Abstract
Objectives
Identifying genetic pathogenic variants improves clinical outcomes for children with developmental and epileptic encephalopathy (DEE) by directing therapy and enabling accurate reproductive and prognostic information for families. We aimed to explore the additional personal utility of receiving a genetic diagnosis for families.
Methods
Semi‐structured interviews were conducted with fifteen families of children with a DEE who had received a genetic diagnosis. The interviews stimulated discussion focusing on the impact of receiving a genetic diagnosis for the family. Interview transcripts were analyzed using the six‐step systematic process of interpretative phenomenological analysis (IPA).
Results
Three key themes were identified: “Importance of the label,” “Relief to end the diagnostic journey,” and “Factors that influence personal utility.” Families reported that receiving a genetic label improved their knowledge about the likely trajectory of the DEE, increased their hope for the future, and helped them communicate with others. The relief of finally having an answer for the cause of their child's DEE alleviated parental guilt and self‐blame as well as helped families to process their grief and move forward. Delay in receipt of a genetic diagnosis diluted its psychological impact.
Significance
To date, the factors associated with the personal utility of a genetic diagnosis for DEEs have been under appreciated. This study demonstrates that identifying a genetic diagnosis for a child's DEE can be a psychological turning point for families. A genetic result has the potential to set these families on an adaptive path toward better quality of life through increased understanding, social connection, and support. Early access to genetic testing is important as it not only increases clinical utility, but also increases personal utility with early mitigation of family stress, trauma, and negative experiences.
Keywords: caregiver, family impact, genetics, psychological well‐being, qualitative
Key Point.
The genetic diagnosis of children with DEEs has important personal value for families, beyond that of their clinical management.
The genetic label increased families’ knowledge and understanding, promoted hope for the future, and facilitated social connection.
Families experienced relief to have diagnostic answers and a reduction in guilt and self‐blame.
Improved access to prompt genetic testing may help families gain maximal personal utility with improved psychological outcomes.
1. INTRODUCTION
The developmental and epileptic encephalopathies (DEEs) are a group of severe epilepsies characterized by seizures and interictal epileptic activity which negatively impacts on neurological development. These catastrophic disorders have a mortality rate of ~ 25% by 20 years, 1 and survivors have varying degrees of intellectual, psychiatric, behavioral, and motor disabilities. 2 DEEs were thought to be acquired disorders until 2001 when de novo variants in SCN1A were found in individuals with Dravet syndrome. 3 There are now over 100 genes reported to be associated with DEE and although the diagnostic yield of clinical genetic testing in DEE varies with the cohort studied, yields of up to ~50% are reported. 4 , 5 , 6 The majority of individuals with DEE have de novo pathogenic variants; however, recessive and X‐linked variants have also been found.
A DEE genetic diagnosis is becoming increasingly important in clinical practice. In addition to potentially guiding management and treatment decisions, it also informs accurate prognostic and reproductive counseling. 7 While the clinical utility of genetic testing is well established, 8 there has been less research on the personal utility. Personal utility refers to the personal psychological and social value of a result to the patient and family. 9 A systematic review identified 15 distinct aspects of personal utility, clustered around personal (affective, cognitive, and behavioral) and social outcomes. 9 Individuals with genetic disorders, such as ovarian cancer, disorders with genetic contributions, such as Alzheimer's disease, and healthy individuals who undertake direct‐to‐consumer genetic testing, report an increased sense of control and ability to prepare for the future due to the knowledge and understanding they gained from their genetic result. 10 , 11 , 12 This suggests personal utility is a multifaceted construct regardless of setting or type of disease. A study of three families with inherited relatively mild epilepsy syndromes reported that receiving a genetic diagnosis empowers families, reduces feelings of isolation, and improves quality of life. 13 As DEEs are severe epilepsies with typically no family history due to de novo variants, it is possible that the personal utility of receiving a genetic diagnosis is far greater for these families. Here, we explore the personal utility of a DEE genetic diagnosis for 15 families using the qualitative approach of interpretative phenomenological analysis (IPA).
2. METHODS
2.1. Participants
All parents of children with a DEE who had received a genetic diagnosis were participating in the “Genetic Basis of Epilepsy” study at the University of Otago Wellington, New Zealand, and fulfilled the inclusion criteria were invited to participate. Inclusion criteria were as follows: contact with the research team within the last 10 years (2010‐2020); fluent spoken English; available within the greater Wellington region for face‐to‐face recruitment. A total of 19 families met the inclusion criteria; three could not be contacted, and one withdrew prior to the interview due to scheduling difficulties, leaving 15 participating families. For 13 families, the mother only was interviewed; and for two families, both parents participated in the interview. No siblings were interviewed. The education level of the interviewees ranged from high school graduate to postgraduate tertiary qualifications. The ethnicities of the interviewees included the following: European, Māori, and Asian.
