Abstract
Introduction:
Post-marketing data have demonstrated the potential for weight gain with integrase inhibitors (INSTI) use in antiretroviral (ART) therapy.
Methods:
A medical chart review evaluated virologically suppressed adult prisoners living with HIV and on a non-INSTI regimen before switching or adding an INSTI. Primary outcome assessed average weight change; Secondary outcomes evaluated change in body mass index (BMI), fasting lipid panel, and development of hypertension. Statistical analysis included paired t-tests and descriptive statistics.
Results:
Among 103 study participants, 95% were men with a median age of 44 years. Each INSTI was associated with an average weight increase of 4.3 kg (p < 0.025). Bictegravir and dolutegravir were also associated with significant increases in BMI, +1.4kg/m2 and +2.8kg/m2, respectively (p = 0.011 and p = 0.001).
Conclusion:
Patients receiving HIV care in a correctional setting and on INSTI-based treatments experienced weight gain and increases in BMI. Future research should focus on the mechanism of development and interventions to prevent weight gain.
Keywords: integrase strand transfer inhibitors (INSTI), weight gain, antiretroviral therapy (ART), HIV/AIDS
What do we already know about this topic?
Post-marketing data has linked the use of certain integrase strand transfer inhibitors (INSTIs) to weight gain in people living with HIV.
How does your research contribute to the field?
Our data evaluates the entire class of INSTIs in a controlled patient population without interruptions to antiretroviral therapy (ART).
What are your research’s implications toward theory, practice, or policy?
Our data shows that all INSTIs were associated with weight gain in incarcerated patients receiving INSTI-based ART.
Introduction
Due to the dynamic and profound evolution of antiretroviral therapies (ART), human immunodeficiency virus (HIV) has become a successfully manageable condition associated with a remarkable decrease in mortality from the early days of the epidemic. With the addition of integrase strand transfer inhibitors (INSTIs) to the growing list of ART, these agents have become the standard of care in HIV treatment. Many people living with HIV are switched to these regimens from non-INSTI ART since INSTIs have shown to be highly effective in achieving virologic suppression.
While weight loss was once a complication of acquiring HIV, the initiation of successful ART has shown to result in increased weight gain, reflecting successful virologic suppression and treatment.1 However, regardless of immune status or comorbid conditions, weight gain may be associated with an increased risk for diabetes or cardiovascular disease.2 Recent data from several studies have linked weight gain to the initiation or addition of INSTIs in treatment-naïve populations and in the switch to INSTI-based regimens in treatment-experienced participants.3-5 While there is increasing data in treatment-naïve patients, further analysis is still needed to assess the extent of the weight gain and its consequences. This study aims to provide additional data in treatment-experienced participants switching to INSTI-based therapy and provide data about newer, less-studied INSTIs, such as bictegravir.
The primary objective of this study was to determine the percentage of individuals within the Illinois Department of Corrections (IDOC) living with HIV who experienced additional weight gain after initiating an INSTI-based antiretroviral regimen and to evaluate average weight change from baseline through post-initiation. Secondary objectives evaluated extent of change from baseline in body mass index (BMI), BMI categorization for all INSTIs, and fasting lipid panel (FLP), along with development of hypertension, impact on viral suppression and whether certain pre- and post-INSTI regimen components had an additional or predisposing effect on weight gain or BMI after INSTI addition. Correctional facilities provide an ideal setting to evaluate the true effects of INSTIs on weight gain and BMI as they are controlled environments with consistent medication access. Factors such as insurance coverage, cost of antiretrovirals, and clinic and medication adherence are much less of an issue in associating the impact of ART on weight gain in such controlled environments.
Methods
Study Design
A single-center, non-randomized retrospective cohort study at the University of Illinois at Chicago was conducted to evaluate incarcerated adults receiving HIV telemedicine care in IDOC. Participants were evaluated across 26 prisons and treatment centers in Illinois, USA, from 1/1/2011 to 12/31/2018. Data collection of patient information occurred through retrospective review of the hospital’s electronic health record on the basis of prescription and refill history of IDOC patients receiving INSTI antiretrovirals. Electronic health records of each patient were accessed to collect data on demographics, medical and prescription history, and ART pre- and post-switch to an INSTI-based regimen.
