSA-only, p < 0.001), higher proportion of Stage N1 sleep (21% vs 13%, p < 0.001), lower proportion of Stage N2 sleep (44% vs 51%, p < 0.001), higher minimum oxygen saturation (84% vs 81%, p < 0.001), lower ODI (15 vs 20, p = 0.026) and lower total sleep time (394 vs 429, p < 0.001). There were no significant cross-group differences in sleep latency, latency to REM, proportion of Stage N3 sleep, proportion of REM sleep, AHI between the two groups. (See Table 1) The proportion of patients with a low arousal threshold was significantly higher in the OSA-PLMS group than in the OSA-only group (58.9% vs 20%, p < 0.001). Among the three variables used to determine arousal threshold, the OSA-PLMS group contained a significant higher proportion of patients meeting the criteria of MinSaO₂ > 82.5% (58.9% vs 34.7%, p < 0.001) and the criteria of FHypopnea > 58.3% (24.2% vs 11.8%, p = 0.001) than that of the OSA-only group. (See Table 2)
Table 2.
Arousal Threshold and Its Predictors in OSA Patients in the OSA-PLMS and OSA-Only Groups
| OSA-Only | OSA-PLMS | χ2 | p | |
|---|---|---|---|---|
| (N = 625) | (N = 95) | |||
| Low ArTH | 125 (20%) | 56 (58.9%) | 66.47 | < 0.001 |
| AHI < 30 | 370 (59.2%) | 63 (66.3%) | 1.742 | 0.187 |
| minSaO₂ > 82.5% | 217 (34.7%) | 56 (58.9%) | 20.563 | < 0.001 |
| FHypopnea > 58.3% | 74 (11.8%) | 23 (24.2%) | 10.826 | < 0.001 |
Note: Values were expressed as count (percentage).
Abbreviations: PLMS, periodic limb movement syndrome; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; minSaO₂, lowest arterial oxygen saturation; ArTH, arousal threshold; FHypopnea, fraction of hypopnea in all respiratory events.
Univariable and Multivariable Logistic Regression
We included all clinical and PSG parameters that showed significant cross-group differences (p<0.05). In the initial univariable analysis of 720 patients, we found that low arousal thresholds were associated with PLMS (p < 0.001). Since the lowest oxygen saturation and FHypopnea were implicitly included in the calculation of low ArTH, these two items were excluded in the multivariable regression analysis. The results of the multivariable regression showed that age, diabetes, ODI, and low arousal threshold (OR=8.78 (4.73–16.30), p<0.001) were correlated with PLMS. (See Table 3, also see Supplemental Material 1 for a sensitivity analysis of this correlation in regard to three components of the low ArTH classifier.)
Table 3.
Regression Analyses of Factors Associated with PLMS Among OSA Participants
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| β | p | OR (95% CI) | β | p | OR (95% CI) | |||
| Age | 0.088 | <0.001 | 1.092 (1.072–1.113) | 0.07 | <0.001 | 1.073 (1.049–1.097) | ||
| Hypertension | 0.907 | <0.001 | 2.478 (1.600–3.836) | −0.04 | 0.888 | 0.961 (0.549–1.682) | ||
| Diabetes | 1.637 | <0.001 | 5.138 (2.748–9.607) | 0.923 | 0.025 | 2.516 (1.123–5.636) | ||
| Arrhythmia | 0.671 | 0.053 | 1.957 (0.991–3.862) | −0.119 | 0.783 | 2.516 (1.123–5.636) | ||
| Stroke | 1.123 | <0.001 | 3.074 (1.714–5.513) | 0.043 | 0.909 | 1.044 (0.499–2.184) | ||
| SleepEff | −0.037 | <0.001 | 0.964 (0.951–0.977) | 0.004 | 0.788 | 1.004 (0.975–1.034) | ||
| NREM 1, % | 0.056 | <0.001 | 1.057 (1.040–1.074) | 0.018 | 0.202 | 1.018 (0.991–1.045) | ||
| NREM 2, % | −0.048 | <0.001 | 0.953 (0.936–0.971) | −0.018 | 0.186 | 0.982 (0.955–1.009) | ||
| ODI | 0.012 | 0.153 | 1.012 (0.996–1.028) | 0.033 | 0.002 | 1.033 (1.012–1.055) | ||
| TST | −0.004 | <0.001 | 0.996 (0.993–0.998) | −0.001 | 0.764 | 0.999 (0.995–1.004) | ||
| MinSaO₂ | 0.034 | 0.049 | 1.035 (1.000–1.071) | Not included | ||||
| Low ArTH | 1.748 | <0.001 | 5.744 (3.650–9.038) | 2.173 | <0.001 | 8.781 (4.73–16.301) | ||
Note: Odds ratios were expressed as estimate (95% confidence interval).
