Abstract
Objective
Circular RNAs (circRNAs) are non-coding RNAs with high cancer-specific expression and the potential for regulating tumorigenesis. CircRNA_100395 is expressed at low levels in many cancers and is involved in the regulation of tumor cell proliferation and metastasis. However, its expression and function in prostate cancer remain unclear.
Methods
Endogenous expression levels of circRNA_100395 and microRNA-1228 (miR-1228) in prostate cancer tissue samples and cell lines were detected by quantitative reverse transcription-polymerase chain reaction. Cell proliferation, invasion, and migration, cell cycle distribution, and epithelial–mesenchymal transition (EMT) were analyzed in circRNA_100395-overexpressing prostate cancer cells by Cell Counting Kit-8, flow cytometry, Transwell assay, and western blotting, respectively.
Results
CircRNA_100395 expression was downregulated in cancerous prostate tissues relative to adjacent normal tissues. CircRNA_100395 expression was negatively correlated with tumor size, Gleason score, tumor stage, and lymph node metastasis. Moreover, circRNA_100395 overexpression inhibited cell proliferation, altered cell cycle distribution, reduced cell migration and invasion abilities, and suppressed EMT in prostate cancer cells. Moreover, miR-1228 was a direct downstream target of circRNA_100395, and the anti-tumor ability of circRNA_100395 was significantly reversed by miR-1228.
Conclusion
This study identified circRNA_100395 as an anti-tumor circRNA and a potential therapeutic target for prostate cancer.
Keywords: CircularRNA_100395, microRNA-1228, epithelial–mesenchymal transition, metastasis, proliferation, prostate cancer
Introduction
Prostate cancer is one of the most common malignant tumors worldwide. Despite the development of traditional prostatectomy and androgen deprivation therapy, the median survival of prostate cancer patients is only 2 to 3 years,1,2 mainly as a result of uncontrolled proliferation and metastasis of prostate cancer cells.3,4 There is thus a crucial need to identify novel therapeutic targets to suppress cell proliferation and metastasis in prostate cancer.
Circular RNAs (circRNAs) are non-coding RNAs that contain specific circular structures with covalent bonds.5 Their circular structure prevents circRNAs from being easily eliminated by RNA exonucleases, thereby increasing their stability in the cytoplasm, and multiple studies have accordingly indicated that circRNAs are enriched in the cytoplasm of eukaryotic cells.6,7 Studies have also explored the functions of circRNAs, including the splicing and transcriptional regulation of specific RNAs and interactions with RNA-binding proteins.8,9 Moreover, emerging studies have revealed that circRNAs can act as microRNA (miRNA) sponges, interfere with the function of miRNAs, and promote tumor development.10 For example, Wang et al.11 found that the circRNA ITCH inhibited prostate cancer progression by increasing HOXB13 expression via spongy miR-17-5p, and Ou et al.12 reported that the circRNA AMOTL1 regulated cell growth in prostate cancer. Overall, these studies indicate that circRNAs play a crucial role in cell survival and may serve as novel therapeutic targets for cancer. CircRNA_100395 is located at chr1: 173726114–17374498.13 Emerging evidence has shown that circRNA_100395 is downregulated in tumors, and its expression pattern was strongly associated with lymph node metastasis, tumor size, and overall survival in patients with lung,13 liver,14 and ovarian cancers.15 However, the relationship between circRNA_100395 and prostate cancer is still unclear.
In this study, we detected the expression of circRNA_100395 in prostate cancer tissues and analyzed its relationship with clinical features in patients with prostate cancer. We also examined the function of circRNA_100395 by inducing its expression in prostate cancer cells, and identified its impact on cell proliferation, metastasis, and epithelial–mesenchymal transition (EMT). In addition, we predicted miRNA (miR)-1228 as a potential downstream target of circRNA_100395, thus validating the molecular mechanism and biological function of circRNA_100395.
Materials and methods
Tissue samples
This research was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Shenzhen Nanshan District Shekou People’s Hospital. Signed informed consent was obtained from all patients or their family members before collecting their tissue samples. Prostate cancer and normal specimens were obtained from patients with prostate cancer who underwent surgical resection or biopsy at Shenzhen Nanshan District Shekou People’s Hospital and Nanfang Hospital from 2012 to 2019. Cancerous prostate tissues were verified and distinguished from normal tissues by three experienced pathologists. After excision or biopsy, the tissues were quickly frozen and stored at −80°C.
Cell culture
The prostate cancer cell lines PC3, DU145, VCaP, 22RV1, C4-2, and LNCaP, and the normal prostate cell line, RWPE, were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 (TransGen Biotech, Beijing, China) supplemented with 10% fetal bovine serum (TransGen Biotech), in Dulbecco’s modified Eagle’s medium (DMEM, TransGen Biotech), or in keratinocyte serum-free medium containing 5 ng/mL epidermal growth factor and 25 mg/mL bovine pituitary extract (K-SFM, Gibco, Waltham, MA, USA) in an incubator containing 5% CO2 at 37°C.
