Abstract
Mannose-binding lectin (MBL) and lectin complement pathway have become targets of increasing clinical interest. Many aspects of MBL have been recently explored, including the structural properties that allow it to distinguish self from non-self/altered-self structures. Experimental evidences have declared the additional 5′- and 3′-variants that in amalgamation with well-known secretor polymorphisms change MBL function and concentration. Moreover, the current review highlights the differential behavior of MBL on exposure with extra/intracellular pathogens and in autoimmune diseases, stressing the fact that “high MBL levels can increase diseases susceptibility,” a paradox that needs justification. Attributable to these discrepancies, no absolute level of MBL deficiency could be defined so far and thus must be interpreted for specific diseases through case–control population-specific designs. Overall, it is evident that further research is needed about MBL and the lectin pathway of complement. Particularly, the transformative role of MBL over evolution is of interest and its role with regard to pathogenesis of different diseases and potential therapeutic targets within the respective pathways should be further explored. Apart from this, it is necessary to adopt an extensive locus-wide methodology to apprehend the clinical significance of MBL2 polymorphisms in a variety of infectious diseases by the future studies.
Keywords: infectious diseases, autoimmune diseases, single nucleotide polymorphisms, functional SNPs, 5′ near gene, 3′UTR, variants, phagocytosis and MBL patents
1. Introduction
Pathogens’ identification by the innate immune system is facilitated by the germ line-encoded host factors [1]. Pattern recognition receptors (PRRs) exemplify these factors, which recognize the conserved bio-molecular structures on the pathogens’ surface, designated as pathogen-associated molecular patterns (PAMPs). The ability to recognize PAMPs and differentiate the self from non-self/altered-self structures is the characteristic and fundamental definition of PRRs. This PRR–PAMP interaction further leads to the generation of immune responses against these pathogens, eventually leading to their clearance from the host [1]. C-type lectin receptors (CLRs) are the best emerging PRRs that are gaining attention because of their numerous functions. These multifunctional properties of CLRs include endocytosis, phagocytosis, complement activation, oxidative burst, immuno-modulation by activating pro-inflammatory and anti-inflammatory responses, immune cells’ recruitment, extravasations, linking innate with adaptive immunity, controlling adaptive immune responses, regulating and collaborating with other PRRs for the optimal immune response generation, clearance of altered self-cells, and so on [2].
CLRs are the collection of asymmetric molecules possessing one or more preserved structures, referred as carbohydrate-recognition domains (CRDs), which bind to the sugars in a Ca2+-dependent manner [3]. On the basis of their molecular structure, these Ca2+-dependent proteins are of two types, such as transmembrane CLRs and soluble CLR. The transmembrane CLRs are further divided into two categories, i.e., type I and II transmembrane proteins based on the number of CRD regions and N-terminal location. The soluble CLR has an oligomeric protein structure with multiple CRDs that binds to the repetitive carbohydrate structural arrangement present on the pathogens’ surface. This soluble CLR includes a liver-based mannose-binding lectin (MBL) protein, which is a significant constituent of the human innate immunity. However, its elevated levels in the liver cirrhosis patients suggested its extra-hepatic synthesis found in the small intestine, testis tissue, and restricted areas of the gastrointestinal and reproductive tracts [4]. MBL binds to the mannose-rich PAMPs with subsequent clearing of pathogens by complement activation and phagocytosis [5].
The genetic polymorphisms of MBL and its varying serum levels are the hot topics of research in many clinical studies. However, the discrepancies observed in describing the normal and abnormal MBL levels could not provide a precision regarding the MBL’s role in health and disease. Therefore, in the current article, an effort has been made to examine the potential clarifications for the so far muddled and ambiguous discoveries reported on MBL’s function in host immunity. It first outlines the role of MBL as a prototypical PRR and the associated roles it plays in human immunity. Further, it explains and strengthens the need of more extensive locus-wide approach by future association studies to apprehend the clinical significance of MBL2 polymorphisms, apart from the standard ones, in a variety of infectious diseases. Finally, we discussed MBL as a friend and foe in different diseases, a paradox that needs to be resolved.
2. MBL structure
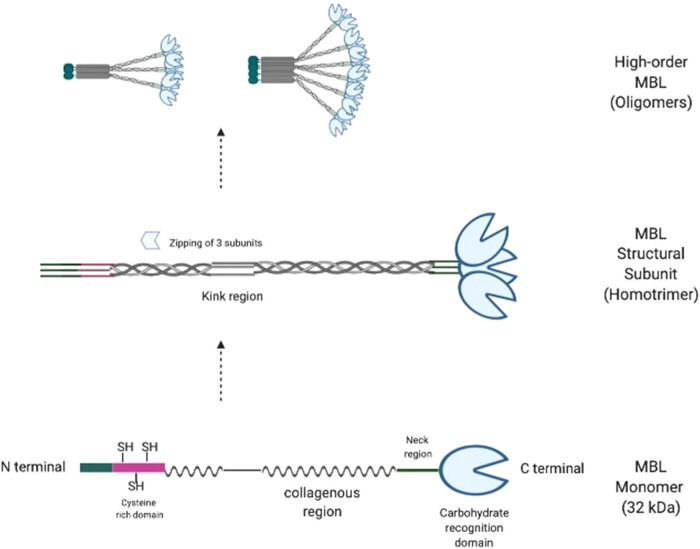
The functional MBL is an extracellular circulatory protein, which is mainly expressed and produced by the liver cells. It is a bouquet-like complex that ranges from minimum two to maximum six sets of homo-trimers formed of monomers of 32 kDa protein as shown in Figure 1 [6]. This 32 kDa MBL monomer is a protein of 248 amino acids, translated from MBL2 gene mapped to 10q11.2-q21. The monomer constitutes cysteine-rich domain, 18–20 tandem repeats of Gly-Xaa-Yaa in a collagen-like region, α-helical neck region, and a CRD from N → C terminal [7]. Owing to the triple helix nature of collagen, three monomers present with their collagen-like region in close proximity creases toward N-terminal resulting tri-meric structural subunit (the homo-trimer) with triple helix and three CRDs at C-terminal [8]. The inter-chain disulfide bonds linking tri-meric subunits further form and stabilize the high order functional oligomers found predominantly in the extracellular fluid [9]. These high order oligomers consist of multiple CRDs that allow concurrent binding of functional MBL with numerous repetitive PAMPs present on the pathogens’ surface ensuring high avidity comparative to the low binding affinity (10−3 M) of individual CRD–PAMP contact [10].
Figure 1.
Structure and assembly of MBL (modified from ref. [113]).
3. MBL, an ideal PRR
Structural studies revealed low affinity (10−3 M) interaction between individual CRD and PAMP residues. However, binding of MBL oligomer to pathogen as a whole has been shown of high affinity (10 × 1010 M) [11]. For these high affinity interactions, it is necessary that the ligand makes a proper geometry by spanning 45 Å in three dimensions of MBL, which is only possible in the case of repetitive carbohydrate structural patterns mainly found on the pathogen surfaces, but hidden inside the self-glycoproteins [12]. The sugar specificity is secured within the Glu-Pro-Asn amino acid sequence of CRD that favors the Ca2+-dependent binding with the 3′- and 4′-hydroxyl groups of carbohydrates including mannose, N-acetyl-d-glucosamine, glucose, fucose and their derivatives, but do not allow binding to galactose and the terminal sialic acid of oligosaccharide chains present on the host cell surface [13,14]. In addition, abnormal glycosylation occurs in the cancerous cells because of oncogenic transformation that leads to the exposure of sufficient sugar ligands in fitting pattern, allowing their recognition and binding by MBL [15]. Besides this, MBL recognizes apoptotic cells with exposed hidden repetitive sugar pattern [16]. Thus, MBL can also recognize altered self-structure and mediates cytotoxicity and clearance by phagocytosis. Therefore, three structural properties, i.e., (i) blocking of oligosaccharide chains with terminal sialic acid residues, (ii) inadequately exposed sugar ligands to allow precise binding configuration, and (iii) hidden repetitive sugar pattern inside glycoproteins, allow MBL to distinguish self from the non-self and altered-self structures, making it an ideal PRR. This prototypical PRR has been shown to bind diverse range of fungal, bacterial, parasitic, and viral species, thereby generating required immune responses [17,18,19,20,21,22].
4. The receptor-mediated effector functions of MBL
The receptor-mediated effector functions of MBL include complement (C) system activation, direct opsono-phagocytosis, MBL-dependent cell-mediated cytotoxicity, apoptosis, pro-inflammatory cytokines release, and ROS production. These are described herewith.
4.1. Activation of complement (C) system
The C system involves the interactions of distinct plasma proteins that opsonize the pathogens and initiates a series of inflammatory reactions for the ultimate clearance of pathogens [23]. Activation of C system on the pathogen surfaces involves three pathways, i.e., the classical, lectin, and alternative, involving different initiatory molecules that eventually converge to produce similar molecules [23]. MBL binding to the pathogen surface activates the so-called MBL-mediated lectin complement pathway. There is a family of serine proteases that bind to MBL and get activated when the complex is associated with the pathogen. These MBL-associated serine proteases (MASPs) include MASP-1 to -3 along with the truncated version of MASP-2 currently named as MAP-2 (also known as Map19 or sMAP), of which MASP-2 has been shown to play an important role in the C system activation [23,24,25,26,27,28]. Following activation, MASP-2 results in the consecutive cleavage of C4 and C2, generating C4b and C2a fragments that interact with each other to form C3 convertase (C4bC2a complex) that will eventually converge with the other C3 convertases produced from both the alternative and the classical pathways. These C3 convertases then start the C cascade activity generating the terminal components of C system that gather to form membrane attack complex and damage the pathogen. Lectin pathway also generates opsonic C3b and other C fragments, which further enhance the phagocytosis as well as activate and infiltrate the additional phagocytes to the spot of C activation.
