Highlights
-
•
Bariatric surgical procedures increase as the prevalence for obesity rises.
-
•
A perforated pyloric ulcer after Roux-en-Y gastric bypass is a rare complication.
-
•
Due to the changed anatomy, no free air was found in the CT-scan in our patient.
-
•
H. Pylori testing was positive in the blood test and negative in the stool sample.
Keywords: Case report, Bariatric surgery, Roux-en-Y gastric bypass, Gastric remnant, Perforated ulcer
Abstract
Introduction and importance
Bariatric or metabolic surgery is an emerging surgical specialty. With the increase of obesity and affiliated complications, the Roux-en-Y gastric bypass became a well-established procedure worldwide.
Case presentation
We present the case of a 46-year-old female patient who presented herself in the emergency department with diffuse abdominal pain, 13 years after a laparoscopic Roux-en-Y gastric bypass. The CT scan found suspicions of an internal hernia. The diagnostic laparoscopy showed a perforated pyloric ulcer of the gastric remnant as well as an internal hernia without any signs of incarceration. The ulcer was repaired by laparoscopic suture and the mesenteric defect at the enteroenterostomy was closed. The testing for H. pylori by different means showed a negative (stool) and a positive (serology) result.
Clinical discussion
The loss of connection of the gastric remnant to the oesophagus poses challenges in the diagnostic process: in regard to the perforated ulcer, free air, the most common sign, is absent, and testing of H. pylori presents limited options.
Conclusion
Bariatric patients remain patients with special considerations even long after undergoing these surgeries because of the drastic change in their anatomy and metabolism. Furthermore, due to the aforementioned reasons, diagnostic by clinical findings and imaging can be difficult and these patients should undergo a diagnostic laparoscopy and multimodal testing for H. pylori.
1. Introduction
Bariatric surgery has been long in existence with its first appearance in the 1950’s. With the surge of laparoscopic surgery technique, these procedures have transformed from being known to have a high mortality and morbidity rate to being a relatively safe operation – despite the high-risk nature of the patients undergoing these procedures [1]. Not only did procedure safety increase, but the worldwide prevalence of obesity nearly tripled from 1975 to 2016 [2]. In 2016, more than 1.9 billion adults aged 18 years and older were overweight. Of these, over 650 million adults were obese [3]. With this increasing problem, the need for bariatric surgery has grown, and especially for the morbidly obese, bariatric surgery has been the most effective way of long-lasting weight loss [4]. The total number of bariatric procedures performed worldwide in 2014 amounted to 579,517 (97.6%) surgical operations and 14,725 (2.4%) endoluminal procedures. The most commonly performed procedure in the world was the sleeve gastrectomy (45.9% of all procedures), followed by Roux-en-Y gastric bypass (RYGB) (39.6%), and adjustable gastric banding (7.4%) [5].
Regarding the safety and efficacy of the bariatric surgery, Reges et al. showed a lower all-cause mortality compared to the usual care nonsurgical obesity management [6], and an observational study performed in Utah comparing patients 7 years post-RYGB and controls showed reduction in all-cause mortality by 40%, cardiovascular death by 49% and type-2 diabetes mellitus related death by 92% [7]. The short-term surgical complications of the Roux-en-Y gastric bypass described include peritonitis due to anastomotic leak (1–6%), bleeding from anastomosis (approx. 2% of all patients undergoing bariatric surgery) and wound infection (3%). Internal hernia (6% after RYGB or biliary pancreatic shunting), anastomotic stenosis (12%), gastric erosion (0.3–7%) and intestinal small bowel obstructions (5%) are longer-term surgical complications [8].
In this case report, we intend to describe a rarer complication after RYGB and demonstrate the difficulties in the diagnostic process.
2. Case
This case report has been reported in line with the SCARE 2020 criteria [9].
A 46-year-old female patient presented herself at our emergency department with diffuse abdominal pain for a duration of approximately 6 h. The pain started quite suddenly, was initially located in the epigastric region and was described as cramps, with an evolution of the localization to the right hemiabdomen. She has a history of episodes of gastric reflux with intake of a proton pump inhibitor (PPI) from approximately 2014 – 2018. Her current body mass index (BMI) was 39.2 kg/m2. Prior to the RYGB in 2007, her BMI measured 46.7 kg/m2, the lowest BMI she reported was in 2009 with 28.7 kg/m2. A preoperative gastroscopy or testing for H. pylori did not take place.
