Abstract
An asymptomatic 3-year-old with Loeys-Dietz Syndrome (LDS) followed for a small patent ductus arteriosus and dilated aorta was found to have a massive ductal aneurysm on routine surveillance cardiac magnetic resonance. The aneurysm was successfully resected. Serial advanced imaging tools are useful in surveillance, diagnosis, and management in patients with LDS.
Keywords: Cardiac magnetic resonance, ductal aneurysm, Loeys-Dietz syndrome
INTRODUCTION
Loeys-Dietz syndrome (LDS) is a connective tissue disorder associated with dilation of the great vessels and diffuse aneurysms.[1] While ductal ampulla aneurysm (DAA) is well-reported, scarce reports of DAA exist in neonates with LDS in whom the presence of the aneurysm prompted further work up for the connective tissue disorder.[2,3,4,5] A multi-institutional series of 24 infant cases of isolated DAA found that only four patients had DAA-related symptoms. While some of the patients had connective tissue disorders, none had LDS. Our case is the first known report of a DAA demonstrating progression over two time points in an asymptomatic child with confirmed LDS.[6]
CLINICAL SUMMARY
A child with LDS Type II (confirmed TGFBR2 mutation) with several typical extracardiac features was followed for a small patent ductus arteriosus (PDA), dilated aortic root (AoR), and ascending aorta (AA). His extracardiac findings included hypertelorism and dysmorphic features with a moderate pectus excavatum deformity. His examination was notable for a soft-continuous murmur at the left infra-clavicular region.
After the diagnosis of the dilated AoR and AA at 17 months of age, losartan was initiated and was later switched to irbesartan. He was clinically asymptomatic, and follow-up echocardiograms were somewhat limited due to agitation. At 3 years of age, routine surveillance cardiac magnetic resonance imaging (MRI) demonstrated a new massive DAA.
Echocardiogram at 17 months of age demonstrated an AoR of 2.7 cm (Z-score + 8.4) and AA of 1.8 cm (Z-score +3.3). Surveillance cardiac MRI at the time confirmed the AoR was severely dilated (2.6 cm × 2.5 cm × 2.5 cm), and the AA was moderately dilated (1.7 cm × 1.8 cm). A small PDA was visualized. Twenty months later, non-contrast cardiac MRI found a large DAA arising from the under surface of the aortic arch measuring 2.3 cm × 2.7 cm that impinged upon the superior aspect of the left pulmonary artery. The AoR and AA measurements were relatively stable at 2.8 cm × 2.9 cm × 3.2 cm and 1.9 cm × 1.8 cm, respectively [Figure 1a and b]. An echocardiogram confirmed severe AoR dilation (3.4 cm, Z-score +11.5) and moderate AA dilation (1.9 cm, Z-score +3.1) with to-and-fro flow within the DAA without evidence of thrombus [Figure 1c].
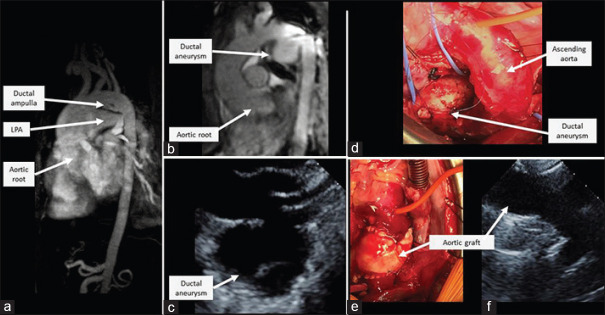
Figure 1.