4.2. Direct opsonophagocytosis by MBL
Besides causing indirect opsonization because of the C components (opsonins) generated as a result of MBL pathway, MBL can directly act as an opsonin, independent of C system activation [29,30,31]. This direct opsonophagocytosis by MBL has been shown to be facilitated by the receptors present on the surface of phagocytic cells (monocytes and neutrophils) [32,33]. Other than this, several other intracellular and extracellular receptors have been defined in the literature, which directly bind to the MBL, although their function is not completely understood. These receptors include cC1qR (calreticulin), C1qRp, complement receptor 1 (CR1), alpha-2-beta-1 integrin, and α-2-macroglobulin receptor (CD91) [34,35,36,37,38,39,40]. This imperative property explains why the low levels of MBL has ultimately been suggested to be responsible for opsonic defects found in the children with recurrent infections, which was earlier thought to be occurring because of the deficiency of other opsonins [41,42].
4.3. MBL-dependent cell-mediated cytotoxicity
Researchers have detected MBL binding to the aberrant carbohydrate structures expressed by the cancerous cells and mediating cytotoxic activity, which was referred as MBL-dependent cell-mediated cytotoxicity [43,44]. It is important to note that this activity was shown by the mutated MBL protein with no opsonic and complement activation activity, suggesting the un-described cytotoxic role of MBL, whose relative importance in tumor immunology is currently not known.
4.4. Role in apoptosis
As mentioned above, MBL can directly bind to the apoptotic cells with exposed repetitive sugar pattern, thereby permitting their recognition and the ultimate clearance of apoptotic cells by phagocytes [16,45]. Low MBL levels have been suggested to be associated with defective apoptotic cell clearance [46].
4.5. Pro-inflammatory cytokine release
MBL has been shown to trigger the release of pro-inflammatory cytokines from the monocytes [47,48]. A study by Jack et al. [49] depicted the elevated production of the pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α from the monocytes at low MBL levels (4 μg/mL). However, the inflammatory production reduces at high MBL concentrations, suggesting the complex role of MBL in modulating the release of pro-inflammatory cytokines with unexplained mechanisms.
4.6. Reactive oxygen species (ROS) production
Other than the above-mentioned functions, MBL mediates ROS production including superoxide and H2O2 from human neutrophils [30,33,50].
Thus, MBL is one of the best examples of a single molecule with multiple functions ranging from an ideal PRR, activation of C system, direct and indirect opsonization, altered self-cells clearance, pro-inflammatory cytokines release to ROS production. These important functional activities explain why the defect in MBL has been shown to be associated with different infectious diseases such as malaria, human immunodeficiency virus (HIV) infection, leprosy, leishmaniasis, schistosomiasis, trypanosomiasis, tuberculosis, systemic lupus erythematosus, rheumatoid arthritis, recurrent vulvovaginal infections (RVVI), filariasis, etc. [51,52,53,54,55,56,57,58].
5. MBL2 structure
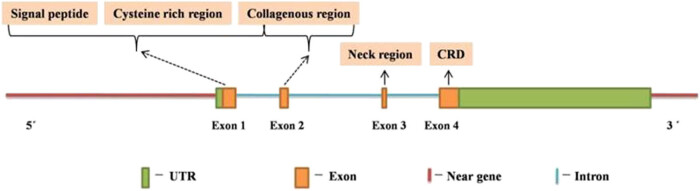
Humans have two MBL genes, MBL1 and MBL2, of which MBL1 is a pseudogene, leaving only one functional gene MBL2 that encodes for MBL protein [59]. MBL2 comprises 7,461 bases, between 5,27,65,380 and 5,27,72,841 bp region of chromosome no. 10 (10q11.2-q21) (NC_000010.11, NCBI), translating into MBL protein of 248 amino acid (NCBI accession number XP_011538118.1) via a 3,570 bp long mRNA (NCBI accession number NM_000242.21). The gene structure of MBL2 is same as that of other eukaryotic genes with each element contributing to the specific function in gene expression. MBL2 comprises four exons and three introns. The exons determine the structure of proteins with exon 1 encoding signal peptide, a cysteine-rich domain, and a portion of glycine-rich collagenous region. Exon 2 encodes the other portion of the collagenous domain, whereas α-helical “neck” region and CRD are encoded by exons 3 and 4, respectively (Figure 2). In addition, the promoter and 3′UTR region of the MBL2 contain various elements that have been shown to regulate MBL2 expression [60,61,62,63].
Figure 2.
Structure of MBL2 (modified from ref. [114]).
6. MBL2 genetics (SNPs with validated functional consequences)
An acute phase protein is the commonly referred term used for MBL accredited to the elevated serum levels’ subsequent infection and under aberrant body changes [64]. However, MBL is not a typical acute phase protein, because its response is generally demonstrated by the individuals’ MBL levels and genetic variations [65,66,67]. Single nucleotide polymorphisms (SNPs) in exon 1 and promoter region of MBL2 have been functionally characterized in regulating serum MBL (sMBL) levels. SNPs in exon 1 include rs5030737/codon 52, rs1800450/codon 54, and rs1800451/codon 57, jointly referred as MBL2 structural variations [68]. SNPs including rs1800450 and rs1800451 involve the glycine replacement with the di-carboxylic acids. In contrast, SNP rs5030737 involves arginine replacement with cysteine in the MBL monomers’ collagenous region consequently forming the variant subunits [69,70]. These variant monomers further impede the synthesis of high order MBL oligomers because of the distortion created in the triple helix collagenous region by codon 54 and 57 variations. Besides this, the additional disulfide bonds that are formed as a result of extra cysteine residues added by codon 52 variation also impede the MBL oligomers synthesis. These structural changes dramatically lower the synthesis, circulating levels, and functional activity of high order MBL oligomers [69,70,71,72,73,74]. Apart from these structural variations, promoter polymorphisms including rs11003125; L/H, rs7096206; Y/X, and rs7095891; P/Q regulate promoter activity of the gene subsequently modulating the MBL protein levels. From this, the biological implication of the genetic variations can be explicated in regard to the MBL2 transcription and translation [75,76,77]. MBL2 promoter and structural polymorphisms are in strong linkage disequilibrium (LD) forming the seven haplotypes, universally referred as secretor haplotypes involving LYPA, HYPA, LXPA, LYQA, LYPB, LYQC, and HYPD. The haplotypes involving HYPA, LYQA, and LYPA confer the high sMBL levels, whereas haplotypes involving LXPA, LYPB, LYQC, and HYPD confer the low sMBL levels [78]. Apart from the seven standard ones, the other haplotypes counting HYQA, HXPA, LXPB, LYQB, and LYPD have been reported by different population-specific studies [79,80,81]. This suggests the emergence of novel haplotypes besides the standard ones, owing to the heterogeneity found in the genetic pool of the various populations and the observed discerning advantage because of the ecological pressures like infections or demographics [82].
However, there are studies that did not find the complete correlation of sMBL levels with the secretor haplotypes signifying the existence of other MBL2 regulating variants [20,78,83]. To reveal this, Bernig’s group in 2004 analyzed the complete MBL2 gene of 10.0 kbp by re-sequencing it and found a great degree of heterozygosity [84]. They further found that the LD pattern of complete MBL2 uncovers a plausible recombination hotspot of 1.6 kbp that lies in the coding region of exon 4 in the 3′ end of the gene. This hotspot divides the whole MBL2 into two separate haplotype blocks in which the markers within each block are in linkage with each other, while the markers of one block are not in linkage with the markers of other. They named these blocks as the 5′ and 3′ haplotype blocks or simply as 5′ and 3′ blocks of MBL2 gene [84]. Moreover, the structural SNPs of exon 1 along with the upstream promoter polymorphisms that form the “secretor haplotypes” were found to lie in the 5′ block and are part of its extended haplotypes, indicating the possibility of additional linked 5′ variants with the possible functional implications. To investigate this, Bernig’s group in another study analyzed both MBL2 blocks in the unrelated Dutch Caucasians [61]. The results of this study suggested that the other variants in 5′ as well as 3′ haplotype block may modify the protein functionality and serum levels (Table 1). To further confirm these findings, the same group presented the preliminary evidence authenticating that 3′UTR variants of MBL2 manipulate the MBL concentration and also interact with the haplotypes of 5′ block that might increase the risk of breast cancer in the African-American women [62]. Other studies by different groups also found that the combined 5′ and 3′ blocks of MBL2 alter sMBL levels and increase risk of postoperative myocardial infarction, colon cancer, and RVVIs in White-Americans, African-Americans, and Indians, respectively [54,55,63,85]. Because of these observed associations, Zanetti et al. [63] functionally characterized the 3′UTR haplotypes of 3′ block and found that the SNP rs10082466 in the 3′UTR haplotype generates a binding site for miR-27a with full complementarity to its seed region. This binding has been shown to hasten the mRNA degradation by microRNAs (miRNAs) resulting in reduced protein translation as depicted by a significant decrease found in the normalized luciferase activity of MBL2 [63]. Overall, this implicates the necessity to adopt an extensive locus-wide methodology by the future association studies to apprehend the clinical significance of MBL2 polymorphisms in a variety of infectious diseases.
