Abstract
Objective
To study changes in T lymphocyte subsets, cytokines, and liver enzymes in patients with malignant obstructive jaundice (MOJ) before and after external biliary drainage (percutaneous transhepatic cholangiography drainage, PTCD) and internal biliary drainage (percutaneous transhepatic insertion of biliary stents, PTIBS).
Methods
MOJ patients undergoing PTCD (n = 44) and PTIBS (n = 38) at our hospital were enrolled in the study from January 2017 until December 2019. Peripheral blood total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), CD3+%, CD4+%, CD4+/CD8+ ratio, interleukin (IL)-2, IL-6, and tumor necrosis factor (TNF)-α were measured before and 1 week after biliary drainage.
Results
There was no significant difference in any parameter between the two groups before biliary drainage. TBIL, DBIL, AST and ALT following PTCD were significantly lower than before PTCD. By contrast, CD3+%, CD4+%, CD4+/CD8+ ratio, IL-2, IL-6 and TNF-α showed no significant difference before and 1 week after PTCD. TBIL, DBIL, AST, ALT, IL-6 and TNF-α were significantly lower following PTIBS than before PTIBS. CD3+%, CD4+%, CD4+/CD8+ ratio and IL-2 were significantly higher following PTIBS than before PTIBS.
Conclusion
Both PTCD and PTIBS were effective for treatment of MOJ, but PTIBS was more beneficial for recovery of immune function.
Keywords: Percutaneous transhepatic cholangiography drainage, percutaneous transhepatic insertion of biliary stent, malignant obstructive jaundice, immune cells, cytokines, liver enzymes
Introduction
Malignant obstructive jaundice (MOJ) is a common disease of the digestive system and is caused by obstruction of the bile ducts by malignant tumors (biliary cancer, pancreatic cancer, and ampulla cancer).1,2 Hyperbilirubinemia and endotoxemia caused by bile duct obstruction can impair liver function as well as immune function.2,3 Suppression of immune function is a key factor leading to high complication rates, high mortality rates and poor prognoses in patients with MOJ.4 Patients with MOJ often experience postoperative infections, which are thought to be associated with weakened immune function, particularly of the cellular immune system.5 Some data have suggested that the mortality rates of MOJ patients were as high as 15% to 20%.1 Therefore, restoring immune function in patients with MOJ quickly and effectively is of great clinical importance to reduce perioperative complications and mortality.
Percutaneous transhepatic cholangiography drainage (PTCD) is a minimally invasive palliative treatment for MOJ.6 Patients undergoing external biliary drainage showed a cumulative bile loss of 800 to 1000 mL or more per day before removal of the drainage tube. Loss of large amounts of bile leads to disturbances of water, electrolyte and pH balance and disrupts digestion of fats, absorption of fat-soluble vitamins, integrity and function of the intestinal mucosal barrier, and homeostasis of the intestinal microflora. Following percutaneous transhepatic insertion of biliary stents (PTIBS), bile can enter the intestine, thus maintaining the physiology of enterohepatic circulation, the balance of water and electrolytes, and the activities and functions of various digestive enzymes in the bile duct, pancreas and intestinal tract. PTIBS has several advantages including fewer complications, enhanced intestinal peristalsis, improved nutritional status, reduced volume of fluid infusion, and reduced financial burden for patients. PTIBS can also reduce the loss of important components of bile such as bile salts and secretory IgA.2
Few studies have investigated whether PTCD and PTIBS can restore the cellular immune function of patients and the production of related cytokines. In this study, changes in T lymphocyte subsets, cytokines and liver enzymes in peripheral blood were analyzed retrospectively before and after biliary drainage. We discuss our results and the evidence they provide for clinical selection of internal and external drainage procedures.
Materials and methods
Patients
Patients with MOJ consulted in our department from January 2017 to December 2019 were treated with either PTCD or PTIBS. Prior to surgery, patients in both groups were unaware of the procedure they would undergo prior to surgery. Tumors causing MOJ included biliary cancer, pancreatic cancer, and ampulla cancer. General and clinical data were collected when patients were admitted to hospital. This study was approved by the Institutional Ethics Committee of Qinhuangdao Municipal No. 1 Hospital (201912B013, 2019-12-18). The patients and/or their families consented to all procedures in the study. We deidentified all patient details.
