Abstract
Background
COVID-19 outbreaks in aged care facilities (ACFs) often have devastating consequences. However, epidemiologically these outbreaks are not well defined. We aimed to define such outbreaks in ACFs by systematically reviewing literature published during the current COVID-19 pandemic.
Methods
We searched 11 bibliographic databases for literature published on COVID-19 in ACFs between December 2019 and September 2020. Original studies reporting extractable epidemiological data as part of outbreak investigations or non-outbreak surveillance of ACFs were included in this systematic review and meta-analysis. PROSPERO registration: CRD42020211424.
Findings
We identified 5,148 publications and selected 49 studies from four continents reporting data on 214,380 residents in 8,502 ACFs with 25,567 confirmed cases of COVID-19. Aged care residents form a distinct vulnerable population with single-facility attack rates of 45% [95% CI 32–58%] and case fatality rates of 23% [95% CI 18–28%]. Of the cases, 31% [95% CI 28–34%] were asymptomatic. The rate of hospitalization amongst residents was 37% [95% CI 35–39%]. Data from 21 outbreaks identified a resident as the index case in 58% of outbreaks and a staff member in 42%. Findings from the included studies were heterogeneous and of low to moderate quality in risk of bias assessment.
Interpretation
The clinical presentation of COVID-19 varies widely in ACFs residents, from asymptomatic to highly serious cases. Preventing the introduction of COVID-19 into ACFs is key, and both residents and staff are a priority group for COVID-19 vaccination. Rapid diagnosis, identification of primary and secondary cases and close contacts plus their isolation and quarantine are of paramount importance.
Funding
Queensland Advancing Clinical Research Fellowship awarded to Prof. Gulam Khandaker by Queensland Health's Health Innovation, Investment and Research Office (HIRO), Office of the Director-General.
Keywords: Coronavirus disease 2019, Covid-19, SARS-CoV-2, Aged care facility, Nursing homes, Clinical features, Epidemiology, Outbreak
Research in context:
Evidence before the study
COVID-19 outbreaks in aged care facilities often have devastating consequences. However, there is no systematic review on the epidemiology and risk factors of COVID-19 in aged care facilities.
Added value of this study
We performed a systematic review looking at epidemiological evidence of COVID-19 outbreaks in aged care facilities and identified 49 observational studies in aged care facilities across 14 countries and four continents, reporting data on at least 214,380 residents in 8502 care homes with 25,567 confirmed cases of COVID-19, of which 36 comparative studies were included in a meta-analysis of a total of 17,856 lab-confirmed cases of COVID-19. Aged care residents form a distinct vulnerable population with single-facility attack rates of 45% [95% CI 32–58%] and case fatality rates of 23% [95% CI 18–28%]. Of the cases reviewed, nearly one third (31% [95% CI 28–34%]) were asymptomatic and over one third (37% [95% CI 35–39%]) of the confirmed cases required hospitalisation. Outbreaks were introduced into aged care facilities by both residents (58%) and staff (42%).
Implications of all the available evidence
As best we know, this is the first comprehensive worldwide evaluation of the epidemiology of COVID-19 outbreaks in aged care facilities (ACFs). We find both high attack and fatality rates, but many asymptomatic cases too; preventing the introduction of COVID-19 infection into ACFs should be an urgent priority. During outbreaks, early case identification through facility-wide serial testing of all residents and staff plus reducing staff movements within and between ACFs are proven strategies to decrease the impact of COVID-19 in ACFs. However, evidence on COVID-19 outbreak prevention and control strategies in ACFs are limited and heterogeneous, so further research on this topic is urgently needed. Aged care residents and staff are a priority group for COVID-19 vaccination.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease-2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a global pandemic on 11 March 2020, by the World Health Organisation, which noted a ‘devastating toll’ on older people living in care homes. [1,2] COVID-19 presents risks to older people, who are particularly vulnerable to respiratory diseases. [3] Avoidance of exposure plus vaccination are critical to prevent or diminish outbreaks in residential aged care facilities (RACFs), [4] and a comprehensive understanding of the epidemiology of COVID-19 outbreaks in RACFs is needed to aid disease prevention.
Since the emergence of SARS-CoV-2, there have been 100 million confirmed cases and more than 2 million deaths across 191 countries worldwide as of January 29, 2021. [5] Numerous devastating outbreaks have been reported in care facilities, with varying fatality rates of 8% in South Korea, 41% in the United States, 44% in the United Kingdom, 75% in Australia, and 80% in Canada. [6] The older adult population suffers from increased rates of hospitalisation and higher case fatality rates compared to the younger population. [7,8] Due to age-related immunosenescence, cognitive and functional impairment, and multiple co-morbid diseases, manifestations of infectious diseases are variable amongst older adult populations. [9] Care facilities across the world forms diverse congregant settings with substantial differences in the dependency level of the residents and provision of wide variety of services with or without delivering skilled nursing care. [10] The gathering of residents, healthcare professionals, and visitors in settings like aged care facilities contributes to an increased risk of disease transmission, making the control of an outbreak with non-pharmacological interventions difficult. [11,12] Moreover, the SARS-CoV-2 has a long incubation period and an individual may remain asymptomatic while infectious. [13] Unlike influenza outbreaks, a COVID-19 outbreak spreads rapidly (i.e. high attack rate and higher R0), is more clinically severe, and involves increased adverse consequences such as mortality. [14,15] Given that there is now a wealth of individual reports of outbreaks and fatalities in aged care facilities, it is possible to review and summarise the epidemiological index of SARS-CoV-2 infection, transmission characteristics, clinical manifestations, and co-morbid risk factors amongst care home residents.
We aimed to define the epidemiology of COVID-19 outbreaks in RACFs to estimate the epidemiological index of the disease for these vulnerable populations. In this ongoing pandemic, care homes are experiencing a lots of challenges to ensure adequate resources to manage outbreaks. [16] Hence, there is an urgent need for real-time data collection to mitigate outbreaks, develop surveillance strategies, and formulate an effective response to COVID-19 clusters in congregate settings.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines and is based on a protocol (PROSPERO 2020 CRD42020211424) registered prospectively on 28 September 2020. [17] An information specialist (CK) searched the following major bibliographic databases to locate literature on COVID-19 in care homes: Ovid Medline, Ovid Embase, CINAHL, SCOPUS, and Web of Science, limiting the publication date from 01 January 2019, to 28 September 2020, consistent with the emergence of SARS-CoV-2. Initial scoping searches were undertaken in Ovid Medline to test and refine the search terms. COVID-19 and SARS-CoV-2 terms were combined with terms synonymous with residential aged care facilities. Where available, both synonyms and equivalent text word terms were used (e.g. ‘Coronavirus’, ‘Coronavirus infections’, ‘nursing homes’ and ‘homes for the aged’). Truncation was used to ensure variant terms were retrieved. No language limits were applied. The full Ovid Medline search strategy, including all terms used is available in the supplementary materials (Supplementary Table 1). Grey literature sources were searched by another team member (MRH) in consultation with the information specialist (CK). These sources included the WHO Global Health Library, Virtual Health Library, Google Scholar, and the Cochrane Library's COVID-19 Study Register (https://covid-19.cochrane.org/). Pre-print databases including medRxiv.org, bioRxiv.org were also searched.
2.2. Study eligibility and quality assessment
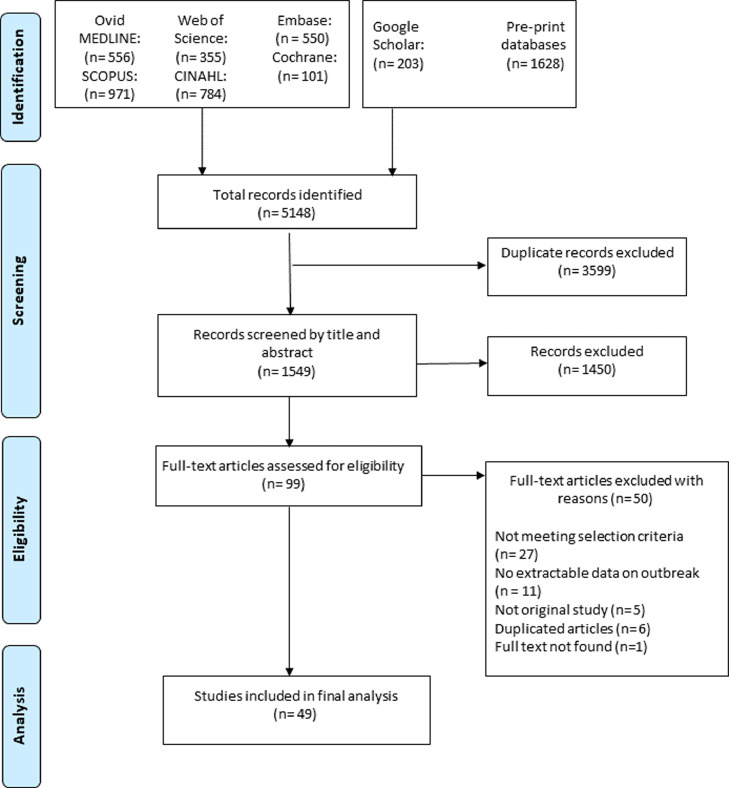
We included original studies reporting extractable epidemiological data on COVID-19 outbreaks as part of outbreak investigations or non-outbreak surveillance of COVID-19 in care home residents. Two independent reviewers (MRH, HOM) initially assessed the relevance of items retrieved from the databases and grey literature searches against the selection criteria. After initial selection, the full text of each report was collected and reviewed for eligibility by two authors (MRH, HOM). Each stage of the screening phase was performed independently, with rigorous checking from both reviewers. Any discordant opinions were escalated, with consensus determined following discussion with the senior author (GK) (Fig. 1). Studies were excluded if they did not report PCR-confirmed SARS-CoV-2 cases from aged care facilities or if the study setting was a hospital or older adults individuals residing in the community. In cases where there was more than one published study from a single data source, the most comprehensive article was prioritised. When there was more than one publication from a single centre, author names, city and country of case recruitment, duration of the study, facility type, and the number of reported cases were cross-checked with caution and duplicate articles were excluded. Articles were also excluded if there was inadequate data to meet the inclusion criteria, if there was non-extractable data, or the full text of the report was inaccessible.
