Abstract
Essential oils are plant-derived aromatic volatile oils, and they contain bioactive compounds that have been shown to improve poultry nutrition. In this study, we investigated the effects of oregano essential oil (OEO) on intestinal antioxidative capacity, immunity, and gut microbiota of young yellow-feathered chickens. A total of nine hundred and sixty 1-d-old female Qingyuan partridge chickens were randomly allocated to four treatment groups with six replicates of 40 birds each, and the feeding trial was lasted for 30 d. The controls were fed on a basal diet without in-feed antibiotics; the birds in the antibiotic group were fed the basal diet supplemented with 20 mg/kg virginiamycin; the remaining birds were fed the basal diet containing 150 or 300 mg/kg OEO, respectively. Dietary supplementation with 150 or 300 mg/kg OEO increased average daily feed intake (P = 0.057) and average daily gain (P < 0.05). The activities of glutathione peroxidase and total antioxidative capacity in plasma, jejuna, and ileal mucosa were increased by OEO supplementation (P < 0.05), with a trend of lower jejunal content of malonaldehyde (P = 0.062). Moreover, dietary OEO increased the content of secretory immunoglobulin A (P = 0.078) and the relative expression of Claudin 1, Mucin 2, and Avain beta-defensin 1 in ileum (P < 0.05). Sequencing data of 16S rRNA indicated that dietary OEO increased the relative abundance of Firmicutes phylum, and Clostridium and Lactobacillus genera, and decreasing that of Romboutsia. Functional analyses indicated that microbial amino sugar and nucleotide sugar metabolism, replication, and repair systems were higher in OEO groups than those of controls and antibiotic treatment. In conclusion, dietary supplementation with OEO enhanced growth performance, alleviated local oxidative stress in intestine, improved production of natural antibodies, and favorably modulated intestinal microbiota composition.
Keywords: immunity, intestinal antioxidative capacity, ileal microbiota, oregano essential oil, yellow-feathered chickens
Introduction
Antibiotics have been extensively used in poultry production to increase productivity and efficiency. The emergence of antimicrobial resistance has become a large threat to the One World Health in the coming decades. In responding to this challenge, many countries have banned or restricted the use of in-feed antibiotics in hope to reduce development of bacterial resistance (Magnusson, 2020). On the other hand, it is urgent to develop reliable and effective alternatives to antibiotics without compromising productivity and animal welfare.
As natural agents, plant extracts have been used to replace antibiotics in broiler diets to improve the safety of by-products (Salaheen et al., 2017; Aziz and Karboune, 2018). Oregano essential oil (OEO), one of plant extracts, has been shown to have antioxidative, anti-inflammatory, antimicrobial, and antiviral characteristics (Leyva-López et al., 2017). OEO contains high concentrations of carvacrol, thymol and their precursors, γ-terpinene, and ρ-cymene. Several studies have shown that feed supplemented with OEO promotes nutrient digestion and improves antioxidative status, immunity, and meat quality in broiler chickens (Franciosini et al., 2016; Ri et al., 2017; Betancourt et al., 2019; Yang et al., 2019). However, the mechanisms for OEO’s growth promoting effect have not been well elucidated, and in particular, the information regarding whether its effects are related to alter intestinal oxidative status and innate immune system is still not available.
It has become increasingly recognized that intestinal bacteria are known to have a strong influence on host metabolism, antioxidation, and immunological activity, thus having potential importance in impacting poultry health and growth (Rowland et al., 2018). Ross-308 broiler chickens drinking water with 0.4 ml/L lavender essential oil decreased the number of pathogenic Escherichia coli and coliforms and increased numbers of probiotic bacteria (Adaszyńska-Skwirzyńska and Szczerbińska, 2019). Bauer et al. (2019) reported that Ross-308 broiler diets supplemented with 1% and 2% oregano powder reduced their jejunal abundance of Proteus, Klebsiella, Staphylococcus, and Bifidobacterium. The meat-type Qingyuan partridge chicken is a native slow-growing breed with superior meat quality of typical economic importance in South China (>100 million birds), whereas these local breeds are considered susceptible to Salmonella, Colibacillus, and Coccidium infection with high morbidity and mortality at starter phase. Until now, very limited information has existed on the effect of chicken breeds on those pathogens susceptibility (Broom and Kogut, 2019). Recently, natural alternatives such as antioxidants, plant extracts, and probiotics have been used to enhance intestinal disease resistance to obtain safe, reliable, and high-quality poultry products without any in-feed medication or antibiotics (Windisch et al., 2008; Park et al., 2016; Suresh et al., 2018). Thus far, there is no research that has evaluated the possible impact of OEO on intestinal antioxidation, immunity, and the related intestinal microflora community in these chickens. The aim of the present study, therefore, was to address these issues and fill the gap of knowledge in this aspect, aiming to demonstrate the potential of OEO as an antibiotic substitute for local yellow-feathered chickens.
Materials and methods
All experimental protocols for the study were approved by the Animal Care Committee of the Institute of Animal Science, Guangdong Academy of Agricultural Sciences, Guangzhou, China (Protocol number: GAASISA-2019-019).
Animal, diets, and experimental design
A total of 960 healthy 1-d-old female Qingyuan chicks were purchased from Guangdong Aijiankang Biotechnology Co. Ltd (Qingyuan, Guangdong, China) and randomly divided into four treatment groups, each with six replicates of 40 birds (n = 240/treatment). During the trial period, each replicate was housed in a galvanized steel floor pen (1.6 × 1.4 × 0.4 m) with eight water nipples and two feeders. All chicks were handled in accordance with the management guidelines for Qingyuan partridge chickens for lighting, ad libitum feeding and availability of antibiotic-free tap water throughout the experiment period (30 d). The room temperature was initially set at 35 °C for the first week and then decreased by 2 to 3 °C per week to a final temperature of 26 °C. Room humidity was maintained between 50% and 60%.
The dietary treatments were as follows: 1) controls (CON) received a basal diet without antibiotics; 2) an in-feed antibiotic treatment (AB) that was the basal diet containing 20 mg/kg virginiamycin; 3) low-level OEO (OEO-150) was the basal diet with 150 mg/kg OEO; and 4) high-level OEO (OEO-300) was the basal diet with 300 mg/kg OEO. The basal diet (Table 1) was formulated to meet the standard nutritional requirements of slow-growing yellow-feathered chickens, as described in the Chinese Feeding Standard of Chicken (MOA, 2004). The Virginiamycin Premix (500 g/kg, virginiamycin) was purchased from Phibro Animal Health Co., Ltd (Shanghai, China). The OEO in form of ORSENTIAL Dry (light yellow to yellow, free-flowing powder) was obtained from Kemin (China) Technologies Co., Ltd (Zhuhai, China). It was a combination of oregano essential oil extracted from Origanum vulgare, which contained a minimum of 22 g/kg of carvacrol and 11 g/kg of thymol. For more details about the O. vulgare, please review the U.S. patent (No. US20140336421A1) online. The defatted rice bran and silica were used as carriers.
Table 1.
