Abstract
Conjugated linoleic acid (CLA) improves oxidative stress and mitochondrial biogenesis in various species but has not been thoroughly investigated in horses. We collected blood and muscle samples from lightly exercising horses before and 6 and 12 wk after receiving either soybean oil (CON; n = 5) or CLA (CLA; n = 5) supplementation. Samples were analyzed for markers of mitochondrial characteristics, antioxidant status, oxidative stress, and muscle damage. Data were analyzed using a linear model with repeated measures. In the triceps brachii (TB), citrate synthase (CS) activity was higher in CON than CLA horses (P = 0.003) but was unaffected by diet in the gluteus medius (GM). Integrative (relative to mg protein) cytochrome c oxidase (CCO) activity was higher in TB than the GM (P < 0.0001), while intrinsic (relative to CS) CCO was lower in the TB than the GM (P = 0.02) and tended to be lower in CON than CLA horses (P = 0.06). Neither CS nor integrative CCO activities were affected by time. In the GM, superoxide dismutase activity tended to increase in CON through week 12 (P = 0.10). Over both muscle groups, glutathione peroxidase activity tended to be higher in CON compared with CLA at week 12 (P = 0.06). Malondialdehyde was higher in the TB than the GM (P = 0.0004) but was unaffected by diet, while serum creatine kinase activity tended to be lower in CLA than CON horses (P = 0.07). These results suggest that CLA supplementation may lead to mitochondrial adaptations and prevent myofiber perturbation in skeletal muscle of young, lightly exercised horses.
Keywords: conjugated linoleic acid, equine, fatty acid, oxidative stress, mitochondria
Introduction
Derived from ruminant food products, conjugated linoleic acids (CLA) are fatty acid stereoisomers of linoleic acid (LNA) with biologically important functions (Bauman et al., 2000; Ali et al., 2012). Isomers cis-9, trans-11 and trans-10, cis-12, as well as the mixture, affect body composition (Ostrowska et al., 1999; Park et al., 1999), behave as anticarcinogens (Ha et al., 1987; Pariza, 2004), activate peroxisome proliferation (Moya-Camarena et al., 1999), and have antioxidant capabilities (Leung and Liu, 2000; Luo et al., 2012; Sharifi et al., 2018). Although the mechanism for CLA antioxidant activity is unknown, it is suggested that high levels of CLA are capable of quenching free radicals (Luo et al., 2012). Furthermore, CLA has been shown to activate nuclear factor erythroid 2-related factor 2 (Nrf2) which regulates expression of antioxidant enzymes during high levels of oxidative stress (Mollica et al., 2014). Oxidative stress results from excess free radical production and can be stimulated by intense exercise. However, limited oxidative stress can incite mitochondrial biogenesis provided that antioxidant sources are not overwhelmed by reactive oxygen species (ROS; Onmaz et al., 2011; Sun et al., 2015). Accumulation of ROS is dangerous to living cells. Their ability to destroy lipid membranes through lipid peroxidation contributes to aging and tumor development (Kehrer, 1993; Martínez-Cayuela, 1995).
Recent work in mice has indicated that CLA enhances proteins involved in mitochondrial biogenesis (Kim and Park, 2015). Peroxisome proliferator-activated receptor-coactivator 1α (PGC-1α), commonly known as the “master regulator” of mitochondrial biogenesis, is the primary protein that initiates expansion of mitochondria (Dorn et al., 2015). Supplementation of CLA reportedly augmented in vitro and in vivo PGC-1α protein expression (Kim and Park, 2015; Kim et al., 2016b). Furthermore, CLA amplified transcription and translation of mitochondrial DNA through activation of nuclear respiratory factor 1 (Nrf1) and mitochondrial transcription factor A (Tfam; Kim and Park, 2015; Kim et al., 2016a, 2016b). Increased mitochondrial biogenesis could increase energy production and enhance endurance capacity, but may also increase ROS production, as ROS are a natural by-product of mitochondrial oxidative respiration.
Mitochondria-dense muscles of equine athletes constantly produce high amounts of adenosine triphosphate (ATP) to accommodate energy needs during exercise (Hodgson and Rose, 1987; Poole, 2004). Consequently, it has been reported that horses involved in high-intensity exercise training are extremely susceptible to high levels of oxidative stress due to overwhelming ROS status (Kirschvink et al., 2008). While horses undergoing moderate exercise do not appear to be as prone to elevated ROS (Valberg et al., 1993; Velázquez-Cantón et al., 2018), dietary supplementation of antioxidants such as vitamin E and selenium has become common in performance horses to maintain equilibrium between oxidant and antioxidant status (Avellini et al., 1999; White et al., 2016; White and Warren, 2017; Velázquez-Cantón et al., 2018). To our knowledge, mitochondrial and antioxidant benefits of CLA in horses have not been extensively investigated. Therefore, the aim of this study was to elucidate CLA effects on lightly exercised horses to determine the value of continuous supplementation. We hypothesized that CLA would mitigate exercise-related oxidative stress and enhance mitochondrial volume density and function.
Materials and Methods
Horses
This study was reviewed and approved by the Sam Houston State University (SHSU) Institutional Animal Care and Use Committee (16-09-08-1008-3-01) prior to the start of the experiment. The muscle tissue collection procedure was reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee (2016-0294) prior to tissue collections. Ten stock type horses (5 castrated males and 5 females) ranging in age from 1 to 3 yr with a mean starting BW of 385 kg (SEM 14) were used in this 12-wk study. All horses were enrolled in SHSU’s Equine Behavior and Training course throughout the duration of the study. In accordance with the course, horses were individually housed in 3 × 3 m stalls with ~30 min/d access to dry lots 5 d/wk at the SHSU Agriculture Center in Huntsville, TX. Horses were exercised on a freestyle mechanical exerciser (Priefert Manufacturing, Mt. Pleasant, TX) 5 d/wk. The first 9 wk of the exercise program consisted of 10 min walk at 1.6 m/s, 10 min trot at 3.1 m/s, then an additional 10 min of walk, switching direction every 5 min for a total of 30 min. Three wk before the final biopsy, exercise intensity slightly increased but total time decreased to 20 min following the Equine Behavior and Training course schedule. Beginning at week 10, horses began and ended the exercise walking 4 min at 1.6 m/s (2 min each direction). Between walking, horses trotted for 6 min at 3.1 m/s (3 min each direction), then increased trotting speed to 4.7 m/s for 6 min (3 min each direction). In addition to exercise on the mechanical exerciser, all horses were lightly exercised in the class 5 d/wk while learning to carry a saddle and first rides (minimal walking and potentially light trotting).
Dietary treatments
Horses were blocked by age, sex, and BW and randomly assigned to receive 1.5% of total daily concentrate of either soybean oil (CON; n = 5) or CLA (CLA; n = 5) top-dressed on their concentrate. The CLA supplement contained 55% CLA with a mixture of cis-9, trans-11 and trans-10, cis-12 isomers (Lutalin, BASF Corp., Florham Park, NJ). The soybean oil was devoid of CLA (Table 1; previously reported by Bradbery et al., 2018). The basal diet consisted of 1% BW/d (as-fed) commercial concentrate (SafeChoice, Cargill Animal Nutrition, Elk River, MN) and 1% BW/d (as-fed) coastal Bermudagrass (Cynodon dactylon) hay. This resulted in a supplementation rate of 82.5 mg CLA/kg BW/d. The daily ration of basal diet plus supplement was split into 2 equal meals offered to horses individually in 3 × 3 m stalls at 12 hr intervals. Diets were isocaloric and isonitrogenous, formulated to meet or exceed NRC requirements for growing horses (NRC, 2007), and were adjusted weekly for changes in BW. All feeds were analyzed prior to beginning the study. Concentrate and hay were analyzed by Equi-Analytical Laboratories (Ithaca, NY) using standard analytical methods (Table 2). This study was performed concurrent with a separate study focused on the impacts of CLA supplementation on body composition in young horses undergoing regular exercise (Miller et al., 2018). The inclusion rate of dietary CLA was based on levels expected to achieve statistically significant differences in body composition outcomes in young, lightly exercised horses (Headley et al., 2012).
