Abstract
Objectives
To study the effect of glucagon-like peptide 1 (GLP-1) on NLR family pyrin domain containing 3 (NLRP3) inflammasome-induced inflammation in perivascular adipose tissue (PVAT) of Zucker diabetic fatty (ZDF) rats and the underlying role of nuclear factor (NF)-κB signalling.
Methods
Thirty ZDF rats were randomly divided into three study groups: DM (0.9% saline, subcutaneously); DM+GLP-1 (liraglutide, s.c.); and NF-κB+GLP-1 (betulinic acid then liraglutide, s.c.). Ten Zucker lean rats were examined as normal controls. PVAT from ZDF (DM) rats was examined for inflammasome mRNA. Protein levels of NLRP3, cleaved caspase-1, caspase-1, gasdermin D (GSDMD), interleukin (IL)-1β and IL-18 in PVAT were compared between control, DM and DM+GLP-1 groups. Protein levels of NLRP3, IL-1β, IL-18 and NF-κB in PVAT were compared between control, DM, DM+GLP-1 and NF-κB+GLP-1 groups.
Results
The inflammasome most abundantly expressed in ZDF rat PVAT was NLRP3. NLRP3, cleaved caspase-1, IL-1β, IL-18, and GSDMD were markedly upregulated in DM versus control tissue, and GLP-1 reversed this effect. Inhibition of NLRP3 inflammasome-associated inflammation by GLP-1 was lost by activation of NF-κB with betulinic acid.
Conclusion
GLP-1 may alleviate NLRP3 inflammasome-dependent inflammation in PVAT by inhibiting NF-κB signalling.
Keywords: GLP-1, NLRP3 Inflammasome, perivascular adipose tissue, NF-κB
Introduction
Obesity has become a worldwide public health problem, and is frequently combined with other medical conditions, particularly type 2 diabetes mellitus (T2DM) and atherosclerosis.1 The prevalence of T2DM in China has been increasing annually.2 Atherosclerosis, including coronary heart disease, is a major complication in patients with T2DM, and the main cause of disability and death in these patients.3 Therefore, emphasizing early prevention and treatment of angiopathy in patients with T2DM is important. The inflammatory response has been accepted to play a decisive role in the development of atherosclerosis. Notably, obesity and diabetes can lead to adipose tissue inflammation. Compared with patients of European descent, Chinese patients develop visceral obesity more easily. Dysfunctional adipose tissue can result in chronic and systemic inflammation, which is closely associated with cardiovascular disease.4
Perivascular adipose tissue (PVAT) is located outside the adventitia, and has a dual effect on the pathophysiology of blood vessels.5 As a specific type of visceral adipose tissue, PVAT regulates the function and structure of blood vessels by secreting adipokines. In patients with obesity and diabetes, levels of PVAT are significantly higher than those in healthy people. Obesity leads to a chronic systemic inflammatory response in PVAT, reduces the production of anti-inflammatory adipokines, and aggravates cell dysfunction, vascular oxidative stress and inflammation. Furthermore, research has shown that PVAT plays an important role in the development of atherosclerosis.6
The inflammasome is a complex composed of cytoplasmic pattern recognition receptors (nucleotide-binding oligomerization domain [NOD]–like receptor [NLR] family pyrin domain containing 1 [NLRP1], NLR family pyrin domain containing 3 [NLRP3], NLR family CARD domain containing 4 [NLRC4], and absent in melanoma 2 [AIM2]), apoptosis-related spot-like protein (ASC) and caspase-1 precursor (procaspase-1). The inflammasome activates caspase-l, which then activates interleukin (IL)-1β and IL-18, promotes inflammatory responses, and activates gasdermin D (GSDMD), causing pyroptosis.7 The NLRP3 inflammasome is the best-studied inflammasome type. NLRP3 is abundantly expressed in the aortic tissue of patients with coronary atherosclerosis and is closely related to the degree of coronary artery stenosis and expression of ASC, caspase-1, IL-1β and IL-18 in unstable and stable plaques.8,9 Knockdown of NLRP3 has been shown to significantly reduce the area of atherosclerosis plaques in apolipoprotein E-deficient mice.10 Pyroptosis is a type of caspase-1-dependent programmed cell death characterized by fast membrane pore formation and release of inflammatory contents, resulting in cell lysis. The best-studied type of pyroptosis is NLRP3 inflammasome-dependent pyroptosis.11