Composition and nutrient levels of the basal diet of yellow-feathered chickens (air-dry basis, %)
| Ingredients | Contents, % | Calculated nutrient levels2 | Contents, % |
|---|---|---|---|
| Corn | 64.00 | AME (MJ/kg)3 | 11.93 |
| Wheat bran | 4.50 | Crude protein | 19.00 |
| Soybean meal | 21.50 | Calcium | 0.95 |
| Corn gluten meal | 5.50 | Available phosphorus | 0.44 |
| l- Lysine | 0.24 | Lysine | 1.04 |
| dl-Methionine | 0.11 | Methionine | 0.44 |
| Limestone | 1.21 | Threonine | 0.70 |
| Dicalcium phosphate | 1.64 | Tryptophan | 0.17 |
| NaCl | 0.30 | Arginine | 1.09 |
| Premixa | 1.00 | Isoleucine | 0.70 |
| Total | 100.00 |
1Vitamins and minerals in the diet were supplied exactly as stated by the Chinese Feeding Standard of Chicken (2004), which provide the followings per kilogram of diet: vitamin A, 15,000 IU; vitamin D3, 3,300 IU; vitamin E, 20 mg; vitamin K3, 5 mg; thiamin, 3.8 mg; riboflavin, 4.0 mg; pyridoxine, 3.5 mg; cyanocobalamin, 0.01 mg; calcium pantothenic, 10 mg; niacin, 25 mg; folic acid, 0.55 mg; biotin, 0.15 mg; choline chloride, 1,300 mg; Fe, 80 mg; Cu, 7 mg; Mn, 60 mg; Zn, 70 mg; I, 0.35 mg; Se, 0.23 mg. The carrier was corn cob meal.
2Values were calculated from data provided by the Feed Database in China (2016).
3Apparent metabolic energy.
Growth performance measurement
The initial and final body weight (BW) of the individual birds were recorded, and the average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated on a per replicate basis between day 1 and day 30 of age. Mortality recorded daily was used to adjust the total number of birds per replicate for correct calculations of ADFI and FCR.
Sample collection
A total of 48 birds, 2 from each replicate (12 per treatment), were euthanized at day 30 by approved methods and exsanguinated. Blood samples were collected from the wing vein in heparinized-evacuated tubes (5 mL), which were then centrifuged at 4,000× g for 10 min at 4 °C; plasma samples were then collected and stored at −80 °C. Ileal digesta samples were immediately collected and stored at −80 °C. After 5-cm segment of the mid-jejunum and mid-ileum was opened lengthwise, rinsed with phosphate-buffered saline (PBS, pH = 7.4), each intestinal mucosa was scraped with a glass slide and placed into sterile tubes, which were quickly plunged into liquid nitrogen, and then stored at −80 °C.
Antioxidative status of plasma and tissue
The plasma activities of glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), and total antioxidative capacity (T-AOC), as well as the concentrations of malonaldehyde (MDA), were carried out in duplicate using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) following the manufacturer’s instruction, similar to those described previously (Huang et al., 2015). The intra-assay coefficient of variation (CV) was 3.6% and interassay CV was 6.8% for GSH-Px; the intra-assay CV was 1.7% for T-SOD; the intra-assay CV was 3.6% and interassay CV was 6.4% for T-AOC; and the intra-assay CV was 2.3% and interassay CV was 5.34% for MDA.
One gram of frozen intestinal mucosal (the jejunal and ileal) tissue and 9 mL of 0.9% ice-cold PBS were homogenized in a T25 Ultra-Turrax homogenizer (Ika Works Inc., Staufen, Germany), then centrifuged at 4,000 × g for 10 min at 4 °C. Supernatants were collected and promptly analyzed, as described above for plasma samples. The results were normalized against total protein concentration in each sample for intersample comparison. The protein content for intestinal tissue was determined using Coomassie Brilliant Blue reagent with bovine serum albumin standards.
Immune indices of plasma and intestine
Plasma concentrations of immunoglobulin A (IgA), immunoglobulin M (IgM), and immunoglobulin G (IgG), transforming growth factor β (TGF-β), and tumor necrosis factor α (TNF-α) were measured using corresponding chicken ELISA kits (Beijing Equation Biotechnology co., Ltd, Beijing, China) following the manufacture’s protocols. The intra-assay CV were below 8% and inter-assay CV were below 10% for IgA, IgM, and IgG (IgY).
Approximately 0.5 g of intestinal mucosa (the jejunum and ileum) tissue was homogenized in PBS (pH 7.4) using an Ultra-Turrax homogenizer for 30 s and then centrifuged at 4,000 × g for 20 min at 4 °C, and supernatant was collected and stored at −20 °C for analysis. The concentrations of secretory immunoglobulin A (SIgA), IgM, IgG, TGF-β, and TNF-α were measured by a spectrophotometer (Biomate 5, Thermo Electron Corporation, Rochester, NY). The assays were conducted in duplicate with the respective chicken ELISA kits (Beijing Equation Biotechnology).
Quantitative real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) from snap-frozen ileal mucosa following the manufacturer’s protocol, and the integrity of RNA was verified by gel electrophoresis. The RNA concentration and purity were quantified by OD260/OD280 (1.8 < ratio < 2.1) using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). One microgram of total RNA from each sample was used to generate cDNA in a final volume of 20 µL using a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa Biotechnology, Dalian, China), following the manufacturer’s instructions. The cDNA was then diluted 10-fold with nuclease-free water and then stored at −20 °C. Primer sequences for the genes located in GenBank used were listed in Table 2. The qPCR was performed in triplicate using an iTaq Universal SYBER Green Supermix (TaKaRa Biotechnology) in a CFX96 Real Time PCR Detection System (Bio-Rad, Hercules, CA). The qPCR program started with denaturation at 95 °C for 30 s, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. The SYBR Green PCR reaction system had a total volume of 20 µL, including 10 µL of 2× SYBR Premix (Bio-Rad, Shanghai, China) Premix, 2 µL of 10× diluted cDNA, 1 µL of each primer, and 7 µL nuclease-free water. The relative mRNA expression of target genes was calculated with the 2−ΔΔCt method as reported (Livak and Schmittgen, 2001) using β-actin as an intrinsic standard; expression was further normalized to that measured in the CON treatment.
Table 2.
