Infants treated with M + P for NOWS have a shorter length of morphine treatment and hospital stay than infants treated with M + C.
Abstract
BACKGROUND AND OBJECTIVES:
Despite the neonatal opioid withdrawal syndrome (NOWS) epidemic in the United States, evidence is limited for pharmacologic management when first-line opioid medications fail to control symptoms. The objective with this study was to evaluate outcomes of infants receiving secondary therapy with phenobarbital compared with clonidine, in combination with morphine, for the treatment of NOWS.
METHODS:
We performed a retrospective cohort study of infants with NOWS from 30 hospitals. The primary outcome measures were the length of hospital stay, duration of opioid treatment, and peak morphine dose. Outcomes were compared by group by using analysis of variance and multivariable linear regression controlling for relevant confounders.
RESULTS:
Of 563 infants with NOWS treated with morphine, 32% (n = 180) also received a secondary medication. Seventy-two received phenobarbital and 108 received clonidine. After adjustment for covariates, length of hospital stay was 10 days shorter, and, in some models, duration of morphine treatment was 7.5 days shorter in infants receiving phenobarbital compared with those receiving clonidine, with no difference in peak morphine dose. Infants were more likely to be discharged from the hospital on phenobarbital than clonidine (78% vs 29%, P < .0001).
CONCLUSIONS:
Among infants with NOWS receiving morphine and secondary therapy, those treated with phenobarbital had shorter length of hospital stay and shorter morphine treatment duration than clonidine-treated infants but were discharged from the hospital more often on secondary medication. Further investigation is warranted to determine if the benefits of shorter hospital stay and shorter duration of morphine therapy justify the possible neurodevelopmental consequences of phenobarbital use in infants with NOWS.
What’s Known on This Subject:
Phenobarbital and clonidine are the most-commonly used secondary medications for the treatment of neonatal opioid withdrawal syndrome. Variation between centers exists, and few studies directly compare the 2 medications in neonatal opioid withdrawal syndrome treatment.
What This Study Adds:
In this multicenter retrospective cohort study of 180 infants, we found that phenobarbital-treated infants had shorter length of stay and, potentially, fewer days of morphine treatment than clonidine-treated infants but were sent home on medication more often.
Because of the ongoing opioid epidemic, >32 000 opioid-exposed infants are now born annually in the United States,1 with the majority showing at least some signs of neonatal opioid withdrawal syndrome (NOWS).2 Many knowledge gaps remain regarding optimal treatment of infants with NOWS. It is generally accepted that nonpharmacologic treatment (swaddling, low-stimulus environment, rooming in, skin-to-skin) should be the first-line therapy, with opioid medication added if this approach fails to adequately control symptoms.3 When opioid therapy is not sufficient, secondary medications must be considered. There is wide variation among institutions with respect to selection and initiation of secondary medication.4,5 Across the country, selection of a secondary medication for NOWS has largely been based on local preference and protocol, possibly dictated by concern for potential side effects.
Phenobarbital and clonidine are the most commonly used secondary medications for NOWS.6 Phenobarbital is a barbiturate and potent sedative. Commercially available phenobarbital formulations contain 13.5% alcohol in the solution and 15% alcohol in the elixir, although compounded alcohol-free solutions are available.7 Clonidine decreases noradrenergic activity and lessens symptoms of withdrawal, such as tachypnea, tachycardia, fever, sweating, sneezing, and yawning.8 Clonidine solution is not commercially available so it must be compounded, which increases risk of dosing errors.9 Few safety, pharmacokinetic, and pharmacodynamic data are available for clonidine,10 and although data exist in these areas for phenobarbital,11 studies comparing the 2 as a treatment of NOWS are lacking. Only 2 small studies of 68 patients12 and 25 patients13 have prospectively compared the effects of phenobarbital and clonidine in infants treated with morphine for NOWS. In both of these studies, infants treated with phenobarbital had significantly shorter morphine treatment days and shorter hospital length of stay. This is despite researchers in the first study having used morphine plus phenobarbital (M + P) or morphine plus clonidine (M + C) as initial treatment and those in the second study having used phenobarbital or clonidine as adjunctive therapy after failure of morphine. However, in both studies, phenobarbital was continued as an outpatient, whereas clonidine was weaned and discontinued before discharge, leading to an overall longer length of treatment of infants given phenobarbital. In contrast, researchers in a single retrospective study comparing different NOWS treatment protocols found that if a secondary medication was needed, using morphine every 3 hours with clonidine was associated with a shorter length of hospitalization and duration of morphine treatment than using morphine every 4 hours with phenobarbital.14
Given the paucity of literature on this topic with conflicting findings, additional studies are needed to compare clonidine and phenobarbital as secondary NOWS agents. The objective with this investigation was to compare the in-hospital outcomes of length of morphine treatment, length of hospital stay, and peak morphine dose in infants treated with M + P versus infants treated with M + C as part of a large multicenter observational study evaluating current practice in infants with prenatal opioid exposure. We hypothesized that infants treated with M + P would have a shorter length of stay than infants treated with M + C but no difference in the length of opioid treatment.