Inclusion criteria for this study was adults (≥ 18 years of age), living with HIV or AIDS, incarcerated in IDOC, receiving stable ART (i.e. ≥6 months and virologically suppressed), documented CD4, HIV-RNA, and weight monitoring at least 6 months prior and post switch or addition of an INSTI.
Exclusion criteria in this population were inmates on INSTIs prior to incarceration, ART-naïve, release or unavailable data before 6 months of weight, CD4 or viral load monitoring, reincarceration, or segregation.
Data collection prior to ART switch included: age, gender, ethnicity, height (inches), concurrent medications prior to switch, comorbid medical conditions, length of incarceration (months), current ART and duration (months). Weight parameters included pre-switch average weight (kilograms, average weight in the preceding 6 months prior to switch (including weight on day of switch), BMI at baseline (using average weight prior to switch), BMI classification, and ideal body weight. Laboratory parameters included measurements of CD4 count and percentage prior to switch, HIV-1 RNA level, hemoglobin A1c (HgbA1C), and fasting lipid panel (FLP) (total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL)). Finally, pre- and post-switch blood pressure data was also collected. Development of hypertension (HTN) was defined as initiation of an antihypertensive medication or ≥3 blood pressure readings ≥130/80 mmHg.
Data collection post ART switch included: INSTI regimen, specific integrase, concomitant medications over study period, 4 measurements of CD4 count and percentage and HIV-1 RNA level after the switch, new medical conditions contributing to weight gain, length of time followed-post INSTI (months), FLP, HgbA1C, and the date individual was released from prison and/or reincarcerated. Post-switch weight parameters included average weight (kg) by averaging weight from 4 follow-up visits (12-months), difference in weighted averages to identify weight gain, BMI, and the difference in pre- and post-BMI.
Statistical Analysis
The chi-squared statistic was used to assess whether weight gain occurred in study participants after switching to an INSTI-based regimen. Paired student t-tests were used to compare differences between weight and BMI among all included study participants as well as for those who switched to their INSTI-based regimens. Paired student t-tests were also used to compare fasting lipid panel data, weight gain from ART prior to switch (i.e. NNRTI or PI based) and whether the regimen contained TAF or TDF. The Wilcoxon Mann-Whitney test was used for continuous variables, including length of time on ART before switch, length of time post-switch, and time in prison. Descriptive statistics were utilized to summarize demographic data as well as the primary outcome.
Ethical Approval and Informed Consent
IRB approval was obtained by University of Illinois at Chicago for protocol # 2020-1007 in order to conduct this research. Informed consent was not required based on the retrospective nature of this manuscript.
Results
Changes in Weight and BMI
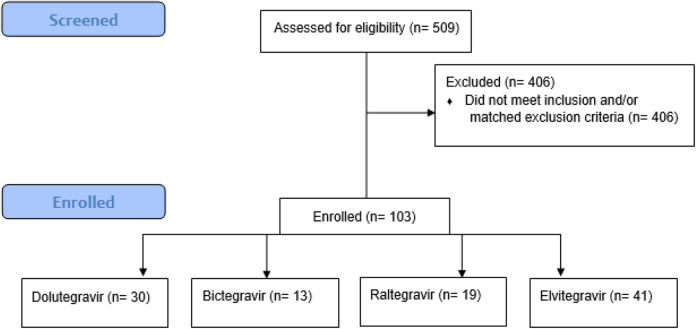
Of 509 participants receiving an INSTI and screened, 103 met criteria for inclusion (Figure 1). Among these 103 individuals analyzed, 86% were black men with an average age of 44 years. On average, participants were stable on ART for 45.4 and 17.2 months prior and post-INSTI switch, respectively (Table 1). Forty-one participants (40%) were switched to elvitegravir-based therapy, followed by 30 (29%) to dolutegravir therapy, 19 (18%) to raltegravir therapy, and 13 (13%) to bictegravir. Weight gain was found with all INSTIs regardless of baseline regimen. Ninety-eight participants were on a TDF-based backbone prior to start of an INSTI. After switch or addition of an INSTI, 35 remained on TDF/emtricitabine (FTC)-based therapy, 31 switched to TAF/FTC, 28 had an abacavir/lamivudine backbone, and 9 were on a nuke-sparing regimen. The average weight gain per person associated with the INSTI class was found to be 4.9 kg. The total average change in BMI from baseline was 1.6 kg/m2. For participants with available height data, 23 experienced an increase in BMI categorization.