Primer sequences for quantitative real-time PCR
| Gene name1 | Primers sequence (5′–3′) | GenBank accession number | |
|---|---|---|---|
| GPX1 | Forward | AAGTGCCAGGTGAACGGGAAGG | NM_001277853.2 |
| Reverse | AGGGCTGTAGCGGCGGAAAG | ||
| SOD1 | Forward | GGTGCTCACTTTAATCCTG | NM_205064.1 |
| Reverse | CTACTTCTGCCACTCCTCC | ||
| HMOX1 | Forward | CTCAAGGGCATTCATTCG | NM_205344.1 |
| Reverse | ACCCTGTCTATGCTCCTGTT | ||
| NRF2 | Forward | ATCACCTCTTCTGCACCGAA | NM_205117.1 |
| Reverse | GCTTTCTCCCGCTCTTTCTG | ||
| ZO-1 | Forward | CCAAAGACAGCAGGAGGAGA | XM_015278981.1 |
| Reverse | TGGCTAGTTTCTCTCGTGCA | ||
| OCLN | Forward | TCATCCTGCTCTGCCTCATCT | NM_205128.1 |
| Reverse | CATCCGCCACGTTCTTCAC | ||
| CLDN1 | Forward | GAGGATGACCAGGTCAAGAAG | NM_001318434.1 |
| Reverse | TGCCCAGCCAATGAAGAG | ||
| MUC2 | Forward | CATTCAACGAGGAGAGCTGC | NM_001318434.1 |
| Reverse | TTCCTTGCAGCAGGAACAAC | ||
| AvBD1 | Forward | GAGTGGCTTCTGTGCATTTCTG | NM_204993.1 |
| Reverse | TTGAGCATTTCCCACTGATGAG | ||
| MYD88 | Forward | GAAGCAGCGTTTGGGAGTG | NM_001030962.4 |
| Reverse | AGCATTACCAGGGCTGAGTT | ||
| TLR4 | Forward | AGTCTGAAATTGCTGAGCTCAAAT | NM_001030693.1 |
| Reverse | GCGACGTTAAGCCATGGAAG | ||
| β-Actin | Forward | GAGAAATTGTGCGTGACATCA | NM_205518 |
| Reverse | CCTGAACCTCTCATTGCCA |
1GPX1, glutathione peroxidase 1; SOD1, superoxidate dismutase 1; HMOX1, hemoxygenase 1; NRF2, nuclear factor erthyroid 2-related factor 2; ZO-1 zona occludin 1; OCLN, occludin; MUC2, mucin 2; AvBD1, avian beta defensin 1; MYD88, myeloid differentiation factor 88; TLR4 toll-like receptor 4.
Sequencing of intestinal bacteria and bioinformatics
Bacterial 16S rRNA sequencing was performed as described previously (Chen et al., 2019). Genomic DNA was extracted from ileal contents using the Mag-Bind Soil DNA Kit (Omega Biotec, Norcross, GA). The V3 to V4 hypervariable regions of the bacterial 16S ribosomal RNA genes were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) using an ABI GeneAmp 9700 PCR thermocycler (ThermoFisher, Waltham, MA). Sequencing used the Illumina MiSeq platform at Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China). Raw data were demultiplexed, quality-filtered, and merged, and operational taxonomic units (OTU) were clustered and annotated using UPARSE with 97% similarity cutoff (version 7.1; http://drive5.com/uparse/). The taxonomy of each OTU representative sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/). Alpha diversity (Species observed, Shannon, Simpson, Chao1, ACE, and Good’s coverage) metrics were then conducted using QIIME, and a beta diversity (between groups) distance matrix based on unweighted the UniFrac metric was calculated and used for principal coordinate analysis (PCoA), and unweighted unifrac full tree method was calculated by analysis of similarities (ANOSIM). Differentially abundant taxa among the treatments were identified using LDA effect-size (LefSe) analysis (α = 0.05, LDA score > 4).
Statistical analysis
The experiment was designed as a completely randomized design, and each pen was considered an experimental unit, n = 6. The variation associated with dietary treatment was considered a fixed effect, whereas replicates considered as a random effect. Data in the study, presented as means along with the pooled SEM, were subjected to one-way ANOVA (SAS, version 9.1 for Windows; SAS Inc.). The differences among treatments were examined using Duncan’s multiple range tests. Chi-square analysis (Fisher’s exact test) was analyzed by PROC FREQ to analyze the difference in the mortality. Differences were considered significant when P < 0.05, and trends (0.05 < P < 0.10) were also presented.
Results
Growth performance
As shown in Table 3, compared with the CON treatment, both OEO-150 and OEO-300 treatments increased 15 and 17 g of ADG (P < 0.01), respectively, and a trend for higher ADFI (P = 0.059). There was no difference in growth performance in the antibiotic control (AB), compared with the CON (P > 0.05). No effect was observed on mortality among treatments (P > 0.05).
Table 3.
Effects of oregano essential oil on growth performance of yellow-feathered chickens in the starter phase1
| Treatments2 | ||||||
|---|---|---|---|---|---|---|
| Variables | CON | AB | OEO-150 | OEO-300 | SEM | P-value |
| 1-d BW, g | 33.50 | 33.58 | 33.52 | 33.55 | <0.01 | 0.961 |
| 30-d BW, g | 289.3b | 295.1b | 304.3a | 306.3a | 3.02 | 0.002 |
| ADFI, g | 18.90b | 18.98b | 19.19ab | 19.91a | 0.270 | 0.059 |
| ADG, g | 8.84c | 9.09bc | 9.34ab | 9.41a | 0.101 | 0.003 |
| FCR | 2.14 | 2.09 | 2.06 | 2.13 | 0.029 | 0.200 |
| Mortality, % | 0.83 | 0.42 | 0.00 | 0.00 | 0.005 | 0.403 |
1Data are means with the SEM derived from ANOVA error mean square for n = 6.
2CON, control diet; AB, control diet containing 20 mg/kg virginiamycin; OEO-150, control diet containing 150 mg/kg organic essential oil; OEO-300, control diet containing 300 mg/kd organic essential oil.
a,b,cMeans within the same row having different superscripts differ significantly (P < 0.05).
Plasma and intestinal enzyme activities
As shown in Table 4, the activities of jejunal T-SOD and T-AOC were increased over CON in AB and OEO-150 treatments (P < 0.05), and the activities of jejunal GSH-Px were increased in OEO-300 treatment (P < 0.05). The activities of ileal GSH-Px and T-AOC were also increased over CON in AB and OEO-150 treatments (P < 0.05). There were no differences among the treatments in plasma activities of GSH-Px, T-SOD, T-AOC, and concentration of MDA (P > 0.05).
Table 4.
Effects of oregano essential oil on antioxidative indices in plasma and intestine of yellow-feathered chickens at 30 d of age1
| Treatments2 | ||||||
|---|---|---|---|---|---|---|
| Variables | CON | AB | OEO-150 | OEO-300 | SEM | P-value |
| Plasma | ||||||
| GSH-Px, units/mL | 613.7b | 605.9b | 645.6a | 634.8ab | 9.72 | 0.033 |
| T-SOD, units/mL | 131.6 | 136.2 | 132.5 | 134.6 | 6.98 | 0.521 |
| T-AOC, units/mL | 2.49 | 3.23 | 3.15 | 2.88 | 0.490 | 0.708 |
| MDA, nmol/mL | 2.83 | 2.07 | 2.40 | 2.67 | 0.224 | 0.115 |
| Jejunum | ||||||
| GSH-Px, units/mg prot | 293.2b | 328.3ab | 314.6ab | 391.4a | 24.13 | 0.041 |
| T-SOD, units/mg prot | 678.0ab | 861.9a | 800.7a | 623.3b | 50.68 | 0.022 |
| T-AOC, units/mg prot | 1.93b | 2.92a | 2.79a | 2.48ab | 0.199 | 0.009 |
| MDA, nmol/mg prot | 1.91a | 1.44ab | 1.42ab | 1.07b | 0.202 | 0.062 |
| Ileum | ||||||
| GSH-Px, units/mg prot | 175.8b | 211.8ab | 225.7a | 193.3ab | 12.35 | 0.078 |
| T-SOD, units/mg prot | 1031.5 | 1180.7 | 1182.2 | 934.7 | 83.83 | 0.134 |
| T-AOC, units/mg prot | 1.78b | 2.01ab | 2.19a | 1.85ab | 0.088 | 0.016 |
| MDA, nmol/mg prot | 0.86 | 0.64 | 0.77 | 0.67 | 0.126 | 0.770 |
1Data are means with the SEM derived from ANOVA error mean square for n = 6.