Table 1.
Concentration of CLA isomers in dietary supplements1
| Fatty acid | Soybean oil | CLA |
|---|---|---|
| cis-9, trans-11 CLA, % | ND2 | 26.18 |
| trans-10, cis-12 CLA, % | ND2 | 26.12 |
1Previously published by Bradbery et al.(2018).
2ND, not detectable (minimum detectable limit 0.003%).
Table 2.
Nutrient composition of basal diet feeds1
| Grain concentrate | Pasture forage | |
|---|---|---|
| DE, Mcal/kg | 3.03 | 2.04 |
| Crude fat, % | 8.36 | 1.4 |
| Crude protein, % | 16.1 | 9.0 |
| Lysine, % | 1.00 | 0.31 |
| NDF, % | 33.5 | 65.3 |
| ADF, % | 18.0 | 36.0 |
| Ca, % | 1.63 | 0.21 |
| P, % | 1.14 | 0.17 |
| Mg, % | 0.48 | 0.11 |
| Zn, ppm | 235 | 42 |
| Cu, ppm | 65 | 9 |
| Fe, ppm | 429 | 119 |
| Mn, ppm | 248 | 138 |
| Se, ppm | 1.07 | 0.08 |
| Co, ppm | 1.8 | 0.8 |
1Values are presented on a 100% dry matter basis.
Sample collection
Skeletal muscle and blood samples were collected at weeks 0, 6, and 12 for analysis of muscle citrate synthase (CS), cytochrome c oxidase (CCO), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities, and malondialdehyde (MDA) concentration, and serum creatine kinase (CK) activity. Collections occurred ~3 to 4 hr before exercise. Muscle tissue samples were collected from the gluteus medius (GM) and triceps brachii (TB) using a tissue collection procedure as previously described (White et al., 2016). Briefly, horses were sedated with detomidine hydrochloride that was administered intravenously at recommended dosages (Dormosedan; Zoetis, Parsippany-Troy Hills, NJ) prior to beginning tissue collection procedures. The collection areas were clipped, scrubbed with a 7.5% povidone-iodine solution, and then rinsed with a 70% ethanol solution. The tissue collection sites were desensitized with 0.5 mL of 2% lidocaine (Vetone, Boise, ID) and a 14-gauge needle was used to create the initial puncture through the skin. Tissue was collected using a 14-gauge, 9-cm tissue collection needle (SuperCore; Argon Medical Devices Inc., Frisco, TX) inserted to a depth of 5 cm. The tissue collection site alternated between left and right muscle groups at each sampling interval. Samples obtained from the same side of the horse were obtained ~2 cm from the previous insertion site. At each sampling interval, ~300 mg (wet weight) of muscle tissue was flash frozen in liquid nitrogen and stored at –80 °C until enzymatic activity analyses were performed. Flash frozen muscle was cryopulverized into a fine powder (Spectrum Bessman Tissue Pulverizer; Thermo Fisher Scientific, Waltham, MA) for evaluation for enzyme activities and MDA concentration. Approximately 10 mL of blood was collected into evacuated containers (Vacutainer; Becton, Dickinson and Co., Franklin Lakes, NJ) containing no anticoagulant for harvesting of serum. Serum samples remained at 25 °C for ~1 hr prior to processing. Samples were then centrifuged at 3,000 × g for 10 min at 4 °C, and serum was harvested and stored at –80 °C until analysis.
Muscle enzyme activities
Muscle samples were analyzed for CS and CCO activities as markers of mitochondrial volume density and function, respectively (Larsen et al., 2012; Meinild Lundby et al., 2018), as previously reported (Spinazzi et al., 2012; Li et al., 2016), using a microplate reader (Synergy H1; Biotek, Winooski, VT). Briefly, CS activity was assessed at 412 nm by measuring the initial rate of reaction of free CoA-SH with DTNB; CCO activity was determined by measuring the maximal, linear rate of oxidation of fully reduced cyt c at 550 nm. Samples were analyzed in triplicate; intra-assay CV was 2.3% and 2.1% and interassay CV was 5.7% and 7.2% for muscle CS and CCO activities, respectively. Enzymatic activities were normalized to protein content, determined using the Coomassie Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Cytochrome c oxidase activity is presented on an integrative (per milligram protein) and intrinsic (per unit CS) basis.
Muscle samples were also analyzed for SOD and GPx activities as measures of antioxidant status using commercially available kits (SOD: NWK-SOD02; GPx: NWK-GPX01; Northwest Life Science Specialties, LLC, Vancouver, WA). Muscle tissue that had been previously cryopulverized and stored at –80 °C was diluted 1 mg tissue (wet weight) to 20 μL extraction buffer (1 mM EGTA, 210 mM mannitol, 70 mM sucrose, pH 7.2). Diluted samples were sonicated using a sonic dismembrator (Fisher Scientific, Hampton, NH) 3 times for 3 s each on ice, and then centrifuged at 10,000 × g for 15 min at 4 °C. Homogenate supernatants were collected and stored at –80 °C until analysis. Muscle samples were analyzed per manufacturer instructions in triplicate for GPx and SOD activity. Intra-assay CV for both GPx and SOD was 4.8%.
Muscle malondialdehyde concentration
Muscle samples were evaluated for concentration of the lipid peroxidation marker, MDA, per manufacturer instructions using a commercially available kit (Northwest Life Science Specialties, LLC) as previously described (White and Warren, 2017). Previously cryopulverized muscle powder was diluted 1 mg tissue (wet weight) to 10 μL assay buffer provided in the kit and sonicated 3 times for 3 s each on ice, and then centrifuged at 11,000 × g for 10 min at 4 °C. Homogenate supernatants were collected and stored at –80 °C until analysis. Samples were analyzed in triplicate with an intra-assay CV of 2.9%. Muscle homogenate total protein was quantified using the Coomassie Protein Assay kit (Thermo Fisher Scientific), and MDA concentration was normalized to sample total protein concentration.
Serum creatine kinase activity
Serum samples were analyzed for CK activity as a marker of muscle damage (Siciliano et al., 1995) using a commercially available kit, following manufacturer instructions (CK Liqui-UV; EKF Diagnostics, Boerne, TX). Samples were analyzed in triplicate with an intra-assay CV of 4.7%.
Statistical analysis
Given the range of ages for horses in this study, we correlated serum CK and muscle SOD, GPx, CS, and CCO activities with starting age in months using PROC CORR in SAS 9.4 (SAS Institute, Inc., Cary, NC) to ensure 1-yr-olds did not present different measures to 3-yr-olds, for example. No variable was correlated with age in months (P > 0.3).