RNA interference
PC3 and DU145 cells were transfected with circRNA_100395 cDNA plasmids (pcDNA3.1; developed by GenePharma, Suzhou, China) and miR-1228 mimic (OE-circRNA_100395) or the relative negative control sequence (miR-1228 NC) using Lipofectamine 3000 transfection reagent (Thermo Fisher, Waltham, MA, USA).
Quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
RNA was isolated from frozen tissues and cell lines using TRIzol reagent (Takara, Dalian, China), according to the manufacturer’s protocol. A total of 500 ng of RNA was then reverse-transcribed into cDNA using Prime Script RT Master Mix (Takara), and the cDNA was amplified with a SYBR-Green PCR kit (Roche, Basel, Switzerland). The expression levels of circRNA_100395 and miR-1228 were analyzed using the 2−ΔΔCt method. The complete sequences of the primers are shown in Table 1.
Table 1.
Sequences of oligomers and primers used in the present research
| Name | Sequence (5′–3′) |
|---|---|
| Circ_100395-forward | AGT GAT GTG GCC CCT ACA AG |
| Circ_100395-reverse | CCA CTG GAG ACC ACT GGT TG |
| miR-1228-forward | AGT GGG CGG GGG CAG G |
| miR-1228-reverse | AGT GCA GGG TCC GAG GTA TT |
| β-actin-forward | TCC GCA AAG ACC TGT ACG A |
| β-actin-reverse | GTA CTT GCG CTC AGG AGG AG |
Cell proliferation assay
CircRNA_100395-overexpressing PC3 and DU145 cells were seeded into 96-well plates. Diluted Cell Counting Kit-8 solution (CCK-8; Sigma-Aldrich, St Louis, MO, USA) was then added to each well after 1, 2, 3, and 4 days of incubation. Optical density values were then measured using a microplate reader (Bio-Rad, CA, USA) at 450 nm.
Flow cytometry
PC3 and DU145 cells were collected after trypsinization and stained using a propidium iodide cell cycle kit (TransGen) according to the manufacturer’s instructions. The cells were analyzed with a FACS Canto II flow cytometer (BD Biosciences, San Jose, CA, USA).
Transwell assays
The metastatic capacities of PC3 and DU145 cells were analyzed using Transwell chambers (0.8 μm; Corning, NY, USA) with or without Matrigel coating. Treated cells were seeded into the top chamber and 500 μL of RPMI 1640 containing 15% fetal bovine serum was added to the bottom chamber. After removing the non-invading and non-migrating cells with cotton-tipped swabs, the remaining cells were fixed and stained with 0.1% crystal violet. Invaded and migrated cells were imaged and counted using a microscope (Bio-Rad).
Western blotting
Proteins were isolated from PC3 and DU145 cells using radioimmunoprecipitation assay lysis buffer (Thermo Fisher) and quantified using a bicinchoninic acid protein assay kit (BioVision, San Francisco, CA, USA). Proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride (Thermo Fisher) membranes. The membranes were incubated in 5% milk followed by incubation with primary antibodies against EMT-related proteins overnight at 4°C, using an Epithelial-Mesenchymal Transition Antibody Sampler Kit (catalog no. 9782; CST, Danvers, MA, USA) at a dilution of 1:1000. The membranes were subsequently incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (BD Biosciences) diluted in 5% bovine serum albumin at 1:5000 and incubated at room temperature for 1 hour. Protein bands were evaluated using SynGene systems with GeneSnap software (Syngene, Frederick, MD, USA).
Dual-luciferase reporter assay
The luciferase reporter vectors were constructed by Miaolingbio (Guangdong, China) and confirmed by sequencing. PC3 cells were incubated in 96-well plates and transfected with pmirGLO-circRNA_100395-wild-type (WT) or pmirGLO-circRNA_100395-mutant (MUT) plasmids and miR-1228 mimics or miR-1228 NC sequentially. Three replicates were set up for each sample. DNA and transfection reagents were prepared at a ratio of firefly:Renilla:transfection reagent of 0.1 μg: 0.01 μg: 0.25 μL. The final RNA concentration was 100 nM for miRNA and transfection reagent (0.25 μL/well). Relative luciferase activity was detected after 48 hours by dual-luciferase reporter assay (Promega, Madison, WI, USA) in WT and MUT cells, with a mutated circRNA-binding site. Renilla luciferase was used as a reference. The degree of activation of the target reporter gene was compared based on the ratio of firefly:Renilla luciferase.