Fig. 1.
Flow diagram of the study selection process.
All included studies were quality-assessed based on the STROBE reporting guidelines for observational studies and utilising the questions from the Meta Quality Appraisal Tool (MetaQAT; Supplementary Table 2). [18] To assess potential bias, methodological flaws, statistical analysis, and the outcome reporting of the studies, quality appraisal was performed following key checklists from the MetaQAT tool, highlighting four criteria: relevancy, reliability, validity, and applicability. Two reviewers (MRH, HOM) performed the quality assessment independently in parallel, and results were reported accordingly (Supplementary Table 2). Dissimilarities were reviewed by the senior author (GK) and consensus reached with further discussion.
2.3. Data abstraction
Independent but parallel data abstraction of all included reports was undertaken by two reviewers (MRH, HOM), with the final results reviewed by the senior author (GK) for resolution of any discrepancies. Microsoft Excel 2010 was utilised for extraction of the following variables from the included studies, when available: author, publication year, journal, city and country of patient recruitment, sample size, setting, number of facilities, and number of outbreaks. Additionally, the method of confirmed diagnosis, definition and duration of the outbreak, and definitions for asymptomatic, symptomatic, and pre-symptomatic cases were extracted. Index case characteristics (age, gender, type of index case, symptom status), the number of residents and staff in the facility, their bed capacity, testing method (symptom-based test, facility-wide mass testing, universal screening), and the total number of tested participants was also collected. Also included were RT-PCR confirmation, demographic characteristics of confirmed cases (age, sex, ethnicity, etc.), R0 value or range, clinical features at the time of diagnosis, co-morbidity status, attack rate, secondary attack rate, case fatality rate and mortality rate amongst the residents, hospitalisation rate, severity grade of the illness, and case outcomes.
2.4. Principle summary measures and synthesis
The primary outcomes of the review were three key summary measures: attack rate (AR), case fatality rate (CFR), and mortality rate (MR). Attack rates were estimated by dividing the number of confirmed cases reported by the number of individuals at risk for SARS-CoV-2 infection. The ‘at-risk’ population was determined based on the group under observation in the study (e.g. all residents, all residents and employees, or residents under surveillance for possible COVID-19 infection). If no such population estimate was reported, the number of beds in the facility was utilised for the number of ‘at-risk’ individuals. All included articles were subjectively sub-grouped into low-fidelity and high-fidelity studies based on the confidence in the reported data. Studies that explicitly reported the total number of at-risk participants in the facility, the total number of RT-PCR-confirmed cases, and the number of individual participants tested during the study period separately were designated as high-fidelity studies. Studies missing any of the key denominators to estimate the principle summary measures were identified as low-fidelity studies. For residents diagnosed with RT-PCR-confirmed SARS-CoV-2 infection, demographic and disease characteristics were descriptively analysed. To estimate the pooled attack rate and case fatality rate for all included studies with available data, a random effects meta-analysis of proportion with a 95% confidence interval was performed due to the substantial heterogeneity in study designs. Proportion meta-analyses for the principle summary measures (AR, CFR) were further sub-grouped based on sample size, study fidelity, and data reported from single centre and cohort groups accordingly. Non-comparative data for rates of clinical manifestation and type of co-morbidities amongst the confirmed cases were pooled as proportions with 95% confidence intervals. We used DerSimonian and Laird random effects meta-analysis following Logit transformation of the data. [19,20] Heterogeneity between studies was measured using the I2 statistic, with substantial heterogeneity considered if I2 > 75%. [21] Publication bias for the outcome measures was visualized by funnel plot, and meta-regression was undertaken for the key summary measures to explore associations amongst variables. Sensitivity analysis were also performed excluding the studies which were identified from the pre-print database (i.e. medRxiv) and were only included if no significant differences were observed when compared with the peer reviewed published papers. We used R (version 4.0.3) for the analysis and ArcGIS 10.6 (Esri, Redlands, CA, USA) for illustrating the map.
2.5. Role of the funding source
The funders had no role in study design, data collection or analysis, writing of the article, or the decision to publish. The corresponding author had access to all the data in the study and final responsibility for decision to submit the article for publication.
3. Results
Our systematic review identified 49 studies from 14 countries across 4 continents reporting data on 214,380 residents in 8502 care homes, with 25,567 confirmed cases of COVID-19 (Fig. 1–2). A total of 5148 articles were retrieved from our search and following screening titles and abstracts, 99 studies were eligible for full-text review. Finally, we included 49 studies for analysis after exclusion of 50 studies accounting for no extractable data on outbreak events, not meeting selection criteria and duplicate studies (Fig. 1). The studies were all observational in nature. [[11], [12], [13],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]] All of the studies reported RT-PCR-confirmed COVID-19 cases amongst the residents.
Fig. 2.
Global distribution of the included studies by country.
Of these 49 studies, 30 articles reported 2999 COVID-19 outbreak events in care homes, with the remaining 19 reporting epidemiological information regarding care-home residents during the non-outbreak surveillance period (Table 1). All outbreak events were reported over a period of four months between March and June 2020. Based on eighteen studies with reliable sex distribution data, the mean proportion of female residents was 55% (n = 3037). [11,26,31,[35], [36], [37],39,40,43,44,46,48,49,55,57,58,63,67] Twenty-two studies reported a mean age, and the pooled mean age of residents was 81.5 years. [11,13,24,26,31,[35], [36], [37],39,40,43,44,46,48,49,52,55,57,58,62,63,67] Thirteen studies involving 21 outbreak events reported information on the index case. In 12 outbreaks (58%), the index case was a resident, and various staff or visitor categories (e.g. healthcare provider, nurse, caregiver, and visitor) were found to be the index case in the remaining events (42%). amongst 2999 care-home COVID-19 outbreaks, the majority (n = 2920, 97%) were reported from long-term care facilities, followed by 63 outbreak events from nursing homes, eight from skilled-nursing facilities, and five in assisted-living facilities. Nearly two-thirds of the studies reported outbreaks from a single facility (n = 17), and the remaining studies reported data either separately or collectively from multiple facilities (n = 13). Demographic characteristics and the epidemiologic index of SARS-CoV-2 amongst the care-home residents are illustrated in Table 1.
Table 1.
Characteristics of included studies and epidemiologic index of SARS-CoV-2 in aged care facilities.
| First Author/ Year | Country | Study design | Study period (month) | Outbreak setting (number of facilities) | Number of outbreak | Participants investigated in the facility | Index case | Total number of residents | Total number of investigated participants (N)⁕ | RT-PCR-confirmed COVID-19 cases (n)⁕ | Sex distribution of COVID-19 cases (M/F)¶ | Age of COVID-19 residents (years)¥ | Attack rate (%) | Case fatality rate (%) | Mortality rate (%) | Fidelity of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilbert/202030 | Australia | Case study | March | NH (1) | 1 | R, S | Staff, Female | 76 | 76 | 17 | NR | NR | 22.·3 | 35·2 | 7·8 | HF |
| Frank/202029 | Belgium | Retrospective cohort | March–April | NH (1) | 1 | R, S | NR | 130 | 118 82 |
40 11 |
NR | NR | 30.·7 | NR | NR | HF |
| Stall/202023 | Canada | Retrospective cohort | March–May | LTC (190) | 190 | R | NR | 22,990 | NR | 5218 | NR | NR | 22·6 | 27·8 | 6·3 | LF |
| Stall/202022 | Canada | Retrospective cohort | April | NH (1) | 1 | R, S | NR | 126 | 126 | 89 | NR | NR | 70·6 | 13·4 | 9·5 | LF |
| Hu/202031 | China | Retrospective cohort | January–April | NH (1) | NA | R | NR | 34 | 34 | 34 | 11/23 | 82·7 (7·4) | NR | NR | NR | LF |
| Blain/202024 | France | Prospective cohort | March | NH (1) | 1 | R, HCP | NR | 79 | 79 34 |
38 8 |
NR | 86 (15·5) |
48·1 | 31·5 | 15·1 | HF |