Table 1.
Functionally characterized polymorphisms of MBL2
| dbSNP identifiera | SNP position (5′ → 3′)b | Chromosome positionb | Secretorc | Region | Nucleotide change | Effect | Characterized function | References |
|---|---|---|---|---|---|---|---|---|
| rs11003125 | −618 | 5,27,72,254 | L/H (aka −550) | 5′ near gene | C/G | Variation in the promoter activity of MBL2 | Alter transcriptional levels | [75,76,77] |
| rs7096206 | −289 | 5,27,71,925 | Y/X (aka −221) | 5′ near gene | G/C | |||
| rs7095891 | −65 | 5,27,71,701 | P/Q (aka +4) | 5′ near gene | C/T | |||
| rs5030737 | +152 | 5,27,71,482 | A/D (codon 52) | Exon 1 | C/T | Distortion in the triple helix collagenous region | Dramatically reduce the formation of functional higher order oligomers | [69,70,71,72,73,74] |
| rs1800450 | +161 | 5,27,71,475 | A/B (codon 54) | Exon 1 | G/A | |||
| rs1800451 | +170 | 5,27,71,466 | A/C (codon 57) | Exon 1 | G/A | Formation of adventitious disulfide bonds | ||
| rs10082466 | +4,455 | 5,27,66,862 | — | 3′UTR | A/G | Generates a binding site for miR-27a | Hasten mRNA degradation by miR-27a with reduced protein translation | [63] |
NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/SNP).
NCBI contig accession number NT_030059.14 (NCBI build 142, locus ID 4153).
Designation extensively used in the literature is also followed in the present study, and these markers form the “secretor haplotypes.”
7. MBL in different diseases and MBL paradox
The important functions of MBL in immune defense led to the following implicit assumption: “High serum MBL (sMBL) levels provide protection and low levels risk to the diseases.” This assumption was initially documented in 1989, when the defect in opsonization because of MBL deficiency (i.e., low sMBL levels) was recognized as the reason of frequent inexplicable infections in children [42]. After this, the number of pediatric population-specific studies started emphasizing the importance of nonspecific innate immunity provided by MBL in the early life. The later was considered as the substitute for the defense offered by the adaptive immunity, which is suggested to be undeveloped in infants. Nonetheless, the studies based on the adults suggested the role of MBL throughout one’s life, emphasizing its role as a first line of defense against pathogens till the adaptive immunity starts responding [86]. A synopsis involving specific examples covering the mushrooming literature regarding MBL in different diseases is discussed below:
7.1. MBL in infectious diseases caused by extracellular pathogens and autoimmune diseases
Low sMBL levels (i.e., MBL deficiency) have been shown to be predisposing individuals to many infectious diseases specifically caused by the extracellular pathogens [87]. One of the earliest evidences in this regard was observed in the AIDS patients with MBL2 structural variant alleles. These patients were found to have a low survival rate attributed to their increased susceptibility to coinfections [88]. In addition, the number of studies have shown the MBL-deficient states in patients with hepatitis B or C indicating that the low sMBL levels increase susceptibility to this disease [89,90]. The same scenario was also observed in the autoimmune diseases where MBL deficiency was shown to predispose individuals to systemic lupus erythematosus [79] and rheumatoid arthritis [91]. In general, a surplus literature can be found vis-à-vis MBL2 genetic variations and their association with low sMBL levels in susceptibility to many diseases including tuberculosis, malaria, filariasis, systemic lupus erythematosus, trypanosomiasis, rheumatoid arthritis, HIV infection, RVVIs, and many more (as afore referred), not to mention the severe acute respiratory syndrome-coronavirus (SARS-CoV) infection.
It has been documented that MBL paucity upsurges the risk of SARS-CoV-1 infection [92] with a higher prevalence of low secretor haplotypes in SARS cases than controls. Of all the viral envelopes to which MBL can bind, both plasma-derived and recombinant human MBL have been shown to directly inhibit SARS-CoV spike (S) glycoprotein (SARS-S)-mediated viral infection [93]. Therefore, MBL binding to SARS-S may intrude the events necessary for the efficient viral entry. This further suggests a possible role of MBL in the coronavirus disease 2019 (COVID-19). In this respect, a recent study suggested that SARS-CoV-2 uses the mannose-rich S protein for binding the angiotensin-converting enzyme 2 (ACE2) receptor of host to mediate host-cell entry [94]. This provides an assumption that MBL may possibly bind and inhibit the S-ACE2 interaction in SARS-CoV-2, as it did for SARS-CoV-1. A recent pre-print by Gao et al. [95] strengthened this speculation, by suggesting the role of MASP-2-mediated complement over-activation on interaction with N protein dimers of SARS-CoV-2 that aggravates the lung injury. Moreover, a case–control study showed higher expression of MBL in the lungs of COVID-19 group than controls [96]. In contrast, comparable plasma MBL levels were found between COVID-19 cases and controls by another study [97]. These two case–control studies together provide the inclusive findings attributed to their underpower study design. Given the prevalence of this disease, it is very important that such case–control studies must be performed in the larger datasets of different populations, so that the role of MBL in COVID-19 can be elucidated. This is of utmost importance as the presence of symptomatic COVID appears to be dependent more on the host factors rather than on the pathogen itself. Therefore, the knowledge of MBL role in COVID-19 may help us devise an efficient immune-based therapy.
7.2. MBL in infectious diseases caused by intracellular pathogens (the paradox)
The paradox, i.e., “high MBL levels increase diseases susceptibility and low MBL levels protect,” came into light when MBL was found to have predisposing effect on the infectious diseases involving leprosy and visceral leishmaniasis, both caused by the intracellular parasites [98,99]. These patients had considerably high sMBL levels in comparison to the controls. In unison, the higher prevalence of variant alleles observed in controls than in patients suggest the advantageous role of functional MBL deficiency in these controls. This unexpected frequency was explained by the fact that intracellular parasite is dependent on the phagocytosis to invade the host cells and use opsonization by complements to enter the phagocytes through complement receptors. Therefore, the presence of low MBL levels in the controls will consequently lower the probability of parasitization because of the reduction in the complement-mediated phagocytosis [98]. This could be the plausible rationale behind the few exceptions that have surplus existence of low MBL phenotypes across the globe, suggesting that these geographic areas must be prone to the intracellular parasitism. Therefore, any mechanism that diminishes C system activation would be favorable conferring the selective advantage to those individuals carrying the variant alleles and low MBL levels [71,100]. The mechanism was proposed to be similar to sickle cell anemia, where the sickle cell gene was found to provide protection against malaria that ultimately leads to the selective advantage to the carriers of sickle cell allele [101]. Overall, this suggests that low sMBL levels provide protection against intracellular pathogens.
Thus, these reports mentioned above have suggested the protective role played by MBL against diseases either through high levels or selectively by low levels. In contrast, there are reports that did not find any significant difference between the MBL levels of cases and controls [102,103]. Moreover, MBL-deficient individuals with no evidence of infections have also been reported, which argues against the defensive role played by the MBL [102,103]. However, these studies cannot be viewed as the evidence of no clinical applicability of sMBL levels. This is because different groups of scientific researchers have provided the evidence verifying the defensive role of MBL under a case–control design. In addition, the studies have also suggested that MBL paucity works in synergism with other humoral immunodeficiencies to cause diseases [104,105]. These are the discrepancies because of which no absolute level of MBL deficiency could be determined. Therefore, it is important that instead of generalizing the MBL’s role, an effort should be made in defining its versatile nature in different diseases.
8. Clinical trials and patents
The plasma-derived MBL has passed phase-I clinical trials and has successfully been used for the treatment of a 2-year-old girl with opsonic defect suffering from the devastating recurrent infections and other patients with cystic fibrosis and lung infections [106,107,108,109,110,111]. Furthermore, patents have been filed for the use of recombinant MBL for the management of melanoma cancer, lymphoma cancer, pancreatic cancer, ovarian cancer, or colorectal cancer (WO2008019322A2, US6846649B1, and WO2004026330A1). However, after some enthusiasm about MBL substitution treatment, there is a lack of well-designed large trials that could have supported the adoption of MBL supplementation. Patient numbers in the references mentioned are small, and there has been no widespread clinical use of MBL ever since. In light of this, the present literature review commands further research using larger datasets of different populations for the use of emerging MBL-supplementation as effectual treatment for different diseases in which its role has already been elucidated to authenticate the medicinal prospects of MBL.
9. Conclusion and future directions
MBL harbors a complex genetic system and several studies have shown the association of its variants with infectious diseases, suggesting its transformative role in innate immunity, exemplifying how polymorphisms were shaped by ecological pressure like infections and demographics [112]. Therefore, further research focused on elucidating MBL and the lectin pathway of complement with regard to the pathogenesis of different diseases and potential therapeutic targets within the respective pathways along with the exploration of the neglected area of “whether pathology due to MBL paucity entails one or more co-existing immune deficits or not” is needed as supported by different studies [68,104,105].