Statistical analyses
All data are shown as mean ± standard deviation. Data were analyzed using GraphPad Prism v7.0 (GraphPad Software Inc., La Jolla, CA, USA) and IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA). All experiments were performed at least three times. P < 0.05 indicated a statistically significant difference.
Results
Patients
Prostate cancer and normal specimens were obtained from 73 patients with prostate cancer (Table 2)
Table 2.
Clinicopathological variables of patients used for detection of circRNA100395 expression in prostate cancer
| Clinicopathological variable | n |
|---|---|
| Age | |
| ≤65 years | 32 |
| >65 years | 41 |
| PSA | |
| Low (0–10 ng/mL) | 12 |
| High (>10 ng/mL) | 61 |
| Gleason score | |
| ≤7 | 43 |
| >7 | 30 |
| Tumor size, cm | |
| ≤1 cm | 40 |
| >1 cm | 33 |
| Lymph-node metastasis | |
| Yes | 34 |
| No | 39 |
| Tumor stage | |
| T1+T2 | 43 |
| T3+T4 | 30 |
| Total | 73 |
PSA, prostate-specific antigen.
Associations of clinicopathological features with circRNA _100395 expression
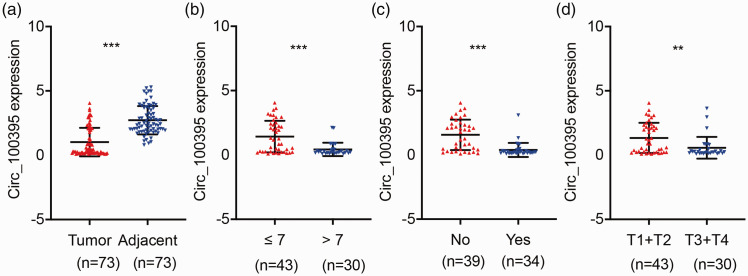
The qPCR results indicated that circRNA_100395 was significantly downregulated in all 73 prostate cancer samples (P < 0.001; Figure 1a). Lower expression of circRNA_100395 was significantly associated with a higher Gleason score (P < 0.001; Figure 1b), lymph node metastasis (P < 0.001; Figure 1c), and advanced tumor stage (P < 0.01; Figure 1d). CircRNA_100395 was reduced in prostate tumor tissues, and may thus be an inhibitory factor in cancer progression.
Figure 1.
Expression of circRNA_100395 in prostate cancer and correlations with clinicopathological parameters. (a) CircRNA_100395 was significantly downregulated in prostate tumor tissues relative to adjacent normal tissues, detected by quantitative reverse transcription-polymerase chain reaction (n = 73). Relative circRNA_100395 expression was lower in patients with (b) higher Gleason score and (c) lymph-node metastasis. (d) Relative circRNA_100395 expression was lower in patients with advanced tumor stage. **P < 0.01, ***P < 0.001.
Effects of circRNA_100395 on cell proliferation and cell cycle distribution in prostate cancer
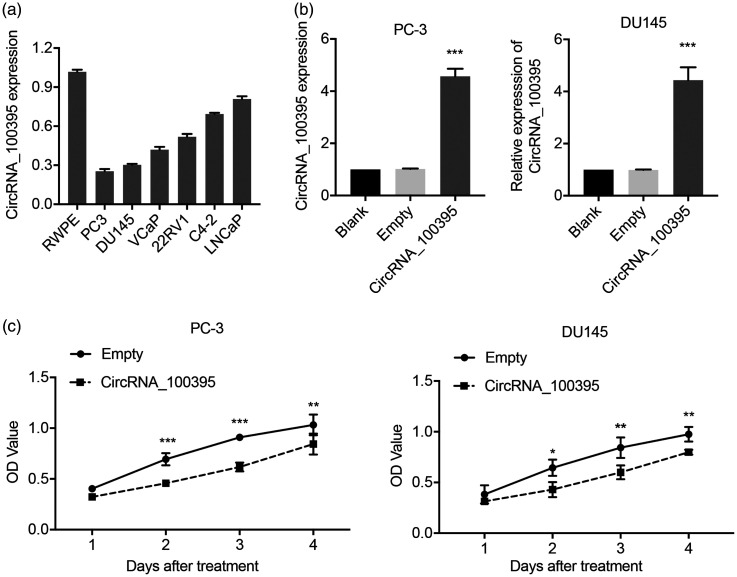
We investigated the biological role of circRNA_100395 in prostate cancer by examining its expression levels in six prostate cancer cell lines (PC3, DU145, LNCaP, C4-2, 22RV1, and VCaP) and in the normal prostate cell line, RWPE. CircRNA_100395 expression levels were downregulated in all prostate cancer cells compared with RWPE cells, as demonstrated by RT-qPCR (Figure 2a). PC3 and DU145 cells exhibited the lowest circRNA_100395 expression levels among the seven tested cell lines and were therefore selected for further cellular experiments. We established circRNA_100395-overexpressing PC3 and DU145 cells via plasmid transfection. CircRNA_100395 expression levels were significantly upregulated by approximately five- and four-fold in PC3 and DU145 cells (OE-circRNA_100395 groups), respectively, compared with non-treated cells (blank) (both P < 0.001), whereas no significant change was observed in the negative control (OE-Control), as determined by RT-qPCR (Figure 2b).