Differences in muscle enzyme activities and MDA concentration, as well as serum CK activity were analyzed using repeated linear models in SAS v9.4. For muscle measures, dietary treatment, time, muscle group, and all interactions were included in the model as fixed effects, and horse within diet was a random effect. For serum CK, dietary treatment, time, and the diet × time interaction were included in the model as fixed effects, and horse within diet was a random effect. For all variables, sex and age in years were initially included in the model as fixed effects but removed due to the lack of statistical significance (P > 0.1). Data were tested for normality and determined to be normally distributed. For GPx and CS activities, week 0 values were determined to significantly affect the model (P ≤ 0.02); therefore week 0 values were included in the model as a covariate. All data are expressed as least squared means ± SEM. Significance was considered at P ≤ 0.05, and trends were acknowledged at P ≤ 0.10.
Results
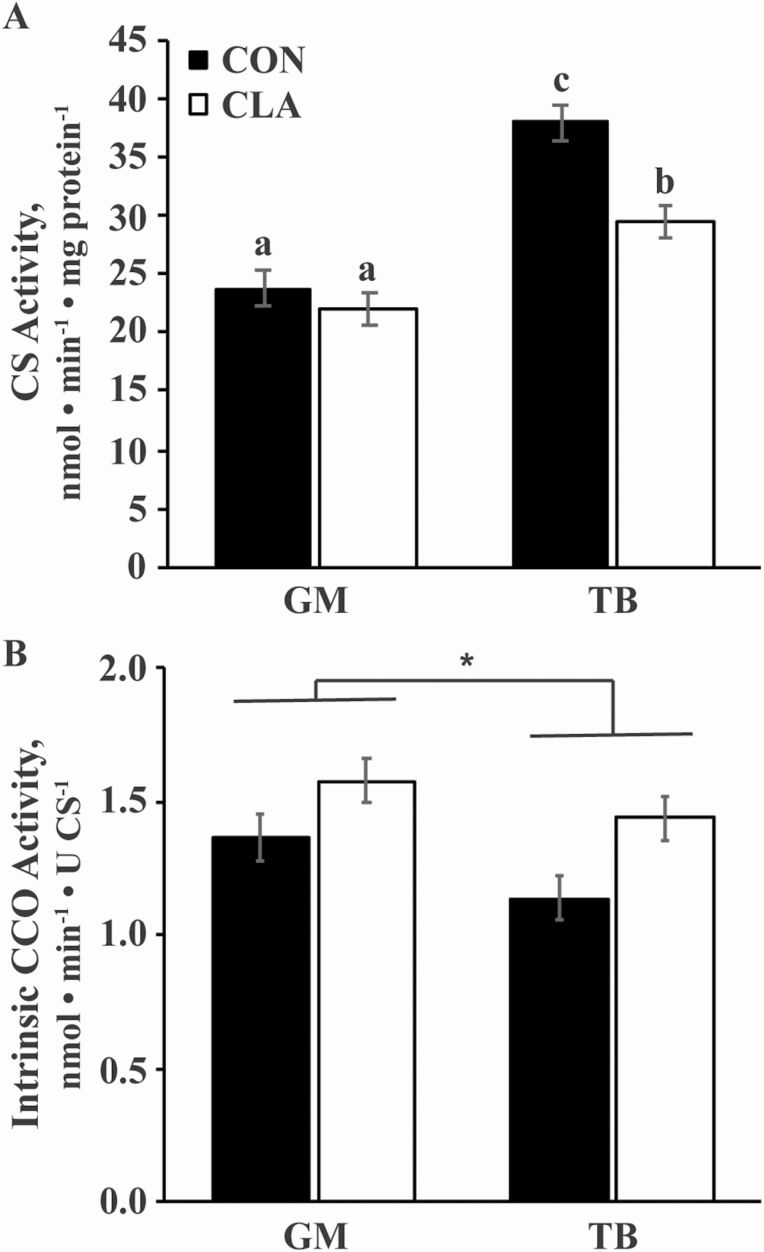
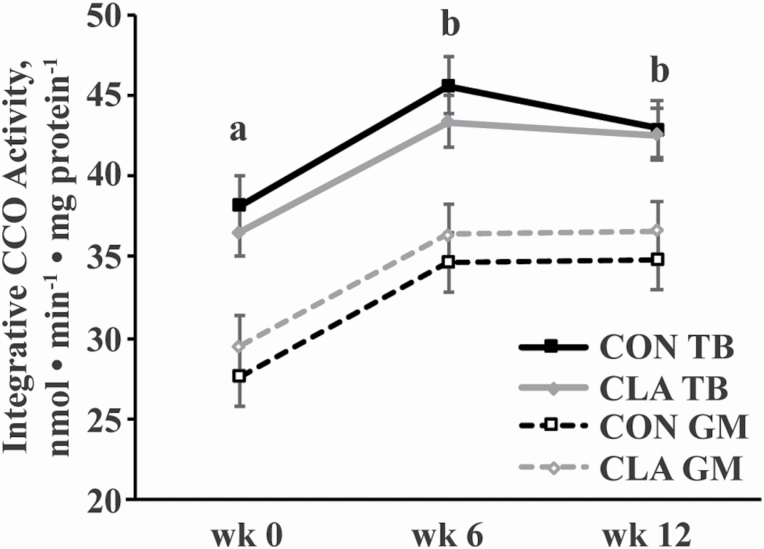
Citrate synthase activity, a marker of mitochondrial volume density (Larsen et al., 2012; Meinild Lundby et al., 2018), was greater in the TB than the GM (P < 0.0001) but was not affected by time (Figure 1A). An interaction of diet and muscle group (P = 0.05) indicated that CS activity was greater in the TB of CON than CLA horses (P = 0.003) but was not different between dietary treatments in the GM (Figure 1A). Intrinsic (relative to CS activity) CCO activity, a marker of mitochondrial function (Larsen et al., 2012), was lower in the TB than GM (P = 0.02) and tended to be lower in CON than CLA horses (P = 0.06), but was unaffected by time (Figure 1B). Similar to CS activity, integrative (relative to mg protein) CCO activity, was also greater in the TB than GM (P < 0.0001) but was not different between dietary treatments (Figure 2). Integrative CCO activity was affected by time (P = 0.007), increasing from weeks 0 to 6 (P = 0.004) but not changing from weeks 6 to 12, resulting in greater activity at week 12 than 0 (P = 0.008; Figure 2).
Figure 1.
(A) CS and (B) intrinsic (relative to CS activity) CCO activities in the GM and TB muscles of young horses receiving either soybean oil (CON; n = 5) or conjugated linoleic acid (CLA; n = 5) for 12 wk. Due to the lack of effect of time and any interactions with time, all time points have been combined. Overall effects of dietary treatment (P = 0.04, P = 0.06), muscle group (P < 0.0001, P = 0.01), and diet × muscle group (P = 0.05, P = 0.5) for panels A and B, respectively, as determined by ANOVA. a,b,cBars with different letters differ (P < 0.05). *GM differs from TB (P = 0.01).
Figure 2.
Integrative (relative to mg protein) CCO activity in the GM and TB muscles of young horses receiving either soybean oil (CON; n = 5) or conjugated linoleic acid (CLA; n = 5) for 12 wk. Overall effects of dietary treatment (P = 0.9), time (P = 0.007), muscle group (P < 0.0001), diet × time (P = 0.9), diet × muscle group (P = 0.4), time × muscle group (P = 0.9), and diet × time × muscle group (P = 0.9) as determined by ANOVA. a,b Time points with different letters differ (P < 0.05).
