Dear Editor,
We read with interest the case that Fowler et al. make for robust near patient testing in the coronavirus disease (Covid-19) pandemic to identify contagious cases.1 The winter peak of Covid-19 in England has seen the highest number of Covid-19 cases and hospital admissions to date, with over 3000 admissions daily, and a peak of 34,015 inpatients with Covid-19.1 Patient triage and cohorting are crucial to reducing nosocomial Covid-19,2 but paucisymptomatic or pre-symptomatic cases limit clinical case detecting,3 and screening with molecular diagnostics introduces delay.4 We piloted the use of point of care antigenic testing for SARS-CoV-2 in patients admitted to hospital for rapid case detection in a period of high disease prevalence.
Between December 23, 2020 and January 30 2021, patients admitted to Oxford University Hospitals NHS Foundation Trust for emergency care were tested for SARS-CoV-2 using both lateral flow device (LFD) and real-time reverse transcription Polymerase chain reaction (PCR) testing. Swabs of the nose and throat were collected by health care workers. LFD testing was performed in the admitting department by staff, using the Innova LFD. Swabs for PCR were transferred to the clinical laboratory in viral transport medium (VTM) and tested by multiplex PCR (Thermo-Fisher TaqPath). 803 patients who had both tests performed with a maximum of 1 day between tests were included for analysis. 732/803 (91%) of patients tested had both tests on the same day. Clinical notes of patients testing positive for SARS-CoV-2 by PCR were reviewed and note made of the reported presence of symptoms of possible Covid-19 (cough, dyspnoea, fever, aguesia or anosmia) as well as admission temperature and oxygen saturation, and previous detection of SARS-CoV-2 by PCR.
Considering PCR results as the reference standard, LFDs showed high specificity (Table 1 ). Of 573 PCR-negative patients, 572 had a negative LFD, and 1 an invalid LFD result, i.e., specificity excluding the invalid result was 100% (exact binomial 95%CI 99.4–100%). Similarly the positive predictive value was high, among 133 patients with a positive LFD results, 133/134 (99.2%, 95%CI 95.9–99.8%) were PCR-positive, with one indeterminate PCR result in a patient testing PCR-positive 5 days later; none were PCR-negative. LFDs also had low rates of invalid results, 2/803 (0.2%).
Table 1.
Sensitivity of Lateral flow device (LFD) compared with Polymerase Chain Reaction (PCR), depending on Cycle threshold (Ct) value (mean Ct of all detected targets).
| All PCR |
Mean Ct across detected PCR targets |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Invalid | <15 | 15–19.9 | 20–24.9 | 25–29.9 | ≥30 | ||
| LFD result | Positive | 133 | 0 | 1 | 59 | 47 | 23 | 4 | 0 |
| Negative | 80 | 572 | 15 | 12 | 15 | 31 | 19 | 3 | |
| Invalid | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Cumulative sensitivity (95% CI) | 83% (72–91) |
80% (72–86) |
69% (62–76) | 63% (56–70) | 62% (56–69) | ||||
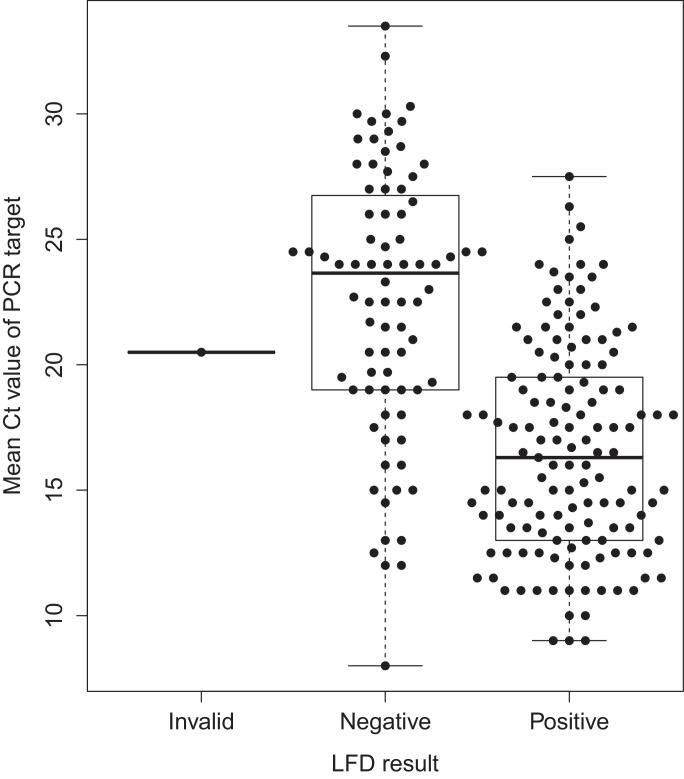
Lateral flow testing showed modest sensitivity, and performed better in those with higher viral loads. Among all 214 SARS-CoV-2 PCR-positive patients, 133 tested positive by LFD, i.e. sensitivity was 62.4% (95% CI 55.6–69.0%), and the negative likelihood ratio was 0.38 (0.32–0.45). 80 patients were LFD-negative, PCR-positive. LFD-negative, PCR-positive individuals had lower viral loads, i.e. higher mean cycle threshold (Ct) values for the detected PCR targets (median 24, IQR 19–27), compared with LFD-positive, PCR-positive patients (median 16, IQR 13–20) (Fig. 1 , Kruskal–Wallis p < 0.001). Sensitivity was greatest in those patients with a mean Ct <20 (78.5%, 95% CI 71.9–85.1%) (Table 1).
