Summary
Proximity and size of the nearest market (‘market gravity’) have been shown to have strong negative effects on coral reef fish communities that can be mitigated by the establishment of closed areas. However, moray eels are functionally unique predators that are generally not subject to targeted fishing and should therefore not directly be affected by these factors. We used baited remote underwater video systems to investigate associations between morays and anthropogenic, habitat, and ecological factors in the Caribbean region. Market gravity had a positive effect on morays, while the opposite pattern was observed in a predator group subject to exploitation (sharks). Environmental DNA analyses corroborated the positive effect of market gravity on morays. We hypothesize that the observed pattern could be the indirect result of the depletion of moray competitors and predators near humans.
Subject areas: environmental science, ecology, biological sciences, zoology, animals, ethology
Graphical Abstract

Highlights
-
•
Baited remote underwater videos and environmental DNA were used to assess morays
-
•
Market gravity had a strong positive effect on moray abundance
-
•
Morays and sharks were negatively associated
-
•
Lack of competitors and predators may explain increased morays on reefs near humans
Environmental science; ecology; biological sciences; zoology; animals; ethology
Introduction
The size and proximity of the nearest market has a strong negative effect on predatory coral reef fishes, with some relief provided by closing reef areas to fishing (Cinner et al., 2018; Graham et al., 2017; MacNeil et al., 2020; Valdivia et al., 2017). This pattern has been observed on coral reefs worldwide, affecting commercially exploited predator taxa ranging from groupers to sharks (Cinner et al., 2018; Graham et al., 2017; MacNeil et al., 2020; Valdivia et al., 2017). Moray eels (Family Muraenidae; hereafter referred to as ‘morays’) are not readily detected in conventional reef surveys, and it is unknown how they respond to human impacts on coral reef systems (Chan, 2017; Gilbert et al., 2005; Mehta and Wainwright, 2007; Mouillot et al., 2013). Morays comprise nearly 200 species of functionally unique reef predators, with their elongate bodies and raptorial pharyngeal jaws enabling them to ambush relatively large fish, crustaceans, and octopuses in habitats with complex structure (Chan, 2017; Gilbert et al., 2005; Mehta and Wainwright, 2007; Mouillot et al., 2013). Morays have little to no commercial value because they are generally unpalatable and can contain toxic levels of ciguatera (Chan, 2017). We hypothesize that size and proximity of markets and closing reefs to fishing would therefore have no effect on morays.
The aim of the present study was to explore relationships between morays and two human-related factors that typically affect predatory reef fish: whether a reef is open or closed to fishing (‘protection status’) and the size and proximity of the nearest market (‘market gravity’), as well as the interaction between these two factors. Market gravity is a measurement of human impact on a location that is a function of both accessibility in terms of travel times to markets and the size of these markets (i.e., human population size [Cinner et al., 2018]). We included reef structural complexity as a factor in the model, which generally has a positive effect on reef fish (MacNeil et al., 2020; Valdivia et al., 2017). We also explored relationships between sharks and these factors to enable comparison between morays and a commercially exploited predator taxon.
Results and discussion
Video sampling throughout the greater Caribbean was conducted as part of the Global FinPrint Project (https://globalfinprint.org/) and involved the deployment of 60-minute baited remote underwater video systems (BRUVS) with ∼1 kg of crushed oily fish in a bait cage within the field of view (see Transparent methods in Supplemental information). A total of 3,157 BRUVS were deployed between June 2015 and April 2019 across 67 reefs in 12 Caribbean nations (Table 1). BRUVS were set in randomized locations on each reef, which was defined in this study as a continuous patch of hard-bottom coral reef habitats of ∼10 km2 across a depth range of 2–40 m. Sightings of morays and sharks were converted to MaxN, which is the maximum number of individuals of each species observed in one frame per 60-minute BRUVS (Harvey et al., 2018). We fit a generalized linear model (GLM) with a Tweedie compound Poisson error structure, which explained 56% of the deviance in mean reef-level moray MaxN (all species combined) and included the factors: log-transformed market gravity (+), protection status (open [+] or closed [–] to fishing), reef complexity (+), and the interaction between log-transformed market gravity and protection status (+) (Table 2, Figure 1A). Reef complexity had the greatest effect (F = 57.91, p < 0.0001), followed by log-transformed market gravity (F = 33.89, p < 0.0001). Protection status (F = 3.22, p = 0.08) and the interaction between protection status and log-transformed market gravity (F = 0.13, p = 0.72) did not significantly affect reef-level moray MaxN. In a model exploring how the same factors affected shark MaxN log-transformed market gravity had the largest effect ([–]; F = 27.50, p < 0.0001), followed by reef complexity ([+]; F = 8.10, p = 0.01). Protection status (open [–] or closed [+] to fishing; F = 0.03, p = 0.87) and the interaction between protection status and log-transformed market gravity ([–]; F = 0.64, p = 0.43) had no significant effect on reef-level shark MaxN (Figures 1B, Table 3). There was no relationship between reef complexity and market gravity in our sampling (Figure 1C), but there was a negative correlation between morays and sharks at both the reef and national scale (Figures 1D and 2). A model where the factor log-transformed market gravity was replaced with reef-level shark MaxN explained 45% of the deviance in mean reef-level moray MaxN, where mean shark MaxN had the greatest effect ([-]; F = 30.81, p < 0.0001), followed by reef complexity ([+]; F = 20.57, p < 0.0001) (Table 4, Figure 1D). Protection status did not significantly affect moray MaxN here either (F = 2.24, p = 0.14). Overall, the GLM with the factor log-transformed market gravity (AIC = −36.63) predicted moray MaxN better than the GLM with the factor shark MaxN (AIC = −22.84). A spatial co-occurrence probability model for shark and moray species was used to determine positive, negative, or random interactions at the reef-level using the R library ‘co-occur’ (Griffith et al., 2016). Spatial co-occurrence between sharks and morays was dominated by negative probabilities (Veech, 2013; Figure 3).