2CON, control diet; AB, control diet containing 20 mg/kg virginiamycin; OEO-150, control diet containing 150 mg/kg organic essential oil; OEO-300, control diet containing 300 mg/kd organic essential oil.
a,bMeans within the same row with different superscripts differ significantly (P < 0.05).
Plasma and intestinal immune indices
Compared with the CON treatment, birds fed OEO-150 and OEO-300 had higher levels of ileal SIgA (P = 0.078); OEO-300 treatment also had the highest plasma content of IgG and lowest mucosal content of ileal TNF-α (P < 0.05); however, there were no differences in the content of jejunal SIgA, IgM, and IgG (P > 0.05). Moreover, no difference of these parameters was observed in AB treatment compared with the CON (P > 0.05) (Table 5).
Table 5.
Effects of oregano essential oil on immunity in plasma and intestine of yellow-feathered chickens at 30 d of age1
| Treatments2 | ||||||
|---|---|---|---|---|---|---|
| Variables | CON | AB | OEO-150 | OEO-300 | SEM | P-value |
| Plasma | ||||||
| IgG, g/L | 478.5b | 518.9b | 562.7ab | 699.3a | 50.41 | 0.031 |
| IgM, g/L | 31.39 | 30.27 | 28.69 | 28.64 | 2.583 | 0.849 |
| TGF-β, ng/L | 152.5a | 139.2ab | 129.1ab | 104.8b | 14.43 | 0.091 |
| TNF-α, ng/L | 30.63 | 31.32 | 28.58 | 30.21 | 1.975 | 0.702 |
| Jejunum | ||||||
| IgG, µg/mg prot | 122.8 | 138.1 | 123.1 | 129.9 | 9. 466 | 0.174 |
| IgM, µg/mg prot | 10.83 | 9.57 | 9.83 | 9.72 | 0.561 | 0.393 |
| SIgA, µg/mg prot | 3.28 | 3.77 | 3.49 | 3.43 | 0.168 | 0.255 |
| TGF-β, ng/mg prot | 28.82 | 21.90 | 26.33 | 30.25 | 2.349 | 0.568 |
| TNF-α, ng/mg prot | 9.60 | 8.67 | 8.89 | 8.29 | 0.840 | 0.501 |
| Ileum | ||||||
| IgG, µg/mg prot | 50.96 | 55.69 | 60.01 | 61.33 | 6.044 | 0.639 |
| IgM, µg/mg prot | 11.23 | 12.13 | 13.24 | 13.72 | 0.798 | 0.152 |
| SIgA, µg/mg prot | 1.36b | 1.26b | 1.50a | 1.54a | 0.132 | 0.078 |
| TGF-β, ng/mg prot | 26.37 | 32.05 | 28.50 | 32.22 | 2.860 | 0.416 |
| TNF-α, ng/mg prot | 9.13a | 8.28ab | 7.83ab | 6.62b | 0.609 | 0.057 |
1Data are means with the SEM derived from ANOVA error mean square for n = 6.
2CON, control diet; AB, control diet containing 20 mg/kg virginiamycin; OEO-150, control diet containing 150 mg/kg organic essential oil; OEO-300, control diet containing 300 mg/kd organic essential oil.
a,bMeans within the same row with different superscripts differ significantly (P < 0.05).
Expression of intestinal antioxidative- and immunity-related genes
As shown in Table 6, mucosal transcript abundance of nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HMOX1), and glutathione peroxidase 1 (GPX1) of ileum were higher in the OEO-150 treatment exceeded that of the CON (P < 0.05). Compared with the CON and AB control treatments, the OEO-300 treatment increased relative expression of claudin 1 (CLDN1) and mucin 2 (MUC2) (P < 0.05) and decreased the mRNA expression of myeloid differentiation primary response 88 (MYD88) (P = 0.085); the OEO-150 treatment increased relative expression of Avain beta-defensin 1 (AvBD1) (P < 0.05). No difference was observed in the ileal mRNA expression of superoxide dismutase 1 (P > 0.05).
Table 6.
Effects of oregano essential oil on gene expression in ileal mucosa of yellow-feathered chickens at 30 d of age1
| Treatments2 | ||||||
|---|---|---|---|---|---|---|
| Variables3 | CON | AB | OEO-150 | OEO-300 | SEM | P-value |
| GPX1 | 1.03b | 1.18b | 1.78a | 1.48ab | 0.090 | 0.031 |
| SOD1 | 1.01ab | 0.56b | 1.35a | 0.63b | 0.108 | 0.048 |
| HMOX1 | 1.03ab | 0.77b | 1.58a | 1.25ab | 0.105 | 0.029 |
| NRF2 | 1.08b | 1.17b | 1.97a | 1.58ab | 0.133 | 0.004 |
| ZO-1 | 1.04 | 1.33 | 1.05 | 0.89 | 0.123 | 0.122 |
| OCLN | 1.01 | 1.15 | 1.05 | 1.00 | 0.084 | 0.575 |
| CLDN1 | 1.03b | 1.88a | 1.66a | 2.18a | 0.093 | 0.007 |
| MUC2 | 1.05b | 1.89a | 1.95a | 1.85a | 0.122 | <0.001 |
| AvBD1 | 1.07b | 0.43c | 1.88a | 1.21ab | 0.201 | 0.002 |
| MYD88 | 1.05ab | 1.21a | 0.96ab | 0.72b | 0.127 | 0.085 |
| TLR4 | 1.08 | 0.98 | 0.92 | 0.86 | 0.130 | 0.344 |
1Data are means with the SEM derived from ANOVA error mean square for n = 6.
2CON, control diet; AB, control diet containing 20 mg/kg virginiamycin; OEO-150, control diet containing 150 mg/kg organic essential oil; OEO-300, control diet containing 300 mg/kd organic essential oil.
3GPX1, glutathione peroxidase 1; SOD1, superoxidate dismutase 1; HMOX1, hemoxygenase 1; NRF2, nuclear factor erthyroid 2-related factor 2; ZO-1 zona occludin 1; OCLN, occludin; CLDN1, claudin 1; MUC2, mucin 2; AvBD1, avian beta defensin 1; MYD88, myeloid differentiation factor 88; TLR4 toll-like receptor 4.
a,b,cMeans within the same row with different superscripts differ significantly (P < 0.05).
Structure of ileal bacterial community
In alpha diversity indexes, there was a decreasing trend on observed species and Chao1 by the effects of OEO supplementation (0.05 < P < 0.1, Supplementary Table 1). Shannon, Simpson, ACE, and Good’s coverage were not influenced in AB, OEO-150, and OEO-300 treatments (P > 0.05, Supplementary Table 1).
