Abstract
Insulin-like growth factor 2 (IGF-2) is a growth factor that is involved in various functions of cells, including stem cells. The effects of IGF-2 on the cellular viability and osteogenic differentiation of stem cell spheroids were investigated in the present study. Stem cell spheroids were formed using concave microwells in the presence of IGF-2 at final concentrations of 0, 10 and 100 ng/ml. Cellular viability was measured qualitatively using a microscope and quantitatively using an assay kit based on water-soluble tetrazolium salt. The level of alkaline phosphatase activity, and an anthraquinone dye assay for calcium deposit evaluation, were used to assess osteogenic differentiation. A quantitative PCR analysis was conducted to evaluate the expression of Runx2 and Col1. Spheroid formation was noticed on day 1 in the microwells, and the spheroidal shape was maintained up to day 7. The cell viability assay values for IGF-2 at 0, 10 and 100 ng/ml at day 1 were 0.193±0.002, 0.191±0.002 and 0.201±0.006, respectively (P>0.05). The absorbance values at 405 nm for the alkaline phosphatase activity assays on day 21 were 0.221±0.006, 0.375±0.010 and 0.280±0.015 for IGF-2 at 0, 10 and 100 ng/ml, respectively. There were significantly higher values for IGF-2 in the 10 and 100 ng/ml groups when compared with the control (P<0.05). Significantly higher Alizarin red staining was noted for IGF-2 in the 10 ng/ml group when compared with the unloaded control at day 21 (P<0.05). Quantitative PCR revealed that mRNA levels of Runx2 and Col1 were significantly higher at 100 ng/ml on day 7. Conclusively, the present study demonstrated that the application of IGF-2 increased alkaline phosphatase activity, Alizarin red staining, and Runx2 and Col1 expression of stem cell spheroids.
Keywords: bone marrow, cell survival, cell differentiation, cellular spheroids, insulin-like growth factor II, osteogenesis, stem cells
Introduction
Insulin-like growth factors (IGFs) are an essential growth factor system connected in both the development of an organism and the sustainment of normal function of various cells of the body (1). IGFs are also reported to possess powerful anti-apoptotic effects (1). Similarly, IGFs and IGF-binding proteins may play an important role in tumor proliferation (2). One kind of IGF, IGF-2, is widely applied for the regulation of various functions of many cells (3,4). IGF-2 has been shown to play a critical role in adult neurogenesis and cognitive function, which results in acting as a memory enhancer (3). IGF-2 enhanced functions of antigen-specific regulatory B cells, which resulted in enhancing the inhibitory effects on allergic inflammation (4).
Recently, IGF-2 has been reported to have modulatory effects on stem cells. IGF-2 modulated hematopoietic stem cell maintenance (5). IGF-2 promoted stemness of neural stem cells, evidenced by increased expression of Oct4, Sox1, and FABP7 mRNA levels (6). IGF-2 induced the differentiation of hematopoietic stem cells (4). Human mesenchymal stem cells derived from human bone marrow can differentiate into epithelial-like cells using various growth factors, including IGF-2, hepatocyte growth factor, keratinocyte growth factor, and epidermal growth factor (7). IGF-2 facilitated the transformation of fibroblasts to myofibroblasts, as well as enhanced the transformation of stem cells into epithelial cells (8). Moreover, IGF-2 has been shown to potentiate osteogenic differentiation (9). Three-dimensional in vitro culturing methods including spheroid culture have been of great interest because they represent in vivo cell biology better (10). Spheroids is known to mimic the solid tissues by simulating the cell-to-cell interaction and secreting their own extracellular matrix along with displaying differential nutrient availability (11). Moreover, spheroid culture is reported to enhance osteogenic differentiation potential of mesenchymal stem cells (12). The hypothesis of this study is that the application of IGF-2 may have beneficial effects on the osteogenic differentiation of stem cells in spheroid formation without application of the scaffold. This study was conducted to evaluate the effects of IGF-2 on the maintenance of morphology, improvements of cellular viability, and enhancement of osteogenic differentiation of stem cell spheroids.
Materials and methods
Cell spheroids using bone marrow mesenchymal stem cells
The present study protocol was reviewed and approved by the Institutional Review Board of Seoul St Mary's Hospital, College of Medicine, the Catholic University of Korea (KC19SESI0234). Informed consent was obtained from the participants. All experiments were performed based on the relevant guidelines and regulations specified in the Declaration of Helsinki.