Footnotes
Author contributions: N. K. collected the literature, designed, and wrote the manuscript. J. S. and M. K. critically checked the manuscript.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Namarta Kalia, Email: kalianamarta62@gmail.com.
Manpreet Kaur, Email: dr.manpreetdhuna@gmail.com.
References
- [1].Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger-and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12(1):21. [DOI] [PMC free article] [PubMed]; Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger-and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12(1):21. doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mayer S, Raulf M-K, Lepenies B. C-type lectins: their network and roles in pathogen recognition and immunity. Histochem Cell Biol. 2017;147(2):223–37. [DOI] [PubMed]; Mayer S, Raulf M-K, Lepenies B. C-type lectins: their network and roles in pathogen recognition and immunity. Histochem Cell Biol. 2017;147(2):223–37. doi: 10.1007/s00418-016-1523-7. [DOI] [PubMed] [Google Scholar]
- [3].Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. [DOI] [PMC free article] [PubMed]; Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1–3 genes. Mol Immunol. 2006;43(7):962–71. [DOI] [PubMed]; Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1–3 genes. Mol Immunol. 2006;43(7):962–71. doi: 10.1016/j.molimm.2005.06.033. [DOI] [PubMed] [Google Scholar]
- [5].Selander B, Mårtensson U, Weintraub A, Holmström E, Matsushita M, Thiel S, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116(5):1425–34. [DOI] [PMC free article] [PubMed]; Selander B, Mårtensson U, Weintraub A, Holmström E, Matsushita M, Thiel S. et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116(5):1425–34. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kurata H, Sannoh T, Kozutsumi Y, Yokota Y, Kawasaki T. Structure and function of mannan-binding proteins isolated from human liver and serum. J Biochem. 1994;115(6):1148–54. [DOI] [PubMed]; Kurata H, Sannoh T, Kozutsumi Y, Yokota Y, Kawasaki T. Structure and function of mannan-binding proteins isolated from human liver and serum. J Biochem. 1994;115(6):1148–54. doi: 10.1093/oxfordjournals.jbchem.a124471. [DOI] [PubMed] [Google Scholar]
- [7].Sunil Singh S, Chi Fai Cheung R, Ho Wong J, Bun Ng T. Mannose binding lectin: a potential biomarker for many human diseases. Curr Med Chem. 2016;23(33):3847–60. [DOI] [PubMed]; Sunil Singh S, Chi Fai Cheung R, Ho Wong J, Bun Ng T. Mannose binding lectin: a potential biomarker for many human diseases. Curr Med Chem. 2016;23(33):3847–60. doi: 10.2174/0929867323666160817162208. [DOI] [PubMed] [Google Scholar]
- [8].Jensen PH, Weilguny D, Matthiesen F, McGuire KA, Shi L, Højrup P. Characterization of the oligomer structure of recombinant human mannan-binding lectin. J Biol Chem. 2005;280(12):11043–51. [DOI] [PubMed]; Jensen PH, Weilguny D, Matthiesen F, McGuire KA, Shi L, Højrup P. Characterization of the oligomer structure of recombinant human mannan-binding lectin. J Biol Chem. 2005;280(12):11043–51. doi: 10.1074/jbc.M412472200. [DOI] [PubMed] [Google Scholar]
- [9].Teillet F, Dublet B, Andrieu J-P, Gaboriaud C, Arlaud GJ, Thielens NM. The two major oligomeric forms of human mannan-binding lectin: chemical characterization, carbohydrate-binding properties, and interaction with MBL-associated serine proteases. J Immunol. 2005;174(5):2870–7. [DOI] [PubMed]; Teillet F, Dublet B, Andrieu J-P, Gaboriaud C, Arlaud GJ, Thielens NM. The two major oligomeric forms of human mannan-binding lectin: chemical characterization, carbohydrate-binding properties, and interaction with MBL-associated serine proteases. J Immunol. 2005;174(5):2870–7. doi: 10.4049/jimmunol.174.5.2870. [DOI] [PubMed] [Google Scholar]
- [10].Iobst ST, Wormald MR, Weis WI, Dwek RA, Drickamer K. Binding of sugar ligands to Ca (2+)-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J Biol Chem. 1994;269(22):15505–11. [PubMed]; Iobst ST, Wormald MR, Weis WI, Dwek RA, Drickamer K. Binding of sugar ligands to Ca (2+)-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J Biol Chem. 1994;269(22):15505–11. [PubMed] [Google Scholar]
- [11].Kawasaki N, Kawasaki T, YAMASHINA I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem. 1983;94(3):937–47. [DOI] [PubMed]; Kawasaki N, Kawasaki T, YAMASHINA I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem. 1983;94(3):937–47. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- [12].Sheriff S, Chang CY, Ezekowitz RAB. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple α-helical coiled-coil. Nat Struct Biol. 1994;1(11):789–94. [DOI] [PubMed]; Sheriff S, Chang CY, Ezekowitz RAB. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple α-helical coiled-coil. Nat Struct Biol. 1994;1(11):789–94. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- [13].Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360(6400):127–34. [DOI] [PubMed]; Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360(6400):127–34. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- [14].Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Mol Immunol. 2006;43(1–2):86–96. [DOI] [PMC free article] [PubMed]; Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Mol Immunol. 2006;43(1–2):86–96. doi: 10.1016/j.molimm.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wei MM, Wang YS, Ye XS. Carbohydrate-based vaccines for oncotherapy. Med Res Rev. 2018;38(3):1003–26. [DOI] [PubMed]; Wei MM, Wang YS, Ye XS. Carbohydrate-based vaccines for oncotherapy. Med Res Rev. 2018;38(3):1003–26. doi: 10.1002/med.21493. [DOI] [PubMed] [Google Scholar]
- [16].Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33(10):2853–63. [DOI] [PubMed]; Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC. et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33(10):2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- [17].Seiler BT, Cartwright M, Dinis AL, Duffy S, Lombardo P, Cartwright D, et al. Broad-spectrum capture of clinical pathogens using engineered Fc-mannose-binding lectin enhanced by antibiotic treatment. F1000Res. 2019;8:108. [DOI] [PMC free article] [PubMed]; Seiler BT, Cartwright M, Dinis AL, Duffy S, Lombardo P, Cartwright D. et al. Broad-spectrum capture of clinical pathogens using engineered Fc-mannose-binding lectin enhanced by antibiotic treatment. F1000Res. 2019;8:108. doi: 10.12688/f1000research.17447.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68(2):688–93. [DOI] [PMC free article] [PubMed]; Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68(2):688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Townsend R, Read R, Turner M, Klein N, Jack D. Differential recognition of obligate anaerobic bacteria by human mannose-binding lectin. Clin Exp Immunol. 2001;124(2):223–8. [DOI] [PMC free article] [PubMed]; Townsend R, Read R, Turner M, Klein N, Jack D. Differential recognition of obligate anaerobic bacteria by human mannose-binding lectin. Clin Exp Immunol. 2001;124(2):223–8. doi: 10.1046/j.1365-2249.2001.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37(11):1496–505. [DOI] [PubMed]; Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37(11):1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- [21].Hammad NM, El Badawy NE, Ghramh HA, Al Kady LM. Mannose-binding lectin: a potential therapeutic candidate against Candida infection. BioMed Res Int. 2018;2813737. [DOI] [PMC free article] [PubMed]; Hammad NM, El Badawy NE, Ghramh HA, Al Kady LM. Mannose-binding lectin: a potential therapeutic candidate against Candida infection. BioMed Res Int. 2018:2813737. doi: 10.1155/2018/2813737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kasperkiewicz K, Swierzko AS, Bartlomiejczyk MA, Cedzynski M, Noszczynska M, Duda KA, et al. Interaction of human mannose-binding lectin (MBL) with Yersinia enterocolitica lipopolysaccharide. Int J Med Microbiol. 2015;305(6):544–52. [DOI] [PubMed]; Kasperkiewicz K, Swierzko AS, Bartlomiejczyk MA, Cedzynski M, Noszczynska M, Duda KA. et al. Interaction of human mannose-binding lectin (MBL) with Yersinia enterocolitica lipopolysaccharide. Int J Med Microbiol. 2015;305(6):544–52. doi: 10.1016/j.ijmm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- [23].Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176(6):1497–502. [DOI] [PMC free article] [PubMed]; Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176(6):1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dobó J, Pál G, Cervenak L, Gál P. The emerging roles of mannose-binding lectin-associated serine proteases (MASPs) in the lectin pathway of complement and beyond. Immunol Rev. 2016;274(1):98–111. [DOI] [PubMed]; Dobó J, Pál G, Cervenak L, Gál P. The emerging roles of mannose-binding lectin-associated serine proteases (MASPs) in the lectin pathway of complement and beyond. Immunol Rev. 2016;274(1):98–111. doi: 10.1111/imr.12460. [DOI] [PubMed] [Google Scholar]