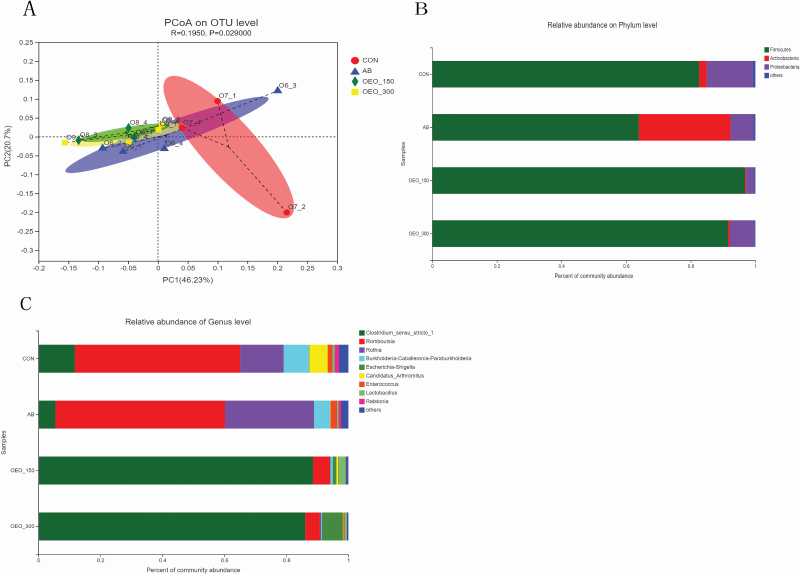
Beta diversity analysis was illustrated by PCoA and ANOSIM (Figure 1A). PCoA of OTU, based on the unweighted unifrac full tree method, indicated the distinct separation of ileal microbiota among the AB, OEO-150, and OEO-300 treatments (P = 0.029).
Figure 1.
Effects of dietary oregano essential oil supplementation on microbiome composition in the ileum of yellow-feathered chickens (n = 4). Principal coordinate analysis (A). On the top five phylum level, relative abundance with statistical difference among four groups (B). Relative abundance of top 10 genus level in the ileum of yellow-feathered chickens (C). CON = the basal diet without antibiotics; AB = the basal diet with 20 mg/kg virginiamycin; OEO-150 = the control diet plus 150 mg/kg oregano essential oil; OEO-300 = the control diet plus 300 mg/kg oregano essential oil.
As shown in Figure 1B, OEO addition increased the relative abundance of ileal Firmicutes with an overall increase of 22.46% (P < 0.05) and reduced the relative abundance of ileal Proteobacteria and Actinobacteria with the percentage of 8.66% and 13.63% loss, respectively (P < 0.05), compared with the CON group. In AB treatment, Actinobacteria were more abundant relative to the CON group, whereas Firmicutes were less abundant (P < 0.05). Birds fed OEO-supplemented diets showed an increase in the relative abundance of ileal Clostridium sensu stricto_1 and Lactobacillus genera with each increase of 72.87% and 2.31% (P < 0.05, Figure 2) compared with birds fed the CON diet. The relative abundance of ileal Romboutsia and Burkholderia–Caballeronia–Paraburkholderia genera was decreased by OEO supplementaion (P < 0.05, Figure 2), as well as an increase in the abundance of Clostridia (class) was observed in the OEO treatments(Supplementary Figure 1).
Figure 2.
LefSe analysis for the enriched microbiota (A), LDA value distribution histogram (B), and KEGG pathway for function prediction of ileal microbiota (C).
The LefSe analysis for determining the differential abundance of bacterial taxa was shown in Figure 3A. Ten bacterial biomarkers were identified among the four treatments. Clostridium sensu stricto_1 and Clostridiaceae_1 were the predominant microbes in OEO-300 diet. Obscuribacterales, Melainabacteria, and Ruminiclostridium_5 were the remarkable microbes in the CON treatment; Romboutsia, Rothia, and Burkholderiaceae were the remarkable microbes in the AB diet. In addition, function prediction exposed changed microbial function with diets containing OEO (OEO-150 and OEO-300), such as increasing amino sugar and nucleotide sugar metabolism, membrane transport, replication, and repair system and decreasing catabolism. The microbiota in the AB treatment predicted increased endocrine metabolism and suppressed the immune system, environmental adaptation, inter alia (Figure 3B).
Discussion
Phytogenics, mainly essential oils, have been reported to improve growth performances in farm animals and are thereby considered as potential key solutions for antibiotic-free livestock nutrition (Windisch et al., 2008). The present study, with Qingyuan partridge chickens, shows that dietary supplementation with 150 to 300 mg/kg OEO had a positive effect on growth performance. This is in agreement with Mathlouthi et al. (2012) who reported that dietary inclusion of 100 mg/kg OEO, comprising 69.55% carvacrol and 4.09% thymol, improved BW, ADG, and FCR in Arbor Acres broiler chickens, and Peng et al. (2016) who showed that dietary supplementation with 300 to 600 mg/kg OEO, containing 2.64% carvacrol and 1.3% thymol, had positive effect on the growth performance and carcass traits of Arbor Acres broilers. It is also reported (Pirgozliev et al., 2019) that addition of essential oils, comprising 5% carvacrol, 3% cinnamaldehyde, and 2% capsicum, improved ADFI, BW gain, and FCR in Ross 308 chicks. In addition, inclusion of 60 to 120 mg/kg OEO, containing an equal level of carvacrol and thymol at 14%, significantly counteracted coccidiosis-induced depression in BW gain and ADFI (Lee et al., 2020). These authors indicated that OEO could serve as a substitute for growth promoters and antibiotics to achieve similar effects in improving broiler production. However, different results were reported by other investigators. For example, Hernández-Coronado et al. (2019) showed that Ross broilers given drinking water containing 400 mg/L of two types of Mexican OEO, presenting 13.8% carvacrol and 28.4% thymol or 60.0% carvacrol and 3.9% thymol, had a slight decrease in ADFI and BW. It is possible that differences may stem from the supplementation levels, route of administration, and chemical composition and source/type of oregano.
In the current study, increased activities of jejunal GSH-Px and T-SOD and both jejunal and ileal T-AOC with decreasing local oxidation product (MDA) were observed in broiler chickens fed 150 mg/kg OEO. In broiler chickens, 150 mg/kg OEO, containing 65% carvacrol, from 15 to 35 d of age increased jejunal activities of GSH-Px and T-SOD; but reduced hepatic activities without affecting lipid peroxidation (Mueller et al., 2012). Also, Broiler diets containing 150 mg/kg oregano powder for 42 d increased the level of T-AOC and suppressed lipid peroxidation, while not altering activity of T-SOD in serum (Ri et al., 2017). Additionally, feeding Pekin ducks with 100 mg/kg OEO, comprising 2.3% carvacrol and 1.2% thymol, from 1 to 35 d did not affect the activities of SOD and T-AOC in serum, liver, or jejunum, whereas concentration of MDA decreased in serum and liver, but not in jejunum (Ding et al., 2020). Increasing inclusion of oregano powder to 10 g/kg in diets of Cherry Valley ducks between day 1 and day 42 had no effect on the activity of GSH-Px in serum and breast muscles and lipid peroxidation, but increased serum SOD activity (Park et al., 2015). In the current study, GPX1, HMOX1, and NRF2 mRNA expression was increased in ileal mucosa of chickens fed the diet supplemented with 150 mg/kg OEO. These findings are consistent with those of Mueller et al. (2012), who revealed that essential oils including OEO increased transcripts of antioxidative enzymes such as GPX1 by upregulating gene expression of HMOX1 and NRF2 in broilers. Therefore, dietary supplementation with OEO appears to enhance systemic and local defense against oxidative stress. It is more obvious to alleviate local oxidative stress in intestine.