Figure 1.
Study profile.
Table 1.
Demographic and Clinical Characteristics.
| Dolutegravir | Bictegravir | Raltegravir | Elvitegravir | |
|---|---|---|---|---|
| (n = 30) | (n = 13) | (n = 19) | (n = 41) | |
| Age, median years (range) | 44 (22-62) | 44 (25-62) | 45 (20-57) | 46 (19-79) |
| Male, n (%) | 28 (97%) | 11 (85) | 18 (95) | 41 (100) |
| Black Race, n (%) | 26 (86%) | 9 (69%) | 15 (79%) | 36 (88%) |
| Median CD4+ Count, cells/mm3 (range) | 646 (316-1115) | 633 (262-1222) | 538 (270-1205) | 648 (203-1504) |
| CD4+ Count < 200 cells/mm3, n (%) | 0 (0) | 1 (8) | 1 (5) | 1 (2) |
| Mean Pre-Switch Time (months)1 | 59 | 50 | 12 | 37 |
| Mean Post-Switch Time (months)2 | 20 | 18 | 15 | 19 |
| Mean BMI at Baseline (kg/m2) + standard deviation | 27.6 + 4.821 | 27.9 + 6.814 | 25.0 + 4.009 | 25.5 + 5.065 |
1 Average time virologically suppressed on ART prior to INSTI switch, (HIV RNA < 200 copies/mL)
2 Average time spent virologically suppressed on ART after INSTI switch, (HIV RNA < 200 copies/mL).
Weight gain was observed with each individual INSTI over a 12-month period (Table 2). Furthermore, each INSTI was associated with change in BMI (Table 2). Dolutegravir was associated with the most weight gain, 6.5 kg (p < 0.0001). Average change in BMI was also highest in this group at 2.8 kg/m2 (p < 0.0001) with 26.7% of dolutegravir participants experiencing an increase in BMI classification. Raltegravir was associated with the lowest amount of weight gain at 3.6 kg (p = 0.009) and within this group, the lowest percentage of participants increased their BMI class to a higher categorization. In terms of actual increase in BMI, elvitegravir was found to have the lowest average increase in BMI at 1 kg/m2 (p = 0.0273). Regardless of INSTI, each was associated with a significant increase in BMI (Table 2). Very few participants lost weight in this study (BIC = 0; DTG = 2; EVG = 7; RAL = 2). Additionally, participants with ≥4.5 kg weight gain over the study period were also observed (BIC = 10; DTG = 15; EVG = 21; RAL = 7).
Table 2.
Integrase Strand Inhibitors (INSTI) Effects on Weight Gain and BMI From Baseline.
| Total | Bictegravir | Dolutegravir | Elvitegravir | Raltegravir | |
|---|---|---|---|---|---|
| (n = 13) | (n = 30) | (n = 41) | (n = 19) | ||
| Average change in weight, kg | -- | +4.9 (p = 0.0036) | +6.5(p < 0.0001) | +4.8(p < 0.0001) | +3.6(p = 0.0090) |
| Average change in BMI, kg/m2 | -- | +1.4(p = 0.011) | +2.8(p ≤ 0.0001) | +1(p = 0.0273) | +1.2(p = 0.0227) |
| Increase in BMI Categorization, n (%) | 23 | 5 (38.5) | 8 (26.7) | 8 (19.5) | 2 (10.5) |
BMI = body mass index; kg = kilogram.
