Abstract
Coriandrum sativum L. is a medicinal and aromatic plant spread around the world, with beneficial properties that are well recognized. Both coriander seeds and leaves are used for pharmaceutical and flavoring purposes. Even though coriander seeds tend to be more popular, the leaves are receiving a consistently growing interest, especially because of popularization of Mexican, Northern African, and Indian cuisines. This increased attention brings about the necessity for providing the product with guaranteed quality, which will retain its valuable characteristics, even after post-harvest treatment. For this reason, it is highly necessary to determine reliable protocols for cilantro preservation, which usually include drying procedures; in order to identify the optimal drying treatments, a spectrum of drying techniques—convective, vacuum-microwave, and a combination of convective and vacuum-microwave—were evaluated. Cilantro-based dried products were examined from the perspectives of volatile organic constituent composition and sensory quality. After headspace solid-phase microextraction-GC/MS analysis and sensory tests, the results demonstrate that convective drying at 70 °C for 120 min followed by vacuum-microwave drying at 360 W and convective drying at 70 °C were the optimal drying methods for preserving cilantro aroma quality, while convective drying at 70 °C for 120 min followed by convective finishing drying at 50 °C decreased cilantro aroma quality.
Keywords: coriander, HS-SPME-GC/MS, napping, VOCs, chemistry behind quality, OACs
1. Introduction
Coriander (Coriandrum sativum L.) is a well-known medicinal and aromatic plant (MAP) from the Apiaceae family, widely cultivated in North and South America (Canada, Mexico, Guatemala, and Argentina), Central and Eastern Europe (Poland, Czechia, Slovakia, Hungary, and Romania) and Asia (Turkey, Iran, Pakistan, and India) [1,2]. The plant is characterized by globular-shaped seeds (fruits) abundant in essential oils (EOs) in which linalool (57.5–75.1%), geranyl acetate (8.9–24.5%), α-pinene (2.3–23.2%), terpineol (0.08–5.3%), geraniol (0.5–2.3%) and citronellol (0.6–1.6%) are indicated as major constituents [1,3]. The total EO yield of coriander seeds ranges between 0.8 and 2.1% [4]. Another extremely valuable part of coriander is the leaves, also called cilantro, the EO content of which varies between 0.1 and 0.29%, and the major constituents of which are (E)-dec-2-enal, (E)-dodec-2-enal, decanol, dodecanol, n-tetradecanol and decanal [5,6]. Both coriander seeds and cilantro, are used as spices, especially in Central and South America, the Middle East and Asia [7], regarding their flavoring and health beneficial properties. The complex chemical composition of coriander plant provides extensive bioactive activities, such as antioxidant, anti-inflammatory, antimicrobial, and analgesic activities [8,9,10].
As a spice, especially in Europe, cilantro seems to be less popular than coriander seeds; however, the cuisines of Mexico, Tex-Mex, Northern Africa, and India value cilantro’s distinct aroma and utilize it as a fresh, chopped spice or as a dried, crushed ingredient of spice blends (masala, curry blends, curcuma blends, and more) [7]. The overall aroma of two commonly used cilantro varieties—C. sativum L. var. vulgare alef and C. sativum L. var. microcarpum DC—is characterized by floral, spicy, pleasant, grassy, herbal, and earthy notes [11]. Cilantro’s odor-active compounds (OACs) mainly include (Z)-3-hexenal (green, cut-grass notes), decanal (green, citrus-peel notes), (E)-2-decenal (green, cut -grass, lettuce notes), (E)-2-dodecenal (green, waxy notes) and other aliphatic hydrocarbons [12]. Those subtle aroma features [13], along with widely reported health promoting properties [14], make cilantro a highly desired MAP.
Undoubtedly, fresh, unprocessed material is the most valuable source of the bioactive and aromatic compounds characteristic of cilantro. Nevertheless, in light of the complicated production and transportation chains, it is impossible to always use it as a fresh ingredient; therefore, it is extremely important to assure the guaranteed quality and safety of cilantro-based products. The most essential quality determinants for MAPs are cultivation conditions (including agronomic practices and weather conditions), harvest time, post-harvest treatments (including material preservation), and plant chemotype [15,16]. Among indicated determinants, post-harvest treatment in the form of drying is the most common processing used for preservation of MAPs quality in a long term [17,18]. The latest studies focused on developing cost-effective, thermo-based drying procedures [19,20], which need to be evaluated on various plant materials [21,22,23,24,25,26,27,28]. The greatest interest is focused on convective drying (CD), microwave drying, vacuum-microwave drying (VMD), freeze-drying, infrared drying, or combined methods such as convective pre-drying followed by vacuum-microwave finish-drying (CPD-VMFD).
In addition, coriander seeds and cilantro were the objects of numerous studies which considered drying proceedings and their influence on the quality of obtained dried products. Some of them were mainly focused on mathematical modelling within cost and energy efficiency evaluation of the drying process [29,30,31], while other studies included evaluation of drying influence on quality in light of volatile organic constituents (VOCs) and non-volatile constituent composition, or even the sensory properties of dried products [32,33,34]. Unfortunately, none of these studies considered a comprehensive experiment design, the intention of which would be to show a complete picture of various drying methods’ influence on VOC composition and their impact on aroma sensory quality of coriander leaves. The most extensive study was designed by Pirbalouti et al., (2017) [32], since it included five drying methods and their influence on cilantro EO composition; however, sensory evaluation was not performed. On the other hand, Fathima et al. (2001) [34] provided a detailed sensory evaluation of cilantro, without testing a wide spectrum of drying methods and VOCs analysis. Therefore, the aim of this study was to investigate the influence of various drying methods on the sensory quality of cilantro and its VOC composition. For this purpose, CD, VMD, and CPD-VMFD with various parameters were applied to obtain dried cilantro-based products, which were evaluated with headspace solid-phase microextraction-GC/MS (HS-SPME-GC/MS) analysis and multiple sensory analyses.
2. Materials and Methods
2.1. Plant Material
Cilantro (Coriandrum sativum L.) plants were delivered by Swedeponic Polska Sp. z o.o. company (Grodzisk Mazowiecki, Poland). The used plants were of a flat-leaf, parsley-like variety, purchased as potted plants (green parts weight 30 ± 2 g). Coriander leaves, immediately after delivery, were chopped, thoroughly mixed, and split for specific purposes—(i) drying procedures, (ii) chemical analyses of fresh material, and (iii) sensory analyses of fresh material. Plant material was vacuum packed and stored at −18 °C awaiting further proceedings.
2.2. Chemicals and Reagents
The analytical grade cyclohexane used during analyses was obtained from UQF (Wrocław, Poland). 2-undecanone, C7-C40 n-alkanes mixture and pure analytical standards (such as decanal, β-pinene, (Z-hex-3-en-1-ol, linalool, (E)-dec-2-enal, (E)-dodec-2-enal, (E)-tridec-2-enal, (E)-tetradec-2-enal and (Z)-tetradec-2-enal) were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
2.3. Drying Methods
Two drying methods (convective drying, vacuum-microwave drying) and their combinations were utilized [35]. In each case, 80 g of fresh cilantro samples were used. Moisture content (Mc) of the samples was determined using a vacuum dryer pre-set at 70 °C and 100 Pa (SPT-200, ZEAMIL Horyzont, Krakow, Poland). Initial moisture content of the material was Mc = 92.62%. The maximum temperature of the sample was determined after each measurement using an infrared camera (Flir Systems AB, Stockholm, Sweden), right after the removal from the dryer. All drying variants were carried out in two technological repetitions and the process lasted until the moisture content was below 10% wet basis (wb).
2.3.1. Convective Drying (CD)
Convective drying was performed at 50, 60 and 70 °C using the equipment engineered at the Institute of Agricultural Engineering (Wroclaw University of Environmental and Life Sciences, Wroclaw, Poland). In addition, two-stage convective drying with temperature adjustment after 120 min was performed (Table 1). The air velocity in each case was 0.5 m/s.
Table 1.