Glucagon-like peptide 1 (GLP-1) is a peptide of 36 or 37 amino acids that is derived from proglucagon and mainly produced by the intestinal L cells. GLP-1 is known to enhance glucose-dependent insulin release and gastric emptying, lower blood glucose, and reduce food intake. Liraglutide is an analogue of GLP-1 that is used as an important therapy in the treatment of diabetes mellitus. GLP-1 improves the adverse clinical outcome of diabetic angiopathy. In clinical studies of the cardiovascular effects of GLP-1, including the LEADER study and the SUSTAIN-6 study, patients with T2DM and cardiovascular disease who used the GLP-1 analogues liraglutide and semaglutide showed a significant reduction in the risk of cardiovascular composite endpoints (comprising cardiovascular death, nonfatal myocardial infarction, and stroke).12,13
In the present study, the effects of GLP-1 on NLRP3 inflammasome-induced inflammation in PVAT from obese diabetic rats was investigated. The hypothesis that nuclear factor (NF)-κB signalling may be the mechanism underlying GLP-1 regulation of NLRP3 inflammasome activation in PVAT was also tested.
Materials and methods
This study included male Zucker diabetic fatty (ZDF) rats and Zucker lean rats (Beijing Charles River Laboratory Animal Technology Co., Ltd.; Beijing, China). Animals were received at the Animal Centre of South China University aged 11 weeks. On arrival to the animal unit, rats were caged pairwise and allowed to acclimatize for 7 days. Rats were housed at 22 ± 1 °C in a 12-h light/12-h dark cycle, with ad libitum access to chow (Purina 5008) and water. Thirty ZDF rats were randomly divided into three groups: DM group, administered 0.9% saline as vehicle, 1 ml/kg body weight, subcutaneously; DM+GLP-1 group, administered 200 µg/kg body weight liraglutide (GLP-1 analogue; Novo Nordisk, Maaloev, Denmark) s.c.; and NF-κB+GLP-1 group, administered 15 µM/kg body weight betulinic acid (NF-κB activator; Sigma-Aldrich, St Louis, MO, USA) then 200 µg/kg body weight liraglutide, s.c. These rats were dosed by s.c. injections once daily for 12 weeks. Additionally, ten Zucker lean rats (obtained at 6 weeks old) were examined as a normal control group. The study groups are summarised in Table 1. All animal experiments were approved by the animal experimental ethics committee of The First Affiliated Hospital of South China University (Hengyang, China), and all animal procedures were conducted in accordance with the institutional animal care and use committee of South China University.
Table 1.
Characteristics of the four study groups.
| Group | Rat type | Treatment |
|---|---|---|
| Control group | Zucker lean rats | None |
| DM group | Zucker diabetic fatty rats | 0.9% saline vehicle, 1 ml/kg, subcutaneous injection |
| DM+GLP-1 group | Zucker diabetic fatty rats | 200 µg/kg liraglutide in 0.9% saline, subcutaneous injection |
| DM+GLP-1+NF-κB group | Zucker diabetic fatty rats | 15 µM/kg betulinic acid followed by 200 µg/kg liraglutide in 0.9% saline, subcutaneous injection |
Real-time quantitative PCR analysis
Perivascular adipose tissue was collected from around the aortic arch and immediately snap frozen. Total RNA was isolated from approximately 0.1–0.2 g of cryopreserved PVAT, that was fully ground before adding TRIzol reagent (Thermo Fisher Scientific; Waltham, MA, USA) at 10 × the volume of the sample, according to the manufacturer’s instructions. A two-step amplifying protocol was performed. Total RNA was reverse transcribed to cDNA using a cDNA Synthesis kit (Thermo Fisher Scientific) with 6 µl (50 pg–5 µg final concentration) RNA template in a total reaction volume of 30 µl, according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) of the cDNA was then performed, with each reaction comprising the following: 1000 ng template cDNA, 12.5 µl SYBR Green Real-time PCR Master Mix (Thermo Fisher Scientific), and 0.5 µl (1 mol/l) forward and reverse primers, respectively, in a 25 µl reaction volume. The primer pair sequences were as follows: NLRP1 forward 5′-AGACAACAATCAGGAGCCGAACAC-3′, and reverse 5′-GTCAACCATCTCAGCAGTCACAGG-3′; NLRP3 forward 5′-GCAGCGATCAACAGGCGAGAC-3′, and reverse 5′-TCCCAGCAAACCTATCCACTCCTC-3′; AIM2 forward 5′-CATCACGGAGGAGGAACTGAAACG-3′, and reverse 5′-AGGTGGCAGAAGCAACAGAACAG-3′; NLRC4 forward 5′-GGGCTCTGCTGCTGAAGTTACAC-3′, and reverse 5′-CCGTGGTGGTGGTGACAATGAC-3′; and β-actin forward 5′-GGTCAGGTCATCACTATCG-3′, and reverse 5′-TGCCACAGGATTCCATAC-3′.