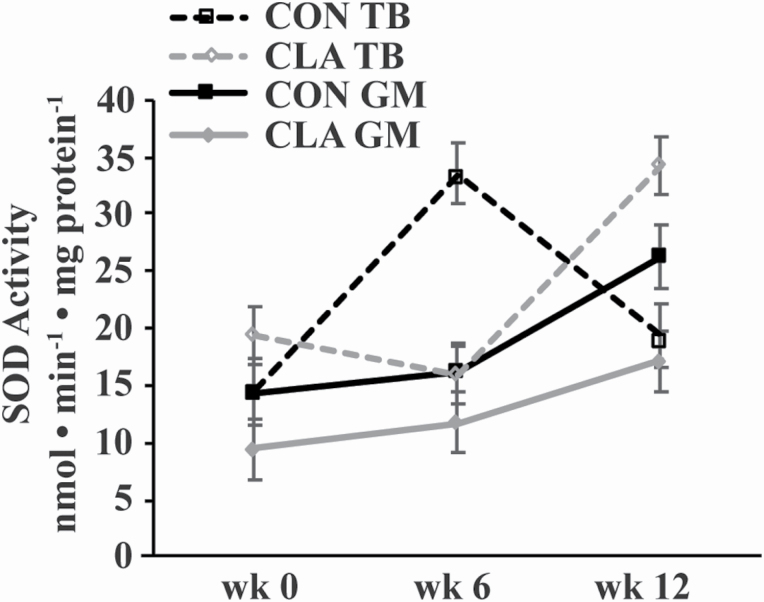
Overall, there was an interaction of dietary treatment, time, and muscle group on muscle SOD activity (P = 0.03; Figure 3). Activity of SOD in the GM of CON horses gradually increased to week 12, resulting in a tendency to be greater at week 12 compared with week 0 (P = 0.1). In contrast, SOD activity in the GM of CLA horses remained unchanged through week 12 (Figure 3). In the TB, SOD activity increased in CON horses at week 6 (P = 0.004) to be greater than CON GM, CLA GM, and CLA TB at week 6 (P ≤ 0.009). Activity of SOD then decreased in CON TB by week 12 (P = 0.05) to be similar to week 0 at week 12. Activity of SOD in the TB of CLA horses, on the other hand, increased by week 12 to be greater than weeks 0 and 6 (P ≤ 0.02). By week 12, SOD activity was greater in the TB of CLA than CON TB and CLA GM (P ≤ 0.04; Figure 3).
Figure 3.
SOD activity in the GM and TB muscles of young horses receiving either soybean oil (CON; n = 5) or conjugated linoleic acid (CLA; n = 5) for 12 wk. Overall effects of dietary treatment (P = 0.4), time (P = 0.02), muscle group (P = 0.01), diet × time (P = 0.09), diet × muscle group (P = 0.2), time × muscle group (P = 0.6), and diet × time × muscle group (P = 0.03) as determined by ANOVA.
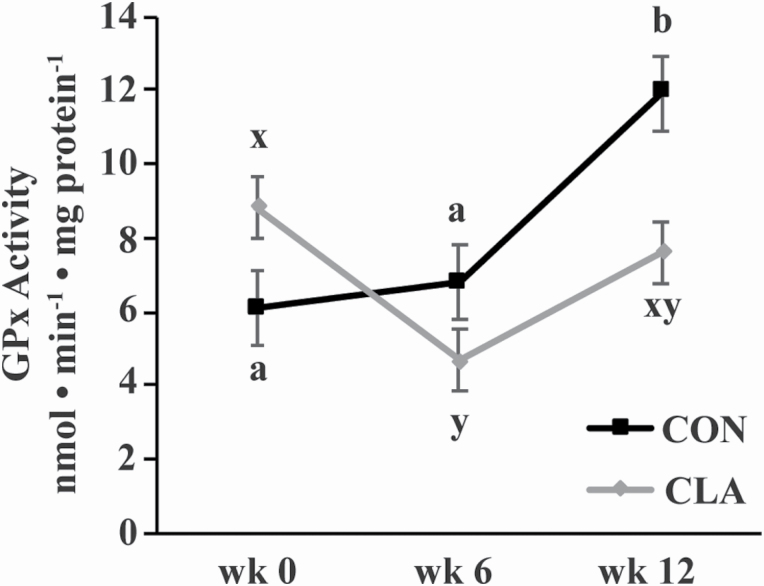
Glutahione peroxidase activity did not differ between the GM and the TB (Figure 4). However, an interaction of dietary treatment and time (P = 0.03) revealed that, over both muscle groups, GPx activity increased in CON horses at week 12 to be greater than week 0 and 6 (P ≤ 0.02; Figure 4). In CLA horses, muscle GPx activity decreased at week 6 (P = 0.03). This resulted in a trend for CON horses to have greater GPx activity than CLA horses at wk 12 (P = 0.06).
Figure 4.
GPx activity in the GM and triceps brachii muscles of young horses receiving either soybean oil (CON; n = 5) or conjugated linoleic acid (CLA; n = 5) for 12 wk. Due to the lack of effect of muscle group and any interactions with muscle group, muscle groups have been combined. Overall effects of dietary treatment (P = 0.4), time (P = 0.02), and diet × time (P = 0.03) as determined by ANOVA. a,b,x,yWithin dietary treatment, time points with different letters differ (P < 0.05).
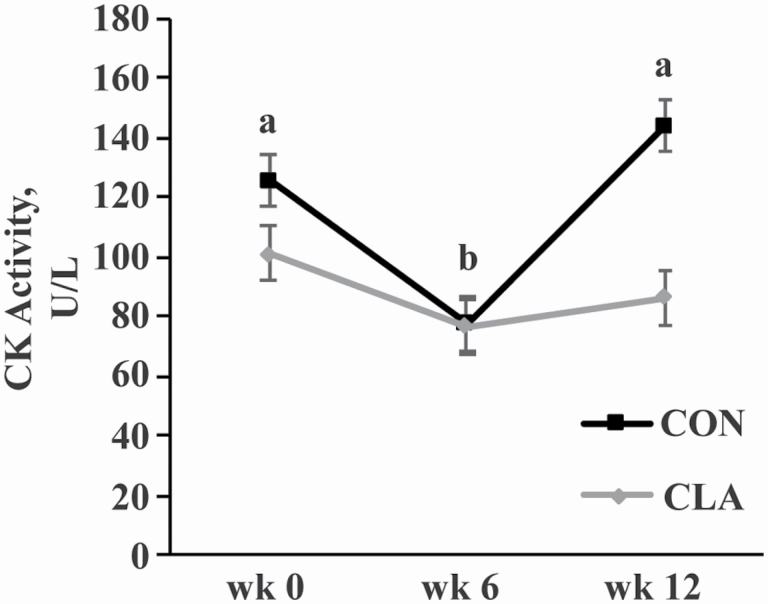
Muscle MDA, a marker of oxidative stress, was higher in the TB than the GM throughout the study (66.0 ± 3.9 vs. 43.9 ± 4.0 pmol/mg protein, respectively; P = 0.0004) but was unaffected by diet, time, or any interaction. Serum CK, a marker of muscle damage, decreased from weeks 0 to 6 (P = 0.04) then increased at week 12 (P = 0.04) to be similar between weeks 0 and 12 (Figure 5). Overall, CON horses tended to have higher serum CK than CLA horses (P = 0.07), but there was no interaction of time and dietary treatment on serum CK (Figure 5).
Figure 5.
Serum CK activity of young horses receiving either soybean oil (CON; n = 5) or conjugated linoleic acid (CLA; n = 5) for 12 wk. Overall effects of dietary treatment (P = 0.07), time (P = 0.05), and diet × time (P = 0.2) as determined by ANOVA. a,bTime points with different letters differ (P < 0.05).