2.2. Procedure
In‐depth semi‐structured interviews were completed at a time and location convenient to participants. Interviews were carried out face to face, or if this was not possible via video conference. Interviews were digitally recorded and transcribed verbatim by the first author, who was not involved in the clinical care of the children. Data collection ran between November 2018 and March 2019. Ethical approval was granted by the New Zealand Health and Disability Ethics Committee.
2.3. Measures
An interview schedule, consisting of three groups of open‐ended questions, was used as a guide for the semi‐structured format. Participants were asked about their general experience of their child's DEE; their experience of receiving a genetic diagnosis; and their experiences surrounding having a genetic diagnosis. The questions were designed to stimulate discussion about what was important to participants, allowing the interviewer to probe or ask follow‐up questions for clarification. The interviews ranged in duration from 32 to 75 minutes, with an average of 56 minutes.
2.4. Data analysis
The study used IPA which is a well‐established qualitative method of analysis within clinical, health, and counseling psychology. 14 It is an iterative, contextual approach that focuses on persons‐in‐context. 15 Using this approach enabled a detailed, in‐depth examination into the lived experiences of parents who have a child or children with a genetic DEE. 14
Patterns of meaning or themes were identified through the processes of familiarization, reflection, integration, interpretation, and thematizing, with the aim of capturing the shared understandings of the experience of receiving a genetic diagnosis for the DEE, while also giving light to each participant's individual variation of the experience. Analysis followed the six rigorous and systematic steps: (a) repeated reading; (b) initial noting; (c) development of emergent themes; (d) identification of connections across emergent themes; (e) identifying recurrent themes across transcripts; and (f) identification of connections/patterns across recurrent themes. 16
3. RESULTS
3.1. Cohort
Fifteen families who met the inclusion criteria consented to participate. Two families had two affected children. The clinical features of the children are reported in Table 1. Children presented with seizures at an average of 1.5 years (ranging from 1 day to 11 years). Developmental delay was noted by three years seven months (range three months to three years seven months). Nine children had drug‐resistant seizures at the time of the study. In 12 families, the variant was de novo, in two it was maternally inherited, and for one child the inheritance was unknown (mother negative, father not tested). The average time between seizure onset and genetic diagnosis was nine years four months (range of 6 months to 23 years seven months).
TABLE 1.
Summary of clinical features of participants’ children
|
Case [Case # in previous publication] |
Age at study (gender) | Epilepsy syndrome | Gene | Variant | Inheritance | Sz onset age [offset] | Developmental delay diagnosis |
Cognitive outcome |
Time elapsed between seizure onset and genetic diagnosis (Year) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 y (F) | West | ALG13 |
c.320A > G p.Asn107Ser |
de novo | 4 mo | 4 mo | Severe ID | 4 y 8 mo (2017) |
| 2 27 [T21213] | 17 y (F) | DEE unspecified | GABRB2 | c.911C > T p.Ala304Val | de novo | 4 y | 6 mo | Profound ID | 11 y (2017) |
| 3 | 6 y (F) | Dravet | SCN1A | c.384‐2 A > G | de novo | 6 mo | 6 mo | Severe ID | 6 mo (2013) |
| 4 | 8 y (F) | DEE unspecified | CDKL5 | Exon 4 Deletion | de novo | 5 wk | 3 mo | Profound ID | 1 y 8 mo (2013) |