Table 1.
BRUVS locations and corresponding anthropogenic and habitat factors
| Location | Reef | Protection status | Market gravity | Reef complexity | No. BRUVS | Moray MaxN | Shark MaxN |
|---|---|---|---|---|---|---|---|
| Antigua and Barbuda | Barbuda – North | Open | 8 | 1.53 | 42 | 0.05 | 0.43 |
| Barbuda – Palasar | Closed | 9 | 2.11 | 45 | 0.13 | 0.71 | |
| Bahamas | Abaco – Bight of Old Robinson Bay | Open | 19.02 | 1.54 | 61 | 0 | 0.1 |
| Abaco – Bight Reef | Open | 18.41 | 1.8 | 68 | 0.03 | 1.04 | |
| Abaco – Elbow Cay Reef | Open | 22.2 | 1.71 | 57 | 0.04 | 0.54 | |
| Abaco – Green Turtle Cay Reef | Open | 19.52 | 1.28 | 61 | 0.05 | 0.79 | |
| Abaco – Guana Cay Reef | Open | 18.03 | 1.41 | 58 | 0 | 1.1 | |
| Abaco – Water Cay Bay | Open | 19.67 | 1.67 | 60 | 0 | 0.1 | |
| Andros – North | Open | 24.57 | 1.13 | 57 | 0.11 | 0.84 | |
| Andros – South | Open | 19.95 | 0.84 | 56 | 0 | 1.09 | |
| Berry Islands – Chub Cay | Open | 20.91 | 0.74 | 39 | 0.05 | 1.03 | |
| Bimini – North | Open | 79 | 1.44 | 53 | 0 | 0.7 | |
| Bimini – South | Open | 79 | 1.43 | 52 | 0 | 0.6 | |
| Conception Island | Closed | 4.4 | 0.23 | 42 | 0 | 0.83 | |
| ∗Eleuthera – South | Open | 9 | N/A | 0 | N/A | N/A | |
| ∗Exumas – Middle | Closed | 8.67 | 0.68 | 67 | 0.01 | 1.3 | |
| Exumas – North | Open | 11.47 | 1.02 | 67 | 0 | 1.21 | |
| Exumas – South | Open | 8 | 0.49 | 42 | 0 | 1.12 | |
| New Providence – North | Open | 1135.84 | 0.47 | 32 | 0.25 | 0.5 | |
| New Providence – South | Open | 134.33 | 0.29 | 38 | 0.08 | 1.03 | |
| ∗San Salvador | Open | 4.81 | 0.41 | 78 | 0.04 | 0.65 | |
| Barbados | Northeast | Open | 124.75 | 0.16 | 29 | 0 | 0.24 |
| Northwest | Open | 173.81 | 1.36 | 31 | 0.52 | 0.06 | |
| Southeast | Open | 295.89 | 2.05 | 35 | 1 | 0.57 | |
| Southwest | Open | 18665.35 | 0.91 | 35 | 0.51 | 0 | |
| Belize | Belize City | Open | 106.64 | 1.46 | 39 | 0.26 | 0 |
| Glover's Reef East | Closed | 80.45 | 2.06 | 123 | 0.23 | 0.92 | |
| Glover's Reef West | Open | 85.1 | 1.52 | 41 | 0.34 | 0.12 | |
| Lighthouse Reef – Halfmoon Caye | Closed | 52.19 | 1.34 | 43 | 0.02 | 1.09 | |
| Lighthouse Reef – Sandbore Caye | Open | 45.65 | 1.77 | 31 | 0.13 | 0.61 | |
| South Water Caye | Open | 119.56 | 1.36 | 106 | 0.09 | 0.23 | |
| British West Indies | Montserrat – West | Open | 20.04 | 1.5 | 48 | 0.15 | 0.17 |
| Colombia | Coastal – Isla Mangle | Open | 133.2 | 1.62 | 50 | 0.16 | 0 |
| Coastal – Isla Tintipan | Open | 126.6 | 1.74 | 50 | 0.14 | 0 | |
| Coastal – Isla Grande | Closed | 287.7 | 2.65 | 49 | 0.51 | 0.04 | |
| Coastal – Isla Tesoro | Closed | 267 | 2.88 | 50 | 0.58 | 0.16 | |