- [25].Stover CM, Thiel S, Thelen M, Lynch NJ, Vorup-Jensen T, Jensenius JC, et al. Two constituents of the initiation complex of the mannan-binding lectin activation pathway of complement are encoded by a single structural gene. J Immunol. 1999;162(6):3481–90. [PubMed]; Stover CM, Thiel S, Thelen M, Lynch NJ, Vorup-Jensen T, Jensenius JC. et al. Two constituents of the initiation complex of the mannan-binding lectin activation pathway of complement are encoded by a single structural gene. J Immunol. 1999;162(6):3481–90. [PubMed] [Google Scholar]
- [26].Takahashi M, Endo Y, Fujita T, Matsushita M. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int Immunol. 1999;11(5):859–63. [DOI] [PubMed]; Takahashi M, Endo Y, Fujita T, Matsushita M. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int Immunol. 1999;11(5):859–63. doi: 10.1093/intimm/11.5.859. [DOI] [PubMed] [Google Scholar]
- [27].Dahl M, Thiel S, Willis A, Vorup-Jensen T, Christensen T, Petersen S, et al. Mannan-binding lectin associated serine protease 3 (MASP-3)-a new component of the lectin pathway of complement activation. Immunopharmacology. 2000;1(49):79.; Dahl M, Thiel S, Willis A, Vorup-Jensen T, Christensen T, Petersen S. et al. Mannan-binding lectin associated serine protease 3 (MASP-3)-a new component of the lectin pathway of complement activation. Immunopharmacology. 2000;1(49):79. [Google Scholar]
- [28].Bohlson SS, Garred P, Kemper C, Tenner AJ. Complement nomenclature-deconvoluted. Front Immunol. 2019;10:1308. [DOI] [PMC free article] [PubMed]; Bohlson SS, Garred P, Kemper C, Tenner AJ. Complement nomenclature-deconvoluted. Front Immunol. 2019;10:1308. doi: 10.3389/fimmu.2019.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuhlman M, Joiner K, Ezekowitz R. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169(5):1733–45. [DOI] [PMC free article] [PubMed]; Kuhlman M, Joiner K, Ezekowitz R. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169(5):1733–45. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91(4):1414–20. [DOI] [PMC free article] [PubMed]; Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA. et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91(4):1414–20. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li D, Dong B, Tong Z, Wang Q, Liu W, Wang Y, et al. MBL-mediated opsonophagocytosis of Candida albicans by human neutrophils is coupled with intracellular Dectin-1-triggered ROS production. PLoS One. 2012;7:12. [DOI] [PMC free article] [PubMed]; Li D, Dong B, Tong Z, Wang Q, Liu W, Wang Y. et al. MBL-mediated opsonophagocytosis of Candida albicans by human neutrophils is coupled with intracellular Dectin-1-triggered ROS production. PLoS One. 2012;7:12. doi: 10.1371/journal.pone.0050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Super M, Gillies S, Foley S, Sastry K, Schweinle J-E, Silverman V, et al. Distinct and overlapping functions of allelic forms of human mannose binding protein. Nat Genet. 1992;2(1):50–5. [DOI] [PubMed]; Super M, Gillies S, Foley S, Sastry K, Schweinle J-E, Silverman V. et al. Distinct and overlapping functions of allelic forms of human mannose binding protein. Nat Genet. 1992;2(1):50–5. doi: 10.1038/ng0992-50. [DOI] [PubMed] [Google Scholar]
- [33].Kawasaki T, Ma Y, Uemura K, Kawasaki N. Mannan-binding protein (MBP)-dependent cell-mediated cytotoxicity (MDCC). Immunopharmacology. 2000;1(49):85.; Kawasaki T, Ma Y, Uemura K, Kawasaki N. Mannan-binding protein (MBP)-dependent cell-mediated cytotoxicity (MDCC) Immunopharmacology. 2000;1(49):85. [Google Scholar]
- [34].Malhotra R, Thiel S, Reid K, Sim R. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990;172(3):955–9. [DOI] [PMC free article] [PubMed]; Malhotra R, Thiel S, Reid K, Sim R. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990;172(3):955–9. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tenner AJ, Robinson SL, Ezekowitz RAB. Mannose binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 1,26,000 Mr component of the Clq receptor. Immunity. 1995;3(4):485–93. [DOI] [PubMed]; Tenner AJ, Robinson SL, Ezekowitz RAB. Mannose binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 1,26,000 Mr component of the Clq receptor. Immunity. 1995;3(4):485–93. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- [36].Klickstein LB, Barbashov SF, Liu T, Jack RM, Nicholson-Weller A. Complement receptor type 1 (CR1, CD35) is a receptor for C1q. Immunity. 1997;7(3):345–55. [DOI] [PubMed]; Klickstein LB, Barbashov SF, Liu T, Jack RM, Nicholson-Weller A. Complement receptor type 1 (CR1, CD35) is a receptor for C1q. Immunity. 1997;7(3):345–55. doi: 10.1016/s1074-7613(00)80356-8. [DOI] [PubMed] [Google Scholar]
- [37].Ghiran I, Barbashov SF, Klickstein LB, Tas SW, Jensenius JC, Nicholson-Weller A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med. 2000;192(12):1797–808. [DOI] [PMC free article] [PubMed]; Ghiran I, Barbashov SF, Klickstein LB, Tas SW, Jensenius JC, Nicholson-Weller A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med. 2000;192(12):1797–808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Edelson BT, Stricker TP, Li Z, Dickeson SK, Shepherd VL, Santoro SA, et al. Novel collectin/C1q receptor mediates mast cell activation and innate immunity. Blood. 2006;107(1):143–50. [DOI] [PMC free article] [PubMed]; Edelson BT, Stricker TP, Li Z, Dickeson SK, Shepherd VL, Santoro SA. et al. Novel collectin/C1q receptor mediates mast cell activation and innate immunity. Blood. 2006;107(1):143–50. doi: 10.1182/blood-2005-06-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–96. [DOI] [PMC free article] [PubMed]; Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA. et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–96. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Duus K, Hansen EW, Tacnet P, Frachet P, Arlaud GJ, Thielens NM, et al. Direct interaction between CD91 and C1q. FEBS J. 2010;277(17):3526–37. [DOI] [PubMed]; Duus K, Hansen EW, Tacnet P, Frachet P, Arlaud GJ, Thielens NM. et al. Direct interaction between CD91 and C1q. FEBS J. 2010;277(17):3526–37. doi: 10.1111/j.1742-4658.2010.07762.x. [DOI] [PubMed] [Google Scholar]
- [41].Turner M, Mowbray J, Roberton D. A study of C3b deposition on yeast surfaces by sera of known opsonic potential. Clin Exp Immunol. 1981;46(2):412. [PMC free article] [PubMed]; Turner M, Mowbray J, Roberton D. A study of C3b deposition on yeast surfaces by sera of known opsonic potential. Clin Exp Immunol. 1981;46(2):412. [PMC free article] [PubMed] [Google Scholar]
- [42].Super M, Lu J, Thiel S, Levinsky R, Turner M. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;334(8674):1236–9. [DOI] [PubMed]; Super M, Lu J, Thiel S, Levinsky R, Turner M. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;334(8674):1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- [43].Ma Y, Uemura K, Oka S, Kozutsumi Y, Kawasaki N, Kawasaki T. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity. Proc Natl Acad Sci. 1999;96(2):371–5. [DOI] [PMC free article] [PubMed]; Ma Y, Uemura K, Oka S, Kozutsumi Y, Kawasaki N, Kawasaki T. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity. Proc Natl Acad Sci. 1999;96(2):371–5. doi: 10.1073/pnas.96.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nakagawa T, Kawasaki N, Ma Y, Uemura K, Kawasaki T. Antitumor activity of mannan-binding protein. Methods Enzymol. 2003;363:26–33. 10.1016/S0076-6879(03)01041-3. [DOI] [PubMed]; Nakagawa T, Kawasaki N, Ma Y, Uemura K, Kawasaki T. Antitumor activity of mannan-binding protein. Methods Enzymol. 2003;363:26–33. doi: 10.1016/S0076-6879(03)01041-3. [DOI] [PubMed] [Google Scholar]
- [45].Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–96. [DOI] [PMC free article] [PubMed]; Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA. et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–96. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RAB. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174(6):3220–6. [DOI] [PubMed]; Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RAB. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174(6):3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- [47].Zhou J, Hu M, Li J, Liu Y, Luo J, Zhang L, et al. Mannan-binding lectin regulates inflammatory cytokine production, proliferation, and cytotoxicity of human peripheral natural killer cells. Mediators Inflamm. 2019;2019:6738286. [DOI] [PMC free article] [PubMed]; Zhou J, Hu M, Li J, Liu Y, Luo J, Zhang L. et al. Mannan-binding lectin regulates inflammatory cytokine production, proliferation, and cytotoxicity of human peripheral natural killer cells. Mediators Inflamm. 2019;2019:6738286. doi: 10.1155/2019/6738286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang F, Li Y, Yang C, Mu Y, Wang Y, Zhang W, et al. Mannan-binding lectin suppresses peptidoglycan-induced TLR2 activation and inflammatory responses. Mediators Inflamm. 2019;2019:1349784. [DOI] [PMC free article] [PubMed]; Wang F, Li Y, Yang C, Mu Y, Wang Y, Zhang W. et al. Mannan-binding lectin suppresses peptidoglycan-induced TLR2 activation and inflammatory responses. Mediators Inflamm. 2019;2019:1349784. doi: 10.1155/2019/1349784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184(9):1152–62. [DOI] [PubMed]; Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184(9):1152–62. doi: 10.1086/323803. [DOI] [PubMed] [Google Scholar]
