Abstract
Presently, water quantity and quality problems persist both in developed and developing countries, and concerns have been raised about the presence of emerging pollutants (EPs) in water. The circular economy provides ways of achieving sustainable resource management that can be implemented in the water sector, such as the reuse of drinking water treatment sludges (WTSs). This study evaluated the potential of WTS containing a high concentration of activated carbon for the removal of two EPs: the steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2). To this end, WTSs from two Portuguese water treatment plants (WTPs) were characterised and tested for their hormone adsorbance potential. Both WTSs showed a promising adsorption potential for the two hormones studied due to their textural and chemical properties. For WTS1, the final concentration for both hormones was lower than the limit of quantification (LOQ). As for WTS2, the results for E2 removal were similar to WTS1, although for EE2, the removal efficiency was lower (around 50%). The overall results indicate that this method may lead to new ways of using this erstwhile residue as a possible adsorbent material for the removal of several EPs present in wastewaters or other matrixes, and as such contributing to the achievement of Sustainable Development Goals (SDG) targets.
Keywords: emerging pollutants, water treatment sludges, adsorption processes, circular economy
1. Introduction
One of the most prominent problems affecting the world’s population is insufficient access to clean water and sanitation. According to Sustainable Development Goal (SDG) 6 “clean water and sanitation” of the Synthesis Report on Water and Sanitation [1], over two billion people are living in countries that are experiencing high water stress conditions. This situation is a result of an overuse of water resources with significant impacts on their sustainability. Concurrently, water quality problems persist in water bodies of both developed and developing countries, such as the loss of the pristine quality conditions, changes in hydromorphological characteristics and an increase in concentrations of emerging pollutants (EPs) [1]. The clearest link between water and the circular economy is to consider drinking water and wastewater treatment plants as resource recovery installations, stimulating the recovery and valorisation of treated water and wastewater materials [2]. The circular economy concept provides ways of advancing towards sustainable water resource management that can be implemented in the water sector to achieve circularity between drinking water and wastewater resources [3].
Conventional wastewater treatment plants (WWTPs) are not entirely effective in the removal of EPs from wastewater (WW) since they were conceptually designed for the removal of macropollutants such as nutrients, suspended solids, pathogenic microorganisms and trace elements. Therefore, EPs such as pharmaceuticals and endocrine disrupting compounds (EDCs) may go through the treatment system unchanged or are only partially removed, leading to their detection in WW-receiving water bodies and WWTP discharges in concentrations ranging from ng/L to mg/L [4,5,6,7,8]. EDCs may lead to the modification of the natural function of the endocrine system in wildlife by (i) blocking or copying the normal effect of hormones; (ii) affecting their synthesis or metabolism; and (iii) changing hormone receptor levels [8,9,10,11]. Among EDCs, estrogens such as 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) have often been indicated as particularly problematic compounds with high associated risks [12,13,14]. E2 is a natural steroid hormone, which is secreted by humans and animals. The synthetic steroid hormone EE2 is based on the natural estrogen E2, which is used in oral contraceptives and hormone replacement therapies. These estrogens are largely excreted by humans and animals through urine and faeces and end up in the environment mainly through the discharge of WWTP effluents and the disposal of animal waste [10,15,16]. Secondary treatment is not effective in the removal of both of these compounds, especially for EE2 due to its recalcitrant nature. As such, it is known that WWTP final discharges are the main source of both E2 and EE2 in the aquatic environment. These chemicals have the potential to bioaccumulate and enter the food chain, posing ecotoxicity to aquatic organisms and implying risks to aquatic ecosystems. Several studies have reported the impact of E2 and EE2 on fish life, such as feminising male fish, reducing testicle size, reducing reproductive fitness, lowering sperm count, inducing the reproduction of vitellogenin, altering other reproductive characteristics and causing behavioural changes [9,17,18]. The uptake by fish and the presence of these compounds in the raw water that is used to produce drinking water to supply human communities also have impacts on human life and have previously been reported. The consumption of these compounds at concentrations above the safety thresholds can increase the risk of cancer and induce cardiovascular diseases [9,16,17].
Several technical solutions for EP removal have previously been developed that allow for their integration with existing treatment processes in an expedient way [7,19]. However, most of the methods are not techno-economically viable for large-scale implementation, and thus, comprehensive research is necessary to develop suitable, low cost, eco-friendly and efficient technologies to remove different kinds of EPs from WW [8,19,20,21,22]. The adsorption process with activated carbon (AC) is considered by many authors to be one of the most promising treatment processes with high EP removal capacity, mainly because (i) it is simple to design and operate; (ii) it has a low investment cost; (iii) it allows reuse and regeneration; and (iv) it does not generate toxic by-products [8,19,21,22,23,24]. Several studies have also already demonstrated that pure AC is able to effectively remove and lower the toxicity of E2 and EE2 in distilled water, drinking water and WW [25,26,27,28,29,30,31,32,33,34,35]. For example, Gökçe and Arayici [33] obtained a removal rate for E2 of 88% with AC produced from sewage sludge. In this referred work [33], the sludges were modified and submitted to several procedures in order to obtain sludge-based adsorbents.
Sludge-based adsorbents have been reported in the literature for the removal of several pollutants from water treatment plants (WTPs) and WWTPs [36,37]. Research efforts, however, have focused on their removal efficacy for compounds like heavy metals [38,39,40,41,42,43], dyes [44,45,46], phenols [47,48], phosphorus and phosphate [49,50,51,52] and antibiotics [53]. Consequently, the adsorbent potential of sludge for compounds like E2 and EE2 remains unknown. Clara et al. [54] tested the capacity of sludge from WWTP (without AC content) to adsorb E2 and EE2 in the mg/L range and noted a high adsorption potential of the tested sludge.