Human bone marrow mesenchymal stem cells (BMSCs; Catholic MASTER cells) were obtained from the Catholic Institute of Cell Therapy (CIC, Seoul, South Korea). Isolation and characterization of the BMSCs were performed following previously reported methods (13). The CIC verified that all samples showed >90% positive CD 73 and CD 90 expression. We plated the cells on a culture dish, and cells that were attached to the dish were removed. We changed the culture medium every 2 or 3 days. The cells were grown in an incubator at 37˚C with 95% air and 5% CO2.
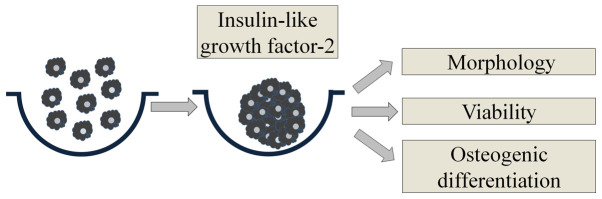
Fig. 1 shows an overview of the study's design. We used commercially available concave microwells (H389600, StemFIT 3D; MicroFIT) to fabricate stem cell spheroids. We loaded a total of 1x106 BMSCs in each well and evaluated the cell response. We treated cell spheroids made of BMSCs with IGF-2 at predetermined concentrations of 0, 10, and 100 ng/ml. We evaluated the morphological characteristics on Days 1, 3, 5, and 7.
Figure 1.
Schematic overview of the present study's design.
Determination of cellular viability
We cultured stem cell spheroids in osteogenic media. We used the commercially available two-color assay based on plasma membrane integrity and esterase activity (Live/Dead Kit assay, Molecular Probes,) for qualitative analysis of the stem cell spheroids on Days 1, 3, 5, and 7(14). We also performed a quantitative cellular viability test using an assay kit based on water-soluble tetrazolium salt (Cell Counting Kit-8, Dojindo) (15).
Level of alkaline phosphatase activity and calcium deposition
The level of alkaline phosphatase activity and an anthraquinone dye assay for calcium deposit evaluation were used to assess osteogenic differentiation (16). We obtained cell spheroids grown on culture plates with osteogenic media on Days 3, 7, 14, and 21. We used a commercially available kit (K412-500, BioVision, Inc.) to evaluate level of alkaline phosphatase activity.
We used an anthraquinone dye assay for calcium deposit evaluation to assess osteogenic differentiation on Days 7, 14, and 21(17). We washed three times with phosphate-buffered saline and then fixed with 4% paraformaldehyde in phosphate-buffered saline at room temperature for 15 min. After that, we carefully removed the fixative and washed three times with deionized water. We added 2% Alizarin Red S Staining solution, and then incubated for 20 min. We removed dye and washed three times with deionized water. The quantification of the bound dyes was performed afterwards by adding 10% cetylpyridinium chloride for 15 min at ambient temperature. Spectrophotometric quantification was performed at 560 nm.
Total RNA extraction and quantification of Runx2 and Col1 by quantitative polymerase chain reaction
We harvested the cells on Day 7(18). We isolated total RNA using purification (Thermo Fisher Scientific, Inc.); RNA was used as a template for reverse transcription using SuperiorScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), and we determined quantities were within ratios of absorbance at 260 and 280 nm spectrophotometrically (ND-2000, Thermo Fisher Scientific, Inc.).
We detected mRNA expression by quantitative polymerase chain reaction (18,19). We designed the sense and antisense primers based on GenBank. The primer sequences were as follows: Runx2 forward 5': AATGATGGTGTTGACGCTGA-3'; reverse 5': TTGATACGTGTGGGATGTGG-3'; Col1 forward 5': CCAGAAGAACTGGTACATCAGCAA-3', reverse 5': CGCCATACTCGAACTGGAATC-3', β-actin forward 5': TGGCACCCAGCACAATGAA-3', and reverse 5': CTAAGTCATAGTCCGCCTAGAAGCA-3'. Normalization was performed by applying β-actin as a housekeeping gene.
Statistical analysis
We presented the data as means ± standard deviations of the experiments. Tests of normality and equality of variances were conducted. We performed two-way analysis of variance to evaluate the effects of concentration and time points with Tukey's post hoc test. We tested the differences among groups by applying one-way analysis of variance with Tukey's post hoc test (SPSS 12 for Windows, SPSS Inc.; P<0.05).
