Abstract
Dried fig is susceptible to infection by Aspergillus flavus, the major producer of the carcinogenic mycotoxins. This fruit may be contaminated by the fungus throughout the entire chain production, especially during natural sun-drying, post-harvest, industrial processing, storage, and fruit retailing. Correct management of such critical stages is necessary to prevent mould growth and mycotoxin accumulation, with temperature being one of the main factors associated with these problems. The effect of different temperatures (5, 16, 25, 30, and 37 °C) related to dried-fig processing on growth, one of the regulatory genes of aflatoxin pathway (aflR) and mycotoxin production by A. flavus, was assessed. Firstly, growth and aflatoxin production of 11 A. flavus strains were checked before selecting two strains (M30 and M144) for in-depth studies. Findings showed that there were enormous differences in aflatoxin amounts and related-gene expression between the two selected strains. Based on the results, mild temperatures, and changes in temperature during drying and storage of dried figs should be avoided. Drying should be conducted at temperatures >30 °C and close to 37 °C, while industry processing, storage, and retailing of dried figs are advisable to perform at refrigeration temperatures (<10 °C) to avoid mycotoxin production.
Keywords: mycotoxin, toxigenic moulds, food safety, figs
1. Introduction
The fig tree originates from the Middle East where it has been cultivated for millennia, probably because of well adaptation to high temperatures and low water regimes, so it has traditionally been cultivated in marginal soils under rain-fed conditions. Its fruit, the common fig (Ficus carica L.), is a typical species of the tropic and subtropic areas, being one of the most important agricultural products in the Middle East and Mediterranean region [1]. Fig is a seasonal fruit that can be harvested twice a year, either during the spring and summer season or in the early and late summer, depending on the cultivar [2,3]. Both fresh and dried figs are extensively consumed worldwide due to their organoleptic characteristics, important nutritional value, and natural sweetness [4]. In addition, in the last decade, production of fresh and dried figs has increased by 44% [5]. However, the high perishability of fresh fruit extremely limits the increase of area and production of this crop in the Mediterranean basin and further exportation to third countries. For this reason, the production of dried fig has been dramatically rising during the last years [5], since drying is a potential agricultural preservation technique, regardless of geographical and other challenges. Drying has proven to be a reliable preservation method for figs, in terms of technical feasibility and nutritional quality [6]. However, when temperature and duration of drying are not extremely controlled, as occurs in natural sun-drying, the hygienic-sanitary quality of figs may be affected.
Natural sun-drying has been practiced widely in tropical and subtropical countries since ancient times [7], with the main objective of ensuring the conservation of figs and extending their shelf life [8]. Apart from inconveniences caused by the uncontrolled temperature and time, the absence of meshes implies drying of figs on the ground, which in turn can lead to their infection by filamentous fungi [9]. The most predominant toxigenic fungi in dried figs are Aspergillus section Nigri, Aspergillus section Flavi, Fusarium spp., and Penicillium species [10,11,12]. Recently, some reports have also informed about the presence of Alternaria spp. in dried figs [13,14]. Some of these filamentous fungi may produce mycotoxins when the environmental factors, especially temperature and water activity (aw), are propitious [15,16,17]. In addition, other critical stages of dried fig processing to take into account are storage, and even during fruit retailing, since when figs are at this phase they are also susceptible to fungal colonisation and further mycotoxin production [11,18].
There are various mycotoxins found in figs including ochratoxin A (OTA), alternariol (AOH), tenuazonic acid (TeA), fumonisin B1, and aflatoxins [11,13,14,19,20,21]. Aflatoxins are the most important and with the highest prevalence found in figs. These mycotoxins have been found in dried figs from Turkey [11,19], Cyprus [22], and China [21]. Among the aflatoxins, aflatoxin B1 is recognized as one of the most potent carcinogens in foods and has been classed by the International Agency of Research for Cancer (IARC) in group 1A [23]. Due to the high toxicity of the aflatoxins and its high incidence in dried figs, the European Union has established maximum limits for aflatoxin contamination in this product at 6 μg/kg AFB1 and 10 μg/kg total aflatoxins (sum of AFB1, AFB2, AFG1, AFG2) [24].
In spite of these precedents, no investigations have yet been conducted about the ecophysiology of A. flavus, mould species producer of aflatoxins, in figs [7,25,26], under different environmental conditions occurring during fig processing. For this reason, this study is of great interest in order to investigate the capacity of A. flavus to grow and produce aflatoxins in a dry fig-based (DFB) medium from both phenotypic and genotypic points of view. These kinds of studies could pave the way to understand changes in the ecological status during the fig drying to comprehend the environmental conditions which favour the growth of A. flavus and aflatoxin production. Thus, the objective of this study was to evaluate the effect of temperature related to fig processing on growth, one of the regulatory genes of aflatoxin pathway (aflR) and mycotoxin production of A. flavus on a DFB agar at 0.96 aw.
2. Results
2.1. Selection of Two Aflatoxigenic Strains: Initial Screening
Initial experiments were performed using eleven A. flavus strains (M30, M42, M43, M55, M93, M111, M112, M115, M116, M144, and M148) to evaluate differences and similarities in their growth, lag time, and mycotoxin production capacity. For this, the A. flavus strains were inoculated on DFB agar 0.96 aw and incubated at 25 °C for 7 days.
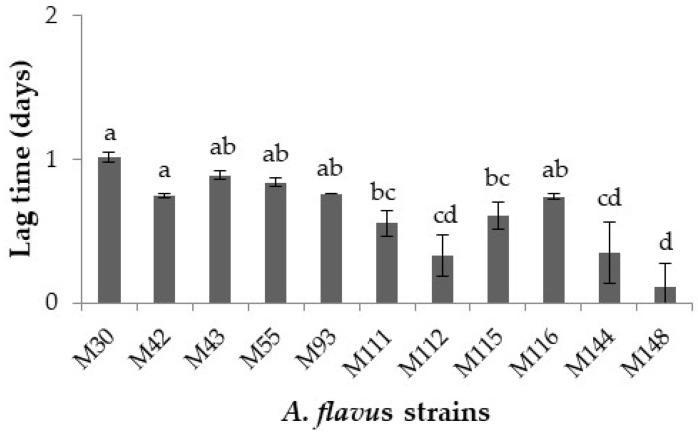
Figure 1 shows the combined effect of temperature, aw, and nutritional composition of the DFB agar on lag times prior to growth of the A. flavus strains tested. The lag times fluctuated between 0.11 (A. flavus M148) and 1.01 (A. flavus M30) days. Although it may appear that they were quite similar, some significant intra-strain differences (p ≤ 0.05) were found.
