Abstract
Introduction
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has profound quality of life and economic consequences for individuals, their family, formal services and wider society. Little is known about which therapeutic interventions are more cost-effective.
Objective
A systematic review was carried out to identify and critically appraise the evidence on the cost-effectiveness of CFS/ME interventions.
Methods
The review protocol was prespecified (PROSPERO: CRD42018118731). Searches were carried out across two databases—MEDLINE (1946–2020) and EMBASE (1974–2020). Additional studies were identified by searching reference lists. Only peer-reviewed journal articles of full economic evaluations examining CFS/ME interventions were included. Trial- and/or model-based economic evaluations were eligible. Data extraction and screening were carried out independently by two reviewers. The methodological quality of the economic evaluation and trial were assessed using the Consensus Health Economic Criteria checklist (CHEC-list) and Risk of Bias-2 (RoB-2) tool, respectively. A narrative synthesis was used to summarise the economic evidence for interventions for adults and children in primary and secondary care settings.
Results
Ten economic evaluations, all based on data derived from randomised controlled trials, met our eligibility criteria. Cognitive behavioural therapy (CBT) was evaluated across five studies, making it the most commonly evaluated intervention. There was evidence from three trials to support CBT as a cost-effective treatment option for adults; however, findings on CBT were not uniform, suggesting that cost-effectiveness may be context-specific. A wide array of other interventions were evaluated in adults, including limited evidence from two trials supporting the cost effectiveness of graded exercise therapy (GET). Just one study assessed intervention options for children. Our review highlighted the importance of informal care costs and productivity losses in the evaluation of CFS/ME interventions.
Conclusions
We identified a limited patchwork of evidence on the cost-effectiveness of interventions for CFS/ME. Evidence supports CBT as a cost-effective treatment option for adults; however, cost-effectiveness may depend on the duration and frequency of sessions. Limited evidence supports the cost effectiveness of GET. Key weaknesses in the literature included small sample sizes and short duration of follow-up. Further research is needed on pharmacological interventions and therapies for children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-021-00635-7.
Key Points for Decision Makers
| Cognitive behavioural therapy appears to represent good value for money for treating adults affected by chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). |
| Evidence on the cost-effectiveness of treatments for young people with CFS/ME is scarce. |
| Productivity losses and informal care costs are substantial and should be included in future analyses. |
Introduction
Background
Chronic fatigue syndrome (CFS), or myalgic encephalomyelitis (ME), is medically unexplained, severe and persistent fatigue that results in impairment and disability [1]. There is no diagnostic test for CFS/ME and many different case definitions are used in research and clinical practice [2]. A recent systematic review estimated a global prevalence of 0.89% (95% confidence interval [CI] 0.60–1.33) for CFS/ME using the Centers for Disease Control and Prevention’s (CDC) 1994 definition, the most commonly used case definition [3].
The syndrome causes a profound loss in quality of life and often results in financial implications to the individual with CFS/ME, their family, formal healthcare services and wider society. Compared with patients with chronic fatigue, patients who meet the diagnostic criteria for CFS/ME (e.g. chronic fatigue and symptoms such as cognitive difficulties and post-exertional malaise) use 22% more healthcare services, experience twice as much lost income, and informal caregivers (i.e. family and friends) will likely provide four times more support [4]. Across Europe (EU-28), CFS/ME has been estimated to cost around €40 billion per year [5]. Productivity loss due to adults with CFS/ME discontinuing employment prior to accessing CFS/ME specialist assessment is estimated to cost the UK economy over £100 million per year and is potentially substantially higher as many adults with CFS/ME do not access specialist support [6]. From the family perspective, many parents of children with CFS/ME experience a loss in monthly income and increase in monthly expenditure, for example travel/transport, diet, leisure, educational items and complementary/other therapies [7].
Novel interventions may be beneficial in part, either by reducing fatigue severity (e.g. measured using the Checklist Individual Strength (CIS) fatigue subscale [8]), or by improving health-related quality of life (e.g. expressed in terms of quality-adjusted life years (QALYs) [9]), or by capability well-being [10]. A number of potential treatment strategies have been proposed (e.g. pharmaceutical, supplements, management strategies and lifestyle changes), but supportive evidence is often limited, of poor quality, or inconclusive [11]. However, two Cochrane reviews have concluded that cognitive behavioural therapy (CBT) is effective in reducing fatigue [12], and that exercise therapy probably has a positive effect on fatigue [13]. In 2007, the UK National Institute for Health and Care Excellence (NICE) issued guidance and recommended CBT and graded exercise therapy (GET), while also recommending further research on different methods of delivering care [14]. More recently, several randomised controlled trials (RCTs) of various interventions have incorporated economic evaluations and report clinical- and cost-effectiveness in both adults and young people.
The aim of this review was to assess the evidence on the cost-effectiveness of interventions for CFS/ME. Furthermore, this review also aims to critically appraise the quality of economic evidence to provide a guide for decision makers to allocate resources for CFS/ME.
Research Question
What evidence is there that interventions for CFS/ME are cost-effective, and is this evidence of sufficient quality to inform policy?
Methods
Protocol and Eligibility Criteria
The methods for this systematic review were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [15] (ESM 5) and were prespecified in a registered protocol (PROSPERO: CRD42018118731). Eligible studies were full economic evaluations comparing both costs and effects of two or more interventions. Eligible analysis methods included cost-effectiveness analyses (CEA), cost-utility analyses (CUA), cost-benefit analyses (CBA), and cost-consequence analyses (CCA). Comparative studies and/or model-based economic evaluations assessing any intervention in any CFS/ME population subgroup were eligible. The review was restricted to peer-reviewed studies as this was expected to yield higher-quality studies.
Search Strategy and Study Selection
Searches (see Table 1 in electronic supplementary material [ESM] 1) were conducted in MEDLINE (via OVID from 1946 to April 2020) and EMBASE (via OVID from 1974 to week 17 2020) and were restricted to the English language. We ran a focused search strategy based on the key terms ‘cost-effectiveness’ and ‘CFS’. In addition, the reference lists of eligible studies and systematic reviews related to CFS/ME were hand searched. We compared the results of our focused search (Table 1 in ESM 2) with a search that used broader terms (Table 2 in ESM 1) in the NHS Economic Evaluation Database (NHS EED). The NHS EED provides access to economic evaluations published between 1968 and December 2014, however our broader search in the NHS EED did not identify any additional studies (Table 2 in ESM 2) and therefore indicated that our focused search in MEDLINE and EMBASE, along with hand searching of reference lists, were likely to have identified all eligible studies. Two reviewers (MC, EM) independently screened the title and abstract of potentially eligible studies against the eligibility criteria, followed by full-text screening if required. Disagreement between reviewers was resolved by consulting a third reviewer (WH).