Inclusion and exclusion criteria
All patients with MOJ seen in our department and diagnosed with malignant tumors by imaging examinations, such as abdominal B-scan ultrasound or computed tomography, from January 2017 to December 2019 were included. Patients did not choose the type of choledochojejunostomy received, and did not receive any chemotherapy, radiotherapy or other anti-tumor treatments. Patients with obstructive jaundice resulting from cholangitis or cholelithiasis, patients with multiple organ failure, and patients with histories of chemotherapy, radiation, or other interventions were excluded.
Biliary drainage
For PTCD, puncture was performed with the assistance of digital subtraction angiography under local anesthesia and with guidance from B-scan ultrasound at the most obvious site of bile duct dilation. At this point, the location and extent of bile duct obstruction was determined by injecting contrast medium, then, using the guide wire expander, applying the interventional exchange technique to fix the drainage tube into the bile duct. After the procedure, patients were deprived of water and kept in bed. Anti-infection, anti-enzyme, hepatoprotective, hemostatic and other treatments were administered. Vital signs were monitored and external drainage was closely observed.
For PTIBS, the bile duct puncture technique was the same as for PTCD. A black loach guide wire was applied to the upper part of the obstructed segment. A single curved duct was introduced along the guide wire, then the guide wire and duct were combined to pass through the narrow segment. An 8- to 10-mm diameter balloon was introduced along the guide wire to expand the narrow segment. After exiting the balloon, a rigid guide wire was used to transport a stent of the proper size to the site of narrowing. Contrast medium was observed to pass smoothly through the narrowing into the duodenum. Finally, the guide wire and catheter were removed to block the puncture channel.
Data collection
Total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), CD3+%, CD4+%, CD4+/CD8+ ratio, interleukin (IL)-2, IL-6 and tumor necrosis factor (TNF)-α were measured 1 day before biliary drainage and 1 week after. Measurements before and after surgery were compared between the two groups. Procedures in patients whose TBIL decreased more than 30% in 1 week or more than 50% in 2 weeks were judged as significantly effective. Procedures in patients whose TBIL decreased less than 30% in 1 week or less than 50% in 2 weeks were judged as effective. All other procedures were judged as ineffective. Total efficacy was calculated as (significantly effective + effective) / total number of cases × 100%. Complications occurring within 2 weeks of procedures were recorded and analyzed. The incidence of acute pancreatitis, acute cholangitis and abdominal hemorrhage was compared between the two groups. Changes in indices of liver function, immunological indices and cytokine levels before and after interventions were compared between the two groups.
Statistical analysis
SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Continuous data were presented as means ± standard deviations and categorical data were presented as counts and percentages. Differences between continuous variables were assessed using the t test and differences between categorical variables were assessed using the χ2 test. Values of p<0.05 were considered statistically significant.
Results
Comparison of patients undergoing PTCD and PTIBS
Among 82 patients with MOJ consulted in our department from January 2017 to December 2019, 44 underwent PTCD and 38 underwent PTIBS. Patients undergoing PTCD were 44 to 71 years old; 21 were women and 23 were men. The tumors causing MOJ were biliary cancer (n = 22), pancreatic cancer (n = 15), and ampulla cancer (n = 7). Patients undergoing PTIBS were 42 to 74 years old; 18 were women and 20 were men. The tumors causing MOJ were biliary cancer (n = 20), pancreatic cancer (n = 13), and ampulla cancer (n = 75). Demographic and clinical data of patients at hospital admission are shown in Table 1. Gender, age, type of malignant tumor, Karnofsky performance score, tumor size, presence of distant metastasis and preoperative chemotherapy were similar in the PTCD and PTIBS groups.
Table 1.
Demographic and clinical characteristics of patients with malignant obstructive jaundice undergoing PTCD and PTIBS.
| PTCD (n = 44) | PTIBS (n = 38) | Statistic | p value | |
|---|---|---|---|---|
| Primary cancer | Biliary cancer (n = 22) | Biliary cancer (n = 20) | χ2 = 0.133 | 0.935 |
| Pancreatic cancer (n = 15) | Pancreatic cancer (n = 13) | |||
| Ampulla cancer (n = 7) | Ampulla cancer (n = 5) | |||
| Age (years) | 57.36 ± 7.99 | 57.29 ± 8.61 | t = 0.040 | 0.968 |
| Sex (F/M) | 21/23 | 18/20 | χ2 = 0.001 | 0.974 |
| Karnofsky performance score | 62.40 ± 1.83 | 62.33 ± 1.70 | t = 0.179 | 0.859 |
| Tumor size (≥5 cm/<5 cm) | 2/42 | 3/35 | χ2 = 0.399 | 0.527 |
| Distant metastasis (Y/N) | 11/33 | 9/29 | χ2 = 0.019 | 0.890 |
| Preoperative chemotherapy (Y/N) | 5/39 | 6/32 | χ2 = 0.344 | 0.558 |
Values are shown as counts or means ± standard deviations.