Results
Formation of cell spheroids with human bone marrow-derived stem cells
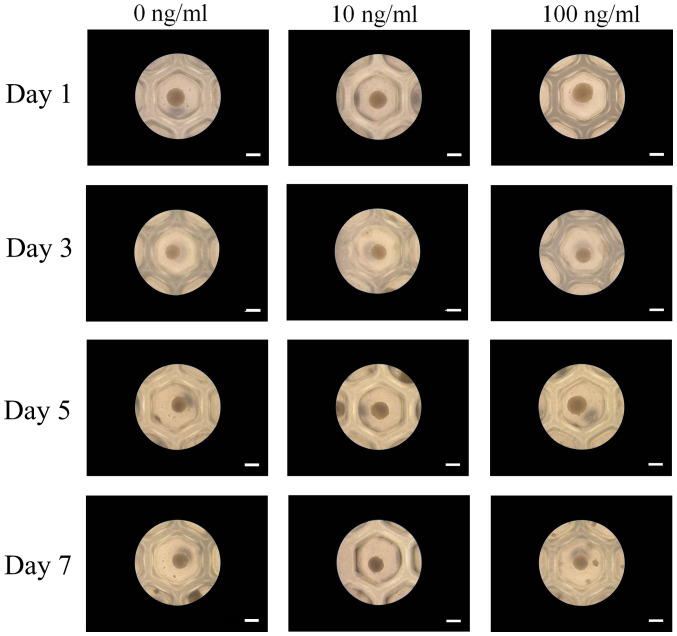
Spheroids were fabricated in each microwell on Day 1 (Fig. 2). The addition of IGF-2 at 10 and 100 ng/ml concentrations did not produce any morphological changes. Extended incubation on Days 3, 5, and 7 did not show any morphological changes.
Figure 2.
Morphology of the stem-cell spheroids at days 1, 3, 5 and 7. Scale bar, 200 µm.
Determination of cellular viability
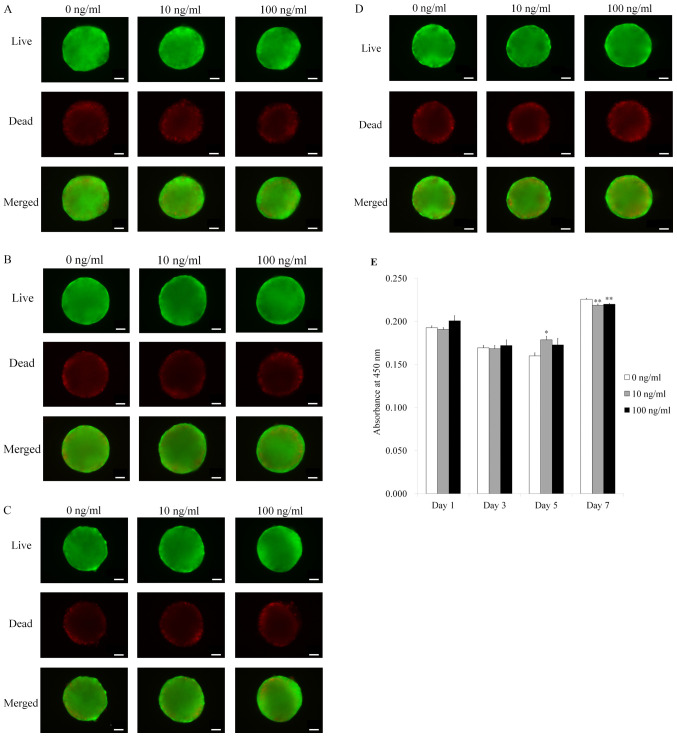
Qualitative results of the viability of cell spheroids were analyzed using a Live/Dead kit assay on Days 1, 3, 5, and 7 (Fig. 3A-D). Most of the cells on the surface produced green fluorescence, indicating live cells. The quantitative values for cellular viability on Days 1, 3, 5, and 7 are shown in Fig. 3E. The relative values for IGF-2 at 0, 10, and 100 ng/ml at Day 1 were 0.193±0.002, 0.191±0.002, and 0.201±0.006, respectively (P>0.05).
Figure 3.
Live, dead and merged images of stem cell spheroids. (A) Live, dead and merged images of stem cell spheroids on day 1. Scale bar, 100 µm. (B) Live, dead and merged images of stem cell spheroids on day 3. Scale bar, 100 µm. (C) Live, dead and merged images of stem cell spheroids on day 5. Scale bar, 100 µm. (D) Live, dead and merged images of stem cell spheroids on day 7. Scale bar, 100 µm. (E) Cellular viability was assessed using a Cell Counting Kit-8 assay on days 1, 3, 5 and 7. *P<0.05 vs. IGF-2 at 0 ng/ml on day 5; **P<0.05 vs. IGF-2 at 0 ng/ml on day 7. IGF-2, insulin-like growth factor 2.