Figure 1.
Lag time prior to growth (days) of the 11 Aspergillus flavus strains over the 7 day incubation period. Different letters indicate significant differences (p ≤ 0.05).
Regarding the mean growth rates of the strains of A. flavus, they are displayed in Figure 2. Growth rates ranged from 5.15 (M115) to 6.49 (M43) mm radius/day. The strains M43, M55, and M93 grew faster than the remaining A. flavus strains checked, excluding the strain M30 (p ≤ 0.05). The strains M111, M112, M115, M116, and M148 showed the slowest growth of the strains evaluated.
Figure 2.
Growth rate (mm/day) of the 11 Aspergillus flavus strains over the 7 day incubation period. Different letters indicate significant differences (p ≤ 0.05).
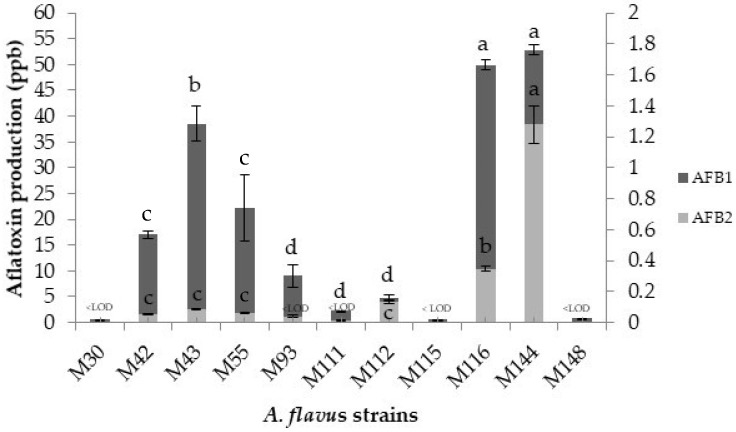
With respect to aflatoxin production by the A. flavus strains at the specific environmental and nutritional conditions evaluated, higher intra-strain differences compared to the other two parameters analysed (lag phase and growth rates) were observed. In Figure 3, it can be observed that, in general, all the strains produced much higher amounts of aflatoxin B1 than aflatoxin B2; even in three of the strains, no aflatoxin B2 production was detected above the limit of detection of the technique (M30, M115, and M148). Regarding the aflatoxin B1, the strains M30, M115, and M148 produced aflatoxin B1 quantities lower than 1 ppb. Three other strains (M93, M111, and M112) synthesised this mycotoxin at levels between 2 and 9 ppb, while the remaining 5 strains produced aflatoxin B1 quantities higher than 17 ppb, the strains M144 and M116 being the highest producers of this mycotoxin. With regard to aflatoxin B2, the maximum amount synthesised was 1.28 ppb by the strain M144. All the other A. flavus produced this mycotoxin at levels below 1 ppb.
Figure 3.
Aflatoxin production (ppb) of the 11 Aspergillus flavus strains over the 7 day incubation period. Different letters indicate significant differences for the same aflatoxins (p ≤ 0.05). * LOD means Limit of Detection.
Based on the results obtained, the strains A. flavus M30 and A. flavus M144 were selected to carry out a more detailed study to study the lag time, growth, aflatoxin contamination, and related gene expression of A. flavus in relation to ecophysiological parameters linked to dried-fig production. These two strains were selected based on their lowest and highest aflatoxin production of the 11 strains isolated from dried figs.
2.2. Effect of Temperature on Lag Times, Growth Rates, Mycotoxin Production and Aflatoxin-Related Gene Expression
2.2.1. Lag Times Prior to Growth
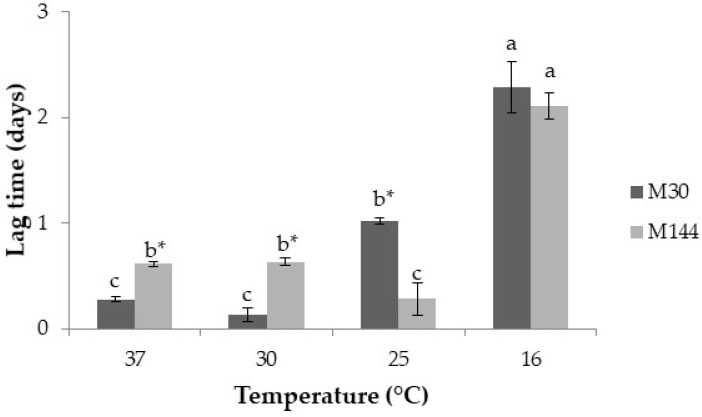
Figure 4 shows the effect of temperature related to the dried-fig processing on lag times prior to growth for both strains of A. flavus (M30 and M144). For both strains, no growth occurred at 5 °C. At the warmer temperatures tested (37 and 30 °C), A. flavus M30 had shorter lag phases than A. flavus M144, while at 25 °C, the latter showed the shortest lag time. At the lowest temperature evaluated, no differences were found between both strains. In addition, for both strains, the length of the lag phase rose substantially as temperature decreased.
Figure 4.
Lag time prior to growth (days) of the Aspergillus flavus M144 and A. flavus M30 at the different temperatures studied over the 12 days incubation period. Different letters indicate significant differences at the different temperatures for the same strain (p ≤ 0.05). Asterisk (*) means significant differences between both strains at the same temperature (p ≤ 0.05).