Dried cilantro samples codes.
| Drying Method | Code |
|---|---|
| Convective drying at 50 °C | CD50 |
| Convective drying at 60 °C | CD60 |
| Convective drying at 70 °C | CD70 |
| Convective drying at 50 °C for 120 min followed by convective finishing drying at 70 °C | CD50/70 |
| Convective drying at 60 °C for 120 min followed by convective finishing drying at 70 °C | CD60/70 |
| Convective drying at 70 °C for 120 min followed by convective finishing drying at 50 °C | CD70/50 |
| Convective drying at 70 °C for 120 min followed by convective finishing drying at 60 °C | CD70/60 |
| Vacuum-microwave drying with power 240 W | VMD 240 |
| Vacuum-microwave drying with power 360 W | VMD 360 |
| Vacuum-microwave drying with power 480 W | VMD 480 |
| Convective pre-drying at 50 °C for 120 min followed by vacuum-microwave drying at 360 W | CPD50-VMFD |
| Convective drying at 60 °C for 120 min followed by vacuum-microwave drying at 360 W | CPD60-VMFD |
| Convective drying at 70 °C for 120 min followed by vacuum-microwave drying at 360 W | CPD70-VMFD |
2.3.2. Vacuum-Microwave Drying (VMD)
Vacuum-microwave drying was carried out at 240, 360, and 480 W (Table 1). The SM-200 dryer (Plazmatronika, Wroclaw, Poland) was used in the study. The pressure in the dryer ranged from 4 to 6 kPa. The samples were weighed after 4, 3 or 2 min in the case of drying at 240, 360, and 480 W, respectively.
2.3.3. Combined Convective Pre-Drying Followed by Vacuum-Microwave Finishing Drying (CPD-VMFD)
Combined drying consisted of 120 min of convective pre-drying at 50, 60 or 70 °C followed by vacuum-microwave finishing drying at 360 W (Table 1) carried out until desired moisture content was obtained.
2.4. Modelling of Drying Kinetics
The drying kinetics were fitted on the basis of mass losses recorded during drying by different methods. Moisture ratio (MR) was calculated according to the simplified equation:
| (1) |
where M(t) is the moisture content at a given time and M0 is the initial moisture content.
The drying model was fitted using Table Curve 2D software (Systat Software, San Jose, California, USA) according to the experimental points obtained in the study. The good fit of the applied model was determined on the basis of the highest values of R2 and the lowest values of root mean square error (RMSE). Preliminary studies found that the best fit was obtained when Page’s model was applied:
| (2) |
where A, k, and n are drying constants and τ is the time taken for drying.
2.5. VOC Profiling
The VOC profile of cilantro was investigated according to Łyczko et al.’s (2019b) [35] protocol, with later slight modifications made by Łyczko et al. (2020) [36]. Briefly, plant material samples were weighed and placed into headspace vials together with internal standard (IS) (2-undecanone), and afterwards VOC extraction was performed with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fiber, needle size 23 ga, StableFlex, for use with manual holder/autosampler, fiber L 2 cm (Supeclo, Bellefonte, PA, USA). Further, GC-MS analysis was carried out on Varian CP-3800/Saturn 2000 apparatus (Varian, Walnut Creek, CA, USA) and analytes were separated by Zebron ZB-5MSi (30 m × 0.25 mm × 0.25 µm) column. GC temperature program: initially 50 °C, then to 130 °C at 4.0 °C/min ratio, then to 180 °C at 20 °C/min ratio; carrier gas: helium with linear velocity 35.0 cm/sec; split ratio 1:10. MS operational conditions: ion source temperature 250 °C; electron impact (EI) ionization at 70 eV; scanning range from 35 to 300 m/z. Analyses were run in three repetitions.
For qualitative analysis, Varian Workstation software was used, whereas for quantitative analysis, ACD/Spectrus Processor (Advanced Chemistry Development, Inc., Toronto, ON, Canada) was utilized. VOCs were identified via determination of linear retention indices (LRIs) and mass spectra comparison (Adams, 2012 [37]; NIST 17 Mass Spectral and Retention Index Library). The LRIs filter was narrowed down to ±15 points and only mass spectra matches with similarity score ≥90% were qualified as hits. The molecular mass of VOCs was confirmed by chemical ionization mode performed with isobutane as the reagent gas. Quantification was done by peak area normalization against IS peak area.
2.6. Sensory Evaluation
The sensory quality evaluation of dried cilantro was carried out, as previously reported by Łyczko et al. (2020) [36], with a trained panel of 20 panellists (age 23–52) from the Food Quality and Safety Group (Escuela Politécnica Superior de Orihuela) of the Universidad Miguel Hernández de Elche (Orihuela, Alicante, Spain). The selection of panelists was performed according to ISO standard 8586-1 [38,39]. Before the actual evaluation, panelists were involved in 3 training meetings in order to adjust the panelist’s senses for required aroma attributes. Sensory analyses were performed in a tasting room with individual booths, controlled light (70–90 fc), and controlled temperature (22 ± 1 °C). Coded cilantro samples were provided for sensory analysis, during which 3 tests were performed— a ranking test, napping test, and descriptive sensory analysis. In the first place, ranking and napping tests were carried out. The ranking test was used to evaluate the intensity of dried products aroma in comparison to fresh cilantro aroma and to reduce the sample number for further steps. The reduction in samples for descriptive sensory analysis was required, since it was impossible to fully study all samples initially available in this study [40,41]. For this, 20 panelists were asked to rank the coriander samples according to perceived intensity of the attribute “fresh coriander”. The panelists used the ascending rank order, in which 1 meant the least and 13 meant the most intense sample, respectively. For descriptive sensory analysis, one sample was selected from each drying treatment/type, having high intensity in the ranking test and having no measurable intensity of off-flavors. For the descriptive sensory analysis, a sensory sheet was developed containing 21 sensory attributes previously selected from the according to the scientific literature, napping test, and orientation sessions. The samples were served in 30 mL covered cups and a random block design was used for sample presentation in order to avoid biases. Each panelist was asked to smell and score the samples using an 11-point numerical scale, where 0 represented none or no intensity and 10 extremely strong, with a 0.5 increment. Additionally, the napping technique was used to group dried samples and to support the reduction in sample number for descriptive sensory analysis. Napping consisted of the position of the products on a A3 sheet of paper according to their similarities by the panelists, who were also asked to add at least 3 descriptors for each group of samples [42]. As the last step, descriptive sensory analysis was carried out by 7 judges who evaluated 4 chosen samples. In this step, the panelists evaluated the basic tastes, aroma, and flavor of dry cilantro according to the lexicon first given by Łyczko et al. (2020) [36] with slight modifications (the full lexicon is available in the Supplementary Materials).
2.7. Statistical Analysis
Statistical analysis was performed with Statistica 13.3 software (StatSoft, Kraków, Poland). The one-way analysis of variance (ANOVA) of HS-SPME-GC/MS data and descriptive sensory analysis were carried out, followed by Tukey’s test (p < 0.05), and for a ranking test, Friedman’s rank-sum analysis (α = 0.05), supported by Tukey’s honest significance difference (p < 0.05), was used. The statistical analysis and graphic interpretation (biplot) of the napping results were carried out with XLSTAT Premium 2016 software (Addinsoft, París, France). The procedure of data handling, collected during napping, consisted of determining the position of each sample on X and Y axis and the sensory description given by each panelist. Check-all-that-apply (CATA) and multifactor data analysis (MFA) analyses were performed to generate the biplot. In all relevant cases, standard deviation (SD) was given.
3. Results
3.1. Drying
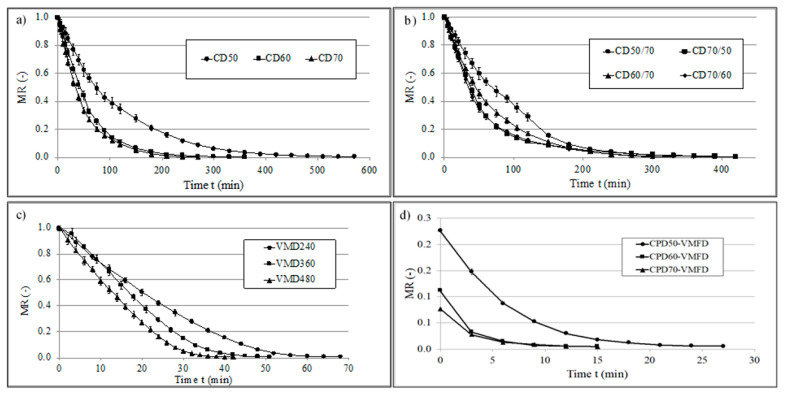
Figure 1 shows the drying kinetics of the cilantro samples treated by different drying methods and Table 2 shows the drying kinetics constants (A, k, n), drying times, maximum temperature of sample heating (Tmax), and final moisture content (Mcwb) of cilantro dried using different methods. It can be seen that during CD with an increase in temperature from 50 to 70 °C, the duration of drying decreased over two times (from 570 min in the case of CD50 to 270 min in case of CD70). On the other hand, application of two-term CD led to an only slightly shorter time of the process than in case of CD50, and even longer times than in case of CD60 or CD70. Furthermore, CD with temperature adjustment after 120 min resulted in higher moisture content than in the case of CD60 or CD70. A smooth transition between temperatures in two-term CD can be noticed, except for CD50/70, where a sudden drop in MR value after 120 min can be seen (Figure 1b).