Abbreviations: PLMS, periodic limb movement syndrome; OSA, obstructive sleep apnea; SleepEff, sleep efficiency; NREM, non-rapid eye movement; ODI, oxygen desaturation index; TST, total sleep time; minSaO₂, lowest arterial oxygen saturation; ArTH, arousal threshold.
Validation Using MrOS Dataset
To validate the relationship between low arousal threshold (ArTH) and PLMS found in this study, we applied the multivariable logistic model to the MrOS dataset (See Table 1 for patient characteristics). Three logistic regression models were used with each adding a set of independent variables. Adjusting for age, BMI and race, Model 1 showed that men with low ArTH had an increased risk of PLMS, with OR = 1.32 (1.08–1.61). Model 2 showed an increased OR of 1.33 (1.07–1.64), after correcting for the proportion of N2 sleep, sleep efficiency, average oxygen saturation and TS90 based on Model 1. Model 3 corrected for Model 3 corrected for concurrent hypertension, diabetes mellitus, atrial fibrillation, stroke and asthma based on Model 2. This final model showed that PLMS was associated with older age, non-African American ethnicity (both p < 0.001), higher proportion of Stage N2 Sleep (p < 0.001), history of diabetes (OR = 1.44, p = 0.008) as well as low ArTH (OR = 1.33 (1.08–1.64), p = 0.009) (See Table 4).
Table 4.
Risk of PLMS by Arousal Threshold in the MrOS Dataset
| Low ArTH | |||
|---|---|---|---|
| β | OR (95% CI) | p | |
| Model 1: Age + BMI + Race | 0.278 | 1.32 (1.08–1.61) | 0.006 |
| Model 2: Model 1 + SleepEff + NREM2 + avgSaO₂ + TS90 | 0.282 | 1.33 (1.07–1.64) | 0.009 |
| Model 3: Model 2 + Hypertension+ Diabetes + Arrhythmia + Asthma + Stroke | 0.285 | 1.33 (1.08–1.64) | 0.009 |
Note: Odds ratios were expressed as estimate (95% confidence interval).
Abbreviations: PLMS, periodic limb movement syndrome; ArTH, arousal threshold; BMI, body mass index; SleepEff, sleep efficiency; NREM, non-rapid eye movement; avgSaO₂, average arterial oxygen saturation; TS90, percentage of total sleep time at oxygen saturation level < 90%.
Discussion
PLMS has thus far been regarded as mostly an incidental concomitant phenomenon found in PSG monitoring in OSA patients since patients rarely present with isolated complaints of PLMS due to the lack of attendant leg discomfort as in RLS. However, this is no coincidence: current literature supports the view that PLMS is more common in OSA patients than in the general population. Taking PLMI ≥ 5 as the standard, Canada and the United States reported that the prevalence of PLMS in OSA patients is 48%11 and 33%,34 respectively, compared to a prevalence of 4–11% in all adults,35 and PLMS is rare among the under-40s.36 Our study included only the Chinese population, where the prevalence was found to be 16.7% using the same PLMI ≥ 5 standard, or 13.2% using the newer PLMI ≥ 15 standard. This suggests that the occurrence of PLMS in the Chinese OSA population may be lower than that in the North American population.