Discussion
The aim of this study was to elucidate antioxidant benefits of CLA in skeletal muscle of young, exercising horses as well as to provide insight on the impact of CLA supplementation on mitochondria. Horses receiving CLA had lower mitochondrial volume density (CS activity) in the TB, but intrinsic mitochondrial function (CCO activity) was greater in CLA-supplemented horses compared with nonsupplemented CON horses across both muscle groups. Similar to previous work (Geelen et al., 2000; White et al., 2017a, 2017b) light exercise training did not invoke mitochondrial biogenesis in these young horses. In general, horses receiving CLA supplementation appeared to show less muscle damage (serum CK activity) but also had lower muscle antioxidant activity.
The current study investigated both the TB and the GM in order to evaluate dietary impact of CLA on muscles with varying metabolic profiles. In the horse, the TB serves more of a postural role and has a greater percentage of oxidative fibers compared with the GM, which is a propulsive muscle, engaged during forward movement, and has a greater percentage of glycolytic fibers (van den Hoven et al., 1985; Galisteo et al., 1992; Sewell et al., 1994; Enríquez et al., 2015). Muscles with a greater proportion of oxidative fibers tend to contain more mitochondria for enhanced oxidative respiration to generate energy, while muscles with a greater proportion of glycolytic fibers contain fewer mitochondria (Mishra et al., 2015). This is supported by data in the current study, which showed the TB had greater mitochondrial volume density and integrative mitochondrial function compared with the GM, and is in agreement with previous research in young Quarter Horses (White et al., 2017a). Also similar to previous reports (White et al., 2017a), intrinsic mitochondrial function, which indicates the function at a mitochondrial unit level rather than a whole tissue level, was lower in the TB compared with the GM. In essence, this indicates that fewer mitochondria may be needed to achieve the same functional output in the GM compared with the TB, which is likely related to the differing functions of the muscle groups.
Interestingly, intrinsic mitochondrial function was greater in CLA horses compared with CON horses, but integrative mitochondrial function and mitochondrial volume density were unaffected by diet. A lack of an effect of CLA supplementation on mitochondrial function was similarly reported in murine skeletal and cardiac muscle (Van Hoose et al., 2016), though at a lower rate of supplementation (20 mg CLA/kg BW/d). However, previous investigation of fatty acid profiles of tissues in mice supplemented with 240 mg CLA/kg BW/d indicated that CLA was integrated into adipose, but not into muscle tissue, which may be the reason muscle mitochondrial function was not impacted by CLA supplementation in those studies (Shen et al., 2013, 2015). Consequently, CCO mRNA and activity were amplified in adipose, but not muscle, in CLA-fed mice. Notably, mRNA levels of peroxisome proliferator-activated receptor γ (PPAR γ), which triggers fatty acid oxidation, were increased in adipose tissue which would enhance substrate availability for oxidative phosphorylation (Shen et al., 2013, 2015). Similar to our results, no change in liver or soleus muscle (an oxidative muscle) CS activity was noted in unexercised mice and rats following supplementation of 80 to 3000 mg CLA/kg BW/d (Javadi et al., 2004; Mollica et al., 2014; Rossignoli et al., 2018). Conversely, when CS activity was presented relative to tissue weight rather than normalized to protein content, CS activity was markedly elevated in CLA-fed animals potentially indicating CLA increased mitochondrial volume density but with a concomitant increase in muscle protein accretion (Javadi et al., 2004; Mollica et al., 2014). In contrast, our study indicated lower CS activity with CLA treatment, even when expressed relative to tissue weight (data not shown). These conflicting results warrant further investigation.
A common concern associated with greater mitochondrial activity is the potential for elevated ROS production. While ROS act as essential signaling molecules, an overproduction of ROS (typically during intense exercise, stress, or disease) may lead to cellular damage. This idea is supported by our data that indicate the TB, which had higher CS activity (mitochondrial volume density) and integrative CCO activity (mitochondrial function) than the GM, also had higher MDA concentrations, indicative of oxidative stress. The TB also had higher SOD activity (an antioxidant) than the GM, which may have served to combat the excess ROS production. Similar results have been reported in exercised rats, where SOD production was higher in the soleus (slow twitch) than in the extensor digitorum longus (fast twitch) muscle (Kwon et al., 2017).
Superoxide dismutase and GPx are antioxidants produced by animals to counteract oxidative stress. Superoxide dismutase catalyzes the reaction of superoxide anions to molecular oxygen and hydrogen peroxide while GPx further reduces hydrogen peroxide to nontoxic water (Bowler et al., 1992). In our study, activities of both SOD and GPx were elevated in the GM of CON horses, which may indicate CLA was sufficient to reduce free radicals in the supplemented group, while CON required increased antioxidants to combat oxidative stress. Additionally, GPx prevents lipid peroxidation which could explain relatively low MDA concentrations in the GM (Hassan Eftekhari et al., 2013; Gaschler and Stockwell, 2017). Similar to our results, in vitro supplementation of 10 ppm CLA to rat hepatocytes decreased SOD and GPx activities (Cantwell et al., 1999). Further, supplementation of as little as 612 mg CLA/100 g diet (compared with 825 mg CLA/100 g diet in the current study) substantially reduced SOD production in rats undergoing wound treatment compared with a placebo diet (Park et al., 2010). Although the inflammatory reaction is not identical during exercise and wound healing, this may still advocate for free radical quenching capabilities of CLA. Contrasting reports showed that during CLA and selenium supplementation to sedentary lambs, CLA had no effect on GPx, while selenium improved GPx protein and gene expression (Ghaderzadeh et al., 2019), which is likely attributable to selenium being included in the molecular composition of GPx. In our study, inconsistencies in the effects of CLA presented themselves in the TB at week 12 where SOD increased in CLA horses but decreased in CON horses. Although the exercise regimen slightly increased in intensity 3 wk before the final muscle collection, it is unlikely this change influenced a surge of SOD in CLA horses since the slight increase in intensity was paired with a decrease in exercise duration and the overall intensity was still very low. These results warrant further investigation.
Given that mitochondrial volume density and integrative mitochondrial function were similar between CLA and CON horses, but intrinsic mitochondrial function was higher in CLA horses, we hypothesized that mitochondria in CLA horses may be more efficient at producing ATP, thereby producing fewer ROS. In fact, MDA concentration was unaffected by CLA supplementation in the current study, indicating no differences in oxidative stress due to diet. However, after 12 wk of supplementation, CLA horses had lower GPx activity compared with CON horses, as well as lower SOD activity in the GM, perhaps suggesting a decreased need for activity of traditional antioxidant enzymes in CLA horses. Previous studies have detailed decreases in MDA due to CLA supplementation in models of stress, including strenuous exercise (Kwon et al., 2017) or compromised immunity (Park et al., 2010; Hassan Eftekhari et al., 2013; Matin et al., 2018). In salmonella-infected chicks, a mixed CLA diet increased SOD activity and decreased MDA (Zhang et al., 2008). Notably, MDA concentrations in our study did not change over time, indicating that the submaximal exercise protocol utilized in the current study was likely not intense enough to induce lipid peroxidation. This is supported by work in moderately exercised horses supplemented with the antioxidants, selenium and vitamin E, which showed no change in MDA concentrations following training (Velázquez-Cantón et al., 2018). In racehorses undergoing intensive training, however, selenium and vitamin E supplementation decreased MDA concentrations (Avellini et al., 1999). Combined, these data support the idea that antioxidant supplementation may not be necessary for healthy horses that are not undergoing prolonged or intensive exercise (Deaton et al., 2010) but may be necessary for stressed, diseased, or heavily exercised animals.
