Abstract
In the present study, the simultaneous and accurate determination of liquid viscosity and surface tension of the n-alkanes n-hexane (n-C6H14), n-octane (n-C8H18), n-decane (n-C10H22), and n-hexadecane (n-C16H34) by surface light scattering (SLS) in thermodynamic equilibrium is demonstrated. Measurements have been performed over a wide temperature range from 283.15 K up to 473.15 K for n-C6H14, 523.15 K for n-C8H18, and 573.15 K for n-C10H22 as well as n-C16H34. The liquid dynamic viscosity and surface tension data with average total measurement uncertainties (k = 2) of (2.0 and 1.7) % agree with the available literature and contribute to a new database at high temperatures. Over the entire temperature range, a Vogel-type equation for the dynamic viscosity and a modified van der Waals equation for the surface tension represent the measured data for the four n-alkanes within experimental uncertainties. By also considering our former SLS data for n-dodecane (n-C12H26) and n-octacosane (n-C28H58), empirical models for the liquid viscosity and surface tension of n-alkanes were developed as a function of temperature and carbon number covering values between 6 and 28. Agreement between these models and reference correlations for further selected n-alkanes which were not included in the development procedure was found.
Graphical Abstract

INTRODUCTION
Linear alkanes with carbon numbers between 6 and 16 are important working fluids in many fields of process and energy engineering. Examples are the catalytic synthesis of n-alkanes with varying chain length during the Fischer-Tropsch process,1 their subsequent separation by distillation,2,3 and utilization in combustion processes.4-6 For the design and modeling of these processes, accurate thermophysical properties of the involved chemicals including viscosity and surface tension are required, in particular at process-relevant conditions above 400 K. In this regard, standard databases for thermophysical properties of pure components and mixtures have been established, for example the Dortmund database7 and the ThermoData Engine (TDE) of the National Institute of Standards and Technology (NIST).8 For the subsequent development of reference correlations of fluids as they are implemented in, e.g., the NIST reference database REFPROP,9 primary experimental data with low measurement uncertainties are ideally required. Data related to this category should be obtained from quasi-primary methods based on a physically sound working equation that connects the experimentally measured parameters with the property of interest.10 For the viscosity and surface tension of n-alkanes with carbon numbers of 10 and larger, there is still a lack of reliable, direct experimental data in the open literature, especially at temperatures above 400 K.11-14
A quasi-primary method for the determination of viscosity of liquids in the medium viscosity range15 is the surface light scattering (SLS) technique. It probes the dynamics of thermal fluctuations at phase boundaries in a contactless way via the temporal analysis of the scattered light intensity and is based on rigorous working equations according to the linearized hydrodynamic description of surface fluctuations.16,17 By taking necessary precautions against instrumental line broadening effects, the technique can be used without any need for a calibration procedure. For systems consisting of a vapor and a liquid phase as well as showing relatively low viscosities, which is true for the systems in this study, the quantities of the surface fluctuations with a defined wavelength directly accessible by SLS are their characteristic dynamics, i.e., damping and frequency. Besides the latter two properties, only information on the liquid and vapor densities as well as the vapor viscosity is necessary for a simultaneous determination of the liquid viscosity and surface tension in macroscopic thermodynamic equilibrium.18-20
During the last two decades, research activities at the Department of Chemical and Biological Engineering (CBI) and at the Erlangen Graduate School in Advanced Optical Technologies (SAOT) of the Friedrich-Alexander-University Erlangen-Nürnberg (FAU) developed SLS as a reliable and accurate instrument in thermophysical property research of fluids covering broad ranges of viscosity and surface or interfacial tension. For various systems at vapor-liquid equilibrium, e.g., reference fluids (toluene,21 diisodecyl phthalate,22 carbon dioxide,18 and n-pentane23), refrigerants,20,24,25 hydrofluoroethers,26 ionic liquids,27-30 linear31 and branched32 alkanes as well as mixtures of n-alkanes with other n-alkanes31,33 or oxygenated derivatives,33 viscosity and surface or interfacial tension have been determined with typical measurement uncertainties (k = 2) below 2 % at temperatures between (233 and 573) K. For binary model systems, the simultaneous analysis of fluctuations at the surface and in the bulk of the fluid allowed for the determination of interfacial tension, viscosity, thermal diffusivity, and mutual diffusivity of the very same sample within one single SLS setup.34 Recently, we demonstrated the applicability of SLS for the investigation of multiphase systems in vapor-liquid-liquid equilibrium, simultaneously measuring the viscosities of the two liquid phases as well as the vapor-liquid and liquid-liquid interfacial tensions.35
In the present study, the simultaneous determination of liquid viscosity and surface tension of the linear alkanes n-hexane (n-C6H14), n-octane (n-C8H18), n-decane (n-C10H22), and n-hexadecane (n-C16H34) by SLS at saturation conditions over a broad temperature range between (283 and 573) K with low measurement uncertainties is shown. Besides a check of the existing literature database for the studied fluids, our measurement data contribute to a new database for the liquid viscosity and surface tension, in particular for n-C10H22 and n-C16H34 at temperatures above 400 K. In the following, first, the theory, experimental procedure, and data evaluation for SLS is described. Then, the experimental results for liquid dynamic viscosity and surface tension of the four studied n-alkanes are correlated as a function of temperature by appropriate fit equations and are discussed in comparison with experimental data and reference correlations given in the literature. Taking also into account our former SLS results for n-dodecane (n-C12H26) and n-octacosane (n-C28H58), empirical models for the liquid viscosity and surface tension of n-alkanes were developed as a function of temperature and carbon number ranging between 6 and 28. Finally, the transferability of these models to the representation of viscosity and surface tension of further selected n-alkanes with carbon numbers within the specified range of validity is tested against literature data.
SURFACE LIGHT SCATTERING
Theoretical background.
Liquid viscosity and surface tension of the n-alkanes under saturation conditions were measured simultaneously by surface light scattering (SLS). With this non-invasive technique, the dynamics of microscopic thermal fluctuations at phase boundaries are probed in macroscopic thermodynamic equilibrium by analyzing the intensity of the scattered light originating from the interaction between the incident light and the fluctuating surface structure. For the studied n-alkanes with relatively small liquid viscosities, surface fluctuations at the phase boundary between a liquid (′) and a vapor (″) phase propagate and show an oscillatory behavior. In this case, the measured data for the dynamics of surface fluctuations, i.e. frequency ωq and damping Γ are, in a first-order approximation, governed by the surface tension σ and the liquid kinematic viscosity ν′. For an accurate determination of liquid viscosity and surface tension by SLS, the exact numerical solution of the dispersion relation for surface fluctuations at the vapor-liquid phase boundary was used in its complete form.16,17 For this, data for ωq and Γ at a defined wave number of surface fluctuations q measured by SLS are combined with reference data for the dynamic viscosity of the vapor phase η″ and density data ρ′ and ρ″ for the liquid and vapor phases. More details on the SLS method and its application for the simultaneous determination of viscosity and surface tension can be found in refs 16, 18, and 19.
Materials and sample preparation
According to the specifications of the suppliers36, all n-alkane samples studied in this work had a purity of 0.99 or higher in terms of mass fraction. Since the samples did not show any particle-like impurities, they could be used without any further purification. Helium, which served as an inert gas in all the experiments, was provided by Linde AG with a mole fraction purity of more than 0.99999. A summary of the investigated chemicals and their properties is given in Table 1.
Table 1.
Specification of the Investigated Chemicals
| substance | source | molar mass M / (g·mol−1) |
specified purity |
critical temperature TC / K |
|---|---|---|---|---|
| n-C6H14 (n-hexane, CAS 110-54-3) | Merck | 86.18 | mass fraction ≥ 0.99a |
507.82c |
| n-C8H18 (n-octane, CAS 111-65-9) | Merck | 114.23 | mass fraction 0.99999a |
568.74d |
| n-C10H22 (n-decane, CAS 124-18-5) | Alfa Aesar |
142.28 | mass fraction 0.995a |
617.70e |
| n-C16H34 (n-hexadecane, CAS 544-76-3) | Alfa Aesar |
226.45 | mass fraction 0.991a |
722.10f |
| Helium (CAS 7440-59-7) | Linde AG | 4.0026 | volume fraction ≥ 0.99999b |
5.1953g |
The experimental SLS setup including the sample cell used in the present study is identical to that employed in our former investigations of vapor-liquid systems.20,21,31-34 For details, refs 18, 20, and 31 are recommended. In the following, only the experimental conditions and procedure relevant for the present study are summarized.
The clean sample cell was filled with about 50 ml of the n-alkane sample under atmospheric conditions and flushed by helium several times to avoid any contamination of the sample with air or other gases. After that, the cell was closed and an initial partial pressure of about 0.1 MPa of helium was adjusted. Owing to the negligible effect of helium on the liquid density, liquid viscosity, and surface tension of n-alkanes at such small pressures,9 all measurements can be considered to be performed at saturation conditions.
For the sample cell placed inside an insulated housing, the temperature T was regulated through resistance heating and measured by calibrated 100 Ω platinum resistance probes with expanded uncertainties (k = 2) of U(T) = 20 mK. During each experimental run, the temperature stability was better than ±2 mK. For each temperature point, six measurements at different q values corresponding to different external angles of incidence ΘE = (3.0, 3.1, and 3.2)° were performed. Misalignment was avoided by irradiating the laser from either side with respect to the axis of observation. Temperatures between (283.15 and 473.15) K for n-C6H14, (283.15 and 523.15) K for n-C8H18, (283.15 and 573.15) K for n-C10H22 as well as (293.15 and 573.15) K for n-C16H34 were investigated, where the temperature was stepwise increased. Here, the temperature step was 10 K between the lowest temperature and 323.15 K, and 25 K between 323.15 K and the largest temperature.
After finishing the measurements up to 423.15 K for each sample, the measurement cell was emptied, cleaned, and refilled with a new sample for the subsequent measurements at larger temperatures. As an orange coloration of the samples was observed via the laser beam in all cases, the measurement time was reduced to a minimum in order to minimize thermal degradation. After the measurements at the largest temperature of (523.15 or 573.15) K, replicates were performed for all samples at a specific temperature in the lower temperature range with reduced laser power. All results from these repeated measurements agreed with those from the first measurements within combined uncertainties, which is an indication for the stability of the systems.
Measurement example and data evaluation.
Assuming heterodyne conditions and negligible line broadening effects (consistent with our experiments), the normalized intensity correlation function for the analysis of surface fluctuations of a defined q number with an oscillatory behavior is described by16
| (1) |
In eq 1, a and b are parameters that depend on the experimental boundary conditions, while the phase term ϕ mainly accounts for the deviations of the spectrum from the Lorentzian form. The correlation time τC and the frequency ωq represent the mean lifetime or the reciprocal of the damping Γ (= 1/τC) of the surface fluctuations and their frequency of propagation.
Figure 1 shows four example correlation functions obtained from the investigation of the vapor-liquid interface of n-C6H14 and n-C16H34 at saturation conditions at temperatures of T = (303.15 and 473.15) K and an incident angle of ΘE = 3°. From a nonlinear regression based on a Levenberg-Marquardt algorithm in which the squared sum of residuals is minimized, the values and expanded uncertainties (k = 2) for τC and ωq are calculated which are given in Figure 1. Within the entire fit range, no systematic deviations of the measured correlation function from eq 1 can be observed, as can be seen from the individual residual plots in Figure 1. For a given modulus of the wave vector q with a relative expanded uncertainty Ur(q) = 0.2 %, the measured values for ωq and τC have average relative expanded uncertainties Ur(ωq) = 0.3 % and Ur(τC) = 1.5 %. At T = 303.15 K in Fig. 1a, n-C16H34 shows a smaller decay time or larger damping than n-C6H16, which can be related to the larger viscosity of n-C16H34. At the increased temperature of 473.15 K shown in Fig. 1b, the viscosities of the n-alkanes decrease, which results in a larger decay times of the surface fluctuations. Here, the frequency related to n-C6H16 is more than a factor of two smaller that related to n-C16H34 because of the smaller surface tension of n-C6H16.