Drinking water treatment plant sludges (WTSs) have been recycled as aggregates, soil improvement agents and environmental remediation materials. The use of WTSs as an adsorbent in WW treatment is related to their high concentration of amorphous aluminium and ferric ions. These ions have a high affinity to phosphors and heavy metals through ion-exchange and complexation mechanisms [55,56,57,58]. The first report indicating the potential use of WTS containing AC was made by Lee et al. [58]. They evaluated the possibility of regenerating the AC and coagulants present in WTS via pyrolysis to produce multifunctional remediation material for the removal of pollutants present in WW.
Considering (i) the capacity of AC to remove EPs in an adsorbent process and (ii) the possibility to reuse WTS for several applications, it is envisaged that there is a potential use of WTS containing AC for the removal of EPs. As detailed above, however, current knowledge in this field remains limited. The main goal of this study was therefore to evaluate the potential of two unmodified WTSs with high content of activated carbon, without reactivation, as an adsorbent for the removal of two selected EPs, namely, E2 and EE2. No additional AC was incorporated in the sludges used nor was any kind of sludge treatment/modification made (including an AC reactivation step). Consequently, the AC content in the test sludges only had its origin from the conventional liquid treatment phase in the drinking WTP process. The elemental and mineral composition, ash content, pH at the point of zero charge (pHpzc), thermogravimetric analysis and textural characterisation of the sludges were determined. The removal potential of the two hormones by the sludges was also assessed, allowing an evaluation of the circularity of this residue and thus the possibility of transforming it into a new adsorbent material.
2. Results and Discussion
2.1. Water Treatment Sludge Characterisation
The elemental analysis, ash content, pHpzc and textural parameters of the two WTSs are presented in Table 1. WTS samples presented high ash contents: 42.9% for WTS1 and 30.9% for WTS2, which was expected given their origin. Nevertheless, these values are slightly lower than those observed by Lee et al. [58], who reported an ash content of around 51.0% for sludge obtained in South Korea. The variation between the ash content of these WTSs could be explained by the different reagents used in the treatment line and therefore the different mineral content. In fact, the presence of some mineral elements could be important because they may promote the formation of strong interactions with organic pollutants and as such promote their removal from water [58,59,60].
Table 1.
Elemental analysis, ash content, pH at the point of zero charge (pHpzc) and textural parameters of the selected drinking water treatment sludges (WTSs). The values presented for the ash content correspond to the mean of duplicates (, n = 2).
| Parameter | WTS1 | WTS2 |
|---|---|---|
| C (w/w%) | 24.31 | 34.09 |
| N (w/w%) | 0.24 | 0.46 |
| H (w/w%) | 2.64 | 2.92 |
| S (w/w%) | 0.16 | 0.18 |
| O (w/w%) * | 29.78 | 31.49 |
| Ash (w/w%) | 42.9 ± 0.2 | 30.8 ± 0.8 |
| Surface area (m2/g) | 127 | 318 |
| Vtotal (cm3/g) | 0.065 | 0.161 |
| pHpzc | 11.29 | 7.46 |
* Obtained by difference (%O = 100 − %C − %H − %N − %S − %Ashes).
The higher content of carbon observed in WTS2 as compared to WTS1 indicates that WTS2 may have a higher incorporation of activated carbon in its composition. The higher carbon content is reflected in the lower ash content of WTS2 and consequently in a higher surface area and porosity (Table 1). On the other hand, since WTS1 presented a higher ash content, it has a lower surface area given that the ash may block the pores in the sludge [58,61]. The pHpzc is directly related to the ash content of the samples: WTS1 is the more alkaline sample because it presented the higher mineral content. WTS2, on other hand, had a more neutral pHpzc, which indicates that WTS2 is more similar to alum sludge [57,62]. According to these results, WTS1 and WTS2 surfaces were positively charged in water solutions with a pH of below 11.29 and 7.46, respectively (Table 1).
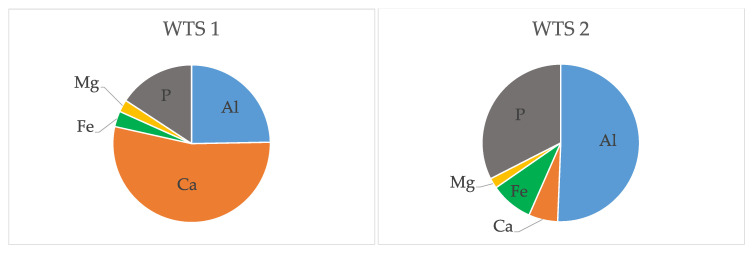
The results from the mineral analysis (Table 2) demonstrate that the elements present in higher concentrations in WTS2 were aluminium (Al; 5.4%) and phosphorous (P; 3.5%), followed by iron (Fe; 1%) and calcium (Ca; 0.6%), as visualised in Figure 1. For WTS1, the elements with the highest concentrations were calcium (Ca; 11.5%), followed by aluminium (Al; 5.3%), phosphorous (P; 3.4%) and iron (Fe; 0.7%) (Table 2; Figure 1).
Table 2.
