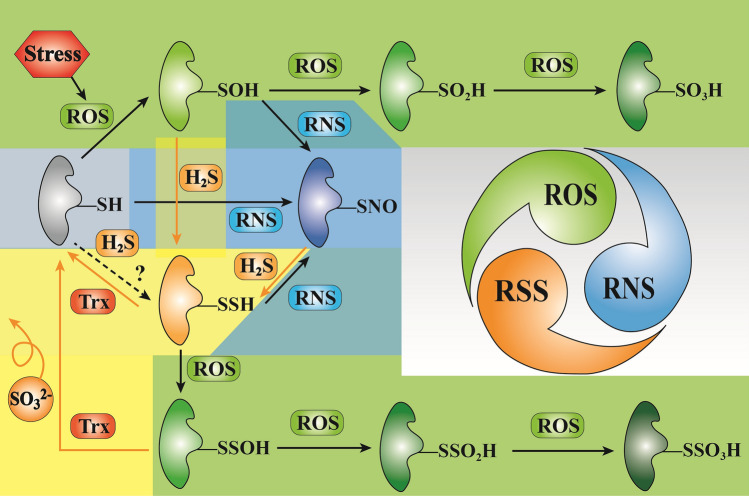
Fig. 4.
Multiple post-translational modifications on cysteine residues of proteins. Protein cysteine thiols (RSH) can be sulfonated to produce RSOH with increasing ROS. Upon continuous exposure to ROS, RSOH could further generate irreversible sulfinic (RSO2H) and sulfonic acids (RSO3H). On the basis of RSOH, H2S and NO can be persulfidated and S-nitrosylated, respectively, to produce RSSH and RSNO. In the presence of nitrotransferase, NO can also react directly with RSH to produce RSNO. Once persulfidated cysteine thiols encounter ROS, RSSH will rapidly react with ROS to form adducts (RSSOH, RSSO2H and RSSO3H). Among them, RSSH and RSSOH can be reduced back to thiols by the action of the thioredoxin (Trx)