| 5 28 [13] | 5 y (F) | DEE unspecified | EEF1A2 | c.1150G > C p.Gly384Arg | de novo | 1 d | 5 mo | Profound ID | 1 y (2015) |
| 6 29 [B3] | 17 y (F) | GCE | PCDH19 | c.1919T > G p.Leu640Arg | de novo | 16 mo [11 y 8mo] | 3 y 7 mo | Borderline ID | 7 y 8 mo (2011) |
| 7a 29 [A1] | 12 y (F) | GCE | PCDH19 | c.497_498insA p.Tyr166* | de novo | 10 mo [5 y2 mo] | 3 y 3 mo | Severe ID | 4 y 2 mo (2011) |
| 7b 29 [A2] | 12 y (F) | GCE | PCDH19 | c.497_498insA p.Tyr166* | de novo | 10 mo [5 y 2 mo] | 3 y 4 mo | Mild ID | 4 y 2 mo (2011) |
| 8 30 [55] | 32 y (M) | Dravet | SCN1A | c.512T > A p.Ile171Lys | de novo | 7 mo | 7 mo | Severe ID | 23 y 5 mo (2010) |
| 9 30 [68] | 32 y (F) | Dravet | SCN1A |
c.4062delT p.Asp1355Thrfs*8 |
de novo | 5 mo | 2 y 6 mo | Severe ID | 23 y 7 mo (2010) |
| 10 | 7 y (M) | Dravet | SCN1A | c.5347G > A p.Ala1783Thr | de novo | 6 mo | 2 y | Moderate ID | 1 y 6 mo (2013) |
| 11 29 [E7] | 26 y (F) | GCE | PCDH19 |
c.2534C > T p.Ser845Asn |
Mother negative, father not tested | 8 mo | 11 mo | Moderate ID | 23 y 4 mo (2017) |
| 12 | 12 y (F) | Epilepsy with Myoclonic atonic seizures | SCN1A | c.32C > A p.Pro11His | Maternally inherited | 2 y [3 y] | 2 y | Normal | 5 y (2013) |
| 13 | 7 y (F) | Early‐onset epileptic encephalopathy | KCNQ2 | c.1700T > A p.Val567Asp | de novo | 4 d [10 mo] | 9 mo | Severe ID | 3 y (2015) |
| 14a 29 [C5] | 31 y (F) | GCE | PCDH19 | Exon 6 deletion | Maternally inherited | 14 mo [22 y] | 1 y 1 mo | Mild ID | 22 y 4 mo (2012) |
| 14b 29 [C4] | 27 y (F) | GCE | PCDH19 | Exon 6 deletion | Maternally inherited | 19 mo [21 y] | 1 y 6 mo | Mild ID | 18 y 3 mo (2012) |
| 15 | 22 y (F) (Died) | DEE unspecified | PAFAH1B1 | Intronic deletion | de novo | 11 y [22 y‐died] | 2 y | Mild ID | 2 y (2005) |
In the case column, the superscript number denotes the reference number of the paper in which this individual has been previously published. The case # denotes the specific patient identification number in that publication.
Abbreviations: d, days; DEE, developmental and epileptic encephalopathy; F, female; GCE, girls clustering epilepsy; ID, intellectual disability; M, male; mo, months; wk, weeks; y, years.
3.2. Thematic analysis
Three key themes emerged and describe parents’ lived experience of receiving a genetic diagnosis as the cause of their child's DEE: (a) importance of the label, (b) relief to end the diagnostic journey, and (c) factors that influence personal utility. All families discussed more than one theme, all themes (eight families), and two themes (seven families; Figures 1 and 2).
FIGURE 1.
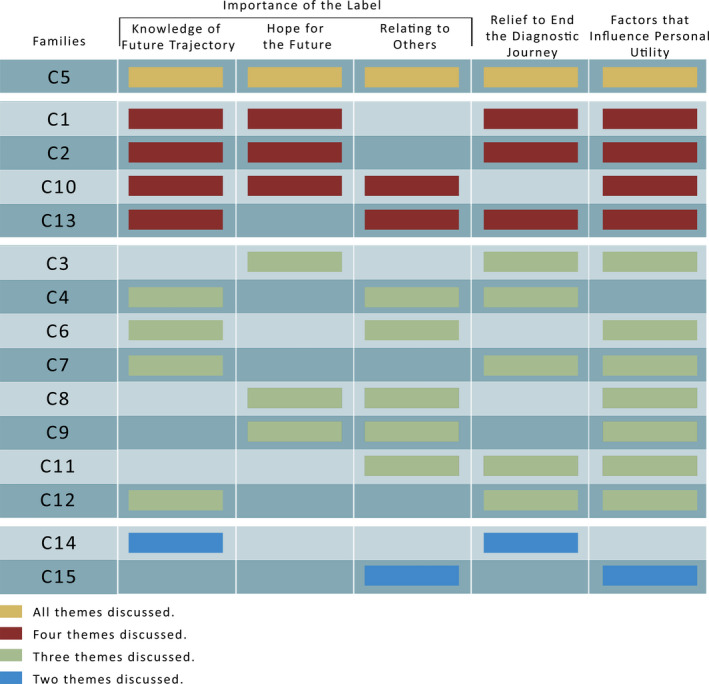
Pattern of responses across all themes and subthemes for each family
FIGURE 2.
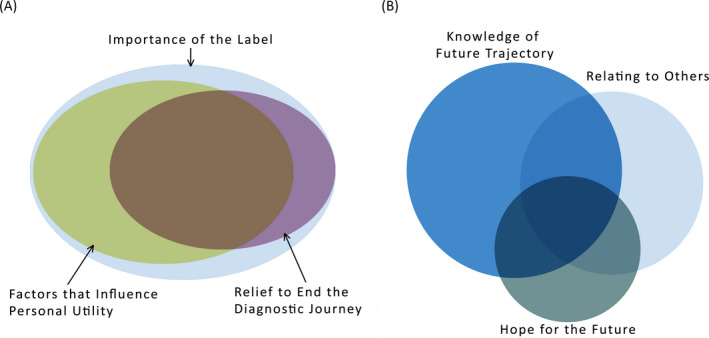
Conceptual venn diagram showing the overlap of themes. A, All themes. B, Subthemes from the 'Importance of the Label' theme. This figure is a conceptualisation of the interaction of both the themes and subthemes. There was considerable overlap in the discussion of the three main themes with all families discussing the 'Importance of the Label'. Within the theme 'Importance of the Label', there was less overlap between the subthemes
3.2.1. Importance of the label
Receiving a genetic label of a DEE was important for all 15 of the families as it validated that their child was not a medical anomaly and that their disorder, while rare, was known to medical professionals. Three subthemes emerged: (a) knowledge of future trajectory, (b) hope for the future, and (c) relating to others. Two families discussed all three subthemes, seven families discussed two subthemes, and six families discussed one subtheme (Figures 1 and 2).