Mineral composition of drinking water treatment sludge (WTS) samples. The values presented correspond to the mean of replicates (, n = 2)
| Mineral Composition (mg/kg) | WTS1 | WTS2 |
|---|---|---|
| Al | 53,000 ± 800 | 54,450 ± 5 350 |
| As | 37.4 ± 0.9 | 65.6 ± 7.9 |
| Ca | 115,350 ± 1650 | 6429.54 ± 733.41 |
| Cd | n.d. | n.d. |
| Cr | n.d. | n.d. |
| Cu | 4.1 ± 0.4 | 10.5 ± 1.1 |
| Fe | 6890.5 ± 502.9 | 9442.5 ± 1328.5 |
| Hg | n.d. | n.d. |
| K | 275.8 ± 10.7 | 119.9 ± 13.0 |
| Mg | 5424.0 ± 112.0 | 2280.3 ± 458.7 |
| Mn | 468.6 ± 19.0 | 150.4 ± 14.1 |
| Mo | 5.3 ± 0.1 | 5.2 ± 0.6 |
| Na | 16.1 ± 0.3 | 5.4 ± 0.5 |
| Ni | n.d. | n.d. |
| P | 33,817.6 ± 588.5 | 34,947.3 ± 4242.3 |
| Pb | 13.1 ± 0.2 | 14.1 ± 2.6 |
| Sb | 37.0 ± 0.6 | 37.4 ± 3.8 |
| Se | 38.4 ± 0.2 | 41.3 ± 3.8 |
| Zn | 21.8 ± 1.4 | 15.5 ± 1.4 |
n.d.—not detected.
Figure 1.
Mineral composition of drinking water treatment sludge (WTS) samples in w/w% for the major elements.
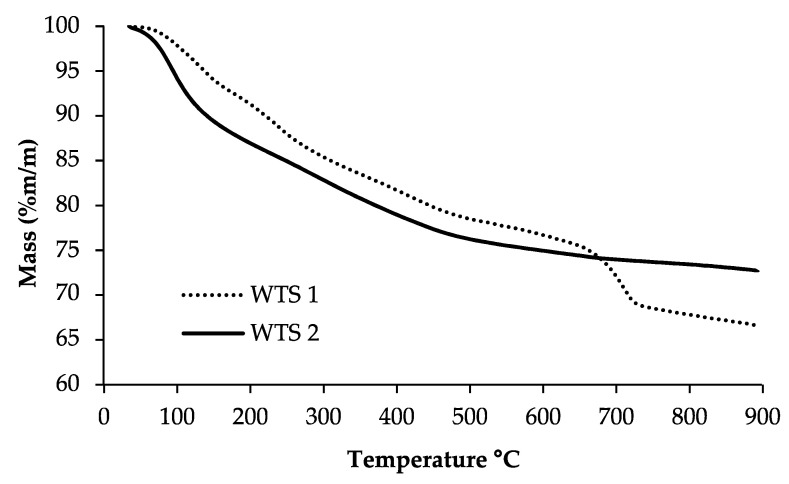
The main difference in mineral composition between the two WTSs was related to the calcium content (Table 2), which could be explained by the addition of insoluble limestone residues in the final stage of the sludge treatment of WTS1. To understand the thermal behaviour of WTS during an activation/regeneration process, it is fundamental to study their thermal decomposition through thermogravimetric analysis (TGA), which is presented in Figure 2.
Figure 2.
Thermogravimetric analysis (TGA) curves for the two water treatment sludges (WTSs) evaluated.
During the TGA analysis, the total mass loss was 34% and 27% for WTS1 and WTS2, respectively. These values may suggest that, through a regeneration/activation process, a significant mass was lost [61]. Both samples presented weight loss associated with water loss at 105 °C: WTS2 humidity was 6.5%, while for WTS1, this was 2.5%. WTS1 also presented a significant mass loss above 700 °C, which could be related to the decomposition of CaCO3 [59,63]. In fact, WTS1 was enriched with calcium that was probably present as carbonates.
2.2. Removal of Emerging Pollutants from Water
The removal efficiency experiment of the adsorbates with the WTS adsorbents was performed by using a dosage of 5 g WTS/L. This dosage was based on the concentrations used by Lee et al. [58] in their adsorption experiment, which were 4, 5 and 20 g/L.
The overall removal efficiency for both hormones was above 50% in both WTSs (Table 3). These results are in line with the removal efficiencies reported in the studies by Yoon et al. [30], Fuerhacker et al. [31], Gökçe and Arayici [33] and Rowsell et al. [32]. It should be noted, however, that these previous studies were performed using pure AC, with higher surface areas and pore volumes. Thus, few studies have been conducted to date using raw (unmodified) WTS (with or without an activation process) for the removal of EPs, as done in the present study. Evidently, such studies would be useful to meet the SDG targets and circular economy commitments.
Table 3.
Final concentrations of the emerging pollutants in the two water treatment sludges (WTSs) evaluated.
| Compound | Initial Concentration (ng/L) | WTS1 | WTS2 |
|---|---|---|---|
| Final Concentration (ng/L) | Final Concentration (ng/L) | ||
| E2 | 600 ± 200 (500 (2)) | 60 ± 20 | <LOQ (1) |
| 400 ± 100 (350 (2)) | 80 ± 30 | <LOQ (1) | |
| 300 ± 100 (200 (2)) | <LOQ (1) | <LOQ (1) | |
| EE2 | 600 ± 200 (500 (2)) | 150 ± 60 | <LOQ (1) |
| 400 ± 100 (350 (2)) | 170 ± 70 | <LOQ (1) | |
| 300 ± 100 (200 (2)) | 100 ± 40 | <LOQ (1) |
(1) LOQ value—50 ng/L; (2) Initial target concentration.
