Abstract
Stingless bee-collected pollen (bee bread) is a mixture of bee pollen, bee salivary enzymes, and regurgitated honey, fermented by indigenous microbes during storage in the cerumen pot. Current literature data for bee bread is overshadowed by bee pollen, particularly of honeybee Apis. In regions such as South America, Australia, and Southeast Asia, information on stingless bee bee bread is mainly sought to promote the meliponiculture industry for socioeconomic development. This review aims to highlight the physicochemical properties and health benefits of bee bread from the stingless bee. In addition, it describes the current progress on identification of beneficial microbes associated with bee bread and its relation to the bee gut. This review provides the basis for promoting research on stingless bee bee bread, its nutrients, and microbes for application in the food and pharmaceutical industries.
Keywords: stingless bee, bee bread, fermented pollen, phenolic compounds, microbes
1. Introduction
Bees have a unique social life. In a social bee community, the queen dominates the reproduction and has a morphology different from other bees. The colonies live for many years (perennial) [1]. Stingless bee is an example of social bees alongside honeybee and bumblebee. It contains more than 50 genera and 600 species worldwide [2]. Stingless bee is different from other bee species. As its name implies, stingless bee′s sting is significantly reduced [2] and is not an effective mechanism for nest protection, but it can still bite to protect its hive from threats.
Bee products are crucial for bee survival, and it is acquired for medicine, offering cosmetic, and everyday uses since 8000 BC [3]. Gradually, modern beekeeping industry emerged, whereby beekeepers collect these products for commercial purposes or personal uses. Stingless bees are widely distributed in countries such as South America, Africa, Southeast Asia, and Australia [1], prompting ventures of stingless bee beekeeping industry in these local regions for socioeconomic development and biodiversity conservation.
Bee bread is one the bee by-products made from pollen collected by the bee added with nectar and bee salivary enzymes before it undergoes lactic acid fermentation in beehives [4]. Bee bread is also referred to as fermented pollen, pot-pollen, stored pollen, or ambrosia [4,5]. Occasionally, among researchers, it is used interchangeably with bee pollen. In this article, the term bee bread is used and strictly referred to as stored pollen collected from inside of the beehives.
Consumer interest and demand for natural products have influenced in-depth research for bee bread nutritional properties. Bee bread is rich in carbohydrate, protein, and lipids and contain other micronutrients such as minerals, vitamins, phenolic compounds, and essential amino acids [6]. It possesses therapeutic values such as anti-inflammatory properties, antioxidant properties, antimicrobial properties, antitumor activity, and antihypertensive activity [6,7]. Bee bread nutritional values are varied as factors such as botanical origin, geographical location, climatic conditions, soil type, beekeeper activities, and bee species contribute towards its chemical composition [8,9].
Research on bee pollen from European honeybee (Apis spp.) has been continuously reported [4,6,10] overshadowing what bee bread can offer. One of the reasons is the difficulty in acquiring honeybee bee bread from the honeycomb [4]. This consequently influenced researchers′ preference to study on bee pollen. However, bee pollen is biochemically different to bee bread [11] as the latter is a product of fermentation. This makes research on bee bread essential to distinguish between these bee products for the development of meliponiculture industry. Apiarists could profit from bee bread production, which could sustain bee-keeping industry by not relying solely on honey as a source of income.
The nutrient richness of bee bread simultaneously promotes the growth of microbes. For the bee, microbes are essential to protect bee colonies against pathogens and provide nutrients for bee growth [12]. There is plenty of studies reported on isolation and identification of bee bread microbes with profound interest on lactic acid bacteria [13,14] because of its significant status as an industrially important group of bacteria. For instance, recent discoveries have identified bacteria from stingless bee bee bread as probiotic [15] and source of antimicrobial compounds and industrial enzymes [16] signifying the potential use of bee bread microbes in the food industry.
In this review paper, bee bread of stingless bee is introduced, highlighting its chemical composition and health benefits. In addition, microbes specifically associated with bee fermentation in relation to the bee gut are discussed.
2. Stingless Bee Bee Bread: From Production to Harvesting
The bee bread raw materials originate from the plant pollens. Pollens are fine granular substances produced by the plant anther containing male gametophyte (sperm cell), which are essential for plant reproduction. It is either distributed through the wind (“anemophilous”—wind-pollinated) or by an insect (“entomophilous”—insect-pollinated). The global production of pollens was estimated as 1.36 million kg per year with China, Australia, and Argentina as the biggest contributors [4].
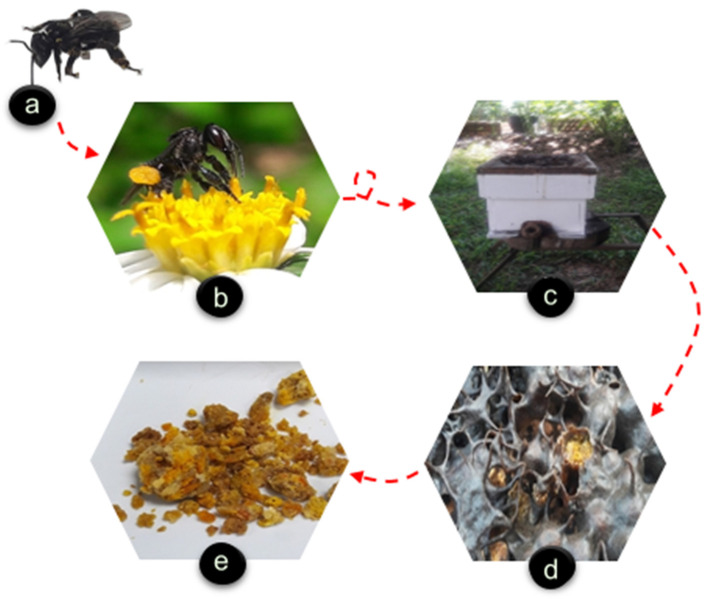
The process of stingless bee making bee bread begins from plant pollen, which is illustrated in Figure 1. Stingless bee can have an average flight range within a radius of 712 m [17] varying with bee species, bee′s body size, and food availability [18], [19]. Stingless bee such as Heterotrigona itama prefers foraging plants closest to their hive especially from white- and cream-coloured flowers [20,21,22] with nectar containing high sugar concentration [19].
Figure 1.
The production of bee bread from flower pollen. (a) Stingless bee, (b) stingless bee visits flower and collects pollen on its hind leg, (c) stingless bee returns to its hive, and (d) stores the pollen inside cerumen pots where lactic acid fermentation occurs to form bee bread. (e) Collected bee bread.
During foraging, forager bees collect nectar and store it in their honey stomach while their bodies are covered in pollen dust. Pollen profile collected by different stingless bees species has been documented in Southeast Asia [21,22,23,24,25,26,27,28] and South America [29,30,31,32,33]. Stingless bees collect pollen from underutilised fruits, tree, and ornamental plants, shrubs, epiphytes, herbs, and lianas [21,31]. They have smaller body size compared to honeybees, which provides an advantage in collecting pollen from small flowers such as Mimosa pudica [22,26] and Mimosa caesalpiniaefolia [33].
As the bees collect pollens, they use their salivary enzymes (amylase and glucosidase) [34] and honey [35] to moisten, agglutinate, and pack the pollen into “pollen basket” on their hind legs [36]. The addition of these substances converts flower pollen into bee pollen. The forager bees transport the collected pollen (bee pollen) with nectar back to their hive. Some beekeepers collect the bee pollen, and this is usually observed in the case of honeybee Apis. Bee pollen is acquired using a pollen trap installed at the hive entrance, which strips the pollen from the bee′s leg by forcing the bees to crawl through a small tight hole [6].
If the bee pollen is not harvested, the worker bees pack the bee pollen inside a cerumen pot (for stingless bee) (Figure 2) or honeycomb cells (for honeybee) made from beeswax and resin. Once the pollen pots are full, the pots are sealed closed [37] before being consumed by larvae or young adult bees as a protein source [2]. During storage, lactic acid fermentation takes place to transform bee pollen into bee bread [38].
Figure 2.
(a) Stingless bee colony and (b) honey and bee bread in separate cerumen pots.
A single stingless bee colony could produce up to 6 kg of bee bread per year depending on the species. In Brazil, the maximum price for bee bread, locally known as “sambura” is 247 USD/kg [39]. In Malaysia, fresh wet bee bread could cost up to 95 USD/kg [22].
Because stingless bee′s bee bread is stored in cerumen pot, its acquisition is different from those of honeybee. Honeybee bee bread is acquired either through manual extraction [40] or usage of a specialised bee bread harvester for large scale production [41]. However, the current method to acquire stingless bee′s bee bread is using forceps, tweezers, or spatula. This imposes challenges especially towards mass production of bee bread for commercialisation [11].
Biochemical Changes from Bee Pollen to Bee Bread
Flower pollen undergoes different development stages to become the end product known as bee bread. The biochemical profiles of flower pollen, bee pollen, and bee bread (Figure 3) are different as several biochemical changes take places at each stage [11,42]. Early studies revealed minor differences between bee bread and bee pollen. Bee bread lacks starch and has low ash content but has higher reducing sugar and fibre than bee pollen [43]. In the last decade, a few studies have attempted to compare the nutrient content of bee pollen and bee bread using more advanced technology. Although Anđelković et al. [42] found higher crude ash, protein, fewer minerals, and lower cellulose in bee bread than bee pollen, the botanical origins of the pollens which could influence the outcomes were not investigated.
Figure 3.

(a) Flower pollen, (b) bee pollen on the stingless bee (Heterotrigona itama) hind leg, and (c) fermented bee pollen (bee bread) collected from the stingless bee cerumen pot.
Comparing the chemical composition of monofloral pollens is more enticing [6]. For example, investigating and comparing the monofloral fresh pollen, bee pollen, and bee bread of Aloe greatheadii var. davyana and Helianthus anthuus (sunflower) showed that bee bread has significantly higher water and carbohydrate content, but lower protein, lipid, and fatty acid content than its fresh pollen [44,45]. Flower pollen chemical profile was assumed to shift after collection because of the addition of bee salivary enzymes and honey [43,45]. However, according to researchers, these changes are considered minor [44,45].
Perhaps the noticeable change between bee pollen and bee bread is the pH. Bee bread is more acidic compared to bee pollen. Studies on pH changes of stingless bee pollen and bee bread are still lacking. However, when looking upon European bee pollen pH, it shows to be significantly reduced from pH 4.7 to 3.97 after transformation into bee bread [46]. Duarte et al. [47] also found high pH values (4.2–5.6) for Melipona asilvai, M. quadrifasciata anthidioides, M. scutellaris, M. subnitida, Tetragona clavipes, and Plebeia sp. bee pollen. The pH for stingless bee bee bread will be discussed in the later section.
Fermentation not only produces chemical changes in bee bread but also has been speculated to improve bee bread digestibility and bioavailability by degrading the outer pollen layer [48,49,50]. Nevertheless, Fernandes-da Silva [51] found no major difference in the nutritional value and digestibility between bee pollen and bee bread collected by Scaptotrigona postica. A recent study also showed bee bread digestibility equivalent to those of fresh pollen even after consumption and digestion by Apis mellifera scutellata [52]. In this sense, microbial fermentation was not able to ferment the highly resistant pollen walls to the fullest.
3. Physicochemical Properties
3.1. Physical Properties
A stingless bee can carry a pollen weight of 10.9 mg [53] and the pollen load decreases as the size of the bee increases [54]. The honeybee is larger than a stingless bee; therefore, it only carries an average of 7.9 mg pollen [55]. Pollen has diverse colours ranging from yellow, orange, brown, black, and purple which could indicate its botanical origin. For instance, bellflower (Nesocodon mauritianum) has purple pollen. Pollen colour tends to change into black once stored in hive due to oxidation [56]. Bee product’s colour is also associated with their phenolic content. The pollen colour is attributed to the presence of flavonoid [57], which relatively depends on the plant source.
Pollen wall has three different layers. The outermost layer is the pollenkitt followed by the outer wall (exine) and inner wall (intine). The exine consists of sporopollenin, while the intine is composed of cellulose and pectin [58]. The exine is the most difficult layer to be digested by bees and animals [59], and the structural integrity remains after storage of bee bread in the hive [51,52].
Bee bread contains accumulated pollen grains from various flowering plants. Although analysis of pollen colour could give an insight into its floral origin, a single pollen colour does not indicate monofloral source [60]. This is confirmed by Modro et al. [56] who suggested accurate pollen analysis for taxa identification by melissopalynology.
Melissopalynology is the microscopic study of pollen grains in bee products such as honey, pollen, propolis, and royal jelly [61]. Pollen grains are studied based on their physical features such as size, shape, aperture, and ornamentation [58]. The pollen grains sizes can be between 2.5 and 250 µm in diameter [10]. The surface of pollen has different structural properties such as clava, perforate, reticulate (mesh), granula, and echinate (spike-like) [58]. Each pollen is classified as either predominant, secondary dominant, or minor based on its frequency [62], which is subsequently important for product identification as either monofloral/unifloral or multifloral.
Pollen morphology is species-specific; therefore, it provides valuable information on the pollen’s botanical origin and plants preferentially visited by the bees [24,63]. This helps beekeepers plan and manage suitable landscape for bee foraging. Identifying plant origin also contributes to a better understanding of bee’s nutritional source, and bee products made from these plants, eventually, give commercial value to it [61].
The taste of bee bread is different across species. For example, Tetragonula angustula, Ptilotrigona and Frieseomelitta doederlini, and F. varia produce sweet bee bread, whereas Melipona and Scaptotrigona produce bitter bee bread [39,64]. To reduce the bee bread sourness, it is marketed mixed with honey or added in vitamins and juices [39].
3.2. Chemical Properties
Importantly, 250 different compounds comprise bee bread, and these are macro- and micronutrients, vitamins, amino acids, fatty acids, and phenolic compounds [65]. Bee bread has been dubbed as the “perfectly complete food” on various occasions because of its nutrient richness [66,67,68]. Bee pollens are consumed mainly as human food in Brazil and European countries such as Bulgaria, Poland, and Switzerland, and therefore, its physicochemical parameters and nutritional values have been standardised there [36]. Campos et al. [36] have proposed a quality criterion for honeybee Apis mellifera pollen to be considered as an international standard. However, unlike bee pollen, bee bread standard has yet to be articulated. Due to this setback, researchers opt to compare the research data of stingless bee bee bread to the international standard of bee pollen Apis spp. [22].