Data Collection Process and Data Items
Data on study design and findings were extracted into a spreadsheet by one reviewer (EM) and checked by a second reviewer (MC). Disagreements were resolved by discussion with a third reviewer (WH). Extracted information included (1) record details (author, title, publication date, journal); (2) study characteristics (trial design, country, sample size, analytical technique, population, intervention and comparator names and descriptions, primary clinical and economic outcome measure, time horizon, study perspective, cost categories, currency, price year); (3) study results (mean costs, mean effects, incremental costs, incremental effects, summary measure of efficiency (e.g. incremental cost-effectiveness ratio [ICER], net monetary benefit [NMB]). Information describing the intervention and comparator groups (including type, frequency and duration) and CFS/ME case definition were added to the data extraction sheet after the review had started.
Assessment of Methodological Quality and Risk of Bias in the Individual Studies
The methodological quality of the economic evaluation was assessed using the Consensus Health Economic Criteria checklist (CHEC-list) [16]. Two reviewers (MC, EM) independently applied the checklist to the included studies. In addition, as all studies identified through the searches were economic evaluations based on single RCTs, we used the Cochrane Risk of Bias-2 (RoB-2) tool [17] for assessing the potential bias in the underlying trials. The RoB-2 tool assesses risk of bias across five domains: randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of reported result. All RoB-2 assessments were judged by one reviewer (MC) and a 10% sample was checked by a second reviewer (EM) [ESM 4].
Summary Measures and Methods of Analysis
A structured narrative synthesis of the included studies was more appropriate than a meta-analysis, given the expected variation in patient groups, interventions and methods of economic analysis between studies. We summarised the primary measure of efficiency (e.g. ICER, NMB). Evidence tables were produced to present key study results. Separate evidence tables were presented for interventions in adults and children, as well as the most common intervention type (CBT). For simplicity, where studies had used both a wide (e.g. societal) and narrow (e.g. health service) study perspective, we presented only the wider perspective findings. Similarly, where studies conducted both CEA and CUA, we presented only the CUA findings.
Results
Study Selection
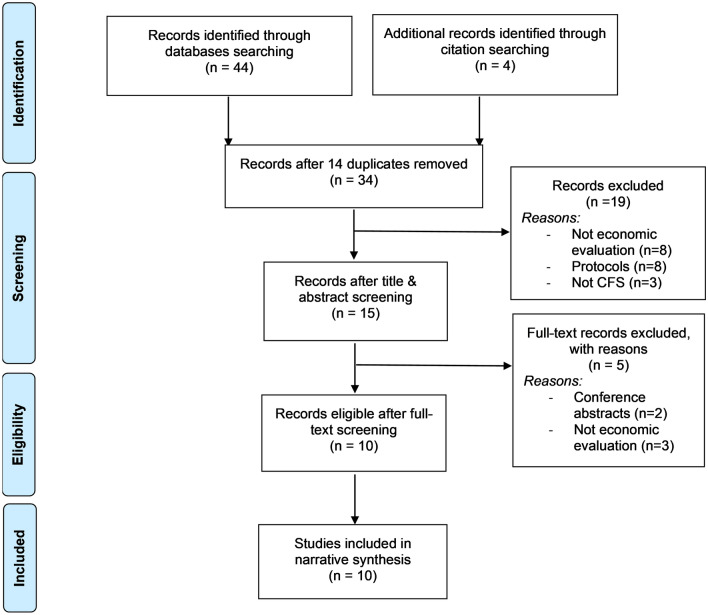
The database and supplementary searches yielded 34 articles after removing duplicates (Fig. 1). After initial screening of titles and abstracts, 15 potentially relevant studies were reviewed in full; 10 studies [18–27] met all eligibility criteria and were included in our analysis. The major reasons for studies not meeting the eligibility criteria were as follows: 11 were not full economic evaluations, three were not specific to chronic fatigue, and eight were protocols of studies including economic evaluations. In 9 of the 10 economic evaluations included in our review, the trial methods were detailed in a separate clinical effectiveness paper [28–36]. In these cases, the RoB-2 assessment was based on both publications.
Fig. 1.
Study selection process. CFS chronic fatigue syndrome
Characteristics of Studies
All economic evaluations were conducted alongside RCTs; in nine studies, the primary RCT results were reported separately[28–36]. Most studies (Table 1) were conducted in the UK (70%, n = 7) [19–21, 23–26], one was conducted in the US [22] and two were conducted in The Netherlands [18, 27]. The sample sizes of the studies ranged from 100 to 640 participants, with a median of 133. Five studies randomised patients to more than two interventions, therefore sample size per arm was often relatively small [20, 23–26]. Only one study focused on children [19]. In the remaining studies, the mean participant age was between 37 and 49 years. There was wide variation in the CFS/ME case definition used to assess participant eligibility, and, in most trials recruiting from primary care, not all participants met the established CFS/ME case definitions. The baseline Chalder Fatigue Questionnaire (CFQ) score (reported in six studies) ranged from 23 [18] to 28 points [20]. Baseline EuroQol 5-Dimension (EQ5D) scores (reported in six studies) typically lay within the range of 0.42 [24] to 0.56 [37]; however, two studies were outliers, one reporting substantially lower scores in children [19] and the other reporting substantially higher scores [25].
Table 1.