PTCD, percutaneous transhepatic cholangiography drainage; PTIBS, percutaneous transhepatic insertion of biliary stents.
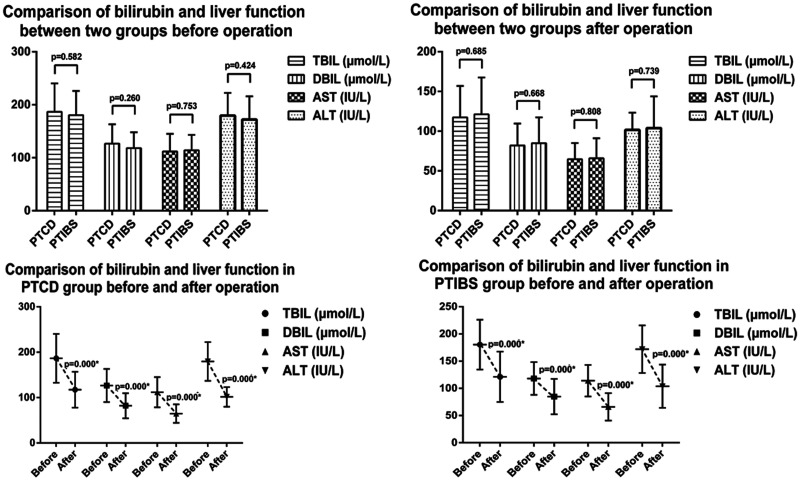
Comparison of bilirubin level and liver function before and after PTCD and PTIBS
Prior to biliary drainage by PTCD, TBIL was 186.56±53.93 µmol/L, DBIL was 126.60±36.60 µmol/L, AST was 111.83 ± 33.32 IU/L, and ALT was 179.72 ± 42.74 IU/L. Prior to biliary drainage by PTIBS, TBIL was 180.40 ± 45.84 µmol/L, DBIL was 118.12 ± 30.02 µmol/L, AST was 114.03 ± 28.98 IU/L, and ALT was 172.04 ± 43.72 IU/L. There were no significant differences in TBIL, DBIL, AST or ALT in the two groups of patients prior to surgery. One week following PTCD, TBIL decreased to 117.35 ± 39.56 µmol/L, DBIL to 82.00 ± 27.64 µmol/L, AST to 64.68 ± 20.41 IU/L, and ALT to 101.61 ± 21.63 IU/L. Differences in all of these parameters before and after PTCD were statistically significant (p<0.05). One week following PTIBS, TBIL decreased to 121.21 ± 46.39 µmol/L, DBIL to 84.86 ± 32.48 µmol/L, AST to 65.90 ± 25.22 IU/L, and ALT to 103.93 ± 39.77 IU/L. Differences in all of these parameters before and after PTIBS were statistically significant (p<0.05). There were no significant differences in TBIL, DBIL, AST and ALT levels between the two groups post-surgery (Figure 1).
Figure 1.
Levels of total bilirubin (TBIL), direct bilirubin (DBIL), asparagine aminotransferase (AST) and alanine aminotransferase (ALT) before and after percutaneous transhepatic cholangiography drainage (PTCD) and percutaneous transhepatic insertion of biliary stents (PTIBS) in patients with malignant obstructive jaundice.