A StepOnePlus™ Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific) was employed for thermocycling, using the following conditions: 95 °C for 10 min; followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 32 s; followed by 95 °C for 15 s, 60 °C for 60 s and 95 °C for 15 s. Expression levels were quantified with the 2-ΔΔCq method.
Western blotting analysis
The protein concentration from PVAT in rats was measured using a BCA Protein Assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). Proteins in each 20 µg protein sample were separated by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. The membrane was blocked with nonfat milk for 1.5 h at room temperature, then incubated with primary antibodies at 4 °C overnight, washed three × 5 min each with tris-buffered saline Tween-20 (TBST), followed by incubation with goat anti-rabbit or anti-mouse secondary antibodies for 1 h at room temperature. After washing three × 15 min each with TBST, the protein bands were visualized using a Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) and the density was quantified with ImageJ software, version 1.46 (NIH, Bethesda, MA, USA). Primary antibodies (and working dilutions) were as follows: anti-NLRP3 (1: 1000; cat. No. ab232401; Abcam, Cambridge, MA, USA); anti-ASC (1: 1000; cat. No. ab175449; Abcam); anti-β actin (1: 10000; cat. No. ab8224; Abcam); anti-cleaved caspase1 (1: 1000; #89332S; Cell Signalling Technology, Danvers, MA, USA); anti-caspase1 (1: 1000; cat. No. ab179515; Abcam); anti-GSDMD (1: 1000; cat. No. ab239377; Abcam); anti-NF-κB (1: 500; cat. No. ab28849; Abcam); anti-IL-1β (1: 1000; cat. No. ab9722; Abcam); and anti-IL-18 (1: 1000; cat. No. ab191860; Abcam). Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG antibody (1: 2000; cat. Nos. SA00001-1 and SA00001-2; Proteintech Group, Rosemont, IL, USA) were used as secondary antibodies.
Statistical analyses
Each experiment was performed three times. Data are presented as mean ± SEM, and all data analyses were conducted using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Between-group statistical analyses were performed using Student’s t-test. One-way analysis of variance (ANOVA) with a Tukey post-hoc test was used for comparisons of more than two groups. A P value < 0.05 was considered to indicate a statistically significant difference.