In our study, patients in the OSA-PLMS group displayed a higher proportion of stage N1 sleep and lower proportion of stage N2 sleep stage (Table 1). As is consistent with the literature,6,7 the disruption of sleep structure is associated with the periodic limb movements. The potential mechanisms of such disruption can be manifold. Some studies have found that PLM is often accompanied by frequent EEG arousals,37 which prevent deeper, more stable stages of sleep. Furthermore, among OSA patients, about one-third are clinically characterized by a low respiratory arousal threshold,38 which is a key factor associated with increased ventilatory instability.39 As shown by both our data and the MrOS external dataset, OSA-PLMS patients demonstrate an elevated tendency to have a low arousal threshold. Low arousal threshold leads to premature airflow recovery and limits the accumulation of respiratory stimuli required to activate pharyngeal dilators.40 Transient hyperventilation response after awakening causes blood CO₂ levels to continue to decline after the end of apnea events, aggravating ventilatory instability and perpetuating the cycle of repetitive arousals.17,39 Indeed, this mechanism of low arousal threshold could offer an explanation for the increased arousals in OSA-PLMS patients, compared to OSA-only patients, which is also seen in some studies,6,7 bridging the gap between the ostensibly unrelated symptoms of PLMS and OSA. Crucially, this negative effect is further compounded by the more recent finding that put into doubt the traditional belief that arousals are necessary for re-opening the obstructed airway,18,40 that is, arousals are not a “protective” mechanism as traditionally suggested and low ArTH contributes to the pathogenesis of severe OSA.41
In our study, the methodology adopted to identify patients with low ArTH was a prediction tool developed by Edwards et al,23 whose study validated this prediction tool against the gold standard epiglottic ArTH measurement in 146 patients. The tool achieved a reasonably high sensitivity (80.4%) and specificity (88.0%), and its robustness is further affirmed through its adoption by many recent studies.24–28 This made possible the retrospective data collection and external dataset validation on a much larger sample of patients than the arguably more accurate invasive epiglottic measurement could. In our sample, 58.9% of patients in the OSA-PLMS group were of the low arousal threshold phenotype, compared to 20% in the OSA-only group (p < 0.001, Table 3). Using multivariable logistic regression, this difference is estimated to represent an odds ratio of 8.78 (4.73–16.3, p < 0.001) for patients with low ArTH. This relationship between low ArTH and PLMS is further validated using the MrOS database (N = 2289) where the odds ratio was calculated at 1.33 (1.08–1.64, p = 0.009). The MrOS cohort had a median age of 76.3 years, while the median age in our study was 45 years. This suggests that low arousal threshold is not only a risk factor for OSA but also and plays an important role in predisposing OSA patients to PLMS in all age groups.
Clinical Significance
Despite its muted significance in patient complaints, PLMS combined with OSA represents notable cardiovascular and cerebrovascular risks. One multisite, longitudinal study by A. Zinchuk et al of 1247 US veterans assessed the relationship between OSA phenotype and cardiovascular outcomes. Based on the polysomnographic features, seven phenotypes were identified among the OSA patients using cluster analysis, namely, “mild”, “periodic limb movements of sleep (PLMS)”, “NREM and arousal”, “REM and hypoxia”, “hypopnea and hypoxia”, “arousal and poor sleep” and “combined severe”. Astonishingly, membership to the “PLMS (N = 119)” cluster was shown to be an even better predictor than AHI categories (AHI ≥ 30 vs AHI < 15) in predicting cardiovascular outcomes, and the PLMS cluster carries the highest risks of negative cardiovascular outcomes (OR = 2.02, 1.32–3.08) among the six clusters.12 In our study, we found that the prevalence of hypertension, arrhythmia, diabetes mellitus, and stroke in the OSA-PLMS group was higher than that in the OSA-only group. Similarly, a study by Koo BB et al demonstrated the relationship between PLMS and CVD in the MrOS sleep study cohort.42 Furthermore, some studies have observed heightened sympathetic activation in PLM patients,43,44 which led to increased heart rate and blood pressure, potentially contributing to the elevated cardiovascular risk.