It is challenging to generalise bee bread nutritional values. Several factors including botanical and geographical origin, climatic condition, soil type, beekeepers’ activities, or storage treatments in commercial production [69,70] contribute to these challenges. The chemical properties of bee bread from stingless bee worldwide are summarised in Table 1.
Table 1.
Chemical analysis of bee bread from stingless bee across different geographical locations.
| Bee Species | Origin | Botanical Origin | Moisture (%) | Ash (%) | Total Lipid (%) | Protein (%) | Crude Fibres (%) | Total Carbohydrate (%) | Water Activity | pH | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterotrigona itama | Malaysia | ND | 8.10 | 1.70 | – | 47.4 | – | 55.1 | – | – | [77] |
| Heterotrigona itama | Malaysia | ND | – | – | 2.17–4.80 | 17.22–18.37 | – | 32.74–58.73 | – | – | [78] |
| Heterotrigona itama | Malaysia | Mimosa pudica, Sphagneticola trilobata, Bidens pilosa, Cassia sp., Areca catechu, Peltophorum pterocarpum, Phaleria capitate, Cassia siamea, Citrus aurantifolia Ageratum conyzoides | 11.09–12.51 a | 2.54 a | 5.3 a | 22.6 a | – | 58.2 a | 0.73–0.85 | – | [22] |
| Heterotrigona itama | Malaysia | ND | 30.45 | 3.28 | 13.43 | 30.43 | – | 22.36 | – | – | [79] |
| Tetragonula laeviceps | Thailand | Trema micrantha, Cocos nucifera | 16.1 a | 2.30 a | 7.4 a | 15.5 a | – | 58.7 a | - | – | [80] |
| Tetragonula testaceitarsis | Cocos nucifera, Acacia sp. | 31.7 a | 2.20 a | 5.4 a | 17.9 a | – | 43.1 a | – | – | ||
| Lepidotrigona terminata | Trema micrantha, Cocos nucifera, Acacia sp | 25.3 a | 1.80 a | 5.3 a | 14.3 a | – | 53.4 a | – | – | ||
| Lepidotrigona flavibasis | Tapirira sp. | 22.8 a | 2.20 a | 4.9 a | 16.7 a | – | 53.3 a | – | – | ||
| Melipona sp. aff. eburnea | Venezuela | Fabaceae Papilionoideae (46%), Malpighiaceae (26%) | 48.54 | 2.33 | 3.19 | 18.32 | – | 27.62 | – | – | [81] |
| Scaptotrigona sp. f. ochrotricha | Malpighiaceae (54%), Fabaceae Papilionoideae (20%), | 43.49 | 1.94 | 6.72 | 16.80 | – | 31.03 | – | – | ||
| Meliponini seminigra | Brazil | ND | 53.39 | 4.03 a | 10.81 a | 37.63 a | 9.30 a | 25.66 a | 0.91 | 3.70 | [82] |
| Meliponini interrupta | ND | 37.12 | 2.74 a | 6.47 a | 25.00 a | 13.65 a | 44.27 a | 0.85 | 3.34 | ||
| Tetragonula biroi Friese | Philippines | ND | 14.15–16.73 | 3.23–3.97 a | 2.64–5.43 a | 19.55–23.55 a | – | 53.05–59.94 a | 0.60–0.67 | 3.73–4.13 | [83] |
| Tetragonula angustula | Venezuela | ND | 23.56–25.45 | 1.90–2.22 | 3.98–5.43 | 18.10–26.31 | – | 42.25–49.4 | – | – | [84] |
| Frieseomelitta sp. aff. Varia | Southern Venezuela | Antigonon sp., Astronium sp., Avicennia sp., Bidens sp., Eupatorium sp., Scrophularia nodosa Solanum americanum | 29.96 | 3.13 | 3.51 | 24.72 | – | 38.68 | – | – | [9] |
| Melipona compressipes | 32.75 | 2.85 | 4.12 | 21.01 | – | 39.27 | – | – | |||
| Melipona eburnean | 35.89 | 2.54 | 6.03 | 18.44 | – | 37.10 | – | – | |||
| Melipona favosa | 29.01 | 2.92 | 4.38 | 22.31 | – | 41.38 | – | – | |||
| Melipona sp. fulva | 31.65 | 2.45 | 5.72 | 19.43 | – | 40.75 | – | – | |||
| Melipona lateralis kangarumensis | 38.32 | 2.76 | 4.80 | 21.77 | – | 32.35 | – | – | |||
| Melipona paraensis | 42.74 | 1.93 | 5.23 | 19.08 | - | 31.02 | – | – | |||
| Melipona mandacaia | Brazil | ND | 36.0 | 4.9 | – | 21.0 | 3.6 | – | 0.86 | 3.49 | [85] |
| Scaptotrigona mexicana | Cañada Blanca, Mexico | Heliocarpus sp., Bursera simaruba, Chamaecrista sp., Desmodium adscendens, Desmodium tortuosum, Eugenia capuli, Bidens pilosa, Vernonia sp., Pouteria sp., Verbesina sp., Coccoloba sp. | 24.6 | 3.1 | 0.46 | 22.01 | – | 31.99 | – | 3.46 | [86] |
| Scaptotrigona mexicana | Manuel León, Mexico |
Bursera simaruba, Parthenium fruticosum, Helianthus sp., Spondias mombin, Solanum sp., Chamaecrista sp., Serjania sp., Heliocarpus sp., Vernonia sp., Pithecellobium sp. Coccoloba sp., Bidens pilosa. |
15.5 | 2.5 | 1.1 | 20.49 | - | 33.10 | – | 3.61 | [86] |
| Scaptotrigona mexicana | Fortín de las Flores, Mexico | Spondias mombin, Dendropanax arboreus, Solanum sp., Verbesina sp., Heliocarpus sp., Pouteria sp., Cordia sp., Vernonia sp., Eugenia sp., Dysphania ambrosioides, | 26.7 | 2.9 | 1.1 | 21.06 | – | 35.02 | – | 3.64 | [86] |
| Melipona scutellaris | Brazil | Tapirira guianensis, Spondias mombin, Syagrus sp., Hylocereus undatus, Pueraria phaseoloides, Mimosa caesalpiniifolia, Pithecellobium dulce, Ossaea sp., Miconia sp., Psidium sp., Corymbia torrelliana, Myrcia obovate, Syzygium samarangense, Campomanesia sp., Eugenia stipitate, Eugenia uniflora, Plinia cauliflora, Solanum macrocarpon, Solanum stipulaceum, Solanum sp., Cestrum sp., Allophylus sp. | 44.71 | 1.84 | 4.25 | 23.88 | 0.87 | 24.48 | 0.92 | 3.75 | [71] |
| Melipona scutellaris | Brazil | Cocos nucifera, Spondias, Tapirira, Bidens, Vernonia, Protium, Terminalia, Erythroxylum, Acalypha, Croton, Acacia, Caesalpinia, Centrosema, Chamaecrista, Desmodium, Leucaena, Macroptilium, Mimosa caesalpiniifolia, Mimosa pudica, Mimosa quadrivalvis, Mimosa tenuiflora. | 47.3–55.7 | 3.45–5.90 | 2.43–7.94 | 10.19–24.02 | – | 10.85–28.89 | – | 3.28–3.99 | [33] |
a Dry weight; ND = not determined.
3.2.1. Water Content, Water Activity, and pH
Bee bread has shown to possess high moisture content. It is attributed to the pollen’s hygroscopic properties, which attracts water from the environment. Bee bread accumulates water from the surrounding and also through the addition of bee saliva and honey resulting in a sticky end product. Storage in closed pots prevents water loss from bee bread [71]. According to the bee pollen standard [36], the water content in pollen should not be more than 6–9/100 g.
Because pollen is hygroscopic, bee bread requires adequate drying parameters to achieve the desired moisture content. Different preservation methods are utilised in the beekeeping industry to preserve bee bread. Oven drying has been proven to be the best method to preserve bee bread over frozen and refrigeration method based on the reduction in moisture content and microbial load [72].
Bee bread has water activity between 0.60 and 0.92 (Table 1) within the range of Aw suitable for microbial colonisation, which is 0.60–1.00. The preservation method such as drying until Aw goes below this level and can help prolong the bee bread shelf life. High Aw of fresh bee bread provides a favourable environment for the growth of beneficial bacteria, pathogenic bacteria, fungi, or mould [15,73,74]. This creates concern over the risk of occurrence of mycotoxins, which has been reported in pollen [75]. Therefore, Aw parameter should be critically observed for quality control during storage.
As previously mentioned, bee bread is more acidic than bee pollen. Duarte et al.′s [47] study on seven species of stingless bee′s bee pollen in Brazil revealed that the pH values were in the range of 4.9–5.9. As shown in Table 1, pH values for stingless bee bee bread were reported to be in the range of 3.28–4.13. The lowest recorded pH was in one sample of bee bread of Melipona scutellaris (pH 3.28) [33]. The pH value dropped as the lactic acid content in bee bread becomes higher (3.06%–3.20%) than bee pollen (0.56%) [76] suggesting a lactic acid fermentation by bacteria and yeast [14] However, the exact mechanism is still poorly understood.
3.2.2. Carbohydrates
Table 1 summarises the carbohydrate content in the stingless bee bee bread, which ranges from 10.85% to 59.94%. Carbohydrates mostly make up bee bread due to the addition of nectar during processing of pollen into bee bread. A significant increase in carbohydrates has been reported in two separate studies on monofloral bee bread [44,45]. The authors found higher carbohydrate content in Aloe greatheadii var. davyana and Helianthus annuus bee bread in comparison to its fresh pollen.
Most bee bread from Southeast Asia (SEA) is reported to have higher carbohydrate values than bee bread from other regions. Thailand′s Tetragonula testaceitarsis bee bread had the lowest value with 43.1% [80], while Tetragonula biroi Friese from the Philippines has the highest value with 59.94% [83] among SEA countries.
The sugar analysis comparison (Table 2) shows reducing sugars such as fructose, glucose, and sucrose were present in high amounts. Mannitol (sugar alcohol) was also found abundant in bee bread of Tetragonula biroi from the Philippines [83], Meliponini subnitida from Brazil [29,87], and Trigona spp. from Malaysia [88]. Other oligosaccharides such as sorbitol, cellobiose, isomaltose, maltose, raffinose, and stachyose were found in minute amount in Tetragonula biroi bee bread [83].
Table 2.
Essential amino acid in stingless bee bee bread (g/100 g).
| Bee Species | Country | Instrument | Phe | Val | His | Met | Iso | Leu | Thr | Ala | Try | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterotrigona itama | Malaysia 1 | HPLC | 1.84–2.80 | 1.00–1.18 | 0.78–1.15 | 0.37–0.53 | 0.76–0.83 | 1.61–1.83 | 1.52–2.00 | 1.00–1.06 | ND | [22] |
| Tetragonula laeviceps | Philippines 1 | MS-GC | 1.63 | 0.65 | 0.96 | 0.12 | 0.88 | 1.62 | 0.17 | 0.40 | 0.96 | [80] |
| Melipona subnitida | Brazil 2 | HPLC-PDA | 0.36–0.41 | 0.29–0.41 | 0.70–0.73 | 0.14–0.17 | 0.14–0.21 | 0.61 | 0.23–0.30 | 0.91–0.93 | 0.70–0.73 | [87] |
Phe: phenylalanine, Val: valine, His: histidine, Met: methionine, Iso: isoleucine, Leu: leucine, Thr: threonine, Ala: alanine, Try: tryptophan; HPLC: high-performance liquid chromatography, PDA: photodiode-array detector, MS: mass spectrometry, GC: gas chromatography; ND = not determined; 1 For botanical origin, refer to Table 1; 2 Botanical origin not determined.
Bee incorporates nectar and its salivary enzymes during pollen agglutination [10]. Depending on bee species, honeybee Apis mellifera L. salivary (thoracic) gland secretes invertase, amylase, and glucosidase [89]. Stingless bee Meliponini, Trigonini, and Scaptotrigona spp. could digest pollen polysaccharides into simple sugar using enzymes α and ß-amylase and α-glucosidase [34]. Simple sugars are hypothesised to be fermented by bacteria during bee bread fermentation resulting in varying degrees of sugar fractions.
3.2.3. Protein
Bee bread is rich in protein content, thus becoming the main protein source for bee development. The protein levels are varied across different geographical locations and between bee species (10.19–47.4%) (Table 1). Pollen with highest protein content was found to have originated from a highly nectariferous plant [90]. Although protein content decreases from fresh pollen to bee bread [44,45], whereas the amino acid concentration remains unchanged in fresh pollen, bee pollen, and bee bread [44,45].
Bee bread is claimed to contain all the essential amino acids–amino acids that cannot be synthesised by an organism [67,69,70], and it is indeed what was observed in the bee bread of stingless bee (Table 1). Belina-aldemita et al. [83] quantified the total free amino acids in Tetragonula biroi from the Philippines and found mean values of only 1.83 g/100 g bee bread. Among the essential amino acids, leucine, and phenylalanine were highly present in the studied bee bread (Table 3).
Table 3.
Sugar content in stingless bee bee bread (g/100 g).
| Bee Species | Country | Botanical Origin | Instrument | Glucose | Fructose | Sucrose | Maltose | Mannitol | References |
|---|---|---|---|---|---|---|---|---|---|
| Heterotrigona itama | Malaysia | Mimosa pudica, Sphagneticola trilobata, Bidens pilosa, Cassia sp., Areca catechu, Peltophorum pterocarpum, Phaleria capitate, Cassia siamea, Citrus aurantifolia Ageratum conyzoides | HPLC-ELSD | 10.27–12.40 | 0.40–1.49 | 0.60–2.10 | 0.70–2.00 | – | [22] |
| Melipona subnitida | Brazil | ND | HPLC-RID | – | – | – | – | 20.80–31.00 | [87] |
| Melipona subnitida | Brazil | Mimosa gemmulata, Mimosa verrucada, Fabaceae, Scrophulariaceae (species not identified) | Column chromatography-TLC-UV | – | – | – | – | 34.90 | [101] |
| Tetragonula biroi | Philippines | ND | HPAEC-PAD | 0.25–1.89 | 4.39–9.58 | 4.29–26.04 | 0.82–2.04 | 5.57–20.85 | [83] |
HPLC: high-performance liquid chromatography, ELSD: evaporative light scattering detector, RID: refractive index detector, TLC: thin-layer chromatography, HPAEC: high-performance anion exchange chromatography, PAD: pulsed amperometric detector.