(a) Baseline cardiac MRI at 17 months of age demonstrated dilated AoR and ductal ampulla. (b) Cardiac MRI at 3 years of age demonstrated a large DAA and progressive AoR and AA dilation. (c) Repeat echocardiogram at 3 years of age on the massive DAA on suprasternal arch view. (d) Intraoperatively, the DAA was isolated and resected. (e) An aortic homograft was placed. (f) Postoperative echocardiogram confirmed an unobstructed aortic arch with no residual duct or aneurysm on suprasternal arch view. LPA: Left pulmonary artery, MRI: Magnetic resonance imaging, DAA: Ductal ampulla aneurysm, AoR: Aortic root
Multimodality cardiac imaging confirmed this clinically asymptomatic and potentially life-threatening finding prompting operative resection of the DAA. The surgical repair was performed through a midline sternotomy approach. After carefully dissecting out the arch vessels, the patient was placed on cardiopulmonary bypass, and then, deep hypothermic circulatory arrest. The PDA was divided and ligated and the ductal aneurysm was resected [Figure 1d]. The aortic arch from the AA to the proximal descending aorta, just distal to the take-off of the ductal aneurysm, was replaced. Aortic homograft was chosen for the arch replacement due to the ability to fit an “adult-sized” graft and was preferred to a Gelweave graft due to concern for kinking secondary to the severe chest wall deformity [Figure 1e]. As a result of his severe pectus deformity and the absence of any aortic insufficiency, we deferred aortic valve-sparing root replacement, which would have required aortic valve resuspension and coronary re-implantation. Given his young age, repair of the pectus deformity was not performed at this time. Post-operative echocardiogram demonstrated an unobstructed aortic arch with no residual duct or aneurysm [Figure 1f]. Follow-up echocardiograms continue to reveal an unobstructed aortic arch and no residual aneurysm without significant progression of aortic root dilatation. The patient will have a follow-up cardiac MRI within the next year.
DISCUSSION
Cardiac imaging revealed rapid evolution of this clinically silent, yet life-threatening lesion in our patient. LDS guidelines recommend at least annual echocardiograms and full vascular imaging at diagnosis and follow-up at 2-year intervals in the absence of dissections or aneurysms.[1] Echocardiography has been reported as the primary modality of DAA diagnosis. Advanced imaging including CT and MRI can be used to elucidate associated thrombus or extravascular compression.[6] As third trimester antenatal ultrasound imaging shows a reported incidence of DAA in 1.5% of the scans, surveillance is important in these patients to predict life-threatening complications like spontaneous rupture.[6] Surgical resection is typically well-tolerated and should be considered early as even a small DAA may spontaneously rupture.[6] Even small silent PDA may need continued surveillance in patients with LDS as we noted one instance of development of DAA in a patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.MacCarrick G, Black JH, 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. Loeys-Dietz syndrome: A primer for diagnosis and management. Genet Med. 2014;16:576–87. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lund JT, Hansen D, Brocks V, Jensen MB, Jacobsen JR. Aneurysm of the ductus arteriosus in the neonate: Three case reports with a review of the literature. [[Last accessed on 2020 Mar 04]];Pediatr Cardiol. 1992 13:222–26. doi: 10.1007/BF00838780. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1518741 . [DOI] [PubMed] [Google Scholar]
- 3.Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur J Cardiothorac Surg. 1991;5:566–70. doi: 10.1016/1010-7940(91)90220-e. [DOI] [PubMed] [Google Scholar]
- 4.Yetman AT, Beroukhim RS, Ivy DD, Manchester D. Importance of the clinical recognition of Loeys-Dietz syndrome in the neonatal period. Pediatr. 2007;119:e1199–202. doi: 10.1542/peds.2006-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta S, Younoszai A, Pietz J, Achanti B. Pharmacological closure of the patent ductus arteriosus. [[Last accessed on 2020 Mar 06]];Images Paediatr Cardiol. 2003 5:1–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22368623 . [PMC free article] [PubMed] [Google Scholar]
- 6.Dyamenahalli U, Smallhorn JF, Geva T, Fouron JC, Cairns P, Jutras L, et al. Isolated ductus arteriosus aneurysm in the fetus and infant: A multi-institutional experience. J Am Coll Cardiol. 2000;36:262–9. doi: 10.1016/s0735-1097(00)00707-5. [DOI] [PubMed] [Google Scholar]