Abstract
Abstract
Hydrogen sulfide (H2S) was initially recognized as a toxic gas and its biological functions in mammalian cells have been gradually discovered during the past decades. In the latest decade, numerous studies have revealed that H2S has versatile functions in plants as well. In this review, we summarize H2S-mediated sulfur metabolic pathways, as well as the progress in the recognition of its biological functions in plant growth and development, particularly its physiological functions in biotic and abiotic stress responses. Besides direct chemical reactions, nitric oxide (NO) and hydrogen peroxide (H2O2) have complex relationships with H2S in plant signaling, both of which mediate protein post-translational modification (PTM) to attack the cysteine residues. We also discuss recent progress in the research on the three types of PTMs and their biological functions in plants. Finally, we propose the relevant issues that need to be addressed in the future research.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s42994-021-00035-4.
Keywords: Hydrogen sulfide, Nitric oxide, Reactive oxygen species, Sulfur metabolism, Biotic and abiotic stresses, Growth and development, Persulfidation, S-Sulfenylation, S-Nitrosylation
Introduction
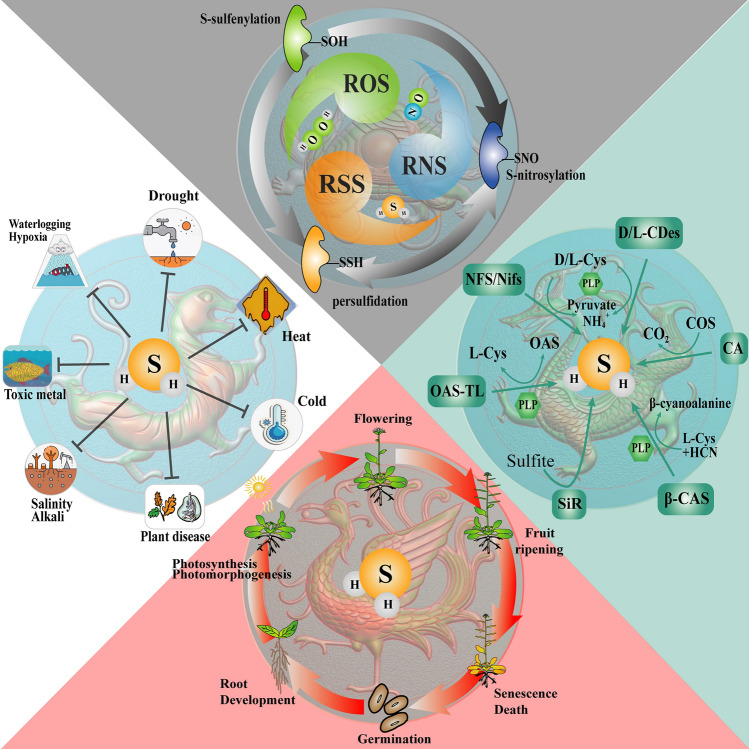
For hundreds of years since its discovery, hydrogen sulfide (H2S) has been regarded as a gas with an unpleasant odor and high toxicity (Fu et al. 2018b; Lefer 2019). In the early research, much attention was paid to the risk of excessive H2S exposure for animals, plants and microorganisms. Until recent decades, H2S was gradually found to act as a signal molecule involved in the regulation of biological and physiological processes. Particularly, with increasing knowledge about the role of nitric oxide (NO) and carbon monoxide (CO) as signaling molecules in mammalian and plant physiology research (Burnett et al. 1992; Snyder 1992; Wu and Wang 2005), the unique identity of H2S as a new gasotransmitter has been gradually revealed (Wang 2002) (Fig. S1). In mammals, endogenous H2S controls a variety of physiological processes and participates in the regulation of the pathogenesis of various diseases, including hypertension, atherosclerosis, angiogenesis and myocardial infarcts (Wang 2012; Wen et al. 2018). There has been increasing evidence showing the signaling role of H2S in plants. Different from the role as a phytotoxin at high concentrations, H2S at low concentrations has been shown to play critical roles in diverse processes of plant life cycle, such as plant growth, development, and biotic and abiotic stress responses (Chen et al. 2011; Fu et al. 2018b; Jin et al. 2013; Luo et al. 2020) (Fig. S2).
Endogenous production of H2S in plants
Understanding the production of endogenous H2S is a critical prerequisite for clarifying the role of H2S in various biological and physiological processes. To analyze the enzymes related to the production of H2S in plants, it is necessary to review the corresponding enzymes in animals first. In mammals, H2S biogenesis is catalyzed by the enzymes in the trans-sulfuration pathway, namely cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), and occurs in the cytoplasm (Kabil and Banerjee 2014). CBS catalyzes the β-substitution of serine with homocysteine to form cystathionine and H2O, which is kinetically the most efficient H2S-producing reaction. When cysteine replaces serine as the substrate, the reaction products will be cysteine and H2S. In addition, CBS also catalyzes cysteine to generate H2S through extra β-substitution reactions (Chiku et al. 2009; Kabil and Banerjee 2014; Kabil et al. 2011; Singh et al. 2009). CSE, a homotetrameric enzyme, can decompose cystathionine to form cysteine, ammonia and α-ketobutyrate. Due to the inclusiveness of its substrate-binding domain, CSE can directly combine and catalyze homocysteine and cysteine to produce H2S (Kabil and Banerjee 2014; Singh et al. 2009). Another enzyme, 3-mercaptopyruvate sulfurtransferase (3-MST), also contributes to the production of endogenous H2S from 3-mercaptopyruvate. Aspartate aminotransferase (AAT)/cysteine aminotransferase catalyzes the transamination reaction between cysteine and α-ketoglutarate (Kimura et al. 2013). Subsequently, 3-MST transfers the sulfur to a nucleophilic cysteine in the active site to yield persulfide and pyruvate (Shibuya et al. 2009). Among the three aforementioned H2S-producing enzymes which have been validated in mammals, CBS and 3-MST have homologs in plants, indicating that they may play potential roles in the production of H2S in plants. However, there is still a lack of solid evidence for the existence of CSE homologous genes in plants.
d/l-Cysteine desulfhydrase (d/l-CDes)
The exploration of H2S-producing enzymes in plants can be traced back to the mid-1960s. Tishel and Mazelis (1966) identified the activity of d/l-cysteine lyase in cabbage leaves, and H2S together with pyruvate and ammonia was found in the homogenate. It was not until 1980 that Harrington and Smith validated the existence of l-cysteine desulfhydrase [l-CDes; EC 4.4.1.28] in tobacco cells by the S35-labeled l-cysteine isotope method (Harrington and Smith 1980). After decades of exploration, d-cysteine desulfhydrase [d-CDes; EC 4.4.1.15] was successfully identified in Arabidopsis (Arabidopsis thaliana) (Papenbrock et al. 2007; Riemenschneider et al. 2005). In Arabidopsis, four cysteine desulfhydrase (CDes) genes have been reported, including l-cysteine desulfhydrase (LCD, At3g62130), l-cysteine desulfhydrase 1 (DES1, At5g28030) (Álvarez et al. 2010), d-cysteine desulfhydrase 1 (DCD1, At1g48420) and d-cysteine desulfhydrase 2 (DCD2, At3g26115) (Hou et al. 2016; Riemenschneider et al. 2005). The common characteristic of these genes is that they all require the participation of coenzyme 5′⁃pyridoxal phosphate (PLP) for the degradation of cysteine to produce H2S, ammonia and pyruvate in a stoichiometric ratio of 1:1:1 (Papenbrock et al. 2007). The only difference lies in the chirality of the substrates: the substrate of LCD and DES1 is l-cysteine, while that of DCD1 is d-cysteine. The most special one is DCD2, which can degrade the two isomers of cysteine (Riemenschneider et al. 2005). The production of endogenous H2S via CDes has been confirmed in various physiological and developmental processes of plants (Kaya and Ashraf 2020; Shen et al. 2013), especially DES1 and LCD, which use l-cysteine as the substrate, are the most widespread in plants. The important functions of these two genes will be discussed in detail later. With the progress in research, more CDes homologues have been cloned in different species, such as OsDCD1 and OsLCD2 in rice (Shen et al. 2019; Zhou et al. 2020), and BnDES1 in Brassica (Brassica napus) (Xie et al. 2013).
In addition, nitrogenase Fe–S cluster (NFS/Nifs) is also a putative H2S-producing enzyme with l-cysteine desulfhydrase-like activity (Pilon-Smits et al. 2002). AtNFS1 (At5g65720) and AtNFS2 (At1g08490) play an important role in the formation of Fe–S clusters, and can produce l-alanine and elemental S with the participation of PLP in Arabidopsis (Leon et al. 2002). H2S can be produced with the availability of an appropriate amount of reducing agent to provide electrons (Jez and Dey 2013; Leon et al. 2002; Pilon-Smits et al. 2002).
O-Acetylserine(thiol)lyase (OAS-TL)
Another enzyme is O-acetylserine(thiol)lyase [OAS-TL; EC 2.5.1.47], which plays a major role in the last step of cysteine synthesis (Tai and Cook 2000). In the process of cysteine synthesis, serine acetyltransferase (SAT) catalyzes acetyl-CoA and serine to form O-acetylserine (OAS); then, OAS-TL catalyzes OAS and sulfide (i.e., H2S) to synthesize cysteine (Álvarez et al. 2010; Heeg et al. 2008; Ravina et al. 2002). The latter step also requires the participation of coenzyme PLP. In Arabidopsis, nine OAS-TL family genes have been identified, including DES1 mentioned above. The main catalysts for cysteine synthesis include OASA1 (At4g41880), OASB (At2g43750) and OASC (At3g03630), which are subcellularly localized in the cytoplasm, chloroplast and mitochondria (Álvarez et al. 2010; Wirtz and Hell 2006), respectively. Other family members have different or unidentified functions. In fact, there is a lack of in vivo experimental evidence for the ability of OAS-TL to generate H2S. Since OAS-TL could generate H2S in some in vitro experiments, some studies have concluded that the enzymatic reaction of OAS-TL is a reversible process that can generate endogenous H2S. In this regard, we speculate that OAS-TL has bidirectional catalytic activity, but its ability to produce endogenous H2S in the plants is almost completely suppressed. This also explains the emergence of DES1 in addition to OAS-TL in Arabidopsis from an evolutionary point of view, and why des1 and oasa1 mutations resulted in opposite phenotypes under toxic metal stress.
β-Cyanoalanine synthase (β-CAS)
Cyanide (CN−), another cytotoxic molecule found after NO, CO and H2S, inhibits the electron transport activity in the chloroplasts and mitochondria via binding to cytochrome oxidase (COX) or other metalloenzymes (Yamasaki et al. 2001). Even so, cyanide is still produced in plants and germs. In higher plants, to detoxify the cyanide emerging within the cells, β-cyanoalanine synthase [β-CAS; EC 4.4.1.9] catalyzes the reaction between l-cysteine and HCN to synthesize β-cyanoalanine and H2S (Yamasaki et al. 2019), which also requires the participation of PLP. As mentioned above, 3-MST in mammals also catalyzes a similar reaction with 3-mercaptopyruvate as the sulfur donor. In Arabidopsis, β-CAS genes, including CYSC1, CYSD1 and CYSD2, are also members of the OAS-TL gene family, but with different active domains (Yamaguchi et al. 2000). The mitochondrial CYSC1 is involved in root hair formation (García et al. 2010) at the early stage of this pathway (Arenas-Alfonseca et al. 2018a, b). Besides, CYSC1 is also considered to be responsive to water deficiency and pathogen infection in Arabidopsis (García et al. 2013; Machingura et al. 2013), which is consistent with the H2S response model introduced later.
Carbonic anhydrase (CA)
Unlike that of the three enzymes mentioned above, the relationship of carbonic anhydrase [CA; EC 4.2.1.1] with H2S seems not to have been fully revealed. However, there have been some earlier reports about the catalysis of CA on carbonyl sulfide (COS), the most abundant sulfur gas in the atmosphere (Watts 2000), to produce carbon dioxide (CO2) and H2S via hydrolytic reaction (Notni et al. 2007). Many green plants can absorb COS in the air through the stomata, and then assimilate and store the required sulfide through CA catalytic reactions (Bloem et al. 2012). When the absorption of sulfate in plant roots is blocked, the above reaction would occur as a compensation (Bloem et al. 2011; Yamasaki and Cohen 2016). Interestingly, COS increases stomatal conductance, but the response was disrupted in CA-deficient antisense lines (Stimler et al. 2012). Through a test of the stomatal responses against COS in 22 plant species, Stimler et al. (2012) proposed that CA is a plausible H2S-producing enzyme in plants. However, there is still a lack of more intuitive evidence on the mechanism of COS uptake and endogenous H2S production in plants.
H2S plays a role in plant sulfur metabolism
As a signal molecule in organisms, H2S is involved in various physiological activities with different signal pathways. Moreover, it is an endogenous sulfide and a key node of sulfur metabolism in organisms. Besides, sulfur is essential for all living organisms on Earth as a key component of amino acids (i.e., cysteine and methionine), polypeptide glutathione (GSH), several group transfer coenzymes and vitamins (Romero et al. 2014). Mammals ingest S-amino acid methionine through the diet, while inorganic sulfur is reduced to cysteine through the assimilation pathway of reducing sulfate in plants.
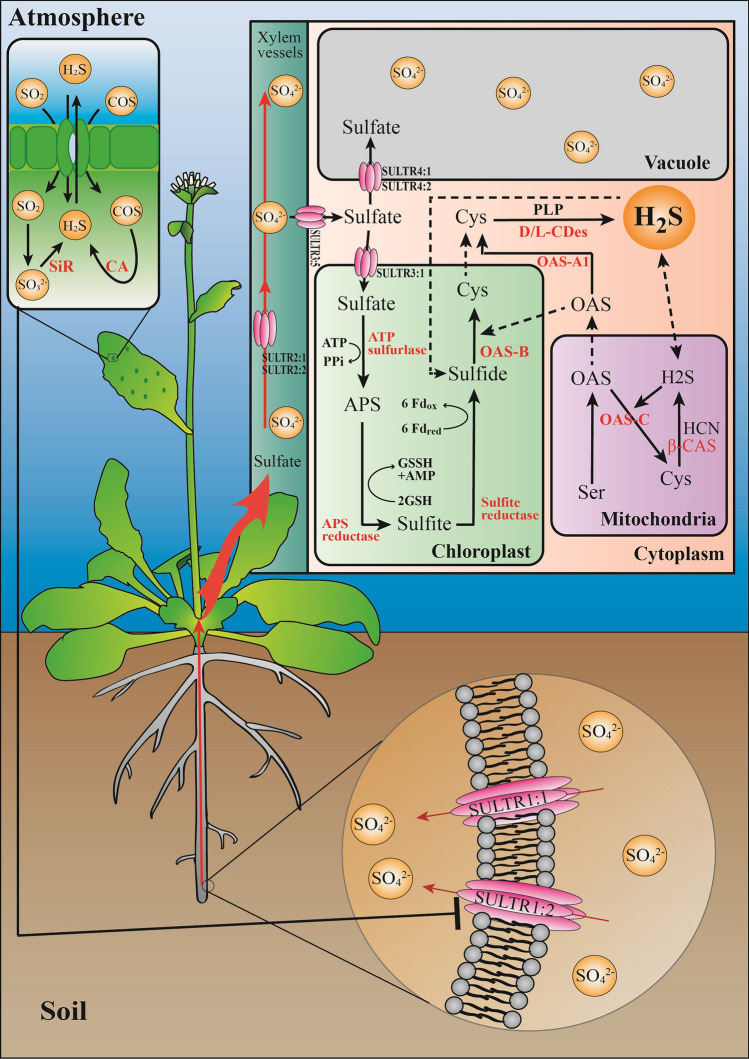
For plants, there are two routes for the absorption of S elements, namely root uptake and gas exchange through stomata (Notni et al. 2007), with the former as the main route (Fig. 1). In agriculture, sulfur is widely applied in the form of sulfate fertilizers (Fuentes-Lara et al. 2019), and transported by a proton/sulfate co-transport mode mediated by sulfate transporters (SULTRs) in root epidermal cells (Buchner et al. 2004). Subsequently, the sulfate is loaded into the xylem vessels and distributed into the entire plant (Leustek et al. 2000), which is stored into vacuoles via SULTR4:1 (Takahashi et al. 2011) or transported to chloroplasts via SULTR3:1 to launch the assimilatory activities (Gotor et al. 2015). After entry into the chloroplasts, sulfate is activated to adenosine 5′-phosphosulfate (APS) under the catalysis by ATP sulfurylase. As an intermediate, APS is further reduced to sulfite via the APS reductase (APR) with GSH as the reducing molecule (Birke et al. 2015). Subsequently, through a six-electron reaction with reduced ferredoxin as a reductant, sulfite is reduced to sulfide under the catalysis by sulfite reductase (SiR) (Fu et al. 2018b). These sulfides are S donors required for the synthesis of cysteine. Since H2S belongs to sulfides, SiR is considered as a major enzyme of H2S production in plastids (Filipovic et al. 2018). Besides sulfur assimilation, cysteine can be degraded to generate H2S. The second route for plants to obtain S is from the atmosphere, and COS is one of the S-containing gases captured by plants through the stomata (Fig. 1). In addition, many other sulfur-containing gases, such as H2S, SO2 and SO3, also sneak into plants in this way. Among them, COS and SO2 can promote the generation of endogenous H2S in plants through different metabolic pathways (Baillie et al. 2016; Notni et al. 2007). It is worth noting that SO2 can also induce stomatal closure like H2S, but in a much less efficient way (Baillie et al. 2016).
Fig. 1.
H2S acts as a node in plant sulfur metabolism. The transport of sulfate from roots is the main way for plants to absorb S elements, which are then transported to all parts of the plant through the xylem vessels. Part of the sulfate entering the cells will be stored in vacuoles, and the other part will enter the assimilation pathway in the chloroplast. After being activated to APS, sulfate is further reduced to sulfite via APS reductase with GSH as the reducing molecule. Then, through a six-electron reaction with reduced ferredoxin, sulfite is reduced to sulfide under the catalysis by SiR. The produced sulfide is a substrate for the synthesis of cysteine. Together with OAS, cysteine is synthesized under the catalysis of OAS-TL enzyme. Cysteine can be degraded to generate H2S by CDes. Another mode for obtaining S elements is from the atmosphere. H2S, COS and SO2 are captured by plants through the stomata. COS can be hydrolyzed to produce H2S under the action of CA, while SO2 can be hydrolyzed into sulfite and enter the assimilation pathway. The two absorption pathways interact and restrain each other
The synthesis of cysteine is closely associated with the function of OAS-TL. In Arabidopsis, OASA1 plays a major catalytic role; OASB and OASC play a redundant role; and OASC mainly maintains the dynamic balance of OAS in mitochondria, which actively catalyzes cysteine synthesis only in the deficiency of both OASA1 and OASB (Heeg et al. 2008). DES1 appears to belong to L-CDes and catalyzes the decomposition of l-cysteine to H2S. Exogenous application of H2S to Arabidopsis would enhance the activity of OAS-TL and the production of cysteine (Khan et al. 2018). The exogenous cysteine also directly promotes the production of endogenous H2S, not only through the decomposition of l-CDes, but also through the increased synthesis of abscisic acid (ABA), resulting in enhanced expression and activity of DES1 (Batool et al. 2018). Under some abiotic stress conditions such as cadmium (Cd) stress, the oasa1 mutant showed significant sensitivity (Lopez-Martin et al. 2008). On the contrary, the des1 mutant showed significantly enhanced tolerance to Cd (Álvarez et al. 2010). This may be related to the different effects of OASA1 and DES1 on intracellular cysteine homeostasis. Evidently, the total intracellular cysteine content was reduced by approximately 35% in the oasa1 mutant and increased by approximately 25% in the des1 mutant relative to the wild type (WT) (Lopez-Martin et al. 2008; Romero et al. 2014). Considering that OAS-TL in Arabidopsis root cells can interact with SULTR2;1 and inhibit its sulfate transporting activity (Shibagaki and Grossman 2010), the inhibition of SULTR expression by the supply of cysteine to plant roots or fumigation with H2S and SO2 could be ascribed to the enhancement of OAS-TL enzyme activity by endogenous H2S (Herschbach et al. 1995; Vauclare et al. 2002). In areas with high levels of atmospheric H2S, the capacity of root sulfate transporters of wild plants and crops is usually weakened, and vice versa.
H2S positively responds to biotic and abiotic stresses in plants
As a small gaseous signaling molecule, H2S readily traverses the intracellular and intercellular domains, and plays a key role in regulating the homeostasis in plant cells (Papanatsiou et al. 2015). H2S has been identified as a brilliant defender against different stresses such as drought, heat, chilling, heavy metals, osmotic and saline (Pandey and Gautam 2020) (Fig. 2). In addition, a growing body of research has revealed the crosstalk between H2S and various signaling pathways, indicating its key role in the protection of plants against stresses (Banerjee et al. 2018). With increasing knowledge about the action and regulation associated with H2S, it becomes possible to generalize the protective role of H2S in plant stress responses.