When cell membranes are damaged by free radicals due to oxidative stress, CK, a relatively large molecule, is able to escape the muscle cell and enter the blood stream (Baird et al., 2012), hence its utilization as a myofiber damage marker. In general, CK activity in CLA horses was lower than in CON horses, which could indicate CLA prevented muscle damage. While CK levels did fluctuate, they remained within reference ranges for resting horses (below 200 U/L; Ostaszewski et al., 2012). Therefore, the exercise regimen in our study was likely not strenuous enough to elicit muscle damage. Recent work has indicated that CK levels in response to exercise are more dependent on the intensity of the exercise than the length of the exercise (Baird et al., 2012). For example, no increase in serum CK was noted before, during, or after a longer exercise of moderate intensity in horses (Valberg et al., 1993). Similar to MDA concentrations, CLA has been shown to decrease circulating CK in animals and humans under high stress (Liu et al., 2008; Terasawa et al., 2017; Rojas et al., 2020). Therefore, the horses in the current study were likely not stressed enough due to the submaximal nature of the exercise program to induce significant muscle fiber perturbation. Future research should investigate the effects of CLA supplementation in horses following more strenuous exercise programs.
Supplementation of CLA to young horses enrolled in a submaximal exercise training program appeared to enhance mitochondrial function in the GM and the TB without increasing mitochondrial volume density, which may have led to a decrease in oxidative stress. This was evidenced by CLA-supplemented horses having lower antioxidant enzyme activities in the GM compared with nonsupplemented control horses but no difference in lipid peroxidation. Further, although serum CK remained within reference ranges for all horses, CLA-supplemented horses had significantly lower activity than control horses. Future research is warranted to delineate beneficial impacts on muscle health and mitochondrial function in stressed, diseased, or intensely exercised horses.
Glossary
Abbreviations
- ATP
adenosine triphosphate
- CCO
cytochrome c oxidase
- CK
creatine kinase
- CLA
conjugated linoleic acid
- CS
citrate synthase
- GM
gluteus medius
- GPx
glutathione peroxidase
- LNA
linoleic acid
- MDA
malondialdehyde
- mRNA
messenger RNA
- Nrf1
nuclear respiratory factor 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- PGC-1α
peroxisome proliferator-activated receptor-coactivator 1α
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TB
triceps brachii
- Tfam
mitochondrial transcription factor A
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ali, Y. M., A. A. Kadir, Z. Ahmad, H. Yaakub, Z. A. Zakaria, and M. N. Abdullah. . 2012. Free radical scavenging activity of conjugated linoleic acid as single or mixed isomers. Pharm. Biol. 50:712–719. doi: 10.3109/13880209.2011.621714. [DOI] [PubMed] [Google Scholar]
- Avellini, L., E. Chiaradia, and A. Gaiti. . 1999. Effect of exercise training, selenium and vitamin E on some free radical scavengers in horses (Equus caballus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 123:147–154. doi: 10.1016/s0305-0491(99)00045-0. [DOI] [PubMed] [Google Scholar]
- Baird, M. F., S. M. Graham, J. S. Baker, and G. F. Bickerstaff. . 2012. Creatine-kinase- and exercise related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012:960363. doi: 10.1155/2012/960363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman, D. E., L. H. Baumgard, B. A. Corl, and J. M. Griinari. . 2000. Biosynthesis of conjugated linoleic acid in ruminants. J. Anim. Sci. 77(suppl_E):1–15. doi: 10.2527/jas2000.77E-Suppl1f [DOI] [Google Scholar]
- Bowler, C., M. V. Montagu, and D. Inze. . 1992. Superoxide dismutase and stress tolerance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 43(1):83–116. doi: 10.1146/annurev.pp.43.060192.000503 [DOI] [Google Scholar]
- Bradbery, A. N., J. A. Coverdale, K. L. Vernon, J. L. Leatherwood, C. E. Arnold, R. A. Dabareiner, M. K. Kahn, A. A. Millican, and T. H. Welsh, Jr. 2018. Evaluation of conjugated linoleic acid supplementation on markers of joint inflammation and cartilage metabolism in young horses challenged with lipopolysaccharide. J. Anim. Sci. 96:579–590. doi: 10.1093/jas/skx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell, H., R. Devery, M. OShea, and C. Stanton. . 1999. The effect of conjugated linoleic acid on the antioxidant enzyme defense system in rat hepatocytes. Lipids 34:833–839. doi: 10.1007/s11745-999-0430-4. [DOI] [PubMed] [Google Scholar]
- Deaton, C. M., D. J. Marlin, C. A. Roberts, N. Smith, P. A. Harris, F. J. Kelly, and R. C. Schroter. . 2010. Antioxidant supplementation and pulmonary function at rest and exercise. Equine Vet. J. Suppl. 34(S34):58–65. doi: 10.1111/j.2042-3306.2002.tb05392.x [DOI] [PubMed] [Google Scholar]
- Dorn, G. W., 2nd, R. B. Vega, and D. P. Kelly. . 2015. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29:1981–1991. doi: 10.1101/gad.269894.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enríquez, V., S. Granados, M. P. Arias, and J. C. Calderón. . 2015. Muscle fiber types of gluteus medius in the Colombian Creole Horse. J. Equine Vet. Sci. 35(6):524–530. doi: 10.1016/j.jevs.2015.02.010 [DOI] [Google Scholar]
- Galisteo, A. M., E. Aguera, J. G. Monterde, and F. Miro. . 1992. Gluteus-medius muscle-fiber type composition in young Andalusion and Arabian horses. J. Equine Vet. Sci. 12(4):254–258. doi: 10.1016/s0737-0806(06)81459-0 [DOI] [Google Scholar]
- Gaschler, M. M., and B. R. Stockwell. . 2017. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482:419–425. doi: 10.1016/j.bbrc.2016.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen, S. N., W. L. Jansen, M. J. Geelen, M. M. Sloet van Oldruitenborgh-Oosterbaan, and A. C. Beynen. . 2000. Lipid metabolism in equines fed a fat-rich diet. Int. J. Vitam. Nutr. Res. 70:148–152. doi: 10.1024/0300-9831.70.3.148 [DOI] [PubMed] [Google Scholar]
- Ghaderzadeh, S., F. Mirzaei Aghjehgheshlagh, S. Nikbin, and B. Navidshad. . 2019. Correlation effects of nano selenium and conjugated linoleic acid on the performance, lipid metabolism and immune system of male Moghani lambs. Iranian J. Appl. Anim. Sci. 9(3):443–451. [Google Scholar]
- Ha, Y. L., N. K. Grimm, and M. W. Pariza. . 1987. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis 8:1881–1887. doi: 10.1093/carcin/8.12.1881 [DOI] [PubMed] [Google Scholar]