Regardless of the cause of such risks, current evidence highlights the importance of early intervention in patients with OSA complicated by PLMS to reduce their risks of cardiovascular events.
Inadequacy of Current Approaches to the Management of OSA-PLMS Patients
There has been a lack of research attention to the specific treatment of PLMS. On the one hand, the clinical symptoms of this group of patients are not obvious or bothersome to patients, due to the absence of strong discomfort as in RLS; on the other hand, the currently used treatments and medications lack robust research trials of effectiveness. Evidently, the underlying link between PLMS and a whole host of comorbidities warrant further investigation into this clinical disorder, despite its lack of frank clinical symptoms. In PLMS patients who are often treated by CPAP for their concomitant OSA, studies have shown that CPAP treatment has no clear impact on the severity of PLMS.6,13 The current treatment approaches of PLMS mainly “borrow” from the pharmacological treatments of RLS, where the current guideline presents a “standard” level of recommendation for pramipexole and ropinirole and a “guideline” level of recommendation for levodopa with dopa decarboxylase inhibitor, opioids, gabapentin enacarbil in the treatment of RLS.45 In particular, a study46 has shown that Pramipexole reduces PLMS but not arousals. Therefore, these drugs reduce PLMS but usually do not eliminate them, which may continue to be directly or indirectly a risk for cardiovascular diseases.47
From the OSA perspective, the mainstay of the current management approach is the use of Continuous Positive Airway Pressure (CPAP) therapy. However, the treatment efficacy of CPAP in the subgroup of combined OSA-PLM patients leaves much to be desired. A study has shown that patients with combined PLMS demonstrate poorer adherence to CPAP treatment.14 Interestingly, A. Zinchuk et al found a markedly poorer adherence to long-term CPAP therapy in the nonobese (BMI < 30) OSA patients with low ArTH when compared to patients with high ArTH.48 In our study, we demonstrated a strong correlation between low arousal threshold and PLMS. This might suggest that these two subgroups of CPAP non-complying patients with low ArTH and PLMS are highly overlapping or even mechanistically linked by a common etiology, such as the heightened sympathetic activation. Given its accompanying cardiovascular risks in these patients, we call for more attention to this low ArTH – PLMS subgroup of OSA.
Pharmacological approaches that raise the arousal threshold may simultaneously improve PLMS, OSA, and CPAP adherence in this cohort. Indeed, one of the foci of research has been the drug therapy for OSA targeting low arousal thresholds. A pilot experiment shows that the application of 3 mg of eszopiclone improved AHI (25 times/h vs 14 times/h) and sleep quality in patients with low arousal thresholds, without worsening hypoxemia.49 In our study, the OSA-PLMS group has a high proportion of low arousal thresholds, and it can be envisaged that the use of this class of drugs in the treatment of OSA may be effective in this population.
Limitations
This retrospective study is purely observational, and the findings could only establish a correlation between PLMS and low arousal threshold, while further research is needed to establish the direction of causality. Admittedly, to enable the collection of a large dataset to make possible the analysis of such subtle clinical correlations, the gold standard epiglottic ArTH measurement would be extremely difficult, if not impossible. As such, the classification of low arousal threshold in this study was based on a validated clinical prediction tool, which in turn, brings the additional utility in terms of potential applications in community screening of OSA patients and their phenotypes. This also enables the validation of our findings in the widely analysed and validated MrOS cohort. It should also be noted that most patients had only one session of PSG, which could be susceptible to the influence of the “first night effect”.
Conclusion
Our study presents, to our knowledge, the first piece of evidence that identifies low arousal threshold as an independent predictor for PLMS in OSA patients in both locally collected primary data and the MrOS cohort dataset. This poses low arousal threshold as a potential link between OSA and PLM, where low ArTH serves as a non-anatomical etiology to the former and as a risk factor for the latter. In light of the elevated risks of cardiovascular and cerebrovascular diseases in PLM patients both in our study and the current literature, we recommend more investigations should be done to gain further understanding into the role of low arousal threshold in treating this group of OSA-PLMS patients, as well as the accompanying cardiovascular comorbidities.