When evaluating whether the pre-INSTI baseline regimen predisposed participants to weight gain or produced a certain impact, no statistically significant change in weight or BMI was observed regardless of whether non-nucleoside reverse transcriptase inhibitor (NNRTI) (p = 0.3208) or protease inhibitor (PI)-based (p = 0.7960) therapy preceded INSTI therapy. Of 98 patients receiving TDF prior to switch, 31 (32%) were switched to TAF-based therapy. When comparing participants on TDF-based therapy at baseline to TAF-based therapy + INSTI post-switch, a significant difference in weight gain was found in the TAF-based group where average weight gain was 6.4 kg (p < 0.0001). In participants who remained on TDF-based therapy for the entire study period, weight gain was observed when an INSTI was added to ART + 2.1 kg (p = 0.0222). Finally, in participants receiving TDF-based therapy at baseline compared to those switching to a non-tenofovir regimen + INSTI, significant weight gain was also observed (+6.0 kg; p < 0.0001). When compared to baseline FLP, elvitegravir-based therapy was associated with a significantly higher TC and LDL (Table 3). Bictegravir data was not included as only 2 participants had evaluable data.
Table 3.
Integrase Strand Inhibitors (INSTI) Effects on Fasting Lipid Panels From Baseline.
| Dolutegravir | Elvitegravir | Raltegravir | |
|---|---|---|---|
| (n = 24) | (n = 25) | (n = 13) | |
| Average change in TC (mg/dL) | +3.42(p = 0.6177) | +16.44(p = 0.0022) | -1.85(p = 0.7858) |
| Average change in TG(mg/dL) | -9.71(p = 0.6951) | -12.04(p = 0.5698) | -15(p = 0.5901) |
| Average change in HDL (mg/dL) | +0.25(p = 0.8706) | +2.08(p = 0.3912) | +3.79(p = 0.2456) |
| Average change in LDL (mg/dL) | +4.13(p = 0.4964) | +12.35(p = 0.0268) | -10(p = 0.2746) |
HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; mg/dL = milligram per deciliter; TC = total cholesterol; TG = triglycerides.
Blood glucose levels were evaluated in all participants prior to and post-initiation of therapy, but only 3 individuals had pre- and post-INSTI data available to assess HbA1C . Participants in this study were only evaluated for the development of diabetes if fasting blood glucose levels were outside of the normal reference value. For the 3 individuals with data, one had preexisting diabetes prior to INSTI-switch while the other 2 s did not develop diabetes post-INSTI switch.
Since blood pressure is evaluated at each clinic visit, 21 out of 103 participants (20.4%) were found to have developed hypertension after the initiation of an INSTI. Dolutegravir was associated with the highest incidence of hypertension dolutegravir (n = 9/30; 30%), followed by elvitegravir (n = 7/41; 17%), bictegravir (n = 2/13; 15%), and raltegravir (n = 1/19; 5%).
Discussion
The addition of or switch to an INSTI and the effects on weight and BMI were evaluated in virologically suppressed individuals living with HIV in a prison setting. This permitted our group to evaluate participants who received consistent follow-up, had excellent access to ART, and had no issues with insurance coverage or ART affordability. By studying a controlled population, we can attribute study results to the medications and regimens themselves, rather than to other confounding components that commonly affect therapeutic outcomes and side effects. Additionally, inmates had minimal changes in physical activity and food intake, did not experience homelessness, and had limited or no substance use while incarcerated. The participants in this population received consistent follow-up for labs and medical care under the supervision of an interdisciplinary telemedicine team and were adherent to medical appointments. In other non-controlled settings of previously conducted retrospective studies, participants may not have consistent access to controlled surveillance or follow-up and may have experienced barriers to continuous care such as transportation or associated-costs of ART.3
Similar to other published studies, dolutegravir was associated with the most weight gain (6.5 kg) over an average 17.2 month period (p < 0.0001).3,6,7 In addition, all INSTIs (bictegravir, elvitegravir, and raltegravir) in our study were associated with significant weight gain. Despite most studies linking INSTI-based therapy to weight gain, a study evaluating treatment-naïve participants did not find any difference in lean mass or regional fat with the use of raltegravir versus ritonavir boosted atazanavir and darunavir.4 Another study evaluating virologically suppressed participants switching to raltegravir or dolutegravir-based therapies, also reported no change in weight gain.8 While the previous studies found no change in weight with raltegravir, use of raltegravir in our study was associated with weight gain, but it was the lowest amount. In addition, due to the small sample size of this group, these results should be interpreted cautiously.