Figure 2.
Overexpression of circRNA_100395 inhibited proliferation of prostate cancer cells. (a) Expression levels of circRNA_100395 in six prostate cancer cell lines (PC3, DU145, LNCaP, C4-2, 22RV1, and VCaP), and the immortalized epithelial cell line (RWPE), detected by quantitative reverse transcription-polymerase chain reaction. (b) Successful construction of circRNA_100395-overexpressing prostate cancer cell lines (OE-CircRNA_100395 PC3 and DU145 cells). (c) Cell proliferation determined by CCK-8 assays in PC3 and DU145 cells after upregulation of circRNA_100395. *P < 0.05, **P < 0.01, ***P < 0.001. OD, optical density
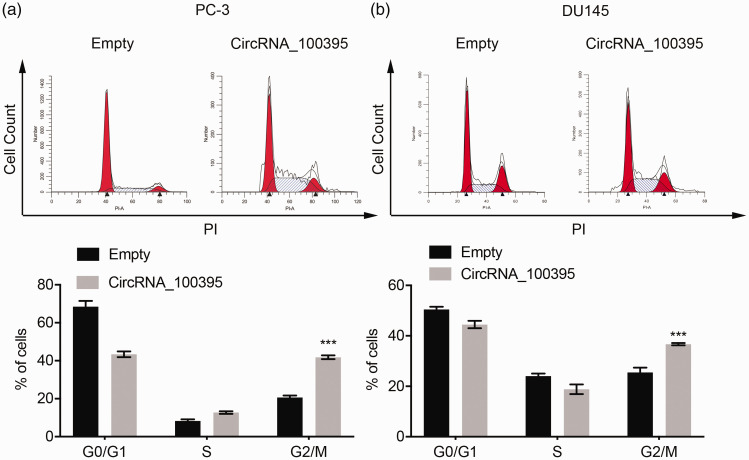
Because circRNA_100395 was closely related to tumor size and prostate cancer stage (Figure 1), we assumed that it may be involved in prostate cancer cell growth. CCK-8 assay revealed that overexpression of circRNA_100395 significantly suppressed the proliferation of PC3 and DU145 cells compared with control cells, based on optical density values (Figure 2c). Moreover, flow cytometric analysis showed that circRNA_100395 overexpression was associated with significantly more PC3 and DU145 cells arrested in the G2/M phase compared with the OE-control group (both P < 0.001; Figure 3a and 3b). These findings indicated that circRNA_100395 potentially exerted anti-growth effects in prostate cancer cell lines.
Figure 3.
Overexpression of circRNA_100395 regulated cycle distribution in prostate cancer cells. Flow cytometry images (left) and static cell cycle distribution (right) in (a) PC3 cells and (b) DU145 cells after upregulation of circRNA_100395. ***P < 0.001. PI, propidium iodide.
Effect of circRNA_100395 on metastatic capacity of prostate cancer cells
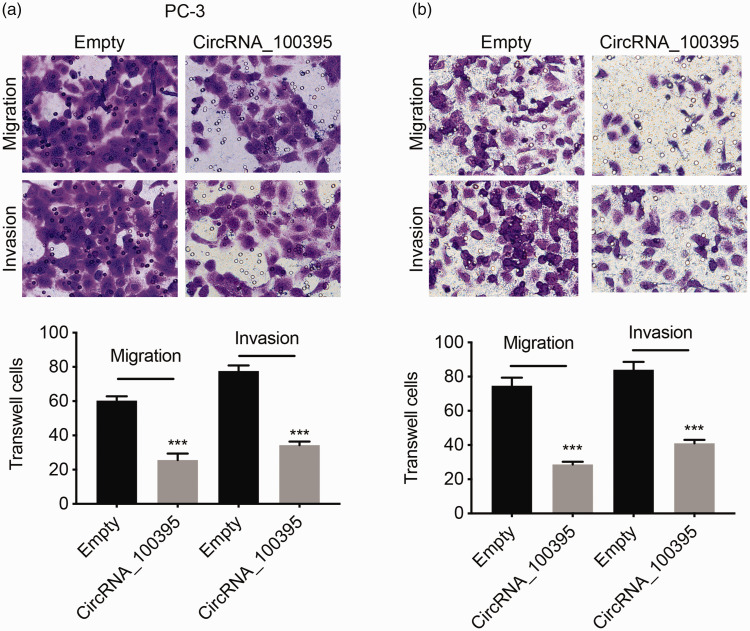
Evaluation of the correlations between circRNA_100395 expression and clinicopathological parameters in patients revealed that circRNA_100395 was significantly associated with lymph node metastasis. We therefore presumed that circRNA_100395 could influence prostate cancer cell metastasis, as a major aspect of tumor progression. CircRNA_100395 overexpression significantly inhibited the migration and invasion abilities of PC3 and DU145 cells in Transwell assays (all P < 0.001; Figure 4a and 4b). These results indicated that circRNA_100395 inhibited prostate cancer cell metastasis.