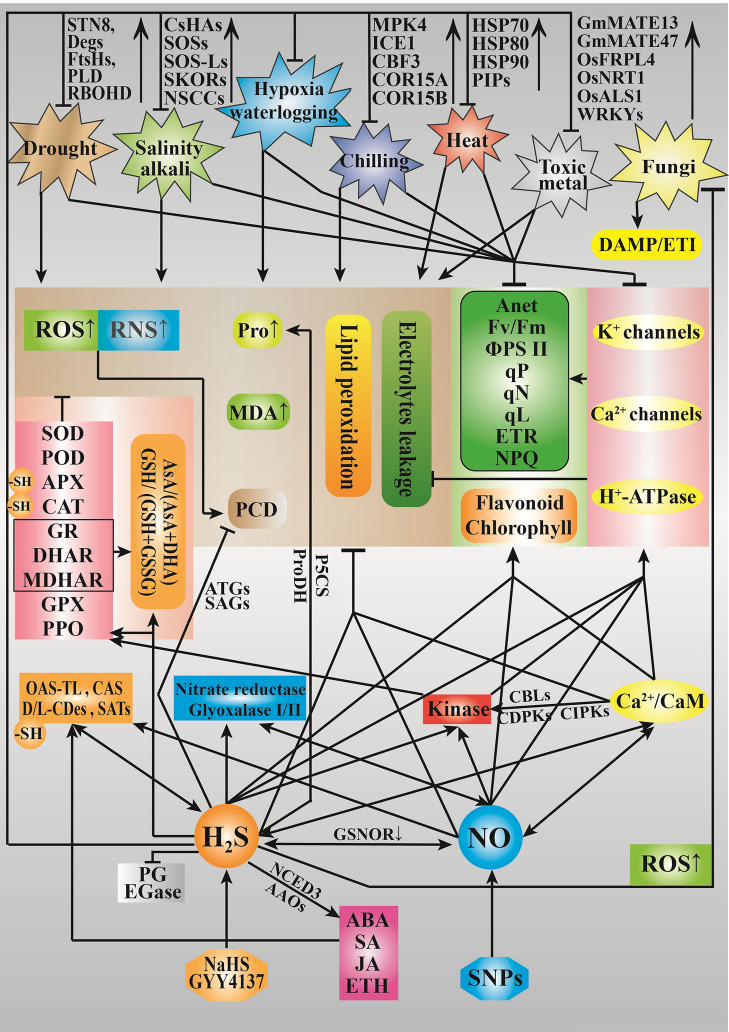
Fig. 2.
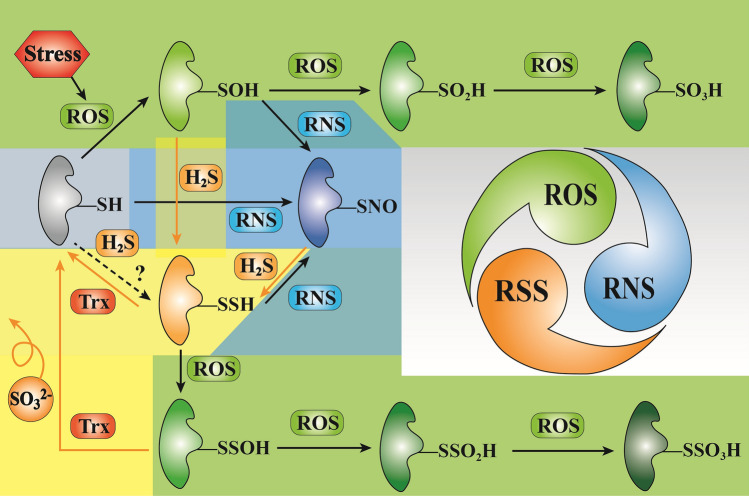
H2S positively responds to biotic and abiotic stresses in plants. The brown shadow is the items related to oxidative damage; yellow shadow is the antioxidant system; green shadow is the photosynthetic system and pigments; and pink shadow is transporters. AAOs, ABA-aldehyde oxidase; ALS, aluminum sensitive; APX, ascorbate peroxidase; ATGs, autophagy proteins; CAS, cyanoalanine synthase; CaM, calmodulin; CAT, catalase; CBF, C-repeat-binding factors; CBL, calcineurin B-like proteins; CDPK, Ca-dependent protein kinase; CIPK, CBL-interacting protein; COR15, cold responsive 15; Deg, D1 protein degradation-related genes; DHAR, dehydroascorbate reductase; d/l-CDes, d/l-cysteine desulfhydrase; EL, electrolyte leakage; EGase, endo-β-1,4-glucanase; ETR, electron transfer rate; Fv/Fm, potential photochemical efficiency; GSNOR, S-nitrosoglutathione reductase; GR, glutathione reductase; HA, proton pump; Hsp, heat shock protein; H2S, hydrogen sulfide; ICE, inducer of CBF expression; MDA, malondialdehyde; MDHAR, monodehydroascorbate reductase; NCED, 9-cis-epoxy-carotenoid dioxygenase; NO, nitric oxide; NPQ, non-photochemical quenching; NRT, nitrate transporter; OAS-TL, O-acetylserine (thiol)lyase; PCD, programmed cell death; PDH: proline dehydrogenase; PG, polygalacturonase; PLD, phospholipase D isoforms; PIPs, aquaporins; POD, peroxidase; PPO, polyphenol oxidase; Pro, proline; P5CS, proline synthase; qN, non-photochemical quenching; qP, photochemical quenching; ROS, reactive oxygen species; RNS, reactive nitrogen species; SATs, serine acetyltransferase; SAGs, senescence-associated genes; SOD: superoxide dismutase; SOS, salt overly sensitive; STN8, D1 protein phosphatase; ΦPS II, actual photochemical efficiency; –SH, persulfidation. Arrowheads indicate positive regulatory interaction and flat arrow heads indicate negative regulation
Classic model for the alleviation of abiotic stress by H2S in plants
H2S can help the plants to resist a variety of abiotic stresses such as drought, cold, heat, salinity, hypoxia and toxic metal to effectively alleviate their damages (Pandey and Gautam 2020; Zhang et al. 2021), which is closely associated with the classic “rescue” mode of H2S. Continuous exposure to any abiotic stress will cause an imbalance of endogenous redox homeostasis. Excessive accumulations of reactive oxygen species (ROS), hydrogen peroxide (H2O2) and superoxide anion (O2−•), will further lead to lipid peroxidation, protein oxidation and damage to plant cells, resulting in autophagy and programmed cell death (PCD) (Da-Silva and Modolo 2018; Hancock 2017). Many studies have demonstrated that exogenous H2S treatment can alleviate oxidative stress by increasing the expression and activities of some enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), polyphenol oxidase (PPO), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and guaiacol peroxidase (GPX) (Aghdam et al. 2018; Christou et al. 2014; Fu et al. 2013; Guo et al. 2018; Khan et al. 2018; Li et al. 2015b, 2019; Luo et al. 2015; Ma et al. 2016; Shan et al. 2018; Shen et al. 2013; Wei et al. 2019; Yang et al. 2016; Ye et al. 2020) (Fig. 2). Recently, H2S was found to enhance the activity of antioxidant enzymes in plants (Amooaghaie et al. 2017; Dawood et al. 2012; Kaya et al. 2018; Li et al. 2012a, 2020b; Sun et al. 2013; Zhang et al. 2015b), which is considered to be related to H2S-mediated post-translational modification (PTM). We will discuss this in detail later.
Moreover, H2S can maintain the redox balance and prevent further apoptosis by dynamic regulation of the NADPH oxidase and antioxidant enzyme systems (Kolupaev et al. 2017; Yang et al. 2016). H2S facilitates the production of more H2O2 by NADPH oxidase through the enhancement of transcription and enzyme activity, and controls antioxidant enzymes to reduce ROS content in a similar way (Christou et al. 2014; Li et al. 2015b; Yang et al. 2016; Ye et al. 2020). Such functional difference seems to be related to the ratio between the ROS and H2S levels. When the accumulation of ROS causes oxidative stress, the increased H2S will reduce the ROS level through enzymatic and non-enzymatic pathways. However, when H2S acts as a driving signal to regulate stomatal movement, RBOHs will be induced to increase endogenous ROS, thus, initiating the downstream signal. Studies of mammals have shown that H2S increases GSH by enhancing the activity of γ-glutamylcysteine (γ-CE) synthetase and cystine transport (Kimura and Kimura 2004). A latest study of plants also demonstrated that H2S increases GSH, the reduced/oxidized GSH (GSH/GSSG) ratio, and the expression of GSH-associated genes (GST Tau, MAAI, APX, GR, GS and MDHAR) under chilling stress (Liu et al. 2020c). In addition, both H2S and H2O2 were also discovered to participate in the up-regulation of ascorbic acid (AsA)-GSH cycle in plant tissues, which acts as the downstream signal for the regulation of H2S on ROS (Mostofa et al. 2015; Shan et al. 2018). Besides, the increases in malondialdehyde (MDA), electrolyte leakage (EL) and proline (Pro) caused by abiotic stress are also important indicators to reflect the oxidative damage of plants. Exogenous spraying or fumigating with H2S on plant seedlings could also significantly inhibit the increase in the activity of MDA, EL and Pro (Da-Silva and Modolo 2018; Kaya and Ashraf 2020; Shan et al. 2018).
The activation of H2S-related enzymes is also a main way for exogenous H2S to alleviate the effect of different stresses. Exogenous application of NaHS can not only increase the activities of d/l-CDes, OAS-TL, CAS and CA enzymes, but also elevate the content of endogenous cysteine and H2S (Khan et al. 2018; Li et al. 2019), which further amplify the physiological effect of H2S. For instance, d/l-CDes, OAS-TL and CAS can be induced by H2S under salt-alkali stress (Jiang et al. 2019; Kaya and Ashraf 2020; Li et al. 2020c). Low temperature stimulation can activate d/l-CDes and increase the content of endogenous H2S (Aghdam et al. 2018; Fu et al. 2013). In addition to amplifying the signal of H2S, the significant increase in endogenous H2S content will further regulate the dynamic S metabolism in plants, thus promoting the production of sulfur derivatives (i.e., cysteine and GSH) and sulfur-containing proteins. H2S can reduce the harm of heat stress by increasing the synthesis of total sulfhydryl compounds, proteins and cysteine in tobacco (Nicotiana tabacum) (Chen et al. 2019; Li and Jin 2016; Li et al. 2015b).
Under drought, salinity/alkali and heat stresses, the contents of chlorophyll and carotenoids in the leaves would decrease dramatically (Christou et al. 2013; Zhang et al. 2010b), as demonstrated by the decrease in potential photochemical efficiency (Fv/Fm), actual photochemical efficiency (ΦPSII), photochemical quenching (qP), electron transfer rate (ETR), and increase in non-photochemical quenching (qN). Intriguingly, these effects could be alleviated by NaHS (Li et al. 2015a). In addition, the enhancement of photosynthetic pigment, photosynthetic quantum yield, gas exchange parameters, SPAD value, and net photosynthetic rate (Pn) could also demonstrate the repair of photosynthesis by H2S under toxic metal stress (Ahmad et al. 2020; Amooaghaie et al. 2017; Bharwana et al. 2014; Dawood et al. 2012; Fu et al. 2019; Kaya et al. 2018, 2020b; Kaya and Aslan 2020; Kushwaha and Singh 2020; Singh et al. 2015; Wang et al. 2020). For example, the expression of the D1 protein, a sensitive target of PSII damage, increased under drought stress. However, upon exposure to NaHS, less D1 protein and phosphorylated D1 protein were detected, which was putatively ascribed to the extra expression of STN8 (catalyze D1 protein phosphorylation) and the genes related to D1 protein degradation, including Deg1, Deg5, Deg8, FtsH2, and FtsH5. These results suggest that H2S alleviates drought-induced PSII damage owing to the fast turnover of D1 protein rather than its high content (Li et al. 2015a). Besides, higher light-saturated CO2 assimilation rate (Asat), net photosynthetic rate (Anet), Fv/Fm and ФPSII as well as the mRNA levels and activities of the key photosynthetic enzymes (Rubisco, TK, SBPase and FBA) were observed in H2S-induced frost tolerance in cucumber (Liu et al. 2020c). In addition to the role in balancing redox homeostasis, H2S may also promote the stability of chloroplast structure and photosynthesis, which will be discussed in the next section.
H2S participates in drought stress response
For many plants in water shortage areas, multiple drought-tolerance mechanisms are essential, and H2S has been identified as a new key factor in plant response to drought. In the early research, botanists found that the spraying of appropriate concentrations of NaHS could effectively improve the resistance of various plants to drought (García-Mata and Lamattina 2010; Zhang et al. 2010b). Such effects were observed in soybean (Glycine max L.), Vicia faba, wheat and Arabidopsis (Jin et al. 2011; Zhang et al. 2010b). Due to this broad-spectrum and beneficial physiological effect, later studies were mainly focused on two aspects: extensive exploration of the endogenous redox balance, ion homeostasis and H2S-producing enzymes of plants, and investigation of the regulatory effect of H2S on stomatal movement.
Water loss is the most intuitive effect of drought on plants. Water in plants evaporates into the air through stomata on the epidermis via transpiration. Therefore, the dynamic regulation of stomata is a significant index of plant water conservation. García-Mata and Lamattina (2010) first revealed the function of stomatal closure induced by H2S in Vicia faba, Arabidopsis and Impatiens walleriana, and connected H2S with ABA by treatment with H2S scavenger (HT). With the suppression of endogenous H2S, stomata would become less sensitive to ABA in WT plants. However, Lisjak et al. (2010, 2011) reported that H2S donor, NaHS and/or GYY4137, could promote stomatal opening of plants, which was ascribed to the reduction of NO accumulation in guard cells caused by H2S. Similar work has been repeated in Capsicum annuum. This contradiction has raised some discussions (Desikan 2010). However, subsequent studies seemed to support the conclusion that stomatal closure is promoted by H2S (Liu et al. 2011; Pandey 2014; Scuffi et al. 2016). Drought-induced hormone (i.e., ABA, salicylic acid (SA), jasmonic acid (JA) and ethylene) and ROS signals vary among plants, which promote the accumulation of H2S in guard cells and initiate the signals downstream of H2S to induce stomatal closure (Deng et al. 2020; García-Mata and Lamattina 2013; Jin et al. 2013; Liu et al. 2011; Scuffi et al. 2014). After that, the endogenous H2S begins to exert its own functions, inducing the movement of guard cells indirectly by affecting the second messenger signals such as NO, H2O2, eATP, Ca2+, phosphatidic acid (PA), carbohydrate, microfilament and microtubules, thus, promoting stomatal closure (Pantaleno et al. 2020). H2S increases the content of NO in guard cells by activating NO-producing enzymes (García-Mata and Lamattina 2013), and the same effect was observed for H2O2. Recent experiments revealed that H2S can increase the content of endogenous H2O2 in guard cells by promoting the production and enzyme activity of NADPH oxidase isoforms and phospholipase D isoforms (Scuffi et al. 2018), which is the most critical step to enhance the persulfidation level of NADPH oxidase isoforms, respiratory burst oxidase homolog D (RBOHD), and activate H2O2 synthesis (Shen et al. 2020). This process is related to the dynamic regulation of ROS by H2S through its own chemical characteristic, and the regulation of a variety of secondary signal initiation enzyme systems by triggering S-persulfidation modification. H2S can persulfidate DES1 and enhance its ability to produce H2S, and then further persulfidates Open Stomata 1 (OST1)/SNF1-Related Protein Kinase2.6 (SnRK2.6) to accelerate the stomatal closure (Chen et al. 2020). Finally, on the one hand, H2S directly or indirectly regulates the ion channels on the guard cell membrane, thus, changing the osmotic potential and turgor pressure of guard cells and resulting in stomatal closure. Using a non-invasive micro-test technique (NMT), it was found that endogenous H2S induces a transmembrane K+ efflux and Ca2+ and Cl− influxes in guard cells, while not affects the flow of H+ (Jin et al. 2017). Detection with two-electrode voltage clamp (TEVC) showed that H2S selectively inhibits inward-rectifying K+ channels of tobacco (Nicotiana tabacum) guard cells (Papanatsiou et al. 2015). In addition, H2S also activates the S-type anion channel (SLAC1) in Arabidopsis guard cells with OST1 and cytosolic free Ca2+ (Wang et al. 2016). On the other hand, it can alter the morphology of guard cells by affecting the stability of cell membrane, cytoplasm and cell wall. H2S inhibits the activities of polygalacturonase (PG) and endo-β-1,4-glucanase (EGase), thus, helping to maintain the integrity of cell wall in Fragaria × ananassa and Actinidia deliciosa (Gao et al. 2013; Zhang et al. 2014). H2S also regulates the stability of microtubules by sulfhydryl actin and tubulin (Li et al. 2018a). Stable cell wall and cytoskeleton structure are essential for the movement of guard cells.
The advancement in omics studies provides a broader horizon of research. A total of 7552 transcripts have been investigated by transcriptome analysis. GO categories of ‘transport’ were enriched under the ‘H2S + drought’ treatment, especially the ion transport categories. The KEGG pathways of ‘ribosome biogenesis in eukaryotes’, ‘protein processing in endoplasmic reticulum’, ‘fatty acid degradation’, and ‘cyanoamino acid metabolism’ were also induced by H2S under drought stress (Li et al. 2017). In general, these results suggest that H2S alleviates drought damage, which is probably related to transport systems, phytohormones signal transduction, protein-processing pathways, and metabolism of fatty acids and amino acids (Li et al. 2017). Similarly, using the isobaric tags for relative and absolute quantitation (iTRAQ) technique, 120 proteins were identified to be significantly regulated by NaHS under drought stress. Functional annotation revealed that nearly all 120 proteins are related to signal transduction, protein synthesis, carbohydrate metabolism, photosynthesis, stress, and secondary metabolism (Ding et al. 2018). Systematic analysis with different omics provides important guidance for future studies to dissect the mechanism for H2S-dependent drought tolerance in plants.
H2S boosts plant resistance to high salinity/alkali
High salinity and alkali conditions lead to osmotic stress and cell toxicity due to excess ions and ultimately nutrition disorders and oxidative stress in plants (Munns and Tester 2008), which will cause considerable yield losses. Recently, H2S was recognized to play a key role in cell signaling during plant response to high salinity and alkali, even nitrate (Christou et al. 2013; Guo et al. 2018; Lai et al. 2014). H2S can ameliorate salt-alkali stress-induced adverse effects (Jiang et al. 2019), which is partially similar to the case of other stresses, and the most distinctive feature is the reestablishment of redox balance (Lai et al. 2014). Besides, the coordination of NO signal is indispensable for the preservation of a stable redox state by H2S (Da-Silva et al. 2018; Janicka et al. 2018). H2S not only increases endogenous NO and total S-nitrosothiols (SNOs) content in plants under salt-alkali stress (Christou et al. 2013; Ziogas et al. 2015), but also enhances the activity of nitrate reductase (NR) and glyoxalase I and II and decreases that of the S-nitrosoglutathione reductase (GSNOR) (Guo et al. 2018; Janicka et al. 2018; Mostofa et al. 2015). NO treatment can also elevate the content of H2S and the activity of H2S-producing enzymes. Similarly, exogenous NaHS and SNP can activate the enzyme activities for the rapid endogenous production of themselves (Ziogas et al. 2015).
Microarray analysis using GeneChip and proteomics analysis showed that nine functional categories consisting of thousands of genes had specific changes in salt-stressed seedlings after NaHS treatment, including metabolism, signal transduction, immune response, transcription factor, protein synthesis and degradation, transporter, cell wall decomposition and polymerization, hormone response, cell death, energy and unknown proteins (Guo et al. 2018; Li et al. 2014a, 2020c). Among them, the change in ion transporters is the most widely concerned. Another adverse effect of salt-alkali stress on plants is the breaking of ion balance, which is as serious as the oxidative burst. Salt stress can lead to the influx of a large amount of Na+ into plant cells, which will directly destroy the membrane potential homeostasis on both sides of the cell membrane, and promote the outflow of intracellular K+ (Zhang and Tielborger 2019; Zhu 2002). As observed in many plants, such as rice, wheat, strawberry, tomato, Medicago sativa, Arabidopsis, Spartina alterniflora, Malus hupehensis, Populus euphratica and Populus popularis, H2S application can reduce the accumulation of intracellular Na+ and the Na+/K+ ratio, and inhibit the exosmosis of intracellular K+ (Ding et al. 2019; Guo et al. 2018; Lai et al. 2014; Li et al. 2020a, c; Mostofa et al. 2015; Wei et al. 2019; Zhao et al. 2018). On the one hand, H2S increases the activity of PM H+-ATPase under salt stress (Chen et al. 2015a; Jiang et al. 2019; Zhao et al. 2018), and induces the expression of several genes encoding the isoforms of the plasma membrane proton pump (CsHA2, CsH4, CsH8, CsH9 and CsHA10) (Janicka et al. 2018). On the other hand, salt overly sensitive (SOS) pathway is activated by the up-regulation of related genes (i.e., SOS1, SOS2, SOS3, SOS2-like, SOS3-like, and SOS4) (Christou et al. 2013; Ding et al. 2019; Li et al. 2020a), which can effectively expel excessive Na+ from the cells. Moreover, overexpression of SKORs and NSCCs and activation of mitogen-activated protein kinase (MPK) pathway also contribute to the rescue of plants by H2S from salt stress (Deng et al. 2016; Jiang et al. 2019; Lai et al. 2014; Li et al. 2020a), which also restrain K+ efflux in plant seedlings.