| ∗Seaflower – Albuquerque | Open | 12 | 1.14 | 50 | 0.02 | 1.28 | |
| Seaflower – Serranilla | Open | 1 | 1.14 | 63 | 0.03 | 1.37 | |
| Seaflower – Providencia | Open | 264.78 | 1.06 | 43 | 0.14 | 0.79 | |
| Cuba | Guanahacabibes – Maria la Gorda | Closed | 70.56 | 3.13 | 40 | 0.15 | 0.2 |
| Guanahacabibes – Reef | Closed | 66.9 | 3 | 40 | 0.08 | 1.8 | |
| Punta Frances North | Closed | 39 | 2.79 | 40 | 0.3 | 0 | |
| Punta Frances South | Closed | 100 | 3.38 | 40 | 0.25 | 0 | |
| Jardines de la Reina | Closed | 33.47 | 2.7 | 62 | 0.34 | 0.71 | |
| Dominican Republic | Bayahibe | Open | 249.23 | 0.49 | 42 | 0.07 | 0 |
| Boca Chica | Open | 2661.95 | 0.56 | 44 | 0.11 | 0 | |
| Buen Hombre | Open | 312.93 | 1.89 | 40 | 0.13 | 0 | |
| Santo Domingo – La Caleta | Closed | 2962 | 2.34 | 40 | 0.38 | 0 | |
| French West Indies | Guadeloupe – Grande cul de Sac | Open | 159.07 | 3.07 | 41 | 0.39 | 0.02 |
| Guadeloupe – Petit Terre | Closed | 95.18 | 2.21 | 48 | 0.42 | 0.08 | |
| Martinique – Reef 1 | Open | 226.9 | 2.96 | 42 | 0.76 | 0 | |
| Martinique – Reef 2 | Open | 137.64 | 3.15 | 47 | 1.02 | 0 | |
| Jamaica | Coastal – Dragon Point | Open | 330.54 | 1.54 | 25 | 0.44 | 0 |
| ∗Coastal – East Portland | Open | 329.92 | 1.68 | 37 | 0.24 | 0 | |
| Coastal – Negril | Closed | 513.64 | 1.44 | 24 | 0.38 | 0 | |
| Coastal – Ocho Rios | Open | 377.82 | 1.24 | 34 | 0.15 | 0 | |
| Pedro Bank | Open | 15.58 | 3.12 | 54 | 0.28 | 0.67 | |
| Turks and Caicos | South Caicos – Back | Open | 67 | 0.19 | 25 | 0 | 0.4 |
| South Caicos – Reserve | Closed | 61.55 | 0.52 | 20 | 0 | 0.8 | |
| U.S.A. | Florida – Miami | Open | 13,785.08 | 0.4 | 38 | 0.03 | 0.18 |
| Florida Keys – Lower Keys | Open | 92.55 | 0.76 | 43 | 0.16 | 0.33 | |
| Florida Keys – Tip | Open | 125.67 | 1.5 | 40 | 0.23 | 0.45 | |
| ∗Florida Keys – Middle Keys 1 | Open | 171.88 | 0.79 | 43 | 0.23 | 0.49 | |
| Florida Keys – Middle Keys 2 | Open | 135.58 | 0.85 | 42 | 0.12 | 0.29 | |
| Florida Keys – Middle Keys 3 | Open | 107.14 | 0.73 | 41 | 0.15 | 0.39 | |
| Florida Keys – Upper Keys 1 | Open | 250.88 | 0.59 | 42 | 0.07 | 0.38 | |
| Florida Keys – Upper Keys 2 | Open | 226.29 | 0.96 | 44 | 0.18 | 0.66 | |
| Florida Keys – Upper Keys 3 | Open | 199.79 | 1.38 | 48 | 0.17 | 0.58 |
Reefs with an asterisk denote where eDNA samples were also collected. Protection status refers to open or closed to fishing. Market gravity score is derived from Cinner et al. (2018) ‘Global Gravity of Coral Reefs Spatial Layer.’ Reef complexity (0–5) is scored for each BRUVS (via BenthoBox online annotation tool [https://benthobox.com/]) and averaged per reef. Moray MaxN and Shark MaxN are the mean per reef.
Table 2.