Figure 1.

Measurement examples of normalized correlation functions including their residuals for n-C6H16 (upper part) and n-C16H34 (lower part) at 303.15 K (a) and 473.15 K (b) as a function of the lag time τ using an incident angle of 3°:  , measurement data;
, measurement data;  , fit according to eq 1.
, fit according to eq 1.
In addition to the measured data for ωq and τC, reference data for the n-alkanes at saturation conditions in the form of the liquid density ρ′, the vapor density ρ″, and the vapor dynamic viscosity η″ with relative expanded uncertainties Ur(ρ′) between (0.2 and 2) %, Ur(ρ″) between (0.5 and 2) %, and Ur(η″) = 10 % were required to solve the dispersion relation.16,17 For this, the composition of the vapor phase as a function of temperature was determined in an ideal approach from the vapor pressure of the corresponding pure n-alkane specified by the REFPROP database9,38-41 and the partial pressure of helium used as inert gas. For helium, the data for the gas density and viscosity were adopted from a Helmholtz equation of state published by Ortiz-Vega42 and the pure fluid model of Arp et al.,43 respectively. For the liquid and vapor densities, the equations of state38-41 implemented in the REFPROP database.9 were used. To describe the vapor density of the binary mixtures consisting of n-alkane and helium, a linear mole-based mixing rule using the densities of the pure substances was applied. For the vapor viscosity η″, the corresponding-states model of Lucas44 allowing predictions of the vapor viscosity of gas mixtures at low pressures within 5 %45 was employed where the required critical pressures of the pure n-alkanes were taken from the REFPROP database9,38-41
By an exact numerical solution of the dispersion equation for surface fluctuations,16,17 the liquid dynamic viscosity η′ (=ν′/ρ′) and surface tension σ were determined with total expanded measurement uncertainties (k = 2) of Ur(η′) = 2.0 % and Ur(σ) = 1.7 %, respectively, averaged over all investigated systems and temperatures. For the calculation of the uncertainties, an error propagation scheme20,31 taking into consideration the uncertainties induced by the primary measured variables and by the adopted reference data was applied.
RESULTS AND DISCUSSION
First, the measurement results for the liquid dynamic viscosity and surface tension of the four investigated n-alkanes are presented and correlated as a function of temperature. Then, the experimental data are compared to available literature in the form of experimental data and reference correlations. Based on the SLS data presented in this work including former results for two further n-alkanes, empirical correlations for the liquid viscosity and surface tension of n-alkanes as function of temperature and carbon number ranging between 6 and 28 are developed and applied to further selected n-alkanes for testing their transferability.
Summary and correlation of liquid viscosity and surface tension data.
The results for the liquid dynamic viscosity η′ and surface tension σ of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 as well as their expanded uncertainties (k = 2) obtained from SLS at saturation conditions between (283.15 and 573.15) K are summarized in Table 2 and are shown in Figure 2. In this figure, our measurement results31 for n-dodecane (n-C12H26) and n-octacosane (n-C28H58) are given for comparison. The data listed in Table 2 are average values of six independent measurements with different angles of incidence ΘE. Furthermore, Table 2 includes the used input data for the liquid density ρ′, vapor density ρ″, and vapor viscosity η″ required for the evaluation of η′ and σ.
Table 2.
Liquid Dynamic Viscosity η′ and Surface Tension σ of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 Obtained by Surface Light Scattering at Saturation Conditions as Function of Temperature T Using Literature Data for the Liquid Density ρ′, Vapor Density ρ″, and Vapor Dynamic Viscosity η″a
| T / K |
ρ′ / (kg·m−3) |
ρ″ / (kg·m−3) |
η″ / (μPa·s) |
η′ / (mPa·s) |
100Ur(η′) |
σ / (mN·m−1) |
100Ur(σ') |
|---|---|---|---|---|---|---|---|
| n-C6H14 | |||||||
| 283.15 | 668.28 | 0.81 | 17.93 | 0.3495 | 2.1 | 19.28 | 1.0 |
| 293.15 | 659.29 | 0.87 | 17.79 | 0.3127 | 1.2 | 18.22 | 0.8 |
| 303.15 | 650.15 | 1.06 | 17.47 | 0.2809 | 1.6 | 17.34 | 1.0 |
| 313.15 | 642.24 | 1.39 | 17.00 | 0.2546 | 1.4 | 16.22 | 1.2 |
| 323.15 | 631.37 | 1.89 | 16.43 | 0.2340 | 1.5 | 15.31 | 0.8 |
| 348.15 | 606.75 | 4.00 | 14.81 | 0.1905 | 1.2 | 12.94 | 0.8 |
| 373.15 | 580.55 | 7.72 | 13.45 | 0.1550 | 1.8 | 10.53 | 0.8 |
| 398.15 | 552.18 | 13.71 | 12.65 | 0.1268 | 1.7 | 8.21 | 0.9 |
| 423.15 | 520.69 | 23.10 | 12.43 | 0.1026 | 1.9 | 5.98 | 0.9 |
| 448.15 | 484.16 | 38.04 | 12.74 | 0.0837 | 2.2 | 3.75 | 1.9 |
| 473.15 | 437.84 | 63.64 | 13.76 | 0.0655 | 3.7 | 1.89 | 1.7 |
| n-C8H18 | |||||||
| 283.15 | 710.56 | 0.18 | 19.32 | 0.6247 | 1.3 | 22.25 | 0.7 |
| 293.15 | 702.53 | 0.20 | 19.36 | 0.5523 | 1.2 | 21.31 | 1.0 |
| 303.15 | 694.43 | 0.25 | 19.40 | 0.4828 | 1.3 | 20.53 | 1.0 |
| 313.15 | 686.26 | 0.32 | 19.45 | 0.4320 | 1.3 | 19.45 | 1.0 |
| 323.15 | 678.00 | 0.42 | 19.32 | 0.3870 | 1.4 | 18.64 | 1.0 |
| 348.15 | 656.89 | 0.92 | 18.18 | 0.3079 | 1.2 | 16.37 | 1.2 |
| 373.15 | 634.92 | 1.92 | 16.15 | 0.2487 | 1.2 | 14.00 | 0.9 |
| 398.15 | 611.81 | 3.77 | 14.05 | 0.2028 | 1.8 | 11.90 | 0.9 |
| 423.15 | 587.18 | 6.91 | 12.50 | 0.1665 | 1.6 | 9.81 | 1.4 |
| 448.15 | 560.47 | 11.93 | 11.62 | 0.1393 | 1.2 | 7.88 | 0.8 |
| 473.15 | 530.83 | 19.71 | 11.33 | 0.1168 | 2.0 | 5.82 | 1.6 |
| 498.15 | 496.74 | 31.76 | 11.58 | 0.0947 | 3.7 | 4.00 | 3.1 |
| 523.15 | 455.09 | 51.27 | 12.51 | 0.0755 | 3.5 | 2.32 | 2.5 |
| n-C10H22 | |||||||
| 283.15 | 738.06 | 0.17 | 19.16 | 1.077 | 1.6 | 23.94 | 2.1 |
| 293.15 | 730.28 | 0.17 | 19.62 | 0.9005 | 1.2 | 23.05 | 2.4 |
| 303.15 | 722.55 | 0.18 | 20.01 | 0.7900 | 0.9 | 22.39 | 2.1 |
| 313.15 | 714.71 | 0.19 | 20.40 | 0.6924 | 1.6 | 21.43 | 2.1 |
| 323.15 | 706.98 | 0.21 | 20.75 | 0.6091 | 1.2 | 20.61 | 2.1 |
| 348.15 | 687.34 | 0.32 | 21.34 | 0.4636 | 0.9 | 18.75 | 2.0 |
| 373.15 | 667.36 | 0.61 | 21.20 | 0.3614 | 1.6 | 16.52 | 2.1 |
| 398.15 | 646.81 | 1.22 | 20.06 | 0.2895 | 1.1 | 14.38 | 2.1 |
| 423.15 | 625.48 | 2.38 | 18.15 | 0.2403 | 1.9 | 12.40 | 2.5 |
| 448.15 | 603.09 | 4.39 | 16.03 | 0.1978 | 1.5 | 10.29 | 2.1 |
| 473.15 | 579.21 | 7.68 | 14.24 | 0.1677 | 2.1 | 8.52 | 2.7 |
| 498.15 | 553.26 | 12.81 | 13.03 | 0.1366 | 2.9 | 6.58 | 2.4 |
| 523.15 | 524.29 | 20.68 | 12.38 | 0.1138 | 3.1 | 4.97 | 2.3 |
| 548.15 | 490.60 | 32.86 | 12.28 | 0.0974 | 4.4 | 3.43 | 3.0 |
| 573.15 | 448.50 | 52.75 | 12.90 | 0.0762 | 6.0 | 1.94 | 4.4 |
| n-C16H34 | |||||||
| 293.15 | 773.70 | 0.16 | 19.56 | 3.486 | 1.8 | 27.38 | 2.3 |
| 303.15 | 766.65 | 0.16 | 20.10 | 2.772 | 2.4 | 26.60 | 2.6 |
| 313.15 | 759.63 | 0.16 | 20.58 | 2.261 | 2.6 | 25.56 | 1.1 |
| 323.15 | 752.63 | 0.16 | 21.02 | 1.840 | 2.1 | 24.28 | 1.6 |
| 348.15 | 735.17 | 0.16 | 22.07 | 1.236 | 2.6 | 22.19 | 1.3 |
| 373.15 | 717.69 | 0.17 | 23.12 | 0.8927 | 1.6 | 20.31 | 1.0 |
| 398.15 | 700.11 | 0.19 | 24.14 | 0.6788 | 1.3 | 18.54 | 0.6 |
| 423.15 | 682.35 | 0.25 | 24.95 | 0.5351 | 1.4 | 16.69 | 1.4 |
| 448.15 | 664.29 | 0.41 | 25.36 | 0.4256 | 0.9 | 15.09 | 0.8 |
| 473.15 | 645.82 | 0.73 | 25.17 | 0.3440 | 1.8 | 13.26 | 1.5 |
| 498.15 | 626.80 | 1.36 | 24.28 | 0.2862 | 1.6 | 11.46 | 1.6 |
| 523.15 | 607.01 | 2.47 | 22.74 | 0.2360 | 2.4 | 9.72 | 2.8 |
| 548.15 | 586.21 | 4.30 | 20.83 | 0.1937 | 3.0 | 8.25 | 2.6 |
| 573.15 | 564.04 | 7.20 | 19.11 | 0.1705 | 3.7 | 7.00 | 4.1 |
Directly measured values for frequency ωq and damping Γ at a defined wave vector q of surface fluctuations were combined with reference data for ρ′, ρ″, and η″ described in the text to determine η′ and σ by an exact numerical solution of the dispersion relation.16,17 The relative expanded uncertainties (k = 2) for the employed properties are Ur(ρ′) between (0.2 and 2) %, Ur(ρ″) between (0.5 and 2) %, and Ur(η″) = 10 % (level of confidence = 0.95). The expanded uncertainty for the temperature is U(T) = 0.02 K. For the liquid dynamic viscosity and surface tension, the relative expanded uncertainties Ur(η′) and Ur(σ) are given in the table.
Figure 2.