Characteristics of the selected studies
| First author, year, country | Economic evaluation, (study type) | Interventions | Setting | Target population; sample size | Case definition | Baseline fatigue and utility score | Perspective; time horizon | Currency, price year | Cost categories | Outcomes measures | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chisholm (2001) [18], UK | CEA (RCT) | COUN vs. CBT | Primary care | Age 16–75 years and fatigue ≥ 3 months; n = 129 | 28% CDC criteria |
CFQ: 22.4–24.2 NR |
Societal; 6 months | GBP, NR | Health care costs, community-based services costs, intervention costs, costs of lost employment and informal care | CFQ | NR |
| Crawley (2018) [19], UK | CUA (RCT) | LP + SpMC vs. SpMC | Secondary care | Age 12–18 years with mild/moderate CFS/ME; n = 100 | NICE criteria |
CFQ: 25.0–25.1 EQ5D: 0.31–0.34 |
Health care and public sector; 12 months | GBP; 2013 | Health care costs, productivity loss of earnings, education service use (e.g. school counsellor), patient health-related travel, family costs, intervention costs | QALY (EQ5D-Y) | Charity |
| McCrone (2012) [20], UK | CUA (RCT) | APT vs. CBT vs. GET vs. SpMC | Secondary care | Age ≥ 18 years and ≥ 6 on the CFQ; n = 640 | Oxford criteria |
CFQ: 27.7–28.5 EQ5D: 0.48–0.54 |
Healthcare and societal; 12 months | GBP; NR | Health care costs, informal care costs, loss of productivity, intervention costs | QALY (EQ5D-3L) | Government |
| McCrone (2004) [21], UK | CEA (RCT) | GET vs. CBT | Primary care | Age 16–75 years and fatigue ≥ 3 months; n = 123 | 29% CDC criteria |
CFQ: 24.7–25.3 NR |
Societal; 8 months | GBP; 2001 | Health care costs, informal care costs, intervention costs | CFQ | Charity |
| Meng (2017) [22], US | CUA (RCT) | FSM vs. UC | Primary care | Age 18–65 years and ≥ 6 months of fatigue; n = 137 | NR |
FSS: 6.45–6.62 NR |
Societal; 12 months | USD; 2014 | Health care costs, health services utilisation, prescription medication, productivity losses, out-of-pocket expenses, intervention costs | QALY (SF-36) | Government |
| O'Dowd (2006) [23], UK | CCA (RCT) | CBT and GAS vs. EAS vs. SMC | Secondary care | Adults with a diagnosis of CFS/ME referred by a GP; n = 127 | CDC criteria |
CFQ: 23.9–25.0 HUI: 0.53–0.63 |
Healthcare; 12 months | GBP; 2002 | Health care costs, medication costs, resource costs, intervention costs | HUI | Government |
| Richardson (2013) [24], UK | CUA (RCT) | PR vs. SL vs. TAU | Primary care | Age ≥ 18 years and ≥ 4 on the Chalder fatigue score; n = 296 | Oxford criteria |
CFQ: 10.3–10.5 (bimodal score) EQ5D: 0.42–0.45 (approximately) |
Healthcare; 70 weeks | GBP; 2009 | Health care costs, intervention costs (descriptive analysis: informal care costs, loss of productivity, private expenditures) | QALY (EQ5D-3L) | Government |
| Sabes-Figuera (2012) [25], UK | CEA (RCT) | GET vs. COUN vs. BUC | Primary care | Age 18–75 years and fatigue ≥ 3 months; n = 163 | NR |
CFQ: 23.4–24.8 EQ5D: 0.64 |
Healthcare; 6 months | GBP; 2009 | Health care costs, social care costs, medication costs, intervention costs | CFQ | Charity |
| Severens (2004) [26], Netherlands | CUA, CEA (RCT) | CBT vs. NC vs. SG | Secondary care | Age 18–60 years and CIS fatigue score ≤ 36; n = 270 | CDC criteria (with one exception) |
CIS: 51.9–52.3 EQ5D: 0.49–0.53 |
Healthcare and societal; 14 months | EUR; 1998 | Health care costs, informal and formal home care support costs, productivity loss, intervention costs |
CIS QALYs (EQ5D-3L) |
Healthcare insurance board |
| Vos-Vromans (2017) [27], Netherlands | CEA, CUA (RCT) | CBT vs. MRT | Secondary care | Age 18–60 years and ≥ 40 on the CIS fatigue subscale; n = 109 | CDC criteria |
CIS: 51.1–51.5 EQ5D: 0.48–0.56 |
Societal; 52 weeks | EUR; 2011 | Health care costs, patient and family costs, costs from productivity losses, intervention costs |
CIS QALY (EQ5D-3L) |
Multiple |
APT adaptive pacing therapy, BUC usual care plus self-help booklet, CBT cognitive behavioural therapy, CCA cost-consequence analysis, CDC Centers for Disease Control and Prevention, CEA cost-effectiveness analysis, CFS chronic fatigue syndrome, CFQ Chalder Fatigue Questionnaire score, CIS Checklist Individual Strength fatigue subscale, COUN counselling, CUA cost-utility analysis, EAS, education and support group, EQ5D EuroQol 5 dimensions, EQ5D-3L EQ5D using three levels quality-of-life questionnaire, EQ5D-Y EQ5D for children and adolescents, EUR Euro, FSM fatigue self-management, FSS Fatigue Severity Scale, GAS graded activity scheduling, GBP British Pound Sterling, GET graded exercise therapy, GP general practitioner, HUI Health Utility Index, LP lightning process, ME myalgic encephalomyelitis, MRT multidisciplinary rehabilitation treatment, NC natural course, NICE National Institute for Health and Care Excellence, NR not reported, PR pragmatic rehabilitation, QALY quality-adjusted life-year, RCT randomised controlled trial, SF-36 Short-Form 36 General Health Questionnaire, SG guided support groups, SL supportive listening, SMC standard medical care, SpMC specialist medical care, TAU treatment as usual, UC usual care, USD US dollars
Five of the studies reported on interventions used in patients recruited in primary care settings [18, 21, 22, 24, 25]. The follow-up period varied from 6 to 17 months, with a median of 12 months. Only two studies had a time horizon > 12 months [24, 26]. Six studies presented an analysis from the societal perspective [18, 20–22, 26, 27].
The most common intervention evaluated was CBT [18, 21, 23, 26, 27]; however, the description of CBT was often brief and there was between-study variation in the personnel involved and the number and duration of sessions. Three trials evaluated GET [20, 21, 25], two evaluated counselling sessions [18, 25], and two evaluated support groups [23, 26]. A number of interventions including fatigue self-management, pragmatic rehabilitation, adaptive pacing therapy, supportive listening, and the lightning process were evaluated in a single trial. Many studies compared a specified intervention to some form of ‘usual care’, which was often not well-described and variously labelled (e.g. treatment as usual, standard medical care).
The studies were similar in terms of their design, the cost elements included, and choice of outcome measures. Six studies used the QALY as the measure of health benefit [19, 20, 22, 24, 26, 27]. Two studies used the CIS fatigue subscale [26, 27] and three studies used the CFQ as the measure of health benefit [18, 21, 25].
