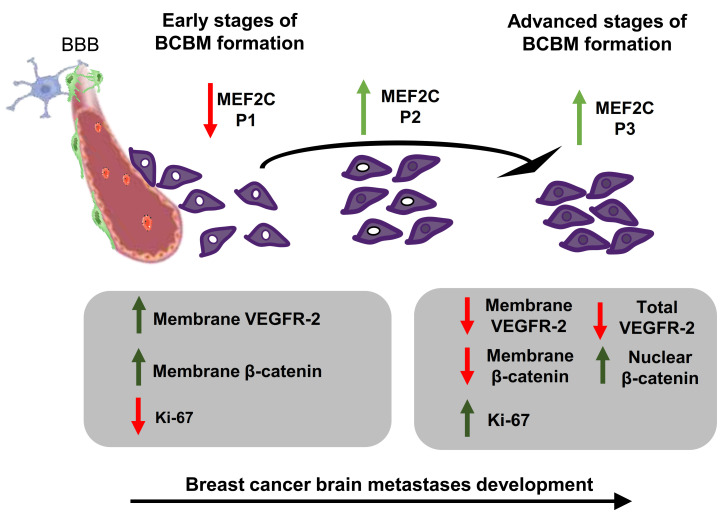
Figure 5.
Schematic representation of the proposed interplay between myocyte enhancer factor 2C, vascular endothelial growth factor receptor 2 (kinase domain insert domain receptor, KDR), and β-catenin (CTNNB1) along with the development of breast cancer brain metastases. In the initial stages of brain metastases, the pattern of myocyte enhancer factor 2C (MEF2C) expression in malignant cells extravasating into the brain and still close to blood–brain barrier (BBB) microvessels is categorized as phenotype (P) 1 (~100% cells with extranuclear location). As metastases progress, the transcription factor progressively translocates into the nucleus giving rise to P2 (~50% cells with extranuclear location and ~50% with overall cell staining), and afterward, to P3 (~100% cells presenting overall cell staining). In parallel, the clear expression of vascular endothelial growth factor receptor 2 (VEGFR-2) at the cell membrane decreases, and an overall loss of the receptor is observed as metastases develop. This is accompanied by a decreased expression of β-catenin in the plasma membrane, which translocates into the nucleus, in line with the role of this molecule in signaling in cancer. Accordingly, an increasing number of Ki-67 positive cells is observed, reflecting the proliferative activity of metastatic cells that accounts for the enlargement of metastases. Collectively, the expression of MEF2C and its translocation into the nucleus is associated with disease severity, which pathogenesis involves VEGFR-2 and β-catenin signaling.