Figure 1.
Drying kinetics of cilantro obtained by (a) convective drying at 50, 60 and 70 °C; (b) convective pre-drying for 120 min at 50, 60 and 70 °C followed by convective finishing drying; (c) vacuum-microwave drying at 240, 360 and 480 W; (d) combined convective pre-drying at 50, 60 and 70 °C followed by vacuum-microwave finishing drying at 360 W—results presented for VMFD part of the treatment.
Table 2.
Model constants (A, k, n), drying times, maximum temperature (Tmax), and final moisture content (Mcwb) of the cilantro samples dried using different methods.
| Drying Conditions | Constants | Statistics | Drying Time [min] | Maximum Temperature | Mcwb [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | k | n | RMSE | R2 | CPD | CD | VMD | Tmax, [°C] | ||
| CD50 | 1 | 0.0091 | 0.995 | 0.0115 | 0.9989 | - | 570 | - | 50 ± 2 | 6.2 ± 0.5 |
| CD60 | 1 | 0.0106 | 1.124 | 0.0206 | 0.9964 | - | 360 | - | 60 ± 2 | 3.3 ± 0.2 |
| CD70 | 1 | 0.0192 | 1.021 | 0.0109 | 0.9989 | - | 270 | - | 70 ± 2 | 3.6 ± 0.3 |
| CD50/70 | 1 | 0.0062 | 1.123 | 0.0188 | 0.9972 | 120 | 270 | - | 70 ± 2 | 4.0 ± 0.3 |
| CD70/50 | 1 | 0.0151 | 1.062 | 0.0215 | 0.9958 | 120 | 300 | - | 50 ± 2 | 8.1 ± 0.5 |
| CD60/70 | 1 | 0.0151 | 1.001 | 0.0075 | 0.9995 | 120 | 180 | - | 70 ± 2 | 6.4 ± 0.4 |
| CD70/60 | 1 | 0.0193 | 1.002 | 0.0227 | 0.9953 | 120 | 210 | - | 60 ± 2 | 9.7 ± 0.6 |
| VMD 240 | 1 | 0.0111 | 1.391 | 0.0242 | 0.9943 | - | - | 68 | 50 ± 2 | 9.0 ± 0.5 |
| VMD 360 | 1 | 0.0063 | 1.671 | 0.0143 | 0.9982 | - | - | 51 | 53 ± 2 | 7.8 ± 0.3 |
| VMD 480 | 1 | 0.0191 | 1.441 | 0.0264 | 0.9932 | - | - | 42 | 56 ± 2 | 8.1 ± 0.2 |
| CPD50-VMFD | 0.2231 | 0.1351 | 1.082 | 0.002 | 0.9992 | 120 | - | 27 | 53 ± 2 | 6.1 ± 1.1 |
| CPD60-VMFD | 0.1111 | 0.5842 | 0.687 | 0.0014 | 0.9982 | 120 | - | 15 | 55 ± 2 | 5.9 ± 0.9 |
| CPD70-VMFD | 0.0768 | 0.4921 | 0.697 | 0.0012 | 0.9972 | 120 | - | 15 | 62 ± 2 | 5.2 ± 0.9 |
CD—convective drying, CPD—convective pre-drying, VMD—vacuum-microwave drying, VMFD—vacuum-microwave finish-drying.
The highest Mcwb was obtained when CD70/60 was used and the highest water reduction, and therefore the lowest Mc, was reached when CD60 was applied. As for VMD, an increase in the power of magnetrons from 240 to 480 W significantly reduced the duration of the process (from 68 min in the case of VMD 240 to 42 min when VMD 480 was used). Overall, VMD resulted in much shorter processing time comparing to convective drying. Furthermore, the maximum temperature of the sample treated by VMD increased when higher power was applied.
Combined drying resulted in shorter time of the process than in the case of CD, but still higher than when only VMD was applied. The use of this method resulted in moderate final Mc of the sample as well as moderate maximum temperature of the material recorded during drying. Page’s model was successfully applied in the study to describe drying kinetics on the basis of high values of determination coefficient (R2 > 0.99 in every case) and low values of RMSE (RMSE < 0.026). This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.2. Aroma Profiling
In total, 61 VOCs were found in the fresh cilantro VOC profile composition, and 59 of them were successfully identified (the mass spectra of the two unknown compounds are given in the supplementary data). As presented in Table 3, (Z)-3-hexen-1-ol (17.38 ± 0.38%), nonane (9.28 ± 1.20%), (Z)-3-hexen-1-ol acetate (34.54 ± 2.08%), limonene (2.43 ± 0.22%), linalool (1.44 ± 0.02%), decanal (4.53 ± 0.13%), decanol (4.76 ± 0.56%), dodecanal (1.76 ± 0.30%), (E)-2-dodecenal (4.18 ± 0.36%), dodecanol (1.35 ± 0.67%) and (Z)-2-tetradecenal (4.77 ± 0.50%) were reported as the most abundant VOC profile constituents. The rest of the VOCs present in cilantro VOC profile occurred in quantities lower than 1%.
Table 3.
Volatile organic constituent composition in fresh cilantro aroma profile.
| Compound | Compound Class | LRIexp 1 | LRIlit 2 | LRIlit 3 | Contribution 4 [%] | The Match Fitting Score 5 [%] |
Odour Description 6 |
|---|---|---|---|---|---|---|---|
| 3-Methyl-1-butanol | alcohol | 736 | 736 | 731 | 0.34 ± 0.01 | 93 | |
| 2-Methyl-1-butanol, | alcohol | 739 | 739 | - | 0.57 ± 0.01 | 91 | |
| (Z)-Hex-3-enal | aldehyde | 803 | 810 | 797 | 0.16 ± 0.04 | 91 | Green/Floral |
| (Z)-Hex-3-en-1-ol | alcohol | 851 | 857 | 850 | 17.38 ± 0.38 | 94 | Green, cut grass |
| (E)-Hex-2-en-1-ol | alcohol | 863 | 862 | - | 0.39 ± 0.02 | 92 | |
| 3-Methylbutyl acetate | ester | 874 | 876 | 869 | 0.12 ± 0.01 | 92 | |
| Allyl Isothiocyanate | other | 884 | 887 | - | 0.17 ± 0.03 | 95 | |
| Nonane | alkane/alkene | 902 | 900 | 900 | 9.28 ± 1.20 | 90 | |
| α-Pinene | terpene/terpenoid | 934 | 937 | 932 | 0.37 ± 0.05 | 95 | |
| Camphene | terpene/terpenoid | 950 | 952 | 946 | tr 7 | 94 | |
| 2-Methylpropyl butanoate | ester | 955 | 955 | - | 0.16 ± 0.02 | 91 | |
| Sabinene | terpene/terpenoid | 974 | 974 | 969 | 0.11 ± 0.01 | 93 | |
| β-Pinene | terpene/terpenoid | 979 | 978 | 974 | 0.14 ± 0.01 | 94 | Mouldy, earthy/Mushroom |
| 6-Methyl-hept-5-ene-2-one | others | 986 | 988 | - | 0.10 ± 0.02 | 90 | |
| Unknown | - | 992 | 0.31 ± 0.01 | ||||
| Decane | alkane/alkene | 1001 | 1000 | 1000 | 0.27 ± 0.04 | 94 | |
| (Z)-Hex-3-en-1-ol, acetate | ester | 1009 | 1005 | 1004 | 34.54 ± 2.08 | 90 | |
| 3-Carene | terpene/terpenoid | 1012 | 1011 | 1008 | 1.57 ± 0.06 | 90 | |