Tweedie GLM to predict moray abundance on BRUVS including market gravity
| Factors | Degrees of freedom (DF) | Deviance | Residual DF | Residual deviance | F-value | p value | Deviance explained |
|---|---|---|---|---|---|---|---|
| Null | 66 | 25.25 | |||||
| log10(Gravity) | 1 | 5.01 | 65 | 20.24 | 33.89 | <0.0001 | 0.20 |
| Protection status | 1 | 0.48 | 64 | 19.77 | 3.22 | 0.08 | 0.02 |
| Reef complexity | 1 | 8.56 | 63 | 11.20 | 57.91 | <0.0001 | 0.34 |
| log10(Gravity) ∗ Protection status | 1 | 0.02 | 62 | 11.18 | 0.13 | 0.72 | 0.001 |
| Sum of deviance explained | 0.56 | ||||||
Analysis of deviance table for the Tweedie GLM to determine the effects of key anthropogenic (log-transformed market gravity score, protection status [open or closed to fishing]) and habitat (mean reef complexity score [0–5]) factors on the reef-level occurrence (mean MaxN) of morays on reefs throughout the greater Caribbean (n = 67 reefs). An asterisk denotes the interaction between factors.
Figure 1.
Relationships between market gravity, morays, sharks, and reef complexity in the Caribbean
(A) Relationship between market gravity and moray occurrence (n = 67 reefs in 12 nations).
(B) Relationship between market gravity and shark occurrence on the same reefs.
(C) Relationship between market gravity and mean reef complexity.
(D) Relationship between moray and shark MaxN. Red lines depict the linear trendlines.
Correlation coefficients and p values calculated via Pearson (A, B, C) and Spearman's rank (D; non-linear relationship).
Table 3.
Tweedie GLM to predict shark abundance on BRUVS
| Factors | Degrees of freedom (DF) | Deviance | Residual DF | Residual deviance | F-value | p value | Deviance explained |
|---|---|---|---|---|---|---|---|
| Null | 66 | 47.61 | |||||
| log10(Gravity) | 1 | 12.47 | 65 | 35.14 | 4.49 | <0.0001 | 0.26 |
| Protection status | 1 | 0.01 | 64 | 35.12 | 2.88 | 0.87 | 0.0003 |
| Reef complexity | 1 | 3.68 | 63 | 31.45 | 28.26 | 0.01 | 0.08 |
| Protection status ∗ log10(Gravity) | 1 | 0.29 | 62 | 31.16 | 0.64 | 0.43 | 0.01 |
| Sum of deviance explained | 0.35 | ||||||
Analysis of deviance table of Tweedie GLM to determine the effects of key anthropogenic (protection status [open or closed to fishing], log-transformed market gravity score) and habitat (mean reef complexity score [0–5]) factors on the reef-level occurrence (mean MaxN) of sharks on reefs throughout the greater Caribbean (n = 67 reefs). An asterisk denotes the interaction between factors.
Figure 2.
Relationship between mean country-level occurrence of sharks and morays on BRUVS
Jurisdictions where all shark fishing and trade is prohibited at the time of sampling are labeled as ‘Shark sanctuaries’. Red circles denote non-shark sanctuaries, blue triangles denote shark sanctuaries, and vertical and horizontal error bars denote standard deviation.
Table 4.
Tweedie GLM to predict moray abundance on BRUVS including shark abundance
| Factors | Degrees of freedom (DF) | Deviance | Residual DF | Residual deviance | F-value | p-value | Deviance explained |
|---|---|---|---|---|---|---|---|
| Null | 66 | 28.00 | |||||
| Shark MaxN | 1 | 7.24 | 65 | 20.76 | 30.81 | <0.0001 | 0.26 |
| Protection status | 1 | 0.53 | 64 | 20.23 | 2.24 | 0.14 | 0.02 |
| Reef complexity | 1 | 4.83 | 63 | 15.40 | 20.57 | <0.0001 | 0.17 |
| Sum of deviance explained | 0.45 | ||||||
Analysis of deviance table for the Tweedie GLM to determine the effects of key anthropogenic (protection status [open or closed to fishing]), habitat (mean reef complexity score [0–5]), and ecological (mean shark MaxN) factors on the reef-level occurrence (mean MaxN) of morays on reefs throughout the greater Caribbean (n = 67 reefs).
Figure 3.
Co-occurrence probability matrix between morays and different shark species on BRUVS
Each cell represents the spatial co-occurrence probability of a pair of species. Significant probabilities are denoted by color (red: negative, blue: positive). Non-significant co-occurrence is denoted by the color gray.