| Belmin/ 202025 | France | Retrospective cohort | March–May | NH (17) | NA | R, S | Resident | 1250 | 1250 794 |
5 6 |
NR | NR | 0·4 | 100 | 0·4 | LF |
| Sacco/202026 | France | Retrospective cohort | March | NH (1) | NA | R, S | NR | 87 | 87 92 |
41 22 |
15/26 | 88·8 (7) | 47·1 | 26·8 | 12·6 | HF |
| Guery/202027 | France | Letter | April | NH (1) | 1 | S | Resident, Female, 80 years | NR | 136 | 3 | NR | NR | NR | 2·2 | NR | HF |
| Klein/202028 | Germany | Prospective cohort | March | NH (1) | 1 | R | NR | 60 | 60 | 39 | NR | NR | 65 | 20·5 | 13·3 | HF |
| Kennelly/ 202032 | Ireland | Cross-sectional | April–May | NH (28) | 21 | R, S | NR | 1741 | 1741 1227 |
710 395 |
NR | NR | 40·8 | 25·8 | 10·5 | HF |
| Nouvenne/ 202033 | Italy | Prospective cohort | April | NH (5) | NA | R | NR | 83 | 83 | 44 | NR | NR | 53·0 | NR | NR | LF |
| Veronese/ 202034 | Italy | Retrospective cohort | April–May | NH (1)` | NA | R | NR | 175 | 175 | 50 | NR | NR | 28·5 | 24·0 | 6·9 | HF |
| Carta/202035 | Italy | Prospective cohort | March–April | LTCF (1) | NA | R | NR | 65 | 65 | 54 | 35/8 | 81 (56–97) | 83·0 | 20·3 | 16·9 | LF |
| Rutten/ 202036 | Netherlands | Prospective cohort | March–April | NH (1) | NA | R | NR | 1969 | 1969 | 857 | 300/557 | 84 (8·7) | 43·5 | NR | NR | LF |
| Van Den Besselaar/ 202037 | Netherlands | Retrospective cohort | May–June | NH (1) | 1 | R, S | NR | 181 | 181 244 |
113 56 |
31/82 | 85 [44–99] | NR | 62·4 | NR | HF |
| Van Buul/202038 | Netherland | Cross-sectional | May | NH (3) | NA | R, HCP | NR | 297 | 297 | 16 | NR | NR | NR | 5·3 | NR | HF |
| Kittang/ 202039 | Norway | Retrospective cohort | March–April | NH (3) | 3 | R, S | Pre-symptomatic staff, Resident | 115 | 115 157 |
40 42 |
14/26 | 86·2 (69–98) | 34·7 | 52·5 | 18·2 | HF |
| Park/202040 | Republic of Korea | Prospective cohort | April | LTCF (3) | 3 | R, S | Caregiver, Female, 45 years; Resident, Female, 50 & 85 years | 569 | 266 47 |
18 6 |
68/109 | 82·4 | 3·1 | 38·9 | 1·2 | HF |
| Song/202041 | Republic of Korea | Cross-sectional | May | NF (5) | 5 | R, S | Daytime worker, Resident | 296 | 137 | 41 | NR | NR | 13·8 | 14·6 | 2·0 | HF |
| Borras-Bermejo/202042 | Spain | Cross-sectional | April | NH (69) | 1 | R, S | NR | 3214 | 3214 2655 |
768 403 |
NR | NR | 23·8 | NR | NR | LF |
| Bearnabeu-Wittel/202068 | Spain | Retrospective cohort | March–April | NH (4) | 4 | R | NR | 457 | 457 | 272 | 67/205 | 87 [81–91] | 59·5 | 22·4 | 13·3 | HF |
| Ladhani/202044 | UK | Prospective cohort | April | NH (6) | 6 | R, S | NR | 264 | 264 | 105 | 23/82 | 88 [85–91] | 39·7 | 16·1 | 3·2 | HF |
| Graham/202045 | UK | Prospective cohort | March–April | NH (4) | 4 | R, S | Resident€ | 394 | 313 70 |
126 3 |
NR | NR | 31·9 | 16·7 | 5·3 | HF |
| Marossy/202046 | UK | Cross-sectional | May | ECH (6), NH (18), RF (13) | 37 | R, S | NR | 1034 | 1034 1421 |
93 67 |
22/71 12/55 |
88·1 | 8·9 | 36·5 | 3·2 | LF |
| Shallcross/202047 | UK | Cross-sectional | April–June | LTC (2724) | 2724 | R, S | NR | 160,033 | 113,160 163,831 |
12,995 7051 |
NR | NR | NR | 8·1 | NR | LF |
| Smith/202048 | UK | Prospective cohort | April–June | ECH (44) | 14 | R, S | NR | 518 | 518 340 |
103 49 |
41/62 | 87·8 (71–104) | 19·8 | 20·3 | 4·0 | HF |
| McMiachael/202011 | USA | Retrospective cohort | March | SNF (1) | 1 | R, HCP, V | Resident, Female, 73 years | 130 | 118 | 101 | 32/69 | 83 (51–−100) | 77·7 | 33·7 | 26·1 | HF |
| Roxby/202012 | USA | Prospective cohort | March | Independent/ALF (1) | 1 | R, S | NR | 80 | 80 62 |
4 2 |
NR | NR | 5 | NR | NR | HF |
| Patel/202049 | USA | Prospective cohort | March | SNF (1) | 1 | R, S | Resident, Female, 57 years | 127 | 126 42 |
35 19 |
11/24 |
82 [75–92] | 16·2 | 28·5 | 7·8 | HF |
| Temkin-Greener/202050 | USA | Retrospective cohort | March–May | ALF (4685) | NA | R | NR | NR | NR | 2647 | NR | NR | NR | 29·3 | NR | LF |
| Weil/202051 | USA | Cross-sectional | March–May | SNF (15), ALF (1) | NA | R, S | NR | 1208 | 1208 1583 |
110 51 |
NR | NR | 9·1 | NR | NR | LF |
| Sanchez/202052 | USA | Cross-sectional | March–May | SNF (26) | NA | R | NR | 2773 | 2773 | 1207 | NR | 72 [64–82] | 43·5 | 23·7 | 10·3 | HF |
| Louie/202053 | USA | Prospective cohort | March–April | SNF (3), ALF (1) | 4 | R, HCP | NR | 156 | 156 147 |
63 23 |
NR | NR | 40·3 | 19 | 7·6 | HF |
| Arons/202013 | USA | Cross-sectional | March | SNF (1) | 1 | R, S | NR | 89 | 76 51 |
48 26 |
NR | 78.6 (9·5) | 53·9 | NR | NR | HF |
| Goldberg/202054 | USA | Cross-sectional | April | SNF (1) | 1 | R, S | NR | 97 | 97 97 |
83 36 |
NR | NR | 85·5 | 28·9 | 24·7 | HF |
| Dora/202055 | USA | Prospective cohort | March–April | SNF (1) | NA | R, S | Resident, Male, > 90 years | 99 | 99 136 |
19 8 |
19/0 | 75 [66–85] | 19·1 | 5·2 | 1·0 | HF |
| Bigelow/202056 | USA | Prospective cohort | April | NH (1) | 1 | R | NR | 170 | 170 | 37 | NR | NR | 21·7 | NR | NR | HF |
| Feaster/202057 | USA | Prospective cohort | April | SNF (8), ALF (1) | NA | R, S | NR | 608 | 582 356 |
408 223 |
171/237 | 78·4 (13) | 67·1 | NR | NR | HF |
| Mills/202058 | USA | Prospective cohort | April | ALF (101) | 3 | R | NR | 1794 | 35 | 7 | 2/5 | 81·9 (11) | 0·03 | 14·2 | 0·05 | LF |
| Harris/202059 | USA | Prospective cohort | April | SNF (1) | 1 | R | NR | 48 | 48 | 41 | NR | NR | 85·4 | 14·6 | 12·5 | HF |
| Shrader/202060 | USA | Prospective cohort | March | LTC (1) | 1 | R, S | Resident, Female, 72 years | 98 | 98 56 |
52 19 |
NR | NR | 53·0 | 9·6 | 5·1 | HF |
| Jatt/202061 | USA | Prospective cohort | March–April | SNF (1) | NA | R | NR | NR | 149 | 18 | NR | NR | 12·0 | NR | NR | LF |
| Escobar/202062 | USA | Prospective cohort | April | NH (1) | 1 | R, S | Resident | 84 | 84 212 |
27 32 |
NR | 86 (15.5) | 32 | NR | NR | HF |
| Rudolph/202063 | USA | Prospective cohort | April | CLC (134) | NA | R | NR | 7325 | 7325 | 443 | 432/11 | 76.3 (10·8) | 6·0 | NR | NR | HF |
| Eckardt/202064 | USA | Cross-sectional | April–May | LTC (1) | 1 | R, S | NR | NR | 276 524 |
10 16 |
NR | NR | 3·6 | NR | NR | HF |
| Quicke/202065 | USA | Prospective cohort | NR | SNF (5) | 4 | S | NR | NR | 351 | 70 | NR | NR | 19·9 | NR | NR | LF |
| Telford/202066 | USA | Prospective cohort | March | SNF, NH, ALF (28) | 28 | R, S | NR | 2868 | 2868 2803 |
821 264 |
NR | NR | 28·6 | 16·2 | 4·5 | HF |
| Shi/202067 | USA | Retrospective cohort | March–May | LTC (1) | 1 | R | NR | 389 | 389 | 146 | 66/80 | 55·9 | 37·5 | 30·1 | 11·3 | HF |
R = resident, S = staff, HCP = healthcare professional, V = visitor, NR = not reported, NA = not applicable, NH = nursing home, SNF = skilled nursing facility, LTC = long-term care, ECH = extra-care home, ALF = assisted-living facility, RT-PCR = reverse transcriptase polymerase chain reaction, ¥ = age in years expressed in mean (SD), median [IQR], or mean (range) as reported. ⁕ = two data entries on the column, first refers to resident and second refers to staff, ¶ = male/female, €=only one facility reported the index case status. HF = high fidelity (studies that explicitly reported the total number of at-risk participants in the facility, total number of RT-PCR-confirmed cases, and number of individual participants tested during the study period separately). LF = low fidelity (studies missing any of the key denominators to estimate one of the principle summary measures). Attack rate expressed as percentage (%): (Number of positive cases/Total number of at-risk population) * 100; case fatality rate expressed as percentage (%): (Number of death cases/Total number of positive cases) * 100; mortality rate expressed as percentage (%): (Number of death cases/Total number of residents in the facility) * 100. All rates were calculated only for residents in the facility.
3.1. Quality assessment of studies
The risk of bias was generally low to moderate after considering the observational nature of the studies (Supplementary Table 2); however, both within and across studies the overall findings were heterogeneous. Most of the studies (89%, n = 44) reported relevant and reliable data, but the validity was of a low quality in a small number (10%, n = 5) of the included studies. Any study reporting relevant valid data without any methodological flaw was considered for inclusion following assessment (Supplementary Table 2).
3.2. Clinical features of COVID-19 patients in care homes
Fourteen studies (28%) reported the symptom status of confirmed cases of COVID-19 amongst residents in care homes. [12,13,24,26,31,36,37,39,43,45,48,49,55,67] amongst these, 31% [95% CI 28–34%] were asymptomatic and 63% [95% CI 60–66%] were symptomatic (Table 2). Only five studies reported symptom status patterns (typical and atypical) in COVID-19-positive residents. [13,24,36,45,49] amongst residents presenting with symptoms, typical and atypical COVID-19 symptoms were observed in 30% [95% CI 24–36%] and 8% [95% CI 6–10%], respectively. Pooled estimates from all reported studies revealed a proportion of 30% [95% CI 22–41%] asymptomatic residents in care homes (Supplementary Fig. 1).
Table 2.