3.2.4. Lipid
Lipid content of stingless bee bee bread is between 0.46% and 14.43% (Table 1). The outer pollen wall (pollenkitt) is made up of lipid [59,91]. Lipid is also present in the pollen grain at a different amount between the same and different species [92]. Bee bread lipid content is similar to bee pollen but significantly lower than flower pollen [45]. Addition of bee glandular enzymes into fresh pollen could alter the lipid composition available in bee pollen and bee bread.
Bee bread has diverse fatty acid profiles, which provides benefits to the bee nutrition and also for human health. According to Szczęsna [93], the most abundant fatty acids in bee pollen are linoleic (omega-6) followed by α-linolenic (omega-3) and palmitic acids. The fatty acid composition is not much different between bee pollen and bee bread [44]. Bee bread is rich in polyunsaturated fatty acids (PUFAs) such as omega-3, fatty acids which cannot be synthesised by our body [94]. Studies on fatty acid composition in stingless bee bee bread is still limited. For instance, bee bread of Melipona scutellaris contains 12 fatty acids (9 saturated and 3 unsaturated), and the most abundant saturated fatty acid was capric acid (1.89–5.66 g/100 g) and the common PUFAs were Omega-6 (α-linoleic acid) (0.50–1.63 g/100 g) and omega-3 (0.30–0.86 g/100 g) [33].
However, in another study, palmitic acid (1.65 g/100 g) was the prevalent fatty acid followed by omega-6 (1.52 g/100 g) in Tetragonula laeviceps bee bread. The ratio of polyunsaturated to saturated fatty acids was 1.59, higher than the ideal ratio of 1, which can slightly reduce HDL cholesterol level [80]. Similarly, palmitic acid was the dominant fatty acid among 15 fatty acids identified in Melipona mandacaia bee bread [85]. Omar et al. [88] analysed the volatile compounds in Malaysia trigona species using gas chromatography (GC-MS). Propanoic acid and palmitic acid were the most abundant saturated fatty acids in Trigona apicalis (4.04%) and Trigona thoracica (1.28%) bee bread, respectively. Meanwhile, minute amounts of omega-3 and omega-6 fatty acids were detected in range of (0.07–0.11%). In addition, analysis of volatile organic compounds (VOCs) found acetic acid as the sole organic acid in Australian Tetragonula carbonaria and Tetragonula hockingsi but absent in Austroplebeia australis [95].
3.2.5. Phenolic Compounds
Phenolic compounds are secondary plant metabolites found widely in plants as a protective mechanism when encountering biotic or abiotic stress. They include phenolic acids, flavonoids, proanthocyanidins, and more. Intake of food containing phenolic compounds is sought to reduce the risk of gaining chronic diseases because of its antioxidant and anti-inflammatory properties [96]. According to findings of Urcan et al. [97], bee pollen and bee bread have similar phenolic profile. In contrast, Kaškonienė et al. [98] revealed that there is increase in flavonoid content (55–135%) after bee bread fermentation. Several studies have quantified the total phenolic content (TPC) and total flavonoid content (TFC) in stingless bee bee bread.
TPC and TFC content varies for different stingless bee species even from the same location [99]. The solvent of extraction also influences extraction efficiency. For instance, ethanolic extract and aqueous extract of Heterotrigona itama bee bread yielded different amounts of polyphenols. Ethanolic extracts were found to have higher TPC (21.32–22.54 mg GAE/g) and TFC (16.48–26.57 mg QE/g) than aqueous extract [78]. When comparing between ethanol and methanol, both solvents showed similar efficiency in extracting phenolic compounds from Australian Austroplebeia spp. and Tetragonula spp. bee bread. [100]. However, TPC and TFC of ethanolic extracts were slightly higher with 1281.2–2683.2 mg GAE/100 g pot-pollen and 282.9–698.0 mg QE/100 g pot-pollen, respectively. By far, ethanol has shown superior extraction for stingless bee bee bread compared to other solvents tested.
In Southern Venezuela, Vit et al. [84] determined the phenolic content and flavonoid contents in ethanolic extract of Tetragonula angustula bee bread. TPC ranged from 1053.1 to 2627.4 mg GAE/100 g pot-pollen, while the TFC was between 104.6 and 676.4 mg QE/100 g pot-pollen. In another study, Vit et al. [9] detected lower TPC and TFC from bee bread of seven stingless species of Frieseomelitta sp. and Melipona spp. with 1018.0–2085.0 mg GAE/100 g pot-pollen and 75.7–656.3 mg QE/100 g pot-pollen, respectively.
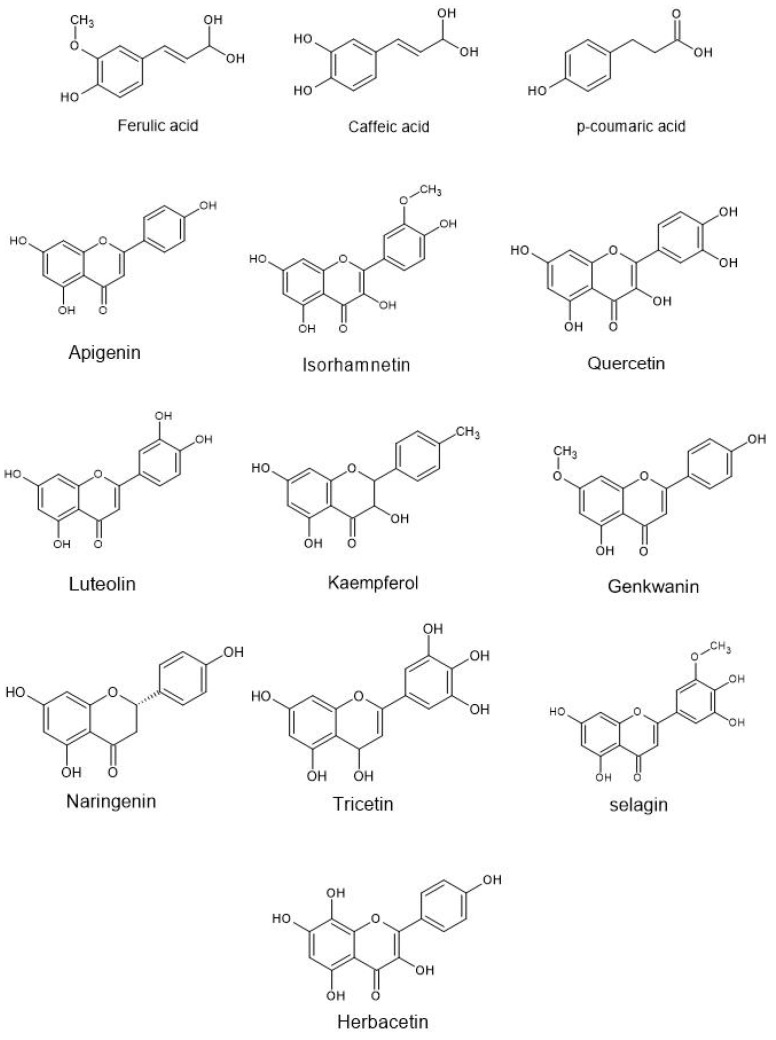
Table 4 summarises the bioactive compounds available in stingless bee bee bread. Figure 4 shows the chemical structures of the bioactive compounds. In general, kaempferol, isorhamnetin, and quercetin were the most frequently detected compounds in bee bread of stingless bee [9,29,78,101]. These molecules are also widely found in bee pollen of Apis spp. [4], fruits and vegetables, with great potential to reduce the risks of cancer [102,103].
Table 4.
Bioactive compounds in stingless bee bee bread.
| Bee Species | Origin | Botanical Origin | Instrument | Compound | References |
|---|---|---|---|---|---|
| Melipona subnitida | Brazil | Mimosa gemmulata, Mimosa verrucada, Fabaceae, Scrophulariaceae (species not identified) | Column chromatography | 6-naringenin, 7-isorhamnetin, 2-tricetin, 3-selagin, 4-8-methoxyherbacetin | [101] |
| Heterotrigona itama | Malaysia | ND | LC-MS | Caffeic acid, ferulic acid, kaempferol, apigenin, and isorhamnetin | [104] |
| Frieseomelitta sp. aff. Varia, | Southern Venezuela | Antigonon sp., Astronium sp., Avicennia sp., Bidens sp., Eupatorium sp., Scrophularia nodosa Solanum americanum | HPLC-UV | Kaempferol, luteolin, quercetin | [9] |
| Melipona compressipes, | Kaempferol, luteolin, 8 methoxykaempferol | ||||
| Melipona eburnean | Genkwanin, 8 methoxykaempferol | ||||
| Melipona favosa, | Quercetin | ||||
| Melipona sp. fulva | Kaempferol, 8 methoxykaempferol, quercetin | ||||
| Melipona lateralis kangarumensis | Kaempferol, luteolin, 8 methoxykaempferol, quercetin | ||||
| Melipona paraensis | Genkwanin, luteolin | ||||
| Melipona rufiventris | Brazil | ND | Column chromatography—NMR | p-hydroxycinnamic acid, dihydroquercetin, isorhamnetin, isorhamnetin-3-O-(6”-O-E-p-coumaroyl)-β-D-glucopyranoside, luteolin, and quercetin | [29] |
| Scaptotrigona affinis postica | Brazil | ND | LC-ESI-IT-MS/MS | Gluconic acid, digalloylshikimic acid, quercetin-3,4-diglucoside, apigenin-6-C-glucoside, isoorientin-2”-O-rhamnoside, kaempferol 3,7-di-O-rhamnoside, ellagic acid, monogalloylglucose, protocatechuic acid 3-glucoside, procyanidin dimmer digallate (a-type) | [105] |
| Melipona fasciculata | Brazil | ND | LC-ESI-IT-MS/MS | Gluconic acid derivate, gluconic acid, kaempeferol derivative, kaempferol, 6-hydroxykaempferol 3,6-diglucoside 7-glucuronide, ellagic acid dimer, quercetin 3,4′-diglucoside, ellagic acid, protocatechuic acid 3-glucoside, linolenic acid, linoleic acid | [106] |
| Tetragonula biroi Friese | Philippines | ND | HPLC-DAD | Rutin, quercetin-3-O-β-D-glucoside, and quercetin | [107] |
LC: liquid chromatography, MS: mass spectrometry, HPLC: high-performance liquid chromatography, UV: ultraviolet, NMR: nuclear magnetic resonance, ESI: electrospray ionisation, IT: ion trap, DAD: photodiode array detection.
Figure 4.
Chemical structures of some of the phenolic compounds found in stingless bee bee bread.
3.2.6. Other Micronutrients
Analysis of bee bread vitamin content is still scarce. Heterotrigona itama bee bread contains an average of 0.1108 mg vitamin C/g bee bread, which is considerably low based on the daily recommended intake [22]. Because vitamin C is unstable, some factors such as the method of preservation, drying parameter, and storage age and condition need to be optimised as they have shown to affect the vitamin content in pollen [60,108]. External factors, for instance, botanical origin, soil type, and climate are also suggested to influence vitamin C in pollen [109].
The most abundant mineral in stingless bee bee bread is potassium followed by phosphorus. From the studies conducted (Table 5), potassium was in the range of 2222.5–13,366.6 mg/kg. Similarly, it is also the most prevalent mineral in stingless bee honey [110]. Other minerals such as boron, rubidium, and strontium have been found in trace amounts in bee bread of Tetragonula biroi [83].
Table 5.
Mineral composition in stingless bee bee bread.
| Bee Species | Country | Instrument | K | P | Na | Mg | Ca | Mn | Fe | Cu | Zn | Se | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterotrigona itama | Malaysia | ICP-MS | 6524.9 | 6402.28 | 139.70 | 1635.4 | 1547.31 | 61.66 | 126.43 | 12.7671 | 60.62 | 0.26 | [22] |
| Tetragonula laeviceps | Brazil | ICP-OES | 5656.0 | – | 89.9 | 1160.0 | 2566.0 | – | – | – | – | – | [80] |
| Tetragonula testaceitarsis | 4594.7 | – | 133.5 | 1318.0 | 2904.0 | – | – | – | – | – | |||
| Lepidotrigona terminata | 4606.3 | – | 77.2 | 1176.0 | 2507.3 | – | – | – | – | – | |||
| Lepidotrigona flavibasis | 5125.7 | – | 81.7 | 1315.3 | 2719.7 | – | – | – | – | – | |||
| Melipona subnitida | Brazil | FAAS | 5918.5–13,366.6 | – | – | 975.4–2166.1 | 1864.1–3424.9 | 35.1–75.0 | 16.4–33.5 | 0.8–1.9 | 36.4–71.2 | – | [87] |
| Scaptotrigona mexicana | Mexico | ICP-OES | 2222.5–2836.9 | 2736.1–3657.3 | – | – | – | – | – | – | – | – | [86] |
| Tetragonula biroi | Philippines | ICP-MS | – | – | – | 1763.58–2407.65 | 3780.28–5186.89 | 42.58–266.90 | 70.37–123.77 | 9.52–12.02 | 45.16–56.25 | – | [83] |
K: potassium, P: phosphorus, Na: sodium, Mg: magnesium, Ca: calcium, Mn: manganese, Fe: iron, Cu: copper, Zn: zinc, Se: selenium; ICP: inductively coupled plasma, MS: mass spectrometry, OES: optical emission spectrometry, FAAS: flame atomic absorption spectrometry.
Apart from minerals, toxic metals such as arsenic, mercury, lead, and cadmium were detected in bee bread of Heterotrigona itama [22] and with a few H. itama samples exceeding the proposed limit for heavy metal [36]. Belina-aldemita et al. also detected these inorganic contaminants in Tetragonula biroi Friese bee bread but at a safe level [74]. Toxic metals accumulation in bee bread imposes high risk to human health but it can also become a good bioindicator of metal pollution in the environment [111] as a result of anthropogenic activities in nearby areas.
4. Health Benefits
There has been a tremendous report published on bee pollen as it is shown to possess therapeutic properties such as antioxidant, anti-inflammatory, antibacterial, anti-fungicidal, hepatoprotective, and anti-atherosclerotic activities [10]. In contrast, only a few publications have been reported on stingless bee bee bread’s biological properties, which are shown in Table 6.
Table 6.