Results
The most abundant inflammasome in PVAT is NLRP3
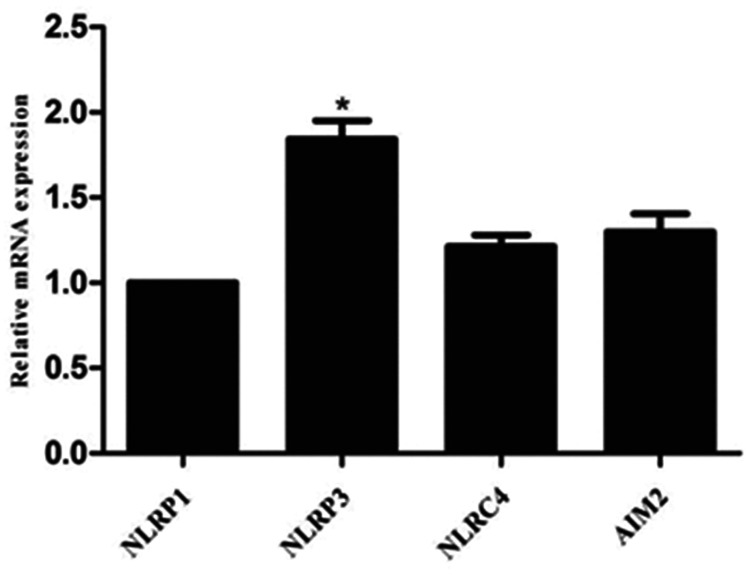
Levels of inflammasome mRNA were examined in PVAT from ZDF rats, and NLRP3 was found to be the most highly expressed compared with NLRP1, NLRC4 and AIM2 (P = 0.022; Figure 1).
Figure 1.
Semiquantitative reverse-transcription real-time polymerase reaction showing expression of NLR family pyrin domain containing 1 (NLRP1); NLR family pyrin domain containing 3 (NLRP3); NLR family CARD domain containing 4 (NLRC4); and absent in melanoma 2 (AIM2) in perivascular adipose tissue from male Zucker diabetic fatty rats (n = 3). NLRP3 was the most highly expressed, P = 0.022 versus other genes.
GLP-1 blunts inflammasome activation in PVAT
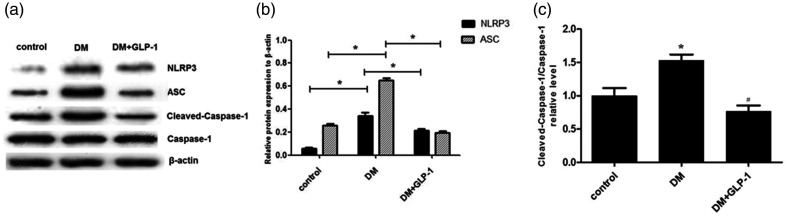
To investigate the effects of GLP-1 on inflammation and NLRP3 inflammasome activation in PVAT, Zucker lean rats (controls) and ZDF rats treated with or without liraglutide for 12 weeks (DM and DM+ GLP-1 groups) were analysed. Protein levels of NLRP3, cleaved caspase-1, caspase-1, and ASC in PVAT were measured using Western blots. As shown in Figure 2, NLRP3, cleaved caspase-1, and ASC were significantly upregulated in the DM group compared with controls (P < 0.05), and this upregulation was effectively reversed by GLP-1 (P < 0.05; DM+GLP-1 group versus DM group).
Figure 2.
Protein levels of NLR family pyrin domain containing 3 (NLRP3), apoptosis-related spot-like protein (ASC), cleaved caspase-1 and caspase-1 in perivascular adipose tissue from Zucker lean rats (controls) and male Zucker diabetic fatty rats, with or without liraglutide for 12 weeks (DM and DM+GLP-1 groups): (a) representative western blots; (b) NLRP3 and ASC levels normalised to β-actin; and (C) ratio of cleaved caspase-1/caspase-1. Data presented as mean ± SEM of 10 rats per group (samples assayed in triplicate); *P < 0.05, DM versus controls, or DM+GLP-1 versus DM group (analysis of variance).
GLP-1 alleviates NLRP3 inflammasome activation in PVAT
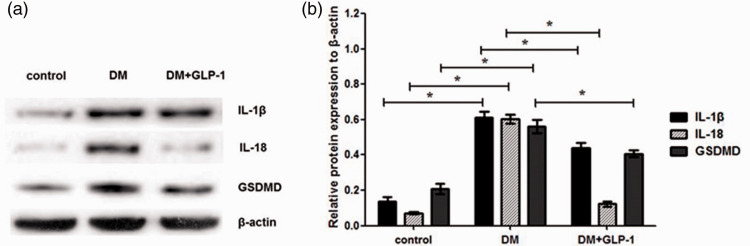
As shown in Figure 3, expression levels of IL-1β, IL-18, and GSDMD in PVAT from ZDF rats (DM group) were higher than those in Zucker lean rats (control group; P < 0.05), suggesting that hyperglycaemia and hyperlipidaemia may induce inflammatory activation in PVAT. Moreover, GLP-1 was shown to significantly reverse the upregulated expression of these indicators (P < 0.05; DM+GLP-1 group versus DM group). Thus, it may be surmised that GLP-1 alleviates the inflammatory activation in PVAT induced by hyperglycaemia and hyperlipaemia in the ZDF rat model.