Rate of clinical manifestations, co-morbidity disorders, and outcomes amongst confirmed COVID-19 residents in aged care homes.
| Characteristics | Number of studies (N) | Confirmed cases (n/N) | Proportion (95% confidence interval) |
|---|---|---|---|
| Symptoms at the time of diagnosis¶ | |||
| Asymptomatic | 13 | 311/985 | 0·31 (0·28–0·34) |
| Symptomatic | 13 | 647/1019 | 0·65 (0·61–0·68) |
| Typical symptoms | 4 | 74/247 | 0·30 (0·24–0·36) |
| Atypical symptoms | 5 | 82/989 | 0·08 (0·06–0·10) |
| Symptoms | |||
| Respiratory symptoms | |||
| Cough | 14 | 850/1876 | 0·45 (0·43–0·47) |
| Dyspnoea | 10 | 448/1497 | 0·29 (0·27–0·32) |
| Sore throat | 6 | 66/1128 | 0·05 (0·04–0·07) |
| Rhinorrhoea | 5 | 70/1331 | 0·05 (0·04–0·06) |
| Hypoxia | 5 | 126/373 | 0·33 (0·29–0·38) |
| Polypnea | 2 | 38/79 | 0·48 (0·37–0·59) |
| Systemic symptoms | |||
| Fever | 13 | 935/1872 | 0·49 (0·47–0·52) |
| Hypothermia | 1 | 4/41 | 0·09 (0·03–0·23) |
| Weakness | 6 | 199/1421 | 0·14 (0·12–0·15) |
| Malaise | 5 | 88/1162 | 0·07 (0·06–0·09) |
| Anorexia | 9 | 250/1712 | 0·14 (0·13–0·16) |
| Myalgia | 5 | 8/256 | 0·03 (0·01–0·06) |
| Hypotension | 1 | 2/41 | 0·04 (0·01–0·17) |
| Chills | 3 | 5/196 | 0·02 (0·01–0·06) |
| Rash | 1 | 2/103 | 0·01 (0·00–0·07) |
| Conjunctivitis | 1 | 1/41 | 0·02 (0·00–0·01) |
| gastrointestinal symptoms | |||
| diarrhoea | 7 | 81/647 | 0·12 (0·10–0·15) |
| Nausea | 6 | 32/528 | 0·06 (0·04–0·08) |
| Vomiting | 6 | 56/1461 | 0·03 (0·03–0·04) |
| Acute gastrointestinal symptoms | 3 | 22/177 | 0·12 (0·08–0·18) |
| Ageusia | 1 | 4/272 | 0·01 (0·00–0·03) |
| Anosmia | 2 | 4/313 | 0·01 (0·00–0·02) |
| Neurological symptoms | |||
| Headache | 7 | 45/1116 | 0·04 (0·03–0·05) |
| Confusion | 5 | 262/1174 | 0·22 (0·20–0·24) |
| Altered mental status | 1 | 4/41 | 0·09 (0·03–0·23) |
| Delirium | 5 | 150/607 | 0·24 (0·21–0·28) |
| Falls | 5 | 52/602 | 0·08 (0·06–0·11) |
| Dizziness | 2 | 5/89 | 0·05 (0·02–0·12) |
| Seizure | 1 | 1/35 | 0·02 (0·00–0·17) |
| Resident characteristics | |||
| Gender | |||
| Male | 18 | 1360/3037 | 0·44 (0·43–0·46) |
| Female | 18 | 1670/3037 | 0·55 (0·53–0·57) |
| Co-morbidities | |||
| Cardiac disease | 11 | 453/1251 | 0·36 (0·33–0·38) |
| Pulmonary disease | 12 | 303/1334 | 0·25 (0·22–0·27) |
| Hypertension | 7 | 662/993 | 0·66 (0·63–0·69) |
| Obesity | 9 | 181/833 | 0·21 (0·19–0·24) |
| Smoker | 2 | 19/67 | 0·28 (0·18–0·40) |
| Diabetes mellitus | 12 | 395/1292 | 0·33 (0·30–0·35) |
| Cancer | 3 | 26/168 | 0·15 (0·10–0·21) |
| Liver disease | 2 | 10/128 | 0·05 (0·02–0·09) |
| Renal disease | 9 | 165/536 | 0·30 (0·27–0·34) |
| Cerebrovascular disease | 5 | 127/512 | 0·24 (0·21–0·28) |
| Dementia | 5 | 550/821 | 0·67 (0·63–0·70) |
| Cognitive dysfunction | 3 | 165/201 | 0·82 (0·76–0·86) |
| Received haemodialysis | 2 | 4/75 | 0·05 (0·02–0·13) |
| dyslipidaemia | 3 | 148/340 | 0·43 (0·38–0·48) |
| neurological disorder | 2 | 67/299 | 0 ·22 (0·18–0·27) |
| Anxiety disorder | 1 | 37/272 | 0·14 (0·10–0·18) |
| Depression | 1 | 72/272 | 0·26 (0·21–0·32) |
| Outcomes | |||
| Hospitalized | 12 | 1057/2829 | 0·37 (0·35–0·39) |
| ICU admission | Not reported | ||
| Invasive ventilation | Not reported | ||
= Studies explicitly reporting symptomatic and asymptomatic cases number were included in the analysis.
The pooled proportion of residents with confirmed COVID-19 infection presenting with the most prevalent clinical symptoms was 49% for fever [95% 47–52%], 45% for cough [95% CI 43–47%], 33% for hypoxia (SaO2 < 90%) [95% CI 29–38%], 29% for dyspnoea [95% CI 27–32%], and 24% for delirium [95% CI 21–28%] (Table 2).
3.3. Attack rate, case fatality rate, and mortality rate amongst COVID-19-positive residents in care homes
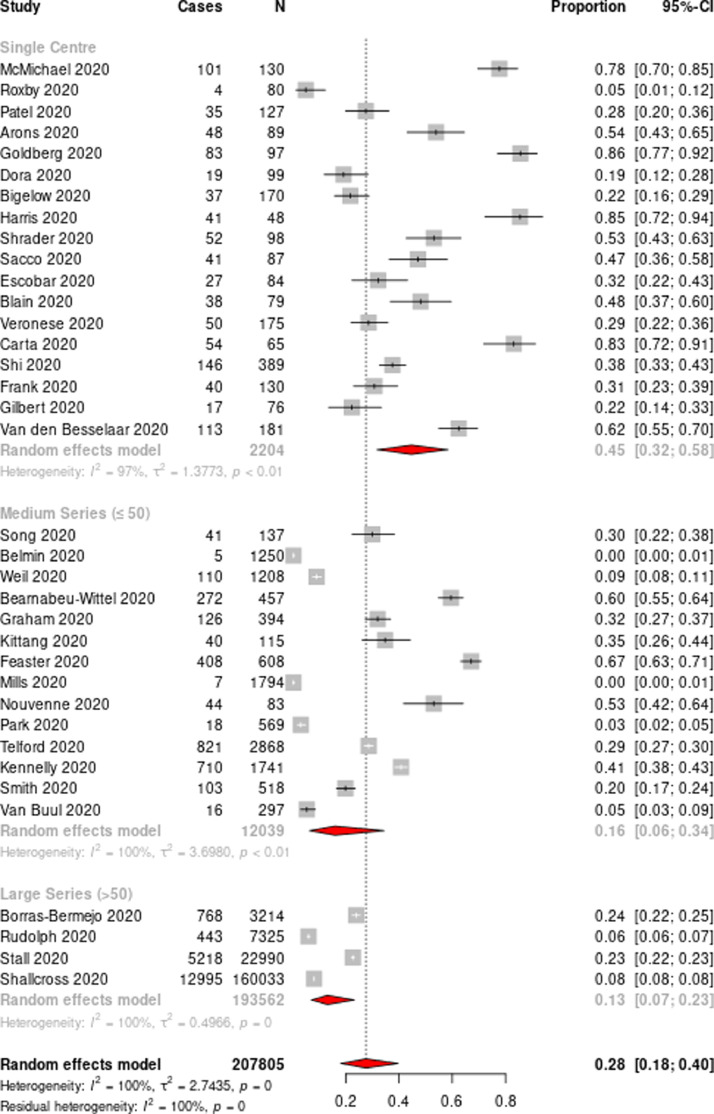
Overall, the pooled attack rate from all included studies was 28% [95% CI 18–40%] (Fig. 3). Sub-group analysis based on the number of facilities showed a variable attack rate ranging from 45% [95% CI 32–58%] for single-facility data and 16% [95% CI 6–34%] for medium series (<=50 facilities) to 13% [95% CI 7–23%] for large series (>50 facilities) (Fig. 3). Regional sub-group analysis based on the distribution of the facilities also revealed a similar attack rate of 29% [95% CI 15–50%] for North America and 30% [95% CI 17–47%] for European region (Supplementary figure 2, Supplementary figure 3a). However, facility sub-group analysis revealed significant differences with highest attack rate in the skilled nursing facility 60% [95% CI 35–81%], followed by multifacility 30% [95% CI 10–64%], long term care facility 27% [95% CI 9–58%] and nursing home 26% [95% CI 17–39%] respectively (Supplementary figure 3b). Random effects meta-analysis demonstrated a total pooled case fatality rate of 22% [95% CI 18–28%] (Fig. 4a). After aggregating data from single-facility studies, the case fatality rate increased to 23% [95% CI 18–28%] (Fig. 4b). Case fatality rates by region showed similar effect size (22% [95% CI 18–28%) (Supplementary figure 4a), however, facility sub-group analysis revealed significant differences with highest case fatality rate in the nursing home 25% [95% CI 19–31%], followed by long term care 25% [95% CI 18–33%], skilled nursing facility 24% [95% CI 22–27%] and multifacility 13% [95% CI 3–40%] respectively (Supplementary figure 4b). Data from 29 studies showed an overall mean mortality rate of 8·9% (range 0·05%–26·1%, median 7·8, interquartile range 9·3). Random effects meta-analysis from single-facility studies revealed a pooled mortality rate of 11% [95% CI 8–15%] (Supplementary figure 5a-5b).