Figure 4.
Overexpression of circRNA_100395 suppressed migration and invasion of prostate cancer cells. Migration and invasion images (upper) and number of Transwell cells (lower) for (a) PC3 and (b) DU145 cells after upregulation of circRNA_100395. ***P < 0.001
Effect of circRNA_100395 on EMT progression in prostate cancer
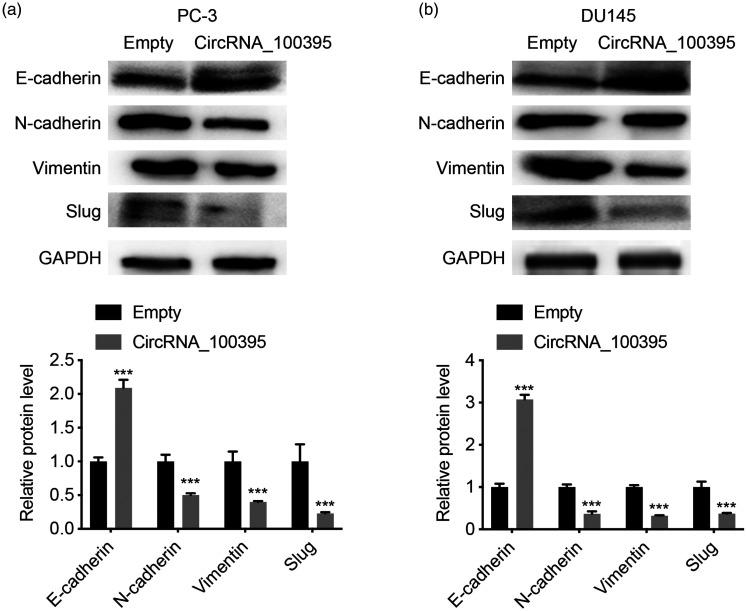
We clarified the mechanism by which circRNA_100395 suppressed prostate cancer cell proliferation and metastasis by detecting EMT-related proteins via western blotting. The epithelial marker protein, E-cadherin, was significantly upregulated in PC3 and DU145 cells overexpressing circRNA_100395, whereas the mesenchymal markers, N-cadherin, vimentin, and Slug, were downregulated (all P < 0.001; Figure 5a and 5b). These results suggest that circRNA_100395 might suppress prostate cancer cell metastasis and proliferation by regulating EMT.
Figure 5.
Overexpression of circRNA_100395 regulated epithelial–mesenchymal transition in prostate cancer cells. Expression levels of E-cadherin, N-cadherin, vimentin and Slug proteins (upper) and static gray values (lower) in (a) PC3 cells and (b) DU145 cells after upregulation of circRNA_100395. ***P < 0.001. GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Role of miR-1228 as a potential regulatory target of circRNA_100395 in prostate cancer
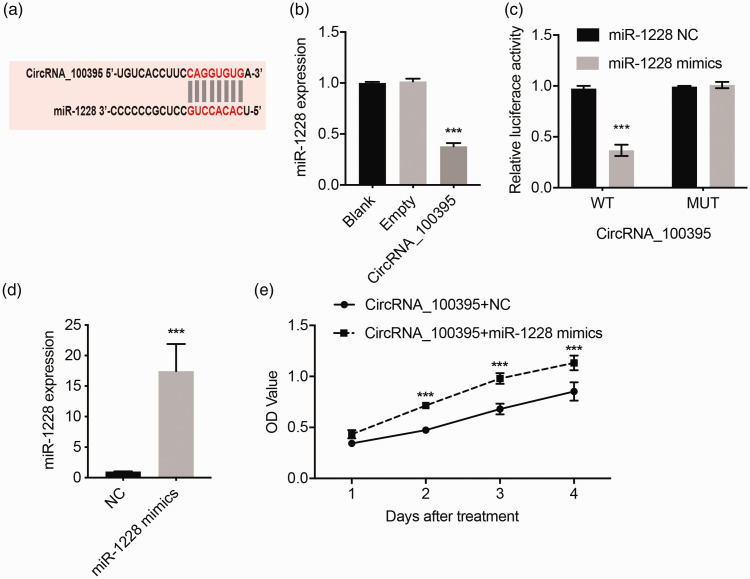
Using the StarBase bioinformatics tool, we identified miR-1228 as a potential downstream effector of circRNA_100395 (Figure 6a). OE-circRNA_100395 PC3 cells showed a 60% decrease in miR-1228 expression compared with the control group (P < 0.001; Figure 6b). We further explored the regulatory relationship between miR-1228 and circRNA_100395 by luciferase reporter assay. Luciferase activity was significantly decreased in WT cells cultured with miR-1228 mimics, but not in cells with a mutated circRNA-binding site (P < 0.001; Figure 6c). These results suggested that circRNA_100395 may bind directly to and inhibit miR-1228 expression.