Systematic studies can help a better understanding of the downstream signal and mechanism for the alleviation effect of H2S on salt-alkali stress. H2S could promote photosynthetic electron transfer, chlorophyll biosynthesis and carbon fixation in Kandelia obovata leaves and cucumber under salt stress (Jiang et al. 2020; Liu et al. 2020d). In addition, the abundance of other proteins related to the metabolic pathways, such as antioxidation (APX, copper/zinc superoxide dismutase, pancreatic and duodenal homeobox 1), protein synthesis (heat-shock protein (HSP), chaperonin family protein 20 and Cysteine synthase 1), nitrogen metabolism (glutamine synthetase 1 and 2), glycolysis (phosphoglycerate kinase and triosephosphate isomerase), and the AsA-GSH cycle (glutathione S-transferase U25-like), was increased by H2S under high salinity (Jiang et al. 2020; Liu et al. 2020d). However, the mechanism underlying the effect of H2S on such huge proteins remains unclear (Guo et al. 2018; Li et al. 2014a, 2020c). Recent studies have revealed that H2S signal acts on the downstream of transcription factors VvWRKY30 and JIN1/MYC2 under salt-alkali stress (Yastreb et al. 2020; Zhu et al. 2019). Hence, it remains to be explored whether there are any other transcriptional regulations or cascade regulations with hormone interference in the future.
H2S helps to resist extreme temperature for plants
Extreme temperature is a severe limiting factor for the growth and productivity of plants (Iba 2002; Suzuki 2019; Wu and Wallner 1984). Different from animals, plants are lack of movability to evade from harmful circumstances. To cope with extreme circumstances, plants have evolved certain effective regulatory mechanisms. H2S is involved in the complex regulatory network of plants to resist extreme environmental temperature.
Damages caused by heat exposure include protein denaturation and aggregation, membrane damage owing to lipid peroxidation, enzyme inactivation, inhibition of protein synthesis, imbalance of redox hemostasis and secondary metabolic disorder (Carmody et al. 2016; Posch et al. 2019; Proveniers and van Zanten 2013). Meanwhile, plants can initiate self-help operations to alleviate the damage caused by high temperature. During this process, the contents of endogenous NO and H2S would be significantly increased, which is largely dependent on the increased expression and initiated activity of the relevant enzymes (Cheng et al. 2018; Li et al. 2013b; Ye et al. 2020). Interestingly, SNP-induced heat tolerance of maize was enhanced by the application of H2S donors (Li et al. 2013b). It seems that H2S acts on the downstream of NO-induced heat tolerance in maize seedlings. Besides, the addition of NaHS leads to dramatic increases in AsA, GSH, flavonoids and carotenoids in maize (Ye et al. 2020). H2S induces the accumulation of endogenous Pro, due to higher Delta (1)-pyrroline-5-carboxylate synthetase (P5CS) activity and lower proline dehydrogenase activity (Li et al. 2013a). H2S also activates trehalose-6-phosphate phosphatase (TPP) and betaine aldehyde dehydrogenase (BADH), and then induces the accumulation of endogenous trehalose and betaine under heat stress (Li et al. 2014d; Li and Zhu 2015). Furthermore, H2S pretreatment also induced the gene expression levels of an array of protective molecules, such as heat shock proteins (HSP70, HSP80, and HSP90) and aquaporins (PIP) (Christou et al. 2014).
Together with H2S, exogenous Ca2+ and CaM can effectively alleviate the damage to plants caused by high temperature stress partly via strengthening the l-CDes activity and H2S accumulation (Li et al. 2015c). The acquisition of H2S-induced heat tolerance requires the transport of extracellular Ca2+ to cytoplasm and the coordination of intracellular CaM (Li et al. 2012c). Methylglyoxal (MG), which is viewed as a toxic by-product of glycolysis and photosynthesis in plants and resembles H2S, participates in the response to abiotic stress. Similar to the scenario of Ca2+ and H2S, application of MG and/or NaHS enhanced the survival and tissue vigor of maize seedlings under heat stress (Li et al. 2018b; Ye et al. 2020). Obviously, there is an interaction between H2S and MG that initiates the thermotolerance in plants. Furthermore, some traditional signals in the regulation of heat-tolerance mechanism also have crosstalk with H2S signals, such as CO, ABA and SA (Li and Gu 2016; Li and Jin 2016; Li et al. 2015d), which can sequentially induce the activation of H2S-producing enzymes and accumulation of endogenous H2S under high-temperature stress.
Low temperature is another extreme temperature condition. Long-term frosty weather affects agricultural production and operations (Furtauer et al. 2019). H2S fumigation can significantly increase the activity of H+-ATPase, Ca2+-ATPase, cytochrome C oxidase (CCO) and succinate dehydrogenase (SDH) related to energy metabolism (Li et al. 2016a). NaHS treatment also increased the content of anthocyanins in wheat seedlings and cucurbitacin C (CuC) in cucumber (Kolupaev et al. 2019; Liu et al. 2019c). The latter may be due to the increase in the S-persulfidation level of bHLH transcription factors (His-Csa5G156220 and His-Csa5G157230) caused by H2S, as well as their binding activity to the promoter of the key synthetase Csa6G088690 for CuC fabrication (Liu et al. 2019c). H2S also amplifies the signal transmission induced by cold via regulating the transcription of genes, such as VvICE1 and VvCBF3 genes in Vitis vinifera (Fu et al. 2013). In Arabidopsis, H2S up-regulates MAPK expression levels, and both H2S and MPK4 regulate the expression levels of the cold responsive genes inducer of CBF expression 1 (ICE1), C-repeat-binding factors 3 (CBF3), cold responsive 15A (COR15A) and COR15B (Du et al. 2017). This result suggests that MPK4 is probably a downstream component of H2S-related cold-stress resistance, which links the H2S signal with the classical cold signal regulated by MAPKs.
H2S rescues plants from hypoxia and waterlogging
Flooding often results in hypoxic conditions around plant roots, which is a serious stress to crops. As hypoxia is the most important consequence of flooding stress, it will be discussed in this section as well. Submerging in water can cause stress to most terrestrial plants, which can result in low availability of light, CO2 and oxygen and hence pose challenges to the normal functions of the plant system (Pandey and Gautam 2020). Flooding increases the emissions of some trace gases such as N2O, N2, CH4 and H2S in the environment around the crops (Kogel-Knabner et al. 2010).
When confronted with the adverse effects caused by hypoxia, plants will carry out some rescue activities. One possibly way is to increase the endogenous H2S content by enhancing the activities of H2S-related enzymes (Cheng et al. 2013; Peng et al. 2016). Exogenous application of low H2S in Pisum sativum and peach can reverse ROS accumulation, cell deaths, electrolyte permeability, rapid synthesis of ethylene and significant reduction of root activity that induced by waterlogging stress (Cheng et al. 2013; Xiao et al. 2020). In addition, there is also a similar relationship between AsA-GSH cycle and H2S (Shan et al. 2020). The mode is similar to that under osmotic stress, which has been introduced in the above section. Hypoxia stress can lead to cell apoptosis, which is related to ethylene synthesis, and there is a parallel relationship between ethylene synthesis and excessive accumulation or apoptosis (Peng et al. 2016). Jia et al. (2018a) showed that H2S reduces ethylene production by inhibiting the activity of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidases (ACOs). H2S induces the persulfidation of LeACO1 and LeACO2 in a dose-dependent manner, thus, inhibiting the activity of LeACO1 and LeACO2 (Jia et al. 2018a). These results provide insights into the general action mode of H2S and contribute to a better understanding of a plant’s response to hypoxia and waterlogging stress.
H2S responses to toxic metal stress
Heavy metals, such as copper (Cu), mercury (Hg), lead (Pd), Cd, arsenic (As), chromium (Cr) and zinc (Zn) (Luo et al. 2020), will cause chronic poisoning when accumulated to a certain extent in organisms. Due to its similar toxicity, Al is also included, and these metals are collectively referred to as toxic metals. Here, we systematically review whether and how H2S alleviates toxic metal stress in plants (Table S1).
Toxic metal stress can increase the death of plant somatic cells as well as reduce survival rate, biomass and yield of crops (Ahmad et al. 2020; Fu et al. 2019; Kaya et al. 2018, 2020b; Kaya and Aslan 2020), which are attributed to the destruction of endogenous redox balance and excessive accumulation of ROS. Exogenous H2S alleviates the stress of toxic metals and improve the survival rate and biomass of plant seedlings, which is also due to the remodeling of the stable redox state by H2S in plants. Notably, this will also increase the species and quantity of microorganisms in the rhizosphere soil (Fang et al. 2019).
Some toxic metals, such as Cu, Co, Cr and Cu, are originally the micronutrients essential for plants, especially in the photosynthetic system, which are involved in the composition of pigments and coenzymes. However, excessive toxic metals will directly destroy the photosynthetic system and organelles in plant cells (Singh et al. 2015). In Brassica and barley, toxic metals could destroy the stability of chloroplast structure in mesophyll cells, making the chloroplasts spongy, increasing thylakoid solvents and starch, and leading to the breakage of other organelles in root, stem and leaf cells (Ali et al. 2013; Qian et al. 2014; Shi et al. 2014). After the application of H2S, increases in the number of mature mitochondria, long endoplasmic reticulum and Golgi bodies could be observed in plant cells (Ali et al. 2014; Qian et al. 2014).
Another mechanism for H2S to alleviate toxic metal stress is to enhance the fixation of toxic metal ions, which is closely associated with the function of cell wall, the regulation of transporters, as well as the cooperation of plant chelators and other signals. Cell wall, the unique structure of plant cells, can bind and fix Cd ions from the extracellular environment to alleviate its toxicity. Exogenous H2S can significantly increase the content of pectin and the activity of pectin methylesterase in Brassica roots, thereby increasing the retention of Cd in pectin fractions (Yu et al. 2019). However, when rice was subjected to Al stress, H2S pretreatment reduced the negative charge in cell walls by decreasing the activity of pectin methylesterase as well as the pectin and hemicellulose contents in roots (Zhu et al. 2018). Plant cells can also alleviate the toxicity by transporting toxic metal ions into vacuoles, which is dependent on the action of H+-ATPase and citrate transporters on the vacuole membrane. This effect will be amplified by the application of H2S, and enhancement of the expression and activity of tonoplast H+-ATPase could reduce cytoplasmic toxic metal ions, which has been reported in crops such as Populus euphratica (Cd2+/H+ antiporters), soybean (H+-ATPase) and barley (Na+/K+-ATPase and W-ATPase) (Chen et al. 2013; Dawood et al. 2012; Sun et al. 2013; Wang et al. 2019). Induction of soybean GmMATE13, GmMATE47 and rice OsFRPL4 by H2S could alleviate Cd and Al stress by increasing citrate exudation (Chen et al. 2013; Yu et al. 2019; Zhu et al. 2018). Under Al stress, rice OsNRT1 and OsALS1 were also induced by H2S, which reduced the content of Al in cytoplasm by transferring Al to vacuoles (Zhu et al. 2018). The most effective strategy for plants to cope with the threat of toxic metals is to temporarily “inactivate” the metal ions through GSH phytochelatins (PCs) and metallothionein (MTs), which is closely related to the sulfur metabolism pathway with H2S-cysteine as the core. Even without exogenous H2S, toxic metal stress can induce the activity of CDes, OAS-TL, CAS and SATs (Cui et al. 2014; Fang et al. 2016, 2017; Jia et al. 2016, 2018b; Lv et al. 2017; Talukdar 2015; Yu et al. 2019), resulting in the production of more endogenous H2S and cysteine (Jia et al. 2016; Shi et al. 2014; Talukdar 2016; Zhang et al. 2010a). Cysteine is the raw material for GSH synthesis through γ-CEs synthetase and GSH synthase (Jobe et al. 2012), and H2S can increase the expression of the genes related to PCs and MTs through transcriptional regulation (Fang et al. 2014a, 2016; Jia et al. 2016; Liu et al. 2016; Valivand et al. 2019a).
There are certain correlation relationships of other signals with H2S under toxic metal stress. NO, the principal partner of H2S, is also endogenously synthesized in response to toxic metal stress just like H2S (Shi et al. 2014). Exogenous application of SNP has a similar action mode to NaHS in alleviating toxic metal stress (He et al. 2019; Zhu et al. 2018), which may be related to the interaction between H2S and NO in the regulation of redox balance (Shivaraj et al. 2020). Ca2+ also assists H2S to alleviate toxic metal stress. Some divalent metal ions, such as Cd2+, Mn2+ and Ga2+, can block the activity of calcium channels in plants, and Ca2+ can also mediate the detoxification process (Fang et al. 2017). For example, CDPK3 could enhance LCD activity in Arabidopsis, and the content of GSSH (S-persulfidation) was significantly lower in lcd and cdpk3 mutants (Qiao et al. 2016). Interestingly, seed priming with NaHS increased the CDPK transcripts in seedling leaves of zucchini under Ni stress (Valivand et al. 2019a, b). In addition, the signals to alleviate toxic metal stress by regulating the synthesis of H2S also include plant hormones (SA, ABA), gas molecules (SO2, H2), elements (Si) and some special organic compounds (Thiamine, Eugenol) (Hu et al. 2018; Kaya et al. 2020a; Kaya and Aslan 2020; Qiao et al. 2015; Zanganeh et al. 2019; Zhu et al. 2015). All of them have been reported to activate the H2S response pathway by increasing the activity of H2S-producing enzymes or endogenous H2S level (Hu et al. 2018; Kaya et al. 2020a; Kaya and Aslan 2020; Qiao et al. 2015; Zanganeh et al. 2019; Zhu et al. 2015). However, cinnamaldehyde was found to alleviate the toxic metal stress in a different action mode, which inhibits the activity of d-CDes in tobacco, and thus, reduces the content of endogenous H2S (Ye et al. 2017).
Recently, the regulatory pattern of trans-acting factors in the promoter region of key genes for H2S synthesis has been reported. WRKY18 and WRKY60 bind to the motif W-box in the promoters of LCD, DCD1, DCD2, DES and NFS2, and WRKY40 binds to the same motif of NFS1. The mRNA levels of the LCD, DES and DCD1 genes were up-regulated, but that of DCD2 was down-regulated in wrky18, whky40 or wrky60 mutants (Liu et al. 2015). Another WRKY family gene, WRKY13, is induced by Cd and thus activates DCD expression to increase the production of H2S (Zhang et al. 2020). Similarly, bZIP transcription factor TGA3 enhances the production efficiency of H2S via combining with the LCD promoter in response to Cr (VI) stress. Ca2+/CaM2 physically interacts with TGA3 to enhance the binding of TGA3 to the LCD promoter (Fang et al. 2017).
Roles of H2S in biotic stress response
Sulfur fertilization can enhance the resistance of crops against fungal pathogens. It was found to obviously increase the contents of total S, sulfate, organic S, cysteine, and GSH in Brassica, but decrease the l-CDes activity (Bloem et al. 2004). Moreover, infection with Pyrenopeziza brassicae increased the cysteine and GSH contents and the l-CDes activity (Bloem et al. 2004). Exposure to fungal infection is accompanied by increased emissions of S-containing gases, including H2S and COS (Bloem et al. 2011, 2012).
Exogenous NaHS can effectively inhibit the merisis of pathogenic bacteria and cure plant diseases. For example, fumigation with H2S could inhibit spore germination, mycelial development and pathogenicity of Monilinia fructicola in peach fruit (Wu et al. 2018), and also significantly inhibited the two fungal pathogens of pear, Aspergillus niger and Penicillium expansum (Tang et al. 2014). These results suggest that H2S can enhance the resistance of plants to pathogen infection, and the production of endogenous H2S is induced by immune signal and exogenous sulfide.
It is interesting to know how H2S helps to resist pathogenic microorganisms as an immune substance in plants. A study on Escherichia coli found that NaHS treatment stimulated the production of ROS and decreased the GSH level in E. coli, resulting in lipid peroxidation and DNA damage (Fu et al. 2018a). Meanwhile, H2S inhibits the antioxidative enzyme activities of SOD, CAT and GR and induces the response of the SoxRS and oxyR regulons in E. coli, which is contrary to the antioxidant pattern of H2S in plants (Fu et al. 2018a). Hu et al. (2014b) isolated three fungal pathogens, including Rhizopus nigricans, Mucor rouxianus and Geotrichum candidum, from sweetpotato infected with black or soft rot. H2S fumigation greatly reduced the percentage of fungal infection upon the inoculation of these three fungi on the surface of sweetpotato slices (Tang et al. 2014). It is marvelous that some pathogens have even evolved certain response mechanisms for resistance against the toxicity of H2S emitted by plants. Plant pathogens Xylella fastidiosa and Agrobacterium tumefaciens employ the BigR operon, which is regulated by the transcriptional repressor BigR and encodes a bifunctional sulfur transferase and sulfur dioxygenase enzyme, to oxidize H2S into sulfite (De Lira et al. 2018). In a feedback mechanism, H2S and polysulfides inactivate BigR and then initiate operon transcription (De Lira et al. 2018). However, the participation of H2S in plant resistance to pathogenic microorganisms is much more complex than what has been known, which is also a field worthy of exploration with interdisciplinary.
H2S contributes to plant growth and development
H2S is also involved in regulating the growth and development process in plant life cycles (Li et al. 2016b). Here, we summarize the existing findings to provide a better understanding on how H2S affects the growth and development of plants.
H2S promotes seed germination
Seed germination is the most critical and flimsy phase of plant life cycle because of its high vulnerability to injury, disease and environmental stress (Rajjou et al. 2012). Recently, a number of studies have elucidated that H2S is involved in the process of seed germination. H2S may promote germination by alleviating the adverse effects of multiple stresses on the seeds. For instance, exogenous NaHS could alleviate the toxic metal stress of wheat seeds (Hu et al. 2015b; Zhang et al. 2010c), the osmotic stress of cucumber seeds (Mu et al. 2018), the high temperature stress of maize seeds and the salinity stress of alfalfa and wheat seeds (Chen et al. 2019; Wang et al. 2012; Zhou et al. 2018). The related mechanisms have been discussed in detail in the previous section. Here, we will focus on the promotion effect of H2S on plant seed germination without stress. H2S affects seed germination in a dose-dependent manner, but too high concentration will lead to inhibition of germination (Baudouin et al. 2016). For cucumber seeds, the germination energy and efficiency and the seedling growth were promoted by H2S (Mu et al. 2018). In bean, corn, wheat, and pea, H2S can increase the germination rate and seedling size and shorten the germination time (Dooley et al. 2013a). Interestingly, endogenous H2S content is enhanced in germinating seeds without exogenous S fertilizer. The increase in H2S is associated with higher activity of d/l-CDes and CAS (Baudouin et al. 2016). Purification and biochemical characterization of CAS expressed in germinating seeds of Sorghum bicolor again confirmed that high CAS activity promotes seed germination (Amiola et al. 2018). However, NaHS treatment was ineffective in breaking seed dormancy since the germination of des1 and WT seeds was inhibited by ABA to almost the same degree (Baudouin et al. 2016). Surprisingly, H2O2 can also promote seed germination, indicating that H2O2 and H2S can synergistically promote seed germination. Soaking with H2O2 greatly improved the germination rate of Jatropha curcas seeds by stimulating the l-CDes activity, which in turn induced the accumulation of H2S (Li et al. 2012b). Conversely, NaHS treatment increased the contents of endogenous H2S and H2O2 in germinating seeds, and the accumulation of H2O2 lagged behind that of H2S, indicating that H2S acts upstream of H2O2 in seed germination of mung bean (Li and He 2015). Either H2S or H2O2 can dramatically stimulate protease activity and production of total free amino acids in cotyledons. These results suggest that both H2S and H2O2 can promote the seed germination of mung bean via mobilizing the storage protein. Actually, the mechanism for the effect of H2S on seed germination still remains elusive. Plant hormone crosstalk, DNA repair, protein PTMs, metabolite synthesis and mRNA transcription are all potentially responsive to H2S signaling.