Health benefits of stingless bee bee bread.
| Stingless Bee Species | Origin | Sample Preparation | Method | Explanation | Reference |
| Antimicrobial Activity | |||||
| Melipona compressipes manaosensis | Maues, Amazonas, Brazil | Bee bread from 3 sampling sites was extracted using hexane (Hex), EtOH, and MetOH | Agar well diffusion and MIC | All sample inhibited Mycobacterium smegmatis (ATCC 607) and Pseudomonas aeruginosa (ATCC 27853). Only bee bread hex, EtOH, and MetOH extract from Maues 3 inhibited Streptococcus oralis (ATCC 10557). C. albicans was sensitive to all extracts. Most of the extracts tested showed MIC greater than 1000 µg/mL. Bee bread EtOH Maues 1 and MetOH Maues 3 showed larvicidal activity C. quinquefasciatus LC50 218.2 and 228.8 µg/mL. | [112] |
| Heterotrigona itama | Malaysia | Bee bread was extracted in hexane and 70% EtOH | Disc diffusion method | Gram-positive bacteria (B. cereus and S. aureus) showed bigger inhibition zone than Gram-negative bacteria (E. coli and Salmonella) | [113] |
| Austroplebeia australis, Tetragonula carbonaria, Tetragonula hockingsi | Australia | Extraction in MetOH and EtOH | Agar well diffusion and MIC | All extracts inhibited Staphylococcus aureus (ATCC 25923), Bacillus subtilis (ATCC 11778), Enterobacter cloacae (ATCC 13047), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) except for A. australis MetOH against P. aeruginosa, T. hockingsi MetOH against E. coli, and T. hockingsi ethanolic extract against E. coli. MIC values of ethanolic extracts were lower than methanolic extracts of pot-pollen | [100] |
| Frieseomelitta sp. aff. Varia, Melipona compressipes, Melipona eburnean, Melipona favosa, Melipona sp. fulva, Melipona lateralis kangarumensis, Melipona paraensis | Australia | Extraction in 95% EtOH | Agar well diffusion | All extracts inhibited Bacillus subtilis (ATCC 11778), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Enterobacter cloacae (ATCC 13047), and Pseudomonas aeruginosa (ATCC 27853). | [114] |
| Antioxidant Activity | |||||
| Heterotrigona itama | Malaysia | Extraction in 95% EtOH | DPPH | IC50: 300 µg/mL | [79] |
| Trigona apicalis, Trigona itama, Trigona thoracica | Malaysia | Extraction in MetOH | DPPH | IC50: Trigona apicalis 1.05 ± 0.01 mg/mL, Trigona itama 3.24 ± 0.03 mg/mL, Trigona thoracica 0.86 ± 0.01 mg/mL | [99] |
| Heterotrigona itama | Malaysia | Bee bread was extracted in hexane and 70% EtOH at concentration 150 µL/mL | DPPH and ABTS | DPPH assay: EtOH bee bread was 93.60 ± 0.0% inhibition and Hex bee bread was 83.81 ± 0.05% inhibition. ABTS assay: EtOH bee bread showed 97.95% inhibition and Hex bee bread was 71.23%. |
[113] |
| Melipona asilvai Moure, M. quadrifasciata anthidioides Lepeletier, M. scutellaris Latreille, M. subnitida Ducke, Frieseomelitta varia Lepeletier, Tetragona clavipes Fabricius and Plebeia sp. | Brazil | Extraction in 70% EtOH at concentration 50 mg/mL | DPPH, FRAP and inhibition of β-carotene | FRAP assay: T. clavipes recorded the highest with 123.4 mgGAE/100 g. Inhibition of β-carotene: M. q. anthidioides (83.3%) was the highest followed by M. scutellaris (76.5%). DPPH values were not stated. |
[47] |
| Frieseomelitta sp. aff. Varia, Melipona compressipes, Melipona eburnean, Melipona favosa, Melipona sp. fulva, Melipona lateralis kangarumensis, Melipona paraensis | Brazil | Extraction in 95% EtOH | ABTS, Fenton type reaction, and hydroxyl radical | Fenton-type reaction: 0.91–1.25 mM uric acid equivalents/100 g pot-pollen. ABTS assays: 193.2 and 771.0 μmoles Trolox equivalents/100 g bee bread. Hydroxyl radical inhibition: 54.08% and 97.32% inhibition/100 g bee bread. The highest antioxidant activity was Melipona lateralis kangarumensis for all three tests. |
[9] |
| Tetragonisca angustula | Venezuela | Extraction in 95% EtOH | ABTS, Fenton type reaction, and hydroxyl radical | Fenton-type reaction: 0.74 and 1.12 mM uric acid equivalent/100 g bee bread. ABTS assays: values were between 401.8 and 500.4 μmoles Trolox equivalents/100 g bee bread. Hydroxyl radical inhibition: 30.0–60.1% inhibition/100 g bee bread. High positive correlations (0.802–0.921) between polyphenol contents and antioxidant activity measured by the three methods. |
[84] |
| Austroplebeia australis, Tetragonula carbonaria, Tetragonula hockingsi | Australia | Bee bread was extracted in MetOH and EtOH | ABTS, Fenton type reaction, and hydroxyl radical | The hydroxyl radical inhibition: 48.6–71.3%/100 g bee bread Fenton-type reaction: 1.00 and 1.05 mM uric acid/100 g bee bread ABTS assay: 229.6 and 426.4 μmol TEAC/100 g of bee bread Overall, EtOH showed higher antioxidant activity than MetOH, but the results were not statistically different. A positive correlation was found between the antioxidant activity measured with three methods and the polyphenol content of bee bread. |
[100] |
| Melipona fasciculata | Brazil | Extraction in 70% EtOH | DPPH, FRAP, and ABTS | DPPH: IC50 117–597.73 µg/mL. FRAP: 0.15–0.99 mmol Fe2+/g ABTS: IC50 34.40–235.19 µg/mL |
[106] |
| Scaptotrigona affinis postica | Brazil | Extraction in 70% EtOH | DPPH, FRAP, and ABTS | DPPH: IC50 273.08 ± 1.43 µg/mL. FRAP: 0.71 ± 0.04 Fe2+/g ABTS: IC50 87.29 ± 0.06 µg/mL |
[105] |
| Tetragonula biroi Friese | Thailand | Extraction in MetOH using two different methods (maceration and sonication) | DPPH, FRAP, ABTS, LPO | DPPH: EC50 3.62–43.04 mg pollen/mL (macerated) and 3.32 to 44.50 mg pollen/mL (sonicated) FRAP: 97.13–251.17 µmol Fe2+ (macerated) and 95.51–256.19 µmol Fe2+ (sonicated). ABTS: 8.90–62.06 µmol TE/g pollen (macerated) and 8.08–62.24 µmol TE/g pollen (sonicated). LPO: 33.97–44.72% (macerated) and 37.09–47.34% (sonicated). |
[107] |
| Others Therapeutic Properties | |||||
| Health Benefits | Animal Models | Research Design | Treatment | Effect | |
| Antidiabetic activity | Thirty-six male Sprague Dawley rats | Study the antiatherogenic effect of bee bread in HFD-induced obese rats. Bee bread of Heterotrigona itama was administered via oral gavage. |
G1: Normal G2: HFD G3: HFD + bee bread (high-fat diet and 0.5 g/kg/day bee bread) G4: HFD + orlistat (high-fat diet and 10 mg/kg/day orlistat) groups |
Bee bread (0.5 g/kg) prevented or suppressed HFD-induced obesity in a manner that was similar or even better than orlistat (at 10 mg/kg). Bee bread treatment; ↓ Lee obesity index, ↓ TC, ↓ LDL, ↓ FAS, ↓ atherogenic index, ↓ oxLDL, ↓ MDA and significantly increased aortic antioxidant enzymes activities (SOD and GPx) in high-fat diet (HFD)-induced obese rats. En face aorta images showed smaller adipocytes sizes, and absence of atherosclerotic plaque in obese rats supplemented with bee bread. |
[104] |
| Antidiabetic activity | Thirty-six male Sprague Dawley rats | Study the bee bread effect on serum renal function parameters, oxidative stress, inflammatory, and Bax in the kidneys of HFD obese rats. Bee bread of Heterotrigona itama was administered via oral gavage. |
G1: Control: received rat diet and water (1 mL/kg); G2: HFD group: received HFD and water (1 mL/kg) G3 and G4: bee bread preventive or orlistat preventive G5 and G6: received HFD and BB (0.5 g/kg) or HFD and orlistat (10 mg/kg); BB or orlistat treatment: received BB (0.5 g/kg) or orlistat (10 mg/kg) |
Bee bread (0.5 g/kg) has shown to ↓ body weight, ↓ BMI, ↓ Lee obesity index, ↓ MDA, ↑ TAA, ↑ SOD, ↑GPx, ↑ GST. ↓ expression of NFkB, TNF-a, IL-1b, and Bax gene. |
[118] |
| Anti-inflammatory activity | Adult male Mus musculus mice with dextran-induced and carrageenan-induced paw oedema | Study the bee bread effect on induced-oedema paw mice model. Bee bread EetOH extract of Melipona fasciculata was administered orally and was monitored for 5 h hourly. |
G1: saline (10 mL/kg) G2: bee bread extract 250 mg/kg G3: bee bread extract 500 mg/kg) G4: cyproheptadine for dextran-induced treatment (10 mg/kg) and indomethacin for carrageenan-induced (10 mg/kg) |
Carrageenan-induced paw oedema test: G3 significantly ↓ mice paw oedema by 52% compared to control in 1 h. After 5 h, G2, G3, and G4 reduced oedema by better (p < 0.001) than G1. Dextran-induced paw oedema test: G3 significantly ↓ mice paw oedema by 84% compared to G4 in 1 h. After 5 h, G2, G3, and G4 reduced oedema better (p < 0.001) than G1. |
[106] |
| Anti-inflammatory activity | Adult male Mus musculus mice with dextran-induced and 1% carrageenan-induced paw oedema | Study bee bread effect on induced-oedema paw mice model. Bee bread EtOH extract of Scaptotrigona affinis postica was administered orally and was monitored for 5 h with 1 h interval |
G1: saline (10 mL/kg) G2: bee bread extract (250 mg/kg) G3: bee bread extract (500 mg/kg) G4: cyproheptadine for dextran-induced treatment (10 mg/kg) and indomethacin for carrageenan-induced (10 mg/kg) |
Carrageenan-induced paw oedema test: After 5 h, G2, and G3 significantly ↓ mice paw oedema by similar to G5, but better than G1. Dextran-induced paw oedema test: After 5 h, G2, and G3 significantly ↓ mice paw oedema by similar to G5 but better than G1. |
[105] |
| Antinociceptive activity | Adult male Mus musculus mice injected with 0.8% acetic acid and 2.5% formalin separately | Bee bread EtOH extract of Melipona fasciculata was administered orally. | G1: saline (10 mL/kg) G2: bee bread extract (250 mg/kg) G3: bee bread extract (500 mg/kg) G4: indomethacin (10 mg/kg) |
Acetic acid writhing test: G3 was more efficient than G4 to reduce abdominal contortions produced, with less than 58% writhing (p < 0.05). Formalin test: In neurogenic phase, G2 and G3 ↓ time that the animals passed licking/biting the induced paw in 42% and 47%, better than G1 and G4. In inflammatory phase, G2, G3, and G4 showed no difference (p > 0.05) to the mice licking/biting time. |
[106] |
| Antinociceptive activity | Adult male Mus musculus mice injected with 0.8% acetic acid and 2.5% formalin separately | Bee bread EtOH extract of Scaptotrigona affinis postica was administered orally. | G1: saline (10 mL/kg) G2: bee bread extract (250 mg/kg) G3: indomethacin (10 mg/kg) |
Acetic acid writhing test: G2 ↓ the number of abdominal writhing’s by 52% compared to G1 (p < 0.05), but equal to G3. Formalin test: In neurogenic phase, G2 ↓ time that the animals passed licking/biting the induced paw by 57%, better than G1 and 51% compared to G3. In inflammatory phase, G2 ↓ licking/biting responses by 99%, better than G1 and 96% compared to G3. |
[105] |
EtOH: ethanol, MetOH: methanol, MIC: minimum inhibitory concentration, DPPH: 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), FRAP: ferric reducing-antioxidant power, LPO: lipid peroxidation, TEAC: Trolox equivalent antioxidant capacity, HFD: high-fed diet, TC: total cholesterol, LDL: low-density lipoprotein, FAS: fatty acid synthase, oxLDL: oxidised-LDL, MDA: malondialdehyde, SOD: superoxide dismutase, GPx: glutathione peroxidase, BMI: body mass index, TAA: total antioxidant activity, GST: glutathione-S-transferase, NFkB: nuclear factor kappa B, TNF-a: tumour necrosis factor alpha, IL-1b: interleukin-1-b, and Bax: B-cell associated protein.
4.1. Antimicrobial Properties
Bee bread extract contains phenolic compounds, which can be attributed to its antimicrobial properties. Therefore, its importance as an antimicrobial agent has to be acknowledged not only towards bacteria but also towards yeast and parasite. Carneiro et al. [112] demonstrated that bee bread extracts of stingless bee M. compressipes manaosensis inhibited P. aeruginosa, M. smegmatis, and Candida albicans efficiently and also mosquito larva C. quinquefasciatus, a vector for human parasitic worm Wuchereria bancrofti, in a concentration-dependent manner.
Akhir et al. [113] used ethanolic extract and hexanoic extract of Heterotrigona itama bee bread against Bacillus cereus, Staphylococcus aureus, Escherichia coli, and Salmonella sp. Gram-positive bacteria were more sensitive towards the bee bread extracts, whereas ethanolic extract demonstrated stronger antibacterial activity compared to hexane extract.
Meanwhile, Perez-Perez et al. [100] examined the antibacterial activities of ethanolic and methanolic extract from bee bread of Austroplebeia australis, Tetragonula carbonaria, and Tetragonula hockingsi in Australia. The extracts were effective against both Gram-positive (Staphylococcus aureus, Bacillus subtilis) and Gram-negative bacteria (Enterobacter cloacae, Escherichia coli, and Pseudomonas aeruginosa). Similarly, extraction using ethanol showed better results as shown by the lowest minimum inhibitory concentration recorded by ethanolic extracts of Tetragonula hockingsi bee bread.
Sulbarán-Mora et al. [114] investigated bee bread ethanolic extracts of Frieseomelitta, Melipona, and Tetragonisca spp. from Venezuela and found that they exhibited antibacterial effects against Bacillus subtilis, Staphylococcus aureus, Enterobacter cloacae, and Pseudomonas aeruginosa but not against Escherichia coli. The antibacterial activities were correlated to the phenolic content.