Consensus Health Economic Criteria Checklist (CHEC-List) Assessment
Overall, the quality of the economic evaluations was good (ESM 3); all studies appropriately addressed the majority of items on the CHEC-list. Nonetheless, for seven items (items 1, 2, 13, 15, 17, 18, 19), methodological quality was affected due to a minority of studies reporting insufficient detail. More specifically, two studies [21, 23] did not report a summary cost-effectiveness result (e.g. ICER, NMB), as specified in item 13.
Risk-of-Bias Assessment
Randomisation Process
Eight of the 10 studies were judged to have a low risk of bias from the randomisation process. In one study, two interventions (CBT and GET) were randomly allocated, but were also compared with a non-randomly allocated usual care group [21]. In another study, although allocation to the groups was random, the authors did not confirm whether the allocation sequence was concealed until participants were enrolled and assigned to the groups [22].
Deviations from Intended Interventions
Just one study stated they were able to blind approximately two thirds (n = 84/127) of participants to the intervention group they had been assigned [23]. The authors reported blinding was not possible for patients randomised to the third group (standard medical care). No other study stated that participants were blinded to the intervention group they were allocated to. Similarly, no studies reported that the staff delivering the intervention had been blinded to the intervention group. Despite this observation, three studies were judged to be low risk of bias as they measured protocol deviations and found a good level of adherence to the therapy protocol [20, 24, 27]. For the remaining seven studies, there was not enough information to judge whether the trial could have led to deviations from the intended intervention.
Missing Outcome Data
With the exception of the study by O’Dowd et al. [23], all studies were judged to be low risk of bias for missing outcome data. Although three studies reported having a sizeable proportion of missing outcome data, the risk of bias was judged to be low as authors partially checked to see if missingness was related to the characteristics of the completers and non-completers [19, 26] or the intervention group [25]. One study reported a high prevalence of missing data, particularly self-reported medication use, direct patient costs and productivity losses [23]. As a consequence, the study authors stated that the economic analysis was constructed on poor-quality data.
Measurement of the Outcome
All studies used participant-reported outcome measures, which raised some concerns around whether the participants’ assessment of the outcome may have been influenced by their knowledge of the intervention status.
Selection of the Reported Result
Just under half of the studies referred to a prespecified analysis plan and confirmed that the plan had been finalised before the outcome data had been analysed [19, 20, 24, 27]. For the remaining six studies, there was insufficient detail to assess the analysis intentions.
Results of Individual Studies and Synthesis of Results
Cognitive Behavioural Therapy (CBT) in Adults
Table 2 presents the results for studies evaluating CBT in adults. The largest trial [20] concluded that CBT was most likely to be cost-effective (likelihood = 59.5%) compared with GET (34.8%), specialist medical care (5.5%) and adaptive pacing (0.2%) at a £30,000 cost per QALY threshold. Two further CUAs found evidence that CBT was more cost-effective than multidisciplinary rehabilitation treatment [27] and guided support groups [26]; however, the evidence did not uniformly support CBT; one CEA reported that CBT was dominated by counselling when the outcome was reported as cost per unit of improved fatigue score [18].
Table 2.
Cognitive behavioural therapy in adults
| First author, year | Intervention | Mean costs (95% CI)/[SD] | Mean outcomes (95% CI)/[SD] | Incremental cost (95% CI)/[SD] | Incremental effect (95% CI)/[SD] | Incremental cost-effectiveness of CBT vs. comparator (unless otherwise stated) | CEAC | CEP |
|---|---|---|---|---|---|---|---|---|
| Chisholm, 2001 [18] | CBT (n = 64) | £4 (− £928 to £822)a | − 7.34 (5.5–9.1)a | NA | NA | NA | N | N |
| COUN (n = 65) | − £176 (− £793 to £410)a | − 8.25 (6.5–10.0)a | − £180 (− £1,103 to £968)a | 0.90 (− 1.80 to 3.60)a | Counselling dominates CBT | |||
| McCrone, 2012 [20] | CBT (n = 143) | £20,288 [£14,363] | 0.60 [0.21] | NA | NA | CBT: 59.5%c | Y | N |
| SpMC (n = 151) | £22,088 [£17,438] | 0.52 [0.25] | − £698b | 0.0492 | SMC: 5.5%c | |||
| APT (n = 146) | £20,935 [£15,531] | 0.53 [0.22] | NR | NR | APT: 0.2%c | |||
| GET (n = 140) | £23,317 [£17,284] | 0.57 [0.23] | NR | NR | GET: 34.8%c | |||
| McCrone, 2004 [21] | CBT (n = 52) | £1970 [£2895] | NR | NA | NA | NA | Y | N |
| GET (n = 50) | £1684 [£2584] | NR | £193 (− £458 to £946)d | 0.71 | NR | |||
| O’Dowd, 2006 [23] | CBT (n = 38) | £699.49 [£480.59]e | 0.047 [0.120]a | NA | NA | NA | N | N |
| EAS (n = 46) | £809.77 [£656.87]e | 0.075 [0.157]a | − £110.28 [£0.366] | − 0.028 [0.363] | NR | |||
| SMC (n = 43) | £451.57 [585.73]e | 0.021 [0.214]a | £247.93 [£0.032] | 0.026 [0.511] | NR | |||
| Severens, 2004 [26] | CBT (n = 37) | NR | 0.0737 QALYsa | NA | NA | NA | Y | Y |
| NC (n = 55) | NR | 0.0458 QALYsa | NR | NR | €21,375 per QALY | |||
| SG (n = 36) | NR | − 0.0018 QALYsa | NR | NR | CBT dominates SG | |||
| Vos-Vromans, 2017 [27] | CBT (n = 52) | €8846 | 0.60 | NA | NA | CBT: 95%c | Y | Y |
| MRT (n = 57) | €14,308 | 0.65 | €5389 (€2488– €8091) | 0.05 |
MRT: 5%c €118,074 per QALY (MRT vs. CBT) |
APT adaptive pacing therapy, CBT cognitive behavioural therapy, CI confidence interval, COUN counselling, EAS education and support group, GET graded exercise therapy, MRT multidisciplinary rehabilitation treatment, N no, NA not applicable, NC natural course, NR not reported, QALYs quality-adjusted life-years, SD standard deviation, SG guided support groups, SMC standard medical care, SpMC specialist medical care, Y yes, CEAC cost-effectiveness acceptability curve, CEP cost-effectiveness plane
aChange in effect/costs from baseline to follow-up
bNo CIs reported
cLikelihood of being the most cost-effective option from a societal perspective
dThis study reports incremental costs adjusted for baseline imbalances with 90% CIs
eHealthcare perspective only
Other Interventions in Adults
Table 3 presents the results for studies evaluating other interventions in adults. One CEA concluded that GET was more effective and cheaper than counselling and was more cost-effective than usual care plus self-help booklet if society is willing to pay more than £987 for a clinically significant improvement in the Chalder fatigue score [25]. A CUA reported that home-based fatigue self-management was cheaper and more effective than treatment as usual in primary care patients with severe chronic fatigue [22]. However, in the third CUA, treatment as usual was cheaper and more effective than both pragmatic rehabilitation and supportive listening in primary care patients [24].