2.2.2. Growth
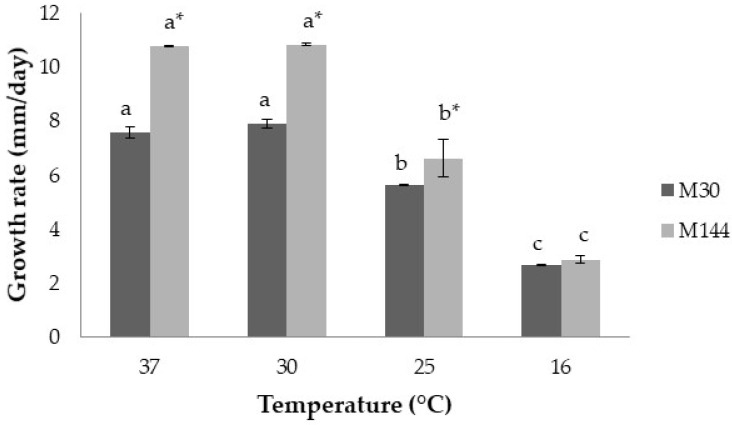
The influence of temperature on the growth of both strains of A. flavus is shown in Figure 5. A. flavus M144 grew faster than A. flavus M30 in most of the conditions tested (p ≤ 0.05), although no significant differences were found at 16 °C (p > 0.05). Optimum growth rates (≈11 and 8 mm/day for A. flavus M144, and A. flavus M30, respectively) were observed at 30 and 37 °C in both strains. Besides, no intra-strain differences were encountered at 30 and 37 °C. Furthermore, the growth of both strains declined as the temperature fell down.
Figure 5.
Growth rate (mm/day) of the Aspergillus flavus M144 and A. flavus M30 at the different temperatures studied over the 12 days incubation period. Different letters indicate significant differences at the different temperatures for the same strain (p ≤ 0.05). Asterisk (*) means significant differences between both strains at the same temperature (p ≤ 0.05).
2.2.3. Aflatoxin Production
Table 1 shows the effect of temperature on AFB1 and AFB2 production by A. flavus M144 and A. flavus M30 after 3, 5, 7, and 12 days of incubation. Neither AFB1 nor AFB2 was produced by the strain A. flavus M30 at the conditions and times evaluated (<LOD: Limit of Detection). Regarding the strain A. flavus M144, it produced much higher quantities of AFB1 than AFB2 in all the conditions tested. However, it should be emphasized that, in spite of the fact that there were differences regarding both mycotoxins produced by the strain A. flavus M144, the tendency was quite similar. Thus, maximum AFB1 and AFB2 production were detected at 25 °C at the four days tested; being detected in general higher amounts as the incubation period increased. At the remaining temperatures studied, AFB1 and AFB2 production was observed at 16 °C by day 12 of incubation.
Table 1.
Aflatoxin B1 and B2 production (ppb) of Aspergillus flavus M144. 1
| Aflatoxin | Days of Incubation | 37 °C | 30 °C | 25 °C | 16 °C |
|---|---|---|---|---|---|
| B1 | 12 | <LOD 2 | 0.25 ± 0.35a3 | 60.63 ± 7.70a1 | 10.15 ± 1.56a2 |
| 7 | <LOD | 0.03 ± 0.01c3 | 58.39 ± 1.93a1 | 0.10 ± 0.07b2 | |
| 5 | <LOD | 0.12 ± 0.04b3 | 2.68 ± 0.51b1 | 0.03 ± 0.01b2 | |
| 3 | <LOD | 0.02 ± 0.01c2 | 1.26 ± 0.83b1 | <LOD | |
| B2 | 12 | <LOD | <LOD | 0.02 ± 0.01b | <LOD |
| 7 | 0.10 ± 0.01a2 | 0.06 ± 0.00 2 | 0.15 ± 0.06a1 | 0.13 ± 0.01 1 | |
| 5 | 0.02 ± 0.01b | <LOD | <LOD | <LOD | |
| 3 | <LOD | <LOD | <LOD | <LOD |
1 The strain M30 did not produce detectable amounts of aflatoxin B1 and B2. 2 LOD: Limit of detection. Different letters along a column indicate significant differences at the different incubation times for the same temperature and for each aflatoxin (B1 and B2) (p ≤ 0.05). Different numbers along a row indicate significant differences at the different temperatures for the same incubation time and the same aflatoxin (p ≤ 0.05).
2.2.4. Gene Expression Studies
The effect of incubation days on aflR gene expression of A. flavus M144 at different temperatures is shown in Figure 6. The incubation temperature of 25 °C was used as a calibrator in this study. As shown in Figure 6, in the case of the expression of aflR gene is inhibited in most cases at temperatures of 16, 30, and 37 °C and all incubation times evaluated, with the exception of day 7 at 16 °C. In the case of the strain M30, no changes in the expression of the tested regulatory gene at the different temperatures evaluated regarding the control occurred (data not shown).
Figure 6.
Effect of the temperature on the expression of the aflR by Aspergillus flavus M144 at different incubation times. The calibrator corresponded to A. flavus when grown at 25 °C.
3. Discussion
This is the first study to examine the impact of temperature on growth, aflR gene expression, and aflatoxin production by A. flavus in a dry fig-based matrix. This species has been encountered in dried figs and can cause accumulation of aflatoxins in this commodity [7,9,11]. Özlüoymak [27] has reported that the critical period for A. flavus for starting to grow is when the ripening of the figs is occurring on the tree and it continues during the over-ripening period. Besides, environmental conditions occurring during processing of dried fig and storage when dried figs are launched into the market where temperatures are rarely controlled also favored growth and development of A. flavus. Once this species colonizes fig, it may synthesise aflatoxins, both on the surface and the inner part of the fig without damaging the skin [28]. It is thus important to understand the ecological conditions for growth, aflatoxin-related gene expression and aflatoxin production by this species in this matrix. This can be useful for targeting control strategies to minimize mycotoxin contamination within the HACCP framework in the dried fig industry.