Recording animals on BRUVS is partially dependent on a positive response to bait, which can be inhibited in predator-rich habitats by predator avoidance behavior (Sherman et al., 2020) and interspecific competition (Sabando et al., 2020). It is therefore important to compare detections from multiple survey methods, including techniques that don't require a behavioral response from the subject (Sherman et al., 2020). Six reefs subject to a range of market gravity (4.81–329.92) were surveyed for moray extra-organismal environmental DNA (eDNA) by vacuum filtering 14–21 replicate 2L water samples collected at random locations per reef across hydrophilic polyethersulfone filters and using universal 12S teleost fish primers (Miya et al., 2015) to generate sequence reads. We reasoned that eDNA surveys would be less sensitive than BRUVS to any difference in moray behavior between reefs because eDNA detection does not depend on individual morays approaching a bait source. A total of 19,168 moray sequence reads, belonging to at least six species, were obtained (Table S1). Each water sample was scored from 0 to 3 representing the minimum number of individual morays detected. We conservatively assumed that all detections of the same species on one filter were from one individual, thus our metric is equivalent to moray species richness per sample. A GLM with a negative binomial error distribution explored the relationship between this metric and ‘high gravity’ (log-transformed market gravity >2; East Portland, Jamaica and Middle Florida Keys, U.S.A.) and ‘low gravity’ categories (log-transformed ∼ 1; Middle Exumas, San Salvador, Cape Eleuthera [Bahamas] and Albuquerque Island in the Seaflower Biosphere Reserve [Colombia]). Market gravity explained 16.9% of the deviance in the minimum number of moray individuals detected per sample (p = 0.01; Table 5). Significantly more individuals were detected in samples collected on reefs subject to high market gravity (at least six moray species were detected overall, with 1–3 individuals in 17.6% of water samples) than reefs subject to lower market gravity (at least two moray species were detected overall, with 1–2 individuals in 3.5% of samples; Table S1).
Table 5.
Negative binomial GLM to predict moray eDNA detection
| Factors | Degrees of freedom (DF) | Deviance | Residual DF | Residual deviance | p-value | Deviance explained |
|---|---|---|---|---|---|---|
| Null | 116 | 38.72 | ||||
| log10(Gravity) | 1 | 6.54 | 115 | 32.19 | 0.01 | 0.17 |
Analysis of deviance table for the negative binomial GLM to determine the effect of log-transformed market gravity (low [~1] or high [> 2]) on the minimum number of moray individuals detected in water samples (n = 118) collected across 6 reefs in the greater Caribbean (U.S.A., Jamaica, Bahamas, Colombia [Seaflower Biosphere Reserve]).
This study is the largest effort to date to assess moray occurrence on coral reefs. The positive effect of increasing reef complexity was expected and likely reflects moray preference for reef structures that provide both refuge and prey ambush opportunities (Gilbert et al., 2005). While a preference for complex structure is typical of Caribbean coral reef predators (Valdivia et al., 2017), the positive effect of market gravity on moray sightings is unusual. This factor or its components (local human population density, proximity to humans) nearly always have a strong negative effect on reef predators (Cinner et al., 2018; Graham et al., 2017; MacNeil et al., 2020; Valdivia et al., 2017). We hypothesize that the positive effect of market gravity on morays could be an indirect result of a local reduction in moray predators, competitors, or both on reefs subject to higher human pressure. The negative correlation between shark and moray MaxN we observed at the reef and national level is consistent with this ‘release’ hypothesis, with uniformly low moray MaxN observed in jurisdictions where sharks are protected at the national level and common on all sampled reefs (‘Shark Sanctuaries’, n = 21 reefs in The Bahamas and Sea Flower Biosphere Reserve, Colombia; Figure 2). Local shark MaxN had a significant negative effect in the moray GLM, which was further supported by the inverse relationship between sharks and morays as demonstrated by their negative co-occurrence probabilities at the reef-level (Figure 3). Morays were less likely to occur on reefs where several shark species (Caribbean reef, nurse, blacknose, tiger, blacktip, unknown) were present. While more research on the ecological relationships between morays, sharks, and other predators is needed to further test this hypothesis, it is mechanistically viable because a wide variety of shark species include morays in their diet (Delorenzo et al., 2015; Estupiñán-Montaño et al., 2017; Lowe et al., 1996; McElroy et al., 2006; Sears and Sikkel, 2016; Torres-Rojas et al., 2010; Wetherbee et al, 1996, 1997) (e.g., Families: Carcharhinidae, Sphyrnidae, Ginglymostomatidae) and probably also compete with them for prey. Anecdotal observations at St. Paul's Rocks (Brazil) linked the decline of sharks around the island with a subsequent increase in sightings of the whitespot moray (Muraena pavonina) (Luiz and Edwards, 2011). Nonetheless, the better fit of the market gravity model than the substituted shark MaxN model suggests that there may be other factors that are contributing to the positive effect of market gravity on morays. It is possible that removals of moray predators and competitors other than sharks (e.g., large teleosts) may also be important. Other viable hypotheses outside of release that could potentially explain this pattern include that prey availability is greater near humans or that human activities benefit morays (e.g., if fishing provides opportunities for morays to scavenge discarded catch).