Fig. 3.
Forest plot of the attack rate of residents in aged care homes
Horizontal lines represent the 95% CIs around the point estimates for each study, and the grey shaded areas are proportional to the weight given to each study.
Fig. 4a.
Forest plot of the case fatality rate of residents in aged care homes (all studies). Horizontal lines represent the 95% CIs around the point estimates for each study, and the grey shaded areas are proportional to the weight given to each study.
Fig. 4b.
Forest plot of the case fatality rate of residents in aged care homes (single-centre studies). Horizontal lines represent the 95% CIs around the point estimates for each study, and the grey shaded areas are proportional to the weight given to each study.
3.4. Clinical outcomes and associated risk factors for COVID-19 patients in care homes
Twelve studies reported hospitalization events for the confirmed COVID-19 residents from care homes. [11,26,39,44,49,52,53, 55,58,59,66,68] None of the included studies reported information on clinical severity grade, rate of intensive care unit treatment, or invasive/non-invasive intervention following diagnosis. Overall, the rate of hospitalization amongst residents was 37% [95% CI 35–39%] for a total 2829 confirmed cases (Table 2).
Multiple co-morbid conditions were reported amongst the confirmed COVID-19 residents in care homes (Table 2), with a prevalence of 36% [95% CI 33–38%] for cardiovascular disease, 66% [95% CI 63–69%] for hypertension, 82% [95% CI 76–86%] for cognitive dysfunction, 67% [95% CI 63–70%] for dementia, 33% [95% CI 30–35%] for diabetes mellitus, and 22% [95% CI 18–27%] for neurological disorders (Table 2).
3.5. Meta-regression and publication bias
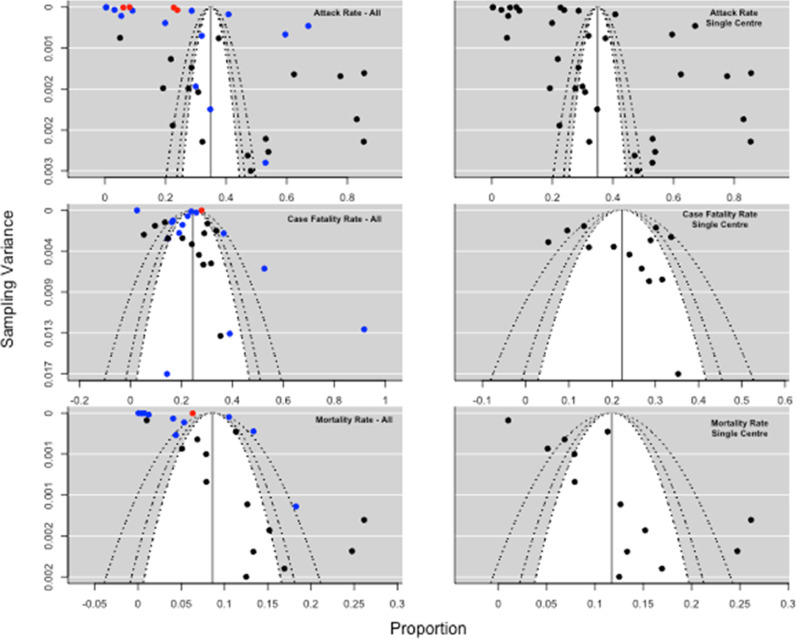
Publication bias was assessed through visual inspection of a funnel plot (Fig. 5) for the pooled attack rate and case fatality rate in single-centre and multi-facility studies. The inverted funnel plot was symmetrical for the pooled case fatality rate. There was considerable heterogeneity in the reporting of attack rates, with multi-facility reports minimally improving when subgroup analysis was performed. Following meta-regression analysis, we found the outbreak size to be the only variable that showed a relationship with the pooled attack rate (i.e. single centre vs medium series (<=50 facilities) or large series (>50 facilities); p-value = 0·04). We did not find any significant relationship with country of study, study design, or type of setting. The meta-regression of pooled case fatality rates did not show any significant relationship with outbreak size, country, type of report, or study design (Supplementary Table 3). To explore the robustness of the results, we performed a leave-one-out sensitivity analysis for the included studies by removing one study at a time and iteratively recalculating the key outcome measures and there were no articles that unduly influenced the findings (Appendix 1).
Fig. 5.
Funnel plot of publication bias based on the pooled attack rate and case fatality rate. The upper-left funnel plot shows the publication bias for all studies; the upper-right funnel plot shows the bias for studies reporting outbreaks in a single centre; the middle-left funnel plot shows the bias for all studies reporting a case fatality rate; the middle-right funnel plot shows the bias for case fatality rates from single-centre outbreaks. the lower-left funnel plot shows the bias for all studies reporting mortality rate; the middle-right funnel plot shows the bias for mortality rates from single-centre outbreaks. Red diamond: large multi-facility reports; blue diamond: medium-sized multi-facility reports; black: single-centre reports. x-axis=proportion, y-axis=sampling variance.
4. Discussion
To the best of our knowledge, this is the first comprehensive report of the attack rate, case fatality rate, mortality rate, clinical features, and risk factors of COVID-19 outbreaks in aged care facilities. We found a high attack rate of 45% [95% CI 32–58%] for SARS-CoV-2 infection, with a devastating case fatality rate of 23% [95% CI 18–28%] in aged care facility outbreaks.
In contrast to influenza, COVID-19 appears to cause outbreaks of greater severity in aged-care facilities. Utsumi et al. reviewed more than three decades of outbreak events and found 49 influenza outbreaks in care homes, with a median attack rate of 33% (range 4–94%) amongst residents and a case fatality rate of 6·5% (range 3–58%). [69] The concomitant use of antiviral drugs and the stringent implementation of infection prevention and control (IPC) practices and seasonal influenza vaccine may contribute to better outcomes in influenza outbreaks. [69] The lack of pharmacological interventions or a readily available vaccine for SARS-CoV-2 are likely factors in the high attack rate (45%) and case fatality rate (23%) amongst RACF residents. The WHO's European Technical Advisory Group of Experts on Immunization (ETAGE) recommended health workers at risk including care workers in hospitals and long-term care facilities, such as nursing homes and residential facilities, older adults and residents of long-term care facilities to be prioritized for COVID-19 vaccination. [70] Several national regulatory authorities have already authorised COVID-19 vaccines and with many candidate vaccines currently in development, prioritisation of high-risk groups would be essential. [71]
Co-morbidities amongst aged care residents, such as cardiovascular diseases, hypertension, and diabetes, also substantially increase their risk of morbidity and mortality, as evidenced from earlier studies. [72,73]
In congregate settings such as cruise ships, correctional facilities, and long-term care homes, asymptomatic and pre-symptomatic individuals are found to accelerate transmission events. [13,[74], [75], [76]] In our review, the pooling of eleven studies showed that nearly one-third of reported residents were asymptomatic, which is consistent with other research. [11,13,74,77,78] The high attack rate amongst asymptomatic aged-care residents and staff highlights the importance of facility-wide testing once a COVID-19 case is diagnosed.
In addition, outbreak events in high-density living facilities can lead to protracted outbreaks causing high death tolls. [11] Across fourteen studies where more than two-thirds of the aged care residents were symptomatic [95% CI 61–68%], significant intra-facility transmission occurred as a result of resident intermingling, despite the implementation of social distancing policies. Restriction of movements amongst residents of an aged care facility with a cognitive disorder is a major challenge. The high case fatality and mortality rates also underscore the importance of enhanced clinical surveillance and extensive testing strategies to identify clusters of infections early. Although, there is limited evidence for environmental surveillance for early warning of clusters or outbreak of COVID-19 infections [79], [80], [81], however closed residential high risk settings like aged care facilities can be quickly adopted for environmental surveillance that can complement established clinical surveillance or launch comprehensive surveillance for the vulnerable populations in the facility. [82]
A multitude of clinical manifestations were found to be prevalent with COVID-19 affected residents. The most common symptoms were fever, cough, dyspnoea, hypoxia (SaO2 < 90 mm Hg), anorexia, diarrhoea, nausea, vomiting, altered mental status with spectrum from confusion, delirium to loss of consciousness. Earlier studies revealed fever and dyspnoea to be highly prevalent amongst critically ill-patients and older adults individuals with COVID-19. [83,84] Diagnosis of clinical infections amongst frail and older people is particularly challenging due to presence of multiple co-morbidities and presentation with atypical constitutional symptoms reported in earlier studies and national guidelines. [85], [86], [87], [88] Similar difficulties have been reported in several studies where residents were asymptomatic at the time of diagnosis and subsequently developed symptoms during the course of illness and required supervised coordinated care. [38,49,55,67] Care homes have comparatively higher rates of dementia, delirium, and cognitive dysfunctional co-morbidities. The challenge of isolating and managing older adult persons with COVID-19 in such environments is critical and requires skilled care and supervision. [89] Where older adult residents with cognitive disorders are housed, staff must maintain heightened vigilance for new symptom onset or exacerbation of existing clinical symptoms and implement IPC precautions immediately. [45,90]
In view of the diverse categories of people accessing aged care facilities (e.g. residents, caregivers, healthcare professionals, support services, office staff, and visitors), there are many ways SARS-CoV-2 can be introduced. In addition, an index case working across multiple facilities has the potential to cause cross-facility transmission. [91] Current evidence on the association between outbreaks in aged-care facilities and the incidence of COVID-19 in surrounding communities also suggests that it is primarily aged care staff and visitors who introduce SARS-CoV-2 into such facilities. [11,92] Vivaldi et al. estimated the increased odds of infection of residents (OR 2·4 95% CI 1·9–3·0) by care-home staff who worked across multiple facilities. [93] The IPC guidelines for care homes in many countries throughout the pandemic have impelled care homes to become relatively confined environments, restricting non-essential visitors and transfer of residents. The guidelines also acknowledge that healthcare workers regularly entering care homes can act as sources of outbreaks. [94] This was also supported by several studies describing healthcare providers as index cases for outbreaks, although it is difficult to causally ascertain this linkage because of the long latency period of SARS-CoV-2 infection and the possibility that an asymptomatic person in the facility may already have spread the disease before the outbreak was identified. [11]
One study indicated that the staff sick-leave policy of a facility can lead to increased attack rates,[95] and certain paid-leave policies can lead to employees working while symptomatic due to fears of financial burden or job loss, ultimately acting as a contributory factor to the high attack rates. [96] Unpaid sick-leave policies also act as a financial disincentive to report symptoms or be tested for SARS-CoV-2. [97] We also found that 42% (9/21) of outbreak investigation reports identified care-home staff as the index case, leading to rapid isolation of the affected individuals and the quarantine of secondary contacts to deter ongoing transmission.
