Abstract
Background
Some experimental studies have established the effect of oysters on the promotion of body growth. Yet, there is a lack of human clinical studies. The objective of this study was to evaluate the effect of a fermented oyster (FO) extract on the increase in the height of children with stature in the 25th percentile by age.
Methods
In total, 100 children (6–11 years old) were randomly divided into two (FO or control) groups. For 24 weeks, the subjects in the FO group took the FO extract once daily before sleeping, whereas the control group took placebo extracts, simultaneously. We evaluated the height gain, height velocity (HV), height standard deviation score (SDS), urine deoxypyridinoline (DPD), growth hormone (GH), insulin-like growth factor (IGF-1), and IGF binding protein 3 (IGFBP-3).
Results
The height gain and height SDS were significantly higher in the FO group than in the placebo group after 24 weeks (height gain: p < 0.001, height SDS: p < 0.005). The HV was also significantly higher in the FO group than in the placebo group after the 6th and 24th week (p = 0.001, p = 0.004). After 24 weeks, we observed a decrease in GH, IGF, and IGFBP-3 in both groups. However, serum IGFBP-3 level in the FO group reduced less than placebo group.
Conclusion
FO supplementation may help to increase the height of children, and the effect might be mediated via effects on the IGFBP-3 levels.
Keywords: Fermented oyster, Growth, RCT, Height, Short stature
1. Introduction
Complex environmental factors, including heredity and nutrition, affect the increase in height in children.1 Short stature is defined as a height that is more than two standard deviations (SD) below the mean, which is near the third percentile.2 However, children in a range that does not correspond to short stature receive growth hormone (GH) treatment because of the increasing interest in height in pediatrics and socio-economic development.3
For more than fifty years, GH treatment has been prescribed to promote an increase in height.4 Although relatively safe, there have been reports of several adverse effects such as rash and pain at the injection site, pre-pubertal gynecomastia, arthralgia.5 Additionally, GH treatment during the pre-pubertal period does not affect the final adult height compared to that of untreated children.6
According to Bonchogangmok (a Chinese book published in 1596), oysters are reported to be effective in removing dampness and phlegm in body, so they have been widely used for the treatment of furuncle, hyperhidrosis and stress.7 Oysters have been known to promote bone generation because of their high calcium content.8 Several studies have indicated the effects of oysters in preventing osteoclast differentiation9 and promoting bone formation.10 Bone formation and the release of GH are important factors influencing the increase in the height of children.11 Thus, the effect of oysters on this increase in height may be expected. Until recently, some experimental studies have established the effect of oysters on the promotion of body growth8, 9; however, no human clinical studies have been reported. Therefore, the purpose of this study was to investigate the effect of fermented oysters (FO) on growth promotion in children with stature in the 25th percentile by age, through the analysis of various growth parameters.
2. Methods
2.1. Participants
This study was conducted in the Korean Medicine Hospital of the Pusan National University. The participants were recruited from April 29, 2019, to April 8, 2020, and children with stature in the 25th percentile by age were eligible to participate in the study according to the inclusion criteria.
2.2. Inclusion and exclusion criteria
We included volunteers who fulfilled the following criteria: (1) healthy children between the ages of 6 and 11 years whose stature was under the 25th percentile, by gender and age, in the “2017 Korean Children and Adolescents Growth Standard”12; (2) voluntary participants who, along with their parents, provided written informed consent.
In line with the laboratory test results, the children who did not meet the following criteria were excluded from the study: those who (1) had an endocrine disease such as GH deficiency, hypothyroidism, type 1 diabetes, or precocious puberty; (2) had an inflammatory disease; (3) had received GH treatment within the previous 4 weeks before screening or any kind of medicinal food for promoting height gain within 2 weeks before screening; (4) had uncontrolled glucose levels and were diabetic; (5) had abnormal laboratory test results for thyroid-stimulating hormone, free thyroxine, creatinine, aspartate aminotransferase, or alanine aminotransferase; (6) had received treatment related to heart disease and attention deficit hyperactivity disorder and (7) wanted to participate in another clinical trial.