Liquid dynamic viscosity and surface tension of investigated n-alkanes at saturation conditions from surface light scattering.  , n-C6H14, SLS, this work;
, n-C6H14, SLS, this work;  , n-C8H18, SLS, this work;
, n-C8H18, SLS, this work;  , n-C10H22, SLS, this work;
, n-C10H22, SLS, this work;  , n-C12H26, SLS, previous work;31
, n-C12H26, SLS, previous work;31
 , n-C16H34, SLS, this work;
, n-C16H34, SLS, this work;  , n-C28H58, SLS, previous work;31
, n-C28H58, SLS, previous work;31
 , fits of the experimental data for viscosity and surface tension data according to eqs 2 and 3.
, fits of the experimental data for viscosity and surface tension data according to eqs 2 and 3.
The upper part of Figure 2 shows that our experimental data for the liquid viscosity of the n-alkanes decrease with decreasing alkyl chain length and increasing temperature. For the representation of the saturated liquid dynamic viscosity of the four investigated n-alkanes as a function of temperature, a Vogel-type equation according to
| (2) |
was used where all experimental data have the same statistical weight in the fitting. In eq 2, T is the temperature in K and 77 η′0, η′1, η′2, η′3, and η′4 are fit coefficients listed in Table 3 for the four studied n-alkanes. As well, the absolute average deviation (AAD) of the experimental data from those calculated with eq 2 is given and between (0.36 and 0.94) %. For all systems and thermodynamic states, the relative deviations of the experimental η′ data from the fits are smaller than the experimental uncertainties of the measurements, as it can be seen in Figure 3.
Table 3.
Coefficients of Equation 2 for the Liquid Dynamic Viscosity η′calc(T) of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 at Saturation Conditions.
| n-C6H14 | n-C8H18 | n-C10H22 | n-C16H34 | |
|---|---|---|---|---|
| η′0 | −2.27934·101 | −2.36227·101 | −1.94144·101 | −9.05711 |
| η′1 / K | 2.24935·104 | 2.65713·104 | 2.18398·104 | 7.13979·103 |
| η′2 / ·K2 | −9.37662·106 | −1.25743·107 | −1.04993·107 | −2.27994·106 |
| η′3 / K3 | 1.79808·109 | 2.74913·109 | 2.36279·109 | 3.47185·108 |
| η′4 / K4 | −1.28243·1011 | −2.24664·1011 | −1.97840·1011 | −9.56343·109 |
| AADa / % | 0.36 | 0.66 | 0.94 | 0.71 |
| T range / K | 283.15–473.15 | 283.15–523.15 | 283.15–573.15 | 293.15–573.15 |
Absolute average deviation of η′ to the fit
Figure 3.

Relative deviations for liquid dynamic viscosity of investigated n-alkanes at saturation conditions from the fits of our experimental data (eq 2,  ) as a function of temperature. (a) n-C6H14:
) as a function of temperature. (a) n-C6H14:  , this work;
, this work;  , reference correlation from Michailiou et al.;46
, reference correlation from Michailiou et al.;46
 , Oliveira and Wakeham;47
, Oliveira and Wakeham;47
 , Assael et al.;48
, Assael et al.;48
 , Berstad;49
, Berstad;49
 , Knapstad et al.;50
, Knapstad et al.;50
 , Grigor’ev et al.;51
, Grigor’ev et al.;51
 , Bauer and Meerlender;52
, Bauer and Meerlender;52
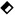 , Dymond and Young;53
, Dymond and Young;53
 , Dymond et al.;54
, Dymond et al.;54
 , Agaev and Golubev.55 (b) n-C8H18:
, Agaev and Golubev.55 (b) n-C8H18:  , this work;
, this work;  , reference correlation from Huber et al.;13
, reference correlation from Huber et al.;13
 , Dymond and Young;53
, Dymond and Young;53
 , Badalyan and Rodchenko;56
, Badalyan and Rodchenko;56
 , Knapstad et al.;50
, Knapstad et al.;50
 , Keramidi and Badalyan;57
, Keramidi and Badalyan;57
 , Oliveria and Wakeham;47
, Oliveria and Wakeham;47
 , Harris et al.;58
, Harris et al.;58
 , Caudwell et al.59 (c) n-C10H22:
, Caudwell et al.59 (c) n-C10H22:  , this work;
, this work;  , reference correlation from Huber et al.;13
, reference correlation from Huber et al.;13
 , Dymond and Young;60
, Dymond and Young;60
 , Knapstad et al.;50
, Knapstad et al.;50
 , Assael et al.;61
, Assael et al.;61
 , Oliveira and Wakeham;47
, Oliveira and Wakeham;47
 , Caudwell et al.59 (d) n-C16H34:
, Caudwell et al.59 (d) n-C16H34:  this work;
this work;  , reference correlation from Meng et al.;14
, reference correlation from Meng et al.;14
 , Nederbragt and 17 Boelhouwer;62
, Nederbragt and 17 Boelhouwer;62
 , Dymond and Young;53
, Dymond and Young;53
 , Dymond et al.;54
, Dymond et al.;54
 , Wakefield and Marsh;63
, Wakefield and Marsh;63
 , Wakefield;64
, Wakefield;64
 , Tanaka et al.;65
, Tanaka et al.;65
 , Wu et al.;66
, Wu et al.;66
 , Mohammed et al.;67
, Mohammed et al.;67
 , Sanchez-Vicente et al.68 Dotted lines (
, Sanchez-Vicente et al.68 Dotted lines ( ) represent the uncertainties of the fits based on the average uncertainty of our experimental data.
) represent the uncertainties of the fits based on the average uncertainty of our experimental data.
For the surface tensions of the studied n-alkanes, decreasing values with decreasing alkyl chain length and increasing temperature are found, as it can be seen in the lower part of Figure 2. The experimental surface tension data are represented by a modified van der Waals equation according to
| (3) |
where all data have the same statistical weight in the fitting. In eq 3, TR (= T/TC) is the reduced temperature and requires information on the critical temperature TC. For the latter property, the data given in Table 1 were employed. Table 4 lists the fit parameters σ0, σ1, and σ2 as well as the AAD of the measured σ data from eq 3 ranging between (0.36 and 0.95) %. The relative deviations of the experimental data from the fits are shown in Figure 4 and are in most cases smaller than the experimental uncertainties of the measured data.
Table 4.
Coefficients of Equation 3 for the Surface Tension σcalc(T) of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 at Saturation Conditions.
| n-C6H14 | n-C8H18 | n-C10H22 | n-C16H34 | |
|---|---|---|---|---|
| σ0 / (mN·m−1) | 51.43 | 56.49 | 44.14 | 68.79 |
| σ1 | 0.5378 | −0.0088 | 0.7697 | −0.9615 |
| σ2 | −0.7079 | −0.1077 | −0.7197 | 0.8507 |
| AADa / % | 0.50 | 0.36 | 0.95 | 0.91 |
| T range / K | 283.15–473.15 | 283.15–523.15 | 283.15–573.15 | 293.15–573.15 |
Absolute average deviation of η′ to the fit
Figure 4.

Relative deviations for the surface tension of investigated n-alkanes at saturation conditions from the fits of our experimental data (eq 3,  ) as a function of temperature. (a) n-C6H14:
) as a function of temperature. (a) n-C6H14:  , this work;
, this work;  , reference correlation from Mulero et al.;11 ;
, reference correlation from Mulero et al.;11 ;  , experimental literature data as given in Table 6. (b) n-C8H18:
, experimental literature data as given in Table 6. (b) n-C8H18:  , this work;
, this work;  , reference correlation from Mulero et al.;11
, reference correlation from Mulero et al.;11
 , experimental literature data as given in Table 6. (c) n-C10H22:
, experimental literature data as given in Table 6. (c) n-C10H22:  , this work;
, this work;  , reference correlation from Mulero et al.;11
, reference correlation from Mulero et al.;11
 , experimental literature data as given in Table 6. (d) n-C16H34:
, experimental literature data as given in Table 6. (d) n-C16H34:  , this work;
, this work;  , reference correlation from Huber;12
, reference correlation from Huber;12
 , experimental literature data as given in Table 6. Dotted lines (
, experimental literature data as given in Table 6. Dotted lines ( ) represent the uncertainties of the fits based on the average uncertainty of our experimental data.
) represent the uncertainties of the fits based on the average uncertainty of our experimental data.
Comparison with literature.
In the following, our experimental data for the saturated liquid dynamic viscosity and surface tension of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 are compared with literature data in the form of the corresponding reference correlations implemented in the REFPROP database9,38-41 as well as experimental data measured in the temperature range of interest in this study. For the viscosity, only the primary experimental data sets used in the development of the respective reference correlations were considered. For the surface tension, all available sources collected by the ThermoData Engine (TDE), version 10.3,8 were employed. This was necessary because it is not clear which surface tension data were used as the basis of the reference correlations of n-C6H14, n-C8H18, and n-C10H22 developed by Mulero et al.11 The relevant details on the literature data considered in this study including the experimental methods used, the specified uncertainties, the number of data points, and the values for the AADs from the fits of our experimental data according to eqs 2 and 3 are summarized in Tables 5 and 6. In Figures 3 and 4, the relative deviations of our SLS results and of the literature data from eqs 2 and 3 are shown as a function of temperature. For visibility purposes, only exemplary error bars representing the total uncertainties of the individual SLS measurements are shown, while error bars related to the literature data are omitted. For the representation of the uncertainty of the fits illustrated by the dotted lines in Figures 3 and 4, the mean uncertainty of our experimental SLS data averaged over the various temperatures was used.
Table 5.
Reference Correlations and Primary Data Sets for the Saturated Liquid Dynamic Viscosity of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 Used for Comparison with the SLS Measurements
| 1st author | year of publication |
technique employeda |
100Ur | number of data points |
AADb / % |
|---|---|---|---|---|---|
| n-C6H14 | |||||
| Reference correlation | |||||
| Michailidou46 | 2013 | 0.5 | |||
| Primary data | |||||
| Oliveira47 | 1992 | VW | 0.5–2.4 | 3 | 0.2 |
| Assael48 | 1991 | VW | 0.5 | 1 | 0.7 |
| Berstad49 | 1989 | OCup | 1.15 | 6 | 1.0 |
| Knapstad50 | 1989 | OCup | 0.33–0.56 | 7 | 0.3 |
| Grigor’ev51 | 1988 | Cap | 0.9c | 4 | 0.7 |
| Bauer52 | 1984 | Cap | 0.3 | 4 | 0.6 |
| Dymond53 | 1980 | Cap | 0.5 | 12 | 0.7 |
| Dymond54 | 1980 | FB | 2 | 4 | 1.5 |
| Agaev55 | 1963 | Cap | 1-3c | 21 | 1.6 |
| n-C8H18 | |||||
| Reference correlation | |||||
| Huber13 | 2004 | 0.8 | |||
| Primary data | |||||
| Dymond53 | 1980 | Cap | 0.5 | 8 | 0.8 |
| Badalyan56 | 1986 | Cap | 1 | 10 | 2.0 |
| Knapstad50 | 1989 | OCup | 0.33–0.56 | 5 | 0.5 |
| Keramidi57 | 1991 | Cap | 1–3d | 6 | 3.2 |
| Oliveira47 | 1992 | VW | 0.5–2.4 | 3 | 0.4 |
| Harris58 | 1997 | FB | 2 | 5 | 0.4 |
| Caudwell59 | 2009 | VW | 2 | 4 | 0.7 |
| n-C10H22 | |||||
| Reference correlation | |||||
| Huber13 | 2004 | 1.6 | |||
| Primary data | |||||
| Dymond60 | 1981 | Cap | 0.5 | 8 | 0.5 |
| Knapstad50 | 1989 | OCup | 0.33–0.56 | 11 | 0.5 |
| Assael61 | 1992 | VW | 0.5 | 6 | 0.5 |
| Oliveira47 | 1992 | VW | 0.5–2.4 | 3 | 0.7 |
| Caudwell59 | 2009 | VW | 2 | 4 | 0.2 |
| n-C16H34 | |||||
| Reference correlation | |||||
| Meng14 | 2018 | 0.9 | |||
| Primary data | |||||
| Nederbragt62 | 1947 | Cap | N/Ae | 5 | 1.0 |
| Dymond53 | 1980 | Cap | 0.5 | 4 | 0.4 |
| Dymond54 | 1980 | FB | 2 | 10 | 0.5 |
| Wakefield63 | 1987 | Cap | 0.5 | 3 | 0.5 |
| Wakefield64 | 1988 | Cap | 0.5 | 2 | 0.2 |
| Tanaka65 | 1991 | TC | 2 | 3 | 1.1 |
| Wu66 | 1998 | Cap | 0.1 | 4 | 1.0 |
Cap, Capillary viscometer; FB, Falling body viscometer; OCup, Oscillating cup viscometer; TC, Torsional crystal viscometer; VW, Vibrating wire viscometer.