The removal efficiency in WTS2 was over 90% (for both E2 and EE2) at any of the initial hormone concentrations tested (Table 3). This result is comparable to the one presented by Yoon et al. [30] using virgin powdered activated carbon (PAC), who achieved a removal efficacy of 99% for both E2 and EE2 with a contact time of 24 h. Ifelebuegu et al. [34] also achieved a 96% removal efficacy of EE2 with 0.1 g/L wood-based granular activated carbon (GAC). Regarding sludge-based carbons, Clara et al. [54] used activated and raw sludge from a WWTP to evaluate the adsorption capacity of E2 and EE2. These authors concluded that both compounds showed a high adsorption affinity to the adsorbent, and within a contact time of 24 h, no difference between activated and inactivated sludge was detected. Some authors have previously suggested that the octanol/water partition coefficient (Log Kow) values for estrogenic compounds, which vary between 2.5 and 4.0, can roughly predict the sorption behaviour. E2 and EE2 are often cited as moderately hydrophobic and to have a tendency to adsorb to the solid phase [9,30,32]. These properties could thus indicate an easy interaction with the adsorbent [35]. The pH value of the test matrix is also an important factor that affects the adsorption capacity of sludge as an adsorbent [37]. In this preliminary study, the pH of the solution with distilled water and hormones was around 3. Previous studies have demonstrated that adsorption is higher at acidic pH and that the highest adsorption of E2 and EE2 occurs at neutral pH (6-7) [25]. Both E2 and EE2 are protonated (with pKa 10.4 and 10.7, respectively), while, according to the WTS pHpzc values, WTS surfaces are positively charged, which may support a positive interaction (no electrostatic repulsion) between adsorbent and adsorbate. Besides the influence of the AC properties on its adsorption capacity, the presence of other elements could also have influenced the adsorption performance of the sludges tested. For example, the high aluminium content of the sludges (Table 2) may have influenced the adsorption process, although there appears little consensus in the literature on this influence [36,38,51,62]. On the one hand, aluminium has been indicated to improve the adsorption capacity of WTS (e.g., Lee et al. [58]). On the other hand, other studies have concluded that aluminium as a coagulant [64,65] in WTS has only a minimal impact on the removal efficacy of steroid compounds [66]. Therefore, it is of utmost importance that further research is conducted to assess the role of aluminium on the adsorption capacity of WTSs containing AC for these hormones.
WTS2 had a higher removal efficiency when compared with WTS1 since the final concentration was lower than the limit of quantification (LOQ) (Table 3), which could be related to a number of factors. The most evident reason is the higher surface area and pore volume of WTS2 as compared to WTS1, which logically also increases the number of available adsorption sites of the former [34]. The better performance of WTS2 may also suggest that a greater portion of AC was available in this sludge for adsorption, and that therefore the reactivation processes may not even be necessary. However, other adsorption mechanisms not evaluated in the present study could also be responsible for the differential interactions between the EPs and the two WTSs evaluated, such as π–π interactions, hydrogen bonding or electrostatic interactions, and this should be explored in future studies [67]. Short-term future prospects for this research are to complete the full characterisation of WTS, including complete textural characterisation and surface chemistry evaluation to identify surface functional groups by Fourier-transform infrared (FT-IR) and X-ray diffraction (XRD) analysis, and also to perform kinetic and equilibrium assays to elucidate the adsorption mechanisms. The pH and temperature dependence of the adsorption process will also be assessed, along with analysis of the stability of the WTS to ensure the safe application of this material. Long-term prospects for this research will include WTS adsorbent capacity analysis by using a real wastewater matrix in order to analyse the removal efficiency of both E2 and EE2 with the aim of bringing the research closer to a real situation.
3. Materials and Methods
3.1. Raw Material
Two WTSs from different WTPs in Portugal were used to evaluate their adsorption potential of E2 and EE2: (i) Santa Águeda WTP in the Castelo Branco region (WTS1) and (ii) Caldeirão WTP in the Guarda region (WTS2). Both these WTPs use powdered AC in their water treatment process to remove flavour and odours from raw water. Therefore, the sludge produced in the sedimentation tanks of these WTPs will always contain AC. Sludges were collected from these WTPs after the final stage of dewatering by filter press, and the collected sludge was kept in the sun for one month to completely remove the remaining water. The obtained material was not subjected to a reactivation process, and the dried material was ground and sieved to obtain a particle size of 45/60 mesh (250–354 µm), between conventional PAC and GAC particle sizes [68,69,70].
3.2. Analytical Methods
3.2.1. WTS Characterisation
Elemental analyses (quantification of carbon, hydrogen, nitrogen and sulphur contents) were performed using an Elemental Thermo Finnigan Analyzer—CE Instruments, model Flash EA 1112 CHNS series (Waltham, MA, USA), based on sample combustion dynamics. The determination of the ash content followed the ASTM D 1762-84 guideline (750 °C) [71]. The pH at the point of zero charge (pHpzc) determination was performed according to the methodology presented by Bernardo et al. [59]. Thermogravimetric analysis (TGA) was performed with Setaram Labsys EVO equipment (Caluire, France) between room temperature and 900 °C with a heating rate of 5 °C/min under argon atmosphere. The mineral analysis was performed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Horiba Jobin-Yvon equipment, Kyoto, Japan) after acidic digestion of the WTS samples for the quantification of the following elements: Al, As, Ca, Cd, Cr, Cu, Fe, Hg, K, Mg, Mn, Mo, Na, Ni, P, Pb, Sb, Se and Zn. Textural parameters such as surface area and total pore volume (Vtotal) were evaluated from the adsorption of N2 at 77 K (ASAP 2010 Micromeritics equipment, Atlanta, GA, USA) by using the single point method at the relative pressure of p/p0 = 0.3.