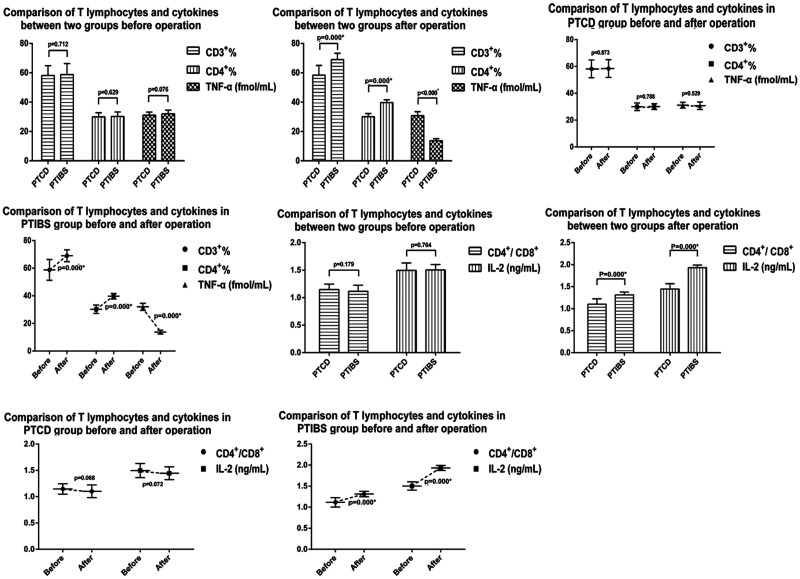
Comparison of T lymphocytes before and after PTCD and PTIBS
Prior to PTCD, the CD3+ T lymphocyte percentage was 58.19 ± 6.65%, the CD4+ T lymphocyte percentage was 29.94 ± 2.80%, and the CD4+/CD8+ ratio was 1.15 ± 0.10. Prior to PTIBS, the CD3+ T lymphocyte percentage was 58.77 ± 7.52%, the CD4+ T lymphocyte percentage was 30.25 ± 3.02%, and the CD4+/CD8+ ratio was 1.12 ± 0.11. There were no significant differences in CD3+ or CD4+ T lymphocyte percentages or in the CD4+/CD8+ ratio between the two groups prior to surgery. One week after PTCD, the CD3+ T lymphocyte percentage was 58.42 ± 6.59%, the CD4+% T lymphocyte percentage was 30.09 ± 2.15%, and the CD4+/CD8+ ratio was 1.10 ± 0.12. There were no significant differences in CD3+ or CD4+ T lymphocyte percentages or in the CD4+/CD8+ ratio before and after PTCD. However, 1 week after PTIBS, the percentage of CD3+ T lymphocytes increased to 69.00 ± 4.29%, the percentage of CD4+ T lymphocytes increased to 39.68 ± 1.98%, and the CD4+/CD8+ ratio to 1.31 ± 0.07. All three of these values were significantly higher than those before surgery (p<0.05). There was a significant difference in postoperative CD3+ T lymphocyte percentages, CD4+ T lymphocyte percentages and the CD4+/CD8+ ratio between patients undergoing PTCD and PTIBS (Figure 2).
Figure 2.
CD3+ T lymphocyte percentages, CD4+ T lymphocyte percentages, CD4+/CD8+ ratio, IL-2 levels and TNF-α levels before and 1 week after percutaneous transhepatic cholangiography drainage (PTCD) and percutaneous transhepatic insertion of biliary stents (PTIBS) in patients with malignant obstructive jaundice.
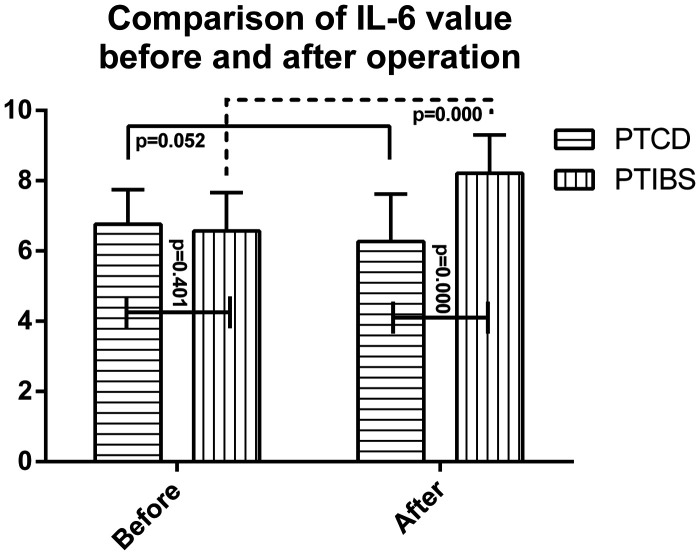
Comparison of cytokines before and after PTCD and PTIBS
IL-2 levels were 1.49 ± 0.13 ng/mL, IL-6 levels were 6.76 ± 0.98 ng/mL, and TNF-α levels were 31.08 ± 2.15 fmol/mL prior to PTCD. IL-2 levels were 1.50 ± 0.10 ng/mL, IL-6 levels were 6.57 ± 1.09 ng/mL, and TNF-α levels were 32.01 ± 2.54 fmol/mL prior to PTIBS. There were no significant difference in IL-2, IL-6 and TNF-α levels between the two groups before surgery. One week after PTCD, IL-2 levels were 1.45 ± 0.12 ng/mL, IL-6 levels were 6.27 ± 1.35 ng/mL, and TNF-α levels were 30.75 ± 2.77 fmol/mL. There were no significant differences between IL-2, IL-6 and TNF-α levels before and after PTCD. However, 1 week after PTIBS, IL-2 levels increased to 1.93 ± 0.06 ng/mL, IL-6 levels decreased to 3.21 ± 1.10 ng/mL, and TNF-α levels decreased to 13.73 ± 1.37 fmol/mL (Figure 3). These differences were statistically significant (p<0.05). There was also a significant difference in postoperative IL-2 and TNF-α levels between the two groups (Figure 2).