- [50].Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin – a soluble pattern recognition molecule. Mol Immunol. 2004;41(2–3):113–21. [DOI] [PubMed]; Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin – a soluble pattern recognition molecule. Mol Immunol. 2004;41(2–3):113–21. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [51].Boschmann SE, Goeldner I, Tuon FF, Schiel W, Aoyama F, de Messias-Reason IJ. Mannose-binding lectin polymorphisms and rheumatoid arthritis: a short review and meta-analysis. Mol Immunol. 2016;69:77–85. [DOI] [PubMed]; Boschmann SE, Goeldner I, Tuon FF, Schiel W, Aoyama F, de Messias-Reason IJ. Mannose-binding lectin polymorphisms and rheumatoid arthritis: a short review and meta-analysis. Mol Immunol. 2016;69:77–85. doi: 10.1016/j.molimm.2015.10.010. [DOI] [PubMed] [Google Scholar]
- [52].Cieslinski J, Skare T, Nisihara R, De Messias-Reason I, Utiyama S. Mannose-binding lectin serum levels in patients with systemic lupus erythematosus: association with thrombocytopaenia and seizure. Lupus. 2018;27(3):372–9. [DOI] [PubMed]; Cieslinski J, Skare T, Nisihara R, De Messias-Reason I, Utiyama S. Mannose-binding lectin serum levels in patients with systemic lupus erythematosus: association with thrombocytopaenia and seizure. Lupus. 2018;27(3):372–9. doi: 10.1177/0961203317722846. [DOI] [PubMed] [Google Scholar]
- [53].Kalia N, Singh J, Sharma S, Arora H, Kaur M. Genetic and phenotypic screening of mannose-binding lectin in relation to risk of recurrent vulvovaginal infections in women of North India: a prospective cohort study. Front Microbiol. 2017;8:75. [DOI] [PMC free article] [PubMed]; Kalia N, Singh J, Sharma S, Arora H, Kaur M. Genetic and phenotypic screening of mannose-binding lectin in relation to risk of recurrent vulvovaginal infections in women of North India: a prospective cohort study. Front Microbiol. 2017;8:75. doi: 10.3389/fmicb.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kalia N, Singh J, Sharma S, Kaur M. SNPs in 3′-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology. 2019;224(1):42–9. [DOI] [PubMed]; Kalia N, Singh J, Sharma S, Kaur M. SNPs in 3′-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology. 2019;224(1):42–9. doi: 10.1016/j.imbio.2018.10.009. [DOI] [PubMed] [Google Scholar]
- [55].Kalia N, Singh J, Sharma S, Kaur M. Impact of SNPs interplay across the locus of MBL2, between MBL and Dectin-1 gene, on women’s risk of developing recurrent vulvovaginal infections. Cell Biosci. 2019;9(1):35. [DOI] [PMC free article] [PubMed]; Kalia N, Singh J, Sharma S, Kaur M. Impact of SNPs interplay across the locus of MBL2, between MBL and Dectin-1 gene, on women’s risk of developing recurrent vulvovaginal infections. Cell Biosci. 2019;9(1):35. doi: 10.1186/s13578-019-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tong X, Wan Q, Li Z, Liu S, Huang J, Wu M, et al. Association between the mannose-binding lectin (MBL)-2 gene variants and serum MBL with pulmonary tuberculosis: an update meta-analysis and systematic review. Microb Pathog. 2019;132:374–80. [DOI] [PubMed]; Tong X, Wan Q, Li Z, Liu S, Huang J, Wu M. et al. Association between the mannose-binding lectin (MBL)-2 gene variants and serum MBL with pulmonary tuberculosis: an update meta-analysis and systematic review. Microb Pathog. 2019;132:374–80. doi: 10.1016/j.micpath.2019.04.023. [DOI] [PubMed] [Google Scholar]
- [57].Tiwari N, Kumar A, Singh AK, Bajpai S, Agrahari AK, Kishore D, et al. Leishmaniasis control: limitations of current drugs and prospects of natural products. Discovery and development of therapeutics from natural products against neglected tropical diseases. Amsterdam, The Netherlands: Elsevier; 2019. p. 293–350.; Tiwari N, Kumar A, Singh AK, Bajpai S, Agrahari AK, Kishore D, Discovery and development of therapeutics from natural products against neglected tropical diseases. Amsterdam, The Netherlands: Elsevier; 2019. Leishmaniasis control: limitations of current drugs and prospects of natural products; pp. p. 293–350. [Google Scholar]
- [58].de Morais VMS, Gonçales JP, Cahú GGdOM, Tozetto-Mendoza TR, Coêlho MRCD. Mannose-binding lectin concentrations in people living with HIV/AIDS infected by HHV-8. BMC Immunol. 2019;20(1):1–6. [DOI] [PMC free article] [PubMed]; de Morais VMS, Gonçales JP, Cahú GGdOM, Tozetto-Mendoza TR, Coêlho MRCD. Mannose-binding lectin concentrations in people living with HIV/AIDS infected by HHV-8. BMC Immunol. 2019;20(1):1–6. doi: 10.1186/s12865-018-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo N, Mogues T, Weremowicz S, Morton CC, Sastry KN. The human ortholog of rhesus mannose-binding protein-A gene is an expressed pseudogene that localizes to chromosome 10. Mamm Genome. 1998;9(3):246–9. [DOI] [PubMed]; Guo N, Mogues T, Weremowicz S, Morton CC, Sastry KN. The human ortholog of rhesus mannose-binding protein-A gene is an expressed pseudogene that localizes to chromosome 10. Mamm Genome. 1998;9(3):246–9. doi: 10.1007/s003359900735. [DOI] [PubMed] [Google Scholar]
- [60].Taylor ME, Brickell P, Craig R, Summerfield J. Structure and evolutionary origin of the gene encoding a human serum mannose-binding protein. Biochem J. 1989;262(3):763–71. [DOI] [PMC free article] [PubMed]; Taylor ME, Brickell P, Craig R, Summerfield J. Structure and evolutionary origin of the gene encoding a human serum mannose-binding protein. Biochem J. 1989;262(3):763–71. doi: 10.1042/bj2620763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bernig T, Breunis W, Brouwer N, Hutchinson A, Welch R, Roos D, et al. An analysis of genetic variation across the MBL2 locus in Dutch Caucasians indicates that 3′ haplotypes could modify circulating levels of mannose-binding lectin. Hum Genet. 2005;118(3–4):404–15. [DOI] [PubMed]; Bernig T, Breunis W, Brouwer N, Hutchinson A, Welch R, Roos D. et al. An analysis of genetic variation across the MBL2 locus in Dutch Caucasians indicates that 3′ haplotypes could modify circulating levels of mannose-binding lectin. Hum Genet. 2005;118(3–4):404–15. doi: 10.1007/s00439-005-0053-5. [DOI] [PubMed] [Google Scholar]
- [62].Bernig T, Boersma BJ, Howe TM, Welch R, Yadavalli S, Staats B, et al. The mannose-binding lectin (MBL2) haplotype and breast cancer: an association study in African-American and Caucasian women. Carcinogenesis. 2007;28(4):828–36. [DOI] [PubMed]; Bernig T, Boersma BJ, Howe TM, Welch R, Yadavalli S, Staats B. et al. The mannose-binding lectin (MBL2) haplotype and breast cancer: an association study in African-American and Caucasian women. Carcinogenesis. 2007;28(4):828–36. doi: 10.1093/carcin/bgl198. [DOI] [PubMed] [Google Scholar]
- [63].Zanetti KA, Haznadar M, Welsh JA, Robles AI, Ryan BM, McClary AC, et al. 3′-UTR and functional secretor haplotypes in mannose-binding lectin 2 are associated with increased colon cancer risk in African Americans. Cancer Res. 2012;72(6):1467–77. [DOI] [PMC free article] [PubMed]; Zanetti KA, Haznadar M, Welsh JA, Robles AI, Ryan BM, McClary AC. et al. 3′-UTR and functional secretor haplotypes in mannose-binding lectin 2 are associated with increased colon cancer risk in African Americans. Cancer Res. 2012;72(6):1467–77. doi: 10.1158/0008-5472.CAN-11-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Thiel S, Holmskov U, Hviid L, Laursen S, Jensenius J. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90(1):31–5. [DOI] [PMC free article] [PubMed]; Thiel S, Holmskov U, Hviid L, Laursen S, Jensenius J. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90(1):31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Dean M, Minchinton R, Heatley S, Eisen D. Mannose binding lectin acute phase activity in patients with severe infection. J Clin Immunol. 2005;25(4):346–52. [DOI] [PubMed]; Dean M, Minchinton R, Heatley S, Eisen D. Mannose binding lectin acute phase activity in patients with severe infection. J Clin Immunol. 2005;25(4):346–52. doi: 10.1007/s10875-005-4702-1. [DOI] [PubMed] [Google Scholar]