2.3. Randomization, allocation concealment
This study was a prospective randomized placebo-controlled trial. The participants were randomly allocated to the treatment and control groups at a 1:1 allocation ratio. We used the block randomization method in SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Since a third-party statistician who was not involved in this study generated the random list, all investigators were unaware of the size and number of blocks. The random list was offered in an opaque sealed envelope, and all participants, investigators, and assessors were blinded to the treatment allocation until the end of the study.
The two study populations were analyzed as the intention to treat (ITT) and per-protocol (PP) populations. All participants for whom at least one primary or secondary endpoint was evaluated were included in the ITT analysis. A PP analysis was conducted on the subjects who continued to take each interventions for more than 3 days and for more than 70% of the total study period.
2.4. Study procedure
At the first visit, the participants were interviewed by the investigator to obtain and record their baseline characteristics (name, age, height, weight, gender), medical history, vital signs, physical examination, primary endpoints, and diet evaluation on the case report form.
Two weeks after randomization (visit 2), the participants received the FO extract or placebo as per the randomization list, according to the study protocol. They also received a questionnaire to record their compliance with the study. Kim or Park (authors of this study) confirmed the results of the questionnaire at each visit. If the compliance rate of a subject was more than 80%, this was considered compliance.
The participants visited every 6 weeks. At the 6th, 12th, and 18th week, we performed a physical examination to obtain the vital signs, medical history, and height. At the 24th week, we obtained and evaluated the height standard deviation score (SDS); height velocity (HV); bone x-ray; laboratory tests, including serum GH, insulin-like growth factor 1 (IGF-1) and IGF binding protein 3 (IGFBP-3); and diet. The diet was evaluated using a 24-hour recall method questionnaire, and it was analyzed using CAN pro version 4.0 (The Korean Nutrition Society, Seoul, Korea). The blood tests were conducted to check the health of the participants and not to evaluate the outcome measures.
2.5. Outcome measurements and assessments
The primary outcome was a change in the height from the baseline to the 6th, 12th, 18th, and 24th week. The height was measured using a stadiometer (GL-150P, G-Tech International Co. Ltd. Kyongki, Korea) at the same time at each visit.
The secondary outcomes were HV, height SDS, bone x-ray, GH, IGF-1, IGFBP-3, osteocalcin and urine deoxypyridinoline (DPD) from the baseline to the 24th week. The HV is commonly used to identify the effect of the intervention in a height-related randomized controlled trial (RCT) since it provides clinicians with an additional tool for distinguishing the abnormal and normal standard of growth.13 Height SDS indicates how far the growth deviates from the normal range by a statistical distribution,14 and this was based on a growth chart, the “2017 Korean Children and Adolescents Growth Standard”,12 in the present study. The serum GH, IGF-1, and IGFBP-3 levels are typical outcome measures in growth-related trials in children because they play an essential role in stimulating the hypertrophy of the growth plate in childhood growth.15
2.6. Intervention
The FO extract and placebo were manufactured by Marin Bioprocess (Busan, Korea). The participants in the FO group were given a 20 ml stick-shaped sachet of 500 mg elemental FO in form of liquid stick (Supplementary Figure 1a). The participant in placebo group were given placebo sachet which had an identical appearance and smell. All participant in both groups were instructed to take 1 stick orally once daily before bedtime for 24 weeks.
In total, 56 sticks, with an extra amount added to the dose for the 6th week, were placed in a box; one box per child was provided to the children's parents at the 2nd, 3rd, 4th, and 5th visits. The box was labeled with a serial number according to the random list (Supplementary Figure 1b).