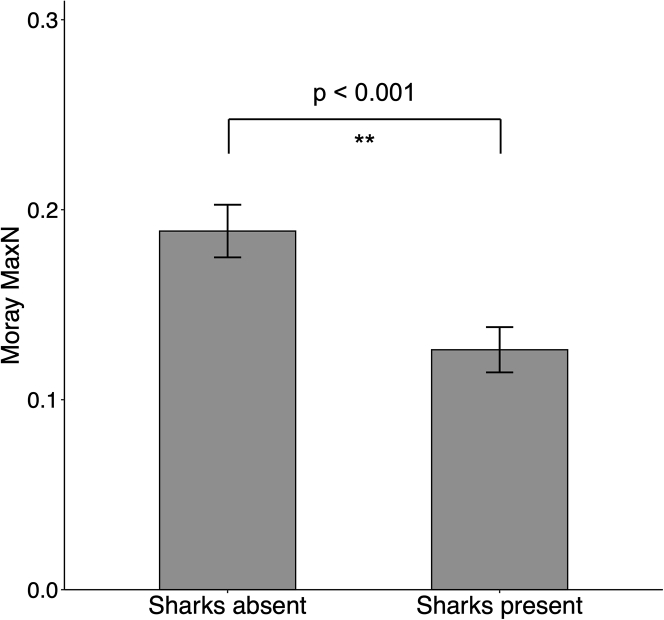
It is possible that morays are less inclined to approach BRUVS on reefs where other predator taxa are common due to elevated predation risk or to avoid aggressive interactions with competitors (Sherman et al., 2020). Indeed, we found that mean moray MaxN was significantly lower on BRUVS sets where at least one shark was also recorded than on sets where sharks were absent (negative binomial GLM, p < 0.001; Figure 4 and Table S2). However, shark presence on BRUVS sets did not always deter moray detection: in 98 analyzed BRUVS when sharks and morays co-occurred, they did not overlap in frame on 76 occasions (76.6%). Moreover, on 23 BRUVS where morays and sharks occurred in frame together, the moray left the field of view and did not return after a shark arrived in only five instances (22%; Video S1). Morays generally ignored sharks when they were able to remain partially hidden in reef structure, when the shark(s) passed in the background, or when the individual shark was small, perhaps because the shark was too small to be a direct threat (Video S2). Detection of moray eDNA also exhibited the same pattern observed in the BRUVS; morays were detected less often in water samples from reefs subject to low market gravity. This indicates that instead of just hiding more often (in which case their eDNA would still be detected), and consequently not being visually detected on BRUVS, morays were also less common at these sites. The combined eDNA and BRUVS results argue for reduced moray populations and changes in their behavior on reefs with lower market gravity and more predators.
Figure 4.
Set-level abundance of morays in the presence or absence of sharks
Mean moray MaxN ±SE on BRUVS sets (n = 2,052 BRUVS across 51 reefs in 11 nations) where sharks were absent or at least one shark was present, on reefs where at least one shark was detected. p value calculated via negative binomial GLM (Table S2).
Morays are functionally unique in that they are able to move undetected within complex reef structure and ambush relatively large prey (Gilbert et al., 2005; Mehta and Wainwright, 2007; Mouillot et al., 2013). Changes in moray abundance and the risk of moray ambush from reef structure could affect prey populations and corresponding risk-sensitive behavior. It may also reduce cooperative hunting with other fishes (e.g., grouper [Epinephelinae]) that capture prey flushed out of structure by morays (Bshary et al., 2006). Yet the ecological roles and importance of morays are not as extensively studied as most other reef predator taxa making it difficult to predict these effects (Gilbert et al., 2005; Mehta and Wainwright, 2007; Mouillot et al., 2013). We show that eDNA and BRUVS are potentially informative survey approaches for these cryptic predators, which are frequently undercounted using underwater visual censuses (Gilbert et al., 2005). Our study indicates that morays are more common on Caribbean coral reefs subject to high market gravity, making them unusual among the major predator taxa (Cinner et al., 2018; Graham et al., 2017; MacNeil et al., 2020; Valdivia et al., 2017). A better understanding of moray ecology is needed to assess the causes and consequences of these profound changes in Caribbean coral reef predator assemblages, while also acknowledging that emerging export markets for morays (Mañez and Paragay, 2013) and human impacts that reduce reef complexity and prey biomass may ultimately deplete morays close to people.
Limitations of the study
This study used a large sample of BRUVS set randomly on reefs distributed throughout the greater Caribbean and provided corroboration of the BRUVS results with more limited eDNA sampling. Our eDNA sampling sites (n = 6) were opportunistically selected and half of the sites were within The Bahamas. Expanding the number and geographic coverage of reefs sampled for moray eDNA could overcome this limitation in the future. Although BRUVS were deployed during daylight hours in this study, it is suggested that some species of reef-associated morays and sharks actively forage during twilight and night hours (Bardach et al., 1959; Chapman et al., 2007; Gruber et al., 1988). Further studies could therefore incorporate nighttime surveys to explore the relationship between these species under low-light conditions. This study focused on testing for associations between morays and three factors that generally have strong effects on reef fish: market gravity, protection status, and reef complexity. While these factors explain much of the deviance in moray sightings on BRUVS there are many other important factors that could be investigated. For example, our model does not include any metric for prey availability. Morays can have broad diets that include a diversity of reef fish, crustaceans, and octopus, some of which are not well sampled using BRUVS and may require targeted sampling efforts using other methods. There remain several potential mechanisms that could be driving the positive association between moray occurrence and market gravity. We discussed a ‘release’ hypothesis in relation to the loss of sharks, but this could also apply to the loss of other potential predators and competitors. Our study only compared morays and sharks due to limitations on the time to annotate BRUVS for other species, and it would be insightful to also model the co-occurrence of morays, sharks, and other large predators on BRUVS in order to resolve the strongest interactions. It is also a viable hypothesis that human activities increase the prey available to morays. Although increased market gravity typically reduces overall reef fish biomass (Cinner et al., 2018), this does not necessarily translate to fewer prey for morays. It is possible that low biomass reefs are composed of more suitable prey species for morays than high-biomass, predator-rich reefs. It is also possible that human activities directly enhance moray presence (e.g., if morays frequently scavenge fishery discards or if SCUBA tour operators feed morays). Overall, future studies should model the relationships of a wider variety of potential moray MaxN covariates to better resolve the key drivers behind the association detected in our analyses. Additional information on moray ecology and their location-specific interactions with humans are necessary to select and quantify the most appropriate potential covariates.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Demian Chapman (dchapman@fiu.edu).