Figure 6.
MiR-1228 reversed anti-proliferation effect of circRNA_100395 in prostate cancer cells. (a) Predicted binding region between circRNA_100395 and miR-1228A. (b) Effect of circRNA_100395 overexpression on miR-1228 expression in PC3 cells. (c) Relative luciferase activity of 293 PC3 cells after co-transfection with pmirGLO-circRNA_100395-WT or pmirGLO-circRNA_100395-MUT, plus miR-1228 or NC mimic. (d) Expression of miR-1228 in OE-circRNA_100395 PC3 cells transfected with miR-1228 NC or miR-1228 mimic. (e) CCK-8 assays of OE-circRNA_100395 PC3 cells transfected with miR-1228 NC or miR-1228 mimic. MUT, mutant; NC, negative control
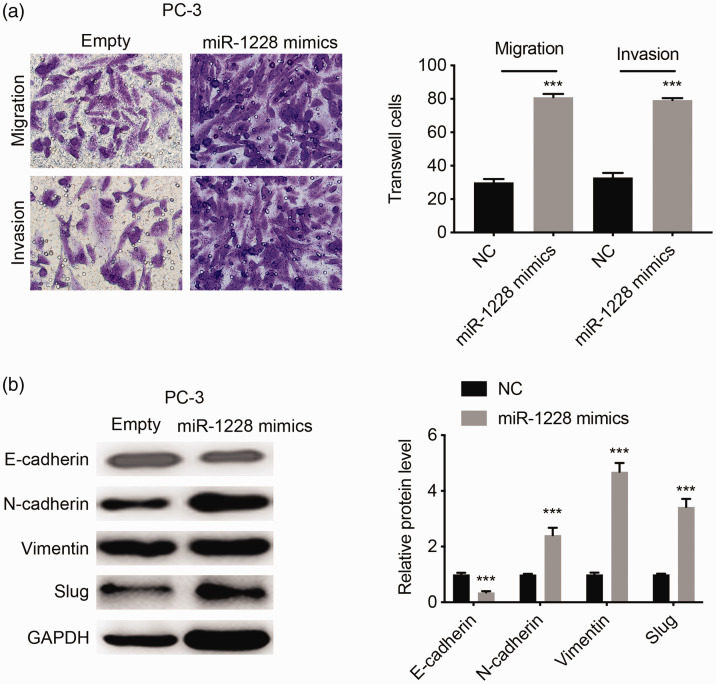
We further explored the relationship between miR-1228 and circRNA_100395 by assessing the expression of miR-1228 in OE-circRNA_100395 PC3 cells (Figure 6d). Cell growth was significantly enhanced after transfection with miR-1228 mimic (P < 0.001; Figure 6e). In addition, cell migration and invasion were significantly inhibited by upregulating miR-1228 according to Transwell assays (both P < 0.001; Figure 7a). Western blot analysis also showed that miR-1228 significantly downregulated the epithelial marker, E-cadherin, and upregulated the mesenchymal markers, N-cadherin, vimentin, and Slug (all P < 0.001; Figure 7b). Overall, these results suggested that miR-1228 was a potential downstream gene of circRNA_100395 in prostate cancer, and that the anti-tumor effect of circRNA_100395 could be reversed by increasing the expression of miR-1228.
Figure 7.
MiR-1228 reversed anti-metastasis and epithelial–mesenchymal transition-regulation effect of circRNA_100395 in prostate cancer cells (crystal violet 1%)(a) Cell migration and invasion assays of OE-circRNA_100395 PC3 cells transfected with miR-1228 NC or miR-1228 mimic (left), and static number of Transwell cells (right). Magnification ×40. (b) Expression of the E-cadherin, N-cadherin, vimentin, and Slug proteins (left) and static gray values (right) in OE-circRNA_100395 PC3 cells transfected with miR-1228 NC or miR-1228 mimic. ***P < 0.001. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, negative control
Discussion
This study identified the tumor-suppressing role of circRNA_100395 in prostate cancer and clarified its underlying mechanism. We demonstrated that expression levels of circRNA_100395 were lower in prostate cancer tissues compared with adjacent normal tissues. We also showed that low circRNA_100395 expression was correlated with Gleason score, lymph node metastasis, and tumor stage. Emerging evidence shows that circRNAs are tightly associated with the occurrence of malignant tumors, and their dysfunction could inhibit tumor growth and metastasis.16,17 For example, Huang et al.18 discovered that circRNA_100876 was dysregulated in esophageal squamous cell carcinoma, and that its expression was closely linked to clinical progression, suggesting that it could be a promising prognostic biomarker. These studies, together with the current results, suggest that circRNA_100395 may be a potential therapeutic biomarker and target in prostate cancer.