Dual effects of H2S on root development
H2S shows dual regulatory effects on root development: it promotes root growth at low concentrations but inhibits root growth at high concentrations. In the previous experiments of our group, Arabidopsis grown on 1/2 MS medium supplemented with 10 ~ 100 μmol/L NaHS had longer roots than the control, while NaHS at concentrations over 200 μmol/L inhibited root elongation, and even suspended root elongation when the concentration exceeded 2 mmol/L. Exogenous application of low concentrations NaHS was found to promote the activity of l-CDes in root cells (Fang et al. 2014c; Hu et al. 2020a), thus, increasing the content of endogenous H2S, which would directly promote the development and growth of roots. The same phenomenon was also observed in strawberry seedlings (Hu et al. 2020a). Specific fluorescent probe WSP-1 was applied to track endogenous H2S in tomato roots in site, and the results further confirmed that H2S accumulation is associated with primordium initiation and lateral root emergence (Li et al. 2014c). Furthermore, fluorescence tracking of endogenous H2S in situ showed that H2S was accumulated exclusively in the outer layer cells of the primary root where lateral roots emerged (Xue et al. 2016). Pharmacological and biochemical approaches were combined to investigate the crosstalk among H2S, NO, CO, indole acetic acid (IAA) and Ca2+ in regulating the development and growth of roots. A rapid increase in H2S and NO was sequentially observed in shoot tips of sweet potato seedlings treated with NaHS. However, the induction effect of H2S on root growth was eliminated by N-1-naphthylphthalamic acid (NPA), an IAA transport inhibitor, and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), an NO scavenger (Zhang et al. 2009). Fang et al. (2014b) observed that down-regulation of SlDES induced by auxin depletion would decrease DES activity and endogenous H2S content, and inhibited lateral root formation. Conversely, treatment with NAA or NaHS could induce endogenous H2S, and thereafter stimulate lateral root formation in the same mode. Subsequently, both NaHS- and NAA-regulated modulation genes of cell cycle, including the up-regulated SlCDKA;1 and SlCYCA2;1, together with the down-regulated SlKRP2, were reversed by HT pretreatment (Fang et al. 2014b). Notably, these results suggest that H2S is a downstream component of auxin signaling to trigger lateral root formation.
For oxidation signals, NO and CO promote root growth similarly to H2S at low concentrations. Exogenous application of NaHS and the heme oxygenase-1 (HO-1) inducer hemin induced lateral root formation in tomato seedlings by triggering intracellular signaling events that involve the induction of tomato HO-1 and the modulation genes of cell cycle, including the up-regulation of SlCDKA;1 and SlCYCA2;1 and down-regulation of SlKRP2 (Fang et al. 2014c). Hence, HO-1/CO might be involved in H2S-induced lateral root formation in tomato. SNP could stimulate the generation of endogenous H2S and the expression of related enzyme genes. HT or PAG partially block the SNP-induced formation of lateral roots and the expression of lateral root-related genes (Li et al. 2014c). Deficiency of H2S could abolish the stimulatory effect of NO on intracellular Ca2+ and CAM1 transcription levels. Moreover, Ca2+ chelator or Ca2+ channel blocker diminished H2S-induced formation of lateral roots (Li et al. 2014c). These findings indicate that the interaction of H2S and Ca2+ signal is downstream of NO signal in the process of promoting root development. In addition, the effect of methane (CH4) on root growth and development was also found to be related to H2S signal, which is also the case for CA (Xue et al. 2016). Exogenous CH4 increased the endogenous H2S level by stimulating the activities of corresponding enzymes, and thus induced the expression of CsDNAJ-1, CsCDPK1, CsCDPK5, CsCDC6 (a cell-division-related gene), CsAux22D-like and CsAux22-like (two auxin-signaling genes) (Kou et al. 2018). Recent research has further confirmed the relation between CH4 and H2S, along with the advancement in transcriptional profiling analysis, increasing representative cell cycle regulatory genes, miRNA and their target genes have been identified, which are mostly involved in the promotion of root development by CH4 and H2S (Mei et al. 2019).
H2S may also act as an inhibitory signal of plant root development and growth at high concentrations in the environment. In this case, there are different regulatory mechanisms compared with the abovementioned pathways. High H2S inhibits the elongation of primary roots by inhibiting the transport of auxin (Jia et al. 2015). Vesicle trafficking and distribution of the PIN proteins are an actin-dependent process, whereas H2S alters the polar subcellular distribution of PIN proteins by controlling the expression of several actin-binding proteins (ABPs) and suppressing the occupancy percentage of filamentous actin (F-actin) bundles in Arabidopsis roots, which eventually inhibits auxin polar transport (Jia et al. 2015). In addition, the effects of H2S on F-actin are partially depleted in T-DNA insertion mutants cpa, cpb and prf3. The density of F-actin bundles and the F-actin/globular actin ratio are lower in overexpressing LCD/OASA1 lines (Li et al. 2018a). Besides, actin protein ACTIN2 (ACT2) is persulfidated at Cys-287, which is adjacent to the D-loop, a core region for hydrophobic and electrostatic interactions, and stabilizes F-actin filaments (Li et al. 2018a). A high accumulation of H2S results in the depolymerization of F-actin bundles and then inhibits root hair growth. Furthermore, a high concentration H2S represses primary root growth by triggering a signal transduction pathway involving ROS burst, MPK6 activation, and NO accumulation (Zhang et al. 2017). Exogenous H2S-induced ROS production is required for NO generation, and MPK6 mediates H2S-induced NO production, suggesting that MPK6 acts downstream of ROS and upstream of NO (Zhang et al. 2017). It remains to be determined whether these vital signals related to the subcellular localization of auxin are inhibited by H2S in the future.
Functions of H2S in photosynthesis and photomorphogenesis
When plants suffer from various abiotic stresses, the imbalance of redox state and the disorder of ion transport will largely restrict the photosynthesis of plants. Exogenous H2S can promote photosynthesis with a higher chlorophyll content in a variety of plants (Chen et al. 2015b; Liu et al. 2020b; Parveen et al. 2017), even in lower algae (Dooley et al. 2013b, 2015; Joshi et al. 2020), suggesting that the promotion of H2S on plant photosynthesis appeared in a very early period of plant evolution to improve plant survival. Chen et al. (2011) have revealed the role of H2S in photosynthesis in Spinacia oleracea. Besides increasing the chlorophyll content, NaHS treatment also promotes seedling growth, soluble protein content, photosynthesis and stacked number of grana lamellae; similarly, the light saturation point (Lsp), maximum net photosynthetic rate (Pmax), carboxylation efficiency (CE), and Fv/Fm all reached their maximal values, whereas the light compensation point (Lcp) and dark respiration (Rd) decreased significantly under NaHS treatment (Chen et al. 2011). H2S also enhances the activity of ribulose-1,5-bisphosphate carboxylase (RuBISCO) and the protein expression of the RuBISCO large subunit, as well as OAS-TL and l-CDes (Chen et al. 2011). Furthermore, H2S positively influences the growth and physiology of rice, including photosynthesis, photorespiration, chlorophyll fluorescence, and stomata. H2S treatment reduced the photosynthesis oxygen sensitivity, CO2 compensation point and glycolate oxidase (GOX) activity, and increased the photosynthetic rate and stomatal conductance (Duan et al. 2015). A recent study revealed that the deletion of either OASB or SERAT2;1 frequently induced antagonistic alterations in biochemical or molecular features (Muller et al. 2017). All of these findings indicate that H2S and the related S metabolism are important for chloroplast photosynthesis and related functions.
It is noteworthy that except for participation in the photosynthetic system from multiple perspectives, the relationship between H2S and light is also reflected in the perception of light signals, plant photomorphogenesis, and even the alleviation of light stress. Exogenous H2S can effectively alleviate the photoinhibition of Dendrobium officinale (Fan et al. 2014). Intriguingly, a similar mode of enhancement occurs in plants at a low light availability (Liu et al. 2019a). Plant photosynthesis is dependent on the plant’s perception of light and related signal transduction. H2S was also found to act downstream of plant light signal, which is induced by light in a specific band. In seedlings of foxtail millet, the H2S content in the hypocotyl increased initially under red, blue or white light, and the duration of increase under white light was longer than that under red or blue light (Liu et al. 2019b). The activity of CDes was increased by red light but decreased by blue and white light. The expression of LCD1 and LCD2 was promoted by red or white light, but inhibited by blue light (Liu et al. 2019b). In contrast, the DES gene was promoted by white light but inhibited by red or blue light. In addition, the activities of LCDs were regulated by the phosphorylation under the mediation of photoreceptors PHYB and CRY1/CRY2 (Liu et al. 2019b). These findings suggest that there are two ways to regulate the production of H2S in light-signaling network: a rapid pattern that involves the phosphorylation occurring on LCDs protein directly or indirectly mediated by photoreceptors, and a slow pattern that involves the regulation of mRNA transcription of LCDs and DES genes. As for photomorphogenesis, H2S promotes the elongation of hypocotyls. NaHS treatment blocked the efflux of the E3 ligase constitutive photomorphogenesis 1 (COP1) from nucleus to cytoplasm and increased the degradation of elongated hypcotyl 5 (HY5), thereby boosting the development of plants by inhibiting the expression of ABI5 (Chen et al. 2019). At present, little is known about whether H2S is involved in photosynthesis, photomorphogenesis or light signal transduction. Considering the importance of photosynthesis in a broad sense, it is promising to carry out in-depth research on H2S function.
H2S resists aging and programmed cell death
The function of H2S in alleviating cell senescence and apoptosis has been widely studied in mammalian cells, such as vascular endothelial cells (Das et al. 2018), neuronal cells (Wu et al. 2019), kidney cells (Chen et al. 2018), and tumor cells (Szadvari et al. 2019). Similar phenomena were observed in plants. Many external factors, such as damage (Zhang et al. 2011), hormone induction (Xie et al. 2014a), and lack of light (Hu et al. 2015a; Li et al. 2015e), can lead to early senescence in plants. Besides, rhythm, climate and seasonal changes also induce the natural aging of plants. It is miraculous that H2S is involved in these signals and reverses this natural process. Exogenous application of NaHS could significantly prolong the survival time of various cut flowers (Zhang et al. 2011), leaves and fruits in vitro (Hu et al. 2015a; Liu et al. 2017), by maintaining the stability of pigment content as well as reducing the respiration rate, oxidative damage and the subsequent PCD process in plant cells. Over accumulation of ROS can induce autophagy in plant cells, and the scavenging ability of H2S on ROS through antioxidant enzymes is dependent on the increase in both transcription and enzyme activities. Naturally, H2S weakens the aging promoting effect of ROS.
For example, H2S treatment alleviated dark-promoted senescence in broccoli florets by sustaining higher activities of GPX, APX, CAT and GR and lower activities of lipoxygenase (LOX), PPO, PAL and protease (Li et al. 2014b, 2015e). Similarly, NaHS treatment on aleurone tissue led to higher transcript levels of the antioxidant genes HvSOD1, HvAPX, HvCAT1 and HvCAT2 and lower transcript levels of HvLOX and cysteine protease genes HvEPA and HvCP3-31 (Zhang et al. 2015a). Exogenous H2S can increase the contents of chlorophyll, carotenoids, anthocyanins and ascorbate through metabolic pathways, and down-regulate the transcription of genes related to chlorophyll degradation (BoSGR, BoCLH2, BoPaO, and BoRCCR), thus inhibiting the etiolation process (Hu et al. 2015a; Li et al. 2014b, 2015e). Hormones such as GA and ethylene can induce aging, and H2S can counteract their signals through potential antagonism. In wheat aleurone cells, H2S alleviates GA-induced PCD via resuming the production of H2S, increasing the content of GSH and NO and the expression of HO-1 and α-amylase (Xie et al. 2014a; Zhang et al. 2015a). The role of GSH in alleviating autophagy has been reported previously in mammalian cells. For example, the deletion of GCLM, a GSH synthesis-related gene, could cause premature aging of fibroblasts and ovarian cells (Chen et al. 2009; Lim et al. 2013). The role of NO in alleviating plant senescence has also been systematically reviewed (Gotor et al. 2013). Meanwhile, d/l-cysteine and H2S can delay the aging time of parsley and peppermint by decreasing ethylene synthesis (Al Ubeed et al. 2019).
Another way to delay aging by H2S is to reduce the respiratory rate and restore and enhance the energy metabolism. H2S can alleviate autophagy induced by carbon starvation (Álvarez et al. 2012). Subsequently, H2S was found to delay senescence by maintaining the energy status in plants (Liu et al. 2017). In Arabidopsis, the mitochondria of des1 were severely damaged and bubbled in older leaves, while OE-DES1 had complete mitochondrial structures and a homogeneous matrix (Jin et al. 2018). In addition, mitochondria isolated from OE-DES1 showed significantly higher H2S production rate, H2S content and ATPase activity level, as well as lower levels of swelling and ATP content compared with the WT and des1 (Jin et al. 2018). Besides, the decrease in H2S caused by DES1 deletion also inhibited the expression of ATPβ-1, 2, 3, while induced that of ATPε (Jin et al. 2018). At the transcriptional level, H2S delays the aging process by regulating senescence-related genes. For instance, H2S alleviates the aging of foliar cells by inhibiting the expression of SAG13, ATG8b and ATG12a while inducing that of SAG12 (Álvarez et al. 2012; Jin et al. 2018; Wei et al. 2017). Recently, it was found that H2S inhibited the abscission of the tomato petiole in a dose-dependent manner, and up-regulated the expression of SlIAA3 and SlIAA4 but down-regulated that of ILR-L3 and ILR-L4 in the earlier stages of the abscission process (Liu et al. 2020a). Moreover, proteomic analysis under ABA treatment showed that persulfidation of the cysteine protease ATG4 could regulate autophagy in Arabidopsis. H2S-induced persulfidation of ATG4 protease directly promotes the post-translational processing of ATG8, which negatively regulates the progress of autophagy (Laureano-Marín et al. 2020). It should also be noted that the action mode for SO2 to alleviate plant senescence is just like that of H2S (Sun et al. 2018; Wang et al. 2017), indicating that the relation between SO2 and H2S is established through the thiometabolism pathway.
H2S delays fruit ripening and prolongs postharvest freshness
Application of H2S donor NaHS or Na2S could significantly inhibit the decay and mildew of postharvest fruits, and prolong their storage time (Ali et al. 2019; Mukherjee 2019; Ziogas et al. 2018). Exogenous H2S can regulate the redox balance, hormone level, thiometabolism and energy metabolism in fruits, maintain the homeostasis of various secondary metabolites and the integrity of cell wall and cell membrane, as well as help to resist the invasion of a variety of fungi by inhibiting the mycelial germination (Table S2).
As mentioned above, H2S can significantly enhance the activities of antioxidant enzymes, including CAT, SOD, APX and POD (Aghdam et al. 2018; Gao et al. 2013; Hu et al. 2012, 2014a; Luo et al. 2015; Yao et al. 2018). Interestingly, the mechanisms by which H2S regulates redox balance are complex and diverse in different fruits. In the fruits of strawberry, kiwifruit, pear and sweet potato, H2S inhibits the oxidation of lipids by reducing the activity of LOX (Gao et al. 2013; Hu et al. 2012, 2014b; Tang et al. 2014). H2S fumigation was found to inhibit the activities of PAL and PPO in apple, banana, tomato and pear fruits (Hu et al. 2014b; Luo et al. 2015; Yao et al. 2018; Zheng et al. 2016). Phenolic compounds are maintained at low levels, which also helps to prevent the oxidative browning of fruits such as lotus root, apples and pears after cutting (Hu et al. 2014b; Sun et al. 2015; Zheng et al. 2016). H2S can also improve the activity of GR in strawberry fruit (Hu et al. 2012) and kiwi fruit (Gao et al. 2013). Aroca et al. (2015) revealed that H2S can directly enhance CAT activity in Arabidopsis through S-persulfidation. Liu et al. (2017) pointed out that H2S enhances the activities of SOD, CAT and APX in Hemerocallis Liliaceae, among which APX may be directly regulated by H2S-induced persulfidation. In most cases, H2S enhances the activity of antioxidant enzymes. Moreover, H2S also increases the expression of SlAPX2, SlCAT1, SlPOD12 and SlCuZn-SOD genes in tomato (Yao et al. 2018). These findings indicate that H2S regulates ROS not only through PTM, but also at the transcriptional level (Begara-Morales et al. 2014; Palma et al. 2020).
Exogenous H2S also affects different secondary metabolic processes. For sulfide and sulfate metabolism, H2S enhances the activity of d/l-CDes, and thus increases the content of endogenous H2S in fruits (Aghdam et al. 2018; Hu et al. 2014a; Liu et al. 2017; Munoz-Vargas et al. 2018). The sugar/acid ratio of plant fruit is considered as an important index of fruit water-holding and storage capacity. During the storage of apple and grape fruits, H2S fumigation could reduce the accumulation of sugars and the content of soluble proteins (Ni et al. 2016; Zheng et al. 2016). However, opposite results were obtained for kiwifruit, strawberry and mulberry fruits (Gao et al. 2013; Hu et al. 2012, 2014a). This may be related to the differences in sugar/acid ratio among different fruit species. Interestingly, H2S treatment also increased the contents of titratable acid and vitamin C in kiwifruit, grape and mulberry fruit (Gao et al. 2013; Ni et al. 2016; Zhu et al. 2014), which is conducive to the reduction of sugar/acid ratio and prolonging of storage time. In different plant species, H2S significantly inhibits the respiration rate of fruits and maintains the stability of energy metabolism during postharvest storage. H2S enhances the activities of H+-ATPase, Ca2+-ATPase, CCO and SDH in banana pulp, and participates in the regulation of energy metabolism in the fruit (Li et al. 2016a).
H2S also delays the change in fruit color (Yao et al. 2018), through delaying the degradation of chlorophyll and inhibiting the production of carotenoids, which has been found in both kiwifruit and banana peels (Gao et al. 2013; Ge et al. 2017). In addition, H2S treatment inhibited the accumulation of anthocyanins (Hu et al. 2014a), which also inhibits the change in fruit color. Meanwhile, H2S affects the levels of flavonoids and phenols in fruits. The contents of flavonoids and phenols in apple and grape fruits were increased after treatment with NaHS (Ni et al. 2016; Zheng et al. 2016), which would delay fruit senescence and decay. In addition, the metabolism of amino acids in fruits is regulated by H2S as well. H2S increases the content of Pro in banana pulp by enhancing the activity of proline synthase P5CS and inhibiting that of SDH (Luo et al. 2015). The metabolism of phenylalanine is affected by H2S, which can increase the activity of PAL, and thus reduce the content of phenylalanine in fruits (Hu et al. 2014b; Zheng et al. 2016). H2S also inhibits the activity of PG (Hu et al. 2012) and EGase (Zhang et al. 2014), indicating that H2S maintains the firmness of fruit by keeping the integrity of cell wall. A recent study showed that endogenous H2S plays a role in fruit ripening in tomato, for the SlLCD1 gene-edited mutant displays accelerated fruit ripening (Hu et al. 2020b).
Inhibition of endogenous ethylene synthesis and signal transduction is one of the important mechanisms for H2S treatment to delay fruit ripening. H2S treatment could prolong the storage time of “Red Fuji” apple, and delay the ripening of apple fruit by suppressing the expression of ethylene synthesis-related genes (MdACS1, MdACS3, MdACO1 and MdACO2) and signal transduction genes (MdETR1, MdERS1, MdERS2, MdERF3, MdERF4 and MdERF5) (Zheng et al. 2016). Similarly, H2S inhibits the expression of ethylene-related genes (SlACO1, SlACO3, SlACO4; SlETR5, SlETR6, SlCRF2, and SlERF2) in tomato (Hu et al. 2019). Compared with the control, H2S treatment down-regulated the ethylene biosynthesis genes (MaACS1, MaACS2, MaACO1 and pectin lyase MaPL), while up-regulated the ethylene receptor genes (MaETR, MaERS1 and MaERS2) in banana fruit (Ge et al. 2017).
NO can bind with ACC oxidase to form a stable “ACC-ACC oxidase-NO” ternary complex in a dose-dependent manner (Mukherjee 2019). This signaling event in turn leads to a decrease in ethylene production in tissues. Ethylene accumulation was reduced in peach fruits under treatment with H2S and NO donors (Zhu et al. 2014). NO-H2S crosstalk showed a stable synergistic effect to inhibit ethylene-induced fruit ripening. Zhang et al. (2014) reported that the combination of exogenous H2S and NO could alleviate ROS stress, improve fruit firmness, and enhance the anti-ripening effect of strawberry fruit. Liu et al. (2011) clarified the downstream position of H2S in the ethylene-NO-H2S signaling pathway. The signal transduction of H2S with ethylene during fruit ripening has been reviewed (Ziogas et al. 2018). In these processes, H2S interacts with ROS and RNS stress signals. The S-persulfidation and S-nitrosylation by H2S and NO directly occur in plants, which is also an important way for them to regulate plant maturation (Huo et al. 2018; Ziogas et al. 2018).