| p-Cymene | terpene/terpenoid | 1025 | 1025 | 1022 | 0.45 ± 0.01 | 91 | |
| Limonene | terpene/terpenoid | 1030 | 1030 | 1024 | 2.43 ± 0.22 | 91 | |
| (Z)-β -Ocimene | terpene/terpenoid | 1038 | 1038 | 1032 | tr | 92 | |
| (E)-β-Ocimene | terpene/terpenoid | 1049 | 1049 | 1044 | 0.15 ± 0.01 | 94 | |
| 3-Methylbutyl butanoate | ester | 1056 | 1056 | 1049 | 0.23 ± 0.01 | 95 | |
| γ-Terpinene | terpene/terpenoid | 1060 | 1060 | 1054 | 0.33 ± 0.03 | 97 | |
| (Z)-Sabinene hydrate | terpene/terpenoid | 1072 | 1064 | 1069 | 0.59 ± 0.04 | 93 | |
| Terpinolene | terpene/terpenoid | 1090 | 1088 | 1086 | 0.45 ± 0.04 | 97 | Mushroom, truffle/Mouldy, earthy |
| Tetrahydrolinalool | terpene/terpenoid | 1099 | 1098 | 1098 | 0.38 ± 0.02 | 93 | |
| Linalool | terpene/terpenoid | 1101 | 1099 | 1095 | 1.44 ± 0.02 | 93 | Citrusy/Floral |
| Limonene epoxide | terpene/terpenoid | 1137 | 1133 | 1137 | 0.15 ± 0.01 | 90 | |
| p-Menthone | terpene/terpenoid | 1157 | 1153 | 1148 | 0.10 ± 0.01 | 90 | |
| Menthol | terpene/terpenoid | 1174 | 1175 | 1167 | 0.51 ± 0.08 | 93 | |
| Terpinen-4-ol | terpene/terpenoid | 1180 | 1177 | 1174 | 0.13 ± 0.01 | 90 | |
| Dill ether | terpene/terpenoid | 1189 | 1186 | 1184 | 0.10 ± 0.01 | 90 | |
| p-Menth-8-en-2-ol | terpene/terpenoid | 1197 | 1195 | 1187 | 0.23 ± 0.02 | 93 | |
| Estragole | other | 1201 | 1196 | 1195 | 0.30 ± 0.02 | 94 | |
| Decanal | aldehyde | 1207 | 1206 | 1201 | 4.53 ± 0.13 | 96 | Floral, citronellol/Fruity |
| Carvone | terpene/terpenoid | 1247 | 1242 | 1239 | 0.65 ± 0.09 | 92 | |
| Linalyl acetate | terpene/terpenoid | 1258 | 1257 | 1254 | 0.27 ± 0.01 | 92 | |
| (E)-De-2-cenal | aldehyde | 1264 | 1263 | 1260 | 0.46 ± 0.14 | 97 | Coriander/Aldehydic/Pungent, spicy |
| (E)-Dec-9-en-1-ol | alcohol | 1270 | 1262 | 1263 | 0.33 ± 0.10 | 90 | |
| Decanol | alcohol | 1274 | 1273 | 1266 | 4.76 ± 0.56 | 91 | Floral/Spicy |
| Bornyl acetate | terpene/terpenoid | 1290 | 1285 | 1283 | 0.26 ± 0.03 | 92 | |
| Undecan-2-ol | alcohol | 1304 | 1308 | 1301 | 0.18 ± 0.05 | 97 | Medicinal/Pungent, spicy |
| Undecanal | aldehyde | 1310 | 1307 | 1305 | 0.29 ± 0.08 | 95 | Fruity/Floral/Spicy |
| Methyl dodecanoate | ester | 1330 | 1325 | 1323 | 0.10 ± 0.02 | 91 | |
| Terpinyl acetate | terpene/terpenoid | 1357 | 1350 | 1346 | 0.20 ± 0.02 | 90 | |
| (E)-Undec-2-enal | aldehyde | 1371 | 1365 | - | 0.18 ± 0.08 | 90 | Fruity/Solvent, chemical |
| (Z)-Tetradec-2-ene, | alkane/alkene | 1380 | 1378 | - | 0.16 ± 0.02 | 91 | |
| Dec-9-enyl acetate | ester | 1398 | 1399 | 1399 | 0.10 ± 0.03 | 92 | |
| Decyl acetate | ester | 1402 | 1408 | 1407 | 0.12 ± 0.03 | 93 | |
| Dodecanal | aldehyde | 1414 | 1409 | 1408 | 1.76 ± 0.30 | 96 | Pungent, spicy/Floral, citronellol |
| Caryophyllene | sesquiterpene | 1420 | 1419 | 1417 | 0.22 ± 0.01 | 95 | |
| Elemene isomer | sesquiterpene | 1444 | 1444 | - | tr | 92 | |
| (Z)-Dodec-2-enal | aldehyde | 1460 | 1467 | - | 0.3 ± 0.07 | 92 | |
| (E)-Dodec-2-enal | aldehyde | 1472 | 1471 | 1468 | 4.18 ± 0.36 | 95 | Coriander/Floral/Pungent |
| Dodecanol | alcohol | 1477 | 1473 | 1469 | 1.35 ± 0.67 | 95 | |
| Eremophilene | sesquiterpene | 1502 | 1499 | - | 0.22 ± 0.03 | 91 | |
| unknown sesquiterpene | sesquiterpene | 1572 | 0.33 ± 0.21 | ||||
| (E)-Tridec-2-enal | aldehyde | 1577 | 1572 | - | 0.30 ± 0.04 | 93 | Coriander/Pungent, spicy |
| (E)-Tetradec-2-enal | aldehyde | 1667 | 1673 | - | 0.26 ± 0.10 | 94 | Pungent, spicy/Aldehydic/Floral |
| (Z)-Tetradec-2-enal | aldehyde | 1682 | 1675 | - | 4.77 ± 0.50 | 91 | Pungent, spicy/Woody |
| Compounds group | Total contribution [%] | ||||||
| Aldehydes | 17.19 ± 0.95 | ||||||
| Alkanes/alkenes | 9.71 ± 0.81 | ||||||
| Esters | 35.47 ± 1.31 | ||||||
| Alcohols | 24.39 ± 1.17 | ||||||
| Others | 0.57 ± 0.02 | ||||||
| Terpenes/terpenoids | 11.01 ± 0.05 | ||||||
| Sesquiterpenes | 0.77 ± 0.18 | ||||||
| SUM | 99.11 | ||||||
1 Experimentally obtained linear retention indices (LRI); 2 LRI according to NIST17 database; 3 LRI according to Adams (2012). 4 Values based on HS-SPME analysis; 5 the match fitting score of obtained mass spectra to mass spectra available in NIST17 database; 6 cilantro odor-active compounds (OACs) and their odor description according to Eyres et al. (2005) [43]; 7 tr < 0.10%.
3.3. Influence of Drying on Cilantro Volatile Organic Constituents Composition
Table 4 illustrates that applying various drying methods caused a significant shift in all OACs’ contributions in the cilantro VOC profile. Regarding the most abundant cilantro OACs (more than 1% of contribution)—(Z)-hex-3-en-1-ol (17.38%), linalool (1.44%), decanal (4.53%, decanol (4.76%), dodecanal (1.76%), (E)-dodec-2-enal (4.18%) and (Z)-tetradec-2-enal (4.77%)—only the share of (Z)-hex-3-en-1-ol decreased in all dried products below 1%. For other most abundant OACs, the pattern of changes was not as clear—particular OACs’ contribution shifts were strongly dependent on applied drying method. The most interesting observation was that, except for (Z)-hex-3-en-1-ol, the contribution of all cilantro OACs was significantly increased by applying drying, regardless of the used method. In addition, it is worth underlining is that drying methods including only VMD caused a significant increase in β-pinene and terpinolene contribution, which are responsible for moldy, earthy, and mushroom aroma notes of dried products.
Table 4.