Tumor cell proliferation, migration, and invasion are hallmarks of tumor development.19,20 Given that circRNA_100395 expression was significantly correlated with tumor size, tumor stage, and lymph node metastasis, we hypothesized that it might be involved in the regulation of prostate cancer cell proliferation and metastasis. We therefore elucidated its function by examining the changes in cell proliferation and metastasis in prostate cancer cells overexpressing circRNA_100395. CCK-8 and flow cytometric analyses consistently revealed that circRNA_100395 overexpression inhibited prostate cancer cell proliferation and altered the cell cycle distribution. Moreover, Transwell assays revealed that OE-circRNA_100395 inhibited metastasis in prostate cancer cells. Numerous previous studies found that circRNAs specifically participated in the regulation of tumor cell development. For example, Kong et al.21 reported that circFOXO3 enhanced prostate cancer cell growth, while Kong et al.22 found that circRNA_0001206 suppressed prostate cancer cell proliferation and metastasis. The findings of these in vitro studies, coupled with the clinical significance of circRNA_100395, indicated that circRNA_100395 inhibited prostate cancer growth and distant metastasis, resulting in favorable outcomes.
EMT is a crucial biological process during cell survival. The pathological features of EMT include loss of cell-to-cell adhesion and cell polarity by epithelioid cells, which then transform into mesenchymal cells with increased proliferative, migratory, and invasive capacities.23,24 In our study, western blot analysis revealed that overexpression of circRNA_100395 increased the expression of the epithelial marker, E-cadherin, and suppressed the mesenchymal proteins, N-cadherin, vimentin, and Slug, in PC3 and DU145 cells. Emerging studies have indicated that cell growth and metastasis are affected by EMT. For example, Shao et al.25 found that NLRP3 promoted colorectal cancer cell proliferation and metastasis by regulating EMT, while Chen et al.14 showed that circRNA_100395 suppressed liver cancer cell proliferation and metastasis by regulating EMT. Collectively, these studies suggest that the mechanism underlying the anti-proliferation and anti-metastatic effects of circRNA_100395 could be related to EMT dysfunction.
Accumulating studies have also indicated that circRNAs can interfere with the expression of miRNAs by functioning as miRNA sponges. CircRNA could collect target miRNA and reduce the intracellular level of target miRNA. thereby regulating cell proliferation through the cavernous mechanism. Zhang et al.26 reported that circRNA_SMAD2 impaired tumor metastasis and EMT by sponging miR-629. The inhibitory role of circRNA_100395 may also depend on unknown miRNAs. In the present study, miR-1228 was predicted as a potential downstream gene of circRNA_100395 using bioinformatics software (Starbase 2.0). Direct binding between circRNA_100395 and miR-1228 was confirmed by dual-luciferase reporter assay, and miR-1228 was found to be downregulated in OE-circRNA_100395 prostate cancer cells. Many studies have confirmed that miR-1228 acts as a tumor inducer, and its overexpression could promote tumor proliferation and metastasis in lung, ovarian, and liver cancers.13–15 Based on the critical regulatory role of miR-1228 in cancer cells, we carried out RT-qPCR, which showed that its expression was increased in prostate cancer cell lines. We also found that miR-1228 reduced the inhibitory effect of circRNA_100395 on cell growth in circRNA_100395-overexpressing PC3 cells, and enhanced cell migration, invasion, and EMT progression.
The major limitation of the current study was the need to clarify the mechanism by which miR-1228 regulates the EMT process. CircRNAs and miRNAs are all epigenetic regulators that directly affect downstream target gene expression. However, we only considered the cell function and detected the protein levels of EMT-related proteins. We were therefore unable to identify the direct target of circ-100395 and miR-1228. Further studies using high-throughput methods, such as RNA-seq, are planned to further explore the molecular mechanisms of circ-100395 and miR-1228.