4.2. Antioxidant Properties
Phenolic compounds are one the most important natural antioxidants, which can be found in fruits, vegetables, tea, herbs, and essential oils. Antioxidant removes overproduced free radicals or reactive oxygen species (ROS), which cause molecular damages on DNA, protein, and lipid. Consequently, it has been linked with onset of diseases such as cancer and inflammation [115]. Stingless bee bee bread contains antioxidant compounds such as phenolic compounds and vitamin C [22]. Therefore, it has been explored for its ability to remove free radicals. Its antioxidant activity using in vitro study has been documented in Malaysia [79,99,113], Brazil [47,105,106], Venezuela [9,84], and Australia [100].
There are multiple methods to assess the antioxidant activity of a plant extract and 2,2,-di-phenyl-2-picryl-hydrazyl (DPPH) is the most common in vitro method applied due to its simplicity, cost effectiveness, and rapidity [116]. However, assessing using more than two in vitro models is more preferable because of the complexity of phytochemicals [117]. Therefore, bee bread antioxidant activity is assessed based on DPPH activity coupled with other antioxidant assays (Table 6).
Antioxidant activity is also affected by solvent extraction. For example, the antioxidant activities of ethanolic extract from H. itama bee bread were determined using DPPH and 2,2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) [113]. The ethanolic extract showed higher DPPH and FRAP radical scavenging activity (93.60 ± 0.03% and 97.95 ± 0.01%. respectively) in comparison to hexanoic extract.
However, Perez-Perez et al. [100] found similar efficiency between ethanolic and methanolic extract of Austroplebeia australis, Tetragonula carbonaria, and Tetragonula hockingsi bee bread when using ABTS, Fenton type reaction, and hydroxyl radical, respectively. The antioxidant activities also varied according to bee species. Comparison between trigona species showed Trigona thoracica bee bread with the highest DPPH inhibition (IC50/0.86 mg/mL) followed by Trigona apicalis (IC50/1.05 mg/mL) and Trigona itama (IC50/3.24 mg/mL) [99].
Bee bread antioxidant ability corresponds to phenolic compounds [84,100,107,119]. Antioxidant activities also depended on the year of pollen collection, botanical origin, and storage time [120].
4.3. Antidiabetic Properties
Obesity is a public health concern. According to World Health Organisation [121], in 2016, more than 1.9 billion adults (18 years and above) were overweight with 650 million obese. A wide array of natural products has been assessed for their ability to combat obesity and that includes bee bread.
For example, Heterotrigona itama bee bread reduced Lee obesity index and levels of total cholesterol (TC), low-density lipoprotein (LDL), fatty acid synthase (FAS) activity, atherogenic index, oxidised-LDL (oxLDL), and malondialdehyde (MDA) and significantly increased aortic antioxidant enzymes activities (superoxide dismutase (SOD) and glutathione peroxidase (GPx)) in high-fat diet (HFD)-induced obese rats. En face aorta images showed smaller adipocytes sizes and absence of atherosclerotic plaque in obese rats supplemented with bee bread [104].
In another research, Eleazu et al. [118] studied bee bread effects on obesity-induced renal pathology. The outcomes showed 0.5 g/kg H. itama bee bread attenuated renal pathology caused by obesity by diminishing oxidative stress and downregulating the expressions of inflammatory markers and bax-mediated proapoptotic condition in the kidney of HFD obese rats.
4.4. Anti-Inflammatory Properties
Some studies also suggest bee bread extract’s ability to reduce inflammation. Melipona fasciculata and Scaptotrigona affinis postica ethanolic bee bread extracts were administered in induced-oedema mice model in two separate studies [105,106]. The anti-inflammatory responses were time and dose independent. After 5 h, the bee bread treatments were able to reduce the paw volume equivalent to the drug treatment of indomethacin (anti-inflammatory) and cyproheptadine (antihistamine). Further analysis identified the possible mechanism of phenolic compounds to inhibit the release of histamine and reduce prostaglandin synthesis [106].
4.5. Antinociceptive Properties
Bee bread extract has the ability to block pain detection through its antinociceptive activity. In vivo studies were conducted on pain-induced mice administered with bee bread ethanolic extract of two different species—Melipona fasciculata and Scaptotrigona affinis postica. Upon investigation, Melipona fasciculata bee bread extract (500 mg/kg) reduced the abdominal contort in mice suffering from acetic acid exposure better than indomethacin. Meanwhile, the formalin test showed bee bread extract efficiency similar to those of indomethacin in reducing biting/licking time in mice [106].
In a different study, Lopes et al. [105] investigated the antinociceptive activity using Scaptotrigona affinis postica ethanolic extract at lower dosage (250 mg/kg). The bee bread treatment showed similar effect to indomethacin in the acetic acid writhing test but significantly better than the drug (p < 0.005) in the formalin test. The presence of polyphenols and flavonoids was suggested to be responsible for these activities [105,106].
5. Microbiology Properties of Bee Bread
Bee pollen conversion into bee bread via lactic acid fermentation is linked with microbial action. Bee bread has been assumed to undergo microbial fermentation to increase its nutritional value [37,48]. This theory is termed as “Bee bread maturation” hypothesis [122], a notion that has been proposed since the mid-1990s but recently has been argued [123]. Through these studies, bee bread was found only to be preserved, and there was lack of evidence in nutrient conversion by microbes.
In this section, understanding the bee bread microbial ecology will include data of bee bread from other bee species such as the honeybee and solitary bee due to limited record on stingless bee bee bread microbes. Beneficial bacteria isolated from bee bread, such as LAB, Bifidobacterium, Bacillus, and yeasts from different bee bread species are summarised in Table 7.
Table 7.
Lactic acid bacteria, nonlactic acid bacteria, and yeasts associated with stingless bee bee bread.
| Bacterium | Bee Species | References |
|---|---|---|
| Lactic Acid Bacteria (LAB) | ||
| Lactobacillus sp. | Apis mellifera 1 | [128,129,134] |
| Apis sp. 1 | [123,126] | |
| Lactobacillus kunkeei | Apis mellifera 1 | [14,128,135] |
| Osmia cornuta 3 | [130] | |
| Apis sp. 1 | [123] | |
| Lactobacillus mucosae | Apis mellifera 1 | [128] |
| Lactobacillus musae | Heterotrigona itama 2 | [15] |
| Lactobacillus crustorum | Heterotrigona itama 2 | [15] |
| Lactobacillus mindensis | Heterotrigona itama 2 | [15] |
| Lactobacillus casei | Apis mellifera 1 | [127] |
| Leuconostoc mesenteroides | Heterotrigona itama 2 | [15] |
| Enterococcus durans | Osmia cornuta 3 | [130] |
| Enterococcus faecalis | Osmia cornuta 3 | [130] |
| Heterotrigona itama 2 | [15] | |
| Enterococcus faecium | Osmia cornuta 3 | [130] |
| Fructobacillus sp. | Apis mellifera 1 | [134] |
| Fructobacillus fructosus | Heterotrigona itama 2 | [15] |
| Weisella sp | Osmia cornuta 3 | [130] |
| Weisella confusa | Osmia cornuta 3 | [130] |
| Weisella viridescens | Osmia cornuta 3 | [130] |
| Oenococcus | Apis sp. 1 | [126] |
| Non-LAB | ||
| Bifidobacterium sp. | Apis mellifera 1 | [14] |
| Apis sp. 1 | [126] | |
| Bifidobacterium asteroides | Apis mellifera 1 | [135] |
| Bacillus sp. | Osmia cornuta 3 | [130] |
| Bacillus megaterium | Tetragonula biroi Friese 2 | [74] |
| Bacillus thuringiensis | Osmia cornuta 3 | [130] |
| Bacillus licheniformis | Osmia cornuta 3 | [130] |
| Apis mellifera 1 | [136] | |
| Bacillus pumilus | Osmia cornuta 3 | [130] |
| Apis mellifera 1 | [136] | |
| Tetragonula biroi Friese 2 | [74] | |
| Heterotrigona itama 2 | [15] | |
| Bacillus flexus | Tetragonula biroi Friese 2 | [74] |
| Bacillus coagulans | Tetragonula biroi Friese 2 | [74] |
| Bacillus subtilis | Osmia cornuta 3 | [130] |
| Tetragonula biroi Friese 2 | [74] | |
| Apis mellifera 1 | [136] | |
| Bacillus safensis | Heterotrigona itama 2 | [15] |
| Bacillus amyloliquefaciens | Heterotrigona itama 2 | [15] |
| Tetragonula biroi Friese 2 | [74] | |
| Bacillus cereus | Heterotrigona itama 2 | [15] |
| Tetragonula biroi Friese 2 | [74] | |
| Yeast | ||
| Starmerella meliponinorum | Tetragonisca angustula 2 | [137,138] |
| Starmerella neotropicalis | Melipona quinquefasciata 2 | [139] |
1 Honeybee, 2 Stingless bee, 3 Solitary bee.
Regardless, isolation and identification of microbes from bee bread have been widely reported. Yet, the study on their functional properties towards the bee ecosystem and most importantly, their potential for industrial application is still incomprehensive [37,124]. Some LAB from honeybee bee bread displayed antifungal activity towards foodborne pathogens Aspergillus niger, Zygosaccharomyces rouxii, and Candida sp. [73]. To date, Ngalimat et al. [16] identified Bacillus spp. from Heterotrigona itama bee bread with enzyme and antimicrobial-producing properties. In addition, Mohammad et al. [15] isolated lactic acid bacteria from the same stingless bee species with probiotic potential in vitro. This provides a basis to promote study for the application of beneficial microbes from bee bread in the food industry.
5.1. Lactic Acid Bacteria
Lactic acid bacteria (LAB) such as Lactobacillus, Enterococcus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Carnobacterium, Aerococcus, Vagococcus, Oenococcus, Tetragenococcus, and Weisella [125] produce lactic acid as a major end product of fermentation. Undeniably, microorganisms such as LAB and yeast are associated with bee bread fermentation process, yet the details of the process are still vague with no conclusive explanation on the exact microorganisms responsible for this process [126]. An attempt to create artificial bee bread using Lactobacillus rhamnosus GG was made [98].
Current literature has conveyed extensive study conferring on microorganisms associated with honeybee bee bread or its ecosystem overall, particularly for Apis mellifera species. Only a few studies have discussed on microorganisms in the stingless bee [37]. The bacterial species found in bee bread across different bee species are diverse. Lactobacillus sp. particularly Lb. kunkeei is commonly found in bee bread [123,127,128,129,130]. About 83.9% of bacteria in bee bread honeybee Apis mellifera belongs to Lactobacillus spp. which is largely composed of Lb. kunkeei [128]. However, Lb. kunkeei was detected in low quantities in fresh bee bread of solitary bee Osmia cornuta and absent in the old bee bread of Osmia coruta and bee bread of H. itama [15,130].
Recently, Mohammad et al. [15] isolated Fructobacillus fructosus from H. itama bee bread. Fructobacillus spp. is a group of fructophilic LAB, which prefer fructose substrate over glucose [131] and can also be found in H. itama honey [132], honeybee Apis mellifera bee pollen, and bee bread [133,134] with the capability to utilise plant lignin in vitro [134].
Culture-independent studies help to provide more useful insight into the bee bread bacterial community, taking into account species which are unculturable under controlled condition. Mattila et al. [126] used 454-pyrosequencing technique to sample the active bacteria in the bee bread of honeybee Apis without cultural bias. Oenococcus and Lactobacillus were the most active microbes in bee bread, comprising 52% and 60% of bacteria, respectively, suggesting its important contribution towards bee pollen conversion.
5.2. Bacillus
Lactic acid bacteria have been the integral focus when it comes to bee bread microbial fermentation, but other bacterial species such as Bacillus seem to hold its importance in the bee bread metabolic conversion. Although Gilliam [136] found Bacillus in honeybee bee bread, the lactic acid production is less efficient than lactic acid bacteria fermentation. The functional role of Bacillus in bee bread was not defined until now. One of the early assumptions of Bacillus role in bee bread production was to produce enzymes for bee pollen conversion [140]. Few Bacillus spp. such as B. subtilis [141], B. megaterium [142], and B. licheniformis [143] have exhibited fermentation ability either using glucose, fructose, or sucrose as substrate. These species have been isolated in bee bread of stingless bee Melipona fasciata [140], H. itama [15,16], Tetragonula biroi Friese [74], honeybee Apis mellifera [136], and solitary bee Osmia cornuta [130]. However, Bacillus fermentation using bee pollen or bee bread is not yet reported.
5.3. Yeast
Fermentation is commonly linked with yeast. Yeasts association with fermentation of bee bread has been proposed by researchers. An early study by Gilliam [144] discovered the least yeast count in Apis mellifera bee bread as opposed to bee pollen and flower pollen. Several studies had also attempted to describe the yeast communities not only in bee bread but also in bee colonies. In Brazil, yeasts such as Starmerella and Candida spp. are the most frequently found in the body, pollen, honey, and propolis of stingless bee species of Tetragonisca angustula, Melipona quadrifasciata, and Frieseomelitta varia [137]. Starmerella spp. also has been highly associated with other stingless bee species. Teixeira et al. [137] found Tetragonisca angustula′s pollen to harbour Starmerella meliponinorum. Meanwhile, Daniel et al. [139] have characterised 25 yeast species with Starmerella neotropicalis as the most abundant in Melipona quinquefasciata bee bread and bee pollen.
5.4. Association of Bee Gut Microbiota and Bee Bread Microbiota
Natural fermentation of bee bread is somehow related to bee gut microbiota [13]. Understanding the bee gut microbiome and the microbial fermenter involved in pollen fermentation could provide valuable information for biotechnological applications [64]. Contribution of bee gut towards bee bread fermentation was first suggested when 6 Lactobacillus and 3 Bifidobacterium strains were isolated from A. mellifera bee bread [129], which are similar to the bacteria isolated from its gut [145].The early assumption was made by theorising bee gut transmission to bee bread through regurgitated honey during bee pollen agglutination process.
The scale of contribution of bee gut towards bee bread microbial inoculation has been studied by Mattila et al. [126]. It was found that only 10% of the bacterial species were commonly shared between bee gut and bee bread. The low impact of bee gut microbiota in contributing a substantial amount of microbes in bee bread fermentation was also supported by Anderson et al. [146] and Corby-Harris et al. [147]. The dominant bacteria in bee bread and honeybee Apis mellifera bee gut were also found in fresh flower and nectar, suggesting common horizontal transmission [146]. Nevertheless, it can be concluded that bee gut and also the hive environment contribute towards the bacteria residency in bee bread.