In a previous study in animals, no toxicity was observed when the animals were administered 100 mg/kg and 200 mg/kg FO extract for 28 days.16 Therefore, an effective dose of 200 mg/kg was converted into the dose appropriate for a child weighing 30 kg based on the body surface area and calculated as 500 mg. The detailed content of each component are presented in Supplementary Table 1. The nutrient composition of FO used in our study is same as that of the previous study.17
2.7. Sample size calculation
Based on one previous clinical study of herbal supplement for height growth in mild short stature children,18 we calculated the difference in the height as 0.51 cm and SD as 0.813, from the baseline to 24 weeks, using G-Power version 3.1.9.2. The previous study reported that the mean difference (MD) in height gain between groups was 0.29 cm and the SD was 0.813. For setting the minimal clinically important difference in our study, we additionally performed the Delphi method with 7 pediatricians. As a result, it was determined that 0.51 cm was appropriate as the MCID between groups. Accordingly, at least 40 subjects per group were required; thus, we estimated that a total of 100 subjects (1:1) would be required, considering a maximum dropout rate of 20%.
2.8. Statistical design and analysis
We analyzed the results based on the ITT analyses. Missing values were inputted via the last observation carried forward method. The analysis was performed using SPSS 22.0 (SPSS Statistics for Windows, version 22.0). The clinical and demographic data were compared between the groups using an independent sample t-test or chi-squared test as appropriate. Changes in the primary and secondary outcomes, between the baseline and 24th week, were compared within each group using a paired t-test. A p value less than 0.05 was considered statistically significant. The baseline characteristics of the participants were analyzed using the independent t-test for comparisons of the two groups (Table 1). We analyzed the differences between each endpoint before and after the treatment and presented effect size (Table 2).
Table 1.
Study Patient's Baseline Characteristics
| Variable | ITT population |
PP population |
||||
|---|---|---|---|---|---|---|
| Control (n = 50) | Experimental (n = 50) | p Value | Control (n = 47) | Experimental (n = 46) | p Value | |
| Gender (male) | 34 (68.0%) | 18 (36.0%) | 0.001** | 33 (70.2%) | 17 (37.0%) | 0.001* |
| Age (yr) | 8.30 ± 1.64 | 8.58 ± 1.79 | 0.417** | 8.28 ± 1.61 | 8.57 ± 1.83 | 0.422** |
| Weight (kg) | 25.12 ± 5.31 | 27.28 ± 6.50 | 0.072** | 24.80 ± 5.18 | 27.28 ± 6.67 | 0.048** |
| Height (cm) | 124.14 ± 9.26 | 126.17 ± 10.61 | 0.311** | 123.86 ± 9.10 | 125.97 ± 10.91 | 0.314** |
The data are expressed as the mean ± SD for the FO and placebo group baseline characteristics in ITT and PP population.
p Values are derived from Chi-square test. **p Values are derived from independent t test (comparing between groups).
ITT, intention to treat; PP, per protocol.