At first, the growth behavior and mycotoxin synthesis ability of 11 A. flavus strains isolated from figs at a fixed temperature (25 °C) in a DFB agar were screened in order to further select 2 strains based on the initial results obtained for in-depth ecophysiological studies. The initial experiment results showed that there were relatively few interspecies significant differences on lag phase and growth, whilst this was not true for aflatoxin production. Regarding the two parameters related to mould growth, the lag phases ranged between 0.11 and 1 days, while mean growth rates varied from 5.15 to 6.49 mm/day. These values indicate that A. flavus starts to grow immediately on a DFB medium and the nutritional composition of this medium based on fig favors the rapid growth of this toxigenic species. This is supported by the comparison of the results of the present study with previous reports informing about the lag phases and growth of A. flavus in different food-based model systems. For instance, Peromingo et al. [29] demonstrated that two strains of A. flavus had little differences on both lag phase prior to growth and growth when growing on two dry-cured meat product-based medium at 25 °C. Casquete et al. [30] observed little differences between three strains of A. flavus at different aw in a cheese model system. With regard to aflatoxin synthesised by the 11 A. flavus strains, there were higher significant differences at strain level, varying aflatoxin B1 amounts produced between 0.6 and 50 ppb, while for aflatoxin B2 they were in the range from <LOD to 1.28 ppb. Previous studies have also shown differences in aflatoxin synthesis by various A. flavus strains at 25 °C in different media [29,31]. Besides, in general, they produced much higher quantities of aflatoxin B1 than aflatoxin B2 in DFB agar. In this study, two A. flavus strains were selected based on their mycotoxin production capacity, being the strains M144 (aflatoxin-producing strain) and M30 (non-aflatoxin-producing strain) used for examining the impact of temperature on growth, aflatoxin-related gene expression and mycotoxin production by A. flavus in DFB agar.
Temperature represents a key environmental factor in the growth and production of aflatoxins [32,33]. For this reason, five different temperatures, which were selected due to their importance during the drying, processing, and retailing of fig fruits, were assessed. For this: 5 °C represents the advisable household and industrial storage temperature; 16, 25 and 30 °C are common minima, average and maximum temperatures during harvest stage at night, respectively, and 37 °C represents extreme temperatures that can occur in the field during the harvest of the fruits (Extremadura a southwest Spanish region in the high summer season; http://redarexplus.gobex.es/RedarexPlus, accessed on 20 December 2020). In addition, 16 and 25 °C are usual intermediate ambient temperatures utilized by both consumers and producers to store dried figs. Also, 25 °C is the usual temperature in the dried fig postharvest. Finally, 37 °C also represents the optimum condition for A. flavus growth [32].
When studying the influence of temperature on growth parameters of the two selected A. flavus strains, overall, both strains were unable to grow at 5 °C over the 12 day incubation period of our experiments. These results are consistent with several investigations that suggest that growth at a temperature below 10 °C does not occur [29,34]. Regarding the other temperatures, despite some differences found between the two strains, in general, the lag phases were shorter and mean growth rates faster as temperature increased (p ≤ 0.05). These results are in accordance with those published by Mohale et al. [35], who investigated the growth of toxigenic and atoxigenic A. flavus strains at 20, 25 and 30 °C, and also with those published by Schmidt-Heydt et al. [32], who showed that the growth optimum for A. flavus was at 37 °C. Pitt and Miscamble [36] reported that the optimum temperature for A. flavus growth was 25 °C in the range from 0.96 to 0.98 aw, 30 °C at 0.985 aw and 37 °C at 0.96 aw. Other previous studies on A. flavus growth on groundnuts suggest aw optima of 0.94 aw at 34 °C [37]. Abdel-Hadi et al. [16] found that optimum growth of A. flavus was 0.99 aw and 35 °C on conducive YES medium. Surprisingly, the strain M144 (aflatoxin-producing strain) initiated its growth slightly later than the other strain tested (M30, non-aflatoxin-producing strain), but its mean growth rate was more rapid at temperatures warmer than 25 °C. Probably, in the case of the strain M144, the synthesis of aflatoxins itself would have been of great help for its adaptation and colonisation of the DFB agar. This phenomenon has been described before [38,39].
Findings from aflatoxins produced by the two strains of interest showed enormous differences at strain and species levels. The aflatoxin produced by both A. flavus at 5 °C was not tested since growth was not observed. The strain M30 did not produce aflatoxins either in temperature or incubation day evaluated. The strain M144 produced both aflatoxin B1 and aflatoxin B2, but the quantities produced of the most carcinogenic were much higher (p ≤ 0.05). As expected, the largest aflatoxin B1 and quantities detected were at 25 °C (p ≤ 0.05); however, also important amounts of such toxin would have been contemplated at 30 °C according to the results reported by Schmidt-Heydt et al. [32], who evaluated the effect of a wide range of aw and temperatures on A. flavus, although this was not observed in this work. At the warmest temperature checked (37 °C), no aflatoxin production was observed, while at 16 °C, at the end of the incubation time the strain synthesised aflatoxin B1 amounts > 10 ppb. These results correlate with those published with Schmidt-Heydt et al. [32]. In the same manner, aflatoxin B2 was more produced by this strain at 25 °C and later at 16 °C. So, it seems that the temperature enormously affects aflatoxin production by A. flavus independently of the substrate where the mould grows. In general, it should be emphasised that the amounts of aflatoxin found in the DFB agar are higher than those found in other culture media, food-based model systems, or food matrices [29,30,31]. The explanation may be that the preferred carbon sources for aflatoxin production are sugars [40], and dried figs provide a rich source of glucose and fructose [7]. Furthermore, the temperature of 25 °C and a 0.96 aw are optimal for the growth of A. flavus [41].
Regarding the assessment of the expression of the aflR gene of the strain M144, the major regulatory gene in the aflatoxin pathway, which activates the aflatoxin structural genes [42], it was observed that, in general, this gene expression was repressed throughout the incubation time and at any of the temperatures evaluated with respect to the calibrator (25 °C). This is in accordance with results obtained in the phenotypic mycotoxin production, where maximum amounts were found at 25 °C. These findings are reasonable since the aflR gene controls are well-correlated with aflatoxin production by A. flavus [32,43,44]. Unsurprisingly, a basal expression of the regulatory gene occurred with no differences between conditions checked in the case of the non-producing strain (A. flavus M30).