Fig. 1.
Lateral flow device (LFD) results and Mean Cycle threshold (Ct) value of Polymerase Chain Reaction (PCR) target detection in 214 patients with SARS-CoV-2 detected. The median (central line), inter-quartile range (box) and range (whiskers) of Ct values are shown.
On a retrospective review of patient notes, we identified at least 11/133 (8%) of LFD positive patients had no Covid-19 symptoms recorded, presenting without cough, dyspnoea, fever, anosmia, ageusia or hypoxia. Furthermore, among LFD-negative PCR-positive patients, 28/80 (35.0%) had a pre-admission SARS-CoV-2 PCR-positive swab, so 161/214 (75.2%) of patients with SARS-CoV-2 detectable by PCR could be identified by either previous results or LFD at admission. The absence of either previous PCR positive swab or a positive LFD at admission had a negative likelihood ratio 0.24 (95% CI 0.19–0.31).
Case identification is critical in reducing nosocomial transmission of SARS-CoV2.2 While Ct values are not a direct measure of infectivity, they do correlate with RNA load and culture positivity and infectious dose.5 , 6 Thus, LFD-positive patients, with higher viral loads, are most likely to represent those patients with the highest infectious risk in the healthcare environment. Additionally, in this cohort, LFDs provided incremental case detection above clinical assessment in asymptomatic adults. LFDs provide a rapid and incremental benefit to clinical triage for case finding.
The excellent specificity seen here corresponds with findings from other evaluation of LFDs,7 as well local experience in testing asymptomatic healthcare workers, where LFDs showed a false positive rate of 0.03% compared with PCR.8 This means a positive LFD can safely be used to triage patients to Covid-19 cohort areas for patients with confirmed infection without exposing these patients to risk of nosocomial acquisition. While a negative result cannot be used in isolation to triage a patient to a COVID-19 free area of the hospital, it does allow earlier identification of positive cases, thus relieving pressure on cohort areas for patients with unconfirmed infection status, which are often the most challenging areas in which to prevent nosocomial transmission.
Despite imperfect sensitivity, when a known COVID diagnosis was taken in account, LFDs in this population had a negative likelihood ratio of 0.24. Therefore in patients where there is a low clinical suspicion of COVID-19, a negative LFD does provide further evidence against infectious SARS-CoV-2 infection, which can also play a role in triage decisions. The sensitivity of LFDs in the emergency hospital setting is lower than that reported in previous evaluations,7 potentially reflecting the challenges of performing LFDs in emergency department settings and the later stage of infection in patients admitted to hospital compared to those attending symptomatic community testing. We did not monitor if all tests were read after the correct time interval, nor were photographs taken of devices at reading to allow for quality assurance. Therefore, reported performance could potentially be improved.
We conclude that LFDs provide a rapid and useful case detection in an acute setting, and are thus a helpful infection control tool.
Declaration of Competing Interest
DWE declares lecture fees from Gilead outside the submitted work. No other authors have a conflict to declare
Acknowledgments
Ethical statement
Patient screening and case reviews were undertaken as part of routine infection control in the hospital.
Funding
BCY is an NIHR Clinical Lecturer. DWE is a Robertson Foundation Fellow.
References
- 1.Fowler V.L., Armson B., Gonzales J.L., et al. A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection. J Infect. Jan 2021;82(1):117–125. doi: 10.1016/j.jinf.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wake R.M., Morgan M., Choi J., Winn S. Reducing nosocomial transmission of COVID-19: implementation of a COVID-19 triage system. Clin Med (Lond) Sep 2020;20(5):e141–e145. doi: 10.7861/clinmed.2020-0411. Epub 2020 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 4.Crozier A., Rajan S., Buchan I., McKee M. Put to the test: use of rapid testing technologies for Covid-19. BMJ. 2021;372:n208. doi: 10.1136/bmj.n208. [DOI] [PubMed] [Google Scholar]
- 5.Jaafar R., Aherfi S., Wurtz N., Grimaldier C., Hoang V.T., Colson P., et al. Correlation between 3790 qPCR positives samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis. 2020:ciaa1491. [Google Scholar]
- 6.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020:ciaa638. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peto T., UK COVID-19 Lateral Flow Oversight Team . 2021. COVID-19: rapid antigen detection for SARS-CoV-@ by lateral flow assay: a national systemic evaluation for mass-testing. medRxiv. [DOI] [Google Scholar]
- 8.Downs L.O., Eyre D.W., O'Donnell D., Jeffery K. Home-based SARS-CoV-2 lateral flow antigen testing in hospital workers. J Infect. 2021 doi: 10.1016/j.jinf.2021.01.008. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]