Inadequate training and lack of personal protective equipment (PPE) monitoring techniques may contribute to disease transmission. A nationwide survey conducted across nursing homes in the US revealed that 36% of care homes did not adhere to hand-hygiene guidelines, and 25% failed to demonstrate appropriate use of PPE. [98] Staff availability to ensure the ongoing provision of appropriate care for residents is paramount during an acute outbreak. [99], [100], [101] Chronic understaffing has long been a global problem in care homes, and the COVID-19 pandemic has reportedly further impacted staff absenteeism and abandonment of jobs in many countries due to inadequate PPE supply, minimal or zero financial incentives, and fear of getting infected. [100,[102], [103], [104]] Li and colleagues found that care homes with higher staff availability experienced a substantial reduction in morbidity and mortality of COVID-19 cases during the early pandemic period. [105] In that review, following a positive diagnosis during universal testing, asymptomatic staff members were unsettled and concerned that they were inadvertently transmitting infections across communities and care homes. The concerns of staff underscore the importance of proper training and disseminating updated information on the novel virus as a key strategy toward adequate preparedness. [27,46,60] Provision of universal paid sick leave, additional financial incentives during a crisis situation, professional development resources, and opportunities for new skill acquisition may encourage staff availability during such global challenges. [47]
Although sick leave policies are important, they are of less value without early identification of asymptomatic and mildly symptomatic cases. This can only be done through enhanced community-wide contact tracing, testing strategies with rapid turnaround times. In the absence of known infection, appropriate PPE use, hand hygiene and other source control methods (e.g. universal mask use, rigorous personal distancing policies) may also be effective to curb the risk of transmission. However, comprehensive evidence on COVID-19 outbreak prevention and control strategies in aged care facilities are limited, and further research is urgently needed to inform policy.
To prevent and control existing and future outbreaks in RACFs, a multidisciplinary team consisting of public health physicians, nurses, administrators, and infection prevention and control experts rigorously enforcing state and national guidelines is essential. [62,106,107] Countries experiencing major havoc across care facilities like England, United States issued national policy in response to surge in infections, however due to rapidly evolving situations implementation of comprehensive actions was less coordinated due to lack of inter-sectoral governance and pre-existing systematic weakness. [108], [109], [110] Reorganization of care homes with regional hospitals, ensuring adequate space to appropriately isolate a person under investigation, periodic screening of staff and residents, and the availability of an adequate staffing pool can significantly minimise further transmission, as demonstrated by countries like Singapore earlier in the pandemic. [90]
Identifying the source of SARS-CoV-2 infection in care-facility outbreaks could be difficult and requires rigorous investigation with real-time data capture and comprehensive mass testing of ‘at-risk’ populations. Development and implementation of risk mitigation strategies such as recording identifiable information on staff, visitors, and residents associated with care facilities could facilitate early intervention and provide opportunities to detect clusters earlier. A careful systematic service-needs assessment amongst older in-house residents is important for the acute provision of care that may be required during outbreaks amongst this highly susceptible and often overlooked group.
This systematic review consolidates epidemiological information on a large number of COVID-19-affected residents in aged care facilities since the emergence of the pandemic. With the inclusion of data on numerous outbreaks from both single-centre and multi-centre studies, our findings offer supporting evidence to inform clinician and public health surveillance strategies to mitigate the burden of COVID-19 across this vulnerable population.
The main limitation of this review is the heterogeneity in the reporting of outcome data due to investigations being carried out on different types of care homes with disparate populations or the reporting of cohort data without detailed descriptions of the characteristics of affected individuals. There was significant variation in the size, number, and patterns of outbreaks reported from single and multi-facility studies. We therefore used a pooled single-centre attack rate as the metric of reference as single-centre reports were less heterogeneous in terms of reporting, were simpler, and had a reduced likelihood of biased assumptions.
Nearly half of the included studies in this review were reported from the care facilities from the North American region, so findings may not be generalizable across other countries care and also older people residing in the community settings. Time constraints in view of the rapid outbreak responses by care homes and state health departments in order to mitigate the burden of disease likely contributed to incomplete reporting. Exploration of the facility factors for example, ownership status, facility size, staff resident ratio, if reported, could have provided additional information which are important in predicting outcome of outbreak events. Following the detection of outbreaks, due to limited testing facilities and resources, differential approaches in testing strategies were taken which resulted in investigating only selective populations or only symptomatic cases, either staff or residents, while in other studies both groups of ‘at-risk’ individuals. Such selective testing approach might have led to underestimation of attack rate and subsequent overestimation of case fatality rate contributing to selection bias in reflecting the true facility attack rate and case fatality rate in the included studies. Besides, determining true typical attack rates and mortality rates in aged care facilities is difficult accounting for publication bias with numerous outbreak events across countries where smaller, non-descript, well-controlled outbreaks later in the pandemic had less likelihood to report, yet still very prevalent. As places learned from early outbreaks that were published in high impact journal, it is feasible that outbreak attack rates may be greatly reduced. Thus, larger studies later in the pandemic may have more heterogeneity but also may be more reflective of the actual situation. Moreover, retrospective data collection based on symptoms assessment may have been subject to recall bias. Symptom reports were often done with a time lag of days to weeks following the identification of outbreaks, and subjects may have been in the post-symptomatic stage at the time of reporting. Moreover, in some studies testing was not done for all of the ‘at-risk’ individuals in a facility, which might have led to an underestimation of the infection rate due to the presence of pre-symptomatic and asymptomatic cases. Index case type was not separately reported in the outbreak events specifying visitors or internal staffs which might provide crucial information in preventing outbreaks in future. The majority of the studies reviewed lacked follow-up testing, further limiting the ability to identify cohorts who were asymptomatic at the time of initial testing and developed symptoms at a later time.
Finally, since we completed our systematic review, we have identified five additional reports from Europe (Spain, Italy, France, Belgium) and the US reporting data between March and June 2020. However, those studies have not been included in this review and, the findings from those individual studies are consistent with our systematic review. [111], [112], [113], [114], [115]
Aged care residents form a distinct vulnerable population with single-facility attack rates of 45% [95% CI 32–58%] and case fatality rates of 23% [95% CI 18–28%]. With such a high attack and case fatality rate preventing the introduction of COVID-19 into aged care facilities is of paramount importance. Aged care residents and staff should be considered as a priority group for COVID-19 vaccine. Comprehensive evidence on COVID-19 outbreak prevention and control strategies in aged care facilities are limited, and future research on this topic is urgently needed.
Declaration of Competing Interest
Professor Robert Booy has received funding from Baxter, CSL, GSK, Merck, Novartis, Pfizer, Roche, Romark and Sanofi Pasteur for conducting research other than this, and for travel to conferences or consultancy work. All other authors declare no competing interests.
Acknowledgments
Contributors
MRH and GK designed the study. MRH, GK, CK, and NS developed the protocol. MRH, GK, and CK designed the literature search. CK and GK supervised the literature search and review process. MRH and HOM reviewed the citations and reports and extracted the data. MRH and NS analysed the data, and NS assisted with meta-regression and visualisation. MRH, NS, and GK interpreted the results. MRH, CK, NS, and GK wrote the first draft of the manuscript. GK, JW, NS, AW, JG, and RB revised successive drafts of the paper, which all authors critically revised. MRH then wrote the final version, which all authors have approved for publication.
Funding
This work was supported by a Queensland Advancing Clinical Research Fellowship awarded to Prof. Gulam Khandaker by Queensland Health's Health Innovation, Investment and Research Office (HIRO), Office of the Director-General. The funders had no role in study design, data collection or analysis, writing of the article, or the decision to publish. The corresponding author had access to all the data in the study and the final responsibility for the decision to submit the article for publication.
Data sharing statement
The authors declare that the data collected was gathered from publicly available databases and is available upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100771.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19–11 March 2020 https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (Accessed on October 25, 2020).
- 2.United Nations . May 2020. Policy brief: the impact of COVID-19 on older persons; p. 3.https://unsdg.un.org/sites/default/files/2020-05/Policy-Brief-The-Impact-of-COVID-19-on-Older-Persons.pdf viewed 11 September 2020. (Accessed October 25, 2020) [Google Scholar]
- 3.Brandén M., Aradhya S., Kolk M. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: a population-based, observational study using individual-level data. The Lancet Healthy Longevity. 2020;1(2):e80–ee8. doi: 10.1016/S2666-7568(20)30016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage R., Nellums L.B. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns Hopkins University. Coronavirus resource center 2020 [cited 2020 October 22, 2020]. https://coronavirus.jhu.edu/. (Accessed on January 29, 2021).