Table 2.
Comparison Between and Within Each Group (ITT Population)
| Variable | Observed value |
Change from baseline |
Effect size | ||
|---|---|---|---|---|---|
| Control (n = 50) | Experimental (n = 50) | Control (n = 50) | Experimental (n = 50) | ||
| Height (cm) | |||||
| visit 2 | 124.14 ± 9.26 | 126.17 ± 10.61 | |||
| visit 3 | 125.09 ± 9.27 | 127.68 ± 11.02 | 0.95 ± 0.76 | 1.51 ± 1.37* | 0.502 |
| visit 4 | 125.80 ± 9.32 | 128.36 ± 11.01 | 1.66 ± 0.79 | 2.19 ± 1.49* | 0.445 |
| visit 5 | 126.37 ± 9.22 | 128.98 ± 11.17 | 2.23 ± 0.76 | 2.81 ± 1.71* | 0.440 |
| visit 6 | 127.06 ± 9.39 | 129.95 ± 11.22 | 2.91 ± 0.84 | 3.78 ± 1.88** | 0.593 |
| HV (cm/year) | |||||
| visit 3 | 8.25 ± 6.60 | 14.21 ± 11.68** | – | – | 0.628 |
| visit 4 | 6.33 ± 5.44 | 6.46 ± 5.48 | – | – | 0.025 |
| visit 5 | 5.34 ± 5.20 | 5.80 ± 4.43 | – | – | 0.096 |
| visit 6 | 6.38 ± 4.72 | 9.10 ± 4.49** | – | – | 0.591 |
| Height SDS | |||||
| visit 2 | −1.39 ± 0.54 | −1.34 ± 0.57 | |||
| visit 3 | −1.33 ± 0.56 | −1.18 ± 0.62 | 0.06 ± 0.15 | 0.16 ± 0.22** | 0.535 |
| visit 4 | −1.31 ± 0.56 | −1.15 ± 0.63 | 0.08 ± 0.15 | 0.19 ± 0.24** | 0.553 |
| visit 5 | −1.31 ± 0.56 | −1.16 ± 0.63 | 0.08 ± 0.14 | 0.18 ± 0.25** | 0.524 |
| visit 6 | −1.30 ± 0.56 | −1.09 ± 0.62 | 0.09 ± 0.15 | 0.25 ± 0.26** | 0.751 |
| Bone ag (mo) | |||||
| visit 2 | 82.26 ± 26.07 | 92.68 ± 24.92* | |||
| visit 6 | 90.32 ± 25.89 | 100.34 ± 24.38* | 8.06 ± 6.86 | 7.66 ± 9.92 | −0.047 |
| GH (ng/mL) | |||||
| visit 2 | 2.26 ± 2.48 | 2.87 ± 3.84 | |||
| visit 6 | 1.89 ± 2.79 | 1.49 ± 1.64 | −0.37 ± 3.42 | −1.38 ± 3.44 | −0.295 |
| IGF-1 (ng/mL) | |||||
| visit 2 | 193.27 ± 69.13 | 214.41 ± 104.02 | |||
| visit 6 | 174.93 ± 71.20 | 203.72 ± 92.03 | −18.33 ± 31.12 | −10.69 ± 44.75 | 0.198 |
| IGFBP-3 (ng/mL) | |||||
| visit 2 | 4580.8 ± 958.79 | 4650.6 ± 948.29 | |||
| visit 6 | 4261.2 ± 930.17 | 4629.4 ± 971.48 | −319.60 ± 523.93 | −21.20 ± 693.32* | 0.486 |
| Osteocalcin (ng/mL) | |||||
| visit 2 | 58.27 ± 14.71 | 64.41 ± 20.26 | |||
| visit 6 | 74.07 ± 22.70 | 80.29 ± 21.90 | 15.80 ± 16.93 | 15.88 ± 21.87 | 0.004 |
| DPD (nM/mM.cre) | |||||
| visit 2 | 18.95 ± 6.57 | 18.11 ± 5.38 | |||
| visit 6 | 15.07 ± 5.54 | 15.46 ± 8.00 | −3.88 ± 6.85 | −2.65 ± 10.36 | 0.140 |
The data is expressed as the mean ± SD for pre and post outcomes and comparison between two groups. *, p < 0.05; **, p < 0.001 comparison between groups.
DPD, Urine Deoxypyridinoline; SD, standard deviation, GH, growth hormone; HV, height velocity; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3; ITT, intention to treat; PP, per protocol.
2.9. Safety assessment
To assess safety, the investigator queried the participants about adverse events at every visit and evaluated laboratory test abnormalities at the baseline and 24th week.
We performed a paired t-test for continuous data and the chi-square or Fisher's exact test for categorical data. A safety assessment was conducted in this study by a pediatrician with more than 10 years of experience.