In chickens, IgA, IgM, and IgG (IgY) are three major classes of immunoglobulins involved in the maintenance of immunity (Ulmer-Franco et al., 2012). Alp et al. (2012) and Mohiti-Asli et al. (2017) showed that 300 mg/kg dietary OEO from 1 to 42 d increased serum concentration of IgG in Ross 308 broilers. Similarly, supplementing broiler diet with 2 g/kg oregano aqueous extracts for 56 d led to significant improvement in the level of IgG (Franciosini et al., 2016), but 125 mg/kg essential oils (mainly oregano mixed with other oils) did not (Hong et al., 2012). It has been reported (Tzora et al., 2017; Mohiti-Asli and Ghanaatparast-Rashti, 2018; Stefanello et al., 2020) that supplemental OEO in poultry diets increased secretion of digestive enzymes, improved intestinal morphology, and consequently utilization of nutrients. It could have led to improved development of body organs including immune organs and production of natural antibodies, which would be beneficial for innate immune responses. In previous studies, bird strain, ages, tissue, and oregano level and form differed, which could influence the properties and effectiveness of OEO (Rodriguez-Garcia et al., 2016). Additionally, dietary OEO supplementation showed a tendency here toward suppressed TNF-α production and downregulated intestinal expression of inflammatory cytokines, consistent with previous studies conducted in broilers and carp fish (Du et al., 2016; Pirgozliev et al., 2019). These collective findings support an anti-inflammatory role for OEO. The intestinal physical barrier is a complete and tightly connected intestinal epithelium structure comprising intestinal mucosal epithelial cells and their tight connections. In the present study, the supplementation with OEO was also seen to increase the gene expression of the CLN1 and MUC2, which is consistent with the previous studies (Liu et al., 2018; Yang et al., 2019). As a primary barrier component of mucus layers, mucin 2 is a target site for SIgA. The SIgA is transported by polymeric immunoglobulin receptor from the lamina propria into luminal mucins to establish the first lines of intestinal defense (Zhang et al., 2015). Rogier et al. (2014) found that mucin 2, but not SIgA, was necessary for excluding gut bacteria from the inner mucus layer. In our study, the abundance of AvBD1 in ileum was upregulated with OEO supplementation. Avian β-defensins are antimicrobial peptides that attack various microorganisms and protect tissues from pathogenic infection. Therefore, it can be speculated that OEO might promote antimicrobial peptides and mucin proteins synthesis, some of which combine with SIgA and further control the number of intraepithelial lymphocytes to protect intestinal integrity.
The complex communities of the intestinal microbiota colonizing the gut of individuals play a crucial role in intestinal nutrient absorption, digestion, intestinal immune regulation, and intestinal health of the host (Tremaroli and Bäckhed, 2012; Sommer et al., 2017; Ruff et al., 2020). Studies have shown that OEO and its principal components, carvacrol and thymol, have antimicrobial properties in poultry production (Lambert et al., 2001). In the Qingyuan partridge chickens studied here, PCoA and ANOSIM analyses revealed that supplementation with 150 or 300 mg/kg OEO had similar composition of the ileal microbiota, but they were distinct from those in birds fed the CON and AB diets. On the phylum level, the diets with OEO led to higher counts of Firmicutes and fewer Proteobacteria and Actinobacteria than in the CON treatment. With the increase of Firmicutes, intestinal barrier functions are strengthened, and inflammatory responses are diminished (Huang et al., 2018). Proteobacteria are a microbial signature of dysbiosis, and its increase has been associated with intestinal diseases (Shin et al., 2015). On the genus level, diets with OEO had a higher relative abundance of Clostridium sensu stricto_1 and lower relative abundance of Romboutsia and Burkholderia–Caballeronia–Paraburkholderia. Clostridium sensu stricto, one of the beneficial bacteria, can produce short-chain fatty acids, indicating a capacity providing energy to intestinal cells and protecting the intestinal barrier (Kong et al., 2019). Dietary supplementation with phytogenics promotes beneficial Clostridia species, resulting in protection against enteric infection (Wlodarska et al., 2015).
Consistent with the findings of previous studies, OEO supplementation increased the abundance of Lactobacillus in addition to some butyrate-producing bacteria, such as Clostridium sensu stricto. Yin et al. (2017) reported that adding blends of essential oils changed the chicken ileal population of microbes by increasing the numbers of L. crispatus, and L. agilis, and decreasing L. salivarius and L. johnsonii. Bauer et al. (2019) reported that Ross-308 broiler diets supplemented with 1% and 2% oregano powder reduced the relative jejunal abundance of Proteus, Klebsiella, Staphylococcus, and Bifidobacterium. Whereas Betancourt et al. (2019) observed no effect of Colombian OEO, mainly represented with 0.9% carvacrol and 78.7% thymol, in cecal phylum and genus in coccidia-challenged broilers, but they found that a positive correlation existed between BW and the Firmicutes:Bacteriodetes ratio. Sidiropoulou et al. (2020) found that feeding broiler chickens a diet with a blend of 50 mg/kg OEO (containing 68.0% carvacrol and 3.7% thymol) and 5 mg/kg garlic essential oil had higher jejunal counts of E. coli and Enterobacteriaceae and lower counts of Clostridium perfringens. These results suggest that OEO may modify the composition of the gut microbial community and improve metabolic outcome in broiler chickens. Bioinformatics analysis suggested that dietary OEO changed the functions of gut microbiota, such as amino sugar and nucleotide sugar metabolism, membrane transport, replication, and repair systems, which may promote nutrient absorption and enhance intestinal barrier function. Reducing the number of intestinal pathogenic bacteria improved the regenerative capacity of epithelial cells, thereby increasing nutrients absorption (Choi et al., 2015; Zeng et al., 2015). From this, further studies are necessary to better elucidate the specific impacts of Clostridium sensu stricto enrichment on the host and to help further understand the role of OEO supplementation in the poultry intestine.
In conclusion, dietary supplementation with 150 to 300 mg/kg OEO (extracted from O. vulgare) enhanced growth performance, alleviated local oxidative stress in intestine, improved production of natural antibodies, and modulated structure of the intestinal microbiota. These findings allow us to gain deeper insights into the potential alternatives to antibiotics for local yellow-feathered chicken production.