3.2.2. Stock Solution and Determination of the Emerging Pollutants
Stock solutions for both 17β-estradiol (E2; Acros Organics, 98% purity, China) and 17α-ethinylestradiol (EE2; Dr. Ehrenstorfer, 97% purity, Germany) were prepared with a concentration of 500 µg/L for each hormone. The stock solutions were stored in a fridge at 4 °C. Each WTS was added to three separate solutions with different hormone concentrations (200, 350 and 500 ng/L) by using a solid/liquid ratio of 5 g/L. The solutions with WTS were submitted to agitation (200 rpm) in jar test equipment for 24 h. After mixing, the samples were filtered through glass microfibre filters (1.2 µm, GF/C—WATERS) under vacuum. The extraction and detection of the studied EPs were performed by solid-phase extraction (SPE) and high-performance liquid chromatography tandem mass spectrometry (HPLC-MS-MS), according to Gaffney et al. [72,73].
4. Conclusions
The results obtained indicate that the tested WTSs present high adsorption potential for both E2 and EE2. Without regeneration or any kind of modification, this adsorbent allowed the achievement of a considerably high, up to a complete, removal of these hormones. These promising results indicate the potential of WTS without addition or activation of AC (i.e., with only AC present from the regular WTP process) in the removal of EPs and may lead to new ways of transforming this erstwhile residue into a possible value-added product. Further studies should be conducted to fully characterise these adsorbent materials through a complete textural characterisation and surface chemistry evaluation to identify surface functional groups and also to perform kinetic and equilibrium assays to elucidate the adsorption mechanisms.
Acknowledgments
The authors would like to thank to EPAL—Empresa Pública de Águas Lives S.A., AdP—Grupo Águas de Portugal for the technical support.
Author Contributions
Conceptualisation, R.M. and P.F.; methodology, R.D., D.S., M.B., V.V.C. and S.S.; validation, M.B., I.M., I.F., V.V.C., R.N.C., P.F., M.A.D. and R.M.; formal analysis, R.D., D.S., M.B., V.V.C. and S.S.; investigation, R.D. and D.S.; resources, R.M., V.V.C., P.F., M.B, I.M. and I.F.; writing—original draft preparation, R.D. and D.S.; writing—review and editing, R.D., R.M. and M.A.D.; visualization, R.D., R.M. and M.A.D.; supervision, R.M. and M.A.D.; funding acquisition, R.D., M.B., P.F., R.M. and M.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CENSE through the Portuguese Foundation for Science and Technology (FCT; UIDB/04085/2020) and through a PhD grant for Rita Dias (SFRH/BD/148793/2019). The authors are also indebted to EPAL AdVT for their financial support to this project. This work was also supported by the Associate Laboratory for Green Chemistry—LAQV, which is financed by national funds from FCT/MCTES (UIDB/50006/2020). Maria Bernardo acknowledges Norma Transitória DL57/2016 Contract (FCT/MCTES).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability MSamples of the compounds E2 and EE2 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations . Sustainable Development Goal 6: Synthesis Report on Water and Sanitation. United Nations; New York, NY, USA: 2018. [DOI] [Google Scholar]
- 2.Nika C.E., Vasilaki V., Expósito A., Katsou E. Water cycle and circular economy: Developing a circularity assessment framework for complex water systems. Water Res. 2020;187:116423. doi: 10.1016/j.watres.2020.116423. [DOI] [PubMed] [Google Scholar]
- 3.Kakwani N.S., Kalbar P.P. Review of circular economy in urban water sector: Challenges and opportunities in India. J. Environ. Manag. 2020;271:111010. doi: 10.1016/j.jenvman.2020.111010. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa M.O., Moreira N.F.F., Ribeiro A.R., Pereira M.F.R., Silva A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016;94:257–279. doi: 10.1016/j.watres.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Tran N.H., Reinhard M., Gin K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018;133:182–207. doi: 10.1016/j.watres.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Ben W., Zhu B., Yuan X., Zhang Y., Yang M., Qiang Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across china: Comparison of wastewater treatment processes. Water Res. 2018;130:38–46. doi: 10.1016/j.watres.2017.11.057. [DOI] [PubMed] [Google Scholar]
- 7.Guillossou R., Le Roux J., Mailler R., Vulliet E., Morlay C., Nauleau F., Gasperi J., Rocher V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere. 2019;218:1050–1060. doi: 10.1016/j.chemosphere.2018.11.182. [DOI] [PubMed] [Google Scholar]
- 8.Rout P.R., Zhang T.C., Bhunia P., Surampalli R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021;753:141990. doi: 10.1016/j.scitotenv.2020.141990. [DOI] [PubMed] [Google Scholar]
- 9.Hamid H., Eskicioglu C. Fate of estrogenic hormones in wastewater and sludge treatment: A review of properties and analytical detection techniques in sludge matrix. Water Res. 2012;46:5813–5833. doi: 10.1016/j.watres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Barreiros L., Queiroz J.F., Magalhães L.M., Silva A.M.T., Segundo M.A. Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices—A review. Microchem. J. 2016;126:243–262. doi: 10.1016/j.microc.2015.12.003. [DOI] [Google Scholar]