Knowledge of future trajectory
For ten families, receiving the genetic result and label was a turning point to a new pathway with a set of expectations about how their child might develop and possible outcomes to anticipate. It brought control back into the family's life, as they could now research and learn about their specific genetic DEE. This knowledge clarified their expectations for prognosis over the course of the child's life and minimized parental rumination on an array of possible negative outcomes. Thus, when the next seizure or comorbidity occurred, participants felt better informed and had made anticipated adjustments to their routine, home environment, or behavior as a way of preparing. This helped them to psychologically and practically cope more effectively.
Participants also used this ability to gather new information to help process their own feelings of loss, uncertainty, and anxiety. For example, receiving the label of CDKL5 was the catalyst allowing case 4’s (Table 2) family to move forward to a life without diagnostic uncertainty and fear. As receiving a genetic label increased scope for knowledge and understanding while decreasing fear of the unknown, families reported spending less time worrying about what they did not know and more time with their child, searching for a positive, adaptive way forward.
TABLE 2.
Representative quotes related to each of the three themes: importance of the label, relief to end the diagnostic journey, and factors to increase personal utility
| Theme (subtheme) | Number of families who discussed each theme | Representative quotes |
|---|---|---|
| Importance of the label | 15/15 | |
| Knowledge of future trajectory | 10/15 | “It was like a huge weight off our shoulders, it was like we have a name and now we can do research on it, now we can find out more about it.” ‐ C13 |
| “I guess it gives you a pathway, you know. You kind of know, yeah it's just that certainty. Kind of weird comfort in knowing that he's going to follow the same lines as what's expected. Just gives you some time to prepare or not be blindsided by what might come next” ‐ C10 | ||
| “I felt like I was standing at a fork in the road and each fork in the road had a door and the CDKL5 one, was, you could see through it, it was like opaque glass but I could see what that looked like… I said ‘you know I feel like you've just given me the key to that door and now we can actually move forward from the fork in the road”‐ C4 | ||
| “If we didn't get that diagnosis, we'd probably be feeling like there's something else lurking around every corner and it still can be a little bit like that with her, but we know why. But if we didn't know, we'd be living our lives on the edge of a sword just thinking what's going to happen next, what are we going to be dealing with in two months’ time, six months’ time, is she going to even be alive in a year?” – C4 | ||
| Hope for the future | 7/15 | “Maybe in the future they might find something that can be done for that specific gene to make things change, I don't know. But if they don't find these things then they can't work on, you know, what can be done about it. And possibly even if it's just preventing other children from having it then that's a good thing too” ‐ C2 |
| “Reading articles like that makes you think 'maybe!', even if they say it's genetic, there's no cure; it just gives you hope that maybe one day. Like some cancers are cured now. Like leprosy before it was not cured, and it's cured now. So yeah, we hope” ‐ C3 | ||
| “You hold onto hope and you go 'well ok if it's so rare, then who knows, you know?'. The future could be bright, it could be gloomy, but it could be bright, so yeah.” ‐ C5 | ||
|
Relating to others |
9/15 | “Just knowing 'oh yes, it is a textbook thing', it's not just a random, she's not just a one off, there's other people out there like us.” ‐ C9 |
| “The label is, for me, for our family, it's been really important to move forward. You can then get onto like forums and connect with other people who've got the same sort of things going on. And that's huge in itself I think… it just makes you feel better, I can't really quantify that. It just does, it's helpful.” ‐ C4 | ||
|
“Yeah it definitely has, just knowing what she has now, has definitely made it a lot easier to explain to people. Because so many people just stare and think ah what's happened? And now it's easier to just say this is what she has, and this is what happened, whereas before we couldn't really say that. We'd just be like 'I don't know; we don't know why she's like this'.” ‐ C13 | ||
| Relief to end the diagnostic journey | 10/15 | |
| “A huge weight lifted (tearful). Yeah, I guess it wasn't anything that I'd done. It was just‐ you know, she's a one off. It was quite a big impact to have had… I think it's just the weight has lifted off the guilt that I was carrying around. Now that I know that it wasn't anything, there was nothing that could've changed it basically.” ‐ C2 | ||
| “Lots of blaming, lots of thinking like ‘who, where'd she get it from?’ Both sides, especially my husband's side, they're more like cultural beliefs it's caused by this, it's caused by that. But luckily, we did the genetic testing, so it was proven from the test that didn't come from both sides. It's a de novo mutation, yeah…it took out that part where you resent, like where you took blame. It's kind of reassuring that there was no cause, you were reassured that this was what happened to her. Unless because you can be thinking your whole life 'what caused it? what caused it?'” – C3 | ||