Contribution of particular cilantro OACs in fresh cilantro and samples subjected to drying.
| Odour Active Compounds | Fresh | CD50 | CD60 | CD70 | CD60/70 | CD50/70 | CD70/50 | CD70/60 | VMD 240 | VMD 360 | VMD 480 | CPD50-VMFD | CPD60-VMFD | CPD70-VMFD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contribution [%] | ||||||||||||||
| (Z)-Hex-3-enal | 0.16f 1 | 0.60bc | 0.14f | 0.41cde | 0.61bc | 0.35def | 0.47bcd | 0.65b | 0.56bcd | 0.65b | 1.70a | 0.24ef | 0.39cde | 0.22ef |
| (Z)-Hex-3-en-1-ol | 17.38a | 0.58bc | 0.12d | 0.26cd | 0.32bcd | 0.24cd | 0.28bcd | 0.30bcd | 0.32bcd | 0.39bcd | 0.64b | 0.25cd | 0.23cd | 0.08d |
| (E)-Hex-2-en-1-ol | 0.39b | 0.24cde | 0.07f | 0.22de | 0.28cd | 0.15ef | 0.24cde | 0.32bc | 0.32bcd | 0.25cd | 0.75a | 0.11f | 0.15ef | 0.07f |
| β-Pinene | 0.14de | 0.33bc | 0.09e | 0.26cd | 0.35bc | 0.22cde | 0.34bc | 0.41b | 0.25cd | 0.36bc | 0.80a | 0.17de | 0.24cd | 0.14de |
| Terpinolene | 0.45bcd | 0.45bc | 0.11f | 0.32cde | 0.45bcd | 0.30de | 0.42bcd | 0.55b | 0.35cde | 0.37cde | 0.70a | 0.23ef | 0.35cde | 0.25ef |
| Linalool | 1.44bc | 1.21bcde | 0.52f | 1.20bcde | 1.37bcd | 1.14bcde | 0.77ef | 1.58b | 0.60f | 0.98cdef | 2.19a | 0.83ef | 0.90ef | 0.93def |
| Decanal | 4.53g | 15.00d | 24.88b | 15.18d | 6.94ef | 20.82c | 4.94fg | 7.92e | 0.32h | 15.05d | 7.82e | 25.25b | 23.34b | 29.10a |
| (E)-Dec-2-enal | 0.46e | 1.35e | 11.90a | 4.17d | 0.58e | 3.91d | 0.31e | 0.56e | 3.41d | 3.25d | 1.34e | 10.85ab | 6.60c | 9.89b |
| Decanol | 4.76b | 2.71cd | 5.93b | 2.17cd | 2.03cd | 4.84b | 1.39d | 3.07c | 4.53b | 8.95a | 1.48d | 5.61b | 4.75b | 5.12b |
| Undecan-2-ol | 0.18bcde | 0.25bc | 0.08e | 0.28b | 0.23bcd | 0.14cde | 0.17bcde | 0.21bcde | 0.24bcd | 0.27bc | 0.64a | 0.11de | 0.15cde | 0.10e |
| Undecanal | 0.29d | 2.23ab | 1.55cd | 2.47ab | 1.54cd | 2.70a | 1.20d | 1.40cd | 0.24d | 1.42cd | 1.81bcd | 1.83bcd | 1.85bcd | 1.90bc |
| (E)-Undec-2-enal | 0.18fg | 0.74de | 3.14a | 1.90c | 0.18fg | 1.88c | 0.14g | 0.21efg | 0.27efg | 0.72def | 0.86d | 2.59b | 1.78c | 1.65c |
| Dodecanal | 1.76h | 5.34bc | 4.36de | 5.86ab | 3.04fg | 6.60a | 2.08h | 2.54gh | 0.33h | 3.37fg | 3.60ef | 4.86cd | 3.79ef | 4.93cd |
| (E)-Dodec-2-enal | 4.18fg | 7.31e | 19.52a | 13.95bc | 2.96g | 14.51b | 1.37h | 1.14h | 0.32h | 2.92g | 4.34f | 14.05bc | 13.07cd | 11.80d |
| (E)-Tridec-2-enal | 0.30f | 0.37ef | 1.53a | 0.94b | 0.22f | 0.66bcd | 0.19f | 0.23f | 0.71bc | 0.30f | 0.63cde | 0.80bc | 0.64cd | 0.42def |
| (E)-Tetradec-2-enal | 0.26bc | 0.80a | 0.19c | 0.24bc | 0.17c | 0.18c | 0.14c | 0.19c | 0.35bc | 0.19c | 0.64ab | 0.25bc | 0.19c | 0.09c |
| (Z)-Tetradec-2-enal | 4.77cd | 2.34fg | 9.46ab | 6.05c | 1.96fg | 8.21b | 1.03gh | 0.20h | 3.24ef | 3.18ef | 0.99gh | 10.76a | 5.54cd | 4.38de |
1 Values followed by the same letters are not statistically different in Tukey’s test and one-way analysis of variance.
3.4. Sensory Analysis
Multiple sensory analyses showed a significant divergence, regarding dried cilantro odor quality on various levels. The very first trial—the ranking test, the results of which are presented in Table 5—evaluated the intensity of the cilantro-like aroma of dried samples. Samples CPD70-VMFD (rank sum 198 points) and CD70 (sum 184 points) were recognized as those with the most intense cilantro-like aroma, while CD50/70 (sum: 100 points), VMD 480 (sum: 98 points) and CD70/50 (sum: 95 points) were pointed out as those with the weakest aroma intensity.
Table 5.
Ranking test results—intensity of cilantro-like aroma of dried cilantro samples in comparison to fresh cilantro leaves.
| Drying Method | Rank 1 |
|---|---|
| CPD70-VMFD | 198a 2 |
| CD70 | 184a |
| CD50 | 181ab |
| CPD60-VMFD | 179ab |
| CD60 | 154abc |
| VMD 360 | 134bcd |
| CD60/70 | 124cd |
| CD70/60 | 122cd |
| CPD50-VMFD | 121cd |
| VMD 240 | 120cd |
| CD50/70 | 100d |
| VMD 480 | 98d |
| CD70/50 | 95d |
1 Maximal rank sum was 260; 2 different letters after each dried sample indicate a significant difference at p < 0.05 in Friedman’s test by the honest significance difference (HSD) test (α).
The napping test, the results of which are illustrated in Figure 2, showed that dried cilantro samples may be divided into five groups. Samples were grouped in the napping test according to the groups established in a clustering dendrogram prepared using Pearson’s correlation based on the unweighted average. The two largest groups, A and C, consisted of three and four samples, respectively, which all included the CD method. Group C, with higher CD initial temperatures, was characterized as “dry grass”, “hay-like”, “parsley-like”, “sweet”, and “chamomile”, while group A, where initial CD temperatures were lower, was characterized as “green”, “lacteal”, “fermented grass”, “wet”, “fresh”, “balsamic”, and with “high intensity”. The next groups, D and E, both contained two samples. Group D, consisting of CPD50-VMFD and CD60/70, was characterized only as “dry leaves”, while group E, consisting of VMD 240 and VMD 480, was characterized as “fermented” and “musty”. One sample—VMD 360—was categorized as group B, characterized as “dry”, “woody”, “infusion”, and “mushrooms”, while the last sample, CD70/50, overall was not characterized with any descriptions.
Figure 2.
Napping test results for cilantro treated with various drying methods presented as biplot generated with MFA, which explains 44.25% of the variance. Samples were grouped in the napping test according to the groups established in a clustering dendrogram prepared using Pearson’s correlation based on the unweighted average.
As the last sensory test, descriptive sensory analysis was performed. For this trial, due to the high number of samples, only one representative for each drying method was selected after ranking and napping techniques—CD60, CD50/70, VMD 240, and CPD60-VMFD. In the case of basic taste descriptors, samples did not show any significant differences, while in case of flavor, pungency and spiciness were the differentiating features. More significant differences were observed among aroma descriptors. As visualized in Figure 3, chamomile, hay-like, herbaceous, vegetable, and woody were the descriptors, which diversified dried cilantro in the case of aroma. CD60 treatment was the one with the strongest intensity of the above-mentioned attributes.
Figure 3.
Descriptive sensory results for cilantro samples from each type of drying treatment. The scale used ranged from 0 = no intensity to 10 = extremely strong intensity; *, **, and *** significant at p < 0.05, 0.01, and 0.001, respectively.
4. Discussion
4.1. Drying Kinetics
The effect of the hot air temperature during CD as well as power of magnetrons during VMD was evaluated in the study. It can be seen that with an increase in temperature during CD, the time taken for drying decreases, maximum recorded temperature increases, and the drying rate of the process accelerates, which is consistent with other studies [26,29,44].
A similar effect was seen in case of VMD, where with an increase in the power of magnetrons, the duration of the process decreases. The same phenomena were also noticed in thyme [45]. Furthermore, increased wattage resulted in an increase in the maximum temperature recorded during VMD, which is consistent with other studies [46].
During CD, the hot air flows around the sample and efficiently removes surface moisture, which is directly correlated with the high drying rate at the initial stage of the CD. Yet, as can be seen in the Figure 1a, over time, the drying rate diminished as a result of slow diffusion of the moisture from the inside of the sample. On the other hand, faster evaporation during VMD is due to the heating in the whole volume of the sample as a result of microwave application, which mitigates the limitations of CD.
The beneficial effect of combined CPD-VMD was due to the phenomena occurring during both CD and VMD, where at the initial stage the surface water is efficiently removed by CD and, then, the VMD is applied to remove the remaining water which is strongly bounded to the plant cellular structure [47]. As a result, a shorter drying time can be obtained.