Figure 3.
IL-6 levels before and after percutaneous transhepatic cholangiography drainage (PTCD) and percutaneous transhepatic insertion of biliary stents (PTIBS).
Comparison of effectiveness and complication rates of PTCD and PTIBS
PTCD was effective in 93.18% of patients, while PTIBS was effective in 94.74% of patients. There was no significant difference in the incidence of postoperative complications between patients undergoing PTCD and PTIBS (Tables 2 and 3).
Table 2.
Effectiveness of PTCD and PTIBS in reducing total bilirubin in patients with malignant obstructive jaundice.
| Significantly effective | Effective | Ineffective | Total efficacy [n (%)] | |
|---|---|---|---|---|
| PTCD (n = 44) | 31 | 10 | 3 | 41 (93.18) |
| PTIBS (n = 38) | 29 | 7 | 2 | 36 (94.74) |
| χ2 | 0.086 | |||
| p value | 0.769 | |||
PTCD, percutaneous transhepatic cholangiography drainage; PTIBS, percutaneous transhepatic insertion of biliary stents.
Table 3.
Complications of PTCD and PTIBS in in patients with malignant obstructive jaundice.
| Abdominal hemorrhage | Pancreatitis | Cholangitis | Death | Total incidence [n (%)] | |
|---|---|---|---|---|---|
| PTCD (n = 44) | 3 | 0 | 2 | 0 | 5 (11.36) |
| PTIBS (n = 38) | 1 | 3 | 1 | 0 | 5 (13.16) |
| χ2 | 0.061 | ||||
| p value | 0.804 | ||||
PTCD, percutaneous transhepatic cholangiography drainage; PTIBS, percutaneous transhepatic insertion of biliary stents.
Discussion
Treatment of biliary obstructions using minimally invasive interventions is important for patients with MOJ.7 Successful treatment can prolong survival and improve quality of life. Percutaneous or endoscopic biliary drainage (EBD) can also be performed, but studies have shown that the incidence of pancreatitis among patients undergoing EBD was higher than in those undergoing PTCD. In this study, PTCD and PTIBS were used for minimally invasive treatment of MOJ.8 Changes in TBIL, DBIL, AST and ALT before and after PTCD and PTIBS were assessed. In both groups, we observed significant decreases in TBIL, DBIL, AST and ALT 1 week post-surgery. We found no difference between the effectiveness of PTCD and PTIBS nor in the incidence of complications among patients undergoing these procedures. Thus, both drainage methods had similar overall effects in relieving jaundice and restoring liver function. However, there were some differences between PTCD and PTIBS. PTCD is relatively simple; however, its disadvantages include the requirement to carry a drainage bag for relatively long periods, loss of a large amount of bile salts causing pH, water and electrolyte imbalances, slow recovery from the procedure, and limited improvement to quality of life.9 The advantages of PTIBS are that bile is able to enter the intestine completely, restoring the biliary tract close to the physiological state. Moreover, there is no need for an in-dwelling drainage tube, so the patient's quality of life improves substantially.10
We found a significant difference in levels of TNF-α before and after biliary drainage in patients undergoing PTIBS, but not in those undergoing PTCD. Levels of TNF-α after PTIBS were significantly lower than those before surgery, in agreement with a previous study.2 One potential explanation for this result is that the lack of bile salts in the intestinal tract during development of MOJ led to imbalances of bacterial flora, production of large amounts of endotoxin, bacterial translocation, entry of endotoxin into the liver through the portal vein,11 and activation of Kuppfer cells to produce large amounts of TNF-α.12,13 In patients undergoing PTIBS, bile could enter the intestine through a narrow segment after drainage, inhibiting the overgrowth of intestinal flora and endotoxin production, maintaining the integrity of mucosal barrier, and preventing endotoxin absorption.