- 11.Coello-Garcia T., Curtis T.P., Mrozik W., Davenport R.J. Enhanced estrogen removal in activated sludge processes through the optimization of the hydraulic flow pattern. Water Res. 2019;164:114905. doi: 10.1016/j.watres.2019.114905. [DOI] [PubMed] [Google Scholar]
- 12.Riva F., Zuccato E., Davoli E., Fattore E., Castiglioni S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in italy. J. Hazard. Mater. 2019;361:103–110. doi: 10.1016/j.jhazmat.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 13.Gosset A., Polomé P., Perrodin Y. Ecotoxicological risk assessment of micropollutants from treated urban wastewater effluents for watercourses at a territorial scale: Application and comparison of two approaches. Int. J. Hyg. Environ. Health. 2020;224:113437. doi: 10.1016/j.ijheh.2019.113437. [DOI] [PubMed] [Google Scholar]
- 14.Díaz-Garduño B., Pintado-Herrera M.G., Biel-Maeso M., Rueda-Márquez J.J., Lara-Martín P.A., Perales J.A., Manzano M.A., Garrido-Pérez C., Martín-Díaz M.L. Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration. Water Res. 2017;119:136–149. doi: 10.1016/j.watres.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Schröder P., Helmreich B., Škrbić B., Carballa M., Papa M., Pastore C., Emre Z., Oehmen A., Langenhoff A., Molinos M., et al. Status of Hormones and Painkillers in Wastewater Effluents across Several European States—Considerations for the EU Watch List Concerning Estradiols and Diclofenac. Environ. Sci. Pollut. Res. 2016;23:12835–12866. doi: 10.1007/s11356-016-6503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ting Y.F., Praveena S.M. Sources, Mechanisms, and Fate of Steroid Estrogens in Wastewater Treatment Plants: A Mini Review. Environ. Monit. Assess. 2017;189:27. doi: 10.1007/s10661-017-5890-x. [DOI] [PubMed] [Google Scholar]
- 17.Adeel M., Song X., Wang Y., Francis D., Yang Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017;99:107–119. doi: 10.1016/j.envint.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Aris AZShamsuddin A.S., Praveena S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014;69:104–119. doi: 10.1016/j.envint.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Luján-Facundo M.J., Iborra-Clar M.I., Mendoza-Roca J.A., Alcaina-Miranda M.I. Pharmaceutical compounds removal by adsorption with commercial and reused carbon coming from a drinking water treatment plant. J. Clean. Prod. 2019:238. doi: 10.1016/j.jclepro.2019.117866. [DOI] [Google Scholar]
- 20.Shah A.I., Din Dar M.U., Bhat R.A., Singh J.P., Singh K., Bhat S.A. Prospectives and challenges of wastewater treatment technologies to combat contaminants of emerging concerns. Ecol. Eng. 2020;152:105882. doi: 10.1016/j.ecoleng.2020.105882. [DOI] [Google Scholar]
- 21.Rizzo L., Malato S., Antakyali D., Beretsou V.G., Đolić M.B., Gernjak W., Heath E., Ivancev-Tumbas I., Karaolia P., Lado Ribeiro A.R., et al. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019;655:986–1008. doi: 10.1016/j.scitotenv.2018.11.265. [DOI] [PubMed] [Google Scholar]
- 22.Sophia A.C., Lima E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018;150:1–17. doi: 10.1016/j.ecoenv.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Mailler R., Gasperi J., Coquet Y., Derome C., Buleté A., Vulliet E., Bressy A., Varrault G., Chebbo G., Rocher V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016 doi: 10.1016/j.jece.2016.01.018. [DOI] [Google Scholar]
- 24.Song J.Y., Bhadra B.N., Jhung S.H. Contribution of H-bond in adsorptive removal of pharmaceutical and personal care products from water using oxidized activated carbon. Microporous Mesoporous Mater. 2017;243:221–228. doi: 10.1016/j.micromeso.2017.02.024. [DOI] [Google Scholar]
- 25.Ifelebuegu A. Removal of steriod hormones by activated carbon adsorption—kinetic and thermodynamic studies. J. Environ. Prot. (Irvine) 2012;3:469–475. doi: 10.4236/jep.2012.36057. [DOI] [Google Scholar]
- 26.Fukuhara T., Iwasaki S., Kawashima M., Shinohara O., Abe I. Adsorbability of estrone and 17β- estradiol in water onto activated carbon. Water Res. 2006;40:241–248. doi: 10.1016/j.watres.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Saha B., Karounou E., Streat M. Removal of 17β-oestradiol and 17α-ethinyl oestradiol from water by activated carbons and hypercrosslinked polymeric phases. React. Funct. Polym. 2010;70:531–544. doi: 10.1016/j.reactfunctpolym.2010.04.004. [DOI] [Google Scholar]
- 28.Polloni-Silva J., Valdehita A., Fracácio R., Navas J.M. Remediation efficiency of three treatments on water polluted with endocrine disruptors: Assessment by means of in vitro techniques. Chemosphere. 2017;173:267–274. doi: 10.1016/j.chemosphere.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Nolasco M.A., Guimarães K.O., Cardoso G. Determination and removal of endocrine disruptors in wastewater by activated carbon. J. Civ. Eng. Archit. 2017:11. doi: 10.17265/1934-7359/2017.07.003. [DOI] [Google Scholar]