Absolute average deviation between the literature data set and eq 2
Uncertainties taken from Reference 46
Uncertainties taken from Reference 13
Uncertainties are not specified
Table 6.
Reference Correlations and Available Experimental Data Sets for Surface Tension of n-C6H14, n-C8H18, n-C10H22, and n-C16H34 Used for Comparison with the SLS Measurements
| 1st author | year of publication |
technique employeda |
100Ur | number of data points |
AADb / % |
|---|---|---|---|---|---|
| n-C6H14 | |||||
| Reference correlation | |||||
| Mulero11 | 2012 | 1.2 | |||
| Primary data | |||||
| Schiff77 | 1884 | CR | 1.75–2.4 | 2 | 1.2 |
| Dutoit70 | 1900 | CR | N/Ac | 2 | 5.0 |
| Morgan78 | 1913 | DW | N/Ac | 2 | 2.4 |
| Harkins71 | 1917 | DW | N/Ac | 1 | 5.3 |
| Harkins79 | 1921 | DW | N/Ac | 4 | 0.8 |
| Hennaut-Roland80 | 1931 | CR | N/Ac | 3 | 0.9 |
| Trieschmann81 | 1935 | MBP | 0.05 | 1 | 2.4 |
| Wibaut82 | 1939 | MBP | 0.15 | 1 | 0.8 |
| Quayle83 | 1944 | MBP | N/Ac | 2 | 0.3 |
| Vogel84 | 1946 | CR | N/Ac | 7 | 1.3 |
| Jasper85 | 1953 | CR | 0.05 | 7 | 0.8 |
| Jasper86 | 1955 | CR | 0.05 | 7 | 0.6 |
| Murphy72 | 1957 | RD | N/Ac | 1 | 7.5 |
| Ben'kovskii87 | 1964 | CR + MBP | 0.5 | 3 | 0.9 |
| Clever88 | 1963 | MBP | N/Ac | 3 | 0.7 |
| Schmidt89 | 1966 | MBP | N/Ac | 4 | 0.7 |
| Ridgway73 | 1967 | RD | 1 | 1 | 3.8 |
| Skripov75 | 1968 | CR | 0.6–1.5 | 6 | 5.8 |
| Skripov74 | 1968 | DV | N/Ac | 3 | 7.5 |
| Bagdasaryan76 | 1979 | N/Ac | N/Ac | 20 | 3.5 |
| Ross90 | 1979 | PD | 0.35 | 1 | 0.9 |
| Grigoryev69 | 1985 | CR | N/Ac | 20 | 0.6 |
| Grigoryev91 | 1992 | CR | N/Ac | 6 | 1.5 |
| Papaioannou92 | 1994 | CR | 0.25 | 1 | 0.2 |
| Pineiro93 | 1999 | DV | 0.2 | 1 | 0.9 |
| Jimenez94 | 2000 | DV | 0.06 | 1 | 0.7 |
| Penas95 | 2000 | DV | 0.15 | 1 | 0.9 |
| Pandey96 | 2001 | CR | 4 | 1 | 1.2 |
| Azizian97 | 2006 | RD | 0.06 | 5 | 1.0 |
| Dominguez-Perez98 | 2006 | DV | 0.06 | 1 | 0.8 |
| Giner99 | 2007 | DV | 0.06 | 7 | 1.4 |
| Azizian100 | 2008 | RD | 0.1 | 5 | 1.0 |
| Giner101 | 2008 | DV | 0.01 | 7 | 1.4 |
| Perez-Gregorio102 | 2009 | DV | 0.06 | 7 | 1.4 |
| Dominguez-Perez103 | 2010 | DV | 0.06 | 1 | 0.8 |
| Perez-Navarro104 | 2010 | DV | 0.06 | 1 | 1.6 |
| Wang105 | 2011 | RD | 0.5 | 5 | 2.0 |
| Mejia106 | 2012 | MBP | 1.1 | 1 | 0.7 |
| Garrido107 | 2014 | PD | 0.06 | 4 | 0.9 |
| Mejia108 | 2014 | MBP | 0.06 | 1 | 0.7 |
| Tahery109 | 2017 | PD | 0.5 | 1 | 0.7 |
| n-C8H18 | |||||
| Reference correlation | |||||
| Mulero11 | 2012 | 0.9 | |||
| Primary data | |||||
| Harkins71 | 1917 | DW | N/Ac | 1 | 1.8 |
| Harkins79 | 1921 | DW | N/Ac | 4 | 1.7 |
| Richards110 | 1924 | CR | N/Ac | 2 | 1.6 |
| Hennau-Roland80 | 1931 | CR | N/Ac | 3 | 1.8 |
| Wibaut82 | 1939 | MBP | 0.15 | 1 | 1.5 |
| Manzoni-Ansidei111 | 1940 | N/Ac | N/Ac | 5 | 1.1 |
| Quayle83 | 1944 | MBP | N/Ac | 4 | 1.5 |
| Smith112 | 1944 | PD | N/Ac | 1 | 1.5 |
| Vogel84 | 1946 | CR | N/Ac | 5 | 1.1 |
| Jasper85 | 1953 | CR | 0.05 | 6 | 1.5 |
| Jasper86 | 1955 | CR | 0.05 | 10 | 0.7 |
| Ben'kovskii87 | 1964 | CR + MBP | 0.5 | 3 | 1.9 |
| Baglay113 | 1988 | CR | 0.5 | 4 | 1.4 |
| Ramkumar114 | 1989 | CR | 7.7 | 3 | 0.2 |
| Grigoryev91 | 1992 | CR | N/Ac | 8 | 2.9 |
| Pineiro93 | 1999 | DV | 0.2 | 1 | 1.3 |
| Penas95 | 2000 | DV | 0.15 | 1 | 1.3 |
| Segade115 | 2003 | DV | N/Ac | 1 | 1.2 |
| Mosteiro116 | 2009 | DV | 0.4 | 1 | 0.4 |
| Mejia117 | 2011 | MBP | 0.5 | 3 | 0.9 |
| Gayol118 | 2013 | DV | 0.5 | 1 | 0.5 |
| Lopez-Lazaro119 | 2015 | DV | 0.15 | 5 | 1.9 |
| Tahery109 | 2017 | PD | 0.5 | 1 | 1.4 |
| n-C10H22 | |||||
| Reference correlation | |||||
| Mulero11 | 2012 | 1.3 | |||
| Primary data | |||||
| Hennaut-Roland80 | 1931 | CR | N/Ac | 3 | 3.3 |
| Quayle83 | 1944 | MBP | N/Ac | 3 | 2.9 |
| Vogel84 | 1946 | CR | N/Ac | 6 | 2.2 |
| Mumford120 | 1950 | MBP | N/Ac | 1 | 3.7 |
| Jasper85 | 1953 | CR | 0.05 | 6 | 2.8 |
| Jasper86 | 1955 | CR | 0.05 | 10 | 1.8 |
| Ben'kovskii87 | 1964 | CR + MBP | 0.5 | 3 | 3.2 |
| Korosi121 | 1981 | CR | 0.15 | 2 | 1.6 |
| Nagarajan122 | 1986 | PD | 2-3 | 2 | 1.0 |
| Pineiro93 | 1999 | PD | 0.2 | 1 | 2.7 |
| Penas95 | 2000 | PD | 0.2 | 1 | 2.4 |
| Domanska123 | 2002 | RD | 0.1 | 2 | 2.7 |
| Gomez-Diaz124 | 2002 | WP | N/Ac | 1 | 5.4 |
| Rolo125 | 2002 | WP | 1 | 5 | 4.7 |
| Queimada126 | 2005 | WP | 0.1 | 6 | 4.0 |
| Mosteiro116 | 2009 | DV | 0.4 | 1 | 1.7 |
| Mohsen-Nia127 | 2010 | MBP | 0.2 | 6 | 2.2 |
| Mejia117 | 2011 | MBP | 0.5 | 3 | 2.8 |
| Gayol118 | 2013 | DV | 0.5 | 1 | 1.0 |
| Luning Prak128 | 2017 | PD | 0.8 | 1 | 3.4 |
| n-C16H34 | |||||
| Reference correlation | |||||
| Huber12 | 2017 | 2.1 | |||
| Primary data | |||||
| Vogel84 | 1946 | CR | N/Ac | 5 | 1.6 |
| Jasper85 | 1953 | CR | 0.05 | 9 | 1.7 |
| Fox129 | 1955 | RD | 0.1 | 1 | 1.2 |
| Jasper86 | 1955 | CR | 0.05 | 9 | 1.1 |
| Koefoed130 | 1958 | CR | 0.10 | 2 | 0.7 |
| Myers131 | 1969 | MBP | 0.15 | 1 | 1.2 |
| Korosi121 | 1981 | CR | 0.15 | 2 | 1.0 |
| Rolo125 | 2002 | WP | 1.00 | 6 | 2.8 |
| Mohsen-Nia132 | 2011 | MBP | 0.16 | 10 | 1.4 |
| Luning Prak133 | 2014 | PD | 1 | 1 | 0.5 |
| Luning Prak134 | 2016 | PD | 1 | 1 | 0.4 |
| Luning Prak128 | 2017 | PD | 1 | 1 | 1.0 |
| Luning Prak135 | 2017 | PD | 1 | 1 | 0.0 |
| Luning Prak136 | 2017 | PD | 1 | 1 | 0.0 |
CR, Capillary rise method; DW, Drop weight method; MBP, Maximum bubble pressure method; RD, Ring detachment method; PD, Pendent drop method; DV, Drop volume method; WP, Wilhelmy plate method
Absolute average deviation (AAD) between the literature data set and eq 3
Uncertainties are not specified
Liquid viscosity.
For the viscosity of n-C6H14 shown in Figure 3a, the reference correlation developed by Michailidou et al.46 together with nine primary data sets47-55 are compared to eq 2. Agreement between our measurements and the reference correlation, which is specified with an uncertainty of (2 and 6) % below and above 450 K, is found within the experimental uncertainties. All primary data sets agree with our fit within the combined expanded uncertainties and show an AAD of 1.7 %. As can be seen from Figure 3a, our data follow the reference correlation at temperatures above 370 K better than the available literature data.
For the liquid viscosity of n-C8H18, Figure 3b shows that the reference correlation developed by Huber et al.13 and specified with an expanded uncertainty of 0.6 % along the saturated liquid line agrees with our experimental data within combined uncertainties over the complete temperature range up to 523.15 K. For the seven primary data sets, agreement with our fit within combined uncertainties is found up to temperatures of 448.15 K. At larger temperatures, the experimental data reported by Badalyan and Rodchenko56 as well as Keramidi and Badalyan57 show increasing negative deviations from eq 2 with maximum values up to −5.3 % at 523.15 K.