4. Conclusions
The effect of temperature during drying and storage of dried figs has a profound effect on lag times prior to growth, relative growth rates, aflR gene expression and aflatoxin production by strains of A. flavus isolated of such fruit. In general, the capacity of colonisation of the dried fig-based model system was similar to all the strains tested; however, their ability to produce aflatoxins varied between strains. Concretely, there are some important differences between the two selected A. flavus (M144, important producing-strain and M30, non-producing strain). Based on the results, mild temperatures and changes in temperature during drying and storage of dried figs should be avoided. Drying should be conducted at temperatures > 30 °C and close to 37 °C, while industry processing, storage, and retailing of dried figs are advisable to perform at refrigeration temperatures (<10 °C) to avoid mycotoxin production.
5. Material and Methods
5.1. Mould Strains
Eleven strains belonging to A. flavus previously isolated from dried figs (Ficus carica L.) from different geographical areas of Extremadura (a southwest region of Spain) were used in this study. Information about the isolate codes, origin, geographical area, and moisture content of the strains is shown in Table 2. Isolation of the strains was made following the protocol described by Ruiz-Moyano et al. [45]. For this, genomic DNA from the 11 moulds isolated was extracted with the quick-DNA Fungal/Bacterial Miniprep Kit (Zymo research) according to the manufacturer’s instructions. The ITS rDNA region was amplified using the primer pairs ITS1 and ITS4 described by White et al. [46]. PCR products were sequenced at the Facility of Bioscience Applied Techniques of SAIUEX (University of Extremadura, Spain) with the same primers used in the amplification steps. Sequencing was performed from both the 5′ and the 3′ ends of each PCR product. The obtained sequences were edited and assembled into a consensus sequence of the corresponding amplicon. To determine the closest known relatives of the obtained ITS rDNA sequences of the isolates, searches were performed against the NCBI nucleotide (nr/nt) database with the Basic Local Alignment Search Tool (BLAST) tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 4 January 2021). All sequences were separately analysed and > 95% similarity was used as the criterion for species identification. The isolates were maintained by regular subculturing in Potato Dextrose Agar (PDA) at 25 °C for 7 days and then kept at 4 °C for short-term storage until required.
Table 2.
Codes, geographical area, moisture content, and origin of the 11 strains of Aspergillus flavus used in the present study.
| Isolate Code | Geographical Area | Origin 1 | Moisture Content (%) |
|---|---|---|---|
| A. flavus M30 | South of Extremadura | Field | 16.78 |
| A. flavus M42 | South of Extremadura | Field | 16.78 |
| A. flavus M43 | South of Extremadura | Field | 16.39 |
| A. flavus M55 | South of Extremadura | Field | 16.78 |
| A. flavus M93 | South of Extremadura | Field | 20.46 |
| A. flavus M111 | South of Extremadura | Field | 20.46 |
| A. flavus M112 | South of Extremadura | Field | 19.01 |
| A. flavus M115 | South of Extremadura | Industry | 27.62 |
| A. flavus M116 | South of Extremadura | Industry | 27.62 |
| A. flavus M144 | North of Extremadura | Field | 36.20 |
| A. flavus M148 | South of Extremadura | Field | 16.39 |
1 Field or industry.
5.2. Culture Medium Preparation
DFB agar was prepared with 30 g of lyophilised dried fig which were added to 300 mL of deionised sterile water and blended with a hand mixer. The remaining deionised sterile water was added to complete 1 L and it was brought to a boil. Subsequently, 20 g of bacteriological agar (Pronadisa, Madrid, Spain) were added and mixed vigorously. The culture medium was sterilised by autoclaving at 121 °C for 20 min (103 KPa). After autoclaving, the DFB agar was shaken, and poured into 9 cm diameter Petri plates. The aw of the DFB agar was measured by using a Novasina LabMaster-aw meter (AG, Lachen, Switzerland).
5.3. Inoculum, Inoculation, and Experimental Settings
For inoculum preparation, the isolates were inoculated by spreading on PDA and incubated at 25 °C for 7 days. The spores of each mould isolate were collected using 10 mL deionised water containing 0.05% Tween 80 and rubbing the surface with a glass rod. The spore suspensions were quantified with the aid of a microscope (Olympus CX 400, Tokyo, Japan) and a Neubauer chamber before their adjustment to 106 spores/mL by diluting with deionised water to be used as inoculum. The spore suspensions were maintained for long-term storage at −80 °C in glycerol solution (50% v/v). New starter cultures were used for each experiment.
Firstly, an initial screening of the mould isolates were done. For this, DFB agar was centrally inoculated with 2 μL of the inoculum of each of the 11 mould isolates and incubated at 25 °C for a period of up to 7 days. The growth assessment and aflatoxin production were tested. The two isolates which obtained the highest (A. flavus M144) and the lowest (A. flavus M30) aflatoxin production were selected to carry out detailed studies on the relationship between ecophysiological factors, growth, gene expression, and aflatoxin contamination.
Secondly, the A. flavus M144 and M30, selected from the initial screening experiment, were 2-point inoculated on DFB agar with 2 μL of each inoculum for growth and aflatoxin production. For gene expression studies, sterile cellophane overlays (Packaging Limited, London, UK) were placed onto DFB agar before inoculation. The agar plates were incubated at 5, 16, 25, 30, and 37 °C for up to 12 days to simulate the wide range of conditions throughout the sun-drying process, industrial processing, storage, and retailing of dried figs. The aw of the medium kept constant during the experiment period. All experiments were done with three replicates per treatment and repeated once.
5.4. Lag Time Prior to Growth and Growth Assessment
Growth was daily recorded by measuring two right angles diameters. Data were analysed using a primary model by plotting colony diameter against time. Data plots showed, after a lag phase, a linear trend with time. The linear part of this graph (linear phase) was used to calculate growth rate (μ, mm/d) [47]. To calculate the lag times (days), the formula of the regression line was equalised to the original inoculum size (diameter, mm).
5.5. Gene Expression Analysis
5.5.1. Sampling and Sample Preparation
For gene expression analysis, samples from strains M144 and M30 were taken at 3, 5, and 7 days of incubation. All experiments were made in triplicate.