Supplementary Material
Acknowledgments
We sincerely thank Prof. Dayong Wu (Tufts University) and W. Bruce Currie (Cornell University) for help in the critical review and edit of this manuscript. This study was partially supported by the Natural Science Foundation of Guangdong Province (2019A1515010912), the Visiting Scholar Program Sponsored by China Scholarship Council (201908440100), the China Agricultural Research System (CARS-41-G10), the Key Project of the Science and Technology Program of Guangzhou City (201804020091), the Key Laboratory of Animal Nutrition and Feed Science in South China, the Ministry of Agriculture and Rural Affairs, the National Key Research and Development Program (2018YFD0501504), the Scientific and Technological Project (2017B020202003) from the Department of Science and Technology of Guangdong Province, the Supporting Program for the Research of State Key Laboratory of Livestock and Poultry Breeding, the Supporting Program for Guangdong Agricultural Research and Development Center of Livestock and Poultry Healthy Breeding, the Guangdong Province Program of withdrawal Technology of in-feed Antibiotics, and the Presidential Foundation of Guangdong Academy of Agricultural Sciences (201805, 201807B, 201809B, and 201908).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- AME
apparent metabolic energy
- ANOSIM
analysis of similarities
- BW
body weight
- FCR
feed conversion ratio
- GSH-Px
glutathione peroxidase
- Ig
immunoglobulin
- LefSe
LDA effect size
- MDA
malonaldehyde
- OEO
oregano essential oil
- PBS
phosphate-buffered saline
- PCoA
principal coordinates analysis
- SIgA
secretory immunoglobulin A
- T-AOC
total antioxidative capacity
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- T-SOD
total superoxide dismutase
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Adaszyńska-Skwirzyńska, M., and D. Szczerbińska. . 2019. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 98:358–365. 10.3382/ps/pey385 [DOI] [PubMed] [Google Scholar]
- Alp, M., M. Midilli, N. Kocabağlı, H. Yılmaz, N. Turan, A. Gargılı, and N. Acar. . 2012. The effects of dietary oregano essential oil on live performance, carcass yield, serum immunoglobulin G level, and oocyst count in broilers. J. Appl. Poult. Res. 21:630–636. doi: 10.3382/japr.2012-00551 [DOI] [Google Scholar]
- Aziz, M., and S. Karboune. . 2018. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 58:486–511. doi: 10.1080/10408398.2016.1194256 [DOI] [PubMed] [Google Scholar]
- Bauer, B. W., A. Radovanovic, N. Willson, Y. S. Bajagai, T. T. H. Van, R. J. Moore, and D. Stanley. . 2019. Oregano: a potential prophylactic treatment for the intestinal microbiota. Heliyon 5:e02625. 10.1016/j.heliyon.2019.e02625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt, L., M. Hume, F. Rodríguez, D. Nisbet, M. U. Sohail, and G. Afanador-Tellez. . 2019. Effects of Colombian oregano essential oil (Lippia origanoides Kunth) and Eimeria species on broiler production and cecal microbiota. Poult. Sci. 98:4777–4786. doi: 10.3382/ps/pez193 [DOI] [PubMed] [Google Scholar]
- Broom, L. J., and M. H. Kogut. . 2019. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 98:1634–1642. doi: 10.3382/ps/pey535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., B. Tan, Y. Xia, S. Liao, M. Wang, J. Yin, J. Wang, H. Xiao, M. Qi, P. Bin, . et al. 2019. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct. 10:366–378. doi: 10.1039/c8fo02161a [DOI] [PubMed] [Google Scholar]
- Choi, K. Y., T. K. Lee, and W. J. Sul. . 2015. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens-a review. Asian-Australas J Anim Sci. 28:1217–1225. doi: 10.5713/ajas.15.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X., X. Wu, K. Zhang, S. Bai, J. Wang, H. Peng, Y. Xuan, Z. Su, and Q. Zeng. . 2020. Dietary supplement of essential oil from oregano affects growth performance, nutrient utilization, intestinal morphology and antioxidant ability in Pekin ducks. J. Anim. Physiol. Anim. Nutr. (Berl). 104:1067–1074. doi: 10.1111/jpn.13311 [DOI] [PubMed] [Google Scholar]
- Du, E., W. Wang, L. Gan, Z. Li, S. Guo, and Y. Guo. . 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens. J. Anim. Sci. Biotech. 7:19. 10.1186/s40104-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feed Database in China . 2016. Tables of feed composition and nutritive values in China 2016 twenty-seventh edition Chinese feed database. China Feed 21:33–43. doi: 10.15906/j.cnki.cn11-2975/s.20162109 [DOI] [Google Scholar]
- Franciosini, M. P., P. Casagrande-Proietti, C. Forte, D. Beghelli, G. Acuti, D. Zanichelli, A. dal Bosco, C. Castellini, and M. Trabalza-Marinucci. . 2016. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous on broiler performance, immune function and intestinal microbial population. J. Appl. Anim. Res. 44:474–479. 10.1080/09712119.2015.1091322 [DOI] [Google Scholar]
- Hernández-Coronado, A. C., R. Silva-Vázquez, Z. E. Rangel-Nava, C. A. Hernández-Martínez, J. R. Kawas-Garza, M. E. Hume, and G. Méndez-Zamora. . 2019. Mexican oregano essential oils given in drinking water on performance, carcass traits, and meat quality of broilers. Poult. Sci. 98:3050–3058. 10.3382/ps/pez094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, J. C., T. Steiner, A. Aufy, and T. F. Lien. . 2012. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 144:253–262. 10.1016/j.livsci.2011.12.008 [DOI] [Google Scholar]
- Huang, C., H. Jiao, Z. Song, J. Zhao, X. Wang, and H. Lin. . 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 93:2144–2153. doi: 10.2527/jas.2014-8739 [DOI] [PubMed] [Google Scholar]
- Huang, Y., X. Shi, Z. Li, Y. Shen, X. Shi, L. Wang, G. Li, Y. Yuan, J. Wang, Y. Zhang, . et al. 2018. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 14:3329–3337. doi: 10.2147/NDT.S188340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C., R. Gao, X. Yan, L. Huang, and H. Qin. . 2019. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 60:175–184. doi: 10.1016/j.nut.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Lambert, R. J., P. N. Skandamis, P. J. Coote, and G. J. Nychas. . 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x [DOI] [PubMed] [Google Scholar]
- Lee, J., D. Kim, Y. Kim, S. Jeong, S. Oh, S. Cho, and K. Lee. . 2020. Dietary encapsulated essential oils improve production performance of coccidiosis-vaccine-challenged broiler chickens. Animals 10:481. 10.3390/ani10030481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-López, N., E. P. Gutierrez-Grijalva, G. Vazquez-Olivo, and J. B. Heredia. . 2017. Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules 22:989. 10.3390/molecules22060989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., M. Song, W. Yun, J. Lee, C. Lee, W. Kwak, N. Han, H. Kim, and J. Cho. . 2018. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 102:1257–1265. doi: 10.1111/jpn.12944 [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and T. D. Schmittgen. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Magnusson, U. 2020. Prudent and effective antimicrobial use in a diverse livestock and consumer’s world. J. Anim. Sci. 98(Suppl 1):S4–S8. doi: 10.1093/jas/skaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathlouthi, N., T. Bouzaienne, I. Oueslati, F. Recoquillay, M. Hamdi, M. Urdaci, and R. Bergaoui. . 2012. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: in vitro antimicrobial activities and effects on growth performance. J. Anim. Sci. 90:813–823. doi: 10.2527/jas.2010-3646 [DOI] [PubMed] [Google Scholar]