3. Results
3.1. Baseline demographic and clinical data of the participants
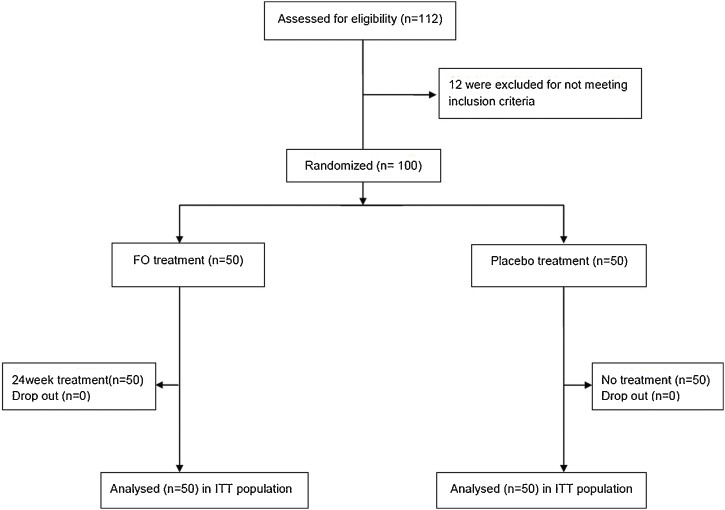
We screened 112 children for eligibility, and 100 participants were deemed eligible for enrollment in the study. The participants were separated into two groups: the FO group (n = 50) or the placebo group (n = 50). Seven participants (FO group: 4, placebo group: 3) were excluded from the study (4 participants: consent withdrawal, 3: failure to visit). Finally, 93 children (52 males, 49 females) participated in this study until the end of the study (Fig. 1).
Fig. 1.
Flow diagram of the randomized clinical trial comparing the FO extract to the placebo for increasing the height of children.
Most of the demographic data, except gender, and clinical data did not significantly differ at the baseline between the two groups (Table 1).
3.2. Primary endpoint
The primary efficacy analysis demonstrated that the FO group showed a significant increase in height gain compared with the placebo group after 24 weeks (p = 0.004, 95% CI: 0.192 to 0.993). After 24 weeks, participants belonging to the FO group were taller than placebo group participants (3.78 ± 1.88 cm and 2.91 ± 0.84, mean difference: 0.87 cm) (Table 2). We concluded that 0.51 cm is the minimally clinical importance difference between group for 24 weeks, which was the number obtained through the process of sample size calculation based on previous study.18 Therefore, it can be considered meaningful when the height growth is greater than this value. Further, after compensating for the gap by gender in the baseline data, the height gain was found to be higher in the FO group than in the placebo group in the ITT analysis (Supplementary Table 2). Our mainly results were shown to the results of ITT analysis, but, our PP analysis also have shown the meaningful results. Therefore, we additionally described the results of PP analysis in Supplementary Table 3.
3.3. Secondary endpoint
The effect size for the MD in the changes in the secondary endpoints are presented in Table 2. The MD in the pre and post-change of HV in the FO group was significantly increased compared with the placebo group at the 6th and 24th week from the baseline. Within each group, the height SDS was significantly increased in all groups at every visit. Furthermore, the increase in the FO group (0.09 ± 0.15) was more significant than that in the placebo group (0.25 ± 0.26). The height SDS was also significantly increased in the FO group compared with the placebo group at the 24th week (p < 0.01; 95% CI: 0.345 to 1.156).
The other secondary endpoints (bone age, GH, IGF-1, osteocalcin, and DPD) were not significantly different between the groups. The serum IGFBP-3 level showed a significant difference between the two groups. Hence, correcting the gender gap between the two groups also revealed a significant difference. The level of IGFBP-3 within each group had decreased during the trial. However, the decrease in the IGFBP-3 level in the FO group (−21.20 ± 693.32) was less than that in the placebo group in the ITT population (−319.60 ± 523.93) (Table 2). All of the primary and secondary endpoints were seen as analogous patterns in the ITT and PP populations.
3.4. Adverse events
In the safety assessment, no severe adverse event (AE) was recorded during this trial, except for one AE that was reported in the FO group (1%). The symptom was urticaria, which resolved naturally. There was no dropout or unblinding owing to the AE, which was considered mild in severity and not likely to be associated with the FO supplementation, according to the judgment of the pediatricians.
4. Discussion
To our knowledge, this is the first placebo RCT investigating the effect of the FO extract on height gain in children with short stature.