Level of alkaline phosphatase activity and calcium deposition
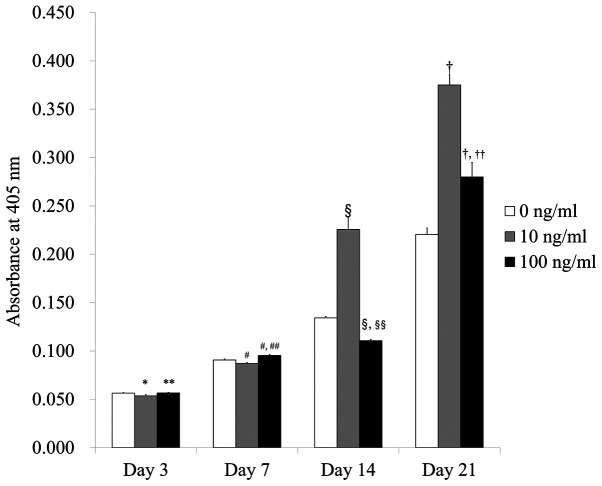
The levels of the alkaline phosphatase activity assays on Days 3, 7, 14, and 21 are shown in Fig. 4. The absorbance values at 405 nm on Day 21 for IGF-2 at 0, 10, and 100 ng/ml were 0.221±0.006, 0.375±0.010, and 0.280±0.015, respectively. There were significantly higher values for IGF-2 in the 10 and 100 ng/ml groups when compared with the control (P<0.05).
Figure 4.
Alkaline phosphatase activity on days 3, 7, 14 and 21. *P<0.05 vs. IGF-2 at 0 ng/ml on day 3; **P<0.05 vs. IGF-2 at 10 ng/ml on day 3; #P<0.05 vs. IGF-2 at 0 ng/ml on day 7; ##P<0.05 vs. IGF-2 at 10 ng/ml on day 7; §P<0.05 vs. IGF-2 at 0 ng/ml on day 14; §§P<0.05 vs. IGF-2 at 10 ng/ml on day 14; †P<0.05 vs. IGF-2 at 0 ng/ml on day 21; ††P<0.05 vs. IGF-2 at 10 ng/ml on day 21. IGF-2, insulin-like growth factor 2.
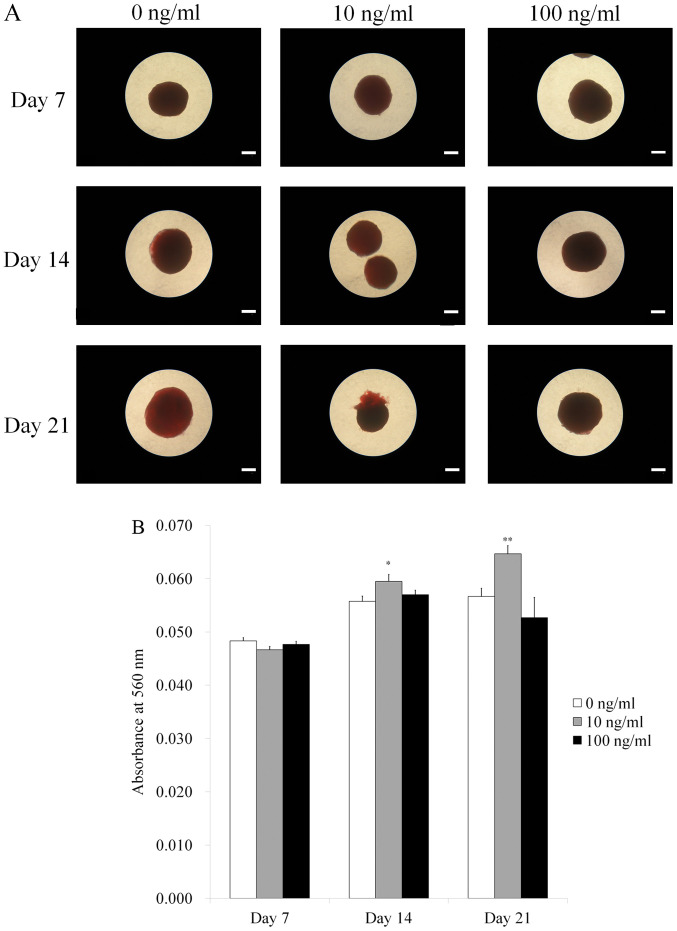
Fig. 5A shows the results for Alizarin Red S staining. Calcium deposits were clearly noted in each group. The quantitative results of the anthraquinone dye assay at Days 7, 14, and 21 are shown in Fig. 5B. The absorbance values at 560 nm on Day 21 for IGF-2 at 0, 10, and 100 ng/ml were 0.057±0.002, 0.065±0.002 and 0.053±0.004, respectively. There were significantly higher values for IGF-2 in the 10 ng/ml group when compared with the unloaded control at Day 21 (P<0.05).