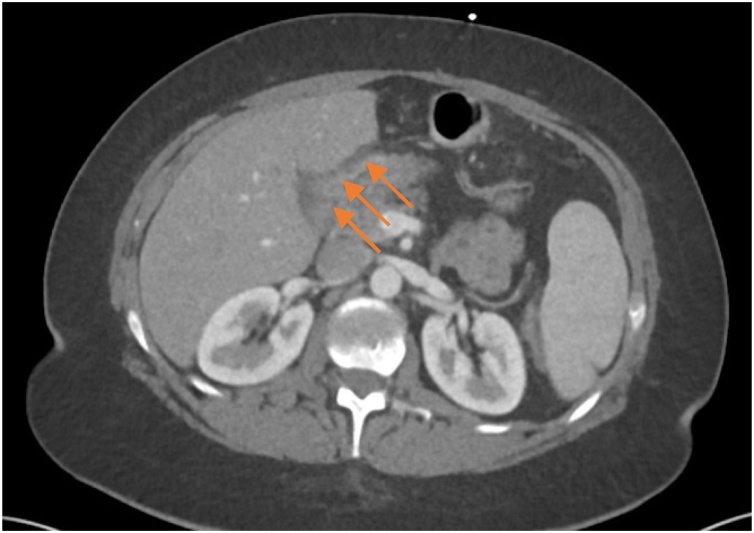
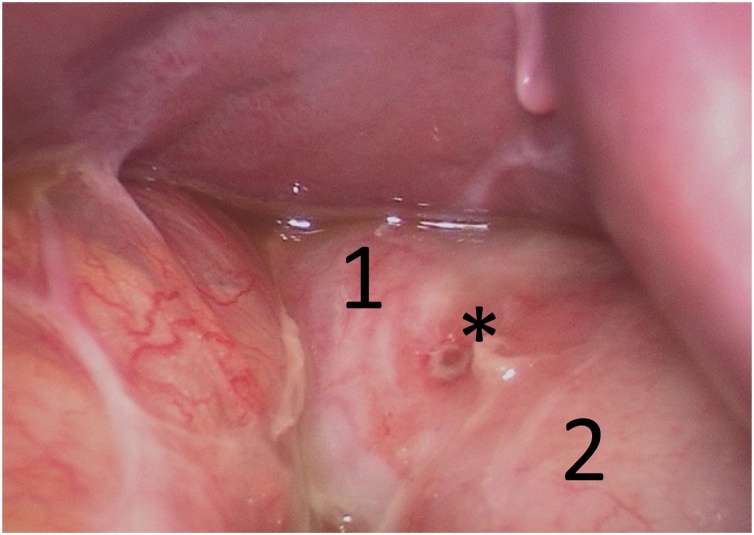
The clinical findings showed a normofrequent, normotensive, afebrile patient with diffuse abdominal tenderness without rebound. Laboratory findings were within normal limits except for a marginally elevated lactate of 2.3 mmol/L (N: 0.5–1.6 mmol/L) in the venous blood gas sample. The computed tomography (CT) of the abdomen during the ER stay showed suspicions of an internal hernia with possible venous congestion as well as some free fluid (Fig. 1). Due to these findings, a diagnostic laparoscopy was discussed with the patient and was performed the same day by a visceral surgeon. The abdomen was systematically explored and surprisingly, a perforated pyloric ulcer on the ventral side of the gastric remnant measuring 4 mm was found (Fig. 2). The ulcer was then closed by laparoscopic, extracorporeally knotted single stitch suture, a biopsy was not taken. The Peterson gap was found to be closed, the mesenterial gap at the jejunojejunostomy showed an internal hernia of 15 cm small intestine of the common channel without any signs of incarceration. The small intestine was repositioned and the mesenterial gap was closed with an Endo Hernia Stapler and PPI therapy was started directly postoperatively. The patient recovered well and was discharged on the seventh postoperative day with PPIs. Stool and blood were tested for H. pylori by monoclonal enzyme immunoassay (EIA) and igG antibody respectively. The stool sample was negative, while the blood sample was positive. Eradication was performed in an outpatient setting with antibiotics in combination with a PPI for 10 days with continuation of the PPI afterwards.
Fig. 1.
CT abdomen, axial plane. Free fluid (arrows), no signs of free air.
Fig. 2.
Perforated pyloric ulcer (*), duodenum (1), gastric remnant (2).