- Hassan Eftekhari, M., F. Aliasghari, M. A. Babaei-Beigi, and J. Hasanzadeh. . 2013. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler. 9:311–318. [PMC free article] [PubMed] [Google Scholar]
- Headley, S., J. A. Coverdale, T. C. Jenkins, C. M. Klein, J. L. Sharp, and K. L. Vernon. . 2012. Dietary supplementation of conjugated linoleic acid in horses increases plasma conjugated linoleic acid and decreases plasma arachidonic acid but does not alter body fat. J. Anim. Sci. 90:4876–4882. doi: 10.2527/jas.2011-4976 [DOI] [PubMed] [Google Scholar]
- Hodgson, D. R., and R. J. Rose. . 1987. Effects of a nine-month endurance training programme on muscle composition in the horse. Vet. Rec. 121:271–274. doi: 10.1136/vr.121.12.271 [DOI] [PubMed] [Google Scholar]
- van den Hoven, R., T. Wensing, H. J. Breukink, A. E. Meijer, and T. A. Kruip. . 1985. Variation of fiber types in the triceps brachii, longissimus dorsi, gluteus medius, and biceps femoris of horses. Am. J. Vet. Res. 46:939–941. [PubMed] [Google Scholar]
- Javadi, M., A. C. Beynen, R. Hovenier, A. Lankhorst, A. G. Lemmens, A. H. Terpstra, and M. J. Geelen. . 2004. Prolonged feeding of mice with conjugated linoleic acid increases hepatic fatty acid synthesis relative to oxidation. J. Nutr. Biochem. 15:680–687. doi: 10.1016/j.jnutbio.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Kehrer, J. P. 1993. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 23:21–48. doi: 10.3109/10408449309104073 [DOI] [PubMed] [Google Scholar]
- Kim, Y., D. Kim, D. J. Good, and Y. Park. . 2016a. Conjugated linoleic acid (CLA) influences muscle metabolism via stimulating mitochondrial biogenesis signaling in adult-onset inactivity induced obese mice. Eur. J. Lipid Sci. Technol. 118(9):1305–1316. doi: 10.1002/ejlt.201500220 [DOI] [Google Scholar]
- Kim, Y., D. Kim, and Y. Park. . 2016. Conjugated linoleic acid (CLA) promotes endurance capacity via peroxisome proliferator-activated receptor δ-mediated mechanism in mice. J. Nutr. Biochem. 38:125–133. doi: 10.1016/j.jnutbio.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Kim, Y., and Y. Park. . 2015. Conjugated linoleic acid (CLA) stimulates mitochondrial biogenesis signaling by the upregulation of PPARγ coactivator 1α (PGC-1α) in C2C12 cells. Lipids 50(4):329–338. doi: 10.1007/s11745-015-4000-5 [DOI] [PubMed] [Google Scholar]
- Kirschvink, N., B. de Moffarts, and P. Lekeux. . 2008. The oxidant/antioxidant equilibrium in horses. Vet. J. 177:178–191. doi: 10.1016/j.tvjl.2007.07.033 [DOI] [PubMed] [Google Scholar]
- Kwon, D., J. Kim, K. Cho, and Y. Song. . 2017. Antioxidative effect of CLA diet and endurance training in liver and skeletal muscles of rat. Biotechnol. Bioproc. E. 22(5):647–652. doi: 10.1007/s12257-017-0119-y [DOI] [Google Scholar]
- Larsen, S., J. Nielsen, C. N. Hansen, L. B. Nielsen, F. Wibrand, N. Stride, H. D. Schroder, R. Boushel, J. W. Helge, F. Dela, . et al. 2012. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, Y. H., and R. H. Liu. . 2000. trans-10,cis-12-conjugated linoleic acid isomer exhibits stronger oxyradical scavenging capacity than cis-9,trans-11-conjugated linoleic acid isomer. J. Agric. Food Chem. 48:5469–5475. doi: 10.1021/jf991163d. [DOI] [PubMed] [Google Scholar]
- Li, C., S. H. White, L. K. Warren, and S. E. Wohlgemuth. . 2016. Effects of aging on mitochondrial function in skeletal muscle of American Quarter Horses. J. Appl. Physiol. 121(1):299–311. doi: 10.1152/japplphysiol.01077.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., P. Chen, J. Li, S. Lin, D. Wang, L. Zhu, and D. Yang. . 2008. Conjugated linoleic acids (CLA) moderate negative responses of heat-stressed cows. Livest. Sci. 118:255–261. doi: 10.1016/j.livsci.2008.02.002 [DOI] [Google Scholar]
- Luo, Z., X.-Y. Tan, C.-X. Liu, X.-D. Li, X.-J. Liu, and W.-Q. Xi. . 2012. Effect of dietary conjugated linoleic acid levels on growth performance, muscle fatty acid profile, hepatic intermediary metabolism and antioxidant responses in genetically improved farmed Tilapia strain of Nile tilapia Oreochromis niloticus. Aquac. Res. 43(9):1392–1403. doi: 10.1111/j.1365-2109.2011.02942.x [DOI] [Google Scholar]
- Martínez-Cayuela, M. 1995. Oxygen free radicals and human disease. Biochimie. 77(3):147–161. doi: 10.1016/0300-9084(96)88119-3 [DOI] [PubMed] [Google Scholar]
- Matin, S., A. Nemati, H. Ghobadi, R. Alipanah-Moghadam, and L. Rezagholizadeh. . 2018. The effect of conjugated linoleic acid on oxidative stress and matrix metalloproteinases 2 and 9 in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 13:1449–1454. doi: 10.2147/COPD.S155985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinild Lundby, A.-K., R. A. Jacobs, S. Gehrig, J. de Leur, M. Hauser, T. C. Bonne, D. Flück, S. Dandanell, N. Kirk, A. Kaech, U. Ziegler, S. Larsen, and C. Lundby. . 2018. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 222(1):e12905. doi: 10.1111/apha.12905 [DOI] [PubMed] [Google Scholar]
- Miller, E. F., J. L. Leatherwood, M. J. Anderson, and M. M. Beverly. . 2018. Evaluation of conjugated linoleic acid (CLA) supplementation on equine body composition. Approaches Poultry Dairy Vet. Sci. 3(3):246–250. doi: 10.31031/apdv.2018.03.000565 [DOI] [Google Scholar]
- Mishra, P., G. Varuzhanyan, A. H. Pham, and D. C. Chan. . 2015. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 22:1033–1044. doi: 10.1016/j.cmet.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica, M. P., G. Trinchese, G. Cavaliere, C. De Filippo, E. Cocca, M. Gaita, A. Della-Gatta, A. Marano, G. Mazzarella, and P. Bergamo. . 2014. c9,t11-Conjugated linoleic acid ameliorates steatosis by modulating mitochondrial uncoupling and Nrf2 pathway. J. Lipid Res. 55:837–849. doi: 10.1194/jlr.M044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Camarena, S. Y., J. P. Vanden Heuvel, and M. A. Belury. . 1999. Conjugated linoleic acid activates peroxisome proliferator-activated receptor α and β subtypes but does not induce hepatic peroxisome proliferation in Sprague–Dawley rats. Biochim. Biophys. Acta. 1436(3):331–342. doi: 10.1016/s0005-2760(98)00121-0 [DOI] [PubMed] [Google Scholar]
- NRC . 2007. Nutrient requirements of horses. 6th rev. ed. Washington, DC: National Academies Press. [Google Scholar]
- Onmaz, A., R. van den Hoven, V. Gunes, M. Cinar, and O. Kucuk. . 2011. Oxidative stress in horses after a 12-hours transport period. Rev. Med. Vet. 162:213–217. [Google Scholar]