Multiple crosstalk of H2S, NO and H2O2 signals in plants
Organisms have evolved metabolisms as well as regulatory mechanisms for adaptation to the changing atmospheric composition during Earth’s history: from H2S to NO to O2, and from ancient to the present (Yamasaki and Cohen 2016). Hence, a great deal of research evidence has shown that the traces of environmental changes left in organisms might marvelously evolve into more complex signal crosstalk and regulatory mechanisms. Either in mammal or plant cells, the special actions, functions and mechanisms of H2S (RSS), NO (RNS) and H2O2 (ROS) are inseparable from their inherent chemical properties and oxidative PTMs. Here, we focus on the clues to help a better understanding of the multiple signals of H2S, NO and H2O2 in plants.
Chemical characteristics and signals: H2S (RSS), NO (RNS) and H2O2 (ROS)
As a gas molecule, H2S has a classic “V-type” molecular structure, which is similar to the molecular structure of H2O. Gaseous H2S has active chemical properties, and is soluble in water (Kimura 2015), forming weak acid known as “hydrogen sulfuric acid”. Its aqueous solution contains hydrogen sulfate HS− (pKa1 = 6.9 in a 0.01–0.1 mol/L solution at 18 °C) and S2− (pKa2, between 12 and 17) (Filipovic et al. 2018), as follows:
At the beginning, hydrogen sulfuric acid is clear, but becomes turbid after being placed for a period of time. This is because hydrogen sulfuric acid will react slowly with oxygen dissolved in water to produce elemental sulfur insoluble in water:
In vivo, the concentration of H2S is low. Hence, inhaling of excessive H2S will promote the oxidation self-rescue of organisms, and oxidize H2S into sulfite and sulfate with low toxicity (Baillie et al. 2016). In addition, channeling of the surplus sulfur to the formation of S-metabolites like thiols is a main way to reduce the toxicity in plants (Baillie et al. 2016). Since S ion in H2S is in the low divalent oxidation state, H2S only undergoes oxidation as a reducing agent (Koppenol and Bounds 2017). Oxidation leads to the formation of sulfate (SO42−), sulfite (SO32−), thiosulfate (S2O32−), persulfides (RSS−), organic (RSSnSR), inorganic (H2Sn) polysulfides, and elemental sulfur (Sn). Compared with the direct oxidation of H2S by O2, which has a strong thermodynamic barrier, ROS is more naturally involved in this process in vivo (Koppenol et al. 2010). The initial oxidation product of H2S is the sulfuryl free radical (HS•) that can react with electron donors including ascorbate and GSH. Importantly, the one-electron oxidation of H2S can initiate oxygen-dependent free radical chain reactions to amplify the initial oxidative event (Carballal et al. 2011; Das et al. 1999). The reaction with hydroperoxides (HOOH) initially forms HSOH, which can react with a second HS− to form HSSH, that is, polysulfide (Carballal et al. 2011; Hoffmann 1977). In the case of H2O2, the final products depend on the initial ratio of H2O2 to H2S and mainly consist of polysulfides, elemental sulfur and sulfate in the presence of excess oxidant (Hoffmann 1977). According to the chemical and computational studies, H2S probably acts as a direct scavenger of oxidants in biological systems. However, compared with that of LMW thiols, the reaction of H2S with some oxidants displayed relatively high rate constants, and thus, the content of H2S (sub micromolar) in the tissues is relatively low (Koike et al. 2017). Besides, H2S cannot compete with thiols to bind one- and two-electron oxidants at such low concentrations (Filipovic et al. 2018). This means that the direct reaction of H2S with oxidants is not fast enough in the biological environment to support a significant scavenging effect unless sufficient exogenous H2S is applied, as mentioned in the previous section. In conclusion, the biological “antioxidant” effects of H2S can be ascribed to the superimposed effect of the direct chemical action of H2S itself and indirect effects via enzymes, transporters and other companions.
In the active form of NO•, NO participates in many physiological processes in mammals, such as immune defense, vasodilation and neuro-transmission (Bogdan 2001; Palmer et al. 1988; Santos et al. 2015), which are mediated by the coordination of NO• with the heme iron in sGC, and then the generation of cyclic guanosine monophosphate (cGMP; a classical second messenger) is activated (Friebe and Koesling 2003; Jahshan et al. 2017). H2S interweaves with NO signaling, either by reacting with NO• or its downstream regulatory network or by modulating NO production and cGMP levels in vivo (Bucci et al. 2010; Cuevasanta et al. 2015b; Da Silva et al. 2017; Hancock and Whiteman 2015; Zhang et al. 2017). The direct reaction between NO and H2S was reported more than a century ago, and gaseous NO can react with gaseous H2S to produce N2O, polysulfides (H2Sn) and elemental sulfur (Dunnicliff et al. 1931; LeConte 1847; Miyamoto et al. 2017; Pierce 1929). However, this is obviously not a one-step reaction, and HNO exists as the actual intermediate of the reaction, which has also been verified in vivo (Yong et al. 2011, 2010). Strangely, single electrons are transferred directly from HS− to NO• to produce HNO and S•− is thermodynamically unreasonable (ΔG 0 ′ = + 102 kJ/mol) (Koppenol and Bounds 2017). An alternative mechanism is the formation of HSNO•−, a powerful reducing agent,
which can initiate a cascade of reactions to result in the formation of N2O, H2Sn and Sn (Arulsamy et al. 1999; Suarez et al. 2015). Interestingly, as a key node in the chemical reaction between NO and H2S, HSNO was also found in the reaction pathways of H2S with “NO+” carriers: acidified nitrite, N2O3, metal nitrosyls, and S-nitrosothiols (Filipovic et al. 2012; Nava et al. 2016). Among them, N2O3 reacts with H2S to produce HSNO, which may be important for intracellular RSNO generation:
This reaction was detected to occur in the lipid bilayer of the cell membrane where a large amount of N2O3 is generated by accumulated NO and O2, which then reacts with the same large amount of H2S (Cuevasanta et al. 2012; Lancaster 2017) (Fig. 3). In the reaction of HSNO and thiols, HSNO acts as an “NO+” carrier that mediates transnitrosation between proteins and across the cell membrane. In addition, the reaction of RSNO and H2S promotes the extracellular formation of HSNO, but on the contrary, HSNO reacts with RSH to release RSNO and H2S once entering the cell (Filipovic et al. 2018), which is also considered as an efficient way for H2S to penetrate the cell membrane (Fig. 3). These findings seem to reveal the profound mechanism of the complementary relationship between NO and H2S in plant physiology. Since the chemical interaction occurring between NO and H2S can produce H2Sn, an enhanced version of H2S signal in mammals (Kimura et al. 2015; Miyamoto et al. 2017), it should be determined whether H2Sn exists or has special physiological effects in plants.
Fig. 3.
Endogenous chemical signals in plants involving H2S, NO and ROS. N2O3 is synthesized by NO and O2 accumulated in the bilayer of cell membrane, and then forms HSNO with H2S. Extracellularly, HSNO can be directly synthesized by H2S and NO. HSNO can enhance the membrane permeability of H2S and NO, which is also the transmembrane transfer mode of cysteine thiols. Intracellularly, H2S undergoes oxidation and generates sulfate (SO42−), sulfite (SO32−), thiosulfate (S2O32−), persulfides (RSS−), organic (RSSnSR), inorganic (H2Sn) polysulfides, and elemental sulfur (Sn), which is controlled by different ratios of H2S and ROS level
Pivotal post-translational modifications: persulfidation, S-nitrosylation and S-sulfenylation
Recent studies have revealed that the persulfidation of protein cysteine residues (RSSH) acts as an important mechanism of H2S signaling in plants. Since the protein PTMs mediated by H2S, NO and ROS occur all by attacking the cysteine residues, we will discuss the persulfidation, S-nitrosylation and S-sulfenylation together in this section.
Protein persulfidation (alternatively called S-sulfhydration) has been identified to be involved in the sulfide-signaling pathway, in which the cysteine thiol (RSH) is persulfidated into a persulfide thiol (RSSH) (Fig. 4). Afterwards, this post-modification may cause functional changes in activities, structures, and subcellular localizations of the target proteins (Aroca et al. 2018). In mammals, persulfidation has been proven to be present on cysteine residues of various proteins, such as KATP channels (Mustafa et al. 2011), TRP channels (Liu et al. 2014), Kelch-like ECH-associated protein 1 (Keap-1) (Hybertson et al. 2011; Wakabayashi et al. 2004; Yang et al. 2013), p66Shc (Xie et al. 2014b), receptor for AGE (RAGE) (Ramasamy et al. 2011; Zhou et al. 2017), Parkin (an E3 ubiquitin ligase) (Vandiver et al. 2013), Nuclear factor κB (NF-κB) (Du et al. 2014; Sen et al. 2012) and Glyceraldehyde Phosphate Dehydrogenase (GAPDH) (Gao et al. 2015; Mustafa et al. 2009). Interestingly, the series of studies of Kimura’s team have revealed that H2Sn can also mediate similar PTMs as H2S in mammalian cells, and is more effective than NaHS (Kimura 2015; Kimura et al. 2015). The occurrence of persulfidation mediates the 3-dimensional conformation of the corresponding proteins by changing the properties of cysteine residues and the disulfide bonds, thus, affecting the activity and function of proteins.
Fig. 4.
Multiple post-translational modifications on cysteine residues of proteins. Protein cysteine thiols (RSH) can be sulfonated to produce RSOH with increasing ROS. Upon continuous exposure to ROS, RSOH could further generate irreversible sulfinic (RSO2H) and sulfonic acids (RSO3H). On the basis of RSOH, H2S and NO can be persulfidated and S-nitrosylated, respectively, to produce RSSH and RSNO. In the presence of nitrotransferase, NO can also react directly with RSH to produce RSNO. Once persulfidated cysteine thiols encounter ROS, RSSH will rapidly react with ROS to form adducts (RSSOH, RSSO2H and RSSO3H). Among them, RSSH and RSSOH can be reduced back to thiols by the action of the thioredoxin (Trx)
In recent years, there has been certain progress in the research on persulfidation in plants due to the advanced detection methods and the corresponding omics analysis. Previously, the biotin switch method (BSM) was widely used for the detection of PTMs by S-nitrosylation (Sell et al. 2008). After three steps of blocking by thiol-blocking reagent methyl methanethiosulfonate (MMTS), reducing by ascorbate and connecting with N-6-(biotinamido) hexyl-3′-(2′-pyridyldithio)-propionamide (biotin-HPDP) to form biotin-labeled proteins, RSNO could finally form biotin-labeled proteins (Mustafa et al. 2009). Aroca et al. (2015) detected a total of 106 persulfidated proteins through the modified BSM. Subsequently, they developed a comparative and quantitative proteomic analysis approach for the detection of endogenous persulfidated proteins in Col-0 and des1 mutant leaves using the tag-switch method. They identified 2330 potential target proteins for persulfidation (Aroca et al. 2017, 2018). KEGG and GO analysis showed that the proteins possibly regulated by persulfidation mainly exist in the cytoplasm and chloroplast, and are involved in processes such as carbon metabolism, abiotic and biotic stress responses, plant growth and development, and RNA translation (Aroca et al. 2017). A differential analysis of the persulfidated proteins of des1 and Col-0 highlighted the importance of H2S produced by DES1, which initiates persulfidation and regulates downstream signals. As expected, DES1-generated H2S-induced S-persulfidation, which was involved in the regulation of stomatal movement in Arabidopsis. In the process of stomatal response to ABA induction, DES1 can self-persulfidate under the action of H2S at Cys44 and Cys205, which is important for the amplification of H2S signal (Shen et al. 2020). Moreover, sustainable H2S accumulation could drive the persulfidation of the NADPH oxidase RBOHD at Cys825 and Cys890, enhancing its ability to produce ROS (Shen et al. 2020). Similarly, SnRK2.6/OST1 has been identified to be persulfidated at Cys131 and Cys137, which activates the kinase during ABA-induced stomatal closure (Chen et al. 2020). It is worth mentioning that Cys137 can also be modified by S-nitrosylation, while the difference is that the NO-mediated PTM inhibits the kinase activity (Wang et al. 2015). All these findings reveal the importance and infinite possibility of cysteine thiol modification, especially sulfhydryl modification, in stomatal movement of plants. In addition, H2S-mediated persulfidation has also been found to regulate other plant signaling pathways. The occurrence of persulfidation induced by H2S can specifically activate cytosolic CAT, APX and GAPDH in cytoplasm (Aroca et al. 2015; Palma et al. 2020), which is also the classic enzyme-dependent pathway of ROS scavenging by H2S. In addition, H2S negatively controls the progress of autophagy through specifically persulfidating Cys170 residue of the ATG4a protease in Arabidopsis (Laureano-Marín et al. 2020). Then, the post-translational processing of ATG8 and the synthesis of autophagosomes are prevented (Laureano-Marín et al. 2020). In addition to positive regulation of ROS scavenging, H2S-mediated persulfidation also has some negative effects. Excessive accumulation of H2S reduces the density of F-actin bundles and the F-actin/globular actin ratio, because persulfidation occurs at the Cys293 residue of ACTIN2, which prevents actin polymerization and then inhibits the development of root hair in Arabidopsis (Aroca et al. 2017; Li et al. 2018a). This coincides with studies in animals. H2S not only dynamically regulates the depolymerization of actin, but also affects the stability of tubulins in mammals (Mustafa et al. 2009). In tomato, H2S treatment could persulfidate the ACC oxidases LeACO1 and LeACO2, and inhibit their activities (Jia et al. 2018a), suggesting that ethylene-induced H2S negatively regulates ethylene biosynthesis by persulfidation of LeACOs. In general, the physiological functions of H2S in plants are far more than we mentioned above, and the biological significance of the persulfidation of protein cysteine mediated by H2S is worth of further exploration.
S-nitrosylation, an NO-mediated protein PTM, is another type of cysteine thiol modification. The concept of S-nitrosylation was first proposed in 1994, meaning that exposure to high concentrations of NO promotes protein cysteine residue thiols (RSH) to form RSNO, which regulates the signal transduction of redox (Stamler 1994) (Fig. 4). RSNO is relatively stable, and is therefore considered as the major form for the storage and transport of NO, but it is sensitive to strong reducing agents (i.e., intracellular GSH and ascorbate), and extremely sensitive to metal ions, especially Fe2+ and Cu2+ (Hogg 2002). Moreover, RSNO can further react with thiols to produce disulfide (RSSR) and HNO (Wong et al. 1998). When reacting with H2S, the product is HSNO. However, an alternative reaction is the formation of HNO and a protein persulfide (RSSH), which is thermodynamically unfavored (Koppenol and Bounds 2017), whereas some protein microenvironments could facilitate this reaction. Different from the similar function of H2S and NO, persulfidation and S-nitrosylation might regulate protein functions differentially. In an omics study of mammals, the persulfide and S-nitrosothiol proteomes were reported to have a 36% overlap (Gao et al. 2015). In plants, the relationship between S-nitrosylation and persulfidation has been gradually revealed (Fig. 4). NO and H2S have a synergistic relationship in many pathways, which can not only promote each other's enzyme activity, but also relieve the effects of various stresses. Surprisingly, relative to S-nitrosylation, persulfidation showed opposite effects on SnRK2.6/OST1 (Wang et al. 2015), actins (Rodriguez-Serrano et al. 2014), APX1 (Begara-Morales et al. 2014) and GAPDH (Vescovi et al. 2013). S-Nitrosylation and persulfidation regulate cysteine thiols at the same site (GAPDH at adjacent locus), but have the opposite effect (inhibiting or promoting) on their activities. In Arabidopsis, a total of 623 candidate proteins were identified to be S-nitrosylated and persulfidated (Aroca et al. 2018), which greatly expands the scope of research on the modification of cysteine thiols.
When exposed to ROS, protein cysteine thiols can be oxidized to sulfenic acid (RSOH), that is S-sulfenylation. Then, RSOH could be further oxidized with the formation of irreversible sulfinic (RSO2H) and sulfonic acids (RSO3H) (Filipovic and Jovanovic 2017) (Fig. 4). Moreover, H2S could react with sulfenic acid to form persulfides (RSSH), and this process is termed as persulfidation. After the completion of persulfidation, RSSH has bidirectional redox ability, and can be reduced back to thiols under the action of thioredoxin (Trx), or rapidly react with ROS/RNS to form an adduct (RSSO3H) under exposure to ROS. The RSSO3H also could be cleaved by Trx to restore free thiol and by-product sulfite (Filipovic and Jovanovic 2017; Wedmann et al. 2016) (Fig. 4). Under the dynamic action of ROS and Trx, RSH and RSSH jointly construct the endogenous cycle of H2S (Wedmann et al. 2016), that is, H2S is recycled and reused by the cells. The formation of RSSO3H is also an adaptive short-term storage method to alleviate the surge of endogenous ROS and RNS in plants. Notably, there is still no solid evidence that H2S acts directly on cysteine thiols during the occurrence of persulfidation (Cuevasanta et al. 2015a), such as the discovery of related catalytic enzymes. Hence, RSOH is considered as an important intermediate for S-sulfenylation, persulfidation and even S-nitrosylation. Besides, H2Sn could be another potential way of H2S-induced persulfidation, which is produced by the oxidation of H2S or by enzymes such as 3-MST to directly S-sulfurate cysteine residues (Kimura et al. 2013, 2015). In mammals, previous studies have revealed that these three oxidative modifications of proteinaceous cysteinyl thiols can be converted to each other, when one of the evoked signals is dominant (Hancock and Whiteman 2016a, b), corresponding modification is more likely to occur on the key cysteine site. These findings show that the modification mode induced by H2S, NO and H2O2 is strongly dose dependent, which may explain why the downstream signal response is much more intense when exogenous donors are used. Intriguingly, without the interference of dose effect, these three kinds of oxidative modifications also have discrepancies in oxidation ability, following the order of S-persulfidation (RSSH) > S-nitrosylation (RSNO) > S-sulfenylation (RSOH) (Hancock and Whiteman 2016a; Olson 2015; Wang 2012), which not only illustrates the mediating role of oxidized cysteine thiols, but also highlights the priority of S-persulfidation in the competition for modifying cysteine residues. Accordingly, it seems that these three kinds of modifications based on cysteine thiols are mutually regulated and transformed. For the mammalian MST, a stable persulfide at cysteine Cys247 can be oxidized by H2O2 to form Cys-thiosulfenate, Cys-thiosulfinate, and Cys-thiosulfonate, and then Trx can convert these modified cysteines to nonmodified cysteines (Nagahara et al. 2012). Recently, a total of 1,537 S-sulfenylated sites on more than 1000 proteins were identified in Arabidopsis. Compared with human S-sulfenylation datasets, 155 conserved S-sulfenylated cysteines were provided, including Cys181 of the Arabidopsis MAPK4 (Huang et al. 2019). RSOH is not only the specific protein regulated by sulfenylation of cysteine thiols, but also the basis of dynamic regulation of the three modifications. Moreover, comparisons across different databases will help to identify the target proteins regulated by the triple regulations.
Perspectives
The impact of gasotransmitter H2S on vegetation is paradoxical, as excessive H2S negatively affects plant growth and development, while plants can utilize low levels of H2S as a dynamic regulator for survival (Ausma and De Kok 2019). H2S can effectively delay the flowering process of plants (Zhang et al. 2011), which is considered as a secondary regulatory pathway to inhibit the PCD process (Romero et al. 2014). However, several recent reports have suggested that H2S is involved in the regulation of plant flowering by transcriptional regulation and PTMs. The more specific mechanism by which H2S affects flowering deserves further exploration.
Many kinds of stress stimuli and growth signals can induce the production of endogenous H2S through enzymatic pathways. For example, exogenous H2S activates DES1 through persulfidation (Chen et al. 2020), and TGA3 promotes the increase in LCD transcription level (Fang et al. 2017). However, little is still known about the regulation mode of the H2S-producing enzymes, which is also worth of more studies.
H2S-induced persulfidation has been proved to be an important PTM in animals and plants. Among all the modified target proteins, ion transporters are undoubtedly an important class. In mammals, various types of ion channels (K+ATP channels, KCa channels, Ca2+ channels, Cl− channels and TPR channels) have been confirmed to be modified and regulated by H2S-induced persulfidation (Lefer 2019; Wang 2012; Yang et al. 2019). In plants, exogenous H2S can inhibit the transport of inward-rectifying K+ channels (IKIN) (Papanatsiou et al. 2015) and activate SLAC1 currents (Wang et al. 2016) in plant guard cells, but it remains unclear whether there is a direct persulfidation process. A large amount of omics data in plants also indicate the potential regulatory effect of persulfidation on the activity of ion channels (Aroca et al. 2017, 2018; Wedmann et al. 2016), but there is still a lack of more intuitive experimental evidence.