| “Yeah, it did make a difference though, because I wasn't constantly in the back of my mind worrying all the time. I was always wondering, was it this? was it that? did I do this? You know, she had a big fall when she was a baby and I often wondered oh god did she have brain trauma? So, I guess that would be for me the biggest result was that I just didn't question it anymore, wasn't looking for a magic cause you know.” ‐ C12 | ||
| Factors that influence personal utility | 13/15 | |
| “It's really important from your researcher perspective to think about the context that came with this diagnosis, which wasn't a whole lot of other detrimental other things, it was just a piece of information… The fact that the gene mutation is there hasn't changed the presentation of what's happening with her physically, therefore there's nothing different yet. Like if all the children started to drop dead at 12 or 13 years old, then that will look completely different for us than it will look if those children are growing normally, not normally, but their normal.”‐ C1 | ||
| “I guess from the information we got that it actually wouldn't make any difference in terms of the treatment. It would just kind of confirm the diagnosis one way or another” ‐ C10 | ||
| “It would've been really nice to have had the genetic diagnosis earlier and it would've saved me 14 years of guilt. Yeah, that would've been really good actually to have had it at the time that she was diagnosed with that and when the epilepsy started and stuff like that, to actually know that it wasn't something that I'd done that it was just a random act of fate… I mean mental health is a big thing at the moment across all sectors. I mean it's not easy having a child with a disability and you see so many people and families just ripped apart by it. If you could know that there's nothing you could've done, then it probably does help.” ‐ C2 | ||
| “Yeah, just wish we could've controlled it maybe a lot earlier and maybe we wouldn't have had to go through so much. Because you only want your best for your children and when you reproduce that's all you want, you know, you want them to be healthy and you don't want anything wrong, you know.” ‐ C7 | ||
| “We always thought we'd have more, but after [affected child] and then after finding out, it's kind of made us think well what if our next kid is like [affected child] as well, like there's no way we could handle two kids like that. So yeah, we decided not to have anymore…I think we just decided that it's just too much to have anymore with all of her needs.” – C13 | ||
| “The other thing is good to know is that it won't affect [sister]. If and when she has children because I guess that's something you always think of. I've always been grateful that A was my second child because if she'd been the first one, she probably would've been the only one. Because I would've been too scared to get pregnant again in case I ended up with two like her.” ‐ C2 | ||
Hope for the future
A genetic label led seven participants to have increased hope that medical professionals would have, or would develop through future research, better effective treatments for their own child and also other children with their genetic disorder. Participants were able to keep up to date on research developments for their child's gene, which gave them a sense of progress and accomplishment and in turn fostered hope and a positive outlook for the future. However, for some participants their newfound hope was short lived when they realized that their child's gene was rare, novel, or did not have a promising long‐term prognosis. Seven of the 15 families discussed the initial difficulty of receiving such information. Despite this, participants tended to “hold onto hope” by drawing meaning that the lack of prognostic knowledge about rare or novel genes did not necessarily mean there was a bad prognosis in the future for their child.
Relating to others
Receiving a genetic diagnostic label also helped reduce nine participants’ sense of isolation and alienation. They were now part of a group of families with variants in the same gene and similar lived experiences. A third of the families described reaching out to other families through support groups, forums, and social media. The support, shared understanding, and advice they received significantly improved participants’ coping and well‐being. The genetic label was also important for families when relating to their external networks. Five families discussed the difficulty of being aware of public perceptions and judgments about their child, and their frustration of not being able to provide an adequate explanation for their child's condition. Receiving a genetic diagnosis meant they could now explain their child's genetic label to others, which was empowering and validating.
3.2.2. Relief to end the diagnostic journey
Most participants had spent many years coping with their child's DEE without knowing the cause. When their genetic cause was identified, nine participants experienced immense relief, as well as a reduction of guilt and self‐blame. Relief was experienced primarily within the context of a de novo genetic variant, where participants were comforted by the information that their child had not inherited the DEE. Participants who learnt that they had passed on the pathogenic variant found it upsetting but also experienced relief that it was caused by something out of their control, not something they had actively done. Furthermore, reducing uncertainty and no longer having to search for answers had a large positive impact for participants.