The study on leaf drying revealed that the quality of leaves is highly dependent on the duration of the process. However, higher temperatures might result in the degradation of bioactive compounds [29]. Two-term CD might mitigate the negative effect of high temperature on the quality of the sample since application of higher temperature of hot air during the first stage of drying does not increase the temperature of the sample. This is due to the intensive evaporation that cools down the sample and the dried material never reaches the temperature of hot air. After 120 min, when the temperature is reduced to 60 or even 50 °C, evaporation slows down and the temperature of the sample stays relatively low. In addition, in the case of CD50/70, the temperature adjustment changed the shape of the curve, which is marked by a sudden drop in the MR after 120 min, and significantly shortened the drying process.
Page’s model applied in this study was previously successfully used to describe the drying kinetics of Origanum majorana [48] and Cassia alata [47].
4.2. Volatile Organic Constituents of Cilantro and Quality of Dried Products
The majority of the most abundant VOCs identified in fresh cilantro were qualified as aliphatic aldehydes and alcohols, what corresponds with de Melo et al. (2019) [49], Nurzyńska-Wierdak (2013) [50], and Padalia et al.’s (2011) [51] findings. Nevertheless, some exceptions were found, such as high contributions of (Z)-hex-3-en-1-ol, (Z)hex-3-en-1 acetate, and limonene—17.38 ± 0.38%, 34.54 ± 2.08%, and 2.43 ± 0.22%, respectively. In addition, in comparison to previous studies on cilantro, the high percentage of linalool was surprising, since it is much more characteristic for coriander seeds; however, some studies, such as the one performed by Shahwar et al., (2012) [51], supported this result. Such results may be reasoned in various plant chemotypes, which is well-documented in the scientific literature for coriander and other MAPs such as chamomile or sweet basil [52,53,54].
In terms of dried cilantro, some earlier studies were conducted; however, none of them included such a wide spectrum of drying methods, evaluated in the light of their impact on the sensory quality of products, as in the present study. Clearly, as shown in Table 5, the highest scores in the ranking test were granted to CPD70-VMFD and CD70 products. Furthermore, those two samples were qualified in the same group (C—“dry grass”, “herbal”, “sweet”, “hay-like”, “parsley-like” and “chamomile”) during the napping test. Regarding the cilantro OACs, according to Cadwallader et al. (1999) [12], (Z)-hex-3-enal and (E)-alk-2-enals are essential for the characteristic cilantro aroma. Moreover, such results were also confirmed by Eyres et al. (2005) [43]. Therefore, focusing on indicated VOCs, the CPD70-VMFD and CD70 products showed some similarities in VOC composition. Both samples showed not statistically significant differences regarding (Z)-hex-3-enal, its derivative (Z)-hex-3-en-1-ol, (E)-undec-3enal, and (E)-tetradec-3-enal contribution, which are responsible for green, cut grass, fruity, solvent-like, chemical, pungent, spicy, aldehydic, and floral aroma notes. On the other hand, the CPD70-VMFD and CD70 products differed in the case of (E)-dec-2-enal, (E)-dodec-2-enal, (E)-tridec-2-enal, and (Z)-tetradec-2-enal. A slight difference in (E)-dodec-2-enal contribution (in favor of CD70 product over CPD70-VMFD—13.95% to 11.80%) and higher difference in (E)-dec-2-enal (in favor of CPD70-VMFD product over CD70 product—9.89% to 4.17%) may be responsible for the marginally higher score of the CPD70-VMFD sample. Such an implication is supported by Tamura et al.’s (2013) [55] findings, which report that among cilantro OACs, (E)-dodec-2-enal, due to the low odor threshold, has the most significant impact on cilantro aroma quality. Admittedly, in this case, the highest scored dried product (CPD70-VMFD) had a lower contribution of (E)-dodec-2-enal than the second in the rank (CD70); however, CPD70-VMFD had a higher content of (E)-dec-2-enal, which has a strongly similar odor description to that of (E)-dodec-2-enal (Eyres et al., 2005) [43]. One of the most surprising experimental findings was that during the descriptive sensory analysis, particular aroma attributes of CD60 scored the highest, while during the ranking test, the CD60 sample was placed lower than the CPD70-VMFD and CD70 samples. Such a result supports the hypothesis that the strongest does not always mean the finest, as was proven in our earlier studies on Thai basil and lavender flowers [23,36]. Furthermore, in the napping test, CD60 was qualified into the group with some off-flavor descriptions (“lacteal”, “wet”, “fermented grass”), which also may be the reason for locating this sample lower in the ranking test than CPD70-VMFD and CD70.
The lowest scores in the ranking test were received by CD50/70, CD70/50, and VMD 480 products. Nevertheless, samples were qualified to groups (A and E) or closely placed next to groups (CD70/50) characterized by MAPs with off-flavor descriptions such as “fermented grass”, “wet”, “lacteal”, “mushrooms”, “fermented” or “musty”. Confronting these results with the cilantro VOC profile composition, it was unambiguous that for ”fermented”, “musty”, and “mushroom” descriptions of drying treatments consisting only of VMD, high contributions of β-pinene and terpinolene were responsible, which, according to Breheret et al. (1997) [56], have a significant role in a wild mushroom aroma. In addition, it is interesting to mention that the compound responsible for the woody notes (Figure 3) of dried cilantro was identified as (Z)-tetradec-2-enal. There was a clear relationship between the woody notes’ intensity and the contents of this compound in the VOC profile analysis.
In earlier studies by Fathima et al., (2001) [43] and Pirbalouti et al., (2017) [32], it was pointed out that the application of microwave and high temperature (up to 60 °C) during cilantro drying decreased the quality of the dried products, which seems to support the part of obtained results in the present study, in terms of the results obtained in the case of using VMD individually. Overall, the lowest scores were granted to drying methods consisting only of VMD. In addition, VMD received the most negative odor descriptions (mushroom) during the napping test. On the other hand, the previously cited studies pointed out that applying high temperatures may also be destructive for cilantro aroma quality. Nevertheless, the current results show that utilizing only CD gave satisfying results, which corresponds to Kamel et al.’s (2013) [33] findings. The differences in quality of dried cilantro by CD may be caused by many factors, such as the humidity of the environment in which the process was performed, drying air velocity, and more. The overall satisfying results of combined drying, consisting of CPD followed by VMFD, applied for herbs was earlier confirmed in Chua et al., (2019b, 2019a) [47,57], Calín-Sánchez et al. (2013, 2011) [45,58] and Ali et al.’s (2020) [59] studies. Furthermore, Pirbalouti et al. (2017) [32], in their study, based the evaluation of the quality of dried cilantro on EO composition, which, as was proven in our earlier studies on lavender [23,35] and Thai basil [36], is not correct for the determination of the quality for whole plants.
5. Conclusions
The growing demand for medicinal and aromatic plants, especially in terms of flavoring purposes, results in an urgent need to determine optimal protocols in the case of preservation. Regarding the cost and time efficiency, drying technologies are leading post-harvest treatments. Earlier studies showed that the universal drying protocol for all kind of medicinal and aromatic plants is questionable, since plants’ morphological differences and specific volatile organic constituent compositions result in different process requirements. In this study, the evaluation of different drying methods for the preservation of Coriandrum sativum L. leaves (cilantro) was performed. The experimental results of the cilantro aroma profile (HS-SPME-GC/MS), multiple sensory analyses of dried products (ranking test, napping test, sensory descriptive analysis), and technological aspects of drying processes show that convective drying at 70 °C for 120 min followed by vacuum-microwave drying at 360 W and convective drying at 70 °C were the optimal drying treatments guaranteeing cilantro-based products the highest quality. The aroma profile of these samples was characterized by low contents of (Z)-hex-3-enal and (Z)-hex-3-en-1-ol and intermediate contents of (E)-dec-2-enal, (E)-dodec-2-enal, (E)-tridec-2-enal, and (Z)-tetradec-2-enal. In contrast, the lowest rated samples, mainly including only vacuum-microwave drying and convective drying at two temperatures, namely vacuum-microwave drying with a power of 480 W or convective drying at 50 °C for 120 min followed by convective finishing drying at 70 °C, were characterized by high contents of β-pinene and terpinolene.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/10/2/403/s1, Figures S1 and S2: unknown volatile compounds mass spectra; Table S1: lexicon for sensory descriptive analysis; Database S1: link to raw data files including cilantro VOCs profiles analysis.