Lastly, due to the complexity of the dynamic changes of ROS, RNS and RSS, it is difficult to predict the modification mode of protein cysteine residues. Although many progresses and conjectures have been made in biochemical studies, there are still no strong evidence and clear understanding on the property of RSH at the molecular level, such as whether the node effect of RSOH is universal, whether H2S forms RSSH directly by the enzymatic way, the dose effect, and competition relationship between RSNO and RSSH. These are undoubtedly challenging research direction in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31670267, 32070214) and the Fundamental Research Funds for the Central Universities (2662020SKY008). Thank the medical workers and volunteers for their dedication and sacrifice in the battle against the Novel Coronavirus in global.
Compliance with ethical standards
Conflict of interest
All the authors state that there is no conflict of interest.
References
- Aghdam MS, Mahmoudi R, Razavi F, Rabiei V, Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci Hort. 2018;238:264–271. [Google Scholar]
- Ahmad R, et al. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plantarum. 2020;168:289–300. doi: 10.1111/ppl.13001. [DOI] [PubMed] [Google Scholar]
- Al Ubeed HMS, Wills RBH, Bowyer MC, Golding JB. Inhibition of postharvest senescence of green leafy vegetables by exogenous D-cysteine and L-cysteine as precursors of hydrogen sulphide. J Hortic Sci Biotech. 2019;94:620–626. [Google Scholar]
- Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang GP. Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem. 2013;32:2234–2239. doi: 10.1002/etc.2309. [DOI] [PubMed] [Google Scholar]
- Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou WJ. Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotox Environ Safe. 2014;110:197–207. doi: 10.1016/j.ecoenv.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Ali S, Nawaz A, Ejaz S, Haider STA, Alam MW, Javed HU. Effects of hydrogen sulfide on postharvest physiology of fruits and vegetables: an overview. Scientia Hort. 2019;243:290–299. [Google Scholar]
- Álvarez C, Calo L, Romero LC, García I, Gotor C. An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 2010;152:656–669. doi: 10.1104/pp.109.147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez C, García I, Moreno I, Perez-Perez ME, Crespo JL, Romero LC, Gotor C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell. 2012;24:4621–4634. doi: 10.1105/tpc.112.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiola RO, Ademakinwa AN, Ayinla ZA, Ezima EN, Agboola FK. Purification and biochemical characterization of a beta-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench. Turk J Biochem. 2018;43:638–650. [Google Scholar]
- Amooaghaie R, Zangene-Madar F, Enteshari S. Role of two-sided crosstalk between NO and H2S on improvement of mineral homeostasis and antioxidative defense in Sesamum indicum under lead stress. Ecotox Environ Safe. 2017;139:210–218. doi: 10.1016/j.ecoenv.2017.01.037. [DOI] [PubMed] [Google Scholar]
- Arenas-Alfonseca L, Gotor C, Romero LC, García I. Beta-cyanoalanine synthase action in root hair elongation is exerted at early steps of the root hair elongation pathway and is independent of direct cyanide inactivation of NADPH oxidase. Plant Cell Physiol. 2018;59:1072–1083. doi: 10.1093/pcp/pcy047. [DOI] [PubMed] [Google Scholar]
- Arenas-Alfonseca L, Gotor C, Romero LC, García I. Role of mitochondrial cyanide detoxification in Arabidopsis root hair development. Plant Signal Behav. 2018;13:e1537699. doi: 10.1080/15592324.2018.1537699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca Á, Serna A, Gotor C, Romero LC. S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol. 2015;168:334–342. doi: 10.1104/pp.15.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca Á, Benito JM, Gotor C, Romero LC. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68:4915–4927. doi: 10.1093/jxb/erx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca Á, Gotor C, Romero LC. Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci. 2018;9:1369. doi: 10.3389/fpls.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulsamy N, Bohle DS, Butt JA, Irvine GJ, Jordan PA, Sagan E. Interrelationships between conformational dynamics and the redox chemistry of S-nitrosothiols. J Am Chem Soc. 1999;121:7115–7123. [Google Scholar]
- Ausma T, De Kok LJ. Atmospheric H2S: impact on plant functioning. Front Plant Sci. 2019;10:743. doi: 10.3389/fpls.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie CK, et al. Detoxification of volcanic sulfur surplus in planta: three different strategies of survival. Environ Exp Bot. 2016;126:44–54. [Google Scholar]
- Banerjee A, Tripathi DK, Roychoudhury A. Hydrogen sulphide trapeze: environmental stress amelioration and phytohormone crosstalk. Plant Physiol Biochem. 2018;132:46–53. doi: 10.1016/j.plaphy.2018.08.028. [DOI] [PubMed] [Google Scholar]
- Batool S, et al. Sulfate is incorporated into cysteine to trigger ABA production and stomatal closure. Plant Cell. 2018;30:2973–2987. doi: 10.1105/tpc.18.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin E, Poilevey A, Hewage NI, Cochet F, Puyaubert J, Bailly C. The significance of hydrogen sulfide for Arabidopsis seed germination. Front Plant Sci. 2016;7:930. doi: 10.3389/fpls.2016.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, et al. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J Exp Bot. 2014;65:527–538. doi: 10.1093/jxb/ert396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, Ahmad MSA. Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut R. 2014;21:717–731. doi: 10.1007/s11356-013-1920-6. [DOI] [PubMed] [Google Scholar]
- Birke H, Kok LJ, Wirtz M, Hell R. The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol. 2015;56:358–367. doi: 10.1093/pcp/pcu166. [DOI] [PubMed] [Google Scholar]
- Bloem E, et al. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J Exp Bot. 2004;55:2305–2312. doi: 10.1093/jxb/erh236. [DOI] [PubMed] [Google Scholar]
- Bloem E, Rubekin K, Haneklaus S, Banfalvi Z, Hesse H, Schnug E. H2S and COS gas exchange of transgenic potato lines with modified expression levels of enzymes involved in sulphur metabolism. J Agron Crop Sci. 2011;197:311–321. [Google Scholar]
- Bloem E, Haneklaus S, Kesselmeier J, Schnug E. Sulfur fertilization and fungal infections affect the exchange of H2S and COS from agricultural crops. J Agr Food Chem. 2012;60:7588–7596. doi: 10.1021/jf301912h. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bucci M, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscl Throm Vas. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- Buchner P, Takahashi H, Hawkesford MJ. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J Exp Bot. 2004;55:1765–1773. doi: 10.1093/jxb/erh206. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TSK, Snyder SH. Nitric oxide—a physiological mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Carballal S, et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radical Bio Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- Carmody M, Waszczak C, Idanheimoa N, Saarinen T, Kangasjarvi J. ROS signalling in a destabilised world: a molecular understanding of climate change. J Plant Physiol. 2016;203:80–94. doi: 10.1016/j.jplph.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Johansson E, Fan Y, Shertzer HG, Vasiliou V, Nebert DW, Dalton TP. Early onset senescence occurs when fibroblasts lack the glutamate-cysteine ligase modifier subunit. Free Radic Biol Med. 2009;47:410–418. doi: 10.1016/j.freeradbiomed.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot. 2011;62:4481–4493. doi: 10.1093/jxb/err145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil. 2013;362:301–318. [Google Scholar]
- Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep. 2015;5:12516. doi: 10.1038/srep12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Hydrogen sulphide improves adaptation of Zea mays seedlings to iron deficiency. J Exp Bot. 2015;66:6605–6622. doi: 10.1093/jxb/erv368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, et al. Exogenous H2S inhibits autophagy in unilateral ureteral obstruction mouse renal tubule cells by regulating the ROS-AMPK signaling pathway. Cell Physiol Biochem. 2018;49:2200–2213. doi: 10.1159/000493824. [DOI] [PubMed] [Google Scholar]
- Chen Z, et al. The hydrogen sulfide signal enhances seed germination tolerance to high temperatures by retaining nuclear COP1 for HY5 degradation. Plant Sci. 2019;285:34–43. doi: 10.1016/j.plantsci.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Chen SS, et al. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol Plant. 2020;13:732–744. doi: 10.1016/j.molp.2020.01.004. [DOI] [PubMed] [Google Scholar]
- Cheng W, et al. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol Bioch. 2013;70:278–286. doi: 10.1016/j.plaphy.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Shi JS, Dong YN, Ma Y, Peng Y, Hu XY, Chen JH. Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul. 2018;84:11–23. [Google Scholar]
- Chiku T, Padovani D, Zhu WD, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot. 2013;64:1953–1966. doi: 10.1093/jxb/ert055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A, Filippou P, Manganaris GA, Fotopoulos V. Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 2014;14:42. doi: 10.1186/1471-2229-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevasanta E, Denicola A, Alvarez B, Moller MN. Solubility and permeation of hydrogen sulfide in lipid membranes. PLoS ONE. 2012;7:e34562. doi: 10.1371/journal.pone.0034562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevasanta E, Lange M, Bonanata J, Coitino EL, Ferrer-Sueta G, Filipovic MR, Alvarez B. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J Biol Chem. 2015;290:26866–26880. doi: 10.1074/jbc.M115.672816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevasanta E, et al. Insights into the mechanism of the reaction between hydrogen sulfide and peroxynitrite. Free Radical Bio Med. 2015;80:93–100. doi: 10.1016/j.freeradbiomed.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Cui WT, et al. Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (Homo) glutathione and reactive oxygen species homeostases. PLoS ONE. 2014;9:e109669. doi: 10.1371/journal.pone.0109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CJ, Batista Fontes EP, Modolo LV. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana Plant Sci. 2017;256:148–159. doi: 10.1016/j.plantsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Das TN, Huie RE, Neta P, Padmaja S. Reduction potential of the sulfhydryl radical: pulse radiolysis and laser flash photolysis studies of the formation and reactions of center ·SH and HSSH·- in aqueous solutions. J Phys Chem A. 1999;103:5221–5226. [Google Scholar]
- Das A, et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Silva CJ, Modolo LV. Hydrogen sulfide: a new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot Bras. 2018;32:150–160. [Google Scholar]
- Da-Silva CJ, Mollica DCF, Vicente MH, Peres LEP, Modolo LV. NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide. 2018;76:164–173. doi: 10.1016/j.niox.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Dawood M, Cao FB, Jahangir MM, Zhang GP, Wu FB. Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater. 2012;209:121–128. doi: 10.1016/j.jhazmat.2011.12.076. [DOI] [PubMed] [Google Scholar]
- De Lira NPV, et al. BigR is a sulfide sensor that regulates a sulfur transferase/dioxygenase required for aerobic respiration of plant bacteria under sulfide stress. Sci Rep. 2018;8:3508. doi: 10.1038/s41598-018-21974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016;79:391–399. [Google Scholar]
- Deng G, Zhou L, Wang Y, Zhang G, Chen X. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta. 2020;251:42. doi: 10.1007/s00425-00019-03334-00429. [DOI] [PubMed] [Google Scholar]
- Desikan R. Sniffing stomata? New Phytol. 2010;188:910–913. doi: 10.1111/j.1469-8137.2010.03529.x. [DOI] [PubMed] [Google Scholar]
- Ding HN, et al. Characterizing physiological and proteomic analysis of the action of H2S to mitigate drought stress in young seedling of wheat. Plant Mol Biol Rep. 2018;36:45–57. [Google Scholar]
- Ding HN, et al. Exogenous hydrogen sulfide alleviates salt stress by improving antioxidant defenses and the salt overly sensitive pathway in wheat seedlings. Acta Physiol Plant. 2019;41:123. [Google Scholar]
- Dooley FD, Nair SP, Ward PD. Increased growth and germination success in plants following hydrogen sulfide administration. PLoS ONE. 2013;8:e62048. doi: 10.1371/journal.pone.0062048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley FD, Wyllie-Echeverria S, Roth MB, Ward PD. Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquat Bot. 2013;105:7–10. [Google Scholar]
- Dooley FD, Wyllie-Echeverria S, Gupta E, Ward PD. Tolerance of Phyllospadix scouleri seedlings to hydrogen sulfide. Aquat Bot. 2015;123:72–75. [Google Scholar]
- Du JB, et al. Hydrogen sulfide suppresses oxidized low-density lipoprotein (Ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the Nuclear Factor kB ( NF-kB) pathway. J Biol Chem. 2014;289:9741–9753. doi: 10.1074/jbc.M113.517995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Jin Z, Liu D, Yang G, Pei Y. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol Biochem. 2017;120:112–119. doi: 10.1016/j.plaphy.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Duan BB, Ma YH, Jiang MR, Yang F, Ni L, Lu W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015;75:33–44. [Google Scholar]
- Dunnicliff HB, Mohammad S, Kishen J. The interaction between nitric oxide and hydrogen sulphide in the presence of water. J Phys Chem. 1931;35:1721–1734. [Google Scholar]
- Fan HH, Guan L, Li TC, Wu QJ, Wu MJ, Cai YP, Lin Y. Hydrogen sulphide alleviates oxidative damage and enhances light energy transformation under high light for Dendrobium officinale. Sci Hort. 2014;177:47–52. [Google Scholar]
- Fang HH, Jing T, Liu ZQ, Zhang LP, Jin ZP, Pei YX. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium. 2014;56:472–481. doi: 10.1016/j.ceca.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Fang T, Cao ZY, Li JL, Shen WB, Huang LQ. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Bioch. 2014;76:44–51. doi: 10.1016/j.plaphy.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Fang T, Li JL, Cao ZY, Chen M, Shen W, Huang LQ. Heme oxygenase-1 is involved in sodium hydrosulfide-induced lateral root formation in tomato seedlings. Plant Cell Rep. 2014;33:969–978. doi: 10.1007/s00299-014-1577-8. [DOI] [PubMed] [Google Scholar]
- Fang HH, Liu ZQ, Jin ZP, Zhang LP, Liu DM, Pei YX. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ Pollut. 2016;213:870–877. doi: 10.1016/j.envpol.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Fang H, et al. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr(VI) tolerance in Arabidopsis. Plant J. 2017;91:1038–1050. doi: 10.1111/tpj.13627. [DOI] [PubMed] [Google Scholar]
- Fang LC, et al. Application of signaling molecules in reducing metal accumulation in alfalfa and alleviating metal-induced phytotoxicity in Pb/Cd-contaminated soil. Ecotox Environ Safe. 2019;182:109459. doi: 10.1016/j.ecoenv.2019.109459. [DOI] [PubMed] [Google Scholar]
- Filipovic MR, Jovanovic VM. More than just an intermediate: hydrogen sulfide signalling in plants. J Exp Bot. 2017;68:4733–4736. doi: 10.1093/jxb/erx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic MR, et al. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic MR, Zivanovic J, Alvarez B, Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev. 2018;118:377–461. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Fu PN, Wang WJ, Hou LX, Liu X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol. 2013;82:295–302. [Google Scholar]
- Fu LH, et al. Hydrogen sulfide inhibits the growth of Escherichia coli through oxidative damage. J Microbiol. 2018;56:238–245. doi: 10.1007/s12275-018-7537-1. [DOI] [PubMed] [Google Scholar]
- Fu Y, et al. Central role of adenosine 5'-phosphosulfate reductase in the control of plant hydrogen sulfide metabolism. Front Plant Sci. 2018;9:1404. doi: 10.3389/fpls.2018.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Dawood M, Wang NH, Wu FB. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019;89:227–237. [Google Scholar]
- Fuentes-Lara LO, et al. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules. 2019;24:2282. doi: 10.3390/molecules24122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtauer L, Weiszmann J, Weckwerth W, Nagele T. Dynamics of plant metabolism during cold acclimation. Int J Mol Sci. 2019;20:21. doi: 10.3390/ijms20215411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SP, et al. Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. HortScience. 2013;48:1385–1392. [Google Scholar]
- Gao XH, et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife. 2015;4:e10067. doi: 10.7554/eLife.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I, Castellano JM, Vioque B, Solano R, Gotor C, Romero LC. Mitochondrial beta-cyanoalanine synthase is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2010;22:3268–3279. doi: 10.1105/tpc.110.076828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I, Rosas T, Bejarano ER, Gotor C, Romero LC. Transient transcriptional regulation of the CYS-C1 gene and cyanide accumulation upon pathogen infection in the plant immune response. Plant Physiol. 2013;162:2015–2027. doi: 10.1104/pp.113.219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010;188:977–984. doi: 10.1111/j.1469-8137.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 2013;201–202:66–73. doi: 10.1016/j.plantsci.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Ge Y, et al. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE. 2017;12:e0180113. doi: 10.1371/journal.pone.0180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotor C, García I, Crespo JL, Romero LC. Sulfide as a signaling molecule in autophagy. Autophagy. 2013;9:609–611. doi: 10.4161/auto.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotor C, Laureano-Marín AM, Moreno I, Aroca Á, García I, Romero LC. Signaling in the plant cytosol: cysteine or sulfide? Amino Acids. 2015;47:2155–2164. doi: 10.1007/s00726-014-1786-z. [DOI] [PubMed] [Google Scholar]
- Guo ZL, Liang YL, Yan JP, Yang E, Li KZ, Xu HN. Physiological response and transcription profiling analysis reveals the role of H2S in alleviating excess nitrate stress tolerance in tomato roots. Plant Physiol Bioch. 2018;124:59–69. doi: 10.1016/j.plaphy.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Hancock JT. Harnessing evolutionary toxins for signaling: Reactive oxygen species, nitric oxide and hydrogen sulfide in plant cell regulation. Front Plant Sci. 2017;8:189. doi: 10.3389/fpls.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JT, Whiteman M. Hydrogen sulfide and reactive friends: the interplay with reactive oxygen species and nitric oxide signalling pathways. Pr Int Plant Sulfur. 2015;2015:153–168. [Google Scholar]
- Hancock JT, Whiteman M. Alone NO longer: interactions of nitric oxide with reactive oxygen species and hydrogen sulfide. Adv Bot Res. 2016;77:1–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Whiteman M. Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann Ny Acad Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- Harrington HM, Smith IK. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980;65:151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Li Y, He LF. Role of nitric oxide and hydrogen sulfide in plant aluminum tolerance. Biometals. 2019;32:1–9. doi: 10.1007/s10534-018-0156-9. [DOI] [PubMed] [Google Scholar]
- Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R. Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell. 2008;20:168–185. doi: 10.1105/tpc.107.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach C, Dekok LJ, Rennenberg H. Net uptake of sulfate and its transport to the shoot in Spinach plants fumigated with H2S or SO2-does atmospheric sulfur affect the inter-organ regulation of sulfur nutrition. Bot Acta. 1995;108:41–46. [Google Scholar]
- Hoffmann MR. Kinetics and mechanism of oxidation of hydrogen-sulfide by hydrogen-peroxide in acidic solution. Environ Sci Technol. 1977;11:61–66. [Google Scholar]
- Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- Hou LX, Zhu D, Ma Q, Zhang DD, Liu X. H2S synthetase AtD-CDes involves in ethylene and drought regulated stomatal movement. Sci Bull. 2016;61:1171–1175. [Google Scholar]
- Hu LY, et al. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J Agric Food Chem. 2012;60:8684–8693. doi: 10.1021/jf300728h. [DOI] [PubMed] [Google Scholar]
- Hu H, Shen W, Li P. Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. Int J Food Sci Tech. 2014;49:399–409. [Google Scholar]
- Hu KD, et al. Hydrogen sulfide prolongs postharvest storage of fresh-cut pears (Pyrus pyrifolia) by alleviation of oxidative damage and inhibition of fungal growth. PLoS ONE. 2014;9:e85524. doi: 10.1371/journal.pone.0085524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Liu D, Li P, Shen W. Hydrogen sulfide delays leaf yellowing of stored water spinach (Ipomoea aquatica) during dark-induced senescence by delaying chlorophyll breakdown, maintaining energy status and increasing antioxidative capacity. Postharvest Biol Tec. 2015;108:8–20. [Google Scholar]
- Hu KD, Bai GS, Li WJ, Yan H, Hu LY, Li YH, Zhang H. Sulfur dioxide promotes germination and plays an antioxidant role in cadmium-stressed wheat seeds. Plant Growth Regul. 2015;75:271–280. [Google Scholar]
- Hu L, et al. Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J Agric Food Chem. 2018;66:9914–9922. doi: 10.1021/acs.jafc.8b03098. [DOI] [PubMed] [Google Scholar]