In our study, the children who received FO supplementation for 24 weeks showed a significant increase in height gain, HV, and height SDS compared with the children who received the placebo treatment. The MD in the height pre- and post-trial within each group was also 0.87 cm greater in the FO group than in the control group. Considering that common children grow about 5–6 centimeters per year,19 our result is considered to be meaningful improvement. Furthermore, one recent RCT for height growth in children, they reported that the MD of height gain in the intervention group was 0.29 cm taller than placebo group.18 Compared to the results of this study, our values of MD in height gain showed larger difference, so it may be considered that FO is effective in height growth of mild short stature children. Furthermore, our gender-adjusted subgroup analysis indicates that the participant in FO group was taller than the participant in placebo group. The MD in height gain was 0.75 cm in male group and 0.66 cm in female group (Supplementary Table 2-1, 2-2, 3-1, 3-2), which shows an interesting result suggesting that FO has a clinical effect in height growth, considering that the females has naturally shown more rapid height growth than males in pre-pubertal ages.20 Here we believe that our findings show a clinical relevance of FO.
Recently, a study reported that the FO extract contains a high GABA content, which can have positive effects on the body length and growth-plate length. Additionally, the administration of the FO extract to rats increased the IGFBP-3 level.17 Thus, our results of an increase in height may be considered to have been mediated by the abovementioned effects.
In our results, although the GH and IGF-1 levels were not significantly different between the groups, we noted that the change in the serum IGFBP-3 level was significantly different between the groups. The decrease of IGFBP-3 level in the FO group was less than that in the placebo group. IGFBP-3 mediates the action of IGF-1 and increases the half-life of IGF while facilitating the binding of free IGF in the blood to the IGF receptors, ultimately promoting growth.21 Recently, a study revealed that IGFBP-3 was an effective key element in predicting the final increase in height in children with short stature.22 Therefore, in our study, the effect of the FO extract on the increase in height may have been mediated by the increase in the serum IGFBP-3 levels.
Unusually, the serum GH, IGF-1, and IGFBP-3 levels were decreased over the 24 weeks in both groups. These factors are important biochemical indexes of the increase in height in children. However, according to several studies, the mean changes in GH and IGF-1 with age have remained controversial. While some previous studies reported that the IGF-1 and IGFBP-3 levels increase throughout childhood,23, 24, 25 others showed that the levels tend to decrease with age, with the declining function of the brain tissue,26 or with the regulation of the GH-IGF-1 axis.27 One clinical trial also reported a decrease in the levels of IGF-1 and IGFBP-3 after the administration of a gonadotropin-releasing hormone agonist.27 This is similar to our result. Despite the decreases in these markers, the mean IGF-1 and IGFBP-3 values were within the normal upper range in the FO group during our trial. Further mechanistic studies investigating the effects of FO and its components on various growth hormones are warranted.
Our study had some limitations. First, there was a lack of restrictions regarding genetic factors or environmental factors such as sleep hours, eating habits, and other external efforts to promote their children's height. Hence, confounding bias may be present. Second, based on prior growth-related study, a higher growth effect was achieved in groups that had been followed for more than 1 year.28 So, it would be needed if there was follow up at 6 months after the end of the study.
From our results, FO can be recommended for short stature children without growth hormone deficiency or other hormonal abnormalities in clinical practice.
In conclusion, we demonstrated the efficacy and safety of FO supplementation for 6 months to increase height in children with stature in the 25th percentile by age: the treatment resulted in a positive HV, and height SDS. Furthermore, IGFBP-3 has significant difference between two groups. Based on above results, we surmise that the effects of FO on height growth may be mediated by increases in serum IGFBP-3 level and it is thought to be meaningful result for the effect of FO on the height growth of children.
Author contributions
Conceptualization: AR and KB; Methodology: JY, JHC, and KB; Validation: AR, JY, JHC and KB; Data curation: JHP and BJ; Investigation: JHP, BJ and KB; Writing and Original draft: AR and KB; Writing – Review & Editing: AR, JY and KB; Supervision: KB; Resources: AR, HY and BC; Project administration: BC, HY and KB; Funding acquisition: KB.
Conflict of interest
The authors declare no conflict of interest. The company of FO provided the intervention and placebo products and it also collected and disposed of the products after the trial was over.
Funding
This research was part of the project titled “Development of functional food products with natural materials derived from marine resources” funded by the Ministry of Oceans and Fisheries, South Korea (grant no.:20170285).