Table 3.
Additional interventions for adults
| First author, year | Intervention | Mean costs (95% CI)/[SD] | Mean outcomes (95% CI)/[SD] | Incremental cost (95% CI)/[SD] | Incremental effect (95% CI)/[SD] | Incremental cost-effectiveness of intervention vs. TAU or BUC | CEAC | CEP |
|---|---|---|---|---|---|---|---|---|
| Meng, 2017 [22] | FSM (n = 89) | − $864a | NR | − $64 (− $206 to $77)a | 0.014 QALYs (− 0.008 to 0.036)a | FSM dominant | Y | Y |
| TAU (n = 48) | − $569a | NR | NA | NA | NA | |||
| Richardson, 2013 [24] | PR (n = 85) | NR | NR | £218 (− £474 to £911) | − 0.012 QALYs (− 0.088 to 0.065) | TAU dominates PR | Y | N |
| SL (n = 97) | NR | NR | £460 (− £250 to £1169) | − 0.042 QALYs (− 0.122 to 0.038) | TAU dominates SL | |||
| TAU (n = 92) | NR | NR | NA | NA | NA | |||
| Sabes-Figuera 2012, [25] | GET (n = 51) | £474b | 10.06 CFSa | £261 (£141 to £382) | 1.1 CFS (− 2.3 to 4.4)a | £987 per clinically significant improvement in CFS | Y | N |
| COUN (n = 58) | £651b | 8.62 CFSa | £423 (£288 to £559) | − 0.1 CFS (− 3.1 to 2.9)a | BUC dominates COU | |||
| BUC (n = 54) | £213b | 8.56 CFSa | NA | NA | NA |
BUC usual care plus self-help booklet, COUN counselling, CFS chronic fatigue syndrome, CI confidence interval, FSM fatigue self-management, GET graded exercise therapy, N no, NA not applicable, NR not reported, PR pragmatic rehabilitation, QALYs quality-adjusted life-years, SD standard deviation, SL supportive listening, TAU treatment as usual, Y yes, CEAC cost-effectiveness acceptability curve, CEP cost-effectiveness plane
aChange in effect/costs from baseline to follow-up
bHealthcare perspective only
Interventions for Young People
Table 4 presents the only economic evaluation of treatment in children. This study, a CUA [19] reported evidence that the lightning process (an intervention that trains individuals to understand how the brain and body interact) with specialist medical care was not only more costly but also more cost-effective than specialist medical care only. The incremental NMB statistic (at a willingness-to-pay threshold of £20,000 per QALY) was positive and the confidence intervals excluded zero.
Table 4.
Interventions for young people (12- to 18-year-olds)
| First author, year | Intervention | Mean costs (95% CI)/[SD] | Mean outcomes (95% CI)/[SD] | Incremental cost (95% CI)/[SD] | Incremental effect (95% CI)/[SD] | Incremental cost-effectiveness | CEAC | CEP |
|---|---|---|---|---|---|---|---|---|
| Crawley, 2018 [19] | LP and SpMC (n = 51) | £2002 [£67]a | 0.628 QALYs [0.021] | £390 (£189–£591) | 0.095 QALYs (0.030–0.160) | £1508 (£148–£2869) iNMB | Y | N |
| SpMC (n = 49) | £1612 [£84]a | 0.533 QALYs [0.025] | NA | NA | NA |
CI confidence interval, iNMB incremental net monetary benefit, N no, NA not applicable, LP lightning process, QALYs quality-adjusted life-year, SD standard deviation, SpMC specialist medical care, Y yes, CEAC cost-effectiveness acceptability curve, CEP cost-effectiveness plane
aHealth care and public sector perspective
Discussion
Principal Findings
We identified a limited patchwork of evidence on the cost-effectiveness of interventions for CFS/ME. CBT was the most commonly evaluated intervention. There was evidence from three trials to support CBT as a cost-effective treatment option for adults; however, the findings for CBT were not uniform, suggesting that (cost-) effectiveness may relate to, for example, the duration and frequency of sessions. There was some evidence from two trials supporting GET as a potentially cost-effective treatment option in adults. One small trial demonstrated the potential for home-based fatigue self-management to be more cost-effective than usual primary care among adults. A wide array of other interventions, including multidisciplinary rehabilitation, support groups, supportive listening, counselling, and adaptive pacing, have been evaluated but have not been demonstrated to be cost-effective. In adolescents, one small, single-centre trial found that the lightning process was probably a cost-effective addition to specialist medical care. Key weaknesses in the literature included small sample sizes and short duration of follow-up in most trials. There are large gaps in the literature, including on pharmacological interventions for symptom control and on all therapies in children. Although most economic evaluations were methodologically robust, heterogeneity in the inclusion of productivity losses, measurement of QALYs and reporting of incremental cost effectiveness hampered evidence synthesis across studies.