3.2.3. Factors that influence personal utility
While receiving a genetic diagnosis alleviated psychological distress and improved participants’ knowledge and hope for the future, 13 of the 15 families also discussed their experience of missed opportunities. Most participants expressed their views that the fact it took many years to receive a genetic diagnosis lessened the personal utility. These families had already experienced the trauma and guilt surrounding their child's health and had to learn to adjust and cope with their child's DEE to the best of their ability with limited information. Participants hypothesized that the positive psychological effect of the genetic diagnosis would have significantly increased if they had received the information closer to the onset of their child's DEE. For one family (Table 2, C7), it took 13 years after seizure onset to receive a genetic diagnosis for their child. Although they acknowledged this was due to the limited knowledge and technology at the time, the family felt they had experienced years of uncertainty, anxiety, trauma, and stress which could have been significantly alleviated with an earlier diagnosis.
Timing was important for families, not only to reduce negative psychological outcomes, but also in relation to their anxiety around family planning. For two thirds of families, finding out whether their child's DEE was inherited (or whether it was a de novo variant in the child alone) had a significant influence on families’ decisions to have more children or not. Without a prompt genetic diagnosis, many families were uncertain whether their future children would also inherit the DEE, which sometimes resulted in the decision not to have more children, despite their desire to do so. Families also had ongoing concerns and anxiety about the possibility of their other children passing on the DEE gene to the next generation when they got older. Finding out that their child with DEE had a de novo variant meant they no longer had to worry about this.
Families discussed that the amount of personal utility they gained from a genetic diagnosis was influenced by family contextual factors. These factors varied depending on the child's specific genetic diagnosis and how the family had coped psychologically and practically during the period from their child's first seizure until the genetic diagnosis. For some, the genetic diagnosis was a confirmation of a suspected epilepsy syndrome such as an SCN1A variant in Dravet syndrome, which was already being managed appropriately. In these cases (C5, C8, and C11), the genetic diagnosis yielded no change in treatment or additional information regarding the child's prognosis. The genetic result therefore had minimal emotional impact for the family. However, for families with a causal variant in a gene which was well established but not suspected until the genetic result, identifying a gene in this context was more impactful. This change from living with uncertainty and limited understanding to finally having answers through their genetic diagnosis was considerable and provided significant personal utility. For families with a novel or rare genetic diagnosis, the initial emotional response was positive and hopeful. However, this was relatively quickly followed by despair with the realization that not much was known about their specific gene and the diagnosis did not lead to new treatments or prognostic information. Over time they became more positive and hopeful as they realized that research was ongoing, and they followed these new developments.
4. DISCUSSION
This is the first study to explore the lived experience of parents after receiving a genetic diagnosis for their child's DEE. We found that due to the impact of receiving a genetic label and relief that they had reached the end of the diagnostic journey, families reported positive and meaningful personal utility from receiving a genetic diagnosis. The genetic label gave these families better insight into possible outcomes, an increased hope for the future and improved ability to communicate about their child's disorder to others (reducing both public and self‐stigma). The extent of positive utility depended on the timing of the diagnosis and contextual factors specific to each family.
Having a child with a DEE can place immense physical, financial, and psychological burden on families. 17 , 18 , 19 Our families reported that receiving a genetic diagnosis relieves guilt and corrects false causative beliefs. Subsequently, they experienced a sense of closure allowing them psychological freedom to process their grief and move forward. This occurred not only for families with de novo variants but also for those with inherited variants, as parents recognized the genetic change was outside of their control. The positive outcomes reported by these families support conjecture and findings from previous epilepsy research. 13 , 20 , 21 , 22 It is also consistent with what has been found in other genetic disorders 23 and direct to consumer genetic testing. 10
Coming to the end of the diagnostic journey and finally receiving a genetic DEE diagnosis reduced our parents’ emotional burden as they no longer dwelt on finding answers and were able to receive support from other families with similar experiences. The discovery of DEE genes has led to the widespread development of patient support groups for specific genes. With the advent and popularity of social networking, these support groups can easily connect families from all over the world. This means that the rarity of their child's genetic condition is no longer a barrier to finding other similar families. These family gene support groups not only provide information for families but also enable access to other families who can provide support, friendship, and a shared sense of purpose. Having a genetic diagnosis allows families to be part of these communities, which our study shows increases personal utility of the diagnosis.
Receiving health diagnoses can have both positive and negative impacts. 24 How an individual perceives a diagnosis is influenced by how the individual perceives their illness. 25 It is likely that a DEE family's positive experience of their child's genetic diagnosis was shaped by their experience of living with fear and uncertainty for periods of time prior to that diagnosis. Our results are similar to those from families with milder inherited epilepsies who also reported feelings of relief, validation, and hope from receiving a genetic result. 13 Some described a lifting of perceived responsibility as the gene transmission was out of their control, even if inherited. However, for others the finding of a gene consolidated their feeling of guilt at having passed the epilepsy on to their children. As these individuals already had knowledge that epilepsy ran in their families and in some the epilepsy was quite mild, knowing the exact gene did not appear to have had the same impact on anxiety as it did in our families with children with DEEs. Indeed, some individuals with milder epilepsies described that receiving a genetic diagnosis had such a minimal impact that they could not even remember receiving it. 13 This contrasts starkly with the DEE families in our study, where most of the families reported positive psychological impact from receiving the genetic diagnosis. When discussing their experience of receiving a genetic diagnosis and its impact, DEE families touched on 13 out of the 15 components of personal utility, 9 such as to enhance coping, mental preparation, feelings of responsibility, knowledge of condition, self‐knowledge, ability for future planning, and communication with relatives. This congruence between our families’ disclosures with the conceptualization of personal utility strongly supports the importance of including personal utility when measuring the overall impact of genetic testing in children with DEE.