Ethical statement
This study was approved by the Institutional Review Board of the Pusan National University Korean Medicine Hospital, Yangsan, South Korea (approval no.: PNUKHIRB-2019002). We conducted this study based on the 1975 Declaration of Helsinki, as revised in 2008, and followed the ethical guidelines of the institution.
Data availability
The data will be made available upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.imr.2020.100691.
Supplementary material
The following are the supplementary material to this article:
References
- 1.Jelenkovic A., Sund R., Hur Y.-M., Yokoyama Y., Hjelmborg JvB, Möller S. Genetic and environmental influences on height from infancy to early adulthood: an individual-based pooled analysis of 45 twin cohorts. Sci Rep. 2016;6:28496. doi: 10.1038/srep28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlaski J., Katanić D., Privrodski J.J., Kavecan I., Vorguicn I., Obrenović M. [idiopathic short stature] Srp Arh Celok Lek. 2013;141:256–261. doi: 10.2298/sarh1304256v. [DOI] [PubMed] [Google Scholar]
- 3.Yang S.W. Management of children with short stature. J Korean Soc Endocrinol. 2003;18:561–570. [Google Scholar]
- 4.Argente J. Challenges in the management of short stature. Horm Res Paediatr. 2016;85:2–10. doi: 10.1159/000442350. [DOI] [PubMed] [Google Scholar]
- 5.Souza F.M., Collett-Solberg P.F. Adverse effects of growth hormone replacement therapy in children. Arq Bras Endocrinol Metabol. 2011;55:559–565. doi: 10.1590/s0004-27302011000800009. [DOI] [PubMed] [Google Scholar]
- 6.Gool S., Kamp G., Odink R., Keizer-Schrama S., Waal H., Oostdijk W. High-dose GH treatment limited to the prepubertal period in young children with idiopathic short stature does not increase adult height. Eur J Endocrinol. 2010;162:653–660. doi: 10.1530/EJE-09-0880. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y.T., Choi B.T., Choi Y.H., Kwang K.H. Development of health assistances for anti stress used with ostreae concha. Korean J Orient Physiol Pathol. 2006;20:1602–1611. [Google Scholar]
- 8.Coringa R., de Sousa E.M., Botelho J.N., Diniz R.S., de Sá J.C., da Cruz M.C.F.N. Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model. PLOS ONE. 2018;13:e0198697. doi: 10.1371/journal.pone.0198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong J.-W., Choi S.H., Han M.H., Kim G.-Y., Park C., Hong S.H. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in raw 264.7 cells. Int J Mol Sci. 2019;20:1439. doi: 10.3390/ijms20061439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molagoda I.M.N., Karunarathne W.A.H.M., Choi Y.H., Park E.K., Jeon Y.-J., Lee B.-J. Fermented oyster extract promotes osteoblast differentiation by activating the wnt/β-catenin signaling pathway, leading to bone formation. Biomolecules. 2019;9:711. doi: 10.3390/biom9110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsey R.C., Mohan S. Skeletal effects of growth hormone and insulin-like growth factor-1 therapy. Mol Cell Endocrinol. 2016;432:44–55. doi: 10.1016/j.mce.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Center for Disease Control and Prevention, the Korean Pediatric Society, the Committee for the Development of Growth Standard for Korean Children and Adolescents . Division of chronic disease surveillance; Seoul: 2017. 2017 Korean children and adolescents growth standard (commentary for the development of 2017 growth chart). [government report online] Available from: https://knhanes.Cdc.Go.Kr/knhanes/sub08/sub08_02.Do. [Google Scholar]