- Hu KD, et al. Hydrogen sulfide inhibits fruit softening by regulating ethylene synthesis and signaling pathway in tomato (Solanum lycopersicum) HortScience. 2019;54:1824–1830. [Google Scholar]
- Hu JT, Li YL, Liu Y, Kang DI, Wei H, Jeong BR. Hydrogen sulfide affects the root development of strawberry during plug transplant production. Agricult-Basel. 2020;10:12. [Google Scholar]
- Hu KD, et al. A nuclear-localized cysteine desulfhydrase plays a role in fruit ripening in tomato. Hortic Res. 2020;7:211. doi: 10.1038/s41438-41020-00439-41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JJ, et al. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. P Natl Acad Sci USA. 2019;116:21256–21261. doi: 10.1073/pnas.1906768116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J, Huang D, Zhang J, Fang H, Wang B, Wang C, Liao W. Hydrogen sulfide: A gaseous molecule in postharvest freshness. Front Plant Sci. 2018;9:1172. doi: 10.3389/fpls.2018.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybertson BM, Gao BF, Bose SK, McCord JM. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Jahshan S, Dayan L, Jacob G. Nitric oxide-sensitive guanylyl cyclase signaling affects CO2-dependent but not pressure-dependent regulation of cerebral blood flow. Am J Physiol-Reg I. 2017;312:R948–R955. doi: 10.1152/ajpregu.00241.2016. [DOI] [PubMed] [Google Scholar]
- Janicka M, Reda M, Czyzewska K, Kabala K. Involvement of signalling molecules NO, H2O2 and H2S in modification of plasma membrane proton pump in cucumber roots subjected to salt or low temperature stress. Funct Plant Biol. 2018;45:428–439. doi: 10.1071/FP17095. [DOI] [PubMed] [Google Scholar]
- Jez JM, Dey S. The cysteine regulatory complex from plants and microbes: What was old is new again. Curr Opin Struc Biol. 2013;23:302–310. doi: 10.1016/j.sbi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Jia H, Hu Y, Fan T, Li J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci Rep. 2015;5:8251. doi: 10.1038/srep08251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, et al. Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci Rep. 2016;6:39702. doi: 10.1038/srep39702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HL, et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front Plant Sci. 2018;9:1517. doi: 10.3389/fpls.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HL, et al. Hydrogen sulfide - cysteine cycle plays a positive role in Arabidopsis responses to copper oxide nanoparticles stress. Environ Exp Bot. 2018;155:195–205. [Google Scholar]
- Jiang JL, Tian Y, Li L, Yu M, Hou RP, Ren XM. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front Plant Sci. 2019;10:678. doi: 10.3389/fpls.2019.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JL, et al. H2S regulation of metabolism in cucumber in response to salt-stress through transcriptome and proteome analysis. Front Plant Sci. 2020;11:1283. doi: 10.3389/fpls.2020.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun. 2011;414:481–486. doi: 10.1016/j.bbrc.2011.09.090. [DOI] [PubMed] [Google Scholar]
- Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem. 2013;62:41–46. doi: 10.1016/j.plaphy.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Jin ZP, et al. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil. 2017;419:141–152. [Google Scholar]
- Jin ZP, Sun LM, Yang GD, Pei YX. Hydrogen sulfide regulates energy production to delay leaf senescence induced by drought stress in Arabidopsis. Front Plant Sci. 2018;9:1722. doi: 10.3389/fpls.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe TO, Sung DY, Akmakjian G, Pham A, Komives EA, Mendoza-Cozatl DG, Schroeder JI. Feedback inhibition by thiols outranks glutathione depletion: A luciferase-based screen reveals glutathione-deficient γ-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J. 2012;70:783–795. doi: 10.1111/j.1365-313X.2012.04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NC, Yadav D, Ratner K, Kamara I, Aviv-Sharon E, Irihimovitch V, Charuvi D. Sodium hydrosulfide priming improves the response of photosynthesis to overnight frost and day high light in avocado (Persea americana Mill, cv. 'Hass') Physiol Plantarum. 2020;168:394–405. doi: 10.1111/ppl.13023. [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick JA, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Ashraf M. The endogenous L-cysteine desulfhydrase and hydrogen sulfide participate in supplemented phosphorus-induced tolerance to salinity stress in maize (Zea mays) plants. Turk J Bot. 2020;44:36–46. [Google Scholar]
- Kaya C, Aslan M. Hydrogen sulphide partly involves in thiamine-induced tolerance to cadmiumtoxicity in strawberry (Fragaria x ananassa Duch) plants. Environ Sci Pollut R. 2020;27:941–953. doi: 10.1007/s11356-019-07056-z. [DOI] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Akram NA. Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper (Capsicum annuum L.) plants exposed to high zinc regime. Environ Sci Pollut R. 2018;25:12612–12618. doi: 10.1007/s11356-018-1510-8. [DOI] [PubMed] [Google Scholar]
- Kaya C, Akram NA, Ashraf M, Alyemeni MN, Ahmad P. Exogenously supplied silicon (Si) improves cadmium tolerance in pepper (Capsicum annuum L.) by up-regulating the synthesis of nitric oxide and hydrogen sulfide. J Biotechnol. 2020;316:35–45. doi: 10.1016/j.jbiotec.2020.04.008. [DOI] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Alyemeni MN, Ahmad P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant. 2020;168:345–360. doi: 10.1111/ppl.13012. [DOI] [PubMed] [Google Scholar]
- Khan MN, AlZuaibr FM, Al-Huqail AA, Siddiqui MH, Ali HM, Al-Muwayhi MA, Al-Haque HN. Hydrogen sulfide-mediated activation of O-Acetylserine (thiol) Lyase and L/D-Cysteine desulfhydrase enhance dehydration tolerance in Eruca sativa mill. Int J Mol Sci. 2018;19:3981. doi: 10.3390/ijms19123981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc Jpn Acad B-Phys. 2015;91:131–159. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. Faseb J. 2013;27:2451–2457. doi: 10.1096/fj.12-226415. [DOI] [PubMed] [Google Scholar]
- Kimura Y, et al. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep. 2015;5:14774. doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel-Knabner I, et al. Biogeochemistry of paddy soils. Geoderma. 2010;157:1–14. [Google Scholar]
- Koike S, Kawamura K, Kimura Y, Shibuya N, Kimura H, Ogasawara Y. Analysis of endogenous H2S and H2Sn in mouse brain by high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radical Biol Med. 2017;113:355–362. doi: 10.1016/j.freeradbiomed.2017.10.346. [DOI] [PubMed] [Google Scholar]
- Kolupaev YE, Firsova EN, Yastreb TO, Lugovaya AA. The participation of calcium ions and reactive oxygen species in the induction of antioxidant enzymes and heat resistance in plant cells by hydrogen sulfide donor. Appl Biochem Micro. 2017;53:573–579. [Google Scholar]
- Kolupaev YE, Horielova EI, Yastreb TO, Ryabchun NI, Kirichenko VV. Stress-protective responses of wheat and rye seedlings whose chilling resistance was induced with a donor of hydrogen sulfide. Russ J Plant Physiol. 2019;66:540–547. [Google Scholar]
- Koppenol WH, Bounds PL. Signaling by sulfur-containing molecules. Quantitaive aspects. Arch Biochem Biophys. 2017;617:3–8. doi: 10.1016/j.abb.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Stanbury DM, Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radical Bio Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kou NH, Xiang ZX, Cui WT, Li LN, Shen WB. Hydrogen sulfide acts downstream of methane to induce cucumber adventitious root development. J Plant Physiol. 2018;228:113–120. doi: 10.1016/j.jplph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Kushwaha BK, Singh VP. Glutathione and hydrogen sulfide are required for sulfur-mediated mitigation of Cr(VI) toxicity in tomato, pea and brinjal seedlings. Physiol Plant. 2020;168:406–421. doi: 10.1111/ppl.13024. [DOI] [PubMed] [Google Scholar]
- Lai D, et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci. 2014;225:117–129. doi: 10.1016/j.plantsci.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Lancaster JR. How are nitrosothiols formed de novo in vivo? Arch Biochem Biophys. 2017;617:137–144. doi: 10.1016/j.abb.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Laureano-Marín AM, et al. Abscisic acid-triggered persulfidation of the cysteine protease ATG4 mediates regulation of autophagy by sulfide. Plant Cell. 2020;32:3902–3920. doi: 10.1105/tpc.20.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeConte MC (1847) Action des hydracides sur les acides oxygénés. Ann Chim Phys:180−183
- Lefer D. Redox pioneer: professor Hideo Kimura. Antioxid Redox Signal. 2019;30:1699–1708. doi: 10.1089/ars.2018.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Touraine B, Briat JF, Lobreaux S. The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastildial cysteine desulphurase. Biochem J. 2002;366:557–564. doi: 10.1042/BJ20020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Gu SP. Hydrogen sulfide as a signal molecule in hematin-induced heat tolerance of tobacco cell suspension. Biol Plant. 2016;60:595–600. [Google Scholar]
- Li ZG, He QQ. Hydrogen peroxide might be a downstream signal molecule of hydrogen sulfide in seed germination of mung bean (Vigna radiata) Biologia. 2015;70:753–759. [Google Scholar]
- Li ZG, Jin JZ. Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tiss Org. 2016;125:207–214. [Google Scholar]
- Li ZG, Zhu LP. Hydrogen sulfide donor sodium hydrosulfide-induced accumulation of betaine is involved in the acquisition of heat tolerance in maize seedlings. Braz J Bot. 2015;38:31–38. [Google Scholar]
- Li L, Wang YQ, Shen WB. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals. 2012;25:617–631. doi: 10.1007/s10534-012-9551-9. [DOI] [PubMed] [Google Scholar]
- Li ZG, Gong M, Liu P. Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas. Acta Physiol Plant. 2012;34:2207–2213. [Google Scholar]
- Li ZG, Gong M, Xie H, Yang L, Li J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012;185:185–189. doi: 10.1016/j.plantsci.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Li ZG, Ding XJ, Du PF. Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol. 2013;170:741–747. doi: 10.1016/j.jplph.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013;36:1564–1572. doi: 10.1111/pce.12092. [DOI] [PubMed] [Google Scholar]
- Li JS, Jia HL, Wang J, Cao QH, Wen ZC. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma. 2014;251:899–912. doi: 10.1007/s00709-013-0592-x. [DOI] [PubMed] [Google Scholar]
- Li SP, et al. Hydrogen sulfide alleviates postharvest senescence of Broccoli by modulating antioxidant defense and senescence-related gene expression. J Agr Food Chem. 2014;62:1119–1129. doi: 10.1021/jf4047122. [DOI] [PubMed] [Google Scholar]
- Li YJ, Chen J, Xian M, Zhou LG, Han FX, Gan LJ, Shi ZQ. site bioimaging of hydrogen sulfide uncovers its pivotal role in regulating nitric oxide-induced lateral root formation. PLoS ONE. 2014;9:e90340. doi: 10.1371/journal.pone.0090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Luo LJ, Zhu LP. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot Stud. 2014;55:20. doi: 10.1186/1999-3110-1155-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gao MQ, Xue RL, Wang D, Zhao HJ. Effect of hydrogen sulfide on D1 protein in wheat under drought stress. Acta Physiol Plant. 2015;37:225. [Google Scholar]
- Li ZG, Long WB, Yang SZ, Wang YC, Tang JH, Chen T. Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. Vitro Cell Dev-Pl. 2015;51:428–437. [Google Scholar]
- Li ZG, Xie LR, Li XJ. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol. 2015;177:121–127. doi: 10.1016/j.jplph.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Li ZR, et al. Hydrogen sulfide alleviates dark-promoted senescence in postharvest Broccoli. HortScience. 2015;50:416–420. [Google Scholar]
- Li ZG, et al. Endogenous hydrogen sulfide regulated by calcium is involved in thermotolerance in tobacco Nicotiana tabacum L. suspension cell cultures. Acta Physiol Plant. 2015;37:219. [Google Scholar]
- Li D, Limwachiranon J, Li L, Du R, Luo Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016;208:272–278. doi: 10.1016/j.foodchem.2016.03.113. [DOI] [PubMed] [Google Scholar]
- Li ZG, Min X, Zhou ZH. Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci. 2016;7:1621. doi: 10.3389/fpls.2016.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li M, Wei XL, Zhang X, Xue RL, Zhao YD, Zhao HJ. Transcriptome analysis of drought-responsive genes regulated by hydrogen sulfide in wheat (Triticum aestivum L.) leaves. Mol Genet Genomics. 2017;292:1091–1110. doi: 10.1007/s00438-017-1330-4. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Hydrogen sulfide disturbs actin polymerization via S-sulfhydration resulting in stunted root hair growth. Plant Physiol. 2018;178:936–949. doi: 10.1104/pp.18.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Long WB, Yang SZ, Wang YC, Tang JH. Signaling molecule methylglyoxal-induced thermotolerance is partly mediated by hydrogen sulfide in maize (Zea mays L.) seedlings. Acta Physiol Plant. 2018;40:76. [Google Scholar]
- Li Z, et al. The hydrogen sulfide, a downstream signaling molecule of hydrogen peroxide and nitric oxide, involves spermidine-regulated transcription factors and antioxidant defense in white clover in response to dehydration. Environ Exp Bot. 2019;161:255–264. [Google Scholar]
- Li H, Shi J, Wang Z, Zhang W, Yang H. H2S pretreatment mitigates the alkaline salt stress on Malus hupehensis roots by regulating Na+/K+ homeostasis and oxidative stress. Plant Physiol Biochem. 2020;156:233–241. doi: 10.1016/j.plaphy.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to copper oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol Biochem. 2020;156:257–266. doi: 10.1016/j.plaphy.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Li JB, Yu ZX, Choo S, Zhao JY, Wang ZZ, Xie RR. Chemico-proteomics reveal the enhancement of salt tolerance in an invasive plant species via H2S signaling. Acs Omega. 2020;5:14575–14585. doi: 10.1021/acsomega.0c01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lawson GW, Nakamura BN, Ortiz L, Hur JA, Kavanagh TJ, Luderer U. Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. Cancer Res. 2013;73:908–917. doi: 10.1158/0008-5472.CAN-12-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisjak M, et al. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem. 2010;48:931–935. doi: 10.1016/j.plaphy.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Lisjak M, Teklic T, Wilson ID, Wood M, Whiteman M, Hancock JT. Hydrogen sulfide effects on stomatal apertures. Plant Signal Behav. 2011;6:1444–1446. doi: 10.4161/psb.6.10.17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hou L, Liu G, Liu X, Wang X. Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin Sci Bull. 2011;56:3547–3553. [Google Scholar]
- Liu Y, et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Fang H, Pei Y, Jin Z, Zhang L, Liu D. WRKY transcription factors down-regulate the expression of H2S-generating genes, LCD and DES in Arabidopsis thaliana. Sci Bull. 2015;60:995–1001. [Google Scholar]
- Liu X, et al. Hydrogen sulfide alleviates zinc toxicity by reducing zinc uptake and regulating genes expression of antioxidative enzymes and metallothioneins in roots of the cadmium/zinc hyperaccumulator Solanum nigrum L. Plant Soil. 2016;400:177–192. [Google Scholar]
- Liu D, Xu S, Hu H, Pan J, Li P, Shen W. Endogenous hydrogen sulfide homeostasis is responsible for the alleviation of senescence of postharvest daylily flower via increasing antioxidant capacity and maintained energy status. J Agric Food Chem. 2017;65:718–726. doi: 10.1021/acs.jafc.6b04389. [DOI] [PubMed] [Google Scholar]
- Liu YH, Zhang XH, Liu BW, Ao B, Liu Q, Wen SY, Xu YF. Hydrogen sulfide regulates photosynthesis of tall fescue under low-light stress. Photosynthetica. 2019;57:714–723. [Google Scholar]
- Liu ZQ, Cao CY, Li YW, Yang GD, Pei YX. Light regulates hydrogen sulfide signalling during skoto- and photo-morphogenesis in Foxtail millet. Funct Plant Biol. 2019;46:916–924. doi: 10.1071/FP19079. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Li YW, Cao CY, Liang S, Ma YS, Liu X, Pei YX. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol Biol. 2019;99:535–544. doi: 10.1007/s11103-019-00834-w. [DOI] [PubMed] [Google Scholar]
- Liu D, Li J, Li Z, Pei Y. Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.) Hortic Res. 2020;7:14. doi: 10.1038/s41438-019-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FJ, Fu X, Wu GX, Feng YQ, Li FD, Bi HG, Ai XZ. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ Exp Bot. 2020;175:104052. [Google Scholar]
- Liu FJ, Zhang XW, Cai BB, Pan DY, Fu X, Bi HG, Ai XZ. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020;291:110363. doi: 10.1016/j.plantsci.2019.110363. [DOI] [PubMed] [Google Scholar]
- Liu YL, Shen ZJ, Simon M, Li H, Ma DN, Zhu XY, Zheng HL. Comparative proteomic analysis reveals the regulatory effects of H2S on salt tolerance of mangrove plant Kandelia obovata. Int J Mol Sci. 2020;21:118. doi: 10.3390/ijms21010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martin MC, Becana M, Romero LC, Gotor C. Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant Physiol. 2008;147:562–572. doi: 10.1104/pp.108.117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Li D, Du R, Mou W. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci Hort. 2015;183:144–151. [Google Scholar]
- Luo SL, et al. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil. 2020;449:1–10. [Google Scholar]
- Lv WJ, Yang LF, Xu CF, Shi ZQ, Shao JS, Xian M, Chen J. Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Front Plant Sci. 2017;8:232. doi: 10.3389/fpls.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DY, et al. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE. 2016;11:e0163082. doi: 10.1371/journal.pone.0163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machingura M, Sidibe A, Wood AJ, Ebbs SD. The β-cyanoalanine pathway is involved in the response to water deficit in Arabidopsis thaliana. Plant Physiol Bioch. 2013;63:159–169. doi: 10.1016/j.plaphy.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Mei YD, et al. L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol Biol. 2019;99:283–298. doi: 10.1007/s11103-018-00817-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, et al. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci Rep. 2017;7:45995. doi: 10.1038/srep45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa MG, Saegusa D, Fujita M, Tran LSP. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front Plant Sci. 2015;6:1055. doi: 10.3389/fpls.2015.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Zhang Y, Wu Y, Zhang Q. Physiological mechanism of exogenous H2S alleviating drought stress injury during cucumber seed germination. Acta Agricult Boreali-occidentalis Sin. 2018;27:1328–1334. [Google Scholar]
- Mukherjee S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide. 2019;82:25–34. doi: 10.1016/j.niox.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Muller SM, et al. The redox-sensitive module of cyclophilin 20–3, 2-cysteine peroxiredoxin and cysteine synthase integrates sulfur metabolism and oxylipin signaling in the high light acclimation response. Plant J. 2017;91:995–1014. doi: 10.1111/tpj.13622. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Munoz-Vargas MA, Gonzalez-Gordo S, Canas A, Lopez-Jaramillo J, Palma JM, Corpas FJ. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide. 2018;81:36–45. doi: 10.1016/j.niox.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1169. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara N, Nirasawa T, Yoshii T, Niimura Y. Is novel signal transducer sulfur oxide involved in the redox cycle of persulfide at the catalytic site cysteine in a stable reaction intermediate of mercaptopyruvate sulfurtransferase? Antioxid Redox Sign. 2012;16:747–753. doi: 10.1089/ars.2011.4468. [DOI] [PubMed] [Google Scholar]
- Nava M, et al. Spontaneous and selective formation of HSNO, a crucial intermediate linking H2S and nitroso chemistries. J Am Chem Soc. 2016;138:11441–11444. doi: 10.1021/jacs.6b05886. [DOI] [PubMed] [Google Scholar]
- Ni ZJ, et al. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid Med Cell Longev. 2016;2016:1–14. doi: 10.1155/2016/4715651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notni J, Schenk S, Protoschill-Krebs G, Kesselmeier J, Anders E. The missing link in COS metabolism: A model study on the reactivation of carbonic anhydrase from its hydrosulfide analogue. ChemBioChem. 2007;8:530–536. doi: 10.1002/cbic.200600436. [DOI] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide as an oxygen sensor. Antioxid Redox Sign. 2015;22:377–397. doi: 10.1089/ars.2014.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JM, Mateos RM, Lopez-Jaramillo J, Rodriguez-Ruiz M, Gonzalez-Gordo S, Lechuga-Sancho AM, Corpas FJ. Plant catalases as NO and H2S targets. Redox Biol. 2020;34:101525. doi: 10.1016/j.redox.2020.101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial-cells synthesize nitric-oxide from L-Arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Pandey S. Hydrogen sulfide: a new node in the abscisic acid-dependent guard cell signaling network? Plant Physiol. 2014;166:1680–1681. doi: 10.1104/pp.114.251686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Gautam A. Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol Plantarum. 2020;168:511–525. doi: 10.1111/ppl.13064. [DOI] [PubMed] [Google Scholar]
- Pantaleno R, Scuffi D, Garcia-Mata C. Hydrogen sulfide as a guard cell network regulator. New Phytol. 2020 doi: 10.1111/nph.17113. [DOI] [PubMed] [Google Scholar]
- Papanatsiou M, Scuffi D, Blatt MR, Garcia-Mata C. Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol. 2015;168:29–35. doi: 10.1104/pp.114.256057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenbrock J, Reimenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - From the field to the test tube and back. Plant Biology. 2007;9:582–588. doi: 10.1055/s-2007-965424. [DOI] [PubMed] [Google Scholar]