Consideration of individual family circumstances and differences is crucial when quantifying the impact of a genetic diagnosis. Our study highlights the importance of prompt diagnosis and understanding contextual factors for families. Families reported that the amount and intensity of stress due to not knowing why their child had a DEE were most profound in the first years following their child's DEE presentation. With time, they found ways to manage this stress as they became more accustomed to having a child with DEE. A genetic diagnosis late in the families’ disease journey, therefore, had less impact as it came too late to alleviate the early psychological trauma and negative experiences. If clinical genetic testing for children with DEE becomes routine early in the diagnostic journey, then our study suggests families would likely experience improved psychological outcomes and a better quality of life.
Limitations of this study relate to sample size and cohort recruitment. This study was a focused in‐depth look at the personal utility of a genetic diagnosis for families with children with DEE. However, due to the nature of IPA methodology, the cohort is small and may not be representative of the larger group of families. SCN1A and PCDH19 were the causative genes for over half of our cases. This is not completely unexpected as SCN1A and PCDH19 are common DEE genes. 26 Given the numbers of our families with these genes, the results may be more consistent with the lived experience of families with established genes, and not as representative of families with rarer or newer genetic DEEs. In addition, as all families had previously consented to participating in genetic research, it is possible they were more inclined to have a positive attitude to a genetic result. Nonetheless, the project has identified themes which could be used to develop an online survey that could be given to a larger number of families to ascertain if these themes are representative of the wider group of families with children who have a DEE. Larger cohorts would also allow a more thorough analysis of the impact of age at DEE presentation, the timing of diagnosis during the diagnostic journey, and different genetic diagnoses on the personal utility of a genetic diagnosis for families.
In conclusion, the personal utility of a genetic diagnosis for people with epilepsy, and in particular DEEs, has been underappreciated and under researched. Using IPA, the present study demonstrates that receiving a genetic diagnosis had high personal utility through increased knowledge and connection for families while simultaneously decreasing guilt and blame. The positive psychological benefits and increased understanding allows families to move forward and better cope with their child's DEE, ultimately improving their quality of life and well‐being. This study shows that a clinical genetic DEE diagnosis has personal utility in addition to the already well‐established clinical utility. While there is benefit of genetic testing for families of all ages, our findings further reinforce the need and importance of early clinical genetic testing in children with DEE.
CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
We thank the children and their families for participating in our research. We gratefully acknowledge support from the Health Research Council of New Zealand and Cure Kids New Zealand, the Ted and Mollie Car Endowment Trust.
Jeffrey JS, Leathem J, King C, Mefford HC, Ross K, Sadleir LG. Developmental and epileptic encephalopathy: Personal utility of a genetic diagnosis for families. Epilepsia Open. 2021;6:149–159. 10.1002/epi4.12458
REFERENCES
- 1. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–15. [DOI] [PubMed] [Google Scholar]
- 2. Sillanpää M, Shinnar S. Long‐term mortality in childhood‐onset epilepsy. N Engl J Med. 2010;363(26):2522–9. [DOI] [PubMed] [Google Scholar]
- 3. Claes L, Del‐Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium‐channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68(6):1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheidley BR, Smith LA, Helbig KL. Genetics of epilepsy in the era of precision medicine: Implications for testing, treatment, and genetic counseling. Curr Genet Med Rep. 2018;6(2):73–82. [Google Scholar]
- 5. Symonds JD, McTague A. Epilepsy and developmental disorders: Next generation sequencing in the clinic. Eur J Paediatr Neurol. 2020;24:15–23. [DOI] [PubMed] [Google Scholar]
- 6. Scala M, Bianchi A, Bisulli F, Coppola A, Elia M, Trivisano M, et al. Advances in genetic testing and optimization of clinical management in children and adults with epilepsy. Expert Rev of Neurother. 2020;20(3):251–69. [DOI] [PubMed] [Google Scholar]