Strengths and Weaknesses of the Study
We believe this is the first systematic review of the economic consequences of interventions to treat CFS/ME. We used two large bibliographic databases, predefined published eligibility criteria and two researchers to screen and extract information from eligible studies to ensure consistent decisions that were independent of the characteristics and findings of the primary studies themselves. We used established tools to identify potential sources of bias in the RCT evidence and the methodological quality of the economic evaluations. Our search strategy was brief and was limited to peer-reviewed publications in the English language. It is possible that other relevant studies have been missed. We mitigated this risk by hand searching the reference lists of eligible studies and systematic reviews relevant to CFS/ME and consider it unlikely that any high-quality economic evaluations have been overlooked. However, it is known that trials with planned economic evaluations are more likely to report effectiveness data than the economic results [38] and therefore our findings might be affected by publication bias. Most studies reported findings from more than one perspective (e.g. societal or health system) or used more than one outcome measure in the economic evaluation (e.g. fatigue score or QALY). Where this was the case, we selected the broadest perspective and reported the outcome measure (i.e. the QALY) that allowed greatest comparability between trials. However, this approach sometimes simplified nuanced study findings; for example, Vos-Vromans et al. [37] reported discordant findings dependent on whether the QALY or the fatigue score was used in the economic evaluation. There were also underlying weaknesses in the primary studies. Due to the nature of the interventions and the objective to evaluate them pragmatically, participants and therapists could not be blind to the therapy randomly assigned. As key costs and outcomes were self-reported, there is a risk of both performance and detection bias [39].
Policy Implications
By design, the scope of our review is broad and included all therapeutic interventions delivered in primary and specialist care settings to patients with chronic fatigue of variously defined severity, duration and diagnostic criteria. This was feasible due to the small volume of literature in this area, but it also cautions against unthinking ‘pooling’ of data across studies, or assuming that findings from one trial can be simply generalised to other settings. For example, in one trial [23], CBT was delivered in eight group sessions, and, in the remainder, the number of planned individual CBT sessions varied from six 45-min sessions [21] to sixteen 60-min sessions [26, 27]. It is notable that the three studies that found CBT to be cost-effective [20, 26, 27] all had a higher number of individual CBT sessions. In these three studies, the cost of CBT itself was in the approximate range of £1000–£2000 per patient, representing a substantial investment. This investment may well represent very good value for money if the initial improvements in fatigue and health-related quality of life are sustained in the long-term, particularly if this is accompanied by lower health service use, informal care needs and higher return to work. Long-term follow-up from the PACE trial [40] demonstrates that the advantage of CBT and GET over the comparator groups on fatigue and physical function narrowed over time, highlighting the need for longer-term economic analyses.
Access to specialist CFS/ME care is already limited in many settings and the type of care provided is highly variable [41]. In order to increase access, novel approaches are needed to provide stepped access to low- or high-intensity CBT and GET provided by appropriately trained therapists in primary care settings [42] and/or using remotely delivered (e.g. internet-based) therapy methods [43]. These new services are being established, partly in response to the coronavirus disease 2019 (COVID-19) pandemic, but they will require careful evaluation to determine whether efficacy is maintained and whether efficiency increased [44, 45]. We note that several economic evaluations of such services in adults [46] and children [47, 48] are currently underway. Good economic evidence to support treatment options in children is particularly important as current therapy is largely based on the assumption that findings from adults can be generalised to children.
Methodological Implications
Our review highlighted the importance of informal care and productivity losses in the evaluation of CFS/ME interventions. These costs frequently dominated the cost estimates. Despite this, not every study quantified productivity or informal care and there was little consistency in how they were measured. There was further methodological uncertainty on the most appropriate way to value productivity losses, with some studies using both the human capital and friction-based methods [49]. Friction-based methods tend to result in smaller productivity loss estimates, but it is unclear how pivotal this would be as most studies did not observe large between-arm differences in productivity. The recent development of new questionnaires to measure informal care costs [50] and productivity losses [51] could increase standardisation within economic evaluations and aid policy makers wishing to synthesize evidence across studies.
The primary fatigue outcome measure used was strongly correlated with the country of origin, making international comparisons problematic. UK studies used the CFQ, studies from The Netherlands used the CIS fatigue subscale, and the US study used the Fatigue Severity Scale. Those studies that did not estimate QALYs typically struggled to translate their results into clear recommendations for policy makers. The EQ5D baseline scores reported in the studies reviewed were substantially lower than working aged population norms in high-income countries, which typically exceed 0.80 [52]. This demonstrates the huge impact of CFS/ME on health-related quality of life and great potential for successful therapies to be cost-effective.
In two studies [19, 20] identified by our review, significant treatment effects on fatigue scores were reflected in differences in EQ5D-derived utility scores and QALYs. This suggests that the QALY metric can be sensitive in detecting clinically important improvements in CFS/ME. However, in a third study, Vos-Vromans et al. [27] report that significant improvements on the CIS fatigue score did not translate into any difference in QALYs. The more recent development of the EQ5D-5L (5 levels) [53] should in theory increase responsiveness to small but important changes in health-related quality of life. However, some doubts remain about the validity of the EQ5D in CFS/ME [54] and, more generally, in all health conditions that fluctuate frequently over time [55].
We did not identify any economic evaluations that used a decision analysis model to compare treatments or extrapolate costs and outcomes over a longer time horizon. This possibly reflects the difficulty of meta-analyzing the findings of the relatively small trials that have compared a wide range of complex interventions in disparate patient populations. It is also due to the lack of longitudinal data on the costs and outcomes of CFS/ME that could be used to estimate trajectories in the long-term.
Future Research
Further research is needed to establish the cost-effectiveness of therapies for CFS/ME, including larger studies to assess whether the potential cost effectiveness of fatigue self-management in adults and the lightning process in children can be replicated. Evidence-based services that provide less intensive or remotely delivered therapy will also be needed to increase access to therapy in the future. Better epidemiological data on cost and outcome trajectories in CFS/ME are essential for developing models to predict the long-term cost effectiveness of interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Derek Pheby, Visiting Professor of Epidemiology (retired) at Buckinghamshire New University, High Wycombe, UK, who provided comments on the final draft of the manuscript.
Declarations
Funding
This research received no specific funding from any funding agency in the public, commercial or not-for-profit sectors. Dominic Trépel and Derek Pheby are members of UK EUROMENE, a network of researchers working on ME/CFS. The network received funding from the European Union to support network activities administered by the COST Association, Brussels, Belgium.
Conflict of interest
William Hollingworth and Esther Crawley were co-authors on one of the studies included in the narrative synthesis. Madeleine Cochrane, Eileen Mitchell, William Hollingworth, Esther Crawley, and Dominic Trépel have no other conflicts of interest or competing interests.
Ethics approval
Ethics approval was not required for this study as the data were not collected from human subjects. The manuscript is a systematic review and therefore draws on secondary data sources.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material (data transparency)
Analytical data are available in the ESM.
Code availability (software application or custom code)
Not applicable.