- MOA . 2004. Feeding standard of chicken, 1st ed. Standards Press of China, Beijing, China. [Google Scholar]
- Mohiti-Asli, M. and M. Ghanaatparast-Rashti. . 2017. Comparison of the effect of two phytogenic compounds on growth performance and immune response of broilers. J. Appl. Anim. Res. 45:603–608. 10.1080/09712119.2016.1243119 [DOI] [Google Scholar]
- Mohiti-Asli, M. and M. Ghanaatparast-Rashti. . 2018. Comparing the effects of a combined phytogenic feed additive with an individual essential oil of oregano on intestinal morphology and microflora in broilers. J. Appl. Anim. Res. 46:184–189. 10.1080/09712119.2017.1284074 [DOI] [Google Scholar]
- Mueller, K., N. M. Blum, H. Kluge, and A. S. Mueller. . 2012. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic- and antioxidant enzymes in broiler chickens. Br. J. Nutr. 108:588–602. doi: 10.1017/S0007114511005873 [DOI] [PubMed] [Google Scholar]
- Park, Y. H., F. Hamidon, C. Rajangan, K. P. Soh, C. Y. Gan, T. S. Lim, W. N. W. Abdullah, and M. T. Liong. . 2016. Application of probiotics for the production of safe and high-quality poultry meat. Korean. J. Food. Sci. Anim. Resour. 31:567–576. 10.5851/kosfa.2016.36.5.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. H., S. N. Kang, D. Shin, and K. S. Shim. . 2015. Antioxidant enzyme activity and meat quality of meat type ducks fed with dried oregano (Origanum vulgare L.) powder. Asian-Australas. J. Anim. Sci. 28:79–85. doi: 10.5713/ajas.14.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Q. Y., J. D. Li, Z. Li, and Z. Y. Duan. . 2016. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed. Sci. Tech. 214:148–153. 10.1016/j.anifeedsci.2016.02.010 [DOI] [Google Scholar]
- Pirgozliev, V., S. C. Mansbridge, S. P. Rose, H. S. Lillehoj, and D. Bravo. . 2019. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 98:3443–3449. doi: 10.3382/ps/pey472 [DOI] [PubMed] [Google Scholar]
- Ri, C., X. Jiang, M. Kim, J. Wang, H. Zhang, S. Wu, V. Bontempo, and G. Qi. . 2017. Effects of dietary oregano powder supplementation on growth performance, antioxidant status and meat quality of broiler chicks. Ital. J. Anim. Sci. 16:246–252. 10.1080/1828051X.2016.1274243 [DOI] [Google Scholar]
- Rodriguez-Garcia, I., B. A. Silva-Espinoza, L. A. Ortega-Ramirez, J. M. Leyva, M. W. Siddiqui, M. R. Cruz-Valenzuela, G. A. Gonzalez-Aguilar, and J. F. Ayala-Zavala. . 2016. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 56:1717–1727. doi: 10.1080/10408398.2013.800832 [DOI] [PubMed] [Google Scholar]
- Rogier, E. W., A. L. Frantz, M. E. Bruno, and C. S. Kaetzel. . 2014. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens 3:390–403. doi: 10.3390/pathogens3020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, I., G. Gibson, A. Heinken, K. Scott, J. Swann, I. Thiele, and K. Tuohy. . 2018. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57:1–24. doi: 10.1007/s00394-017-1445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, W. E., T. M. Greiling, and M. A. Kriegel. . 2020. Host-microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18:521–538. doi: 10.1038/s41579-020-0367-2 [DOI] [PubMed] [Google Scholar]
- Salaheen, S., S. W. Kim, B. J. Haley, J. A. S. Van Kessel, and D. Biswas. . 2017. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 8:2088. doi: 10.3389/fmicb.2017.02088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, N. R., T. W. Whon, and J. W. Bae. . 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503. doi: 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Sidiropoulou, E., I. Skoufos, V. Marugan-Hernandez, L. Giannenas, E. Bonos, K. Aguiar-Martins, D. Lazari, D. P. Blake, and A. Tzora. . 2020. In vitro anticoccidial study of oregano and garlic esstential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 7:420. 10.3389/fvets.2020.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, F., J. M. Anderson, R. Bharti, J. Raes, and P. Rosenstiel. . 2017. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15:630–638. doi: 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- Stefanello, C., D. P. Rosa, Y. K. Dalmoro, A. L. Segatto, M. S. Vieira, M. L. Moraes, and E. Santin. . 2020. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 6:491. doi: 10.3389/fvets.2019.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh, G., R. K. Das, S. Kaur Brar, T. Rouissi, A. Avalos Ramirez, Y. Chorfi, and S. Godbout. . 2018. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 44:318–335. doi: 10.1080/1040841X.2017.1373062 [DOI] [PubMed] [Google Scholar]
- Tremaroli, V., and F. Bäckhed. . 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. doi: 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- Tzora, A., I. Giannenas, A. Karamoutsios, N. Papaioannou, D. Papanastasiou, E. Bonos, S. Skoufos, T. Bartzanas, and I. Skoufos. . 2017. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. J. Poult. Sci. 54:218–227. doi: 10.2141/jpsa.0160071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer-Franco, A. M., G. Cherian, N. Quezada, G. M. Fasenko, and L. M. McMullen. . 2012. Hatching egg and newly hatched chick yolk sac total IgY content at 3 broiler breeder flock ages. Poult. Sci. 91:758–764. doi: 10.3382/ps.2011-01757 [DOI] [PubMed] [Google Scholar]
- Windisch, W., K. Schedle, C. Plitzner, and A. Kroismayr. . 2008. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 86(14 Suppl):E140–E148. doi: 10.2527/jas.2007-0459 [DOI] [PubMed] [Google Scholar]
- Wlodarska, M., B. P. Willing, D. M. Bravo, and B. B. Finlay. . 2015. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 5:9253. doi: 10.1038/srep09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Y. Liu, F. Yan, C. Yang, and X. Yang. . 2019. Effects of encapsulated organic acids and essential oils on intestinal barrier. Poult. Sci. 98:2858–2865. 10.3382/ps/pez031 [DOI] [PubMed] [Google Scholar]
- Yin, D., E. Du, J. Yuan, J. Gao, Y. Wang, S. E. Aggrey, and Y. Guo. . 2017. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringens in chickens. Sci. Rep. 7:7334. doi: 10.1038/s41598-017-07420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Z., S. Zhang, H. Wang, and X. Piao. . 2015. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 6:7. doi: 10.1186/s40104-015-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., S. D. Eicher, and T. J. Applegate. . 2015. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 94:172–180. doi: 10.3382/ps/peu064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.