- 7. Palmer EE, Sachdev R, Kandula T, Macintosh R, Kirk E, Bye A. Genetics of Epileptic Encephalopathies, eLS. Chichester: John Wiley & Sons Ltd; 2017. p. 1–11. [Google Scholar]
- 8. Pitini E, De Vito C, Marzuillo C, D’Andrea E, Rosso A, Federici A, et al. How is genetic testing evaluated? A systematic review of the literature. Eur J Hum Genet. 2018;26(5):605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25(6):662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wasson K, Sanders TN, Hogan NS, Cherny S, Helzlsouer KJ. Primary care patients’ views and decisions about, experience of and reactions to direct‐to‐consumer genetic testing: a longitudinal study. J Community Genet. 2013;4(4):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gooding HC, Linnenbringer EL, Burack J, Roberts JS, Green RC, Biesecker BB. Genetic susceptibility testing for Alzheimer disease: motivation to obtain information and control as precursors to coping with increased risk. Patient Educ Couns. 2006;64(1–3):259–67. [DOI] [PubMed] [Google Scholar]
- 12. Fox E, McCuaig J, Demsky R, Shuman C, Chitayat D, Maganti M, et al. The sooner the better: Genetic testing following ovarian cancer diagnosis. Gynecol Oncol. 2015;137(3):423–9. [DOI] [PubMed] [Google Scholar]
- 13. Vears DF, Dunn KL, Wake SA, Scheffer IE. “It's good to know”: Experiences of gene identification and result disclosure in familial epilepsies. Epilepsy Res. 2015;112:64–71. [DOI] [PubMed] [Google Scholar]
- 14. Smith JA, Osborn M. Interpretative phenomenological analysis as a useful methodology for research on the lived experience of pain. Br J Pain. 2015;9(1):41–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larkin M, Watts S, Clifton E. Giving voice and making sense in interpretative phenomenological analysis. Qual Res Psychol. 2006;3(2):102–20. [Google Scholar]
- 16. Smith JA, Flowers P, Larkin M. Interpretative phenomenological analysis: theory method and research. London: SAGE Publications Ltd; 2009. [Google Scholar]
- 17. Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52:95–101. [DOI] [PubMed] [Google Scholar]
- 18. Camfield P, Camfield C, Nolan K. Helping families cope with the severe stress of Dravet syndrome. Can J Neurol Sci. 2016;43(suppl 3):S9–S12. [DOI] [PubMed] [Google Scholar]
- 19. Gallop K, Wild D, Nixon A, Verdian L, Cramer JA. Impact of Lennox‐Gastaut Syndrome (LGS) on health‐related quality of life (HRQL) of patients and caregivers: literature review. Seizure. 2009;18(8):554–8. [DOI] [PubMed] [Google Scholar]
- 20. Poduri A, Sheidley BR, Shostak S, Ottman R. Genetic testing in the epilepsies—developments and dilemmas. Nat Rev Neurol. 2014;10(5):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shostak S, Zarhin D, Ottman R. What’s at stake? Genetic information from the perspective of people with epilepsy and their family members. Soc Sci Med. 2011;73(5):645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berkovic SF. Genetics of epilepsy in clinical practice. Epilepsy Curr. 2015;15(4):192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosell AM, Pena LD, Schoch K, Spillmann R, Sullivan J, Hooper SR, et al. Not the end of the odyssey: parental perceptions of whole exome sequencing (WES) in pediatric undiagnosed disorders. J Genet Couns. 2016;25(5):1019–31. [DOI] [PubMed] [Google Scholar]
- 24. Gillman M, Heyman B, Swain J. What's in a name? The implications of diagnosis for people with learning difficulties and their family carers. Disabil Soc. 2000;15(3):389–409. [Google Scholar]
- 25. Plug L, Sharrack B, Reuber M. Seizure, fit or attack? The use of diagnostic labels by patients with epileptic or non‐epileptic seizures. Appl Linguist. 2010;31(1):94–11. [Google Scholar]
- 26. Symonds JD, Zuberi SM, Stewart K, McLellan A, O‘Regan M, MacLeod S, et al. Incidence and phenotypes of childhood‐onset genetic epilepsies: a prospective population‐based national cohort. Brain. 2019;142(8):2303–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamdan FF, Myers CT, Cossette P, Lemay P, Spiegelman D, Laporte AD, et al. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am J Hum Genet. 2017;101(5):664–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carvill GL, Helbig KL, Myers CT, Scala M, Huether R, Lewis S, et al. Damaging de novo missense variants in EEF1A2 lead to a developmental and degenerative epileptic‐dyskinetic encephalopathy. Hum Mutat. 2020;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadleir LG, Kolc KL, King C, Mefford HC, Dale RC, Gecz J, et al. Levetiracetam efficacy in PCDH19 Girls Clustering Epilepsy. Eur J Paediatr Neurol. 2020;24:142–7. [DOI] [PubMed] [Google Scholar]
- 30. Harkin LA, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM, et al. The spectrum of SCN1A‐related infantile epileptic encephalopathies. Brain. 2007;130(3):843–52. [DOI] [PubMed] [Google Scholar]