- 6.Comas-Herrera A Z.J., Lemmon E. CPEC-LSE; 2020. Mortality associated with COVID-19 in care homes: international evidence. article in LTCcovCPEC-LSEid.org, international long-term care policy network. 14 October. (Accessed on October 26, 2020) [Google Scholar]
- 7.Sun H., Ning R., Tao Y. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. 2020;68(6):E19–E23. doi: 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Chakhtoura N.G., Bonomo R.A., Jump R.L.P. Influence of aging and environment on presentation of infection in older adults. Infect Dis Clin North Am. 2017;31(4):593–608. doi: 10.1016/j.idc.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarkson P., Hays R., Tucker S., Paddock K., Challis D. Healthcare support to older residents of care homes: a systematic review of specialist services. Qual Ageing Older Adults. 2018;19(1):54–84. [Google Scholar]
- 11.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxby A.C.G., Hatfield A.L. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med. 2020;180(8):1101–1105. doi: 10.1001/jamainternmed.2020.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coburn B.J., Wagner B.G., Blower S. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1) BMC Med. 2009;7(1):30. doi: 10.1186/1741-7015-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infect. Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley D.D., Dick A., Agarwal M., Jones K.M., Mody L., Stone P.W. COVID-19 preparedness in nursing homes in the midst of the pandemic. J Am Geriatr Soc. 2020;68(6):1164–1166. doi: 10.1111/jgs.16520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosella L., Bowman C., Pach B., Morgan S., Fitzpatrick T., Goel V. The development and validation of a meta-tool for quality appraisal of public health evidence: meta quality appraisal tool (MetaQAT) Public Health. 2016;136:57–65. doi: 10.1016/j.puhe.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Lipsey M.W., Wilson D.B. Sage Publications, Inc; Thousand Oaks, CA, US: 2001. Practical meta-analysis. [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stall N.M., Farquharson C., Fan-Lun C. A hospital partnership with a nursing home experiencing a COVID-19 outbreak: description of a multiphase emergency response in Toronto, Canada. J Am Geriatr Soc. 2020;68(7):1376–1381. doi: 10.1111/jgs.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stall N.M., Jones A., Brown K.A., Rochon P.A., Costa A.P. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ. 2020;192(33):E946–EE55. doi: 10.1503/cmaj.201197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blain H., Rolland Y., Tuaillon E. Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J Am Med Dir Assoc. 2020;21(7):933–936. doi: 10.1016/j.jamda.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belmin J.U.-.D., Donadio N., C. Coronavirus disease 2019 outcomes in french nursing homes that implemented staff confinement with residents. JAMA Network Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacco G., Foucault G., Briere O., Annweiler C. COVID-19 in seniors: findings and lessons from mass screening in a nursing home. Maturitas. 2020;141:46–52. doi: 10.1016/j.maturitas.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guery R., Delaye C., Brule N. Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of medicalized nursing home staff after a first case of COVID-19 in a resident. Med Mal Infect. 2020;50(8):748–750. doi: 10.1016/j.medmal.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein A., Edler C., Fitzek A. [The first COVID-19 hotspot in a retirement home in Hamburg] Rechtsmedizin (Berl) 2020:1–7. doi: 10.1007/s00194-020-00404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buntinx F., Claes P., Gulikers M. Added value of anti-SARS-CoV-2 antibody testing in a Flemish nursing home during an acute COVID-19 outbreak in. Acta Clin Belg. 2020;2020:1–6. doi: 10.1080/17843286.2020.1834285. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert G.L. COVID-19 in a Sydney nursing home: a case study and lessons learnt. Med. J. Aust. 2020;213(9):393. doi: 10.5694/mja2.50817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L., Zhen W., Xie X. Clinical and epidemiological features of 34 nursing home elderly with coronavirus disease 2019 (COVID-19) in Wuhan, China: an observational cohort study. Lancet. 2020 doi: 10.1016/S1473-3099(20)30198-5. https://ssrn.com/abstract=3582760 (preprint April 18) (Accessed on October 25, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennelly S.P., Dyer A.H., Martin R. Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2021;50(1):49–54. doi: 10.1093/ageing/afaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouvenne A.T., Parise A., A. Point-of-care chest ultrasonography as a diagnostic resource for COVID-19 outbreak in nursing homes. J Am Med Dir Assoc. 2020;21(7):919–923. doi: 10.1016/j.jamda.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veronese N., Sbrogio L.G., Valle R., Marin L., Boscolo Fiore E., Tiozzo A. Prognostic value of lung ultrasonography in older nursing home residents affected by COVID-19. J Am Med Dir Assoc. 2020;21(10):1384–1386. doi: 10.1016/j.jamda.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carta M., Bragagnolo L., Tramarin A. Anti SARS-CoV-2 antibodies monitoring in a group of residents in a long term care facility during COVID-19 pandemic peak. Diagnosis (Berl) 2020;7(4):395–400. doi: 10.1515/dx-2020-0094. [DOI] [PubMed] [Google Scholar]
- 36.Rutten J.J.S., van Loon A.M., Joling K.J., Smalbrugge M., van Buul L.W., Hertogh C. [COVID-19 in nursing homes a study of diagnosis, symptomatology and disease course] Ned Tijdschr Geneeskd. 2020;164(07):20. [PubMed] [Google Scholar]
- 37.van den Besselaar J.H., Sikkema R.S., Koene F.M. 2020. A COVID-19 nursing home transmission study: sequence and metadata from weekly testing in an extensive nursing home outbreak. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Buul L.W., van den Besselaar J.H., Koene F. Asymptomatic cases and limited transmission of SARS-CoV-2 in residents and healthcare workers in three dutch nursing homes. Gerontol Geriatr Med. 2020;6 doi: 10.1177/2333721420982800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kittang B.R.H., Solheim S.V., Kruger S.P., Loland K., Jansen K.K., K. Outbreak of COVID-19 at three nursing homes in Bergen. Tidsskrift for Den Norske Laegeforening. 2020;140(11):18. doi: 10.4045/tidsskr.20.0405. [DOI] [PubMed] [Google Scholar]
- 40.Park S.Y., Choi G., Lee H. Early intervention reduces the spread of COVID-19 in long-term care facilities in the Republic of Korea. Osong Public Health Res Perspect. 2020;11(4):259–264. doi: 10.24171/j.phrp.2020.11.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song R., Kim H.S., Yoo S.J. COVID-19 in nursing facilities: experience in Republic of Korea. Osong Public Health Res Perspect. 2020;11(4):164–169. doi: 10.24171/j.phrp.2020.11.4.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borras-Bermejo B., Martinez-Gomez X., San Miguel M.G. Asymptomatic SARS-CoV-2 infection in nursing homes, Barcelona, Spain, April 2020. Emerg Infect Dis. 2020;26(9) doi: 10.3201/eid2609.202603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernabeu-Wittel M., Ternero-Vega J.E., Diaz-Jimenez P. Death risk stratification in elderly patients with covid-19. A comparative cohort study in nursing homes outbreaks. Arch Gerontol Geriatr. 2020:91. doi: 10.1016/j.archger.2020.104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladhani S.N., Chow J.Y., Janarthanan R. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham N.S.N., Junghans C., Downes R. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81(3):411–419. doi: 10.1016/j.jinf.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marossy A., Rakowicz S., Bhan A. A study of universal SARS-CoV-2 RNA testing of residents and staff in a large group of care homes in South London. J Infect Dis. 2020;05 doi: 10.1093/infdis/jiaa565. 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shallcross L., Burke D., Stat O.A.C. Risk factors associated with SARS-CoV-2 infection and outbreaks in long term care facilities in England: a national survey. The Lancet Healthy Longevity Forthcoming. 2020 doi: 10.1016/S2666-7568(20)30065-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith E., Aldus C.F., Brainard J. 2020. Testing for SARS-CoV-2 in care home staff and residents in english care homes: a service evaluation. medRxiv: the Preprint Server for Health Sciences. [DOI] [Google Scholar]
- 49.Patel M.C., Chaisson L.H., Borgetti S. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020;71(11):2920–2926. doi: 10.1093/cid/ciaa763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temkin-Greener H., Guo W., Mao Y., Cai X., Li Y. COVID-19 pandemic in assisted living communities: results from seven states. J Am Geriatr Soc. 2020;68(12):2727–2734. doi: 10.1111/jgs.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weil A.A.N., Ong K.L., Davidson T.D., G. Cross-sectional prevalence of SARS-CoV-2 among skilled nursing facility employees and residents across facilities in seattle. J Gen Intern Med. 2020;01 doi: 10.1007/s11606-020-06165-7. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez G.V., Biedron C., Fink L.R. Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities - Detroit, Michigan, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):882–886. doi: 10.15585/mmwr.mm6927e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Louie J.K., Scott H.M., DuBois A. Lessons from mass-testing for COVID-19 in long term care facilities for the elderly in San Francisco. Clin Infect Dis. 2020;20:20. doi: 10.1093/cid/ciaa1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg S.A.L., Klompas J., Mark M., E. Presymptomatic transmission of SARS-CoV-2 amongst residents and staff at a skilled nursing facility: results of real-time PCR and serologic testing. Clin Infect Dis. 2020;15:15. doi: 10.1093/cid/ciaa991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dora A.V.W., Jatt A., Davar L.P., K. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans - Los Angeles, California, 2020. MMWR - Morbidity Mortality Weekly Rep. 2020;69(21):651–655. doi: 10.15585/mmwr.mm6921e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bigelow B.F., Tang O., Toci G.R. Transmission of SARS-CoV-2 involving residents receiving dialysis in a nursing home - Maryland, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1089–1094. doi: 10.15585/mmwr.mm6932e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feaster M., Goh Y.Y. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis. 2020;26(10):2416–2419. doi: 10.3201/eid2610.202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills W.R., Buccola J.M., Sender S. Home-based primary care led-outbreak mitigation in assisted living facilities in the first 100 days of coronavirus disease 2019. J Am Med Dir Assoc. 2020;21(7):951–953. doi: 10.1016/j.jamda.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris D.A., Archbald-Pannone L., Kaur J. Rapid telehealth-centered response to COVID-19 outbreaks in postacute and long-term care facilities. Telemed J E Health. 2021;27(1):102–106. doi: 10.1089/tmj.2020.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrader C.D.A., Pilkerton S., Ashcraft C.S., A. M. Responding to a COVID-19 outbreak at a long-term care facility. Journal of Applied Gerontology. 2021;40(1):14–17. doi: 10.1177/0733464820959163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jatt L.P., Winnett A., Graber C.J., Vallone J., Beenhouwer D.O., Goetz M.B. Widespread severe acute respiratory coronavirus virus 2 (SARS-CoV-2) laboratory surveillance program to minimize asymptomatic transmission in high-risk inpatient and congregate living settings. Infect Control Hosp Epidemiol. 2020;41(11):1331–1334. doi: 10.1017/ice.2020.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Escobar D.J.L., Saberi M., Love P., R. Mitigation of a COVID-19 outbreak in a nursing home through serial testing of residents and staff. Clinical Infectious Diseases. 2020;20:20. doi: 10.1093/cid/ciaa1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudolph J.L., Halladay C.W., Barber M. Temperature in nursing home residents systematically tested for SARS-CoV-2. J Am Med Dir Assoc. 2020;21(7):895–899. doi: 10.1016/j.jamda.2020.06.009. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckardt P.G., Hennemyre R., Arikupurathu J., R. Hospital affiliated long term care facility COVID-19 containment strategy by using prevalence testing and infection control best practices. Am J Infect Control. 2020;03:03. doi: 10.1016/j.ajic.2020.06.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quicke K.G., Sexton E., Young N., M. Longitudinal surveillance for SARS-CoV-2 RNA among asymptomatic staff in five colorado skilled nursing facilities: epidemiologic, virologic and sequence analysis. MedRxiv. 2020;09:09. [Google Scholar]
- 66.Telford C.T.O., Holland U., Turner D., K. Mass screening for SARS-CoV-2 infection among residents and staff in twenty-eight long-term care facilities in Fulton County, Georgia. MedRxiv. 2020;02:02. [Google Scholar]
- 67.Shi S.M., Bakaev I., Chen H., Travison T.G., Berry S.D. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21(10):1378–1383. doi: 10.1016/j.jamda.2020.08.027. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernabeu-Wittel M., Ternero-Vega J.E., Nieto-Martin M.D. Effectiveness of a on-site medicalization program for nursing homes with COVID-19 outbreaks. J Gerontol A Biol Sci Med Sci. 2020;01:01. doi: 10.1093/gerona/glaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Utsumi M., Makimoto K., Quroshi N., Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing. 2010;39(3):299–305. doi: 10.1093/ageing/afq029. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization/Europe | Vaccines and immunization Available from: https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/news/news/2020/11/health-workers-at-risk,-older-adults-and-residents-of-long-term-care-facilities-to-be-prioritized-for-covid-19-vaccination (Accessed on December 12, 2020).