Another benefit of our study is that treatment-experienced participants were virologically suppressed demonstrating a true representation of active use of ART providing valid data for this evaluation. This eliminated the getting back to health effect and demonstrated that most individuals were not immunocompromised as only 3 participants had a CD4 under 200 cells/mm3. Similar to our results, Norwood and colleagues found ART-experienced individuals who switched from efavirenz/tenofovir disoproxil fumarate/emtricitabine to an INSTI-based regimen of either RAL, DTG, or EVG experienced an average weight of 2.9 kg over a period of 18 months (p = 0.81).3 Furthermore, the participants in this cohort who were switched from EFV/TDF/FTC to a regimen containing DTG, specifically DTG/ABC/3TC, gained the most weight of any of the INSTI-based regimens, for an average of 5.3 kg over 18 months (p = 0.001). In the current study, participants who switched to a DTG-containing regimen also experienced the most prominent amount of weight gain over a 12-month period regardless of nucleoside reverse transcriptase backbone.
Increases in BMI and classification results closely mirrored that of the weight gain results; again, dolutegravir was associated with the largest increase in BMI, followed by bictegravir, raltegravir, and elvitegravir. It should be noted that baseline BMI was high in all study groups where, at baseline, the average BMI was greater than 25 which is already considered overweight. In addition, patients switching to dolutegravir or bictegravir regimens had higher baseline BMIs. Twenty-three individuals experienced increases in BMI categorization which increases the risk for dyslipidemia, hypertension, diabetes, and many other comorbidities.9 Unfortunately, not everyone had height data captured in order to calculate BMI which limited the number of evaluable individuals. Additionally, due to the timeframe of this observation, bictegravir was not well-represented in our data since only 13 participants were switched to this agent by the end of 2018, the year bictegravir was introduced to the market.
Regardless of INSTI use and similar to other emerging data, our data was associated with weight gain when participants were switched from TDF to TAF-containing INSTI regimens.5,10,11 In the current study, weight gain associated with dolutegravir could be attributed to the combination with TAF/FTC, however, participants who were on the DTG with TDF/FTC still experienced weight gain.
As weight gain may have consequences on other physiological processes in the cardiovascular and endocrine systems, the development of comorbidities was also assessed. Although recommended as part of baseline laboratory data prior to initiating ART, FLP data was inconsistently obtained prior to and post-INSTI initiation of INSTI-based therapy. For those with available FLP, elvitegravir therapy was associated with significant changes in total cholesterol and LDL similar to the findings of other studies.12 Although not significant, switching from an NNRTI or PI-based regimen to raltegravir demonstrated improvement in lipid parameters.
Blood glucose levels were evaluated in all participants prior to and post-initiation of therapy. Additional work-up was only evaluated if fasting blood glucose levels were outside of the normal reference value.13 During our study, no one developed type 2 diabetes mellitus yet, a case report demonstrated the development of diabetes mellitus in a patient switched from efavirenz to raltegravir with abacavir/lamivudine.14 Based on the paucity of data and updated diabetes recommendations, future studies should evaluate whether or not INSTIs can contribute to the development of diabetes given associated weight gain and traditional risk factors for diabetes.
The development of hypertension was also a secondary outcome evaluated in this study.15 While 18% of the study population developed hypertension (dolutegravir > elvitegravir > bictegravir > raltegravir), the majority of our population was Black which is a risk factor for the development of hypertension with a median age of 44 years. Similar findings were reported in a study in INSTI-naïve patients living in Spain, where the median age was also 44 years. This study found INSTI use was associated with a significant increase in systolic blood pressure (adjusted increase, 7.0 mmHg; 95% CI, 0.3-13.7; P = .039) when correlated with weight gain (r = 0.13; 95% CI, 0.10-0.16; P < 0.001).16 It is unclear whether switching or adding an INSTI contributed to the development of hypertension in our virologically suppressed population but future long-term analyses should evaluate any association.
While many parts of this study were strong, it does not come without limitations. The relatively short follow-up time and strict patient enrollment reduced our overall sample size. Furthermore, the majority of participants enrolled in this study were male, making it difficult to extrapolate these results to the female population. Additionally, bictegravir was approved in 2018 and the study had limited follow-up data on participants switched to bictegravir.17 That being said, this study is one of the first that analyzes weight gain for individuals on bictegravir.