Figure 5.
Results of Alizarin Red S staining on days 7, 14 and 21. (A) Microscopic evaluation. Scale bar, 200 µm. (B) Quantitative analysis of Alizarin Red S staining. *P<0.05 vs. IGF-2 at 0 ng/ml on day 14; **P<0.05 vs. IGF-2 at 10 ng/ml on day 14; #P<0.05 vs. IGF-2 at 0 ng/ml on day 21; ##P<0.05 vs. IGF-2 at 10 ng/ml on day 21. IGF-2, insulin-like growth factor 2.
Evaluation of Runx2 and Col1 by reverse transcription-quantitative polymerase chain reaction
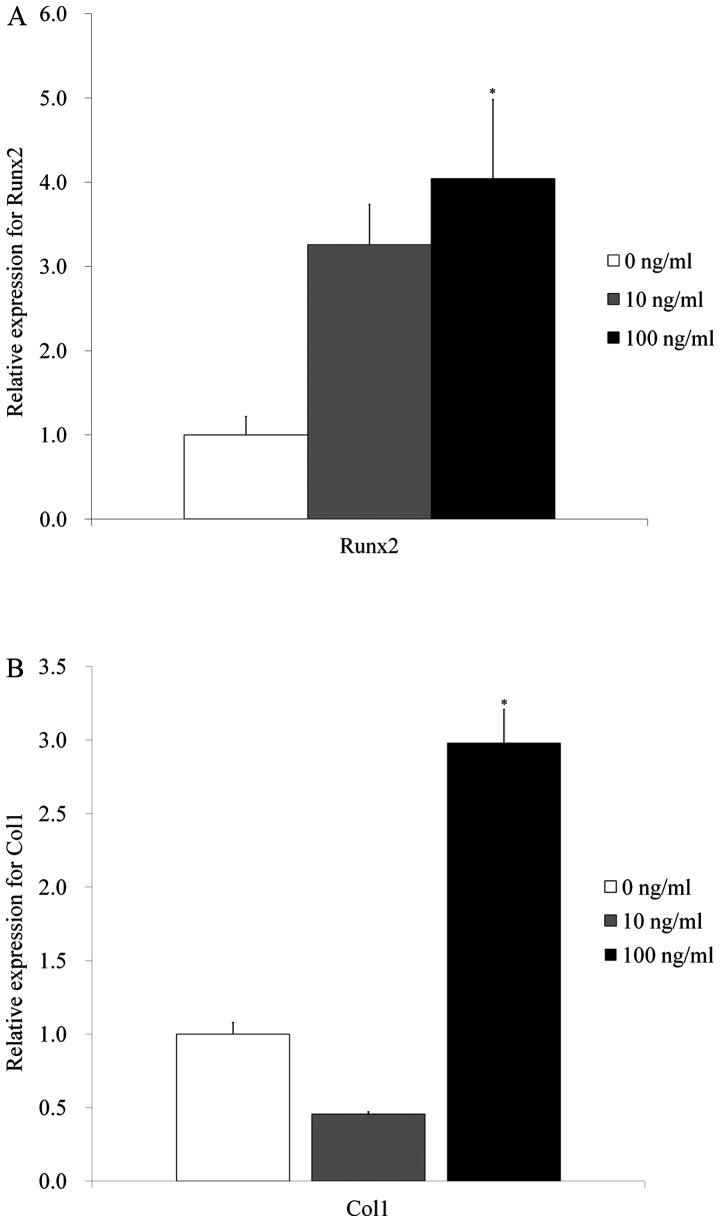
Quantitative real-time polymerase chain reaction revealed that mRNA levels of Runx2 were 1.0±0.2, 3.3±0.5, and 4.0±0.9 for IGF-2 at 0, 10, and 100 ng/ml, respectively. The results showed that the addition of IGF-2 produced a significant increase of Runx2 in 100 ng/ml group (P<0.05) (Fig. 6A).