Values for the liquid viscosity of n-C10H22 at saturation conditions are compared in Figure 3c with the reference correlation from Huber et al.13 specified with an uncertainty of 1 % and with the five underlying primary data sets47,50,53,56-59 obtained at temperatures up to 423.15 K. Within the temperature range between (283.15 and 423.15) K, agreement between our data, the reference correlation, and the primary experimental data within combined uncertainties is found. Above 423.15 K, the reference correlation yields slightly larger viscosity values than our fit with maximum relative deviations up to 4.6 %. This trend seems to be caused by the extrapolative character of the reference correlation which is based on primary data for the saturated liquid viscosity measured only up to 423.15 K.
For the liquid viscosity of n-C16H34, the reference correlation developed by Meng et al.14 with a specified uncertainty of 1 % as well as the seven primary data sets along the saturation line are available for data comparison in Figure 3d. Over the entire temperature range, all literature data agree with our correlation, eq 2, within combined uncertainties, resulting in AAD values below 1.1 %. As it can be seen in Figure 3d, the reference correlation follows the experimental data from Nederbragt and Boelhouwer62 who performed measurements at temperatures between (298.15 and 518.15) K.
Surface tension.
For the surface tension of n-C6H14 shown in Figure 4a, agreement between our data and the reference correlation from Mulero et al.11 within relative absolute deviations of 2 % is found over the entire temperature range between (283.15 and 473.15) K. The same statement holds also for most of the totally 41 different experimental data sets, including the measurements of Grigoryev et al.69 which follow our data and the reference correlation up to 473.15 K. At temperatures below 340 K, larger positive and negative deviations from eq 3, which are up to 7.5 % and outside combined uncertainties, are observed for the data measured by Dutoit and Friedrich,70 Harkins et al.,71 Murphy et al.,72 and Ridgway and Butler.73 At temperatures above 400 K, the three further data sets from Skripov and Sinitsyn,74 Skripov and Firsov,75 and Bagdasaryan76 show increasing negative relative deviations from the present work with values up to −9 %.
Figure 4b shows the comparison of surface tension data of n-C8H18. With the exception of the data reported by Grigoryev et al.,91 all further 22 experimental data sets and the reference correlation from Mulero et al.11 agree well with our measurement results with an AAD of 1.4 %. It should be mentioned that the measurements of Grigoryev et al.91 match with our correlation, eq 3, inside combined uncertainties for temperatures below 400 K, before larger relative deviations occur at larger temperatures. While the surface tension data of Grigoryev et al.91 deviates by +6.3 % at 428.15 K, a further temperature increase results from positive deviations crossing eq 3 to negative deviations of −10.4 % at 522.48 K. The same trend seems also to be reflected in the behavior of the reference correlation from Mulero et al.11 relative to our data at temperatures above 450 K.
In connection with the comparison of the surface tension of n-C10H22 illustrated in Figure 3c, the experimental literature data available up to 373 K as well as the reference correlation of Mulero et al.11 are slightly larger than our experimental data. For temperatures between (283.15 and 373.15) K, the AAD of all literature sources from eq 3 is 2.7 %. Due to the lack of literature data above 373 K, the reference correlation is purely predictive where its extrapolation can represent the temperature-dependent trend of our SLS data.
The situation for the surface tension of n-C16H34 shown in Figure 4d is similar to that for n-C10H22. For temperatures up to 373 K where experimental data are present, the literature data including the reference correlation from Huber12 are generally larger than our surface tension values represented by eq 3 with an AAD of 1.4 %. The increasing deviations of the reference correlation from our fit at temperatures above 500 K can again be related to the extrapolation at states where no experimental data could be considered in the development of the correlation.
Development of empirical models for liquid viscosity and surface tension of n-alkanes.
In this section, simple empirical models for the saturated liquid viscosity and surface tension of n-alkanes as a function of temperature and number of carbon atoms are presented. For this purpose, our experimental results for n-C6H14, n-C8H18, n-C10H22, and n-C16H34 presented in this work in Table 2 as well as for n-C12H26 and n-C28H58 reported in our previous study31 were used as target set. In a first step, the data for the viscosity η′ and surface tension σ were fitted as a function of temperature for the six n-alkanes. In a second step, the fitting parameters obtained from the first step were then fitted as a function of the number of carbon atoms in the respective n-alkanes.
For the correlation of the liquid dynamic viscosity of n-alkanes, we used an empirical correlation in terms of the reduced temperature TR (= T/TC) according to
| (4) |
where η′i,C are fitting parameters which are a function of the number of carbon atoms ranging between 6 and 28. In comparison with the data correlation in eq 2, we found that it is appropriate to terminate the polynomial extension after the third term to obtain fitting parameters which show a clear dependency of the number of carbon atoms. For the critical temperature TC of the n-alkanes, the REFPROP database9 was employed. The coefficients η′i,C are fitted by a second order polynomial as a function of the number of carbon atoms nC according to
| (5) |
where Ai, Bi, and Ci are fitting parameters given in Table 7. In the fitting procedures, the same statistical weight of each measured data point for η′ is assumed. Based on the empirical fit equations according to eqs 4 and 5, the totally 72 experimental data points of the six n-alkanes used as training set can be represented with an AAD of 1.9 %.
Table 7.
Parameters in the Empirical Models for the Liquid Dynamic Viscosity η′i,C and Surface Tension σi,C of n-Alkanes at Saturation Conditions as a Function of Temperature and Number of Carbon Atoms Ranging Between 6 and 28
| Parameters for η′i,C in eq 5 used in eq 4 | ||
|---|---|---|
| A0 = −1.1147·10−1 | B0 = 1.1829 | C0 = −1.7983·10−2 |
| A1 = −1.4660 | B1 = −4.1141 | C1 = 4.8808·10−2 |
| A2 = −2.7482 | B2 = 5.0579 | C2 = −3.5541·10−2 |
| A3 = 1.3182 | B3 = −2.1079 | C3 = 1.4650·10−3 |
| Parameters for σi,C in eq 7 used in eq 6 | ||
| D = 5.6792·101 | E = −4.5458·10−1 | F = 6.0013·10−3 |
For the correlation of the surface tension of n-alkanes, the van der Waals equation137
| (6) |
was found to be appropriate for the representation of the measured σ data over a wide temperature range. Though eq 6 with the universal critical exponent 1.26 is derived from the scaling theory for the description of the surface tension in vicinity of the critical point, we found that it can also be used for a broader range of surface tensions as studied in this work. In eq 6, the parameters σC is fitted as a function of the number of carbon atoms nC via
| (7) |
The coefficients in eq 7 are summarized in Table 7. The same statistical weight for each data point entering into the fitting procedures was assumed. With the developed correlation given by eqs 6 to 7, the 72 surface tension data of the considered six n-alkanes are modeled with an AAD of 1.6 %.
For probing the transferability of the developed correlations to n-alkanes that were not considered in the model development, the four n-alkanes n-heptane (n-C7H16), n-nonane (n-C9H20), n-undecane (n-C11H24) and n-eicosane (n-C20H42) with carbon numbers between 7 and 20 were used as a validation set. For these systems, the critical temperatures required in the empirical correlations were taken from the REFPROP database.9,12,40,138,139 The results for liquid viscosity and surface tension predicted by eqs 4 and 6 including their corresponding fit parameters are compared with available reference correlations. For n-C7H16, n-C9H20, and n-C11H24, the corresponding reference correlations11-13,140-142 for η′ and σ implemented in REFPROP9 were adopted. In detail, the reference correlations developed by Michailidou et al.140 for the viscosity and by Mulero et al.11 for the surface tension were used for n-C7H16. For n-C9H20, the correlations reported by Huber et al.13 and Mulero et al.11 for viscosity and surface tension were employed. While the reference correlations from Assael et al.141 and Mulero and Cachadiña142 were used for the viscosity and surface tension of n-C11H24, those reported by Huber12 were considered for n-C20H42.
For the comparison between the empirical correlations developed in this work and the reference correlations, temperature ranges between (283.15 and 520.15) K for n-C7H16, between (283.15 and 573.15) K for n-C9H20 and n-C11H24 as well as between (313.15 and 573.15) K for n-C20H42 were taken into account. The smaller temperature ranges for n-C7H16 and n-C20H42 are required to exclude the regions in vicinity of the critical point of n-C7H16 (TC = 540.2 K)9 and the melting point of n-C20H42 at T = 309.85 K.143 For the four n-alkanes used as a validation set, agreement between the correlations according to eqs 4 and 6 as well as the reference correlations was found, resulting in AAD values of (4.8 and 2.3) % for the liquid dynamic viscosity and the surface tension, respectively. This indicates the transferability of the developed empirical correlations to n-alkanes with varying carbon number.
CONCLUSIONS
The present study has contributed to a reliable experimental database for the liquid viscosity and surface tension of n-hexane, n-octane, n-decane, and n-hexadecane at saturation conditions over a broad temperature range from (283 up to 573) K. With surface light scattering in thermodynamic equilibrium, total average measurement uncertainties (k = 2) of (2.0 and 1.7) % could be achieved for the liquid dynamic viscosity and surface tension. For the studied n-alkanes, agreement between the experimental data, which were represented by a Vogel-type equation for the dynamic viscosity as well as a modified van der Waals equation for the surface tension as a function of temperature, and the available literature data was found. Based on the measured data, empirical models for the liquid viscosity and surface tension of n-alkanes were developed as a function of temperature and carbon number ranging between 6 and 28. Their transfer to further selected n-alkanes which were not considered in the development procedure showed reasonable agreement.
ACKNOWLEDGMENTS
This work was financially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through funding the Erlangen Graduate School in Advanced Optical Technologies (SAOT) within the German Excellence Initiative and via the project Grant FR 1709/15-1.
Footnotes
A contribution of the National Institute of Standards and Technology, not subject to copyright in the U.S.
REFERENCES
- (1).Yao Y; Hildebrandt D; Glasser D; Liu X Fischer–Tropsch Synthesis Using H2/CO/CO2 Syngas Mixtures over a Cobalt Catalyst. Ind. Eng. Chem. Res 2010, 49, 11061–11066. [Google Scholar]
- (2).Li S; Tang Z; Zhou F; Li W; Yuan X Separation of Primary Alcohols and Saturated Alkanes from Fisher–Tropsch Synthesis Products. Chinese J. Chem. Eng 2014, 22, 980–983. [Google Scholar]
- (3).Lu Y; Han X n-Alkane Distributions, Wax Settling Levels, and Responses to Flow Improvers in Diesel Distillations. Pet. Sci. Technol 2014, 32, 2109–2114. [Google Scholar]
- (4).Tekawade A; Oehlschlaeger MA Spray Ignition Experiments for Alkylbenzenes and Alkylbenzene/n-Alkane Blends. Fuel 2017, 195, 49–58. [Google Scholar]
- (5).Dirbude S; Eswaran V; Kushari A Numerical Modelling of Droplet Evaporation with Convection for n-Alkanes and Kerosene Fuel. 2011, 21, 787–798. [Google Scholar]
- (6).Tekawade A; Oehlschlaeger MA An Experimental Study of the Spray Ignition of Alkanes. Fuel 2016, 185, 381–393. [Google Scholar]
- (7).Dortmund Data Bank. www.ddbst.com (accessed July 30, 2019).