- Parveen M, Asaeda T, Rashid MH. Hydrogen sulfide induced growth, photosynthesis and biochemical responses in three submerged macrophytes. Flora. 2017;230:1–11. [Google Scholar]
- Peng RY, et al. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.) Plant Cell Rep. 2016;35:2325–2340. doi: 10.1007/s00299-016-2037-4. [DOI] [PubMed] [Google Scholar]
- Pierce JA. A study of the reaction between nitric oxide and hydrogen sulphide. J Phys Chem. 1929;33:22–36. [Google Scholar]
- Pilon-Smits EAH, et al. Characterization of a NifS-Like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiol. 2002;130:1309–1318. doi: 10.1104/pp.102.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch BC, et al. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J Exp Bot. 2019;70:5051–5069. doi: 10.1093/jxb/erz257. [DOI] [PubMed] [Google Scholar]
- Proveniers MCG, van Zanten M. High temperature acclimation through PIF4 signaling. Trends Plant Sci. 2013;18:59–64. doi: 10.1016/j.tplants.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Qian P, Sun R, Ali B, Gill RA, Xu L, Zhou WJ. Effects of hydrogen sulfide on growth, antioxidative capacity, and ultrastructural changes in oilseed rape seedlings under aluminum toxicity. J Plant Growth Regul. 2014;33:526–538. [Google Scholar]
- Qiao ZJ, Jing T, Liu ZQ, Zhang LP, Jin ZP, Liu DM, Pei YX. H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil. 2015;393:137–146. [Google Scholar]
- Qiao ZJ, et al. CDPKs enhance Cd tolerance through intensifying H2S signal in Arabidopsis thaliana. Plant Soil. 2016;398:99–110. [Google Scholar]
- Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D. Seed germination and vigor. Annu Rev Plant Biol. 2012;63:507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Year Diabetes Obesity. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravina CG, et al. The sac mutants of Chlamydomonas reinhardtii reveal transcriptional and posttranscriptional control of cysteine biosynthesis. Plant Physiol. 2002;130:2076–2084. doi: 10.1104/pp.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider A, Wegele R, Schmidt A, Papenbrock J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. Febs J. 2005;272:1291–1304. doi: 10.1111/j.1742-4658.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Serrano M, Pazmino DM, Sparkes I, Rochetti A, Hawes C, Romero-Puertas MC, Sandalio LM. 2,4-Dichlorophenoxyacetic acid promotes S-nitrosylation and oxidation of actin affecting cytoskeleton and peroxisomal dynamics. J Exp Bot. 2014;65:4783–4793. doi: 10.1093/jxb/eru237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LC, Aroca MA, Laureano-Marin AM, Moreno I, Garcia I, Gotor C. Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant. 2014;7:264–276. doi: 10.1093/mp/sst168. [DOI] [PubMed] [Google Scholar]
- Santos AI, Martinez-Ruiz A, Araujo IM. S-nitrosation and neuronal plasticity. Br J Pharmacol. 2015;172:1468–1478. doi: 10.1111/bph.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D, Alvarez C, Laspina N, Gotor C, Lamattina L, Garcia-Mata C. Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014;166:2065–2076. doi: 10.1104/pp.114.245373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D, Lamattina L, García-Mata C (2016) Decoding the interaction between nitric oxide and hydrogen sulfide in stomatal movement. In: Gasotransmitters in Plants. Signaling and communication in plants, pp 271–287. 10.1007/978-3-319-40713-5_13
- Scuffi D, et al. Hydrogen sulfide increases production of NADPH oxidase-dependent hydrogen peroxide and phospholipase D-derived phosphatidic acid in guard cell signaling. Plant Physiol. 2018;176:2532–2542. doi: 10.1104/pp.17.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, et al. Hydrogen sulfide-linked sulfhydration of NF-kappa B mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S, Lindermayr C, Durner J. Identification of S-nitrosylated proteins in plants. Methods Enzymol. 2008;440:283–293. doi: 10.1016/S0076-6879(07)00818-X. [DOI] [PubMed] [Google Scholar]
- Shan CJ, Zhang SL, Ou XQ. The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma. 2018;255:1257–1262. doi: 10.1007/s00709-018-1213-5. [DOI] [PubMed] [Google Scholar]
- Shan CJ, Wang BS, Sun HL, Gao S, Li H. H2S induces NO in the regulation of AsA-GSH cycle in wheat seedlings by water stress. Protoplasma. 2020;257:1487–1493. doi: 10.1007/s00709-020-01510-3. [DOI] [PubMed] [Google Scholar]
- Shen JJ, Xing TJ, Yuan HH, Liu ZQ, Jin ZP, Zhang LP, Pei YX. Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS ONE. 2013;8:e77047. doi: 10.1371/journal.pone.0077047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, et al. A putative rice L-cysteine desulfhydrase encodes a true L-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul. 2019;89:217–226. [Google Scholar]
- Shen J, et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell. 2020;32:1000–1017. doi: 10.1105/tpc.19.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HT, Ye TT, Chan ZL. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol Bioch. 2014;74:99–107. doi: 10.1016/j.plaphy.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Grossman AR. Binding of cysteine synthase to the STAS domain of sulfate transporter and its regulatory consequences. J Biol Chem. 2010;285:25094–25102. doi: 10.1074/jbc.M110.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-Mercaptopyruvate Sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- Shivaraj SM, et al. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol Plant. 2020;168:437–455. doi: 10.1111/ppl.13028. [DOI] [PubMed] [Google Scholar]
- Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Singh S, Kumar J, Prasad SM. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J Plant Physiol. 2015;181:20–29. doi: 10.1016/j.jplph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Nitric-Oxide - 1st in a new class of neurotransmitters. Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stamler JS. Redox signaling—nitrosylation and related target interactions of nitric-oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Stimler K, Berry JA, Yakir D. Effects of carbonyl sulfide and carbonic anhydrase on stomatal conductance. Plant Physiol. 2012;158:524–530. doi: 10.1104/pp.111.185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SA, et al. Nitric oxide is reduced to HNO by proton-coupled nucleophilic attack by ascorbate, tyrosine, and other alcohols. A new route to HNO in biological media? J Am Chem Soc. 2015;137:4720–4727. doi: 10.1021/ja512343w. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang RG, Zhang X, Yu YC, Zhao R, Li ZY, Chen SL. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol Bioch. 2013;65:67–74. doi: 10.1016/j.plaphy.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang W, Zeng T, Nie Q, Zhang F, Zhu L. Hydrogen sulfide inhibits enzymatic browning of fresh-cut lotus root slices by regulating phenolic metabolism. Food Chem. 2015;177:376–381. doi: 10.1016/j.foodchem.2015.01.065. [DOI] [PubMed] [Google Scholar]
- Sun KK, Zhu DB, Yao GF, Hu KD, Zhang H. Sulfur dioxide acts as an antioxidant and delays programmed cell death in wheat aleurone layers upstream of H2S and NO signaling pathways. Biol Plant. 2018;62:809–816. [Google Scholar]
- Suzuki N. Temperature stress and responses in plants. Int J Mol Sci. 2019;20:2001. doi: 10.3390/ijms20082001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szadvari I, et al. Sodium/calcium exchanger is involved in apoptosis induced by H2S in tumor cells through decreased levels of intracellular pH. Nitric Oxide. 2019;87:1–9. doi: 10.1016/j.niox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Tai CH, Cook PF. O-acetylserine sulfhydrylase. Adv Enzymol. 2000;74:185. doi: 10.1002/9780470123201.ch5. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol. 2011;62(62):157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- Talukdar D. Functional interplay between glutathione and hydrogen sulfide in regulation of thiol cascade during arsenate tolerance of common bean (Phaseolus vulgaris L.) genotypes. 3 Biotech. 2015;5:819–829. doi: 10.1007/s13205-015-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar D. Glutathione deficiency in a grass pea (Lathyrus sativus L.) mutant reveals major reshuffle in up-stream thiol cascade and down-stream antioxidant defense under arsenate stress. Braz J Bot. 2016;39:55–66. [Google Scholar]
- Tang J, Hu KD, Hu LY, Li YH, Liu YS, Zhang H. Hydrogen sulfide acts as a fungicide to alleviate senescence and decay in fresh-cut sweetpotato. HortScience. 2014;49:938–943. [Google Scholar]
- Tishel M, Mazelis M. Enzymatic degradation of L-cystine by cytoplasmic particles from cabbage leaves. Nature. 1966;211:745. doi: 10.1038/211745a0. [DOI] [PubMed] [Google Scholar]
- Valivand M, Amooaghaie R, Ahadi A. Interplay between hydrogen sulfide and calcium/calmodulin enhances systemic acquired acclimation and antioxidative defense against nickel toxicity in zucchini. Environ Exp Bot. 2019;158:40–50. [Google Scholar]
- Valivand M, Amooaghaie R, Ahadi A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol Bioch. 2019;143:286–298. doi: 10.1016/j.plaphy.2019.09.016. [DOI] [PubMed] [Google Scholar]
- Vandiver MS, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauclare P, et al. Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5'-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J. 2002;31:729–740. doi: 10.1046/j.1365-313x.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- Vescovi M, Zaffagnini M, Festa M, Trost P, Lo-Schiavo F, Costa A. Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed arabidopsis roots. Plant Physiol. 2013;162:333–346. doi: 10.1104/pp.113.215194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? Faseb J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Li L, Cui WT, Xu S, Shen WB, Wang R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil. 2012;351:107–119. [Google Scholar]
- Wang PC, et al. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci USA. 2015;112:613–618. doi: 10.1073/pnas.1423481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wan R, Shi Y, Xue S. Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ modules. Mol Plant. 2016;9:489–491. doi: 10.1016/j.molp.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Wang SS, Zhang YX, Yang F, Huang ZQ, Tang J, Hu KD, Zhang H. Sulfur dioxide alleviates programmed cell death in barley aleurone by acting as an antioxidant. PLoS ONE. 2017;12:e0188289. doi: 10.1371/journal.pone.0188289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Ji F, Zhang YY, Hou JJ, Liu WW, Huang JJ, Liang WH. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ. 2019;42:2340–2356. doi: 10.1111/pce.13555. [DOI] [PubMed] [Google Scholar]
- Wang HR, Che YH, Huang D, Ao H. Hydrogen sulfide mediated alleviation of cadmium toxicity in Phlox paniculata L. and establishment of a comprehensive evaluation model for corresponding strategy. Int J Phytoremediat. 2020;22:1085–1095. doi: 10.1080/15226514.2020.1730299. [DOI] [PubMed] [Google Scholar]
- Watts SF. The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos Environ. 2000;34:761–779. [Google Scholar]
- Wedmann R, et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem Sci. 2016;7:3414–3426. doi: 10.1039/c5sc04818d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, et al. Functional analysis of the role of hydrogen sulfide in the regulation of dark-induced leaf senescence in Arabidopsis. Sci Rep. 2017;7:2615. doi: 10.1038/s41598-017-02872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GQ, Zhang WW, Cao H, Yue SS, Li P, Yang HQ. Effects hydrogen sulfide on the antioxidant system and membrane stability in mitochondria of Malus hupehensis under NaCl stress. Biol Plant. 2019;63:228–236. [Google Scholar]
- Wen YD, Wang H, Zhu YZ. The drug developments of hydrogen sulfide on cardiovascular disease. Oxid Med Cell Longev. 2018;2018:4010395. doi: 10.1155/2018/4010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz M, Hell R. Functional analysis of the cysteine synthase protein complex from plants: structural, biochemical and regulatory properties. J Plant Physiol. 2006;163:273–286. doi: 10.1016/j.jplph.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wong PSY, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochem. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- Wu MT, Wallner SJ. Heat-stress responses in cultured plant cells—heat tolerance induced by heat-shock versus elevated growing temperature. Plant Physiol. 1984;75:778–780. doi: 10.1104/pp.75.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LY, Wang R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhang C, Chen L, Li G, Wang Q, Shi J. Inhibition of hydrogen sulfide and hypotaurine on Monilinia fructicoladisease in peach fruit. Acta Hort. 2018;2018:257–266. [Google Scholar]
- Wu L, et al. Hydrogen sulfide inhibits high glucose-induced neuronal senescence by improving autophagic flux via up-regulation of SIRT1. Front Mol Neurosci. 2019;12:00194. doi: 10.3389/fnmol.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YS, Wu XL, Sun MX, Peng FT. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front Plant Sci. 2020;11:00696. doi: 10.3389/fpls.2020.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Lai DW, Mao Y, Zhang W, Shen WB, Guan RZ. Molecular cloning, characterization and expression analysis of a novel gene encoding L-Cysteine desulfhydrase from Brassica napus. Mol Biotechnol. 2013;54:737–746. doi: 10.1007/s12033-012-9621-9. [DOI] [PubMed] [Google Scholar]
- Xie YJ, Zhang C, Lai DW, Sun Y, Samma MK, Zhang J, Shen WB. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol. 2014;171:53–62. doi: 10.1016/j.jplph.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Xie ZZ, Shi MM, Xie L, Wu ZY, Li G, Hua F, Bian JS. Sulfhydration of p66Shc at Cysteine59 mediates the antioxidant effect of hydrogen sulfide. Antioxid Redox Sign. 2014;21:2531–2542. doi: 10.1089/ars.2013.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YF, Zhang M, Qi ZQ, Li YQ, Shi ZQ, Chen J. Cinnamaldehyde promotes root branching by regulating endogenous hydrogen sulfide. J Sci Food Agri. 2016;96:909–914. doi: 10.1002/jsfa.7164. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Nakamura T, Kusano T, Sano H. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and β-cyanoalanine synthase. Plant Cell Physiol. 2000;41:465–476. doi: 10.1093/pcp/41.4.465. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Cohen MF. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide. 2016;55–56:91–100. doi: 10.1016/j.niox.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Shimoji H, Ohshiro Y, Sakihama Y. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide. 2001;5:261–270. doi: 10.1006/niox.2001.0353. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Ogura MP, Kingjoe KA, Cohen MF. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: Implications for the linkage between D-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants-Basel. 2019;8:411. doi: 10.3390/antiox8090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GD, et al. Hydrogen sulfide protects against cellular senescence via S-Sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Sign. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.) J Integr Agr. 2016;15:2745–2758. [Google Scholar]
- Yang K, Coburger I, Langner JM, Peter N, Hoshi T, Schonherr R, Heinemann SH. Modulation of K+ channel N-type inactivation by sulfhydration through hydrogen sulfide and polysulfides. Pflugers Arch. 2019;471:557–571. doi: 10.1007/s00424-018-2233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao GF, et al. Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J Agric Food Chem. 2018;66:10380–10387. doi: 10.1021/acs.jafc.8b03951. [DOI] [PubMed] [Google Scholar]
- Yastreb TO, Kolupaev YE, Havva EN, Horielova EI, Dmitriev AP. Involvement of the JIN1/MYC2 transcription factor in inducing salt resistance in Arabidopsis plants by exogenous hydrogen sulfide. Cytol Genet. 2020;54:96–102. [Google Scholar]
- Ye XF, et al. Cinnamaldehyde ameliorates cadmium-inhibited root elongation in tobacco seedlings via decreasing endogenous hydrogen sulfide production. Molecules. 2017;22:15. doi: 10.3390/molecules22010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XY, Qiu XM, Sun YY, Li ZG. Interplay between hydrogen sulfide and methylglyoxal initiates thermotolerance in maize seedlings by modulating reactive oxidative species and osmolyte metabolism. Protoplasma. 2020;257:186. doi: 10.1007/s00709-020-01516-x. [DOI] [PubMed] [Google Scholar]
- Yong QC, Hu LF, Wang SH, Huang DJ, Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- Yong QC, et al. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Sign. 2011;14:2081–2091. doi: 10.1089/ars.2010.3572. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhou XY, Zhu ZH, Zhou KJ. Sodium hydrosulfide mitigates cadmium toxicity by promoting cadmium retention and inhibiting its translocation from roots to shoots in Brassica napus. J Agr Food Chem. 2019;67:433–440. doi: 10.1021/acs.jafc.8b04622. [DOI] [PubMed] [Google Scholar]
- Zanganeh R, Jamei R, Rahmani F. Modulation of growth and oxidative stress by seed priming with salicylic acid in Zea mays L. under lead stress. J Plant Interact. 2019;14:369–375. [Google Scholar]
- Zhang RC, Tielborger K. Facilitation from an intraspecific perspective—stress tolerance determines facilitative effect and response in plants. New Phytol. 2019;221:2203–2212. doi: 10.1111/nph.15528. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol. 2009;51:1086–1094. doi: 10.1111/j.1744-7909.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu LY, Li P, Hu KD, Jiang CX, Luo JP. Hydrogen sulfide alleviated chromium toxicity in wheat. Biol Plant. 2010;54:743–747. [Google Scholar]
- Zhang H, Jiao H, Jiang CX, Wang SH, Wei ZJ, Luo JP, Jones RL. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol Plant. 2010;32:849–857. [Google Scholar]
- Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol. 2010;52:556–567. doi: 10.1111/j.1744-7909.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Tec. 2011;60:251–257. [Google Scholar]
- Zhang C, Shi JY, Zhu LQ, Li CL, Wang QG. Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. Int J Agr Biol Eng. 2014;7:114–122. [Google Scholar]
- Zhang YX, et al. The hydrogen sulfide donor NaHS delays programmed cell death in barley aleurone layers by acting as an antioxidant. Oxid Med Cell Longev. 2015;2015:11. doi: 10.1155/2015/714756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LP, et al. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa (L. ssp pekinensis) Oxid Med Cell Longev. 2015;2015:804603. doi: 10.1155/2015/804603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Luo Q, Wang RL, Xu J. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci Rep. 2017;7:868. doi: 10.1038/s41598-017-01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cai W, Ji TT, Ye L, Lu YT, Yuan TT. WRKY13 enhances cadmium tolerance by promoting D-cysteine desulfhydrase and hydrogen sulfide production. Plant Physiol. 2020;183:345–357. doi: 10.1104/pp.19.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. Hydrogen sulfide (H2S), a signaling molecule in plant stress responses. J Integr Plant Biol. 2021;63:146–160. doi: 10.1111/jipb.13022. [DOI] [PubMed] [Google Scholar]
- Zhao N, et al. Hydrogen sulfide mediates K+ and Na+ homeostasis in the roots of salt-resistant and salt-sensitive poplar species subjected to NaCl stress. Front Plant Sci. 2018;9:1366. doi: 10.3389/fpls.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Hu LY, Hu KD, Wu J, Yang F, Zhang H. Hydrogen sulfide alleviates senescence of fresh-cut apple by regulating antioxidant defense system and senescence-related gene expression. HortScience. 2016;51:152–158. [Google Scholar]
- Zhou H, Ding L, Wu ZY, Cao X, Zhang QC, Lin L, Bian JS. Hydrogen sulfide reduces RAGE toxicity through inhibition of its dimer formation. Free Radical Bio Med. 2017;104:262–271. doi: 10.1016/j.freeradbiomed.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Wang Y, Ye XY, Li ZG. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front Plant Sci. 2018;9:1288. doi: 10.3389/fpls.2018.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. Cloning and characterization of a gene encoding true D-cysteine desulfhydrase from Oryza sativa. Plant Mol Biol Rep. 2020;38:95–113. [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Wang W, Shi J, Zhang W, Shen Y, Du H, Wu S. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J Sci Food Agric. 2014;94:2699–2704. doi: 10.1002/jsfa.6613. [DOI] [PubMed] [Google Scholar]
- Zhu DB, et al. Sulfur dioxide enhances endogenous hydrogen sulfide accumulation and alleviates oxidative stress induced by aluminum stress in germinating wheat seeds. Oxid Med Cell Longev. 2015;2015:1–11. doi: 10.1155/2015/612363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CQ, et al. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in rice. Front Plant Sci. 2018;9:294. doi: 10.3389/fpls.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Hou LX, Xiao PL, Guo Y, Deyholos MK, Liu X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019;280:132–142. doi: 10.1016/j.plantsci.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Ziogas V, Tanou G, Belghazi M, Filippou P, Fotopoulos V, Grigorios D, Molassiotis A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol Biol. 2015;89:433–450. doi: 10.1007/s11103-015-0379-x. [DOI] [PubMed] [Google Scholar]
- Ziogas V, Molassiotis A, Fotopoulos V, Tanou G. Hydrogen sulfide: a potent tool in postharvest fruit biology and possible mechanism of action. Front Plant Sci. 2018;9:1375. doi: 10.3389/fpls.2018.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.