Figure 6.
Quantification of mRNA expression by reverse transcription- quantitative polymerase chain reaction on day 7. (A) Expression of Runx2. (B) Expression of Col1. *P<0.05 vs. insulin-like growth factor 2 at 0 and 10 ng/ml. Col1, type I collagen; Runx2, runt-related transcription factor 2.
Reverse transcription-quantitative polymerase chain reaction revealed that mRNA levels of Col1 were 1.0±0.1, 0.5±0.1, and 2.4±0.3 for IGF-2 at 0, 10, and 100 ng/ml, respectively. The results showed that application of IGF-2 produced a significant increase of Col1 in 100 ng/ml group (P<0.05) (Fig. 6B).
Discussion
This study evaluated the effects of IGF-2 on the maintenance of morphology, improvements of cellular viability, and enhancement of osteogenic differentiation of stem cell spheroids. Collectively, this study shows that the addition of IGF-2 increased the osteogenic differentiations and Runx2 and Col1 expression of stem cell spheroids.
IGFs are reported to be key regulators for bone growth, bone repair, and bone remodeling, and IGF-2 has been shown to be a chemotactic factor for human mesenchymal progenitor cells (20). IGF-2 rescued microRNA-repressed osteogenic differentiation (21). An increase in IGF-2 expression can aid in osteogenic differentiation of BMSCs (22). The enhanced differentiation of osteoprogenitor cells by IGF-2 was obtained in a dose-dependent pattern (23). In this report, the IGF-2 significantly produced higher values for alkaline phosphatase activity and anthraquinone dye assay. Moreover, IGF-2 is reported to be involved in the promotion of cell proliferation and survival (24,25). It was reported that the effects of IGF-2 on cellular behavior are mediated by IGF type I receptor and it transports survival signals to the cell through a complex network of signaling mechanisms (26).
The effects of concentration of IGF-2 were tested in previous reports. While subphysiologic doses of IGF-2 caused a modest stimulation of erythropoiesis, addition of a physiologic concentration (100 ng/ml) resulted in up to a 4-fold enhancement in erythroid colony formation (27). Stimulation of cell migration was noticed with the addition of IGF-2 from 1 to 100 ng/ml in a dose-dependent way (20). In this study, significantly higher values for alkaline phosphatase activity assays were seen at 10 and 100 ng/ml. Alizarin Red S staining data showed that higher values for IGF-2 were seen at 10 ng/ml. The highest gene expressions of Runx2 and Col1 were noted at 100 ng/ml. In general, the application of IGF-2 increased alkaline phosphatase activity, Alizarin red staining, and Runx2 and Col1 expression of stem cell spheroids. The differences in the overall effects or maximal effective dose may be due to the culturing condition and culturing period (28).
The effects of IGF-2 may be enhanced synergistically by applying additional molecules. IGF-2 in combination with platelet-derived growth factor and neurotrophin-3 resulted in induction of trans differentiation of muscle-derived cells into Schwann cell-like cells (29). IGF-2 was delivered with bone morphogenetic proteins to evaluate the synergistic effects on osteogenic differentiation (30).
IGF-2 has been proposed to act on via various pathways (5,23,31-33). IGF-2 appeared to exert effects on human marrow erythroid progenitors via a direct mechanism involving the IGF-1 receptor (31). IGF-2 augmented in vitro hematopoiesis primarily through its interaction with IGF-1 and possibly insulin receptors, rather than IGF-2/cation-independent mannose 6-phosphate receptors (32). IGF-2 promoted stemness of stem cells via the A isoform of the insulin receptor and not through activation of either the IGF-1 or the IGF-2 receptors (33). IGF-2 regulates hematopoietic stem cell cycle by upregulation of p57(5). Moreover, IGF-stimulated osteoprogenitor differentiation is mediated through IGF-1 receptor (23). The change in the Runx2 expression can be used as the evaluation tool for the osteogenic differentiation potential for spheroids (19,34,35). Further research including the mitogen-activated protein kinase pathways and Smad signaling pathways may be warranted to evaluate the underlying mechanism (26,36).
This study evaluated the effects of IGF-2 on the maintenance of morphology, improvements of cellular viability, and enhancement of osteogenic differentiation of stem cell spheroids. Collectively, this study shows that the addition of IGF-2 increased the osteogenic differentiation and Runx2 and Col1 expression of stem cell spheroids. Future studies are warranted for the application of IGF-2 along with stem cell spheroids to various models, including in vivo studies using bony defects models in calvaria, mandible and femur.