- 71.World Health Organization/Coronavirus disease (COVID-19): Vaccines/Available from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (Accessed on December 12, 2020).
- 72.Clark A., Jit M., Warren-Gash C. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1e17. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holman N., Knighton P., Kar P. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimball A., Hatfield K.M., Arons M. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moriarty L.F., Plucinski M.M., Marston B.J. Public Health Responses to COVID-19 Outbreaks on Cruise Ships - Worldwide, February-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallace M., Hagan L., Curran K.G. COVID-19 in correctional and detention facilities - United States, February-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):587–590. doi: 10.15585/mmwr.mm6919e1. [DOI] [PubMed] [Google Scholar]
- 77.Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tong Z.D., Tang A., Li K.F. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu F., Zhang J., Xiao A. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4):e00614–e00620. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed W., Angel N., Edson J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization . WHO; 2020. Public health surveillance for COVID-19.https://www.who.int/publications/i/item/WHO-2019-nCoV-sci-brief-environmentalSampling-2020-1 Available from: (Accessed on 07 December, 2020) [Google Scholar]
- 83.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.CDC; 2020. Interim guidance for influenza outbreak management in long-term care and post-acute care facilities.https://www.cdc.gov/flu/professionals/infectioncontrol/ltc-facility-guidance.htm (Accessed on October 26, 2020) [Google Scholar]
- 86.HMSO; 2017. Public health england infection prevention and control: an outbreak information pack for care homes. the “care home pack.https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2019/10/phe-sw-care-home-pack-oct19.pdf [updated October 2019. (Accessed on October 26, 2020) [Google Scholar]
- 87.Stone N.D., Ashraf M.S., Calder J. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965–977. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eke-Usim A.C., Rogers M.A., Gibson K.E., Crnich C., Mody L. Targeted infection prevention study T. constitutional symptoms trigger diagnostic testing before antibiotic prescribing in high-risk nursing home residents. J Am Geriatr Soc. 2016;64(10):1975–1980. doi: 10.1111/jgs.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seijo-Martinez M., Cancela J.M., Ayan C., Varela S., Vila H. Influence of cognitive impairment on fall risk among elderly nursing home residents. Int Psychogeriatr. 2016;28(12):1975–1987. doi: 10.1017/S1041610216001113. [DOI] [PubMed] [Google Scholar]
- 90.Tan L.F.S., Santhosh . Wiley-Blackwell; Malden, Massachusetts: 2020. Preventing the spread of COVID-19 to nursing homes: experience from a singapore geriatric centre; p. 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ursic T., Miksic N.G., Lusa L., Strle F., Petrovec M. Viral respiratory infections in a nursing home: a six-month prospective study. BMC Infect Dis. 2016;16(1):637. doi: 10.1186/s12879-016-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fisman D.N.B., Lapointe-Shaw I., McCready L., Tuite J., A. R. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in Long-term Care Facilities in Ontario, Canada. JAMA Network Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Office for National Statistics . ONS; 2020. Impact of coronavirus in care homes in england.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/impactofcoronavirusincarehomesinenglandvivaldi/26mayto19june2020 26 May to 19 June 2020. (Accessed on October 26, 2020) [Google Scholar]
- 94.Chow E.J., Schwartz N.G., Tobolowsky F.A. Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washington. JAMA. 2020;323(20):2087–2089. doi: 10.1001/jama.2020.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar S., Quinn S.C., Kim K.H., Daniel L.H., Freimuth V.S. The impact of workplace policies and other social factors on self-reported influenza-like illness incidence during the 2009 H1N1 pandemic. Am J Public Health. 2012;102(1):134–140. doi: 10.2105/AJPH.2011.300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palme M., Persson M. Sick pay insurance and sickness absence: some european cross-country observations and a review of previous research. J Econ Surv. 2019;34(1):85–108. [Google Scholar]
- 97.Pichler S., Wen K., Ziebarth N.R. COVID-19 emergency sick leave has helped flatten the curve in the United States: study examines the impact of emergency sick leave on the spread of COVID-19. Health Aff. 2020:10–1377. doi: 10.1377/hlthaff.2020.00863. [DOI] [PubMed] [Google Scholar]
- 98.Center for Medicare & Medicaid Services . CMS; 2020. Trump administration issues key recommendations to nursing homes, state and local governments.https://www.cms.gov/newsroom/press-releases/trump-administration-issues-key-recommendations-nursing-homes-state-and-local-governments Available at. (Accessed on October 25, 2020) [Google Scholar]
- 99.Min A., Hong H.C. Effect of nurse staffing on rehospitalizations and emergency department visits among short-stay nursing home residents: a Cross-sectional study using the US Nursing Home Compare database. Geriatr Nurs. 2019;40(2):160–165. doi: 10.1016/j.gerinurse.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 100.Bowblis J.R., Roberts A.R. Cost-effective adjustments to nursing home staffing to improve quality. Med Care Res Rev. 2020;77(3):274–284. doi: 10.1177/1077558718778081. [DOI] [PubMed] [Google Scholar]
- 101.Stone P.W., Clarke S.P., Cimiotti J., Correa-de-Araujo R. Nurses' working conditions: implications for infectious disease. Emerg Infect Dis. 2004;10(11):1984–1989. doi: 10.3201/eid1011.040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oecd . OECD; 2016. Health at a glance: europe 2016: state of health in the eu cycle. [Google Scholar]
- 103.McGarry B.E., Grabowski D.C., Barnett M.L. Severe staffing and personal protective equipment shortages faced by nursing homes during the COVID-19 pandemic. Health Aff (Millwood) 2020;39(10):1812–1821. doi: 10.1377/hlthaff.2020.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abrams H.R., Loomer L., Gandhi A., Grabowski D.C. Characteristics of US nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653–1656. doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y., Temkin-Greener H., Shan G., Cai X. COVID-19 infections and deaths among connecticut nursing home residents: facility correlates. J Am Geriatr Soc. 2020;68(9):1899–1906. doi: 10.1111/jgs.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rios P., Radhakrishnan A., Williams C. Preventing the transmission of COVID-19 and other coronaviruses in older adults aged 60 years and above living in long-term care: a rapid review. Syst Rev. 2020;9(1):218. doi: 10.1186/s13643-020-01486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.University of Leeds/news/niche-leeds-and-national-care-forum-publish-less-covid-19-report/Available from: https://niche.leeds.ac.uk/wp-content/uploads/sites/56/2020/10/LESS-COVID-19-SPILSBURY-ET-AL-2020.pdf (Accessed on December 08, 2020).
- 108.American Geriatrics S. American geriatrics society policy brief: COVID-19 and nursing homes. J Am Geriatr Soc. 2020;68(5):908–911. doi: 10.1111/jgs.16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen A.T., Ryskina K.L., Jung H.Y. Long-term care, residential facilities, and COVID-19: an overview of federal and state policy responses. J Am Med Dir Assoc. 2020;21(9):1186–1190. doi: 10.1016/j.jamda.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daly M. COVID-19 and care homes in England: what happened and why? Soc Policy Adm. 2020;54(7):985–998. doi: 10.1111/spol.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mas Romero M., Avendano Cespedes A., Tabernero Sahuquillo M.T. COVID-19 outbreak in long-term care facilities from Spain. Many lessons to learn. PLoS ONE. 2020;15(10) doi: 10.1371/journal.pone.0241030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trecarichi E.M., Mazzitelli M., Serapide F. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci Rep. 2020;10(1):20834. doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Psevdos G., Papamanoli A., Barrett N. Halting a SARS-CoV-2 outbreak in a US veterans affairs nursing home. Am J Infect Control. 2021;49(1):115–119. doi: 10.1016/j.ajic.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ly T.D.A., Zanini D., Laforge V. Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int J Antimicrob Agents. 2020;56(6) doi: 10.1016/j.ijantimicag.2020.106219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoxha A., Wyndham-Thomas C., Klamer S. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.