Author Contributions
Conceptualization, J.Ł. and A.S.; methodology, J.Ł., K.M., L.L., H.I.; K.L., Á.A.C.-B. and A.S.; validation, J.Ł., K.M., L.L., K.L., Á.A.C.-B. and A.S.; formal analysis, J.Ł., K.M., L.L., Á.A.C.-B. and A.S.; investigation, J.Ł., K.M., L.L., K.L., H.I. and Á.A.C.-B.; resources, J.Ł., K.L., Á.A.C.-B. and A.S.; data curation, J.Ł., K.M. and L.L.; writing—original draft preparation, J.Ł., K.M., L.L., K.L., Á.A.C.-B. and A.S.; writing—review and editing, J.Ł., L.L., H.I., Á.A.C.-B. and A.S.; visualization, J.Ł., K.M. and L.L.; supervision, K.L., Á.A.C.-B. and A.S.; project administration, J.Ł.; funding acquisition, J.Ł. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland) as the Ph.D. research program “Innowacyjny Doktorat”, no. B020/0009/19.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; London, UK: 2016. [Google Scholar]
- 2.Laribi B., Kouki K., Hamdi M.M., Bettaieb T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015;103:9–26. doi: 10.1016/j.fitote.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Rajeshwari U., Andallu B. Medicinal benefits of coriander (Coriandrum Sativum L.) Spat. DD. 2011;1:51–58. doi: 10.5455/spatula.20110106123153. [DOI] [Google Scholar]
- 4.Wei J.-N., Liu Z.-H., Zhao Y.-P., Zhao L.-L., Xue T.-K., Lan Q.-K. Phytochemical and Bioactive Profile of Coriandrum sativum L. Food Chem. 2019;286:260–267. doi: 10.1016/j.foodchem.2019.01.171. [DOI] [PubMed] [Google Scholar]
- 5.Mandal S., Mandal M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015;5:421–428. doi: 10.1016/j.apjtb.2015.04.001. [DOI] [Google Scholar]
- 6.Padalia R.C., Karki N., Sah A.N., Verma R.S. Constituents of Leaf and Seed Essential Oil of Coriandrum sativum L. J. Essent. Oil Bear. Plants. 2011;14:610–616. doi: 10.1080/0972060X.2011.10643979. [DOI] [Google Scholar]
- 7.Raghavan S. Handbook of Spices, Seasoning and Flavorings. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 8.Prachayasittikul V., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018;105:305–323. doi: 10.1016/j.foodres.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Zangeneh M.M., Zangeneh A., Moradi R., Shahmohammadi A. Chemical Characterization and Antibacterial Activity of the Essential Oil of Coriandrum sativum Leaves in the West of Iran (Kermanshah) J. Essent. Oil Bear. Plants. 2018;21:1349–1358. doi: 10.1080/0972060X.2018.1526130. [DOI] [Google Scholar]
- 10.Yildiz H. Chemical Composition, Antimicrobia, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. Int. J. Food Prop. 2016;19:1593–1603. doi: 10.1080/10942912.2015.1092161. [DOI] [Google Scholar]
- 11.Priyadarshi S., Khanum H., Ravi R., Borse B.B., Naidu M.M. Flavour characterisation and free radical scavenging activity of coriander (Coriandrum sativum L.) foliage. J. Food Sci. Technol. 2016;53:1670–1678. doi: 10.1007/s13197-015-2071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadwallader K.R., Surakarnkul R., Yang S.-P., Webb T.E. Character-Impact Aroma Components of Coriander (Coriandrum Sativuml.) Herb K. In: Shahidi F., editor. Flavor Chemistry of Ethnic Foods. Springer; Boston, MA, USA: 1999. [Google Scholar]
- 13.Das L., Raychaudhuri U., Chakraborty R. Supplementation of Common White Bread by Coriander Leaf Powder. Food Sci. Biotechnol. 2012;21:425–433. doi: 10.1007/s10068-012-0054-9. [DOI] [Google Scholar]
- 14.Priyadarshi S., Naidu M.M. A comparative study on nutritional, fatty acids, carotenoids, aroma and antioxidative characteristics of Microcarpum DC and Vulgare alef varieties of coriander foliage. Indian J. Tradit. Knowl. 2019;18:458–467. [Google Scholar]
- 15.Başer K.H.C., Buchbauer G., editors. Handbook of Essential Oils. Science, Technology, and Applications. 2nd ed. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 16.Oztekin S., Martinov M., editors. Medicinal and Aromatic Crops Harvesting, Drying, and Processing. Haworth Food Articultural Products Press; Binghamton, NY, USA: 2007. [Google Scholar]
- 17.Chua L.Y.W., Chong C.H., Chua B.L., Figiel A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019;12:450–476. doi: 10.1007/s11947-018-2227-x. [DOI] [Google Scholar]
- 18.Rocha R.P., Melo E.C., Radünz L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plants Res. 2012;5:7076–7084. doi: 10.5897/JMPRX11.001. [DOI] [Google Scholar]
- 19.Jin W., Mujumdar A.S., Zhang M., Shi W. Novel Drying Techniques for Spices and Herbs: A Review. Food Eng. Rev. 2018;10:34–45. doi: 10.1007/s12393-017-9165-7. [DOI] [Google Scholar]
- 20.Rahath Kubra I., Kumar D., Jagan Mohan Rao L. Emerging Trends in Microwave Processing of Spices and Herbs. Crit. Rev. Food Sci. Nutr. 2016;56:2160–2173. doi: 10.1080/10408398.2013.818933. [DOI] [PubMed] [Google Scholar]
- 21.El-Zaeddi H., Martínez-Tomé J., Calín-Sánchez Á., Burló F., Carbonell-Barrachina Á. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods. 2016;5:41. doi: 10.3390/foods5020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choo C.O., Chua B.L., Figiel A., Jałoszyński K., Wojdyło A., Szumny A., Łyczko J., Chong C.H. Hybrid Drying of Murraya koenigii Leaves: Energy Consumption, Antioxidant Capacity, Profiling of Volatile Compounds and Quality Studies. Processes. 2020;8:240. doi: 10.3390/pr8020240. [DOI] [Google Scholar]
- 23.Łyczko J., Jałoszyński K., Surma M., García-Garví J.-M., Carbonell-Barrachina A.A., Szumny A. Determination of Various Drying Methods’ Impact on Odour Quality of True Lavender (Lavandula angustifolia Mill.) Flowers. Molecules. 2019;24:2900. doi: 10.3390/molecules24162900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mierzwa D., Szadzińska J. The microwave-assisted convective drying of kale (Brassica oleracea L. var. sabellica L.) using continuous and changeable power radiation. J. Food Process Eng. 2019;42:1–14. doi: 10.1111/jfpe.13004. [DOI] [Google Scholar]
- 25.Nabissi M., Fiorini D., Molle A., Santini G., Maggi F., Benelli G. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2018;128:581–589. [Google Scholar]
- 26.Politowicz J., Lech K., Sánchez-Rodríguez L., Figiel A., Szumny A., Grubor M., Carbonell-Barrachina Á.A. Volatile composition and sensory profile of oyster mushroom as affected by drying method. Dry. Technol. 2018;36:685–696. doi: 10.1080/07373937.2016.1274903. [DOI] [Google Scholar]
- 27.Jayashree E., Visvanathan R., Zachariah T.J. Quality of dry ginger (Zingiber officinale) by different drying methods. J. Food Sci. Technol. 2014;51:3190–3198. doi: 10.1007/s13197-012-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antal T., Figiel A., Kerekes B., Sikolya L. Effect of drying methods on the quality of the essential oil of spearmint leaves (Mentha spicata L.) Dry. Technol. 2011;29:1836–1844. doi: 10.1080/07373937.2011.606519. [DOI] [Google Scholar]
- 29.Babu A.K., Kumaresan G., Aroul V.A., Velraj R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018;90:536–556. doi: 10.1016/j.rser.2018.04.002. [DOI] [Google Scholar]
- 30.Yilmaz A., Alibas I. Determination of Microwave and Convective Drying Characteristics of Coriander Leaves. J. Biol. Environ. Sci. 2017;11:75–85. [Google Scholar]
- 31.Sarimeseli A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011;52:1449–1453. doi: 10.1016/j.enconman.2010.10.007. [DOI] [Google Scholar]