- Ostaszewski, P., A. Kowalska, E. Szarska, P. Szpotanski, A. Cywinska, B. Balasinska, and T. Sadkowski. . 2012. Effects of β-hydroxy-β-methylbutyrate and γ-oryzanol on blood biochemical markers in exercising thoroughbred race horses. J. Equine Vet. Sci. 32:542–551. doi: 10.1016/j.jevs.2012.01.002 [DOI] [Google Scholar]
- Ostrowska, E., M. Muralitharan, R. F. Cross, D. E. Bauman, and F. R. Dunshea. . 1999. Dietary conjugated linoleic acids increase lean tissue and decrease fat deposition in growing pigs. J. Nutr. 129:2037–2042. doi: 10.1093/jn/129.11.2037. [DOI] [PubMed] [Google Scholar]
- Pariza, M. W. 2004. Perspective on the safety and effectiveness of conjugated linoleic acid. Am. J. Clin. Nutr. 79(6 Suppl.):1132S–1136S. doi: 10.1093/ajcn/79.6.1132S. [DOI] [PubMed] [Google Scholar]
- Park, Y., J. M. Storkson, K. J. Albright, W. Liu, and M. W. Pariza. . 1999. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 34(3):235–241. doi: 10.1007/s11745-999-0358-8 [DOI] [PubMed] [Google Scholar]
- Park, N.-Y., G. Valacchi, and Y. Lim. . 2010. Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediators Inflamm. 2010:342328. doi: 10.1155/2010/342328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, D. C. 2004. Current concepts of oxygen transport during exercise. Equine Comp. Exerc. Physiol. 1(1):5–22. doi: 10.1079/ECP20036 [DOI] [Google Scholar]
- Rojas, M. M., D. M. Villalpando, M. Ferrer, A. Alexander-Aguilera, and H. S. García. . 2020. Conjugated linoleic acid supplemented diet influences serum markers in orchidectomized Sprague-Dawley rats. Eur. J. Lipid Sci. Technol. 122(3):1900098. doi: 10.1002/ejlt.201900098 [DOI] [Google Scholar]
- Rossignoli, C. P., C. R. P. Dechandt, A. O. Souza, I. H. Sampaio, T. M. Vicentini, B. G. Teodoro, M. P. C. Neto, G. D. Ferrari, C. A. Couto-Lima, and L. C. Alberici. . 2018. Effects of intermittent dietary supplementation with conjugated linoleic acid and fish oil (EPA/DHA) on body metabolism and mitochondrial energetics in mice. J. Nutr. Biochem. 60:16–23. doi: 10.1016/j.jnutbio.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Sewell, D. A., R. C. Harris, and D. J. Marlin. . 1994. Skeletal muscle characteristics in 2 year-old race-trained thoroughbred horses. Comp. Biochem. Physiol. Comp. Physiol. 108:87–96. doi: 10.1016/0300-9629(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Sharifi, M., M. Bashtani, A. A. Naserian, H. Farhangfar, and A. Emami. . 2018. The effect of grapeseed oil on performance, rumen fermentation, antioxidant status and subcutaneous adipose fatty acid profile in lambs. J. Anim. Physiol. Anim. Nutr. (Berl.). 102:157–165. doi: 10.1111/jpn.12673. [DOI] [PubMed] [Google Scholar]
- Shen, W., J. Baldwin, B. Collins, L. Hixson, K. T. Lee, T. Herberg, J. Starnes, P. Cooney, C. C. Chuang, R. Hopkins, . et al. 2015. Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice. J. Nutr. Biochem. 26:616–625. doi: 10.1016/j.jnutbio.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W., C. C. Chuang, K. Martinez, T. Reid, J. M. Brown, L. Xi, L. Hixson, R. Hopkins, J. Starnes, and M. McIntosh. . 2013. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J. Lipid Res. 54:909–922. doi: 10.1194/jlr.M030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano, P. D., L. M. Lawrence, K. Danielsen, D. M. Powell, and K. N. Thompson. . 1995. Effect of conditioning and exercise type on serum creatine kinase and aspartate aminotransferase activity. Equine Vet. J. 27(S18):243–247. doi: 10.1111/j.2042-3306.1995.tb04929.x| [DOI] [Google Scholar]
- Spinazzi, M., A. Casarin, V. Pertegato, L. Salviati, and C. Angelini. . 2012. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- Sun, Y., Z. Qi, Q. He, D. Cui, S. Qian, L. Ji, and S. Ding. . 2015. The effect of treadmill training and N-acetyl-l-cysteine intervention on biogenesis of cytochrome c oxidase (COX). Free Radic. Biol. Med. 87:326–335. doi: 10.1016/j.freeradbiomed.2015.06.035. [DOI] [PubMed] [Google Scholar]
- Terasawa, N., K. Okamoto, K. Nakada, and K. Masuda. . 2017. Effect of conjugated linoleic acid intake on endurance exercise performance and anti-fatigue in Student Athletes. J. Oleo Sci. 66:723–733. doi: 10.5650/jos.ess17053. [DOI] [PubMed] [Google Scholar]
- Valberg, S., L. Jönsson, A. Lindholm, and N. Holmgren. . 1993. Muscle histopathology and plasma aspartate aminotransferase, creatine kinase and myoglobin changes with exercise in horses with recurrent exertional rhabdomyolysis. Equine Vet. J. 25:11–16. doi: 10.1111/j.2042-3306.1993.tb02893.x. [DOI] [PubMed] [Google Scholar]
- Van Hoose, P. M., N. Q. Kelm, K. M. Piell, and M. P. Cole. . 2016. Conjugated linoleic acid and nitrite attenuate mitochondrial dysfunction during myocardial ischemia. J. Nutr. Biochem. 34:8–16. doi: 10.1016/j.jnutbio.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Velázquez-Cantón, E., N. Cruz-Rodríguez, L. Zarco, A. Rodríguez-Cortez, J. C. Angeles-Hernandez, J. Ramírez-Orejel, and A. Ramírez-Pérez. . 2018. Effect of selenium and vitamin E supplementation on lactate, cortisol, and malondialdehyde in horses undergoing moderate exercise in a polluted environment. J. Equine Vet. Sci. 69:136–144. doi: 10.1016/j.jevs.2018.07.005 [DOI] [Google Scholar]
- White, S. H., S. E. Johnson, J. M. Bobel, and L. K. Warren. . 2016. Dietary selenium and prolonged exercise alter gene expression and activity of antioxidant enzymes in equine skeletal muscle. J. Anim. Sci. 94:2867–2878. doi: 10.2527/jas.2016-0348. [DOI] [PubMed] [Google Scholar]
- White, S. H., L. K. Warren, C. Li, and S. E. Wohlgemuth. . 2017a. Submaximal exercise training improves mitochondrial efficiency in the gluteus medius but not in the triceps brachii of young equine athletes. Sci. Rep. 7:14389. doi: 10.1038/s41598-017-14691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S. H., and L. K. Warren. . 2017. Submaximal exercise training, more than dietary selenium supplementation, improves antioxidant status and ameliorates exercise-induced oxidative damage to skeletal muscle in young equine athletes. J. Anim. Sci. 95:657–670. doi: 10.2527/jas.2016.1130. [DOI] [PubMed] [Google Scholar]
- White, S. H., S. Wohlgemuth, C. Li, and L. K. Warren. . 2017b. Rapid communication: dietary selenium improves skeletal muscle mitochondrial biogenesis in young equine athletes. J. Anim. Sci. 95:4078–4084. doi: 10.2527/jas2017.1919 [DOI] [PubMed] [Google Scholar]
- Zhang, H. J., Y. D. Tian, Y. M. Guo, and J. M. Yuan. . 2008. Dietary conjugated linoleic acid improves antioxidant capacity in broiler chicks. Br. Poult. Sci. 49:213–221. doi: 10.1080/00071660801989836. [DOI] [PubMed] [Google Scholar]