Figure 3.
Protein levels of interleukin (IL)-1β, IL-18 and gasdermin D (GSDMD) in perivascular adipose tissue from Zucker lean rats (controls) and male Zucker diabetic fatty rats, with or without liraglutide for 12 weeks (DM and DM+GLP-1 groups): (a) representative western blots; and (b) IL-1β, IL-18 and GSDMD levels normalised to β-actin. Data presented as mean ± SEM of 10 rats per group (samples assayed in triplicate); *P < 0.05, DM versus controls, or DM+GLP-1 versus DM group (analysis of variance).
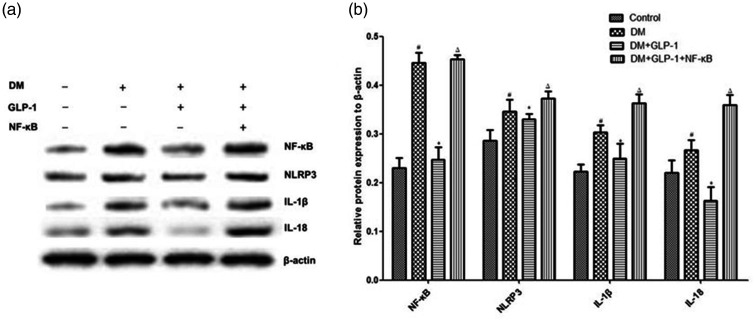
To further analyse the mechanism underlying suppression of the NLRP3 inflammasome by GLP-1, the level of NF-κB was examined. As depicted in Figure 4, expression of NF-κB in PVAT was significantly enhanced in the DM group versus controls (P < 0.05), and this increase was markedly reversed by GLP-1 (P = 0.035; DM+GLP-1 group versus DM group). To further validate the role of NF-κB signalling in this process, inflammatory protein levels in rats randomly divided into four groups: control, DM, DM+GLP-1, and DM+ GLP-1+NF-κB activators, were assessed by western blots. Inhibition of NLRP3 inflammasome-mediated inflammation in the GLP-1-treated group was lost after activation of NF-κB (P < 0.05; DM+GLP-1+NF-κB group versus DM+GLP-1 group; Figure 4). These findings suggest that GLP-1 may participate in reduction of the NLRP3 inflammasome by inhibiting NF-κB signalling.
Figure 4.
Protein levels of nuclear factor (NF)-κB, NLR family pyrin domain containing 3 (NLRP3), interleukin (IL)-1β and IL-18 in perivascular adipose tissue from Zucker lean rats (controls) and male Zucker diabetic fatty rats, with or without liraglutide for 12 weeks (DM or DM+GLP-1 groups) or with liraglutide plus NF-κB activator betulinic acid (DM+GLP-1+NF-κB group): (a) representative western blots; and (b) NF-κB, NLRP3, IL-1β and IL-18 levels normalised to β-actin. Data presented as mean ± SEM of 10 rats per group (samples assayed in triplicate); #P < 0.05, DM versus controls (analysis of variance); *P < 0.05, DM+GLP-1 versus DM; ΔP < 0.05, DM+GLP-1+NF-κB versus DM+GLP-1 (one-way analysis of variance with a Tukey post hoc test).
Discussion
This study generated two significant findings: (1) GLP-1 attenuated activation of the NLRP3 inflammasome in PVAT from a rat model of obesity and diabetes, and (2) NF-κB signalling may be the mechanism underlying the regulation of NLRP3 inflammasome activation by GLP-1 in PVAT. These novel findings suggest a potential clinical application for GLP-1 in the prevention of atherosclerosis.