The current results consistently indicated that circRNA_100395 plays a significant role in the control of prostate cancer cell proliferation, metastasis, and EMT by regulating miR-1228. However, further studies are needed to address the functional relationship between circRNA_100395 and miR-1228 in greater detail.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Qiang Wei https://orcid.org/0000-0001-5925-6922
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci 2019: 1–4. [DOI] [PubMed] [Google Scholar]
- 3.Li SS, Jiang WL, Xiao WQ, et al. KMT2D deficiency enhances the anti-cancer activity of L48H37 in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2019; 11: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahajan K, Malla P, Lawrence HR, et al. ACK1/TNK2 regulates histone H4 Tyr88-phosphorylation and AR gene expression in castration-resistant prostate cancer. Cancer Cell 2017; 31: 790–803.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CX, Li X, Nan F, et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell 2019; 177: 865–880.e21. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Zhang Q, Pan F, et al. Expression of circular RNAs in gynecological tumors: A systematic review. Medicine (Baltimore) 2019; 98: e15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang KW, Dong M. Role of circular RNAs in gastric cancer: recent advances and prospects. World J Gastrointest Oncol 2019; 11: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacazette É, Diallo LH, Tatin F, et al. Do circular RNAs play tricks on us? Med Sci (Paris) 2020; 36: 38–43. [DOI] [PubMed] [Google Scholar]
- 9.Lei M, Zheng G, Ning Q, et al. Translation and functional roles of circular RNAs in human cancer. Mol Cancer 2020; 19: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skrzypek K, Majka M. Interplay among SNAIL transcription factor, microRNAs, long non-coding RNAs, and circular RNAs in the regulation of tumor growth and metastasis. Cancers 2020; 12: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan L, Zhang L, Fan K, et al. Circular RNA-ITCH suppresses lung cancer proliferation via inhibiting the Wnt/β-catenin pathway. Biomed Res Int 2016; 2016: 1579490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou R, Lv J, Zhang Q, et al. CircAmotl1 motivates Amotl1 expression to facilitate cervical cancer growth. Mol Ther Nucleic Acids 2020; 19: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Ma W, Ke Z, et al. CircRNA hsa_circ_100395 regulates miR-1228/TCF21 pathway to inhibit lung cancer progression. Cell Cycle 2018; 17: 2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Chen Z, Cao S, et al. Role of CircRNAs_100395 in proliferation and metastases of liver cancer. Med Sci Monit 2019; 25: 6181–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Lin S, Mo Z, et al. CircRNA_100395 inhibits cell proliferation and metastasis in ovarian cancer via regulating miR-1228/p53/epithelial-mesenchymal transition (EMT) axis. J Cancer 2020; 11: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X, Liu C, Gao C, et al. circRNA‐associated ceRNA network construction reveals the circRNAs involved in the progression and prognosis of breast cancer. J Cell Physiol 2020; 235: 3973–3983. [DOI] [PubMed] [Google Scholar]
- 17.Yang SJ, Wang DD, Zhou SY, et al. Identification of circRNA–miRNA networks for exploring an underlying prognosis strategy for breast cancer. Epigenomics 2020; 12: 101–125. [DOI] [PubMed] [Google Scholar]
- 18.Cao S, Chen G, Yan L, et al. Contribution of dysregulated circRNA_100876 to proliferation and metastasis of esophageal squamous cell carcinoma. Onco Targets Ther 2018; 11: 7385–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Xu M, Wang S, et al. Gain-of-function E76K-mutant SHP2 promotes cell proliferation, metastasis, and tumor growth in glioblastoma through activation of the ERK/CREB pathway. Onco Targets Ther 2019; 12: 9435–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Lin P, Zou J, et al . MiR-216a-5p act as a tumor suppressor, regulating the cell proliferation and metastasis by targeting PAK2 in breast cancer. Eur Rev Med Pharmacol Sci 2019; 23: 2469–2475. [DOI] [PubMed] [Google Scholar]
- 21.Kong Z, Wan X, Lu Y, et al. Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR‐29a‐3p. J Cell Mol Med 2020; 24: 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Z, Zhuo Z, Ma Z, et al. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol 2019; 47: 2449–2464. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Yu D, Wang X, et al. Epithelial to mesenchymal transition is involved in ethanol promoted hepatocellular carcinoma cells metastasis and stemness. Mol Carcinog 2018; 57: 1358–1370. [DOI] [PubMed] [Google Scholar]
- 24.Zhu F, Dai SN, Xu DL, et al. EFNB2 facilitates cell proliferation, migration, and invasion in pancreatic ductal adenocarcinoma via the p53/p21 pathway and EMT. Biomed Pharmacother 2020; 125: 109972. [DOI] [PubMed] [Google Scholar]
- 25.Shao X, Lei Z, Zhou C. NLRP3 promotes colorectal cancer cell proliferation and metastasis via regulating epithelial mesenchymal transformation. Anticancer Agents Med Chem 2020; 20: 820–827. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Luo P, Jing W, et al. circsMaD2 inhibits the epithelial–mesenchymal transition by targeting mir-629 in hepatocellular carcinoma. Onco Targets Ther 2018; 11: 2853–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]