3. Discussion
The above-mentioned complication is rare, described only in individual cases. With the increasing numbers of bariatric operations, we estimate that these long-term complications will increase as well. Due to the remaining changes in their metabolism and anatomy, these patients offer special challenges in the diagnosis and treatment. One of the challenges is faced by the radiological department in the diagnostic of postoperative complications. Reliable CT features of perforated ulcers in patients without RYGB consist of extraluminal gas (97%), fluid or fat strand along the gastroduodenum (89%), ascites (89%), wall defect and/or ulcer (84%), and wall thickening (72%) [10]. As there is no connection of the gastric remnant to the oesophagus, the most frequent sign of perforation cannot develop in patients with a RYGB. As for the internal hernia, which was also diagnosed in our patient by CT scan and confirmed intraoperatively, the diagnostic accuracy varies widely in the literature and up to 20% of patients with internal hernia have CT findings negative for internal hernia [11].
Our case showed furthermore that detection of H. pylori in the gastric remnant is challenging; there are several tests available. Due to the anatomy of the patients, the urea breath test could not be performed and no biopsy was taken intraoperatively. Invasive testing to gain a result by histology as well as the rapid urease test could have been performed by special endoscopy, but this procedure was not available at our hospital. A stool antigen test (SAT) is the other noninvasive method with good sensitivity and specificity, in our case an EIA monoclonal test was performed. There are two types of methodes, the enzyme immunoassay (EIA) and the immunochromatography assay (ICA), either using monoclonal or polyclonal antibodies. In general, EIA-based tests provide more reliable results than ICA-based tests and monoclonal antibody-based tests are more accurate than polyclonal antibody-based tests [12].
However, the accuracy of SAT is influenced by several factors such as antibiotics, PPI, N-acetylcysteine, bowel movement and upper gastrointestinal bleeding [12]. Our patient was receiving PPIs when the test was taken. Serology testing for the detection of initial infection has been reported with a wide variety of accuracy, revealing sensitivity ranged from 57.8%–100% and specificity ranged from 58.7%–96.8% in EIA-based tests; sensitivity ranged from 55.6%–97.8% and specificity ranged from 60.3%–96.8% in ICA-based tests [13]. However, seropositivity does not confirm current H. pylori infection because these antibodies persist for an extended period of time, often greater than half a year [14]. In our case, the antigen test in the stool was negative in contrast to the positive serology. This could be due to a false-negative respectively false-positive result on one of these tests. Furthermore, since the antibody test in the serum does not distinguish between an old or a recent infection, a positive result does not reflect an active infection in every case. The negative result in the stool sample could be due to the lack of passage of the gastric remnant, the diminished accuracy of the test by the PPI treatment started directly postoperatively, or the possibility that the specific antigen was not or not in a sufficient quantity found in the stool sample.
This leads to the question whether a routine gastroscopy and eradication of H. pylori should be performed preoperatively. In a multivariable analysis of a registry cohort, Smelt et al. identified H. pylori status to be the most important independent predictor of marginal ulceration in patients undergoing RYGB, but having little impact on the outcome of other bariatric operations [15]. In 2020, we identified two statement papers addressing this subject; the IFSO position statement recommends a routine preoperative consideration for oesophago-gastro-duodenoscopy in populations where the community incidence of significant gastric and oesophageal pathology is high, particularly when the procedure will lead to part of the stomach being inaccessible [16]. The 2020 clinical guidelines of the European Association for Endoscopic Surgery make a conditional recommendation for either routine eradication of H. pylori or alternative practice due to the indirectness of the evidence and imprecision of effect estimates [17].
4. Conclusion
This case report illustrates the difficulties faced by healthcare providers in diagnosing and managing a rare complication after bariatric surgery. The aim was to demonstrate the multidisciplinary challenges these patients present due to the anatomical and metabolical changes they have undergone. As illustrated in our case, negative radiological findings should not exclude diagnosis of stomach perforation. We therefore recommend a diagnostic laparoscopy with a systematic exploration by a bariatrically skilled surgeon as a first-line diagnostic in patients with acute abdominal pain after bariatric surgery. Furthermore, we would recommend the acquisition of a biopsy intraoperatively since the detection of a H. pylori infection can be challenging. Multimodal testing should be considered, there is no clear recommendation on preoperative routine eradication.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
On the basis of this being a case report and with a present handwritten signed consent of the patient, this case report is exempt from ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Cornelia Gnägi: data collection, report design, writing the paper and publishing.
Michael Durband: data collection, report design, review.
Thomas Kinsbergen: report design, review.
Registration of research studies
Not applicable.
Guarantor
Cornelia Gnägi, Michael Durband, Thomas Kinsbergen.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Contributor Information
Cornelia Regula Gnägi, Email: cornelia.gnaegi@szb-chb.ch.