Understanding bee gut microbiome could get complicated because of diverse bacterial communities [145]. By far, the gut microbiota of honeybee Apis spp. is widely discussed [148,149] while only a few researchers have attempted to characterise the stingless bee gut microbiota. For instance, the culture-dependent analysis performed on stingless bee Meliponini beecheii (Central America), M. bocandei (Africa), and Trigona sp. (Borneo and Thailand) revealed their honey stomach harbours Lactobacillus and Bifidobacterium species [150].
The gut microbiome not only varies between species but also between colonies. For example, Leonhardt and Kaltenpoth [151] focused on LAB-bee associated with Australian stingless bee species (Austroplebeia australis, Tetragonula carbonaria, and Tetragonula hockingsi). Interestingly, the microbiome of these stingless bee is different intercolonies but almost similar to the microbiome of honeybee Apis mellifera [150]. Comparison between three different eusocial bees (honeybee, bumblebee, and stingless bee) found Gilliamella, Bifidobacterium, Lactobacillus Firm-4 and Lactobacillus Firm-5 comprising the core of the gut’s microbiome, with Meliponini as the most diverse one [152].
Bee gut microbiota is maternally inherited [66], acquired through bee faeces or oral trophallaxis [153] and environmental sources such as hive and flower [66]. Functional studies of A. mellifera gut microbiota through metagenomic analysis showed a possible link of these microbes to bee protection against pathogens and nutrition acquisition by degrading the pollen wall [12]. Zheng et al. [154] found enrichment of galacturonic acid, a pectin constituent in the honeybee ileum, which suggests pectin degradation. However, no in vitro and in vivo studies have yet to identify the bee gut species responsible for this fermentation process.
Understanding the functional role of bee gut, especially in bee bread fermentation, could be useful. According to Anderson et al. [116], fermentation helps to preserve bee bread environment rather than provide nutrient enrichment. Vásquez et al. [150] demonstrated the ability of LAB strains found in bee bread and honeybee Apis mellifera to protect bee bread against bee pathogen M. plutonius in vivo and in vitro. However, these studies were heavily based upon honeybee Apis sp. More studies should be conducted on stingless bee, whether it will have similar outcomes or not.
6. Conclusions
This review paper has summarised the physicochemical properties and health benefits of stingless bee bee bread and has discussed the microbial ecology of bee bread in general in relation to the bee gut. Bee bread is one of the overlooked stingless bee products, highly competing with Apis bee pollen in the current market and research focus. The food industry has seen tremendous changes in food trends from processed food to natural products with scientifically proven health benefits. The collective pieces of evidence in recent years have shown the potential of the stingless bee bee bread to be developed as a food ingredient, feed, or a supplement. It is rich in micronutrients, minerals, and phenolic compounds. However, research on their therapeutic values is still scarce. There are wide research opportunities to delve in on the bioactive compounds and their biological properties in vitro and in vivo. More research should be conducted so that an international standard for bee bread of stingless bee could be developed. More research on stingless bee bee bread can add to its commercial value and push to develop the meliponiculture industry. Bee bread has emerged as a reservoir to isolate beneficial microbes that contribute to bee bread preservation and can also provide newfound application in the industries.
Author Contributions
Conceptualisation, N.Z.; writing—original draft preparation, S.M.M.; writing—review and editing, S.M.M., N.Z., N.-K.M.A.-R.; visualisation, S.M.M.; supervision, N.Z.; funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universiti Putra Malaysia under the Putra Grant Scheme: GP/2017/9568900.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heard T. The Australian Native Bee Book: Keeping Stingless Bee Hives for Pets, Pollination and Delectable Sugarbag Honey. Sugarbag Bees; Queensland, Australia: 2016. [Google Scholar]
- 2.Michener C.D. The Bees of the World. Volume 85 JHU Press; Baltimore, MD, USA: 2000. [Google Scholar]
- 3.Mizrahi A., Lensky Y. Bee Products: Properties, Applications, and Apitherapy. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 4.Kieliszek M., Piwowarek K., Kot A.M., Błażejak S., Chlebowska-Śmigiel A., Wolska I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018;71:170–180. doi: 10.1016/j.tifs.2017.10.021. [DOI] [Google Scholar]
- 5.Vit P., Pedro S.R.M., Roubik D.W. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. [Google Scholar]
- 6.Thakur M., Nanda V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020;98:82–106. doi: 10.1016/j.tifs.2020.02.001. [DOI] [Google Scholar]
- 7.Mărgăoan R., Stranț M., Varadi A., Topal E., Yücel B., Cornea-Cipcigan M., Campos M.G., Vodnar D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants. 2019;8:568. doi: 10.3390/antiox8120568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feas X., Vazquez-Tato M.P., Estevinho L., Seijas J.A., Iglesias A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules. 2012;17:8359–8377. doi: 10.3390/molecules17078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vit P., Albore G.R.D., Barth O.M., Peña-Vera M., Pérez-Pérez E. Characterization of pot-pollen from Southern Venezuela. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 361–375. [Google Scholar]
- 10.Denisow B., Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- 11.Khalifa S.A.M., Elashal M., Kieliszek M., Ghazala N.E., Farag M.A., Saeed A., Sabir J.S.M., Battino M., Xiao J., Zou X., et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020;97:300–316. doi: 10.1016/j.tifs.2019.08.021. [DOI] [Google Scholar]
- 12.Engel P., Martinson V.G., Moran N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA. 2012;109:11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos O.Y., Basualdo M., Libonatti C., Vega M.F. Current status and application of lactic acid bacteria in animal production systems with a focus on bacteria from honey bee colonies. J. Appl. Microbiol. 2020;128:1248–1260. doi: 10.1111/jam.14469. [DOI] [PubMed] [Google Scholar]
- 14.Vásquez A., Olofsson T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009;48:189–195. doi: 10.3896/IBRA.1.48.3.07. [DOI] [Google Scholar]
- 15.Mohammad S.M., Mahmud-Ab-Rashid N.K., Zawawi N. Probiotic properties of bacteria isolated from bee bread of stingless bee Heterotrigona itama. J. Apic. Res. 2020;0:1–16. doi: 10.1080/00218839.2020.1801152. [DOI] [Google Scholar]
- 16.Ngalimat M.S., Rahman R.N.Z.R.A., Yusof M.T., Syahir A., Sabri S. Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis. PeerJ. 2019;7:e7478. doi: 10.7717/peerj.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith J.P., Heard T.A., Beekman M., Gloag R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae) Austral Entomol. 2016;56:50–53. doi: 10.1111/aen.12206. [DOI] [Google Scholar]
- 18.Araujo E.D., Costa M., Chaud-Netto J., Fowler H.G. Body size and flight distance in stingless bees (Hymenoptera: Meliponini): Inference of flight range and possible ecological implications. Braz. J. Biol. 2004;64:563–568. doi: 10.1590/S1519-69842004000400003. [DOI] [PubMed] [Google Scholar]
- 19.Basari N., Ramli S.N., Khairi N.S.M. Food reward and distance influence the foraging pattern of stingless bee, Heterotrigona itama. Insects. 2018;9:138. doi: 10.3390/insects9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiew R., Muid M. Beekeeping in Malaysia: Pollen Atlas. Malaysian Beekeeping Research and Development Team; Serdang, Malaysia: 1991. [Google Scholar]
- 21.Ghazi R., Zulqurnain N.S., Azmi W.A. Melittopalynological Studies of Stingless Bees from the East Coast of Peninsular Malaysia. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 77–88. [Google Scholar]
- 22.Mohammad S.M., Mahmud-Ab-Rashid N.K., Zawawi N. Botanical Origin and Nutritional Values of Bee Bread of Stingless Bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020;2020:15–17. doi: 10.1155/2020/2845757. [DOI] [Google Scholar]
- 23.Lob S., Afiffi N., Razak S.B.A., Ibrahim N.F., Nawi I.H.M. Composition and identification of pollen collected by stingless bee (Heterotrigona itama) in forested and coastal area of Terengganu, Malaysia. Malays. Appl. Biol. 2017;46:227–232. [Google Scholar]
- 24.Thakodee T., Deowanish S., Duangmal K. Melissopalynological analysis of stingless bee (Tetragonula pagdeni) honey in Eastern Thailand. J. Asia. Pac. Entomol. 2018;21:620–630. doi: 10.1016/j.aspen.2018.04.003. [DOI] [Google Scholar]
- 25.Zubaidah A.H., Bahri A.R.S., Sanusi J., Adam N.A., Azura A., Hamzah A.H. Application of scanning electron microscope in palynology study of floral resources by indo-malayan stingless bees genus tetragonula. Malays. J. Microsc. 2018;14:95–102. [Google Scholar]
- 26.Zaki N.N.M., Razak S.B.A. Pollen profile by stingless bee (Heterotrigona itama) reared in rubber smallholding environment at Tepoh, Terengganu. Malays. J. Microsc. 2018;14:38–54. [Google Scholar]
- 27.Selvaraju K., Vikram P., Soon J.M., Krishnan K.T., Mohammed A. Melissopalynological, physicochemical and antioxidant properties of honey from West Coast of Malaysia. J. Food Sci. Technol. 2019;56:2508–2521. doi: 10.1007/s13197-019-03728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majid M., Ellulu M.S., Bakar M.F.A. Melissopalynological Study, Phenolic Compounds, and Antioxidant Properties of Heterotrigona itama Honey from Johor, Malaysia. Scientifica. 2020;2020:1–9. doi: 10.1155/2020/2529592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva T.M.S., Camara C.A., Lins A.C.S., de Fátima Agra M., Silva E.M.S., Reis I.T., Freitas B.M. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela) An. Acad. Bras. Cienc. 2009;81:173–178. doi: 10.1590/S0001-37652009000200003. [DOI] [PubMed] [Google Scholar]
- 30.Absy M.L., Rech A.R., Ferreira M.G. Pollen Collected by Stingless Bees: A Contribution to Understanding Amazonian Biodiversity. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 29–46. [Google Scholar]
- 31.Roubik D.W., Patiño J.E.M. The Stingless Honey Bees (Apidae, Apinae: Meliponini) in Panama and Pollination Ecology from Pollen Analysis. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 47–66. [Google Scholar]
- 32.Barth O.M., da Silva de Freitas A., Vanderborgth B. Pollen Storage by Melipona quadrifasciata anthidioides in a Protected Urban Atlantic Forest Area of Rio de Janeiro, Brazil. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 103–109. [Google Scholar]
- 33.De Jesus Oliveira D., dos Santos D.R., Andrade B.R., do Nascimento A.S., da Silva M.O., da Cruz Mercês C., Lucas C.I.S., da Silva S.M.P.C., de Carvalho P.D., de Lima Silva F., et al. Botanical origin, microbiological quality and physicochemical composition of the Melipona scutellaris pot-pollen (‘samburá’) from Bahia (Brazil) Region. J. Apic. Res. 2020:1–13. doi: 10.1080/00218839.2020.1797271. [DOI] [Google Scholar]
- 34.Vit P., Pulcini P. Diastase and invertase activities in Meliponini and Trigonini honeys from Venezuela. J. Apic. Res. 1996;35:57–62. doi: 10.1080/00218839.1996.11100913. [DOI] [Google Scholar]
- 35.Leonhardt S.D., Dworschak K., Eltz T., Blüthgen N. Foraging loads of stingless bees and utilisation of stored nectar for pollen harvesting. Apidologie. 2007;38:125–135. doi: 10.1051/apido:2006059. [DOI] [Google Scholar]
- 36.Campos M.G.R., Bogdanov S., De Almeida-Muradian L.B., Szczesna T., Mancebo Y., Frigerio C., Ferreira F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008;47:154–161. doi: 10.1080/00218839.2008.11101443. [DOI] [Google Scholar]
- 37.Menezes C., Vollet-Neto A., Andrés F., León F., Venturieri G.C., Imperatriz-Fonseca V.L. Pot-Honey: A Legacy of Stingless Bees. Springer; New York, NY, USA: 2013. The Role of Useful Microorganisms to Stingless Bees and Stingless Beekeeping; pp. 153–171. [Google Scholar]
- 38.Ivanišová E., Kačániová M., Frančáková H., Petrová J., Hutková J., Brovarskyi V., Velychko S., Adamchuk L., Schubertová Z., Musilová J. Bee bread—Perspective source of bioactive compounds for future. Potravinarstvo. 2015;9:592–598. doi: 10.5219/558. [DOI] [Google Scholar]
- 39.De Oliveira Alves R.M., Carvalho C.A.L. Pot-Pollen ‘Samburá’ Marketing in Brazil and Suggested Legislation. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 435–443. [Google Scholar]
- 40.Akhmetova R., Sibgatullin J., Garmonov S., Akhmetova L. Technology for extraction of bee-bread from the honeycomb. Procedia Eng. 2012;42:1822–1825. doi: 10.1016/j.proeng.2012.07.577. [DOI] [Google Scholar]
- 41.Wilara Pollen and Bee Bread Harvesting. [(accessed on 6 October 2020)]; Available online: https://wilara.lt/en/bitininkyste/bee-bread-pollen-and-propolis-harvesting/pollen-and-bee-bread-harvesting/
- 42.Anđelković B., Jevtić G., Mladenović M., Marković J., Petrović M., Nedić N. Quality of pollen and honey bee bread collected in spring. J. Hyg. Eng. Des. 2012;1:275–277. [Google Scholar]