- 32.Pirbalouti A.G., Salehi S., Craker L. Effect of drying methods on qualitative and quantitative properties of essential oil from the aerial parts of coriander. J. Appl. Res. Med. Aromat. Plants. 2017;4:35–40. doi: 10.1016/j.jarmap.2016.07.006. [DOI] [Google Scholar]
- 33.Kamel S.M., Thabet H.A., Algadi E.A. Influence of Drying Process on the Functional Properties of Some Plants. Chem. Mater. Res. 2013;3:1–8. [Google Scholar]
- 34.Fathima A., Begum K., Rajalakshmi D. Microwave drying of selected greens and their sensory. Plant Food Hum. Nutr. 2001;56:303–311. doi: 10.1023/A:1011858604571. [DOI] [PubMed] [Google Scholar]
- 35.Łyczko J., Jałoszyński K., Surma M., Masztalerz K., Szumny A. HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules. 2019;24:764. doi: 10.3390/molecules24040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Łyczko J., Masztalerz K., Lipan L., Lech K., Carbonell-Barrachina Á.A., Szumny A. Chemical determinants of dried Thai basil (O. basilicum var. thyrsiflora) aroma quality. Ind. Crop. Prod. 2020;155:112769. doi: 10.1016/j.indcrop.2020.112769. [DOI] [Google Scholar]
- 37.Adams R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy. Academic Press; San Diego, CA, USA: 2012. [Google Scholar]
- 38.International Standard Organization . Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors—Part 1: Selected Assessors. ISO 8586-1:1993. International Standard Organization; Geneva, Switzerland: 1993. [Google Scholar]
- 39.Meilgaard M.C., Civille G.V., Carr B.T. Sensory Evaluation Techniques. 5th ed. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 40.Louw L., Malherbe S., Naes T., Lambrechts M., van Rensburg P., Nieuwoudt H. Validation of two Napping® techniques as rapid sensory screening tools for high alcohol products. Food Qual. Prefer. 2013;30:192–201. doi: 10.1016/j.foodqual.2013.05.016. [DOI] [Google Scholar]
- 41.Pagès J., Cadoret M., Lê S. The sorted napping: A new holistic approach in sensory evaluation. J. Sens. Stud. 2010;25:637–658. doi: 10.1111/j.1745-459X.2010.00292.x. [DOI] [Google Scholar]
- 42.Perrin L., Symoneaux R., Maître I., Asselin C., Jourjon F., Pagès J. Comparison of three sensory methods for use with the Napping® procedure: Case of ten wines from Loire valley. Food Qual. Prefer. 2008;19:1–11. doi: 10.1016/j.foodqual.2007.06.005. [DOI] [Google Scholar]
- 43.Eyres G., Dufour J.-P., Hallifax G., Sotheeswaran S., Marriott P.J. Identification of character-impact odorants in coriander and wild coriander leaves using gas chromatography-olfactometry (GCO) and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry (GCxGC–TOFMS) J. Sep. Sci. 2005;28:1061–1074. doi: 10.1002/jssc.200500012. [DOI] [PubMed] [Google Scholar]
- 44.Politowicz J., Lech K., Sánchez-Rodríguez L., Szumny A., Carbonell-Barrachina Á.A. Volatile composition and sensory profile of Cantharellus cibarius Fr. as affected by drying method. J. Sci. Food Agric. 2017;97:5223–5232. doi: 10.1002/jsfa.8406. [DOI] [PubMed] [Google Scholar]
- 45.Calín-Sánchez Á., Figiel A., Lech K., Szumny A., Carbonell-Barrachina Á.A. Effects of Drying Methods on the Composition of Thyme (Thymus vulgaris L.) Essential Oil. Dry. Technol. 2013;31:224–235. doi: 10.1080/07373937.2012.725686. [DOI] [Google Scholar]
- 46.Calín-Sánchez Á., Lech K., Szumny A., Figiel A., Carbonell-Barrachina Á.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012;48:217–225. doi: 10.1016/j.foodres.2012.03.015. [DOI] [Google Scholar]
- 47.Chua L.Y.W., Chua B.L., Figiel A., Chong C.H., Wojdyło A., Szumny A., Lech K. Characterisation of the convective hot-air drying and vacuum microwave drying of Cassia alata: Antioxidant activity, essential oil volatile composition and quality studies. Molecules. 2019;14:1625. doi: 10.3390/molecules24081625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calín-Sánchez Á., Figiel A., Lech K., Szumny A., Martínez-Tomé J., Carbonell-Barrachina Á.A. Dying methods affect the aroma of Origanum majorana L. analyzed by GC-MS and descriptive sensory analysis. Ind. Crops Prod. 2015;74:218–227. doi: 10.1016/j.indcrop.2015.04.067. [DOI] [Google Scholar]
- 49.de Melo A.C.G.R., dos Santos M.D.V., de Carvalho Neto M.F., Takarashi J.A., Ferraz V.P., Chagas E.A., Chagas P.C., de Melo Filho A.A. Phytochemical Trial and Bioactivity of the Essential Oil from Coriander Leaves (Coriandrum sativum) on Pathogenic Microorganisms. Chem. Eng. Trans. 2019;75:403–408. [Google Scholar]
- 50.Nurzyńska-Wierdak R. Essential Oil Composition of the Coriander (Coriandrum sativum L.) Herb Depending on the Development Stage. ACTA Agrobot. 2013;66:53–60. doi: 10.5586/aa.2013.006. [DOI] [Google Scholar]
- 51.Shahwar M.K., El-Ghorab A.H., Anjum M., Butt M.S., Hussain S., Butt M.S., Hussain S., Characterization M.N. Characterization of Coriander (Coriandrum sativum L.) Seeds and Leaves: Volatile and Non Volatile Extracts. Int. J. Food Prop. 2012;15:736–747. doi: 10.1080/10942912.2010.500068. [DOI] [Google Scholar]
- 52.Farouk A., Fikry R., Mohsen M. Chemical Composition and Antioxidant Activity of Ocimum basilicum L. Essential Oil Cultivated in Madinah Monawara, Saudi Arabia and its Comparison to the Egyptian Chemotype. J. Essent. Oil Bear. Plants. 2016;19:1119–1128. doi: 10.1080/0972060X.2016.1149112. [DOI] [Google Scholar]
- 53.Orav A., Arak E., Raal A. Essential Oil Composition of Coriandrum sativum L. Fruits from Different Countries. J. Essent. Oil Bear. Plants. 2011;14:118–123. doi: 10.1080/0972060X.2011.10643910. [DOI] [Google Scholar]
- 54.Rubiolo P., Belliardo F., Cordero C., Liberto E., Sgorbini B., Bicchi C. Headspace—Solid-phase Microextraction Fast GC in Combination with Principal Component Analysis as a Tool to Classify Different Chemotypes of chamomile flower-heads (Matricaria recutita L.) Phytochem. Anal. 2006;17:217–225. doi: 10.1002/pca.919. [DOI] [PubMed] [Google Scholar]
- 55.Tamura H., Maeyama K., Yoshida E., Kori M. Aroma Character of Coriander (Coriandrum Sativum L.) Leaves: Limited Odor Unit Method and Sensory Perception in Preference. In: Ho C.-T., Mussinan C., Shahidi F., Contis E.T., editors. Nutrition, Functional and Sensory Properties of Foods. RCS Publishing; Cambridge, UK: 2013. [Google Scholar]
- 56.Breheret S., Talou T., Rapior S., Bessière J.-M. Monoterpenes in the aromas of fresh wild mushrooms (Basidiomycetes) J. Agric. Food Chem. 1997;45:831–836. doi: 10.1021/jf960417h. [DOI] [Google Scholar]
- 57.Chua L.Y.W., Chua B.L., Figiel A., Chong C.H., Wojdyło A., Szumny A., Łyczko J. Drying of Phyla nodiflora Leaves: Antioxidant Activity, Volatile and Phytosterol Content, Energy Consumption, and Quality Studies. Processes. 2019;7:210. doi: 10.3390/pr7040210. [DOI] [Google Scholar]
- 58.Calín-Sánchez Á., Szumny A., Figiel A., Jałoszyński K., Adamski M., Carbonell-Barrachina Á.A. Effects of vacuum level and microwave power on rosemary volatile composition during vacuum-microwave drying. J. Food Eng. 2011;103:219–227. doi: 10.1016/j.jfoodeng.2010.10.018. [DOI] [Google Scholar]
- 59.Ali A., Choong C., Lin B., Figiel A., Hwa C., Wojdylo A., Piotr I., Szumny A., Jacek Ł. Volatile and polyphenol composition, anti-oxidant, anti-diabetic and anti-aging properties, and drying kinetics as affected by convective and hybrid vacuum microwave drying of Rosmarinus officinalis L. Ind. Crops Prod. 2020;151:112463. doi: 10.1016/j.indcrop.2020.112463. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.