- 13.Kelly A., Winer K.K., Kalkwarf H., Oberfield S.E., Lappe J., Gilsanz V. Age-based reference ranges for annual height velocity in us children. J Clin Endocrinol Metab. 2014;99:2104–2112. doi: 10.1210/jc.2013-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole T.J. A simple chart to identify non-familial short stature. Arch Dis Child. 2000;82:173–176. doi: 10.1136/adc.82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriström B., Lundberg E., Jonsson B., Albertsson-Wikland K., group obots IGF-1 and growth response to adult height in a randomized GH treatment trial in short non-GH-deficient children. J Clin Endocrinol Metab. 2014;99:2917–2924. doi: 10.1210/jc.2014-1101. [DOI] [PubMed] [Google Scholar]
- 16.Ihn H.J., Kim J.A., Lim S., Nam S.H., Hwang S.H., Lim J. Fermented oyster extract prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis. Nutrients. 2019:11. doi: 10.3390/nu11061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H., Hwang-Bo H., Ji S.Y., Kim M.Y., Kim S.Y., Woo M. Effect of fermented oyster extract on growth promotion in sprague-dawley rats. Integr Med Res. 2020;9:100412. doi: 10.1016/j.imr.2020.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D., Lee S.H., Song J., Jee H.J., Cha S.H., Chang G.T. Effects of astragalus extract mixture HT042 on height growth in children with mild short stature: a multicenter randomized controlled trial. Phytother Res. 2018;32:49–57. doi: 10.1002/ptr.5886. [DOI] [PubMed] [Google Scholar]
- 19.Voss L.D.M.J. Normal growth in the short normal prepubertal child: the wessex growth study. J Med Screen. 1998;5:127–130. doi: 10.1136/jms.5.3.127. [DOI] [PubMed] [Google Scholar]
- 20.Biernat M. Gender and height: developmental patterns in knowledge and use of an accurate stereotype. Sex Roles. 1993;29:691–713. [Google Scholar]
- 21.Ahmed S.F., Farquharson C. The effect of gh and IGF1 on linear growth and skeletal development and their modulation by socs proteins. J Endocrinol. 2010;206:249–259. doi: 10.1677/JOE-10-0045. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Colon S., Lazareva O., Purushothaman R., Malik S., Ten S., Bhangoo A. Baseline IGFBP – 3 as the key element to predict growth response to growth hormone and IGF – 1 therapy in subjects with non – GH deficient short stature and IGF – 1 deficiency. Clin Biochem. 2018;16:e58928–e. doi: 10.5812/ijem.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong A.P.S., Wong G.W.K., Choi K.-C., Ho C.-S., Chan M.H.M., Lam C.W.K. Reference values for serum levels of insulin-like growth factor (IGF-1) and igf-binding protein 3 (IGFBP-3) and their ratio in Chinese adolescents. Clin Biochem. 2007;40:1093–1099. doi: 10.1016/j.clinbiochem.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Bereket A., Turan S., Omar A., Berber M., Ozen A., Akbenlioglu C. Serum IGF-1 and IGFBP-3 levels of turkish children during childhood and adolescence: establishment of reference ranges with emphasis on puberty. Horm Res. 2006;65:96–105. doi: 10.1159/000091301. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield M.J., Rudlin C.R., Crigler J.F., Jr., Karol K.A., Crawford J.D., Boepple P.A. Changes in growth and serum growth hormone and plasma somatomedin-c levels during suppression of gonadal sex steroid secretion in girls with central precocious puberty. J Clin Endocrinol Metab. 1988;66:3–9. doi: 10.1210/jcem-66-1-3. [DOI] [PubMed] [Google Scholar]
- 26.Sonntag W., Brunso-Bechtold J., Riddle D. Age-related decreases in growth hormone and insulin-like growth factor (IGF)-1: implications for brain aging. J Anti Aging Med. 2001;4:311–329. [Google Scholar]
- 27.Yi K.H. Serum igf-1 and igfbp-3 levels in central precocious puberty girls treated with gonadotropin releasing hormone agonist (GnRHa) J Korean Soc Pediatr Endocrinol. 2011;16:20–23. [Google Scholar]
- 28.Chung W.Y., Yoo H.W., Hwang J.S., Ko C.W., Kim H.S., Jin D.K. Effect of growth hormone therapy on height velocity in korean children with idiopathic short stature: a phase iii randomised controlled trial. Horm Res. 2018;90:44–53. doi: 10.1159/000491016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon reasonable request.