Acknowledgment
The authors thank Fei Han and Hao Gui for conducting PSG scoring and Delu Wang for providing helpful suggestions for the study protocol.
Funding Statement
National Natural Science Foundation of China (NSFC 81770085, 82070095); Discipline Construction Program of the Second Affiliated Hospital of Soochow University (XKTJ-TD202003). MrOS Dataset: The National Heart, Lung, and Blood Institute provided funding for the ancillary MrOS Sleep Study and the National Sleep Research Resource.
Ethics approval
Approved by the Research Ethics Committee of the Second Affiliated Hospital of Soochow University, Suzhou, China (JD-LK-2018-004-02). Our study was conducted in accordance with the Declaration of Helsinki.
Consent for participation and publication
Informed consent was sought from all patients involved, with the understanding that anonymized data could be used for research and publications.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. The two first authors have contributed equally to the manuscript.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.American Academy of Sleep Medicine. The International Classification of Sleep Disorders (ICSD-3). 2014. [Google Scholar]
- 2.Salas RE, Rasquinha R, Gamaldo CE. All the wrong moves: a clinical review of restless legs syndrome, periodic limb movements of sleep and wake, and periodic limb movement disorder. Clin Chest Med. 2010;31(2):383–395. doi: 10.1016/j.ccm.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol. 2016;79(3):464–474. doi: 10.1002/ana.24593 [DOI] [PubMed] [Google Scholar]
- 4.Haba-Rubio J, Staner L, Krieger J, Macher JP. Periodic limb movements and sleepiness in obstructive sleep apnea patients. Sleep Med. 2005;6(3):225–229. doi: 10.1016/j.sleep.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 5.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164(8):1454–1458. doi: 10.1164/ajrccm.164.8.2011062 [DOI] [PubMed] [Google Scholar]
- 6.Budhiraja R, Javaheri S, Pavlova MK, et al. Prevalence and correlates of periodic limb movements in OSA and the effect of CPAP therapy. Neurology. 2020;94(17):e1820–e1827. doi: 10.1212/WNL.0000000000008844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferri R, Rundo F, Zucconi M, et al. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. Sleep. 2015;38(6):919–924. doi: 10.5665/sleep.4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung. 2013;42(5):353–360. doi: 10.1016/j.hrtlng.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep. 2017;40(3). doi: 10.1093/sleep/zsx013 [DOI] [PubMed] [Google Scholar]
- 10.Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–65. doi: 10.1002/mds.870120111 [DOI] [PubMed] [Google Scholar]
- 11.Al-Alawi A, Mulgrew A, Tench E, Ryan CF, Prevalence R. Factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2(3):281–287. doi: 10.5664/jcsm.26587 [DOI] [PubMed] [Google Scholar]
- 12.Zinchuk AV, Jeon S, Koo BB, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. doi: 10.1136/thoraxjnl-2017-210431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baran AS, Richert AC, Douglass AB, et al. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26(6):717–720. doi: 10.1093/sleep/26.6.717 [DOI] [PubMed] [Google Scholar]
- 14.Mwenge GB, Rougui I, Rodenstein D. Effect of changes in periodic limb movements under cpap on adherence and long term compliance in obstructive sleep apnea. Acta Clin Belg. 2018;73(3):191–198. doi: 10.1080/17843286.2017.1405137 [DOI] [PubMed] [Google Scholar]
- 15.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea – new pathways for targeted therapy. Sleep Med Rev. 2018;37:45–59. doi: 10.1016/j.smrv.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol. 2014;116(3):302–313. doi: 10.1152/japplphysiol.00649.2013 [DOI] [PubMed] [Google Scholar]
- 18.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–633. doi: 10.1517/14728220903005608 [DOI] [PubMed] [Google Scholar]
- 19.Berry RB, Albertario CL, Harding SM, et al. The AASM Manual for the Scoring of Sleep and Associated Events. 2018. [Google Scholar]
- 20.Flemons WW, Buysse D, Redline S, et al. Sleep–related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 21.Ferri R, Fulda S, Manconi M, et al. World association of sleep medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the international and the european restless legs syndrome study groups (IRLSSG and EURLSSG). Sleep Med. 2016;26:86–95. doi: 10.1016/j.sleep.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. J Clin Sleep Med. 2007. [Google Scholar]