- 30.Yoon Y., Westerhoff P., Snyder S.A., Esparza M. HPLC-Fluorescence detection and adsorption of bisphenol a, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003 doi: 10.1016/S0043-1354(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 31.Fuerhacker M., Dürauer A., Jungbauer A. Adsorption isotherms of 17β-estradiol on granular activated carbon (gac) Chemosphere. 2001 doi: 10.1016/S0045-6535(00)00543-9. [DOI] [PubMed] [Google Scholar]
- 32.Rowsell V.F., Pang D.S.C., Tsafou F., Voulvoulis N. Removal of steroid estrogens from wastewater using granular activated carbon: Comparison between virgin and reactivated carbon. Water Environ. Res. 2009;81:394–400. doi: 10.2175/106143008X357093. [DOI] [PubMed] [Google Scholar]
- 33.Gökçe C.E., Arayici S. Adsorption of 17β-estradiol and estrone by activated carbon derived from sewage sludge. Desalin. Water Treat. 2016;57:2503–2514. doi: 10.1080/19443994.2015.1034183. [DOI] [Google Scholar]
- 34.Ifelebuegu A.O., Lester J.N., Churchley J., Cartmell E. Removal of an endocrine disrupting chemical (17α-ethinyloestradiol) from wastewater effluent by activated carbon adsorption: Effects of activated carbon type and competitive adsorption. Environ. Technol. 2006;27:1343–1349. doi: 10.1080/09593332708618748. [DOI] [PubMed] [Google Scholar]
- 35.Kovalova L., Knappe D.R.U., Lehnberg K., Kazner C., Hollender J. Removal of highly polar micropollutants from wastewater by powdered activated carbon. Environ. Sci. Pollut. Res. 2013;20:3607–3615. doi: 10.1007/s11356-012-1432-9. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad T., Ahmad K., Alam M. Sustainable management of water treatment sludge through 3′R’ concept. J. Clean. Prod. 2016:1–13. doi: 10.1016/j.jclepro.2016.02.073. [DOI] [Google Scholar]
- 37.Devi P., Saroha A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017;578:16–33. doi: 10.1016/j.scitotenv.2016.10.220. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y.F., Haynes R.J. A comparison of water treatment sludge and red mud as adsorbents of as and se in aqueous solution and their capacity for desorption and regeneration. Water. Air. Soil Pollut. 2012;223:5563–5573. doi: 10.1007/s11270-012-1296-0. [DOI] [Google Scholar]
- 39.Zhou Y.F., Haynes R.J. Water treatment sludge can be used as an adsorbent for heavy metals in wastewater streams. WIT Trans. Ecol. Environ. 2010;140:379–389. doi: 10.2495/WM100341. [DOI] [Google Scholar]
- 40.Gibbons M.K., Gagnon G.A. Adsorption of arsenic from a nova scotia groundwater onto water treatment residual solids. Water Res. 2010;44:5740–5749. doi: 10.1016/j.watres.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 41.Agrafioti E., Kalderis D., Diamadopoulos E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manage. 2014;133:309–314. doi: 10.1016/j.jenvman.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Mao S., Chen H., Huang L., Qiu R. Pb(II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour. Technol. 2013;147:545–552. doi: 10.1016/j.biortech.2013.08.082. [DOI] [PubMed] [Google Scholar]
- 43.Liu H., Yuan B., Zhang B., Hu H., Li A., Luo G., Yao H. Removal of mercury from flue gas using sewage sludge-based adsorbents. J. Mater. Cycles Waste Manag. 2014;16:101–107. doi: 10.1007/s10163-013-0145-6. [DOI] [Google Scholar]
- 44.Tong D.S., Liu M., Li L., Lin C.X., Yu W.H., Xu Z.P., Zhou C.H. Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red g dye. Appl. Clay Sci. 2012;70:1–7. doi: 10.1016/j.clay.2012.08.001. [DOI] [Google Scholar]
- 45.Kayranli B. Adsorption of textile dyes onto iron based waterworks sludge from aqueous solution; isotherm, kinetic and thermodynamic study. Chem. Eng. J. 2011;173:782–791. doi: 10.1016/j.cej.2011.08.051. [DOI] [Google Scholar]
- 46.Utomo H.D., Ong X.C., Lim S.M.S., Ong G.C.B., Li P. Thermally processed sewage sludge for methylene blue uptake. Int. Biodeterior. Biodegrad. 2013;85:460–465. doi: 10.1016/j.ibiod.2012.12.004. [DOI] [Google Scholar]
- 47.Zou J., Dai Y., Wang X., Ren Z., Tian C., Pan K., Li S., Abuobeidah M., Fu H. Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity. Bioresour. Technol. 2013;142:209–217. doi: 10.1016/j.biortech.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 48.Monsalvo V.M., Mohedano A.F., Rodriguez J.J. Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents. Chem. Eng. Res. Des. 2012;90:1807–1814. doi: 10.1016/j.cherd.2012.03.018. [DOI] [Google Scholar]
- 49.Boyer T.H., Persaud A., Banerjee P., Palomino P. Comparison of low-cost and engineered materials for phosphorus removal from organic-rich surface water. Water Res. 2011;45:4803–4814. doi: 10.1016/j.watres.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y.S., Zhao Y.Q., Sorohan B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination. 2011;271:150–156. doi: 10.1016/j.desal.2010.12.014. [DOI] [Google Scholar]
- 51.Mohammed W.T., Rashid S.A. Phosphorus removal from wastewater using oven-dried alum sludge. Int. J. Chem. Eng. 2012;2012:11. doi: 10.1155/2012/125296. [DOI] [Google Scholar]
- 52.Chen T.C., Shih Y.J., Chang C.C., Huang Y.H. Novel adsorbent of removal phosphate from TFT LCD wastewater. J. Taiwan Inst. Chem. Eng. 2013;44:61–66. doi: 10.1016/j.jtice.2012.09.008. [DOI] [Google Scholar]