Acknowledgements
BMSCs (Catholic MASTER cells) were supplied by Catholic Institute of Cell Therapy (CIC, Seoul, Korea). The cells were derived from human bone marrow donated by healthy donors after informed consent was obtained from the participants.
Funding Statement
Funding: The present study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant no. 2020R1A2C4001624). The present study was also supported by Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea.
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Authors' contributions
SKM, MK and JBP collaborated to design the study. SKM, MK and JBP were responsible for data access and analysis. SKM, MK and JBP performed the experiments. SKM, MK and JBP wrote the manuscript. SKM, MK and JBP reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study protocol was reviewed and approved by the Institutional Review Board of Seoul St Mary's Hospital, College of Medicine, the Catholic University of Korea (KC19SESI0234). Informed consent for participation was obtained from the participants. All experiments were performed in accordance with relevant guidelines and regulations specified in the Declaration of Helsinki.
Patient consent for publication
Patient consent for publication was obtained from the participants.
Competing interests
The authors declare that they have no competing interests.
References
- 1.LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 2.Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I,-II and IGFBP-2,-3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transplant. 2003;32:411–415. doi: 10.1038/sj.bmt.1704149. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto T, Ouchi Y. Emerging evidence of insulin-like growth factor 2 as a memory enhancer: A unique animal model of cognitive dysfunction with impaired adult neurogenesis. Rev Neurosci. 2014;25:559–574. doi: 10.1515/revneuro-2014-0010. [DOI] [PubMed] [Google Scholar]
- 4.Geng XR, Yang G, Li M, Song JP, Liu ZQ, Qiu S, Liu Z, Yang PC. Insulin-like growth factor-2 enhances functions of antigen (Ag)-specific regulatory B cells. J Biol Chem. 2014;289:17941–17950. doi: 10.1074/jbc.M113.515262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DD, Sommer AG, Balazs AB, Beerman I, Murphy GJ, Rossi D, Mostoslavsky G. Insulin-like growth factor 2 modulates murine hematopoietic stem cell maintenance through upregulation of p57. Exp Hematol. 2016;44:422–433.e1. doi: 10.1016/j.exphem.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, Wood TL, Levison SW. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30:1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Păunescu V, Deak E, Herman D, Siska IR, Tănasie G, Bunu C, Anghel S, Tatu CA, Oprea TI, Henschler R, et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007;11:502–508. doi: 10.1111/j.1582-4934.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Ju Z, Zhang J, Liu X, Tian J, Mu G. Effects of insulin-like growth factor 2 and its receptor expressions on corneal repair. Int J Clin Exp Pathol. 2015;8:10185–10191. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25:2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verjans ET, Doijen J, Luyten W, Landuyt B, Schoofs L. Three-dimensional cell culture models for anticancer drug screening: Worth the effort? J Cell Physiol. 2018;233:2993–3003. doi: 10.1002/jcp.26052. [DOI] [PubMed] [Google Scholar]
- 11.Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: A breakthrough in vivo. Int J Mol Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moritani Y, Usui M, Sano K, Nakazawa K, Hanatani T, Nakatomi M, Iwata T, Sato T, Ariyoshi W, Nishihara T, Nakashima K. Spheroid culture enhances osteogenic potential of periodontal ligament mesenchymal stem cells. J Periodontal Res. 2018;53:870–882. doi: 10.1111/jre.12577. [DOI] [PubMed] [Google Scholar]
- 13.Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int. 2014;2014(129145) doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang SH, Park JB, Kim I, Lee W, Kim H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J Periodontal Implant Sci. 2019;49:258–267. doi: 10.5051/jpis.2019.49.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BB, Tae JY, Ko Y, Park JB. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp Ther Med. 2019;18:3425–3430. doi: 10.3892/etm.2019.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Son J, Min SK, Na CB, Yi G, Koo H, Park JB. A study of the effects of doxorubicin-containing liposomes on osteogenesis of 3D stem cell spheroids derived from gingiva. Materials (Basel) 2019;12(2693) doi: 10.3390/ma12172693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Park JB. Dimethyl sulfoxide leads to decreased osteogenic differentiation of stem cells derived from gingiva via Runx2 and collagen I expression. Eur J Dent. 2019;13:131–136. doi: 10.1055/s-0039-1694904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Lee H, Na CB, Park JB. The effects of simvastatin on cellular viability, stemness and osteogenic differentiation using 3-dimensional cultures of stem cells and osteoblast-like cells. Adv Clin Exp Med. 2019;28:699–706. doi: 10.17219/acem/94162. [DOI] [PubMed] [Google Scholar]