- [66].Herpers BL, Endeman H, De Jong BA, De Jongh BM, Grutters JC, Biesma DH, et al. Acute-phase responsiveness of mannose-binding lectin in community-acquired pneumonia is highly dependent upon MBL2 genotypes. Clin Exp Immunol. 2009;156(3):488–94. [DOI] [PMC free article] [PubMed]; Herpers BL, Endeman H, De Jong BA, De Jongh BM, Grutters JC, Biesma DH. et al. Acute-phase responsiveness of mannose-binding lectin in community-acquired pneumonia is highly dependent upon MBL2 genotypes. Clin Exp Immunol. 2009;156(3):488–94. doi: 10.1111/j.1365-2249.2009.03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Perez-Castellano M, Penaranda M, Payeras A, Mila J, Riera M, Vidal J, et al. Mannose-binding lectin does not act as an acute-phase reactant in adults with community-acquired pneumococcal pneumonia. Clin Exp Immunol. 2006;145(2):228–34. [DOI] [PMC free article] [PubMed]; Perez-Castellano M, Penaranda M, Payeras A, Mila J, Riera M, Vidal J. et al. Mannose-binding lectin does not act as an acute-phase reactant in adults with community-acquired pneumococcal pneumonia. Clin Exp Immunol. 2006;145(2):228–34. doi: 10.1111/j.1365-2249.2006.03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17(11):532–40. [DOI] [PubMed]; Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17(11):532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- [69].Sumiya M, Tabona P, Arai T, Summerfield J, Super M, Levinsky R, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337(8757):1569–70. [DOI] [PubMed]; Sumiya M, Tabona P, Arai T, Summerfield J, Super M, Levinsky R. et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337(8757):1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- [70].Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40(1):37–44. [DOI] [PubMed]; Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S. et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40(1):37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- [71].Lipscombe R, Sumiya M, Hill A, Lau Y, Levinsky R, Summerfield J, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1(9):709–15. [DOI] [PubMed]; Lipscombe R, Sumiya M, Hill A, Lau Y, Levinsky R, Summerfield J. et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1(9):709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- [72].Wallis R, Cheng JY. Molecular defects in variant forms of mannose-binding protein associated with immunodeficiency. J Immunol. 1999;163(9):4953–9. [PubMed]; Wallis R, Cheng JY. Molecular defects in variant forms of mannose-binding protein associated with immunodeficiency. J Immunol. 1999;163(9):4953–9. [PubMed] [Google Scholar]
- [73].Terai I, Kobayashi K, Matsushita M, Miyakawa H, Mafune N, Kikuta H. Relationship between gene polymorphisms of mannose-binding lectin (MBL) and two molecular forms of MBL. Eur J Immunol. 2003;33(10):2755–63. [DOI] [PubMed]; Terai I, Kobayashi K, Matsushita M, Miyakawa H, Mafune N, Kikuta H. Relationship between gene polymorphisms of mannose-binding lectin (MBL) and two molecular forms of MBL. Eur J Immunol. 2003;33(10):2755–63. doi: 10.1002/eji.200323955. [DOI] [PubMed] [Google Scholar]
- [74].Larsen F, Madsen HO, Sim RB, Koch C, Garred P. Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem. 2004;279(20):21302–11. [DOI] [PubMed]; Larsen F, Madsen HO, Sim RB, Koch C, Garred P. Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem. 2004;279(20):21302–11. doi: 10.1074/jbc.M400520200. [DOI] [PubMed] [Google Scholar]
- [75].Madsen HO, Garred P, Thiel S, Kurtzhals J, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155(6):3013–20. [PubMed]; Madsen HO, Garred P, Thiel S, Kurtzhals J, Lamm LU, Ryder LP. et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155(6):3013–20. [PubMed] [Google Scholar]
- [76].Naito H, Ikeda A, Hasegawa K, Oka S, Uemura K, Kawasaki N, et al. Characterization of human serum mannan-binding protein promoter. J Biochem. 1999;126(6):1004–12. [DOI] [PubMed]; Naito H, Ikeda A, Hasegawa K, Oka S, Uemura K, Kawasaki N. et al. Characterization of human serum mannan-binding protein promoter. J Biochem. 1999;126(6):1004–12. doi: 10.1093/oxfordjournals.jbchem.a022543. [DOI] [PubMed] [Google Scholar]
- [77].Jüliger S, Luckner D, Mordmüller B, May J, Weierich A, Lell B, et al. Promoter variants of the human mannose-binding lectin gene show different binding. Biochem Biophys Res Commun. 2000;275(2):617–22. [DOI] [PubMed]; Jüliger S, Luckner D, Mordmüller B, May J, Weierich A, Lell B. et al. Promoter variants of the human mannose-binding lectin gene show different binding. Biochem Biophys Res Commun. 2000;275(2):617–22. doi: 10.1006/bbrc.2000.3343. [DOI] [PubMed] [Google Scholar]
- [78].Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161(6):3169–75. [PubMed]; Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161(6):3169–75. [PubMed] [Google Scholar]
- [79].Sullivan KE, Wooten C, Goldman D, Petri M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39(12):2046–51. [DOI] [PubMed]; Sullivan KE, Wooten C, Goldman D, Petri M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39(12):2046–51. doi: 10.1002/art.1780391214. [DOI] [PubMed] [Google Scholar]
- [80].Oudshoorn A-MJ, van den Dungen FA, Bach KP, Koomen I, Fetter WP, Catsburg A, et al. Mannose-binding lectin in term newborns and their mothers: genotypic and phenotypic relationship. Hum Immunol. 2008;69(6):344–8. [DOI] [PubMed]; Oudshoorn A-MJ, van den Dungen FA, Bach KP, Koomen I, Fetter WP, Catsburg A. et al. Mannose-binding lectin in term newborns and their mothers: genotypic and phenotypic relationship. Hum Immunol. 2008;69(6):344–8. doi: 10.1016/j.humimm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- [81].Jha AN, Sundaravadivel P, Singh VK, Pati SS, Patra PK, Kremsner PG, et al. MBL2 variations and malaria susceptibility in Indian populations. Infect Immun. 2014;82(1):52–61. [DOI] [PMC free article] [PubMed]; Jha AN, Sundaravadivel P, Singh VK, Pati SS, Patra PK, Kremsner PG. et al. MBL2 variations and malaria susceptibility in Indian populations. Infect Immun. 2014;82(1):52–61. doi: 10.1128/IAI.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Søborg C, Madsen HO, Andersen ÅB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188(5):777–82. [DOI] [PubMed]; Søborg C, Madsen HO, Andersen ÅB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188(5):777–82. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- [83].Biezeveld MH, Kuipers IM, Geissler J, Lam J, Ottenkamp JJ, Hack CE, et al. Association of mannose-binding lectin genotype with cardiovascular abnormalities in Kawasaki disease. Lancet. 2003;361(9365):1268–70. [DOI] [PubMed]; Biezeveld MH, Kuipers IM, Geissler J, Lam J, Ottenkamp JJ, Hack CE. et al. Association of mannose-binding lectin genotype with cardiovascular abnormalities in Kawasaki disease. Lancet. 2003;361(9365):1268–70. doi: 10.1016/S0140-6736(03)12985-6. [DOI] [PubMed] [Google Scholar]
- [84].Bernig T, Taylor J, Foster C, Staats B, Yeager M, Chanock S. Sequence analysis of the mannose-binding lectin (MBL2) gene reveals a high degree of heterozygosity with evidence of selection. Genes Immun. 2004;5(6):461–76. [DOI] [PubMed]; Bernig T, Taylor J, Foster C, Staats B, Yeager M, Chanock S. Sequence analysis of the mannose-binding lectin (MBL2) gene reveals a high degree of heterozygosity with evidence of selection. Genes Immun. 2004;5(6):461–76. doi: 10.1038/sj.gene.6364116. [DOI] [PubMed] [Google Scholar]
- [85].Collard CD, Shernan SK, Fox AA, Bernig T, Chanock SJ, Vaughn WK, et al. The MBL2 ‘LYQA secretor’haplotype is an independent predictor of postoperative myocardial infarction in whites undergoing coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):I-106–12. [DOI] [PMC free article] [PubMed]; Collard CD, Shernan SK, Fox AA, Bernig T, Chanock SJ, Vaughn WK. et al. The MBL2 ‘LYQA secretor’haplotype is an independent predictor of postoperative myocardial infarction in whites undergoing coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):I-106–12. doi: 10.1161/CIRCULATIONAHA.106.679530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Summerfield J, Ryder S, Sumiya M, Thursz M, Gorchein A, Monteil MA, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345(8954):886–9. [DOI] [PubMed]; Summerfield J, Ryder S, Sumiya M, Thursz M, Gorchein A, Monteil MA. et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345(8954):886–9. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- [87].Koch A, Melbye M, Sørensen P, Homøe P, Madsen HO, Mølbak K, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285(10):1316–21. [DOI] [PubMed]; Koch A, Melbye M, Sørensen P, Homøe P, Madsen HO, Mølbak K. et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285(10):1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- [88].Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349(9047):236–40. [DOI] [PubMed]; Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J. et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349(9047):236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- [89].Matsushita M, Hijikata M, Ohta Y, Iwata K, Matsumoto M, Nakao K, et al. Hepatitis C virus infection and mutations of mannose-binding lectin gene MBL. Arch Virol. 1998;143(4):645–51. [DOI] [PubMed]; Matsushita M, Hijikata M, Ohta Y, Iwata K, Matsumoto M, Nakao K. et al. Hepatitis C virus infection and mutations of mannose-binding lectin gene MBL. Arch Virol. 1998;143(4):645–51. doi: 10.1007/s007050050320. [DOI] [PubMed] [Google Scholar]
- [90].Yuen MF, Lau CS, Lau YL, Wong WM, Cheng CC, Lai CL. Mannose binding lectin gene mutations are associated with progression of liver disease in chronic hepatitis B infection. Hepatology. 1999;29(4):1248–51. [DOI] [PubMed]; Yuen MF, Lau CS, Lau YL, Wong WM, Cheng CC, Lai CL. Mannose binding lectin gene mutations are associated with progression of liver disease in chronic hepatitis B infection. Hepatology. 1999;29(4):1248–51. doi: 10.1002/hep.510290417. [DOI] [PubMed] [Google Scholar]