- 53.Ding R., Zhang P., Seredych M., Bandosz T.J. Removal of antibiotics from water using sewage sludge- and waste oil sludge-derived adsorbents. Water Res. 2012;46:4081–4090. doi: 10.1016/j.watres.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Clara M., Strenn B., Saracevic E., Kreuzinger N. Adsorption of bisphenol-a, 17β-estradiole and 17α- ethinylestradiole to sewage sludge. Chemosphere. 2004;56:843–851. doi: 10.1016/j.chemosphere.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad T., Ahmad K., Alam M. Sludge quantification at water treatment plant and its management scenario. Environ. Monit. Assess. 2017;189:1–10. doi: 10.1007/s10661-017-6166-1. [DOI] [PubMed] [Google Scholar]
- 56.Babatunde A.O., Zhao Y.Q. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses. Crit. Rev. Environ. Sci. Technol. 2007;37:129–164. doi: 10.1080/10643380600776239. [DOI] [Google Scholar]
- 57.Muisa N., Nhapi I., Ruziwa W., Manyuchi M.M. Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review. J. Water Process. Eng. 2020 doi: 10.1016/j.jwpe.2020.101187. [DOI] [Google Scholar]
- 58.Lee Y.E., Shin D.C., Jeong Y., Kim I.T., Yoo Y.S. Pyrolytic valorization of water treatment residuals containing powdered activated carbon as multifunctional adsorbents. Chemosphere. 2020:252. doi: 10.1016/j.chemosphere.2020.126641. [DOI] [PubMed] [Google Scholar]
- 59.Bernardo M., Correa C.R., Ringelspacher Y., Becker G.C., Lapa N., Fonseca I., Esteves I.A.A.C., Kruse A. Porous carbons derived from hydrothermally treated biogas digestate. Waste Manag. 2020;105:170–179. doi: 10.1016/j.wasman.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Ouyang J., Zhou L., Liu Z., Heng J.Y.Y., Chen W. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review. Sep. Purif. Technol. 2020;253:117536. doi: 10.1016/j.seppur.2020.117536. [DOI] [Google Scholar]
- 61.Salvador F., Martin-Sanchez N., Sanchez-Hernandez R., Sanchez-Montero M.J., Izquierdo C. Regeneration of carbonaceous adsorbents. Part. I: Thermal regeneration. Microporous Mesoporous Mater. 2015;202:259–276. doi: 10.1016/j.micromeso.2014.02.045. [DOI] [Google Scholar]
- 62.Yang Y., Zhao Y.Q., Babatunde A.O., Wang L., Ren Y.X., Han Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006;51:193–200. doi: 10.1016/j.seppur.2006.01.013. [DOI] [Google Scholar]
- 63.Tomeczek J., Palugniok H. Kinetics of mineral matter transformation during coal combustion. Fuel. 2002;81:1251–1258. doi: 10.1016/S0016-2361(02)00027-3. [DOI] [Google Scholar]
- 64.Auriol M., Filali-Meknassi Y., Tyagi R.D., Adams C.D., Surampalli R.Y. Endocrine disrupting compounds removal from wastewater, a new challenge. Process. Biochem. 2006;41:525–539. doi: 10.1016/j.procbio.2005.09.017. [DOI] [Google Scholar]
- 65.Bodzek M., Dudziak M. Removal of natural estrogens and synthetic compounds considered to be endocrine disrupting substances (EDS) by coagulation and nanofiltration. Polish J. Environ. Stud. 2006;15:35–40. [Google Scholar]
- 66.Kulandaivelu J., Choi P.M., Shrestha S., Li X., Song Y., Li J., Sharma K., Yuan Z., Mueller J.F., Wang C., et al. Assessing the removal of organic micropollutants from wastewater by discharging drinking water sludge to sewers. Water Res. 2020;181:115945. doi: 10.1016/j.watres.2020.115945. [DOI] [PubMed] [Google Scholar]
- 67.Qureshi U.A., Hameed B.H., Ahmed M.J. Adsorption of endocrine disrupting compounds and other emerging contaminants using lignocellulosic biomass-derived porous carbons: A review. J. Water Process. Eng. 2020;38:101380. doi: 10.1016/j.jwpe.2020.101380. [DOI] [Google Scholar]
- 68.Mailler R., Gasperi J., Coquet Y., Buleté A., Vulliet E., Deshayes S., Zedek S., Mirande-Bret C., Eudes V., Bressy A., et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016;542:983–996. doi: 10.1016/j.scitotenv.2015.10.153. [DOI] [PubMed] [Google Scholar]
- 69.Krahnstöver T., Wintgens T. Separating powdered activated carbon (PAC) from wastewater—Technical process options and assessment of removal efficiency. J. Environ. Chem. Eng. 2018:5744–5762. doi: 10.1016/j.jece.2018.09.001. [DOI] [Google Scholar]
- 70.Guillossou R., Le Roux J., Mailler R., Morlay C., Vulliet E., Nauleau F., Rocher V., Gasperi J. Influence of the properties of 7 micro-grain activated carbons on organic micropollutants removal from wastewater effluent. Chemosphere. 2020;243:125306. doi: 10.1016/j.chemosphere.2019.125306. [DOI] [PubMed] [Google Scholar]
- 71.Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International; West Conshohocken, PA, USA: 2013. [(accessed on 7 December 2020)]. ASTM D1762-84(2013) Available online: www.astm.org. [Google Scholar]
- 72.Gaffney V.D.J., Cardoso V.V., Rodrigues A., Ferreira E., Benoliel M.J., Almeida C.M.M. Análise de fármacos em águas por SPE-UPLC-ESI-MS/MS. Quim. Nova. 2014;37:138–149. doi: 10.1590/S0100-40422014000100023. [DOI] [Google Scholar]
- 73.Gaffney V.D.J., Almeida C.M.M., Rodrigues A., Ferreira E., Benoliel M.J., Cardoso V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015;72:199–208. doi: 10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.