- 19.Tae JY, Lee H, Lee H, Ko Y, Park JB. Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells. Exp Ther Med. 2018;16:2287–2294. doi: 10.3892/etm.2018.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler J, Brill C, Blum WF, Brenner RE. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2006;345:1177–1183. doi: 10.1016/j.bbrc.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Ding W, Li J, Singh J, Alif R, Vazquez-Padron RI, Gomes SA, Hare JM, Shehadeh LA. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc Res. 2015;106:131–142. doi: 10.1093/cvr/cvv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han LC, Qi MC, Sun H, Hu J, Zou SJ, Li JH. Response of bone marrow mesenchymal stem cells to mechanical stretch and gene expression of transforming growth factor-beta and insulin-like growth factor-II under mechanical strain. Hua Xi Kou Qiang Yi Xue Za Zhi. 2009;27:381–385. (In Chinese) [PubMed] [Google Scholar]
- 23.Jia D, Heersche JN. Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone. 2000;27:785–794. doi: 10.1016/s8756-3282(00)00400-2. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran RB, Bachar Raveh T, Barakat MT, Lee EY, Scott MP. Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice. Cancer Res. 2008;68:8788–8795. doi: 10.1158/0008-5472.CAN-08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillaud-Bataille M, Ragazzon B, de Reyniès A, Chevalier C, Francillard I, Barreau O, Steunou V, Guillemot J, Tissier F, Rizk-Rabin M, et al. IGF2 promotes growth of adrenocortical carcinoma cells, but its overexpression does not modify phenotypic and molecular features of adrenocortical carcinoma. PLoS One. 2014;9(e103744) doi: 10.1371/journal.pone.0103744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44 (Suppl 1):S109–S116. [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders M, Sorba S, Dainiak N. Insulin-like growth factors stimulate erythropoiesis in serum-substituted umbilical cord blood cultures. Exp Hematol. 1993;21:25–30. [PubMed] [Google Scholar]
- 28.Lee H, Son J, Yi G, Koo H, Park JB. Cellular viability and osteogenic differentiation potential of human gingiva-derived stem cells in 2D culture following treatment with anionic, cationic, and neutral liposomes containing doxorubicin. Exp Ther Med. 2018;16:4457–4462. doi: 10.3892/etm.2018.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, He H, Cheng N, Song Y, Ding W, Zhang Y, Zhang W, Zhang J, Peng H, Jiang H. PDGF, NT-3 and IGF-2 in combination induced transdifferentiation of muscle-derived stem cells into Schwann cell-like cells. PLoS One. 2014;9(e73402) doi: 10.1371/journal.pone.0073402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acil Y, Ghoniem AA, Wiltfang J, Gierloff M. Optimizing the osteogenic differentiation of human mesenchymal stromal cells by the synergistic action of growth factors. J Craniomaxillofac Surg. 2014;42:2002–2009. doi: 10.1016/j.jcms.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Merchav S, Silvian-Drachsler I, Tatarsky I, Lake M, Skottner A. Comparative studies of the erythroid-potentiating effects of biosynthetic human insulin-like growth factors-I and -II. J Clin Endocrinol Metab. 1992;74:447–452. doi: 10.1210/jcem.74.2.1730818. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GN, Warren MK, Sakano K, Szabo JM, Kessler SW, Pashapour A, Gress RE, Perdue JF. Comparative effects of insulin-like growth factor II (IGF-II) and IGF-II mutants specific for IGF-II/CIM6-P or IGF-I receptors on in vitro hematopoiesis. Stem Cells. 1996;14:337–350. doi: 10.1002/stem.140337. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler AN, Chidambaram S, Forbes BE, Wood TL, Levison SW. Insulin-like growth factor-II (IGF-II) and IGF-II analogs with enhanced insulin receptor-a binding affinity promote neural stem cell expansion. J Biol Chem. 2014;289:4626–4633. doi: 10.1074/jbc.M113.537597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41–51. doi: 10.1016/j.diff.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S, Yang S, Zhang Y, Li X, Gao D, Zhong Y, Cao L, Ma H, Liu Y, Li G, et al. LncRNA ODIR1 inhibits osteogenic differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis. 2019;10(947) doi: 10.1038/s41419-019-2148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: Implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.