Dear editor,
We read with interest the study by Zhen et al.,1 showing that there was co-infection of SARS-CoV-2 and influenza virus in the early stages of COVID-19 in Wuhan, China. Symptoms of COVID-19 are similar to influenza-like illness in which both diseases are characterized by high fever, cough, myalgia and headache. Given the similarity of symptoms, the overlap of COVID-19 with the influenza peak season and the lack of testing at the initial phase of the pandemic, Zhen et al. indicated concern that some SARS-CoV-2 cases could have been missed during the emerging phase of the pandemic. Recent evidence suggests that there were multiple cases of COVID-19 co-infection with influenza which was undetected until the discovery of SARS-CoV-2.2 , 3
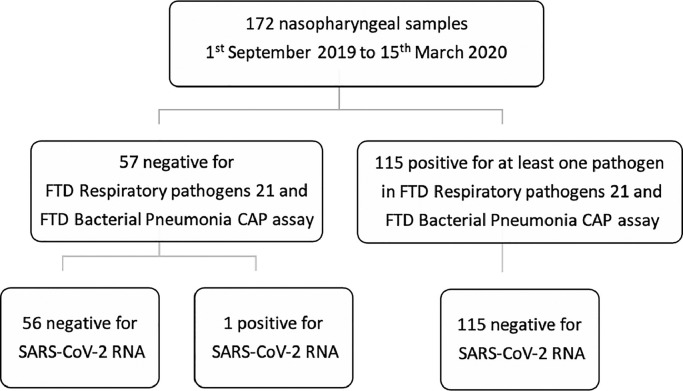
After the declaration of COVID-19 as a pandemic by the World Health Organization in March 2020, global health surveillance was quickly implemented to identify cases of the disease. The official index case of COVID-19 in Northern Cyprus was reported on 10th March 2020 as a tourist from Germany, who arrived at the country via air travel on 8th March 2020.4 Although global surveillance of SARS-CoV-2 was implemented quickly after the declaration of the pandemic, there are reports from several European countries which suggest potential virus circulation prior to the detection of first cases via surveillance.5, 6, 7 In the light of this information, we re-tested 172 nasopharyngeal samples collected from patients who were admitted to Near East University Hospital in Nicosia, Cyprus between 1st September 2019 to 15th March 2020 with respiratory disease symptoms (fever, cough, rhinitis, sore throat or myalgia) for SARS-CoV-2 RNA. These patients were initially screened for viral respiratory RT-PCR panel (FTD Respiratory pathogens 21, Fast Tract Diagnostics, Luxembourg) and bacterial respiratory RT-PCR panel (FTD Bacterial Pneumonia CAP, Fast Tract Diagnostics, Luxembourg) (Fig. 1 ) upon admission with 115 samples positive for at least one pathogen and 57 were negative for all pathogens.
Fig. 1.
Selection of respiratory samples for retrospective SARS-CoV-2 testing.
For each patient sample, nucleic acid extraction was performed using GeneAll Ribospin VRD nucleic acid isolation kit. RT-PCR was performed using Diagnovital SARS-CoV-2 Real-Time PCR kit (RTA Laboratories Inc.), specific to the SARS-CoV-2 envelope gene (E-gene) and the polymerase gene (RdRP), according to the manufacturer's protocol on Insta-Q96 Plus Real-Time PCR System (HiMedia). Confirmation of RT-PCR positive sample was performed using Sansure COVID-19 Nucleic Acid Test Kit (Sansure Biotech Inc.) specific for SARS-CoV-2 ORF1ab and nucleocapsid protein gene (N-gene) on Rotor-Gene Q (Qiagen, Germany), and on QIAstat-Dx Respiratory SARS-CoV-2 Panel RT-PCR system (Qiagen, Germany).
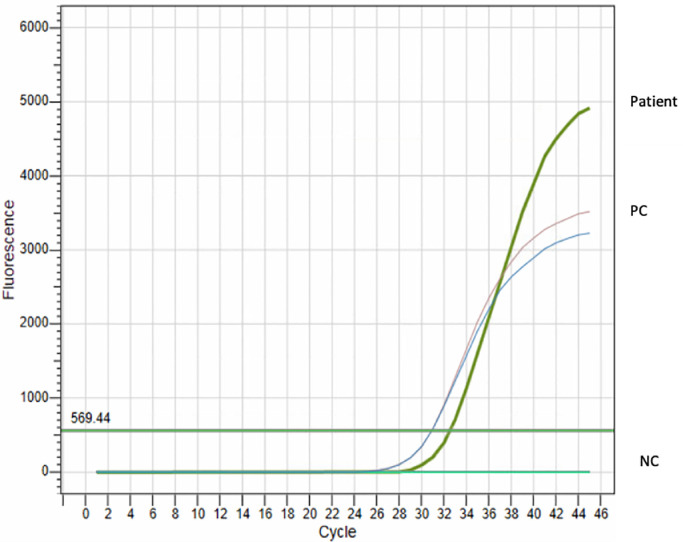
Among 172 enrolled patients 90 were females and 82 were males, respectively. One sample collected from a patient was detected to be positive for SARS-CoV-2 by three different RT-PCR kits (Fig. 2). The positive sample was taken from a 17-year-old Cypriot male teenager who lives in Famagusta region. Patient's nasopharyngeal sample was received at the hospital on 11th March 2020, 1 day after the official announcement of the index case on 10th March 2020 in Northern Cyprus. The patient presented with fever, cough, headache, chest pain and severe myalgia, and was admitted as an in-patient. Both the viral and bacterial respiratory tract panels by RT-PCR testing were negative for the pathogens tested. The last travel history of the patient was in December 2019. After the detection of the positive test result the patient and immediate family members (mother and father) were contacted and tested for SARS-CoV-2 IgM/IgG (AdviseDx SARS-CoV-2 IgM/IgG Immunoassay, Abbott Laboratories Inc.). The patient was detected to be positive for SARS-CoV-2 IgG.