- 23.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. doi: 10.1164/rccm.201404-0718OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmickl CN, Li Y, Orr JE, et al. Effect of venlafaxine on apnea-hypopnea index in patients with sleep apnea. Chest. 2020;(May):1–11. doi: 10.1016/j.chest.2020.02.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu X, Li J, Wu -J-J, et al. Reduced cortical arousability to nocturnal apneic episodes in patients with wake-up ischemic stroke. Sleep Med. 2020;66:252–258. doi: 10.1016/j.sleep.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 26.Lee RWW, Sutherland K, Sands SA, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22(5):1015–1021. doi: 10.1111/resp.13022 [DOI] [PubMed] [Google Scholar]
- 27.Smith PR, Sheikh KL, Costan-Toth C, et al. Eszopiclone and zolpidem do not affect the prevalence of the low arousal threshold phenotype. J Clin Sleep Med. 2017;13(1):115–119. doi: 10.5664/jcsm.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Solh A, Lawson Y, Wilding GE. Impact of low arousal threshold on treatment of obstructive sleep apnea in patients with post-traumatic stress disorder. Sleep Breath. 2020;0–7. [DOI] [PubMed] [Google Scholar]
- 29.IBM Corp. IBM SPSS Statistics for Windows. 2010:1 [Google Scholar]
- 30.Blank JB, Cawthon PM, Carrion-Petersen ML. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study — a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 32.Zhang G-Q, Cui L, Mueller R, et al. The national sleep research resource: towards a sleep data commons. J Am Med Inform Assoc. 2018;25(10):1351–1358. doi: 10.1093/jamia/ocy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2011;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javaheri S. Prevalence of obstructive sleep apnoea and periodic limb movement in 45 subjects with heart transplantation. Eur Heart J. 2004;25(3):260–266. doi: 10.1016/j.ehj.2003.10.032 [DOI] [PubMed] [Google Scholar]
- 35.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–177. doi: 10.1016/j.smrv.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Pennestri MH, Whittom S, Adam B, et al. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29(9):1183–1187. doi: 10.1093/sleep/29.9.1183 [DOI] [PubMed] [Google Scholar]
- 37.Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12(1):47–55. doi: 10.1016/j.sleep.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 38.Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13(1):81–88. doi: 10.5664/jcsm.6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlin NL. Brain circuitry mediating arousal from obstructive sleep apnea. Curr Opin Neurobiol. 2013;23(5):774–779. doi: 10.1016/j.conb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younes M, Loewen AHS, Ostrowski M, et al. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol. 2012;112(2):249–258. doi: 10.1152/japplphysiol.00312.2011 [DOI] [PubMed] [Google Scholar]
- 41.Jordan AS, White DP, Lo Y-L, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32(3):361–368. doi: 10.1093/sleep/32.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men. Circulation. 2011;124(11):1223–1231. doi: 10.1161/CIRCULATIONAHA.111.038968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30(6):755–766. doi: 10.1093/sleep/30.6.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sieminski M, Pyrzowski J, Partinen M. Periodic limb movements in sleep are followed by increases in EEG activity, blood pressure, and heart rate during sleep. Sleep Breath. 2017;21(2):497–503. doi: 10.1007/s11325-017-1476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses. Sleep. 2012;35(8):1037. doi: 10.5665/sleep.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71(6):834–844. doi: 10.1002/ana.23565 [DOI] [PubMed] [Google Scholar]
- 47.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6(6):337–346. doi: 10.1038/nrneurol.2010.55 [DOI] [PubMed] [Google Scholar]
- 48.Zinchuk A, Edwards BA, Jeon S, et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male United States veterans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(05):809–817. doi: 10.5664/jcsm.7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci. 2011;120(12):505–514. doi: 10.1042/CS20100588 [DOI] [PMC free article] [PubMed] [Google Scholar]