After each incubation time, the cellophane disks containing the whole colonies were collected under sterile conditions and quickly frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
5.5.2. RNA Extraction
For RNA extraction, frozen mycelia were ground to fine powder in a pre-frozen mortar and pestle. Next, approximately 50 mg of frozen mycelia were weighed in a sterile Eppendorf, and the RNA extraction was carried out using the SpectrumTM Plant Total RNA Kit (SigmaAldrich, St. Louis, MO, USA). The RNA concentration and purity (A260/A280 ratio) were determined spectrophotometrically using a 1.5 μL aliquot on a NanoDrop (Thermo Scientific™ NanoDrop 2000). Samples were diluted to a concentration of 0.1 μg/μL and treated with DNAse I (Thermo Fisher Scientific, Waltham, MA, USA) in order to remove genomic DNA. Then RNA was kept at −80 °C until reverse transcription (RT) reaction.
5.5.3. RT-qPCR Reactions and Relative Quantification
RT-qPCR assays were used to amplify the aflR gene as target gene, and the β-tubulin gene as endogenous gene.
Primers
The primer pair aflRtaq1/aflRtaq2 previously designed from the aflR gene associated with the aflatoxin biosynthesis pathway [43], and the primer pair F-TubJD/R-TubJD designed from the β-tubulin gene [43] were used.
-
2.
cDNA synthesis
The RT reaction was conducted by using 5 μL of total RNA (100 ng) according to the instructions of PrimeScript™ RT Reagent Kit (Takara Bio Inc., Kusatsu, Shiga, Japan). cDNA samples were stored at −20 °C for subsequent qPCR analysis.
-
3.
Real-time PCR reactions
The real-time PCR (qPCR) reactions were performed in the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the SYBR Green system. Reaction mixtures were dispensed into wells of MicroAmp Optical 96-Well Reaction Plates and sealed with optical adhesive covers (Applied Biosystems). Three replicates of a RNA control sample together with a template-free negative control were also included in the runs. The reaction mixture for each gene consisted of 7.5 μL NZY qPCR Green Master Mix 2x (NZYTech, Lisbon, Portugal), 300 nM of each primer and 2.5 μL of cDNA in a final volume of 12.5 μL. PCR reaction conditions included a first step of 10 min at 95 °C, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. After the final PCR cycle, the melting curve of the PCR products was analysed according to the following protocol: slow ramp between 60 and 95 °C in 0.5 °C increments for 5 s. The value of the quantification cycle (Cq), which corresponds to the intersection between each fluorescence curve and a threshold line was automatically calculated by the 7300 Fast System Software (Applied Biosystems). Three technical repetitions were made.
-
4.
Relative gene expression
Relative quantification of the expression of the aflR gene expression was calculated following the 2−ΔΔCT method [48]. The β-tubulin gene was used as the endogenous control to normalise the quantification of the cDNA target added to each reaction. The calibrator corresponded to A. flavus when grown at 25 °C, a usual temperature in the dried fig postharvest, storage, and harvesting.
5.6. Mycotoxin Analysis
5.6.1. Sampling and Sample Preparation
After 3, 5, 7, and 12 days of incubation, the agar plates containing the whole colonies were immediately stored at −20 °C until use. Aflatoxin content could not be determined at 5 °C since no growth of A. flavus occurred.
5.6.2. Aflatoxin Extraction and Quantification
All solvents used for aflatoxin were HPLC grade and purchased from Thermo Fisher Scientific (Runcorn, UK). The isolation and purification of aflatoxins was conducted following the method described by Rodríguez et al. [49]. Then, the dry extracts were redissolved in 1 mL of HPLC-grace acetonitrile (Fisher Scientific) and filtered through a 0.22 PTFE membrane filter, in vials for quantification. The aflatoxin analysis was performed using an Agilent 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a FLD detector (Agilent G1321A) fitted at 360 nm and using a C18 HPLC column (250 × 4.6 mm, 5 μm particle size; Supelco, Bellefonte, PA, USA). The injection volumen was 100 μL and the flow rate was 1 mL/min. The mobile phase used for the separation contained HPLC grade water (solvent A) and HPLC grade acetonitrile (solvent B), in a gradient mode established from 15% B in the initial phase to 100% B after 30 min. Standard curves for calibration purpose were performed using standards of aflatoxin B1 and B2 acquired from Sigma-Aldrich.
5.7. Statistical Análisis
Data on lag phase, growth rates, aflR gene expression and toxin production were tested for normality using the Shapiro–Wilk test. A statistical analysis of the parameters was performed using one-way ANOVA. The differences among means values were separated by Tukey’s honestly significant difference test (p ≤ 0.05) in SPSS for Windows version 21.0.
Acknowledgments
Mariano Cabrera and Juan Hernández-Barneto are acknowledged for their excellent technical support.