Author’s contribution
Joint first co-authors, Madeleine Cochrane and Eileen Mitchell, carried out the search, screening, data extraction and critical appraisal procedures. Conceptualisation and design of the review was carried out by William Hollingworth and Dominic Trépel. All authors contributed to the narrative synthesis and preparation of the article for publication.
Footnotes
M. Cochrane and E. Mitchell are co-first authors on this work.
References
- 1.Institute of Medicine . Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.Strand EB, Nacul L, Mengshoel AM, Helland IB, Grabowski P, Krumina A, et al. Myalgic encephalomyelitis/chronic fatigue Syndrome (ME/CFS): investigating care practices pointed out to disparities in diagnosis and treatment across European Union. PLoS One. 2019;14(12):e0225995. doi: 10.1371/journal.pone.0225995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) J Transl Med. 2020;18(1):100. doi: 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic syndrome in UK primary care. Psychol Med. 2003;33:253–261. doi: 10.1017/S0033291702006980. [DOI] [PubMed] [Google Scholar]
- 5.Pheby DFH, Araja D, Berkis U, Brenna E, Cullinan J, de Korwin J-D, et al. The Development of a Consistent Europe-Wide Approach to Investigating the Economic Impact of Myalgic Encephalomyelitis (ME/CFS): a report from the European Network on ME/CFS (EUROMENE) Healthcare. 2020;8:88. doi: 10.3390/healthcare8020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin SM, Crawley E, May MT, Sterne JA, Hollingworth W. The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res. 2011;11:217. doi: 10.1186/1472-6963-11-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Missen A, Hollingworth W, Eaton N, Crawley E. The financial and psychological impacts on mothers of children with chronic fatigue syndrome (CFS/ME) Child Care Health Dev. 2012;38(4):505–512. doi: 10.1111/j.1365-2214.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 8.Vercoulen JHMM, Swanink CMA, Fennis JFM, Galama JMD, van der Meer JWM, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–392. doi: 10.1016/0022-3999(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm D, Healey A, Knapp M. QALYs and mental health care. Soc Psychiatry Psychiatr Epidemiol. 1997;32(2):68–75. doi: 10.1007/BF00788923. [DOI] [PubMed] [Google Scholar]
- 10.Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. 2012;21:167–176. doi: 10.1007/s11136-011-9927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Marrero J, Sáez-Francàs N, Santillo D, Alegre J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol. 2017;174(5):345–369. doi: 10.1111/bph.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev. 2008;2008(3):CD001027. doi: 10.1002/14651858.CD001027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2019;10(10):CD003200. doi: 10.1002/14651858.CD003200.pub8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NICE. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) in adults and children. NICE. 2007. https://www.nice.org.uk/guidance/cg53. Accessed 10 Jul 2020.
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21(2):240–245. doi: 10.1017/S0266462305050324. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Chisholm D, Godfrey E, Ridsdale L, Chalder T, King M, Seed P, et al. Chronic fatigue in general practice: economic evaluation of counselling versus cognitive behaviour therapy. Br J Gen Pract. 2001;51(462):15. [PMC free article] [PubMed] [Google Scholar]
- 19.Crawley EM, Gaunt DM, Garfield K, Hollingworth W, Sterne JAC, Beasant L, et al. Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomised controlled trial. Arch Dis Child. 2018;103(2):155–164. doi: 10.1136/archdischild-2017-313375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCrone P, Sharpe M, Chalder T, Knapp M, Johnson AL, Goldsmith KA, et al. Adaptive pacing, cognitive behaviour therapy, graded exercise, and specialist medical care for chronic fatigue syndrome: a cost-effectiveness analysis. PLoS One. 2012;7(8):e40808. doi: 10.1371/journal.pone.0040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCrone P, Ridsdale L, Darbishire L, Seed P. Cost-effectiveness of cognitive behavioural therapy, graded exercise and usual care for patients with chronic fatigue in primary care. Psychol Med. 2004;34(6):991–999. doi: 10.1017/s0033291704001928. [DOI] [PubMed] [Google Scholar]
- 22.Meng H, Friedberg F. Cost-utility of home-based fatigue self-management versus usual care for the treatment of chronic fatigue syndrome. Fatigue Biomed Health Behav. 2017;5(4):202–214. doi: 10.1080/21641846.2017.1343171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A. Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme. Health Technol Assess. 2006;10(37):iii–121. doi: 10.3310/hta10370. [DOI] [PubMed] [Google Scholar]
- 24.Richardson G, Epstein D, Chew-Graham C, Dowrick C, Bentall RP, Morriss RK, et al. Cost-effectiveness of supported self-management for CFS/ME patients in primary care. BMC Fam Pract. 2013;14:12. doi: 10.1186/1471-2296-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabes-Figuera R, McCrone P, Hurley M, King M, Donaldson AN, Ridsdale L. Cost-effectiveness of counselling, graded-exercise and usual care for chronic fatigue: evidence from a randomised trial in primary care. BMC Health Serv Res. 2012;12:264. doi: 10.1186/1472-6963-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severens JL, Prins JB, van der Wilt GJ, van der Meer JWM, Bleijenberg G. Cost-effectiveness of cognitive behaviour therapy for patients with chronic fatigue syndrome. QJM. 2004;97(3):153–161. doi: 10.1093/qjmed/hch029. [DOI] [PubMed] [Google Scholar]
- 27.Vos-Vromans D, Evers S, Huijnen I, Koke A, Hitters M, Rijnders N, et al. Economic evaluation of multidisciplinary rehabilitation treatment versus cognitive behavioural therapy for patients with chronic fatigue syndrome: a randomized controlled trial. PLoS One. 2017;12(6):e0177260. doi: 10.1371/journal.pone.0177260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawley E, Mills N, Beasant L, Johnson D, Collin SM, Deans Z, et al. The feasibility and acceptability of conducting a trial of specialist medical care and the Lightning Process in children with chronic fatigue syndrome: feasibility randomized controlled trial (SMILE study) Trials. 2013;14:415. doi: 10.1186/1745-6215-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedberg F, Adamowicz J, Caikauskaite I, Seva V, Napoli A. Efficacy of two delivery modes of behavioral self-management in severe chronic fatigue syndrome. Fatigue Biomed Health Behav. 2016;4(3):158–174. doi: 10.1080/21641846.2016.1205876. [DOI] [Google Scholar]