- (8).Diky V; Chirico RD; Frenkel M; Bazyleva A; Magee JW; Paulechka E; Kazakov A; Lemmon EW; Muzny CD; Smolyanitsky AY; Townsend S; Kroenlein K ThermoDataEngine, version 10.3; National Institute of Standards and Technology: Gaithersburg, MD, USA: 2018. [Google Scholar]
- (9).Lemmon EW; Bell IH; Huber ML; McLinden MO REFPROP, Standard Reference Data Program, version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA: 2018. [Google Scholar]
- (10).Nieto de Castro CA; Santos FJV; Fareleira JMNA; Wakeham WA Metrology of Viscosity: Have We Learned Enough? J. Chem. Eng. Data 2009, 54, 171–178. [Google Scholar]
- (11).Mulero A; Cachadiña I; Parra MI Recommended Correlations for the Surface Tension of Common Fluids. J. Phys. Chem. Ref. Data 2012, 41, 043105. [Google Scholar]
- (12).Huber ML Preliminary Models for Viscosity, Thermal Conductivity, and Surface Tension of Pure Fluid Constituents of Selected Diesel Surrogate Fuels, NIST Technical Note 1949; National Institute of Standards and Technology: Gaithersburg, MD, USA: 2017. [Google Scholar]
- (13).Huber ML; Laesecke A; Xiang HW Viscosity correlations for minor constituent fluids in natural gas: n-octane, n-nonane and n-decane. Fluid Phase Equilibr. 2004, 224, 263–270. [Google Scholar]
- (14).Meng XY; Sun YK; Cao FL; Wu JT; Vesovic V Reference Correlation of the Viscosity of n-Hexadecane from the Triple Point to 673 K and up to 425 MPa. J. Phys. Chem. Ref. Data 2018, 47, 033102. [Google Scholar]
- (15).Santos FJV; Nieto de Castro CA; Dymond JH; Dalaouti NK; Assael MJ; Nagashima A Standard Reference Data for the Viscosity of Toluene. J. Phys. Chem. Ref. Data 2006, 35, 1–8. [Google Scholar]
- (16).Langevin D Light Scattering by Liquid Surfaces and Complementary Techniques. Marcel Dekker: New York, 1992. [Google Scholar]
- (17).Lucassen-Reynders EH; Lucassen J Properties of Capillary Waves. Adv. Colloid Interfac 1970, 2, 347–395. [Google Scholar]
- (18).Fröba AP Simultane Bestimmung von Viskosität und Oberflächenspannung transparenter Fluide mittels Oberflächenlichtstreuung. Dr.-Ing. Thesis, Friedrich-Alexander-University Erlangen-Nuremberg, 2002. [Google Scholar]
- (19).Fröba AP; Will S Light Scattering by Surface Waves – Surface Light Scattering. In Experimental Thermodynamics, Vol. IX: Advances in Transport Properties of Fluids, Assael MJ; Goodwin ARH; Vesovic V; Wakeham WA, Eds. Royal Society of Chemistry: Cambridge, U.K., 2014; p 22–35. [Google Scholar]
- (20).Fröba AP; Leipertz A Accurate Determination of Liquid Viscosity and Surface Tension Using Surface Light Scattering (SLS): Toluene Under Saturation Conditions Between 260 and 380 K. Int. J. Thermophys 2003, 24, 895–921. [Google Scholar]
- (21).Fröba AP; Leipertz A Viscosity and Surface Tension of Saturated Toluene from Surface Light Scattering (SLS). Int. J. Thermophys 2001, 22, 41–59. [Google Scholar]
- (22).Fröba AP; Leipertz A Viscosity of Diisodecyl Phthalate by Surface Light Scattering (SLS). J. Chem. Eng. Data 2007, 52, 1803–1810. [Google Scholar]
- (23).Fröba AP; Pellegrino LP; Leipertz A Viscosity and Surface Tension of Saturated n-Pentane. Int. J. Thermophys 2004, 25, 1323–1337. [Google Scholar]
- (24).Fröba AP; Botero C; Leipertz A Thermal Diffusivity, Sound Speed, Viscosity, and Surface Tension of R227ea (1,1,1,2,3,3,3-Heptafluoropropane). Int. J. Thermophys 2006, 27, 1609–1625. [Google Scholar]
- (25).Fröba AP; Kremer H; Leipertz A; Flohr F; Meurer C Thermophysical Properties of a Refrigerant Mixture of R365mfc (1,1,1,3,3-Pentafluorobutane) and Galden® HT 55 (Perfluoropolyether). Int. J. Thermophys 2007, 28, 449–480. [Google Scholar]
- (26).Rausch MH; Kretschmer L; Will S; Leipertz A; Fröba AP Density, Surface Tension, and Kinematic Viscosity of Hydrofluoroethers HFE-7000, HFE-7100, HFE-7200, HFE-7300, and HFE-7500. J. Chem. Eng. Data 2015, 60, 3759–3765. [Google Scholar]
- (27).Fröba AP; Kremer H; Leipertz A Density, Refractive Index, Interfacial Tension, and Viscosity of Ionic Liquids [EMIM][EtSO4], [EMIM][NTf2], [EMIM][N(CN)2], and [OMA][NTf2] in Dependence on Temperature at Atmospheric Pressure. J. Phys. Chem. B 2008, 112, 12420–12430. [DOI] [PubMed] [Google Scholar]
- (28).Kolbeck C; Lehmann J; Lovelock KRJ; Cremer T; Paape N; Wasserscheid P; Fröba AP; Maier F; Steinrück HP Density and Surface Tension of Ionic Liquids. J. Phys. Chem. B 2010, 114, 17025–17036. [DOI] [PubMed] [Google Scholar]
- (29).Koller TM; Ramos J; Schulz PS; Economou IG; Rausch MH; Fröba AP Thermophysical Properties of Homologous Tetracyanoborate-Based Ionic Liquids Using Experiments and Molecular Dynamics Simulations. J. Phys. Chem. B 2017, 121, 4145–4157. [DOI] [PubMed] [Google Scholar]
- (30).Koller T; Rausch MH; Schulz PS; Berger M; Wasserscheid P; Economou IG; Leipertz A; Fröba AP Viscosity, Interfacial Tension, Self-Diffusion Coefficient, Density, and Refractive Index of the Ionic Liquid 1-Ethyl-3-methylimidazolium Tetracyanoborate as a Function of Temperature at Atmospheric Pressure. J. Chem. Eng. Data 2012, 57, 828–835. [Google Scholar]
- (31).Koller TM; Klein T; Giraudet C; Chen J; Kalantar A; van der Laan GP; Rausch MH; Fröba AP Liquid Viscosity and Surface Tension of n-Dodecane, n-Octacosane, Their Mixtures, and a Wax between 323 and 573 K by Surface Light Scattering. J. Chem. Eng. Data 2017, 62, 3319–3333. [Google Scholar]
- (32).Klein T; Cui J; Kalantar A; Chen J; Rausch MH; Koller TM; Fröba AP Viscosity and Surface Tension of Branched Alkanes 2-Methylnonane and 4-Methylnonane. J. Chem. Eng. Data 2018, 63, 2833–2839. [Google Scholar]
- (33).Klein T; Cui J; Kalantar A; Chen J; Rausch MH; Koller TM; Fröba AP Liquid Viscosity and Interfacial Tension of Binary and Ternary Mixtures Containing n-Octacosane by Surface Light Scattering. J. Chem. Eng. Data 2019, 64, 817–826. [Google Scholar]
- (34).Koller TM; Klein T; Chen J; Kalantar A; van der Laan GP; Rausch MH; Froba AP Simultaneous Analysis of Equilibrium Fluctuations at the Surface and in the Bulk of a Binary Liquid Mixture by Dynamic Light Scattering. J Phys Chem B 2017, 121, 10950–10956. [DOI] [PubMed] [Google Scholar]
- (35).Koller TM; Prucker T; Cui J; Klein T; Fröba AP Interfacial tensions and viscosities in multiphase systems by surface light scattering (SLS). J. Colloid Interf. Sci 2019, 538, 671–681. [DOI] [PubMed] [Google Scholar]
- (36).Commercial equipment, instruments, or materials are identified only in order to adequately specify certain procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the products identified are necessarily the best available for the purpose.
- (37).Helium Datasheet. https://produkte.linde-gase.de/db_neu/helium_5.0.pdf (accessed May 24, 2019).
- (38).Thol M; Wang Y; Lemmon EW; Span R Fundamental Equations of State for Hydrocarbons. Part II. n-Hexane. to be published 2018. [Google Scholar]
- (39).Beckmueller R; Thol M; Lemmon EW; Span R Fundamental Equation of State for n-Octane. to be submitted to Int. J. Thermophys 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lemmon EW; Span R Short Fundamental Equations of State for 20 Industrial Fluids. Journal of Chemical & Engineering Data 2006, 51, 785–850. [Google Scholar]
- (41).Romeo R; Lemmon EW to be submitted 2018. [Google Scholar]
- (42).Ortiz-Vega DO A New Wide Range Equation of State for Helium-4. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2013. [Google Scholar]
- (43).Arp VD; McCarty RD; Friend DG Thermophysical Properties of Helium-4 from 0.8 to 1500 K with Pressures to 2000 MPa, Technical Note 1334 (revised); National Institute of Standards and Technology: Boulder, 1998. [Google Scholar]
- (44).Lucas K; Luckas M Berechnungsmethoden für Stoffeigenschaften. In VDI-Wärmeatlas, Abschnitt DA; Verein Deutscher Ingenieure: Düsseldorf, Germany: 1984. [Google Scholar]
- (45).Poling B; Prausnitz JM; O'Connell JP The Properties of Gases and Liquids. 5th ed.; McGraw-Hill: New York: 2001. [Google Scholar]
- (46).Michailidou EK; Assael MJ; Huber ML; Perkins RA Reference Correlation of the Viscosity of n-Hexane from the Triple Point to 600 K and up to 100 MPa. J. Phys. Chem. Ref. Data 2013, 42, 033104. [Google Scholar]
- (47).Oliveira CMBP; Wakeham WA The viscosity of five liquid hydrocarbons at pressures up to 250 MPa. Int. J. Thermophys 1992, 13, 773–790. [Google Scholar]
- (48).Assael MJ; Papadaki M; Dix M; Richardson SM; Wakeham WA An absolute vibrating-wire viscometer for liquids at high pressures. Int. J. Thermophys 1991, 12, 231–244. [Google Scholar]
- (49).Berstad DA Viscosity and Density of n-Hexane, Cyclohexane and Benzene, and Their Binary Mixtures with Methane. Ph.D. thesis, Universitetet i Trondheim, Institutt for Uorganisk Kjemi Norges; Tekniske Hegskole, 1989. [Google Scholar]
- (50).Knapstad B; Skjoelsvik PA; Oeye HA Viscosity of pure hydrocarbons. J. Chem. Eng. Data 1989, 34, 37–43. [Google Scholar]
- (51).Grigor’ev BA; Keramidi AS; Rodchenko SI; Grachev VK Viscosity of normal hexane at low temperature. Izv. Vyssh. Uchebn. Zaved., Neft Gaz 1988, 31, 53–55. [Google Scholar]
- (52).Bauer H; Meerlender G Precise viscosity measurements of Newtonian liquids with v < 1 mm2/s for the selection of suitable standards. Rheol. Acta 1984, 23, 514–521. [Google Scholar]