Author Contributions
Conceptualization, A.R. and M.d.G.C.; formal analysis, A.I.G. and A.M-D.; funding acquisition: M.d.G.C.; investigation: A.I.G. and A.M.-D.; methodology: A.I.G., A.R., A.M., M.d.G.C.; project administration: M.d.G.C.; supervision: A.R. and M.d.G.C.; validations: A.I.G., A.R., A.M. and M.J.S.; visualization: A.I.G., A.R. and A.M., resources: M.d.G.C.; writing—original draft preparation: A.I.G. and A.R.; writing—review and editing: A.I.G., A.R., A.M., M.J.S., A.M.-D. and M.d.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by INIA-EAI (Instituto Nacional de Investigaciones Agrarias y Alimentarias- Agencia Estatal de Investigación, Spain), Grant number RTA2017-00032-C02-01 and by the Junta de Extremadura (Spain); and the European Regional Development Fund, Grant number GR15166. A.I. Galván is a recipient of a fellowship from the Spanish Ministerio de Economía, Industria y Competitividad [BES-2017-079830].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Key Contribution
Correct management and control of temperature during drying, storage, industrial processing, and fruit retailing avoid infection of dried fig by A. flavus and their toxic metabolites (aflatoxins).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arvaniti O.S., Samaras Y., Gatidou G., Thomaidis N.S., Stasinakis A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019;119:244–267. doi: 10.1016/j.foodres.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Ouchemoukh S., Hachoud S., Boudraham H., Mokrani A., Louaileche H. Antioxidant activities of some dried fruits consumed in Algeria. LWT. 2012;49:329–332. doi: 10.1016/j.lwt.2012.07.022. [DOI] [Google Scholar]
- 3.Vallejo F., Marín J.G., Tomás-Barberán F.A. Phenolic compound content of fresh and dried figs (Ficus carica L.) Food Chem. 2012;130:485–492. doi: 10.1016/j.foodchem.2011.07.032. [DOI] [Google Scholar]
- 4.FAOSTAT. [(accessed on 20 December 2020)]; Available online: http://www.fao.org/faostat/es/
- 5.Encuesta sobre Superficies y Rendimientos Cultivos (ESYRCE) [(accessed on 20 December 2020)]; Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/
- 6.Mat Desa W.N., Mohammad M., Fudholi A. Review of drying technology of fig. Trends Food Sci. Technol. 2019;88:93–103. doi: 10.1016/j.tifs.2019.03.018. [DOI] [Google Scholar]
- 7.Gilbert J., Senyuva H. Fungal and mycotoxin contamination of dried figs-a review. Mycotoxins. 2008;58:73–82. doi: 10.2520/myco.58.73. [DOI] [Google Scholar]
- 8.Nuroğlu E., Öz E., Bakırdere S., Bursalıoğlu E.O., Kavanoz H.B., İçelli O. Evaluation of magnetic field assisted sun drying of food samples on drying time and mycotoxin production. Innov. Food Sci. Emerg. Technol. 2019;52:237–243. doi: 10.1016/j.ifset.2019.01.004. [DOI] [Google Scholar]
- 9.Trucksess M.W., Scott P.M. Mycotoxins in botanicals and dried fruits: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008;25:181–192. doi: 10.1080/02652030701567459. [DOI] [PubMed] [Google Scholar]
- 10.Turkoz Bakirci G. Investigation of aflatoxins levels in commercial dried figs from western Turkey. Int. Food Res. J. 2020;27:245–251. [Google Scholar]
- 11.Dilek Heperkan Z., Moretti A., Daskaya Dikmen C., Heperkan D., Moretti A., Dikmen C.D., Logrieco A.F. Toxigenic fungi and mycotoxin associated with figs in the Mediterranean area mycotoxins and biosynthetic genes View project prevention of Cronobacter sakazakii in baby foods View project Toxigenic fungi and mycotoxin associated with figs in the Mediterran. Phytopathol. Mediterr. 2012;51:119–130. [Google Scholar]
- 12.Javanmard M. Occurrence of Mould Counts and Aspergillus Species in Iranian Dried Figs at Different Stages of Production and Processing. J. Agric. Sci. Technol. 2010;12:331–338. [Google Scholar]
- 13.López P., Venema D., Mol H., Spanjer M., de Stoppelaar J., Pfeiffer E., de Nijs M. Alternaria toxins and conjugates in selected foods in the Netherlands. Food Control. 2016;69:153–159. doi: 10.1016/j.foodcont.2016.04.001. [DOI] [Google Scholar]
- 14.López P., Venema D., de Rijk T., de Kok A., Scholten J.M., Mol H.G.J., de Nijs M. Occurrence of Alternaria toxins in food products in The Netherlands. Food Control. 2016;60:196–204. doi: 10.1016/j.foodcont.2015.07.032. [DOI] [Google Scholar]
- 15.Aldars-García L., Marín S., Sanchis V., Magan N., Medina A. Assessment of intraspecies variability in fungal growth initiation of Aspergillus flavus and aflatoxin B1 production under static and changing temperature levels using different initial conidial inoculum levels. Int. J. Food Microbiol. 2018;272:1–11. doi: 10.1016/j.ijfoodmicro.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Hadi A., Schmidt-Heydt M., Parra R., Geisen R., Magan N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface. 2012;9:757–767. doi: 10.1098/rsif.2011.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner P.C., Sylla A., Gong Y.Y., Diallo M.S., Sutcliffe A.E., Hall A.J., Wild C.P. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: A community-based intervention study. Lancet. 2005;365:1950–1956. doi: 10.1016/S0140-6736(05)66661-5. [DOI] [PubMed] [Google Scholar]
- 18.Devreese M., De Baere S., De Backer P., Croubels S. Quantitative determination of the Fusarium mycotoxins beauvericin, enniatin A, A1, B and B1 in pig plasma using high performance liquid chromatography–tandem mass spectrometry. Talanta. 2013;106:212–219. doi: 10.1016/j.talanta.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 19.Food and Feed Safety Alerts (RASFF) [(accessed on 20 December 2020)]; Available online: https://ec.europa.eu/food/safety/rasff_.
- 20.Zohri A.A., Abdel-Gawad K.M. Survey of mycoflora and mycotoxins of some dried fruits in Egypt. J. Basic Microbiol. 1993;33:279–288. doi: 10.1002/jobm.3620330413. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.J., Nie J.Y., Yan Z., Li Z.X., Cheng Y., Farooq S. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agric. 2018;17:1676–1690. doi: 10.1016/S2095-3119(18)61966-5. [DOI] [Google Scholar]
- 22.Ioannou-Kakouri E., Aletrari M., Christou E., Ralli A., Koliou A. Occurrence of mycotoxins in local food in Cyprus. COST Action. 2001;835:13–18. [Google Scholar]
- 23.International Agency for Research on Cancer (IARC) [(accessed on 20 December 2020)]; Available online: https://www.iarc.fr/
- 24.Diario Oficial de la Unión Europea Reglamento (UE) No 1058/2012 de la Comisión. [(accessed on 20 December 2020)]; Available online: https://www.boe.es/doue/2012/313/L00014-00015.pdf.