- 30.Prins JB, Bleijenberg G, Bazelmans E, Elving LD, de Boo TM, Severens JL, et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet. 2001;357(9259):841–847. doi: 10.1016/s0140-6736(00)04198-2. [DOI] [PubMed] [Google Scholar]
- 31.Ridsdale L, Godfrey E, Chalder T, Seed P, King M, Wallace P, et al. Chronic fatigue in general practice: is counselling as good as cognitive behaviour therapy? A UK randomised trial. Br J Gen Pract. 2001;51(462):19–24. [PMC free article] [PubMed] [Google Scholar]
- 32.Ridsdale L, Darbishire L, Seed PT. Is graded exercise better than cognitive behaviour therapy for fatigue? A UK randomized trial in primary care. Psychol Med. 2004;34(1):37–49. doi: 10.1017/S0033291703001247. [DOI] [PubMed] [Google Scholar]
- 33.Ridsdale L, Hurley M, King M, McCrone P, Donaldson N. The effect of counselling, graded exercise and usual care for people with chronic fatigue in primary care: a randomized trial. Psychol Med. 2012;42(10):2217–2224. doi: 10.1017/S0033291712000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos-Vromans DC, Smeets RJ, Huijnen IP, Köke AJ, Hitters WM, Rijnders LJ, et al. Multidisciplinary rehabilitation treatment versus cognitive behavioural therapy for patients with chronic fatigue syndrome: a randomized controlled trial. J Intern Med. 2016;279(3):268–282. doi: 10.1111/joim.12402. [DOI] [PubMed] [Google Scholar]
- 35.Wearden AJ, Dowrick C, Chew-Graham C, Bentall RP, Morriss RK, Peters S, et al. Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: randomised controlled trial. BMJ. 2010;340:c1777. doi: 10.1136/bmj.c1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vos-Vromans DCWM, Smeets RJEM, Huijnen IPJ, Koke AJA, Hitters WMGC, Rijnders LJM, et al. Multidisciplinary rehabilitation treatment versus cognitive behavioural therapy for patients with chronic fatigue syndrome: a randomized controlled trial. J Intern Med. 2016;279(3):268–282. doi: 10.1111/joim.12402. [DOI] [PubMed] [Google Scholar]
- 38.Thorn JC, Noble SM, Hollingworth W. Timely and complete publication of economic evaluations alongside randomized controlled trials. Pharmacoeconomics. 2013;31(1):77–85. doi: 10.1007/s40273-012-0004-7. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). Cochrane. 2011. https://handbook-5-1.cochrane.org/front_page.htm. Accessed 26 Jun 2020.
- 40.Sharpe M, Goldsmith KA, Johnson AL, Chalder T, Walker J, White PD. Rehabilitative treatments for chronic fatigue syndrome: long-term follow-up from the PACE trial. Lancet Psychiatry. 2015;2(12):1067–1074. doi: 10.1016/S2215-0366(15)00317-X. [DOI] [PubMed] [Google Scholar]
- 41.Collin SM, Crawley E. Specialist treatment of chronic fatigue syndrome/ME: a cohort study among adult patients in England. BMC Health Serv Res. 2017;17(1):488. doi: 10.1186/s12913-017-2437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rimes KA, Wingrove J, Moss-Morris R, Chalder T. Competences required for the delivery of high and low-intensity cognitive behavioural interventions for chronic fatigue, chronic fatigue syndrome/ME and irritable bowel syndrome. Behav Cogn Psychother. 2014;42(6):760–764. doi: 10.1017/S1352465814000290. [DOI] [PubMed] [Google Scholar]
- 43.Nijhof SL, Bleijenberg G, Uiterwaal CS, Kimpen JL, van de Putte EM. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet. 2012;379(9824):1412–1418. doi: 10.1016/s0140-6736(12)60025-7. [DOI] [PubMed] [Google Scholar]
- 44.Haig-Ferguson A, Loades M, Whittle C, Read R, Higson-Sweeney N, Beasant L, et al. "It's not one size fits all"; the use of videoconferencing for delivering therapy in a Specialist Paediatric Chronic Fatigue Service. Internet Interv. 2019;15:43–51. doi: 10.1016/j.invent.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson E, Parslow R, Hollingworth W, Mills N, Beasant L, Gaunt D, et al. Recruiting adolescents with chronic fatigue syndrome/myalgic encephalomyelitis to internet-delivered therapy: internal pilot within a randomized controlled trial. J Med Internet Res. 2020;22(8):e17768. doi: 10.2196/17768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark LV, McCrone P, Ridge D, Cheshire A, Vergara-Williamson M, Pesola F, et al. Graded exercise therapy guided self-help trial for patients with chronic fatigue syndrome (GETSET): protocol for a randomized controlled trial and interview study. JMIR Res Protoc. 2016;5(2):e70. doi: 10.2196/resprot.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baos S, Brigden A, Anderson E, Hollingworth W, Price S, Mills N, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (fatigue in teenagers on the interNET in the NHS) compared to activity management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): protocol for a randomised controlled trial. Trials. 2018;19(1):136. doi: 10.1186/s13063-018-2500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brigden A, Beasant L, Hollingworth W, Metcalfe C, Gaunt D, Mills N, et al. Managed activity graded exercise iN teenagers and pre-adolescents (MAGENTA) feasibility randomised controlled trial: study protocol. BMJ Open. 2016;6(7):e011255. doi: 10.1136/bmjopen-2016-011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: a review. Appl Health Econ Health Policy. 2018;16(6):765–778. doi: 10.1007/s40258-018-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landfeldt E, Zethraeus N, Lindgren P. Standardized questionnaire for the measurement, valuation, and estimation of costs of informal care based on the opportunity cost and proxy good method. Appl Health Econ Health Policy. 2019;17(1):15–24. doi: 10.1007/s40258-018-0418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van RL. The iMTA Productivity Cost Questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18(6):753–758. doi: 10.1016/j.jval.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20(2):205–216. doi: 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality Life. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haywood KL, Staniszewska S, Chapman S. Quality and acceptability of patient-reported outcome measures used in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. Qual Life Res. 2012;21(1):35–52. doi: 10.1007/s11136-011-9921-8. [DOI] [PubMed] [Google Scholar]
- 55.Sanghera S, Coast J. Measuring quality-adjusted life-years when health fluctuates. Value Health. 2020;23(3):343–350. doi: 10.1016/j.jval.2019.09.2753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.