- [91].Graudal NA, Madsen HO, Tarp U, Svejgaard A, Jurik AG, Graudal HK, et al. The association of variant mannose-binding lectin genotypes with radiographic outcome in rheumatoid arthritis. Arthritis Rheum Off J Am Coll Rheumatol. 2000;43(3):515–21. [DOI] [PubMed]; Graudal NA, Madsen HO, Tarp U, Svejgaard A, Jurik AG, Graudal HK. et al. The association of variant mannose-binding lectin genotypes with radiographic outcome in rheumatoid arthritis. Arthritis Rheum Off J Am Coll Rheumatol. 2000;43(3):515–21. doi: 10.1002/1529-0131(200003)43:3<515::AID-ANR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [92].Ip WE, Chan KH, Law HK, Tso GH, Kong EK, Wong WH, et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–704. [DOI] [PMC free article] [PubMed]; Ip WE, Chan KH, Law HK, Tso GH, Kong EK, Wong WH. et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhou Y, Lu K, Pfefferle S, Bertram S, Glowacka I, Drosten C, et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84(17):8753–64. [DOI] [PMC free article] [PubMed]; Zhou Y, Lu K, Pfefferle S, Bertram S, Glowacka I, Drosten C. et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84(17):8753–64. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–3. [DOI] [PMC free article] [PubMed]; Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–3. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020:20041962. 10.1101/2020.03.29.20041962. [DOI]; Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H. et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020:20041962. doi: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- [96].Malaquias MAS, Gadotti AC, Junior JdSM, Martins APC, Azevedo MLV, Benevides APK, et al. The role of the lectin pathway of the complement system in SARS-CoV-2 lung injury. Transl Res. 2020;S1931–5244(20):30259–60. [DOI] [PMC free article] [PubMed]; Malaquias MAS, Gadotti AC, Junior JdSM, Martins APC, Azevedo MLV, Benevides APK. et al. The role of the lectin pathway of the complement system in SARS-CoV-2 lung injury. Transl Res. 2020;S1931–5244(20):30259–60. doi: 10.1016/j.trsl.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci. 2020;117(40):25018–25. [DOI] [PMC free article] [PubMed]; Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR. et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci. 2020;117(40):25018–25. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Int J Immunogenet. 1994;21(2):125–31. [DOI] [PubMed]; Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Int J Immunogenet. 1994;21(2):125–31. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- [99].de Miranda Santos IK, Costa CH, Krieger H, Feitosa MF, Zurakowski D, Fardin B, et al. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infect Immun. 2001;69(8):5212–5. [DOI] [PMC free article] [PubMed]; de Miranda Santos IK, Costa CH, Krieger H, Feitosa MF, Zurakowski D, Fardin B. et al. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infect Immun. 2001;69(8):5212–5. doi: 10.1128/IAI.69.8.5212-5215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7(2):85–94. [DOI] [PubMed]; Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7(2):85–94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- [101].Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. Br Med J. 1954;1(4857):290. [DOI] [PMC free article] [PubMed]; Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. Br Med J. 1954;1(4857):290. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tacx A, Groeneveld A, Hart M, Aarden L, Hack C. Mannan binding lectin in febrile adults: no correlation with microbial infection and complement activation. J Clin Pathol. 2003;56(12):956–9. [DOI] [PMC free article] [PubMed]; Tacx A, Groeneveld A, Hart M, Aarden L, Hack C. Mannan binding lectin in febrile adults: no correlation with microbial infection and complement activation. J Clin Pathol. 2003;56(12):956–9. doi: 10.1136/jcp.56.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dahl M, Tybjærg-Hansen A, Schnohr P, Nordestgaard BG. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med. 2004;199(10):1391–9. [DOI] [PMC free article] [PubMed]; Dahl M, Tybjærg-Hansen A, Schnohr P, Nordestgaard BG. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med. 2004;199(10):1391–9. doi: 10.1084/jem.20040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Turner M, Super M, Singh S, Levinsky R. Molecular basis of a common opsonic defect. Clin Exp Allergy. 1991;21:182–8. [DOI] [PubMed]; Turner M, Super M, Singh S, Levinsky R. Molecular basis of a common opsonic defect. Clin Exp Allergy. 1991;21:182–8. doi: 10.1111/j.1365-2222.1991.tb01725.x. [DOI] [PubMed] [Google Scholar]
- [105].Aittoniemi J, Baer M, Soppi E, Vesikari T, Miettinen A. Mannan binding lectin deficiency and concomitant immunodefects. Arch Dis Child. 1998;78(3):245–8. [DOI] [PMC free article] [PubMed]; Aittoniemi J, Baer M, Soppi E, Vesikari T, Miettinen A. Mannan binding lectin deficiency and concomitant immunodefects. Arch Dis Child. 1998;78(3):245–8. doi: 10.1136/adc.78.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Valdimarsson H, Stefansson M, Vikingsdottir T, Arason GJ, Koch C, Thiel S, et al. Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBL-deficient humans. Scand J Immunol. 1998;48(2):116–23. [DOI] [PubMed]; Valdimarsson H, Stefansson M, Vikingsdottir T, Arason GJ, Koch C, Thiel S. et al. Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBL-deficient humans. Scand J Immunol. 1998;48(2):116–23. doi: 10.1046/j.1365-3083.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- [107].Valdimarsson H, Vikingsdottir T, Bang P, Saevarsdottir S, Gudjonsson J, Oskarsson O, et al. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59(1):97–102. [DOI] [PubMed]; Valdimarsson H, Vikingsdottir T, Bang P, Saevarsdottir S, Gudjonsson J, Oskarsson O. et al. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59(1):97–102. doi: 10.1111/j.0300-9475.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- [108].Valdimarsson H. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Biochem Soc Trans. 2003;31(Pt 4):768–9. 10.1042/bst0310768. [DOI] [PubMed]; Valdimarsson H. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Pt 4. Vol. 31. Biochem Soc Trans; 2003. pp. 768–9. [DOI] [PubMed] [Google Scholar]
- [109].Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Høiby N, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104(4):431–7. [DOI] [PMC free article] [PubMed]; Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Høiby N. et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104(4):431–7. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Garred P, Pressler T, Lanng S, Madsen HO, Moser C, Laursen I, et al. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr Pulmonol. 2002;33(3):201–7. [DOI] [PubMed]; Garred P, Pressler T, Lanng S, Madsen HO, Moser C, Laursen I. et al. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr Pulmonol. 2002;33(3):201–7. doi: 10.1002/ppul.10064. [DOI] [PubMed] [Google Scholar]
- [111].Bang P, Laursen I, Thornberg K, Schierbeck J, Nielsen B, Valdimarsson H, et al. The pharmacokinetic profile of plasma-derived mannan-binding lectin in healthy adult volunteers and patients with Staphylococcus aureus septicaemia. Scand J Infect Dis. 2008;40(1):44–8. [DOI] [PubMed]; Bang P, Laursen I, Thornberg K, Schierbeck J, Nielsen B, Valdimarsson H. et al. The pharmacokinetic profile of plasma-derived mannan-binding lectin in healthy adult volunteers and patients with Staphylococcus aureus septicaemia. Scand J Infect Dis. 2008;40(1):44–8. doi: 10.1080/00365540701522959. [DOI] [PubMed] [Google Scholar]
- [112].Verdu P, Barreiro LB, Patin E, Gessain A, Cassar O, Kidd JR, et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum Mol Genet. 2006;15(17):2650–8. [DOI] [PubMed]; Verdu P, Barreiro LB, Patin E, Gessain A, Cassar O, Kidd JR. et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum Mol Genet. 2006;15(17):2650–8. doi: 10.1093/hmg/ddl193. [DOI] [PubMed] [Google Scholar]
- [113].Miller A, Phillips A, Gor J, Wallis R, Perkins SJ. Near-planar solution structures of mannose-binding lectin oligomers provide insight on activation of lectin pathway of complement. J Biol Chem. 2012;287(6):3930–45. [DOI] [PMC free article] [PubMed]; Miller A, Phillips A, Gor J, Wallis R, Perkins SJ. Near-planar solution structures of mannose-binding lectin oligomers provide insight on activation of lectin pathway of complement. J Biol Chem. 2012;287(6):3930–45. doi: 10.1074/jbc.M111.320341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kalia N, Sharma A, Kaur M, Kamboj SS, Singh J. A comprehensive in silico analysis of non-synonymous and regulatory SNPs of human MBL2 gene. Springerplus. 2016;5(1):811. [DOI] [PMC free article] [PubMed]; Kalia N, Sharma A, Kaur M, Kamboj SS, Singh J. A comprehensive in silico analysis of non-synonymous and regulatory SNPs of human MBL2 gene. Springerplus. 2016;5(1):811. doi: 10.1186/s40064-016-2543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]