- 43.Herbert E.W., Bee B., Shimanuki H. Chemical composition and nutritive value of bee-collected and bee-stored pollen. Apidologie. 1978;9:33–40. doi: 10.1051/apido:19780103. [DOI] [Google Scholar]
- 44.Nicolson S.W., Human H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae) Apidologie. 2012;44:144–152. doi: 10.1007/s13592-012-0166-5. [DOI] [Google Scholar]
- 45.Human H., Nicolson S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae) Phytochemistry. 2006;67:1486–1492. doi: 10.1016/j.phytochem.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Degrandi-Hoffman G., Eckholm B.J., Huang M.H. A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie. 2013;44:52–63. doi: 10.1007/s13592-012-0154-9. [DOI] [Google Scholar]
- 47.Duarte A.W.F., Vasconcelos M.R.D.S., Oda-Souza M., de Oliveira F.F., López A.M.Q. Honey and bee pollen produced by meliponini (Apidae) in alagoas, brazil: Multivariate analysis of physicochemical and antioxidant profiles. Food Sci. Technol. 2018;38:493–503. doi: 10.1590/fst.09317. [DOI] [Google Scholar]
- 48.Gilliam M. Identifiation and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiol. Lett. 2000;155:1–10. doi: 10.1016/S0378-1097(97)00337-6. [DOI] [Google Scholar]
- 49.Habryka C., Kruczek M., Drygas B. Bee products used in apitherapy. World Sci. News. 2016;48:254–258. [Google Scholar]
- 50.Urcan A.C., Al Marghitas L., Dezmirean D.S., Bobis O., Bonta V., Muresan C.I., Margaoan R. Chemical Composition and Biological Activities of Beebread—Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2017;74:6–14. doi: 10.15835/buasvmcn-asb:12646. [DOI] [Google Scholar]
- 51.Fernandes-da Silva P.G., Serrão J.E. Nutritive value and apparent digestibility of bee-collected and bee-stored pollen in the stingless bee, Scaptotrigona postica Latr. (hymenoptera, apidae, meliponini) Apidologie. 2000;31:39–45. doi: 10.1051/apido:2000100. [DOI] [Google Scholar]
- 52.Nicolson S.W., Da Silva Das Neves S., Human H., Pirk C.W.W. Digestibility and nutritional value of fresh and stored pollen for honey bees (Apis mellifera scutellata) J. Insect Physiol. 2018;107:302–308. doi: 10.1016/j.jinsphys.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Ramalho M., Imperatriz-Fonseca V.L., Giannini T.C. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera) Apidologie. 1998;29:221–228. doi: 10.1051/apido:19980302. [DOI] [Google Scholar]
- 54.Ramalho M., Giannini T.C., Malagodi-braga K.S., Vera L., Imperatriz-fonseca V.L., Pollen V.L. Pollen Harvest by Stingless Bee Foragers Pollen harvest by stingless bee foragers (Hymenoptera, Apidae, Meliponinae) Grana. 2009;33:239–244. doi: 10.1080/00173139409429005. [DOI] [Google Scholar]
- 55.García-García M.C., Ortiz P.L., Dapena M.J.D. Variations in the weights of pollen loads collected by Apis mellifera L. Grana. 2004;43:183–192. doi: 10.1080/00173130410020350. [DOI] [Google Scholar]
- 56.Modro A.F.H., Silva I.C., Luz C.F.P., Message D. Analysis of pollen load based on color, physicochemical composition and botanical source. An. Acad. Bras. Cienc. 2009;81:281–285. doi: 10.1590/S0001-37652009000200014. [DOI] [PubMed] [Google Scholar]
- 57.Bogdanov S. Pollen: Production, Nutrition and Health: A Review. [(accessed on 13 February 2019)];Bee Product Science. 2017 :1–36. Available online: https://www.bee-hexagon.net/
- 58.Halbritter H., Ulrich S., Grimsson F., Weber M., Zetter R., Hesse M., Buchner R., Svojtka M., Frosch-Radivo A. Pollen Terminology: An Illustrated Handbook. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2009. [Google Scholar]
- 59.TRoulston H.I., Cane J.H. Plant Systematics Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000;222:187–188. doi: 10.1007/BF00984102. [DOI] [Google Scholar]
- 60.Almeida-Muradian L.B., Pamplona L.C., Coimbra S., Barth O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005;18:105–111. doi: 10.1016/j.jfca.2003.10.008. [DOI] [Google Scholar]
- 61.De Souza R.R., de Abreu V.H.R., de Novais J.S. Melissopalynology in Brazil: A map of pollen types and published productions between 2005 and 2017. Palynology. 2018;43:690–700. doi: 10.1080/01916122.2018.1542355. [DOI] [Google Scholar]
- 62.Louveaux J., Maurizio A., Vorwohl G. Methods of Melissopalynology. Bee World. 1978;59:139–157. doi: 10.1080/0005772X.1978.11097714. [DOI] [Google Scholar]
- 63.Ibrahim I.F., Balasundram S.K., Abdullah N.A.P., Alias M.S., Mardan M. Morphological Characterization of Pollen Collected by Apis Dorsata from Tropical Rainforest. Int. J. Bot. 2012;8:96–106. doi: 10.3923/ijb.2012.96.106. [DOI] [Google Scholar]
- 64.Villegas-Plazas M., Figueroa-Ramírez J., Portillo C., Monserrate P., Tibatá V., Sánchez O.A., Junca H. Yeast and Bacterial Composition in Pot-Pollen Recovered from Meliponini in Colombia: Prospects for a Promising Biological Resource. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 263–279. [Google Scholar]
- 65.Komosinska-Vassev K., Olczyk P., Kaźmierczak J., Mencner L., Olczyk K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complement. Altern. Med. 2015;2015:297425. doi: 10.1155/2015/297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McFrederick Q.S., Wcislo W.T., Taylor D.R., Ishak H.D., Dowd S.E., Mueller U.G. Environment or kin: Whence do bees obtain acidophilic bacteria? Mol. Ecol. 2012;21:1754–1768. doi: 10.1111/j.1365-294X.2012.05496.x. [DOI] [PubMed] [Google Scholar]
- 67.Kostić A.Ž., Barać M.B., Stanojević S.P., Milojković-Opsenica D., Tešić Ž.L., Šikoparija B., Radišić P., Prentović M., Pešić M.B. Physicochemical composition and techno-functional properties of bee pollen collected in Serbia. LWT Food Sci. Technol. 2015;62:301–309. doi: 10.1016/j.lwt.2015.01.031. [DOI] [Google Scholar]
- 68.Szczęsna T. Protein content and amino acid composition of bee-collected pollen from selected botanical origins. J. Apic. Sci. 2006;50:81–90. doi: 10.1016/j.sjbs.2017.06.003. [DOI] [Google Scholar]
- 69.Ares A.M., Valverde S., Bernal J.L., Nozal M.J., Bernal J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018;147:110–124. doi: 10.1016/j.jpba.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Pascoal A., Rodrigues S., Teixeira A., Feás X., Leticia M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014;63:233–269. doi: 10.1016/j.fct.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 71.De Oliveira Alves R.M., da Silva Sodré G., Carvalho C.A.L. Chemical, microbiological and palynological composition of the ‘Sambura’ Melipona scutellaris pot pollen. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 349–360. [Google Scholar]
- 72.Combey R. Microbial and Qualitative Analyses of Stingless Bee Bread using Dry Preservation Methods. Eur. J. Zool. Res. 2017;5:45–50. [Google Scholar]
- 73.Janashia I., Choiset Y., Jozefiak D., Déniel F., Coton E., Moosavi-Movahedi A.A., Chanishvili N., Haertlé T. Beneficial Protective Role of Endogenous Lactic Acid Bacteria Against Mycotic Contamination of Honeybee Beebread. Probiotics Antimicrob. Proteins. 2018;10:638–646. doi: 10.1007/s12602-017-9379-2. [DOI] [PubMed] [Google Scholar]
- 74.Belina-Aldemita M.D., Fraberger V., Schreiner M., Domig K.J., D’Amico S. Safety aspects of stingless bee pot-pollen from the Philippines. Die Bodenkultur J. Land Manag. Food Environ. 2020;71:87–100. doi: 10.2478/boku-2020-0009. [DOI] [Google Scholar]
- 75.Kostić A.Ž., Milinčić D.D., Petrović T., Krnjaja V., Stanojevic S.P., Barać M.B., Tešić Ž.L., Pešić M.B. Mycotoxins and Mycotoxin Producing Fungi in Pollen: Review. Toxins. 2019;11:64. doi: 10.3390/toxins11020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barene I., Daberte I., Siksna S. Investigation of Bee Bread and Development of Its Dosage Forms. Med. Teor. Pract. 2015;21:16–22. doi: 10.15591/mtp.2015.003. [DOI] [Google Scholar]
- 77.Ismail W.I.W., Hussin N.N., Mazlan S.N.F., Hussin N.H., Radzi M.N.F.M. Physicochemical Analysis, Antioxidant and Anti Proliferation Activities of Honey, Propolis and Beebread Harvested from Stingless Bee. IOP Conf. Ser. Mater. Sci. Eng. 2018;440:1–7. doi: 10.1088/1757-899X/440/1/012048. [DOI] [Google Scholar]
- 78.Othman Z., Ghazali W.S.W., Nordin L., Omar N., Mohamed M. Nutritional, Phytochemical and Antioxidant Analysis of Bee Bread from Different Regions of Malaysia. Indian J. Pharm. Sci. 2019;81:955–960. doi: 10.36468/pharmaceutical-sciences.590. [DOI] [Google Scholar]
- 79.Mohd K.S., Zin N.B. Chemical and Biological Investigation of Apiculture Products from Stingless Bees Heterotrigona itama. J. Agrobiotechnol. 2020;11:7–19. doi: 10.37231/jab.2020.11.1.183. [DOI] [Google Scholar]
- 80.Chuttong B., Phongphisutthinant R., Sringarm K., Burgett M., Barth O.M. Pot-Honey: A Legacy of Stingless Bees. Springer; Cham, Switzerland: 2018. Nutritional Composition of Pot-Pollen from Four Species of Stingless Bees (Meliponini) in Southeast Asia; pp. 313–324. [Google Scholar]
- 81.Vit P., Santiago B., Pedro S.R., Perez-Perez E., Pena-Vera M. Chemical and bioactive characterization of pot-pollen produced by Melipona and Scaptotrigona stingless bees from Paria Grande, Amazonas State, Venezuela. Emir. J. Food Agric. 2016;28:78–84. doi: 10.9755/ejfa.2015-05-245. [DOI] [Google Scholar]
- 82.Rebelo K.S., Ferreira A.G., Carvalho-Zilse G.A. Physicochemical characteristics of pollen collected by Amazonian stingless bees. Ciência Rural. 2016;46:927–932. doi: 10.1590/0103-8478cr20150999. [DOI] [Google Scholar]
- 83.Belina-aldemita M.D., Opper C., Schreiner M., D’Amico S. Nutritional composition of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Compos. Anal. 2019;82:103215. doi: 10.1016/j.jfca.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Vit P., Santiago B., Peña-Vera M., Pérez-Pérez E. Chemical Characterization and Bioactivity of Tetragonisca angustula Pot-Pollen from Mérida, Venezuela. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 339–347. [Google Scholar]
- 85.Bárbara M.S., Machado C.S., Sodré G.D.S., Dias L.G., Estevinho L.M., de Carvalho C.A.L. Microbiological assessment, nutritional characterization and phenolic compounds of bee pollen from Mellipona mandacaia Smith, 1983. Molecules. 2015;20:12525–12544. doi: 10.3390/molecules200712525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contreras-Oliva A., Perez-Sato J.A., Gómez-Merino F.C., López-Garay L.A., Villanueva-Gutiérrez R., Crosby-Galván M.M., Trejo-Téllez L.I. Characterization of Scaptotrigona mexicana pot-pollen from Veracruz, Mexico. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 325–337. [Google Scholar]
- 87.da Silva G.R., da Natividade T.B., Camara C.A., da Silva E.M.S., dos Santos F.D.A.R., Silva T.M.S. Identification of Sugar, Amino Acids and Minerals from the Pollen of Jandaíra Stingless Bees (Melipona subnitida) Food Nutr. Sci. 2014;5:1015–1021. doi: 10.4236/fns.2014.511112. [DOI] [Google Scholar]
- 88.Omar W.A.W., Yahaya N., Ghaffar Z.A., Fadzilah N.H. GC-MS Analysis of Chemical Constituents in Ethanolic Bee Pollen Extracts from Three Species of Malaysian Stingless Bee. J. Apic. Sci. 2019;62:275–284. doi: 10.2478/jas-2018-0022. [DOI] [Google Scholar]
- 89.Al-Sherif A.A., Mazeed A.M., Ewis M.A., Nafea E.A., Hagag E.S.E., Kamel A.A. Activity of salivary glands in secreting honey-elaborating enzymes in two subspecies of honeybee (Apis mellifera L) Physiol. Entomol. 2017;42:397–403. doi: 10.1111/phen.12213. [DOI] [Google Scholar]
- 90.Vossler F.G. Broad Protein Spectrum in Stored Pollen of Three Stingless Bees from the Chaco Dry Forest in South America (Hymenoptera, Apidae, Meliponini) and Its Ecological Implications. Psyche. 2015;2015:13–15. doi: 10.1155/2015/659538. [DOI] [Google Scholar]
- 91.Manning R. Fatty acids in pollen: A review of their importance for honey bees. Bee World. 2001;82:37–41. doi: 10.1080/0005772X.2001.11099504. [DOI] [Google Scholar]
- 92.Mărgăoan R., Mărghitaş L.A., Dezmirean D.S., Dulf F.V., Bunea A., Socaci S.A., Bobiş O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014;62:6306–6316. doi: 10.1021/jf5020318. [DOI] [PubMed] [Google Scholar]
- 93.Szczęsna T. Long-chain fatty acids composition of honeybee-collected pollen. J. Apic. Sci. 2006;50:65–79. [Google Scholar]
- 94.Kaplan M., Karaoglu Ö., Eroglu N., Silici S. Fatty acid and proximate composition of bee bread. Food Technol. Biotechnol. 2016;54:497–504. doi: 10.17113/ftb.54.04.16.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massaro C.F., Villa T.F., Hauxwell C. Metabolomics Analysis of Pot-Pollen from Three Species of Australian Stingless Bees (Meliponini) In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 401–417. [Google Scholar]
- 96.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 97.Urcan A.C., Criste A.D., Dezmirean D.S., Mărgăoan R., Caeiro A., Campos M.G. Similarity of data from bee bread with the same taxa collected in India and Romania. Molecules. 2018;23:2491. doi: 10.3390/molecules23102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaškonienė V., Katilevičiūtė A., Kaškonas P., Maruška A. The impact of solid-state fermentation on bee pollen phenolic compounds and radical scavenging capacity. Chem. Pap. 2018;72:2115–2120. doi: 10.1007/s11696-018-0417-7. [DOI] [Google Scholar]