- (53).Dymond JH; Young KJ Transport properties of nonelectrolyte liquid mixtures—I. Viscosity coefficients for n-alkane mixtures at saturation pressure from 283 to 378 K. Int. J. Thermophys 1980, 1, 331–344. [Google Scholar]
- (54).Dymond JH; Young KJ; Isdale JD Transport properties of nonelectrolyte liquid mixtures—II. Viscosity coefficients for the n-hexane + n-hexadecane system at temperatures from 25 to 100°C at pressures up to the freezing pressure or 500 MPa. Int. J. Thermophys 1980, 1, 345–373. [Google Scholar]
- (55).Agaev NA; Golubev IF Viscosity of n-hexane in liquid and gaseous phases at high pressures and various temperatures. Dokl. Akad.Nauk SSSR 1963, 151, 597–600. [Google Scholar]
- (56).Badalyan AG; Rodchenko SI -. Izv. Vyssh. Uchebn. Zaved., Neft iGaz 1986, 29, 61–64. [Google Scholar]
- (57).Keramidi ASB, A. G. Viscosity of n-Octane during Enrichment. Izv. Vyssh. Uchebn. Zaved., Neft Gaz 1991, 3, 58–60. [Google Scholar]
- (58).Harris KR; Malhotra R; Woolf LA Temperature and Density Dependence of the Viscosity of Octane and Toluene. J. Chem. Eng. Data 1997, 42, 1254–1260. [Google Scholar]
- (59).Caudwell DR; Trusler JPM; Vesovic V; Wakeham WA Viscosity and Density of Five Hydrocarbon Liquids at Pressures up to 200 MPa and Temperatures up to 473 K. J. Chem. Eng. Data 2009, 54, 359–366. [Google Scholar]
- (60).Dymond JH; Young KJ Transport properties of nonelectrolyte liquid mixtures—V. Viscosity coefficients for binary mixtures of benzene plus alkanes at saturation pressure from 283 to 393 K. Int. J. Thermophys 1981, 2, 237–247. [Google Scholar]
- (61).Assael MJ; Dymond JH; Papadaki M Viscosity coefficients of binary n-heptane+n-alkane mixtures. Fluid Phase Equilibr. 1992, 75, 287–297. [Google Scholar]
- (62).Nederbragt GW; Boelhouwer JWM Viscosity data and relations of normal and isoparaffins. Physica 1947, 13, 305–318. [Google Scholar]
- (63).Wakefield DL; Marsh KN Viscosities of nonelectrolyte liquid mixtures. I. n-hexadecane + n-octane. Int. J. Thermophys 1987, 8, 649–662. [Google Scholar]
- (64).Wakefield DL Viscosities of nonelectrolyte liquid mixtures. III. Selected binary and quaternary mixtures. Int. J. Thermophys 1988, 9, 365–381. [Google Scholar]
- (65).Tanaka Y; Hosokawa H; Kubota H; Makita T Viscosity and density of binary mixtures of cyclohexane with n-octane, n-dodecane, and n-hexadecane under high pressures. Int. J. Thermophys 1991, 12, 245–264. [Google Scholar]
- (66).Wu J; Shan Z; Asfour A-FA Viscometric Properties of Multicomponent Liquid n-Alkane Systems. Fluid Phase Equilibr. 1998, 143, 263–274. [Google Scholar]
- (67).Mohammed M; Ciotta F; Trusler JPM Viscosities and Densities of Binary Mixtures of Hexadecane with Dissolved Methane or Carbon Dioxide at Temperatures from (298 to 473) K and at Pressures up to 120 MPa. J. Chem. Eng. Data 2017, 62, 422–439. [Google Scholar]
- (68).Sanchez-Vicente Y; Emerson I; Glover R; Herbage O; Susial Martin R; Trusler JPM Viscosities of Liquid Hexadecane at Temperatures between 323 K and 673 K and Pressures up to 4 MPa Measured Using a Dual-Capillary Viscometer. J. Chem. Eng. Data 2019, 64, 706–712. [Google Scholar]
- (69).Grigor'ev BA; Nemzer BV; Tatavosov GD Experimental study of the surface tension of n-pentane, n-hexane, and n-heptane. Izv. Vyssh. Uchebn. Zaved., Neft Gaz 1985, 28, 53–58. [Google Scholar]
- (70).Dutoit PF, Surface L Tension of Liquids. Arch. Sc. phys. nat. Geneva 1900, 9, 105–132. [Google Scholar]
- (71).Harkins WD; Brown FE; Davies ECH The Structure of the Surfaces of Liquids, and Solubility as Related to the Work done by the Attraction of Two Liquid Surfaces as they Approach each Other. [Surface Tension V.]. J. Am. Chem. Soc 1917, 39, 354–364. [Google Scholar]
- (72).Murphy NF; Lastovica JE; Fallis JG Correlation of Interfacial Tension of Two-Phase Three-Component Systems. Ind. Eng. Chem 1957, 49, 1035–1042. [Google Scholar]
- (73).Ridgway K; Butler PA Physical Properties of the Ternary System Benzene-Cyclohexane-Hexane. J. Chem. Eng. Data 1967, 12, 509–515. [Google Scholar]
- (74).Skripov VP; Sinitsyn EN Nuclation and surface tension in overheated liquids. Zh. Fiz. Khim 1968, 42, 309–312. [Google Scholar]
- (75).Skripov VP; Firsov VV Surface tension of Perfluoroalkanes. Russ. J. Phys. Chem 1968, 42, 653–656. [Google Scholar]
- (76).Bagdasaryan SS Calculation of Surface Tension of Alkanes and Arenes. Russ. J. Phys. Chem 1979, 53, 557. [Google Scholar]
- (77).Schiff R The Capillary Constants of Liquids at their Boiling-Points. Justus Liebigs Ann. Chem 1884, 223, 618–619. [Google Scholar]
- (78).Morgan JLR; Chazal PM The Weight of a Falling Drop and the Laws of Tate, XV. The Drop Weights of Certain Organic Liquids and the Surface Tensions and Capillary Constants Calculated from them. J. Am. Chem. Soc 1913, 35, 1821–1834. [Google Scholar]
- (79).Harkins WD; Cheng YC The Orientation of Molecules in Surfaces. VI. Cohesion, Adhesion, Tensile Strength, Tensile Energy, Negative Surface Energy, Interfacial Tension, and Molecular Attraction. J. Am. Chem. Soc 1921, 43, 35–53. [Google Scholar]
- (80).Hennaut-Roland ML, M Methods and Equipment Used by the Bureau of Physicochemical Standards: IV Surface Tension in a Series of Organic Substances. B. Soc. Chim. Belg 1931, 40, 177–188. [Google Scholar]
- (81).Trieschmann HG Surface Tension and Solvation. Z. Phys. Chem 1935, 29, 328. [Google Scholar]
- (82).Wibaut JP; Hoog H; Langedijk SL; Overhoff J; Smittenberg J A study on the preparation and the physical constants of a number of alkanes and cycloalkanes. Recl. Trav. Chim. Pays-Bas 1939, 58, 329–377. [Google Scholar]
- (83).Quayle OR; Day RA; Brown GM A Study of Organic Parachors. VII. A Series of Saturated Hydrocarbons1. J. Am. Chem. Soc 1944, 66, 938–941. [Google Scholar]
- (84).Vogel AI 38. Physical properties and chemical constitution. Part IX. Aliphatic hydrocarbons. J. Chem. Soc 1946, 133–139. [DOI] [PubMed] [Google Scholar]
- (85).Jasper JJ; Kerr ER; Gregorich F The Orthobaric Surface Tensions and Thermodynamic Properties of the Liquid Surfaces of the n-Alkanes, C5 to C28. J. Am. Chem. Soc 1953, 75, 5252–5254. [Google Scholar]
- (86).Jasper JJ; Kring EV The Isobaric Surface Tensions and Thermodynamic Properties of the Surfaces of a Series of n-Alkanes, C5 to C18, 1-Alkenes, C6 to C16, and of n-Decylcyclopentane, n-Decylcyclohexane and n-Dcylbenzene. J. Phys. Chem.-US 1955, 59, 1019–1021. [Google Scholar]
- (87).Ben'kovskii VG; Bogoslovskaya TM; Kiiko LD; Nauruzov MK Surface tension of hydrocarbons at low temperatures. Petrol. Chem. U.S.S.R 1964, 3, 86–90. [Google Scholar]
- (88).Clever HL; Chase WE Thermodynamics of Liquid Surfaces: Surface Tension of n-Hexane-Cyclohexane Mixtures at 25°, 30°, and 35° C. J. Chem. Eng. Data 1963, 8, 291–292. [Google Scholar]
- (89).Schmidt RL; Randall JC; Clever HL The Surface Tension and Density of Binary Hydrocarbon Mixtures: Benzene-n-Hexane and Benzene-n-Dodecane. J. Phys. Chem.-US 1966, 70, 3912–3916. [Google Scholar]
- (90).Ross S; Patterson RE Surface and interfacial tensions of conjugate solutions in ternary systems. J. Chem. Eng. Data 1979, 24, 111–115. [Google Scholar]
- (91).Grigoryev BA; Nemzer BV; Kurumov DS; Sengers JV Surface Tension of Normal Pentane, Hexane, Heptane, and Octane. Int. J. Thermophys 1992, 13, 453–464. [Google Scholar]
- (92).Papaioannou D; Panayiotou CG Surface Tensions and Relative Adsorptions in Hydrogen-Bonded Systems. J. Chem. Eng. Data 1994, 39, 457–462. [Google Scholar]
- (93).Piñeiro Á; Brocos P; Amigo A; Pintos M; Bravo R Surface tensions and refractive indices of (tetrahydrofuran + n-alkanes) at T = 298.15 K. J. Chem. Thermodyn 1999, 31, 931–942. [Google Scholar]
- (94).Jiménez E; Casas H; Segade L; Franjo C Surface Tensions, Refractive Indexes and Excess Molar Volumes of Hexane + 1-Alkanol Mixtures at 298.15 K. J. Chem. Eng. Data 2000, 45, 862–866. [Google Scholar]
- (95).Penas A; Calvo E; Pintos M; Amigo A; Bravo R Refractive Indices and Surface Tensions of Binary Mixtures of 1,4-Dioxane + n-Alkanes at 298.15 K. J. Chem. Eng. Data 2000, 45, 682–685. [Google Scholar]
- (96).Pandey JD; Soni NK; Singh VK; Sanguri V Surface Tension of Ternary Liquid Mixtures. Phys. Chem. Liq 2001, 39, 763–772. [Google Scholar]
- (97).Azizian S; Bashavard N; Yahyaei B Surface Properties of Dilute Solutions of Alkanes in Benzyl Alcohol. J. Chem. Eng. Data 2006, 51, 56–59. [Google Scholar]
- (98).Domínguez-Pérez M; Segade L; Cabeza O; Franjo C; Jiménez E Densities, Surface Tensions, and Refractive Indexes of Propyl Propanoate + Hexane + m-Xylene at 298.15 K. J. Chem. Eng. Data 2006, 51, 294–300. [Google Scholar]
- (99).Giner B; Villares A; Martín S; Artigas H; Lafuente C Study of the Temperature Dependence of Surface Tensions of Some Alkanol + Hexane Mixtures. J. Chem. Eng. Data 2007, 52, 1904–1907. [Google Scholar]
- (100).Azizian S; Bashavard N Surface Tension of Dilute Solutions of Alkanes in Cyclohexanol at Different Temperatures. J. Chem. Eng. Data 2008, 53, 2422–2425. [Google Scholar]
- (101).Giner B; Bandrés I; Giner I; Montaño DF; López MC Temperature dependence of surface tension of 2-methyl-1-propanol and 2-methyl-2-propanol+n-hexane mixtures. Phys. Chem. Liq 2008, 46, 643–652. [Google Scholar]
- (102).Pérez-Gregorio V; Montaño D; Giner B; Lafuente C; Royo FM Surface and bulk behaviour of some (n-hexane+chloroalkane) mixtures. J. Chem. Thermodyn. 2009, 41, 553–559. [Google Scholar]