- 25.Drusch S., Aumann J. Mycotoxins in Fruits: Microbiology, Occurrence, and Changes during Fruit Processing. Adv. Food Nutr. Res. 2005;50:33–78. doi: 10.1016/s1043-4526(05)50002-0. [DOI] [PubMed] [Google Scholar]
- 26.Molyneux R.J., Mahoney N., Kim J.H., Campbell B.C. Mycotoxins in edible tree nuts. Int. J. Food Microbiol. 2007;119:72–78. doi: 10.1016/j.ijfoodmicro.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Özlüoymak Ö.B. Development of a UV-based imaging system for Real-Time detection and separation of dried figs contaminated with aflatoxins. J. Agric. Sci. 2014;20:302–316. doi: 10.15832/tbd.87873. [DOI] [Google Scholar]
- 28.Ait Mimoune N., Arroyo-Manzanares N., Gámiz-Gracia L., García-Campaña A.M., Bouti K., Sabaou N., Riba A. Aspergillus section Flavi and aflatoxins in dried figs and nuts in Algeria. Food Addit. Contam. Part B Surveill. 2018;11:119–125. doi: 10.1080/19393210.2018.1438524. [DOI] [PubMed] [Google Scholar]
- 29.Peromingo B., Rodríguez A., Bernáldez V., Delgado J., Rodríguez M. Effect of temperature and water activity on growth and aflatoxin production by Aspergillus flavus and Aspergillus parasiticus on cured meat model systems. Meat Sci. 2016;122:76–83. doi: 10.1016/j.meatsci.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Casquete R., Benito M.J., Córdoba M.D.G., Ruiz-Moyano S., Martín A. The growth and aflatoxin production of Aspergillus flavus strains on a cheese model system are influenced by physicochemical factors. J. Dairy Sci. 2017;100:6987–6996. doi: 10.3168/jds.2017-12865. [DOI] [PubMed] [Google Scholar]
- 31.Bernáldez V., Córdoba J.J., Magan N., Peromingo B., Rodríguez A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT Food Sci. Technol. 2017;83:283–291. doi: 10.1016/j.lwt.2017.05.030. [DOI] [Google Scholar]
- 32.Schmidt-Heydt M., Abdel-Hadi A., Magan N., Geisen R. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 2009;135:231–237. doi: 10.1016/j.ijfoodmicro.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Abbas H.K., Wikinson J.R., Zablotowicz R.M., Accinelli C., Abel C.A., Bruns H.A., Weaver M.A. Ecology of Aspergillus flavus, regulation of aatoxin production, and management strategies to reduce aatoxin contamination of corn. Toxin Rev. 2009;28:142–153. doi: 10.1080/15569540903081590. [DOI] [Google Scholar]
- 34.Mousa W., Ghazali F.M., Jinap S., Ghazali H.M., Radu S. Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. J. Appl. Microbiol. 2011;111:1262–1274. doi: 10.1111/j.1365-2672.2011.05134.x. [DOI] [PubMed] [Google Scholar]
- 35.Mohale S., Magan N., Medina A. Comparison of growth, nutritional utilisation patterns, and niche overlap indices of toxigenic and atoxigenic Aspergillus flavus strains. Fungal Biol. 2013;117:650–659. doi: 10.1016/j.funbio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Pitt J.I., Miscamble B.F. Water Relations of Aspergillus flavus and Closely Related Species. J. Food Prot. 1995;58:86–90. doi: 10.4315/0362-028X-58.1.86. [DOI] [PubMed] [Google Scholar]
- 37.Sanchis V., Magan N. Environmental conditions affecting mycotoxins. Mycotoxins Food. 2004:174–189. doi: 10.1533/9781855739086.2.174. [DOI] [Google Scholar]
- 38.Geisen R., Touhami N., Schmidt-Heydt M. Mycotoxins as adaptation factors to food related environments. Curr. Opin. Food Sci. 2017;17:1–8. doi: 10.1016/j.cofs.2017.07.006. [DOI] [Google Scholar]
- 39.Delgado J., Rodríguez A., García A., Núñez F., Asensio M. Inhibitory Effect of PgAFP and Protective Cultures on Aspergillus parasiticus Growth and Aflatoxins Production on Dry-Fermented Sausage and Cheese. Microorganisms. 2018;6:69. doi: 10.3390/microorganisms6030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gourama H., Bullerman L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic Fungi of Concern in Foods and Feeds. J Food Prot. 1995;58:1395–1404. doi: 10.4315/0362-028X-58.12.1395. [DOI] [PubMed] [Google Scholar]
- 41.Martín Castaño S., Medina A., Magan N. Comparison of dry matter losses and aflatoxin B1 contamination of paddy and brown rice stored naturally or after inoculation with Aspergillus flavus at different environmental conditions. J. Stored Prod. Res. 2017;73:47–53. doi: 10.1016/j.jspr.2017.06.004. [DOI] [Google Scholar]
- 42.Chang P.K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genom. 2003;268:711–719. doi: 10.1007/s00438-003-0809-3. [DOI] [PubMed] [Google Scholar]
- 43.Peromingo B., Rodríguez M., Delgado J., Andrade M.J., Rodríguez A. Gene expression as a good indicator of aflatoxin contamination in dry-cured ham. Food Microbiol. 2017;67:31–40. doi: 10.1016/j.fm.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Accinelli C., Abbas H.K., Zablotowicz R.M., Wikinson J.R. Aspergillus flavus aflatoxin occurrence and expression of aflatoxin biosynthesis genes in soil. Can J. Microbiol. 2008;54:371–379. doi: 10.1139/W08-018. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Moyano S., Benito M.J., Martín A., Aranda E., Hernández A., Córdoba M.G. Characterization of molds isolated from smoked paprika by PCR-RFLP and micellar electrokinetic capillary electrophoresis. Food Microbiol. 2009;26:776–782. doi: 10.1016/j.fm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.White T.J., Bruns T., Lee S.J., Taylor J. PCR Protocols: A Guide to Methods and Applications. Academic Press; Cambridge, MA, USA: 1990. 38-Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- 47.Rodríguez A., Medina Á., Córdoba J.J., Magan N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014;178:113–119. doi: 10.1016/j.ijfoodmicro.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez A., Rodríguez M., Luque M.I., Martín A., Córdoba J.J. Real-time PCR assays for detection and quantification of aflatoxin producing molds in foods. Food Microbiol. 2012;31:89–99. doi: 10.1016/j.fm.2012.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.