Conclusion
The addition of or switch to INSTI-based therapy in incarcerated INSTI-naïve PLWH was associated with significant increases in weight and BMI in virologically suppressed participants. Dolutegravir was associated with the highest weight gain, while raltegravir was associated with the lowest within this controlled population. Future research should assess long-term impacts on the development of comorbid conditions along with the mechanism behind weight gain.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study was approved by The University of Illinois at Chicago Institutional Review Board (approval no. 2020-1007). Informed consent was not deemed necessary due to the retrospective nature of this study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sarah M. Michienzi, PharmD  https://orcid.org/0000-0003-1182-7997
https://orcid.org/0000-0003-1182-7997
Melissa E. Badowski, PharmD, MPH  https://orcid.org/0000-0001-7564-0131
https://orcid.org/0000-0001-7564-0131
References
- 1. Center for Drug Evaluation and Research. Get drug information for adult patients. U.S. Food and drug administration. Published 2020. https://www.fda.gov/drugs/hiv-treatment/hiv-treatment-information-adults (accessed 21 December 2020).
- 2. Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with HIV in the US and Canada. Clin Infect Dis. 2020;ciaa1403. doi:10.1093/cid/ciaa1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norwood J, Turner M, Bofill C, et al. Brief Report. JAIDS J Acquir Immune Defic Syndr. 2017;76(5):527–531. doi:10.1097/qai.0000000000001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mccomsey GA, Moser C, Currier J. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260 s. Clin Infect Dis. 2016;62(7):853–862. doi:10.1093/cid/ciw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taramasso L, Berruti M, Briano F, Di Biagio A. The switch from TDF to TAF determines weight gain in patients on rilpivirine-based regimen [published online ahead of print, 2020 February 5]. AIDS. 2020;34(6):877–881. doi:10.1097/QAD.0000000000002496 [DOI] [PubMed] [Google Scholar]
- 6. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274. doi:10.1093/cid/ciz407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vizcarra P, Vivancos MJ, Pérez-Elías MJ, Moreno A, Casado JL. Weight gain in people living with HIV switched to dual therapy: changes in body fat mass. AIDS. 2020;34(1):155–157. doi:10.1097/QAD.0000000000002421 [DOI] [PubMed] [Google Scholar]
- 8. Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS. 2020;34(1):109–114. [DOI] [PubMed] [Google Scholar]
- 9. Bangalore S, Fayyad R, Laskey R, DeMicco D, Messerli F, Waters D. Body-weight fluctuations and outcomes in coronary disease. New Engl J Med, 2017;376(14):1332–1340. [DOI] [PubMed] [Google Scholar]
- 10. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi:10.1056/NEJMoa1902824 [DOI] [PubMed] [Google Scholar]
- 11. Ruane P, Clarke A, Post F. Phase 3 randomized, controlled DISCOVER study of daily emtricitabine/tenofovir alafenamide (F/TAF) or emtricitabine/tenofovir disoproxil fumarate (F/TDF) for HIV pre-exposure prophylaxis: week 96 results. 17th European AIDS Conference, Basel, poster presentation PE3.16, 2019.
- 12. Ku PH, Sun HY, Chuang YC, Wu PY, Liu WC, Hung CC. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cboicistat/ emtricitabine/ tenofovir alafenamide. IJID. 2020;92:71–77. doi:10.1016/j.ijid.2019.12.029 [DOI] [PubMed] [Google Scholar]
- 13. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2019;43(Supplement 1):S14–S31. doi:10.2337/dc20-s002 [DOI] [PubMed] [Google Scholar]
- 14. Fong PS, Flynn DM, Evans CD, Korthuis PT. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS. 2017;28(6):626–628. doi:10.1177/0956462416675107 [DOI] [PubMed] [Google Scholar]
- 15. Arnett D, Blumenthal R, Albert M. Correction to: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e60. doi:10.1161/cir.0000000000000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galdamez R, García JA, Fernández M, et al. Short-term increase in risk of overweight and concomitant systolic blood pressure elevation in treatment-naïve persons starting insti-based antiretroviral therapy. Open Forum Infect Dis. 2019;6(12):ofz491. Published November 13, 2019. doi:10.1093/ofid/ofz491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. BIKTARVY® (bictegravir, emtricitabine, and tenofovir alafenamide) tablets, for oral use. Food and drug administration package insert. 2018.