T lymphocytes in the peripheral blood are a sensitive indicator of cellular immune status. In the healthy body, T lymphocyte subpopulations coordinate with one another to maintain normal immune function. Perturbation of the numbers or function of T lymphocyte populations can lead to immune disorders. The CD4+/CD8+ ratio can indirectly reflect the level of cellular immune function, and is positively correlated with cellular immune function.2 Previous studies showed that obstructive jaundice affected T lymphocyte function in the early stages of the disease; however, some effects lasted longer, and the main manifestations were increased numbers of lymphocytes and decreased numbers of CD4+ T lymphocytes.14 A negative association was observed between elevated lymphocyte levels in the peripheral blood and the risk of serious infection post-treatment.15 Therefore, to reduce the risk of serious infection, lymphocyte function must be restored as early as possible following treatment.
IL-2 is produced by T lymphocytes, mainly CD4+ cells, and promotes proliferation of T lymphocytes, lymphocyte maturation, and immune function.16–19 In this study, patients undergoing PTIBS showed more complete functional recovery of lymphocytes than patients undergoing PTCD within 1 week post-surgery. CD3+ T lymphocyte percentages, CD4+ T lymphocyte percentages, the CD4+/CD8+ ratio and IL-2 levels were significantly increased following PTIBS, but not following PTCD. This finding was consistent with the results of previous studies.2,14 One potential explanation of this result was that following PTCD, bile cannot enter the intestine. Thus, the intestinal tract continues to overproduce TNF-α, which can elicit neutrophil inflammatory mediators that inhibit T lymphocyte proliferation, activation and IL-2 secretion. Therefore, changes in CD3+ T lymphocyte percentages, CD4+ T lymphocyte percentages, the CD4+/CD8+ ratio and IL-2 levels were not obvious following PTCD.5,20 IL-6 is mainly produced by activated mononuclear phagocytes, including Kuppfer cells. During MOJ, levels of TNF-α and the number of Kupffer cells increased, resulting in significant IL-6 production.21 In patients undergoing PTIBS, production of TNF-α was reduced after bile entered the intestine and numbers of Kupffer cells decreased, resulting in decreased levels of IL-6 and weaker inhibitory effects of TNF-α on activation of T lymphocytes. Therefore, CD3+ T lymphocyte percentages, CD4+ T lymphocyte percentages, the CD4+/CD8+ ratio and IL-2 levels were upregulated.
In summary, biliary drainage using PTCD and PTIBS was effective in treating patients with MOJ. Both procedures improved jaundice, achieved biliary drainage, and restored liver function similarly. Compared with PTCD, PTIBS may improve the immune function of patients sooner after surgery. These results provide a theoretical basis for the choice of minimally invasive surgery for MOJ. However, there were several limitations to our study. First the number of cases included in the study was small, and our findings should be replicated in larger prospective studies. Second, our study did not include other minimally invasive methods for treatment of jaundice, such as EBD. Third, this was a retrospective analysis, and thus prone to some degree of bias. We hope that future research can replicate and expand upon our findings.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: JL A, YC D, HT N, JF S and ZB Z contributed to the design of the study and the development of the study protocol, YC D and YG L coordinated the study. HT N and JF S reviewed literature and performed data collection and data analysis. All authors contributed to data interpretation, manuscript drafting and review. XY H drafted the first version of the manuscript.