Materials availability
No materials were newly generated for this paper.
Data and code availability
The BRUVS sampling locations and corresponding ecological, anthropogenic, and habitat factors are listed in Table 1. The number of moray eel sequence reads obtained from water samples are listed in Table S1.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This work is a contribution of the Global FinPrint Project funded by the Paul G. Allen Family Foundation (grant number 11861) and the Shark Conservation Fund. Earthwatch Institute, Moore Bahamas Foundation, Reuter Foundation, Waitt Foundation, and the Moore Family Foundation also provided support. We are grateful to the many staff, students, and volunteers who contributed to BRUVS and eDNA collection and video annotation and Mathew Wyatt for the use of BenthoBox. We thank Joseph Craine, Jessica Devitt, and Vasco Elbrecht from Jonah Ventures. This is contribution #201 from the Coastlines and Oceans Division of the Institute of Environment at Florida International University.
Author contributions
D.D.C. and K.I.F. conceived the BRUVS moray study. D.D.C., M.R.H., E.S.H., M.H., C.A.S., M.A.M, and M.G.M. led Global FinPrint. D.D.C., B.D.P., and J.B. conceived of the eDNA study. All authors contributed to the design and execution of Global FinPrint and/or eDNA sampling. J.B. and B.D.P. conducted eDNA laboratory work and eDNA bioinformatics. BRUVS modeling was conducted by G.M.C. with guidance from E.A.B. eDNA modeling was conducted by K.I.F. with guidance from E.A.B. The manuscript was initially written by G.M.C., J.B., and D.D.C. All authors read and commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102097.
Supplemental information
References
- Bardach J.E., Winn H.E., Menzel D.W. The role of the senses in the feeding of the nocturnal reef predators Gymnothorax moringa and G. Vicinus. Am. Soc. Ichthyol. Herpetol. 1959;1959:133–139. [Google Scholar]
- Bshary R., Hohner A., Ait-el-Djoudi K., Fricke H. Interspecific communicative and coordinated hunting between groupers and giant moray eels in the red sea. PLoS Biol. 2006;4:2393–2398. doi: 10.1371/journal.pbio.0040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T.Y.K. Regional variations in the risk and severity of ciguatera caused by eating moray eels. Toxins (Basel) 2017;9:1–11. doi: 10.3390/toxins9070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D.D., Pikitch E.K., Babcock E.A., Shivji M.S. Deep-diving and diel changes in vertical habitat use by Caribbean reef sharks Carcharhinus perezi. Mar. Ecol. Prog. Ser. 2007;344:271–275. [Google Scholar]
- Cinner J.E., Maire E., Huchery C., Aaron MacNeil M., Graham N.A.J., Mora C., McClanahan T.R., Barnes M.L., Kittinger J.N., Hicks C.C. Gravity of human impacts mediates coral reef conservation gains. Proc. Natl. Acad. Sci. U S A. 2018;115:E6116–E6125. doi: 10.1073/pnas.1708001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo D.M., Bethea D.M., Carlson J.K. An assessment of the diet and trophic level of Atlantic sharpnose shark Rhizoprionodon terraenovae. J. Fish Biol. 2015;86:385–391. doi: 10.1111/jfb.12558. [DOI] [PubMed] [Google Scholar]
- Estupiñán-Montaño C., Estupiñán-Ortiz J.F., Cedeño-Figueroa L.G., Galván-Magaña F., Polo-Silva C.J. Diet of the bull shark, Carcharhinus leucas, and the tiger shark, Galeocerdo Cuvier, in the eastern Pacific ocean. Turk. J. Zool. 2017;41:1111–1117. [Google Scholar]
- Gilbert M., Rasmussen J.B., Kramer D.L. Estimating the density and biomass of moray eels (Muraenidae) using a modified visual census method for hole-dwelling reef fauna. Environ. Biol. Fishes. 2005;73:415–426. [Google Scholar]
- Graham N.A.J., McClanahan T.R., MacNeil M.A., Wilson S.K., Cinner J.E., Huchery C., Holmes T.H. Human disruption of coral reef trophic structure. Curr. Biol. 2017;27:231–236. doi: 10.1016/j.cub.2016.10.062. [DOI] [PubMed] [Google Scholar]
- Griffith D.M., Veech J.A., Marsh C.J. cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016;69:1–17. doi: 10.18637/jss.v069.c02. [DOI] [Google Scholar]