- (103).Domínguez-Pérez M; Rilo E; Segade L; Franjo C; Cabeza O Surface Tension Deviations and Excess Molar Volumes on the Ternary System Propyl Propanoate + Hexane + p-Xylene at 298.15 K. J. Chem. Eng. Data 2010, 55, 1317–1321. [Google Scholar]
- (104).Pérez-Navarro M; Bandrés I; Giner I; Gascón I; Lafuente C Surface Tensions of the Ternary Mixtures Containing an Isomeric Butanol + n-Hexane + 1-Chlorobutane at 298.15 K. J. Chem. Eng. Data 2010, 55, 3532–3537. [Google Scholar]
- (105).Wang J-Y; Zhang X-J; Liu Y-M; Hu Y-Q Interfacial Tensions of Imidazolium-Based Ionic Liquids with n-Alkanes and Cyclohexane. J. Chem. Eng. Data 2011, 56, 3734–3737. [Google Scholar]
- (106).Mejía A; Segura H; Cartes M; Coutinho JAP Vapor–Liquid Equilibrium, Densities, and Interfacial Tensions of the System Hexane + 2,5-Dimethylfuran. J. Chem. Eng. Data 2012, 57, 2681–2688. [Google Scholar]
- (107).Garrido JM; Cifuentes L; Cartes M; Segura H; Mejía A High-pressure interfacial tensions for nitrogen+ethanol, or hexane or 2-methoxy-2-methylbutane: A comparison between experimental tensiometry and Monte Carlo simulations. J. Supercrit. Fluid 2014, 89, 78–88. [Google Scholar]
- (108).Mejía A; Segura H; Cartes M Vapor–liquid equilibrium and interfacial tensions of the system ethanol+hexane+tetrahydro-2H-Pyran. Fluid Phase Equilibr. 2014, 361, 229–236. [Google Scholar]
- (109).Tahery R Surface tension and density of mixtures of m-xylene + n-alkane at 293.15 K: Analysis under the extended Langmuir and Shereshefsky models. J. Chem. Thermodyn 2017, 106, 95–103. [Google Scholar]
- (110).Richards TW; Speyers CL; Carver EK The Determination of Surface Tension with Very Small Volumes of Liquid, and the Surface Tensions of Octanes and Xylenes at Several Temperatures. J. Am. Chem. Soc 1924, 46, 1196–1207. [Google Scholar]
- (111).Manzoni-Ansidei R Parachors of Branched Chain Paraffin Hydrocarbons. Boll. Sci. Fac. Chim. Ind. Bologna 1940, 201–207. [Google Scholar]
- (112).Smith GW The Measurement of Boundary Tension by the Pendent-drop Method. II. Hydrocarbons. J. Phys. Chem.-US 1944, 48, 168–172. [Google Scholar]
- (113).Baglay AK; Gurarii LL; Kuleshov GG Physical properties of compounds used in vitamin synthesis. J. Chem. Eng. Data 1988, 33, 512–518. [Google Scholar]
- (114).Ramkumar DHS; Kudchadker AP Mixture Properties of the Water + γ-Butyrolactone +Tetrahydrofuran System. Part 2. Viscosities and Surface Tensions of γ-Butyrolactone + Water at 303.15-343.15 K and γ-Butyrolactone + Tetrahydrofuran at 278.15-298.15 K. J. Chem. Eng. Data 1989, 34, 463–465. [Google Scholar]
- (115).Segade L; Jiménez de Llano J; Domínguez-Pérez M; Cabeza Ó; Cabanas M; Jiménez E Density, Surface Tension, and Refractive Index of Octane + 1-Alkanol Mixtures at T = 298.15 K. J. Chem. Eng. Data 2003, 48, 1251–1255. [Google Scholar]
- (116).Mosteiro L; Casás LM; Legido JL Surface tension, density, and speed of sound for the ternary mixture {diethyl carbonate+p-xylene+decane}. J. Chem. Thermodyn 2009, 41, 695–704. [Google Scholar]
- (117).Mejía A; Cartes M; Segura H Interfacial tensions of binary mixtures of ethanol with octane, decane, dodecane, and tetradecane. J. Chem. Thermodyn. 2011, 43, 1395–1400. [Google Scholar]
- (118).Gayol A; Casás LM; Andreatta AE; Martini RE; Legido JL Surface Tension of Dialkyl Carbonates + (Alkanes or 1,4-Dimethylbenzene) and 1,4-Dimethylbenzene + Alkanes Binary Mixtures at T = 308.15 K. J. Chem. Eng. Data 2013, 58, 758–763. [Google Scholar]
- (119).López-Lázaro J. d. l. S.; Iglesias-Silva GA; Estrada-Baltazar A; Barajas-Fernández J Density and Surface Tension of Binary Mixtures of 2,2,4-Trimethylpentane + n-Heptane, 2,2,4-Trimethylpentane + n-Octane, Ethyl Acetate + Benzene, and Butanenitrile + Benzene from (293.15 to 323.15) K. J. Chem. Eng. Data 2015, 60, 1823–1834. [Google Scholar]
- (120).Mumford SA; Phillips JWC 19. The physical properties of some aliphatic compounds. J. Chem. Soc 1950, 75–84. [Google Scholar]
- (121).Korosi G; Kovats ES Density and surface tension of 83 organic liquids. J. Chem. Eng. Data 1981, 26, 323–332. [Google Scholar]
- (122).Nagarajan N; Robinson RL Equilibrium phase compositions, phase densities, and interfacial tensions for carbon dioxide + hydrocarbon systems. 2. Carbon dioxide + n-decane. J. Chem. Eng. Data 1986, 31, 168–171. [Google Scholar]
- (123).Domańska U; Kozłowska MK; Rogalski M Solubilities, Partition Coefficients, Density, and Surface Tension for Imidazoles + Octan-1-ol or + Water or + n-Decane. J. Chem. Eng. Data 2002, 47, 456–466. [Google Scholar]
- (124).Gómez-Díaz D; Mejuto JC; Navaza JM; Rodríguez-Álvarez A Viscosities, Densities, Surface Tensions, and Refractive Indexes of 2,2,4-Trimethylpentane + Cyclohexane + Decane Ternary Liquid Systems at 298.15 K. J. Chem. Eng. Data 2002, 47, 872–875. [Google Scholar]
- (125).Rolo LI; Caco AI; Queimada AJ; Marrucho IM; Coutinho JAP Surface Tension of Heptane, Decane, Hexadecane, Eicosane, and Some of Their Binary Mixtures. J. Chem. Eng. Data 2002, 47, 1442–1445. [Google Scholar]
- (126).Queimada AJ; Caço AI; Marrucho IM; Coutinho JAP Surface Tension of Decane Binary and Ternary Mixtures with Eicosane, Docosane, and Tetracosane. J. Chem. Eng. Data 2005, 50, 1043–1046. [Google Scholar]
- (127).Mohsen-Nia M; Rasa H; Naghibi SF Experimental and theoretical study of surface tension of n-pentane, n-heptane, and some of their mixtures at different temperatures. J. Chem. Thermodyn 2010, 42, 110–113. [Google Scholar]
- (128).Luning Prak DJ; Lee BG; Cowart JS; Trulove PC Density, Viscosity, Speed of Sound, Bulk Modulus, Surface Tension, and Flash Point of Binary Mixtures of Butylbenzene + Linear Alkanes (n-Decane, n-Dodecane, n-Tetradecane, n-Hexadecane, or n-Heptadecane) at 0.1 MPa. J. Chem. Eng. Data 2017, 62, 169–187. [Google Scholar]
- (129).Fox HW; Hare EF; Zisman WA Wetting Properties of Organic Liquids on High-Energy Surfaces. J. Phys. Chem.-US 1955, 59, 1097–1106. [Google Scholar]
- (130).Koefoed J; Villadsen JV Surface Tension of Liquid Mixtures - A Micro-Method Applied to the Systems: Chloroform-Carbo-tetrachloride, Benzene-Diphenylmethane and Hepta-Hexadecane. Acta Chem. Scand 1958, 12, 1124–1135. [Google Scholar]
- (131).Myers RS; Clever HL Surface tension of octamethylcyclotetrasiloxane and hexamethyldisilazane and their solutions with carbon tetrachloride and n-hexadecane. J. Chem. Eng. Data 1969, 14, 161–164. [Google Scholar]
- (132).Mohsen-Nia M Measurement and Modelling of Surface Tensions of Systems Containing n-Hexadecane, n-Heptane and n-Pentane. Phys. Chem. Liq 2011, 49, 608–614. [Google Scholar]
- (133).Luning Prak DJ; Cowart JS; McDaniel AM; Trulove PC Density, Viscosity, Speed of Sound, Bulk Modulus, Surface Tension, and Flash Point of Binary Mixtures of n-Hexadecane + Ethylbenzene or + Toluene at (293.15 to 373.15) K and 0.1 MPa. J. Chem. Eng. Data 2014, 59, 3571–3585. [Google Scholar]
- (134).Luning Prak DJ Density, Viscosity, Speed of Sound, Bulk Modulus, Surface Tension, and Flash Point of Binary Mixtures of Butylcyclohexane with Toluene or n-Hexadecane. J. Chem. Eng. Data 2016, 61, 3595–3606. [Google Scholar]
- (135).Luning Prak DJ; Luning Prak PJ; Cowart JS; Trulove PC Densities and Viscosities at 293.15–373.15 K, Speeds of Sound and Bulk Moduli at 293.15–333.15 K, Surface Tensions, and Flash Points of Binary Mixtures of n-Hexadecane and Alkylbenzenes at 0.1 MPa. J. Chem. Eng. Data 2017, 62, 1673–1688. [Google Scholar]
- (136).Luning Prak DJ; Ye S; McLaughlin M; Cowart JS; Trulove PC Density, Viscosity, Speed of Sound, Bulk Modulus, Surface Tension, and Flash Point of Selected Ternary Mixtures of n-Butylcyclohexane + a Linear Alkane (n-Hexadcane or n-Dodecane) + an Aromatic Compound (Toluene, n-Butylbenzene, or n-Hexylbenzene). J. Chem. Eng. Data 2017, 62, 3452–3472. [Google Scholar]
- (137).Miqueu C; Broseta D; Satherley J; Mendiboure B; Lachaise J; Graciaa A An extended scaled equation for the temperature dependence of the surface tension of pure compounds inferred from an analysis of experimental data. Fluid Phase Equilibr. 2000, 172, 169–182. [Google Scholar]
- (138).Aleksandrov IS; Gerasimov AA; Grigor’ev BA Using fundamental equations of state for calculating the thermodynamic properties of normal undecane. Thermal Engineering 2011, 58, 691. [Google Scholar]
- (139).Tenji D; Thol M; Lemmon EW; Span R Fundamental Equation of State for n-Heptane. to be submitted to Int. J. Thermophys 2018. [Google Scholar]
- (140).Michailidou EK; Assael MJ; Huber ML; Abdulagatov IM; Perkins RA Reference Correlation of the Viscosity of n-Heptane from the Triple Point to 600 K and up to 248 MPa. J. Phys. Chem. Ref. Data 2014, 43, 023103. [Google Scholar]
- (141).Assael MJ; Papalas TB; Huber ML Reference Correlations for the Viscosity and Thermal Conductivity of n-Undecane. J. Phys. Chem. Ref. Data 2017, 46, 033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Mulero A; Cachadiña I Recommended Correlations for the Surface Tension of Several Fluids Included in the REFPROP Program. J. Phys. Chem. Ref. Data 2014, 43, 023104. [Google Scholar]
- (143).Domanska U; Morawski P (Solid + liquid) equilibria of (nalkanes + ethyl 1,1-dimethylpropyl ether). The Journal of Chemical Thermodynamics 2001, 33, 1215–1226. [Google Scholar]