ORCID iD: Yanchao Dong https://orcid.org/0000-0002-2006-6723
References
- 1.Sha J, Dong Y, Niu H. A prospective study of risk factors for in-hospital mortality in patients with malignant obstructive jaundice undergoing percutaneous biliary drainage. Medicine (Baltimore) 2019; 98: e15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang ZK, Kou ZP, Li SX, et al. To evaluate the correlation between the change of immune system function before and after the treatment of malignant obstructive type jaundice treated with biliary stent. Eur Rev Med Pharmacol Sci 2018; 22: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 3.Ljungdahl M, Osterberg J, Ransjo U, et al. Inflammatory response in patients with malignant obstructive jaundice. Scand J Gastroenterol 2007; 42: 94–102. [DOI] [PubMed] [Google Scholar]
- 4.Tang K, Sui LL, Xu G, et al. Effects of different palliative jaundice reducing methods on immunologic functions in patients with advanced malignant obstructive jaundice. Anticancer Res 2017; 37: 4665–4670. [DOI] [PubMed] [Google Scholar]
- 5.Katz SC, Ryan K, Ahmed N, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol 2011; 187: 1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrashekhara SH, Gamanagatti S, Singh A, et al. Current status of percutaneous transhepatic biliary drainage in palliation of malignant obstructive jaundice: A review. Indian J Palliat Care 2016; 22: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan F, Cui L, Bai Y, et al. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: a systematic review and meta-analysis. Cancer Imaging 2017; 17: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo A, Ricci AD, Frega G, et al. How to choose between percutaneous transhepatic and endoscopic biliary drainage in malignant obstructive jaundice: An updated systematic review and meta-analysis. In Vivo 2020; 34: 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai AG, Zheng CS, Zhou GF, et al. Comparison of the therapeutic effects of PTBD and PTBS in treatment of malignant obstructive jaundice. Zhonghua Zhong Liu Za Zhi 2010; 32: 456–458. [PubMed] [Google Scholar]
- 10.Niu HT, Huang Q, Zhai RY. Percutaneous transhepatic biliary stenting in patients with intradiverticular papillae and biliary strictures caused by ampullary carcinoma: A case report. Oncol Lett 2014; 7: 1257–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlidis ET, Pavlidis TE. Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int 2018; 17: 17–21. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CH, Yeh CH, Sheen-Chen SM, et al. The kinetic expression of lipopolysaccharide-binding protein and CD14 gene in obstructive jaundice. J Invest Surg 2015; 28: 18–23. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wu F, Long Y, et al. Glutathione supplementation attenuates oxidative stress and improves vascular hyporesponsiveness in experimental obstructive jaundice. Oxid Med Cell Longev 2015; 2015: 486148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Chen X. Change and significance of T-cell subsets and TNF-alpha in patients with advanced malignant obstructive jaundice treated by percutaneous transhepatic biliary external and internal drainage. Front Med China 2007; 1: 364–368. [DOI] [PubMed] [Google Scholar]
- 15.Loof TG, Sohail A, Bahgat MM, et al. Early lymphocyte loss and increased granulocyte/lymphocyte ratio predict systemic spread of Streptococcus pyogenes in a mouse model of acute skin infection. Front Cell Infect Microbiol 2018; 8: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Fang J, Peng X, et al. Effects of sodium selenite on aflatoxin B1-induced decrease of ileac T cell and the mRNA contents of IL-2, IL-6, and TNF-alpha in broilers. Biol Trace Elem Res 2014; 159: 167–173. [DOI] [PubMed] [Google Scholar]
- 17.Grottesi A, Sette M, Palamara T, et al. The conformation of peptide thymosin alpha 1 in solution and in a membrane-like environment by circular dichroism and NMR spectroscopy. A possible model for its interaction with the lymphocyte membrane. Peptides 1998; 19: 1731–1738. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Gaspar M, Santolaria F, Jarque-Lopez A, et al. Prognostic value of cytokines in SIRS general medical patients. Cytokine 2001; 15: 232–236. [DOI] [PubMed] [Google Scholar]
- 19.Haga Y, Tempero MA, Kay D, et al. Intracellular accumulation of unconjugated bilirubin inhibits phytohemagglutin-induced proliferation and interleukin-2 production of human lymphocytes. Dig Dis Sci 1996; 41: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 20.Gadzhiev DN, Tagiev EG, Gadzhiev ND. Directed cytokine therapy in complex treatment of patients with obstructive jaundice of cholelithic genesis. Vestn Khir Im I I Grek 2016; 175: 67–70. [PubMed] [Google Scholar]
- 21.Manohar M, Verma AK, Venkateshaiah SU, et al. Chronic pancreatitis associated acute respiratory failure. MOJ Immunol 2017; 5: 00149. [DOI] [PMC free article] [PubMed] [Google Scholar]