- Gruber S.H., Nelson D.R., Morrissey J.F. Patterns of activity and space utilization of lemon sharks, Negaprion brevirostris, in a shallow Bahamian lagoon. Bull. Mar. Sci. 1988;43:61–76. [Google Scholar]
- Harvey E.S., Santana-Garcon J., Goetze J., Saunders B.J., Cappo M. The use of stationary underwater video for sampling sharks. In: Carrier J., Simpfendorfer C., Heithaus M., editors. Shark Research: Emerging Technologies and Applications for the Field and Laboratory. CRC Press; 2018. pp. 111–132. [Google Scholar]
- Lowe C.G., Wetherbee B.M., Crow G.L., Tester A.L. Ontogenetic dietary shifts and feeding behavior of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ. Biol. Fishes. 1996;47:203–211. [Google Scholar]
- Luiz O.J., Edwards A.J. Extinction of a shark population in the Archipelago of Saint Paul’s Rocks (equatorial Atlantic) inferred from the historical record. Biol. Conserv. 2011;144:2873–2881. [Google Scholar]
- MacNeil M.A., Chapman D.D., Heupel M., Simpfendorfer C.A., Heithaus M., Meekan M., Harvey E., Goetze J., Kiszka J., Bond M.E. Global status and conservation potential of reef sharks. Nature. 2020;583:801–806. doi: 10.1038/s41586-020-2519-y. [DOI] [PubMed] [Google Scholar]
- Mañez K.S., Paragay S.H. First evidence of targeted moray eel fishing in the spermonde archipelago, south sulawesi, Indonesia. TRAFFIC Bull. 2013;25:4–7. [Google Scholar]
- McElroy W.D., Wetherbee B.M., Mostello C.S., Lowe C.G., Crow G.L., Wass R.C. Food habits and ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in Hawaii. Environ. Biol. Fishes. 2006;76:81–92. [Google Scholar]
- Mehta R.S., Wainwright P.C. Raptorial jaws in the throat help moray eels swallow large prey. Nature. 2007;449:79–82. doi: 10.1038/nature06062. [DOI] [PubMed] [Google Scholar]
- Miya M., Sato Y., Fukunaga T., Sado T., Poulsen J.Y., Sato K., Minamoto T., Yamamoto S., Yamanaka H., Araki H. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015;2:150088. doi: 10.1098/rsos.150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillot D., Bellwood D.R., Baraloto C., Chave J., Galzin R., Harmelin-Vivien M., Kulbicki M., Lavergne S., Lavorel S., Mouquet N. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 2013;11:e1001569. doi: 10.1371/journal.pbio.1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabando M.A., Rieucau G., Bradley D., Caselle J.E., Papastamatiou Y.P. Habitat-specific inter and intraspecific behavioral interactions among reef sharks. Oecologia. 2020;193:371–376. doi: 10.1007/s00442-020-04676-y. [DOI] [PubMed] [Google Scholar]
- Sears W.T., Sikkel P.C. Field observation of predation on an adult Caribbean purplemouth moray eel by a nurse shark. Coral Reefs. 2016;35:971. [Google Scholar]
- Sherman C., Heupel M., Moore S., Chin A., Simpfendorfer C. When sharks are away, rays will play: effects of top predator removal in coral reef ecosystems. Mar. Ecol. Prog. Ser. 2020;641:145–157. [Google Scholar]
- Torres-Rojas Y.E., Hernández-Herrera A., Galván-Magaña F., Alatorre-Ramírez V.G. Stomach content analysis of juvenile, scalloped hammerhead shark Sphyrna lewini captured off the coast of Mazatlán, Mexico. Aquat. Ecol. 2010;44:301–308. [Google Scholar]
- Valdivia A., Cox C.E., Bruno J.F. Predatory fish depletion and recovery potential on Caribbean reefs. Sci. Adv. 2017;3:1–12. doi: 10.1126/sciadv.1601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013;22:252–260. [Google Scholar]
- Wetherbee B., GL C., CG L. Distribution, reproduction and diet of the gray shark Carcharhinus amblyrhinchus in Hawaii. Mar. Ecol. Prog. Ser. 1997;151:181–189. [Google Scholar]
- Wetherbee B.M., Crow G.L., Lowe C.G. Biology of the galapagos shark, Carcharhinus galapagensis, in hawai’i. Environ. Biol. Fishes. 1996;45:299–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The BRUVS sampling locations and corresponding ecological, anthropogenic, and habitat factors are listed in Table 1. The number of moray eel sequence reads obtained from water samples are listed in Table S1.