- 99.Fadzilah N.H., Jaapar M.F., Jajuli R., Adnan W., Omar W. Total phenolic content, total flavonoid and antioxidant activity of ethanolic bee pollen extracts from three species of Malaysian stingless bee. J. Apic. Res. 2017;8839:1–6. doi: 10.1080/00218839.2017.1287996. [DOI] [Google Scholar]
- 100.Pérez-Pérez E., Sulbarán-Mora M., Barth O.M., Massaro C.F., Vit P. Bioactivity and Botanical Origin of Austroplebeia and Tetragonula Australian Pot-Pollen. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 377–390. [Google Scholar]
- 101.Da Silva T.M., Camara C.A., Lins A.C.D.S., Filho J.M.B., Da Silva E.M.S., Freitas B.M., DOS Santos F.D.A.R. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Compos. Anal. 2006;19:507–511. doi: 10.1016/j.jfca.2005.12.011. [DOI] [Google Scholar]
- 102.Chen A.Y., Chen Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2014;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, Inflammation and Immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Othman Z.A., Ghazali W.S.W., Noordin L., Yusof N.A.M., Mohamed M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants. 2020;9:33. doi: 10.3390/antiox9010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopes A.J.O., Vasconcelos C.C., Garcia J.B.S., Pinheiro M.S.D., Pereira F.A.N., Camelo D.D.S., De Morais S.V., Freitas J.R.B., Da Rocha C.Q., de Sousa Ribeiro M.N., et al. Anti-inflammatory and antioxidant activity of pollen extract collected by Scaptotrigona affinis postica: In silico, in vitro, and in vivo studies. Antioxidants. 2020;9:103. doi: 10.3390/antiox9020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lopes A.J.O., Vasconcelos C.C., Pereira F.A.N., Silva R.H.M., Dos Santos Queiroz P.F., Fernandes C.V., Garcia J.B.S., Ramos R.M., Da Rocha C.Q., De Jesus Rodrigues Moreira Lima S.T., et al. Anti-Inflammatory and activity and antinociceptive activity of pollen extract collected by stingless bee Melipona fasciculata. Int. J. Mol. Sci. 2019;20:4512. doi: 10.3390/ijms20184512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Belina-Aldemita M.D., Schreiner M., D’Amico S. Characterization of phenolic compounds and antioxidative potential of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Biochem. 2020;44:e13102. doi: 10.1111/jfbc.13102. [DOI] [PubMed] [Google Scholar]
- 108.Ide Melo L.P., de Almeida-Muradian L.B. Stability of antioxidants vitamins in bee pollen samples. Quim. Nova. 2010;33:514–518. doi: 10.1590/S0100-40422010000300004. [DOI] [Google Scholar]
- 109.Oliveira K.C.L.S., Moriya M., Azedo R.A.B., Teixeira E.W., Alves M.L.T.M.F., Moreti A.C.D.C.C., De Almeida-Muradian L.B. Relationship between Botanical origin and Antioxidants Vitamins of Bee-Collected Pollen. Quim. Nova. 2009;32:1099–1102. doi: 10.1590/S0100-40422009000500003. [DOI] [Google Scholar]
- 110.Nordin A., Sainik N.Q.A.V., Chowdhury S.R., Saim A.B., Idrus R.B.H. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J. Food Compos. Anal. 2018;73:91–102. doi: 10.1016/j.jfca.2018.06.002. [DOI] [Google Scholar]
- 111.Aldgini H.M.M., Al-Abbadi A.A., Abu-Nameh E.S.M., Alghazeer R.O. Determination of metals as bio indicators in some selected bee pollen samples from Jordan. Saudi J. Biol. Sci. 2019;26:1414–1422. doi: 10.1016/j.sjbs.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carneiro A.L.B., Gomes A.A., Da Silva L.A., Alves L.B., Da Silva E.C., Pinto A.C.D.S., Tadei W.P., Pohlit A.M., Teixeira M.F.S., Gomes C.C., et al. Antimicrobial and Larvicidal Activities of Stingless Bee Pollen from Maues, Amazonas, Brazil. Bee World. 2019;96:98–103. doi: 10.1080/0005772X.2019.1650564. [DOI] [Google Scholar]
- 113.Akhir R.A.M., Bakar M.F.A., Sanusi S.B. Antioxidant and antimicrobial activity of stingless bee bread and propolis extracts. AIP Conf. Proc. 2018;42:72–79. doi: 10.1063/1.5005423. [DOI] [Google Scholar]
- 114.Sulbarán-Mora M., Pérez-Pérez E., Vit P. Antibacterial Activity of Ethanolic Extracts of Pot-Pollen Produced by Eight Meliponine Species from Venezuela. In: Vit P., Pedro S.R.M., Roubik D.W., editors. Pot-Pollen in Stingless Bee Melittology. Springer International Publishing; Cham, Switzerland: 2018. pp. 391–399. [Google Scholar]
- 115.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer. Free Radic. Biol. Med. 2011;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr. J. Microbiol. Res. 2009;3:981–996. [Google Scholar]
- 118.Eleazu C., Suleiman J.B., Othman Z.A., Zakaria Z., Nna V.U., Hussain N.H.N., Mohamed M. Bee bread attenuates high fat diet induced renal pathology in obese rats via modulation of oxidative stress, downregulation of NF-kB mediated inflammation and Bax signalling. Arch. Physiol. Biochem. 2020:1–17. doi: 10.1080/13813455.2020.1752258. [DOI] [PubMed] [Google Scholar]
- 119.Oltica S., Dezmirean D., Cluj-Napoca V.M. Examination of Antioxidant Capacity of Beebread Extracts by Different Complementary Assays. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. 2007;64:204–207. doi: 10.15835/buasvmcn-asb:64:1-2:2247. [DOI] [Google Scholar]
- 120.Hudz N., Ivanova R., Brindza J., Grygorieva O., Schubertová Z., Ivanišová E. Approaches to the determination of antioxidant activity of extracts from bee bread and safflower leaves and flowers. Potravin. Slovak J. Food Sci. 2017;11:480–488. doi: 10.5219/786. [DOI] [Google Scholar]
- 121.World Health Organization Obesity and Overweight. [(accessed on 16 October 2020)];2020 Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 122.Carroll M.J., Brown N., Goodall C., Downs A.M., Sheenan T.H., Anderson K.E. Honey bees preferentially consume freshly stored Pollen. PLoS ONE. 2017;12:e0175933. doi: 10.1371/journal.pone.0175933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson K.E., Carroll M.J., Sheehan T., Mott B.M., Maes P., Corby-Harris V. Hive-stored pollen of honey bees: Many lines of evidence are consistent with pollen preservation, not nutrient conversion. Mol. Ecol. 2014;23:5904–5917. doi: 10.1111/mec.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ngalimat M.S., Rahman R.N.Z.R.A., Yusof M.T., Hamzah A.S.A., Zawawi N., Sabri S. A review on the association of bacteria with stingless bees. Sains Malays. 2020;49:1853–1863. doi: 10.17576/jsm-2020-4908-08. [DOI] [Google Scholar]
- 125.Narvhus J.A., Axelsson L. The Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Cambridge, MA, USA: 2003. Lactic acid bacteria; pp. 3465–3472. [Google Scholar]
- 126.Mattila H.R., Rios D., Walker-sperling V.E., Roeselers G., Newton I.L.G. Characterization of the Active Microbiotas Associated with Honey Bees Reveals Healthier and Broader Communities when Colonies are Genetically Diverse. PLoS ONE. 2012;7:e32962. doi: 10.1371/journal.pone.0032962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janashia I., Carminati D., Rossetti L., Zago M., Fornasari M.E., Haertlé T., Chanishvili N., Giraffa G. Characterization of fructophilic lactic microbiota of Apis mellifera from the Caucasus Mountains. Ann. Microbiol. 2016;66:1387–1395. doi: 10.1007/s13213-016-1226-2. [DOI] [Google Scholar]
- 128.Asama T., Arima T.-H., Gomi T., Keishi T., Tani H., Kimura Y., Tatefuji T., Hashimoto K. Lactobacillus kunkeei YB38 from honeybee products enhances IgA production in healthy adults. J. Appl. Microbiol. 2015;119:818–826. doi: 10.1111/jam.12889. [DOI] [PubMed] [Google Scholar]
- 129.Vásquez A., Olofsson T.C., Sammataro D., Vásquez A., Olofsson T.C., Sammataro D. A scientific note on the lactic acid bacterial flora in honeybees in the USA—A comparison with bees from Sweden. Apidologie. 2009;40:26–28. doi: 10.1051/apido:2008063. [DOI] [Google Scholar]
- 130.Lozo J., Berić T., Terzić-Vidojević A., Stanković S., Fira D., Stanisavljević L. Microbiota associated with pollen, bee bread, larvae and adults of solitary bee Osmia cornuta (Hymenoptera: Megachilidae) Bull. Entomol. Res. 2015;105:470–476. doi: 10.1017/S0007485315000292. [DOI] [PubMed] [Google Scholar]
- 131.Endo A., Futagawa-Endo Y., Dicks L.M.T. Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst. Appl. Microbiol. 2009;32:593–600. doi: 10.1016/j.syapm.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Yaacob S.N.S., Huyop F., Ibrahim R.K.R., Wahab R.A. Identification of Lactobacillus spp. and Fructobacillus spp. isolated from fresh Heterotrigona itama honey and their antagonistic activities against clinical pathogenic bacteria. J. Apic. Res. 2018;57:395–405. doi: 10.1080/00218839.2018.1428047. [DOI] [Google Scholar]
- 133.Endo A., Salminen S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013;36:444–448. doi: 10.1016/j.syapm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 134.Rokop Z.P., Horton M.A., Newton I.L.G. Interactions between Cooccurring Lactic Acid Bacteria in Honey. J. ASM. 2015;81:7261–7270. doi: 10.1128/AEM.01259-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janashia I., Alaux C. Specific immune stimulation by endogenous bacteria in honey bees (Hymenoptera: Apidae) J. Econ. Entomol. 2016;109:1474–1477. doi: 10.1093/jee/tow065. [DOI] [PubMed] [Google Scholar]
- 136.Gilliam M. Microbiology of Pollen and Bee Bread: The Genus Bacillus. Apidologie. 1979;10:269–274. doi: 10.1051/apido:19790304. [DOI] [Google Scholar]
- 137.Rosa C.A., Lachance M.-A., Silva J.O., Teixeira A.C.P., Marini M.M., Antonini Y., Martins R.P. Yeast communities associated with stingless bees. FEMS Yeast Res. 2003;4:271–275. doi: 10.1016/S1567-1356(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 138.Teixeira A.C.P., Marini M.M., Nicoli J.R., Antonini Y., Martins R.P., Lachance M.-A., Rosa C.A. Starmerella meliponinorum sp. nov., a novel ascomycetous yeast species associated with stingless bees. Int. J. Syst. Evol. Microbiol. 2003;53:339–343. doi: 10.1099/ijs.0.02262-0. [DOI] [PubMed] [Google Scholar]
- 139.Daniel H.-M., Rosa C.A., Thiago-Calaça P.S.S., Antonini Y., Bastos E.M.A.F., Evrard P., Huret S., Fidalgo-Jiménez A., Lachance M.-A. Starmerella neotropicalis f. a., sp. nov., a yeast species found in bees and pollen from Brazil and Cuba. Int. J. Syst. Evol. Microbiol. 2013;63:3896–3903. doi: 10.1099/ijs.0.055897-0. [DOI] [PubMed] [Google Scholar]
- 140.Gilliam M., Rubik D., Lorenz B. Microorganisms associated with pollen, honey and brood provisions in the nest of a stingless bee, Melipona fasciata. Apidologie. 2000;21:89–97. doi: 10.1051/apido:19900201. [DOI] [Google Scholar]
- 141.Ramos H.C., Hoffmann T., Marino M., Nedjari H., Presecan-Siedel E., Dreesen O., Glaser P., Jahn D. Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. J. Bacteriol. 2000;182:3072–3080. doi: 10.1128/JB.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aqeel B.M., Umar D.M. Effect of Alternative Carbon and Nitrogen Sources on Production of Alpha-amylase by Bacillus megaterium. World Appl. Sci. J. 2010;8:85–90. doi: 10.1016/j.jbiotec.2008.07.1864. [DOI] [Google Scholar]
- 143.Akcan N. High Level Production of Extracellular α-Amylase from Bacillus licheniformis ATCC 12759 in Submerged Fermentation. Rom. Biotechnol. Lett. 2011;16:6833–6840. [Google Scholar]
- 144.Gilliam M. Microbiology of Pollen and Bee bread: The Yeasts. Apidologie. 1979;10:43–53. doi: 10.1051/apido:19790106. [DOI] [Google Scholar]
- 145.Olofsson T.C., Vásquez A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008;57:356–363. doi: 10.1007/s00284-008-9202-0. [DOI] [PubMed] [Google Scholar]
- 146.Anderson K.E., Sheehan T.H., Mott B.M., Maes P., Snyder L., Schwan M.R., Walton A., Jones B.M., Corby-Harris V. Microbial Ecology of the Hive and Pollination Landscape: Bacterial Associates from Floral Nectar, the Alimentary Tract and Stored Food of Honey Bees (Apis mellifera) PLoS ONE. 2013;8:e83125. doi: 10.1371/journal.pone.0083125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Corby-Harris V., Maes P., Anderson K.E. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE. 2014;9:e95056. doi: 10.1371/journal.pone.0095056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kwong W.K., Moran N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016;14:374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moran N.A. Genomics of the honey bee microbiome. Curr. Opin. Insect Sci. 2015;10:22–28. doi: 10.1016/j.cois.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vásquez A., Forsgren E., Fries I., Paxton R.J., Flaberg E., Szekely L., Olofsson T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE. 2012;7:e33188. doi: 10.1371/annotation/3ac2b867-c013-4504-9e06-bebf3fa039d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leonhardt S.D., Kaltenpoth M. Microbial Communities of Three Sympatric Australian Stingless Bee Species. PLoS ONE. 2014;9:e105718. doi: 10.1371/journal.pone.0105718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwong W.K., Medina L.A., Koch H., Sing K.-W., Soh E.J.Y., Ascher J.S., Jaffé R., Moran N.A. Dynamic microbiome evolution in social bees. Sci. Adv. 2017;3:1–17. doi: 10.1126/sciadv.1600513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Powell J.E., Martinson V.G., Urban-Mead K., Moran N.A. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 2014;80:7378–7387. doi: 10.1128/AEM.01861-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng H., Nishida A., Kwong W.K., Koch H., Engel P., Steele M.I., Moran N.A. Metabolism of toxic sugars by strains of the bee gut symbiont Gilliamella apicola. mBio. 2016;7:1–9. doi: 10.1128/mBio.01326-16. [DOI] [PMC free article] [PubMed] [Google Scholar]