Atherosclerosis is a process of chronic inflammation, and inflammation is reportedly associated with decreased expression of lipogenic factors in the omental depot from patients with diabetes.14 PVAT-induced inflammation plays an important role in atherosclerosis. Notably, adipocytes and macrophages in PVAT regulate vascular inflammation by transmitting signals to endothelial and inflammatory cells in the arterial wall.15 PVAT inflammation and arterial inflammation share a mutual influence and promotion, causing inflammation of the blood vessels, plaque instability and even rupture. Endogenous genes expressed in human adipose tissue are reported to be relevant to obesity and T2DM.16 Furthermore, PVAT phenotyping would help to establish potential risks, including atherosclerosis, in obese individuals.17 Therefore, PVAT is recognized as a novel therapeutic target of atherosclerosis.18
The present study found that the NLRP3 inflammasome is highly expressed in the PVAT of a rat model of obesity and diabetes, demonstrating that hyperglycaemia and hyperlipidaemia may induce inflammatory activation in PVAT. GSDMD, the key inducer of pyroptosis, was suppressed by GLP-1, demonstrating that GLP-1 may regulate pyroptosis in PVAT. In a high-fat diet-induced obese mouse model, NLRP3, ASC and caspase-1 expression in adipose tissue was shown to be upregulated, and IL-1β was also increased.19 Moreover, knockdown of NLRP3 and caspase-1 genes may improve adipose tissue inflammation, alleviating high-fat diet-induced insulin resistance.20
Endothelial cell inflammatory activation and pyroptosis mediated by the NLRP3 inflammasome has been demonstrated to play an important role in melatonin-mediated atherosclerosis.21 In addition, the GLP-1 analogue liraglutide has been shown to significantly reduce cardiovascular and renal adverse outcomes independent of blood glucose and body weight.22 GLP-1 regulates the macrophage inflammatory response by inhibiting NLRP3 inflammasome activity,23 and a previous study reported that GLP-1 inhibits activation of the NLRP3 inflammasome in peripheral tissues, such as the kidney, lung and liver.24 Furthermore, GLP-1 has been reported to affect the NLRP3 inflammasome in macrophages and myocardial stem cells.25 In addition, Salim et al.26 found that teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated the proinflammatory phenotype of PVAT and inhibited atherogenesis in normoglycemic apolipoprotein E-deficient mice. GLP-1 has also been reported to suppress proinflammatory activation of macrophages and adipocytes in PVAT.27 However, whether GLP-1 affects inflammation mediated by the NLRP3 inflammasome in PVAT has not been determined. To the best of the authors’ knowledge, the present study is the first to determine that GLP-1 attenuates inflammatory activation by the NLRP3 inflammasome in PVAT.
Activation of the NLRP3 inflammasome requires two steps: priming and then assembly of the inflammasome complex. The priming step is initiated by pattern recognition receptors, cytokine receptors, or any factor able to induce the activation of NF-κB, and these initiations result in the upregulation of NLRP3 to a functional level and pro-IL-1β expression.28 The priming step of the NLRP3 inflammasome is initiated by the activation of NF-κB.29 Furthermore, melatonin alleviates NLRP3 inflammasome-induced inflammation and pyroptosis through inhibiting NF-κB/GSDMD signalling in adipose tissue.19 Zhu et al.30 found that PVAT aggravates adventitial remodelling in obese mini pigs by NLRP3 inflammasome/IL-1 signalling. In the present experiment, GLP-1 is surmised to participate in reduction of the NLRP3 inflammasome by inhibiting the NF-κB signal.
In summary, the results of the present study suggest that GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in PVAT through inhibiting the NF-κB signalling pathway. The present data reveal a novel function of GLP-1 in the inflammatory response in PVAT, suggesting a new potential therapy for atherosclerosis. Further studies involving in vitro experiments will shed light on the mechanism of GLP-1.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060521992981 for GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway by Xiangheng Chen, Qiuling Huang, Juling Feng, Zhongsheng Xiao, Xiaoling Zhang and Lei Zhao in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81900488), Natural Science Foundation of Hunan Province (grant No. 2019JJ50549), Technological Innovation Guidance Program of Hunan Province (grant No. 2018SK51605) and Hengyang Science and Technology Board Program (grant No. HKF2019Y47N207).