Fig. 2.
Amplification plot for the SARS-CoV-2 positive patient sample by RT-PCR. Curve designation; patient: patient sample, PC: positive control, NC: negative control.
This study reports a local SARS-CoV-2 infected patient 1 day after the official announcement of the index case on 10th March 2020.4 Identification of a prior infected patient has a great impact on our knowledge regarding SARS-CoV-2 circulation and its spread in Northern Cyprus. Although the transmission history of the positive case is unknown, this positive result indicates that SARS-CoV-2 was not imported into Northern Cyprus via the German tourist group as previously reported, and that the local transmission began prior to the arrival of the German tourists.
Our results suggest that the SARS-CoV-2 was already in circulation in the country in early March. Similar retrospective studies were performed in other countries to determine the presence of SARS-CoV-2 in pre-pandemic period. While studies performed in United Kingdom, Italy and France demonstrated undetected COVID-19 cases in the prepandemic period,5, 6, 7 no SARS-CoV-2 positivity was observed in respiratory samples collected prior to the first reported case in Scotland, Germany and the US.8, 9, 10 Further retrospective studies with a larger sample size are required to interpret the actual onset of SARS-CoV-2 in the Northern Cyprus and rule out earlier community transmission.
This study has limitations. Firstly, only a single centre was enrolled. Secondly, although the samples were stored at −80 °C to ensure integrity, false-negative results due to the quality of the samples according to the storage conditions cannot be ruled out. Despite this, our results highlight the importance of timely and extensive community testing to prevent future widespread viral transmission in the case of a pandemic.
Declaration of Competing Interest
Authors state no conflict of interest.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Approval
This study was approved by the Institutional Review Board at Near East University (YDU/2021/87–1262).
References
- 1.Xin Zheng, Hua Wang, Zhengyuan Su, Wei Li, Dongliang Yang, Fei Deng. Co-infection of SARS-CoV-2 and influenza virus in early stage of the COVID-19 epidemic in Wuhan, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.041. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuhei Azekawa, Ho Namkoong, Keiko Mitamura, Yoshihiro Kawaoka, Fumitake Saito. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020 doi: 10.1016/j.idcr.2020.e00775. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruei Huang Bo, Lan Lin Ya, Kang Wan Chih, Torng Wu Jiin, Yu Hsu Chih, Huang Chiu Ming. Co-infection of influenza B virus and SARS-CoV-2: a case report from Taiwan. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazife Sultanoglu, Buket Baddal, Kaya Suer, Tamer Sanlidag. Current situation of covid-19 in northern cyprus. East Mediterr Heal J. 2020 doi: 10.26719/emhj.20.070. Doi: [DOI] [PubMed] [Google Scholar]
- 5.Chappell Joseph G., Theocharis Tsoleridis, Gemma Clark, Louise Berry, Nadine Holmes, Christopher Moore. Retrospective screening of routine respiratory samples revealed undetected community transmission and missed intervention opportunities for SARS-CoV-2 in the United Kingdom. MedRxiv. 2020 doi: 10.1101/2020.08.18.20174623. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deslandes A., Berti V., Tandjaoui-Lambotte Y., Chakib Alloui, Carbonnelle E., Zahar J.R. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106006. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovanni Apolone, Emanuele Montomoli, Alessandro Manenti, Mattia Boeri, Federica Sabia, Inesa Hyseni. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori. 2020 doi: 10.1177/0300891620974755. Doi: [DOI] [Google Scholar]
- 8.Marcus Panning, Julius Wiener, Kathrin Rothe, Jochen Schneider, Pletz Mathias W., Gernot Rohde. No SARS-CoV-2 detection in the German CAPNETZ cohort of community acquired pneumonia before COVID-19 peak in March 2020. Infection. 2020 doi: 10.1007/s15010-020-01471-y. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomb Rachael M., MacLean Alasdair R., Gunson Rory N. Retrospective screening for SARS-CoV-2 in greater Glasgow and clyde ICUs between December 2019 and February 2020. J Clean Prod. 2020 doi: 10.1016/j.jinf.2020.06.022. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan Catherine A., Natasha Garamani, Sahoo Malaya K., Hong Huang Chun, James Zehnder, Pinsky Benjamin A. Retrospective screening for SARS-CoV-2 RNA in California, USA, late 2019. Emerg Infect Dis. 2020 doi: 10.3201/eid2610.202296. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]