Michael Durband, Email: m.durband@sro.ch.
Thomas Kinsbergen, Email: t.kinsbergen@sro.ch.
References
- 1.Phillips B.T., Shikora S.A. The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism. 2018;79:97–107. doi: 10.1016/j.metabol.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obesity and overweight (n.d.) https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed 10 December 2020).
- 4.C.D.C. Panel NIH conference: gastrointestinal surgery for severe obesity. Ann. Intern. Med. 1991;115(12):956–961. [PubMed] [Google Scholar]
- 5.Angrisani L., Santonicola A., Iovino P., Vitiello A., Zundel N., Buchwald H., Scopinaro N. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes. Surg. 2017;27(9):2279–2289. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reges O., Greenland P., Dicker D., Leibowitz M., Hoshen M., Gofer I., Rasmussen-Torvik L.J., Balicer R.D. Association of bariatric surgery using laparoscopic banding, roux-en-y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. J. Am. Med. Assoc. 2018;319(3):279–290. doi: 10.1001/jama.2017.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams T.D., Gress R.E., Smith S.C., Halverson R.C., Simper S.C., Rosamond W.D., LaMonte M.J., Stroup A.M., Hunt S.C. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007;357(8):753–761. doi: 10.1056/nejmoa066603. [DOI] [PubMed] [Google Scholar]
- 8.Kassir R., Debs T., Blanc P., Gugenheim J., Ben Amor I., Boutet C., Tiffet O. Complications of bariatric surgery: presentation and emergency management. Int. J. Surg. 2016;27:77–81. doi: 10.1016/j.ijsu.2016.01.067. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukhejree I., McCaul J.A., Chi-Yong Ngu J., Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., De Wilde R.L., Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K., Kasivisvanathan V., Mei Z. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) Guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Lee D., Park M.H., Shin B.S., Jeon G.S. Multidetector CT diagnosis of non-traumatic gastroduodenal perforation. J. Med. Imaging Radiat. Oncol. 2016;60(2):182–186. doi: 10.1111/1754-9485.12408. [DOI] [PubMed] [Google Scholar]
- 11.Higa K.D., Ho T., Boone K.B. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes. Surg. 2003;13(3):350–354. doi: 10.1381/096089203765887642. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.K., Kuo F.C., Liu C.J., Wu M.C., Shih H.Y., Wang S.S.W., Wu J.Y., Kuo C.H., Huang Y.K., Wu D.C. Diagnosis of helicobacter pylori infection: current options and developments. World J. Gastroenterol. 2015;21:11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burucoa C., Delchier J.C., Courillon-Mallet A., de Korwin J.D., Mégraud F., Zerbib F., Raymond J., Fauchère J.L. Comparative evaluation of 29 commercial helicobacter pylori serological kits. Helicobacter. 2013;18:169–179. doi: 10.1111/hel.12030. [DOI] [PubMed] [Google Scholar]
- 14.Testerman T.L., Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014;20:12781–12808. doi: 10.3748/wjg.v20.i36.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smelt H.J.M., Smulders J.F., Gilissen L.P.L., Said M., Ugale S., Pouwels S. Influence of Helicobacter pylori infection on gastrointestinal symptoms and complications in bariatric surgery patients: a review and meta-analysis. Surg. Obes. Relat. Dis. 2018;14(10):1645–1657. doi: 10.1016/j.soard.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Brown W.A., Johari Halim Shah Y., Balalis G., Bashir A., Ramos A., Kow L., Herrera M., Shikora S., Campos G.M., Himpens J., Higa K. IFSO position statement on the role of esophago-gastro-duodenal endoscopy prior to and after bariatric and metabolic surgery procedures. Obes. Surg. 2020;30:3135–3153. doi: 10.1007/s11695-020-04720-z. [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo N., Antoniou S.A., Batterham R.L., Busetto L., Godoroja D., Iossa A., Carrano F.M., Agresta F., Alarçon I., Azran C., Bouvy N., Balaguè Ponz C., Buza M., Copaescu C., De Luca M., Dicker D., Di Vincenzo A., Felsenreich D.M., Francis N.K., Fried M., Gonzalo Prats B., Goitein D., Halford J.C.G., Herlesova J., Kalogridaki M., Ket H., Morales-Conde S., Piatto G., Prager G., Pruijssers S., Pucci A., Rayman S., Romano E., Sanchez-Cordero S., Vilallonga R., Silecchia G. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020;34(6):2332–2358. doi: 10.1007/s00464-020-07555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]