ORCID iD: Lei Zhao https://orcid.org/0000-0002-3755-4344
References
- 1.Mzayek F, Wang LE, Relyea G, et al. Impact of abdominal obesity on proximal and distal aorta wall thickness in African Americans: the Jackson Heart Study. Obesity (Silver Spring) 2019; 27: 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes 2018; 67: 3–11. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res 2016; 118: 1771–1785. [DOI] [PubMed] [Google Scholar]
- 4.Crujeiras AB, Cordero P, Garcia-Diaz DF, et al. Molecular basis of the inflammation related to obesity. Oxid Med Cell Longev 2019; 2019: 5250816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Alfonso MS, Gil-Ortega M, Aranguez I, et al . Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol 2017; 174: 3561–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhalil M, Edmond E, Edgar L, et al. The relationship of perivascular adipose tissue and atherosclerosis in the aorta and carotid arteries, determined by magnetic resonance imaging. Diab Vasc Dis Res 2018; 15: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016; 41: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng F, Xing S, Gong Z, et al. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 2013; 22: 746–750. [DOI] [PubMed] [Google Scholar]
- 9.Jin Y, Fu J. Novel insights into the NLRP3 inflammasome in atherosclerosis. J Am Heart Assoc 2019; 8: e012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng F, Xing S, Gong Z, et al. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm 2014; 2014: 507208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 2015; 25: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Villalobos NA, Trevino-Alvarez AM, Gonzalez-Gonzalez JG. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 1797–1798. [DOI] [PubMed] [Google Scholar]
- 13.Ipp E, Genter P, Childress K. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376: 890–891. [DOI] [PubMed] [Google Scholar]
- 14.Poulain-Godefroy O, Lecoeur C, Pattou F, et al. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1–R7. [DOI] [PubMed] [Google Scholar]
- 15.Akoumianakis I, Tarun A, Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br J Pharmacol 2017; 174 :3411–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalán V, Gómez-Ambrosi J, Rotellar F, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res 2007; 39: 495–500. [DOI] [PubMed] [Google Scholar]
- 17.Blundell JE, Dulloo AG, Salvador J, et al. Beyond BMI–phenotyping the obesities. Obes Facts 2014; 7: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee TK, Aronow BJ ,Tong WS, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics 2013; 45: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Gan L, Xu Y, et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res 2017; 63: e12414. [DOI] [PubMed] [Google Scholar]
- 20.Tartey S, Kanneganti TD. Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology 2019; 156: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu X, Bai X, et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res 2018; 64: e12449. [DOI] [PubMed] [Google Scholar]
- 22.Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839–848. [DOI] [PubMed] [Google Scholar]
- 23.Dai Y, Dai D, Wang X, et al. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther 2014; 28: 425–432. [DOI] [PubMed] [Google Scholar]
- 24.Zhu W, Feng PP, He K, et al. Liraglutide protects non-alcoholic fatty liver disease via inhibiting NLRP3 inflammasome activation in a mouse model induced by high-fat diet. Biochem Biophys Res Commun 2018; 505: 523–529. [DOI] [PubMed] [Google Scholar]
- 25.Chen A, Chen Z, Xia Y, et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun 2018; 499: 267–272. [DOI] [PubMed] [Google Scholar]
- 26.Salim HM, Fukuda D, Higashikuni Y, et al. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vascul Pharmacol 2017; 96–98: 19–25. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Liu G, Yuan Y, et al. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis 2019; 10: 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahid A, Li B, Kombe AJK, et al. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol 2019; 10: 2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YG, Kim SM, Kim KP, et al. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells 2019; 8: 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Zhang HW, Chen HN, et al. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin 2019; 40: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060521992981 for GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway by Xiangheng Chen, Qiuling Huang, Juling Feng, Zhongsheng Xiao, Xiaoling Zhang and Lei Zhao in Journal of International Medical Research