Methods
The Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) Current Experience study is a multicenter observational cross-sectional study of infants with NOWS from 30 hospitals across the United States from July 1, 2016, to June 30, 2017. The ACT NOW Collaborative is a partnership between the Environmental influences on Child Health Outcomes Institutional Development Award States Pediatric Clinical Trials Network and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) and is part of the National Institutes of Health’s trans-agency Helping to End Addiction Long-term Initiative. A central Institutional Review Board at the University of Arkansas for Medical Sciences approved the study.
Data were selected for abstraction from medical records by trained research personnel if infants were ≥36 weeks’ gestation and had either NOWS scoring with the Finnegan scoring system or modified Finnegan scoring system within the first 120 hours of life or documented prenatal opioid exposure (maternal history, maternal positive toxicology screen during second or third trimester of pregnancy, and/or infant positive toxicology screen). The Medical Record Abstraction Quality Assurance and Control Framework was used to improve accuracy of data abstraction.15,16 For this retrospective cohort analysis, we included only infants with both documented NOWS scoring and documented prenatal opioid exposure. Additionally, infants had to have been given morphine as first-line treatment to isolate the effects of phenobarbital versus clonidine as secondary medications in infants with NOWS. All sites dosed morphine every 3 hours, although some sites did have caveats in their morphine-weaning protocols that if the infant was sleeping at the 3-hour mark, the dose could be delayed up to 1 additional hour. The choice of clonidine or phenobarbital was based on each individual center’s usual care and protocol. We chose to include the group of infants treated with morphine only as a reference group.
Descriptive statistics were used to characterize and summarize infant and maternal characteristics overall and by infant groups (morphine alone, M + P, and M + C). The mean and SD were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Additionally, the initial descriptive statistics were investigated by using one-way analysis of variance (ANOVA) and χ2 test, as appropriate. No adjustments for multiple comparisons were made for the descriptive analysis. Outcomes of interest included length of morphine treatment in days, length of inpatient hospital stay in days, and peak morphine dose in milligrams per kilogram. For each outcome, we compared the group means using one-way ANOVA followed by multivariable linear regression. Covariates with possible impact on each outcome based on previous literature were selected for the multivariable model.2,17–19 The covariates included were race and ethnicity (defined as non-Hispanic white and other), sex, maternal polysubstance use (defined as mother or infant with a history or positive toxicology screen for substances other than opioids during the second and third trimester, including gabapentin, selective serotonin reuptake inhibitors, and benzodiazepines), maternal opioid (buprenorphine only, methadone only, or other), and hospital NOWS volume (based on number of NOWS cases each site contributed to this study). For our main analysis, we did not include birth weight or gestational age as covariates because all infants in the study were ≥36 weeks’ gestation at birth. We did conduct a sensitivity analysis including birth weight and gestational age as additional confounders. The results of this model (provided in Supplemental Tables 4 and 5) were unchanged from the results of the main analysis, and therefore birth weight and gestational age were not included to achieve the most parsimonious model. Consistent with the National Institutes of Health initiative to consider sex in data analyses,20 we examined the potential moderation effect of sex on the relationship between group and each outcome (ie, group-by-sex interaction). Our main analysis used hospital NOWS volume as a fixed effect because there were only a few sites that used both phenobarbital and clonidine, and using a random site effect would place substantial weight on the limited number of observations within those few hospitals, resulting in broad SEs. However, to be sure, we also conducted a subsequent analysis that used a linear mixed model to account for infants nested within site to help determine which observed results from the fixed effects model might be due to unobserved site level differences. To evaluate whether differences in any observed effect would exist across sites, we compared the site with the largest number of M + P observations with the site with the largest number of M + C observations, as well as grouping M + P observations in smaller sites together and comparing them with M + C observations in smaller sites. All outcome variables were tested for normality and departures from normality were minimal. Nonetheless, we conducted sensitivity analyses for all regressions using log-transformed outcomes. The results did not differ from those of the main analysis, so for clarity, the regression results of the nontransformed outcomes are presented.
Results
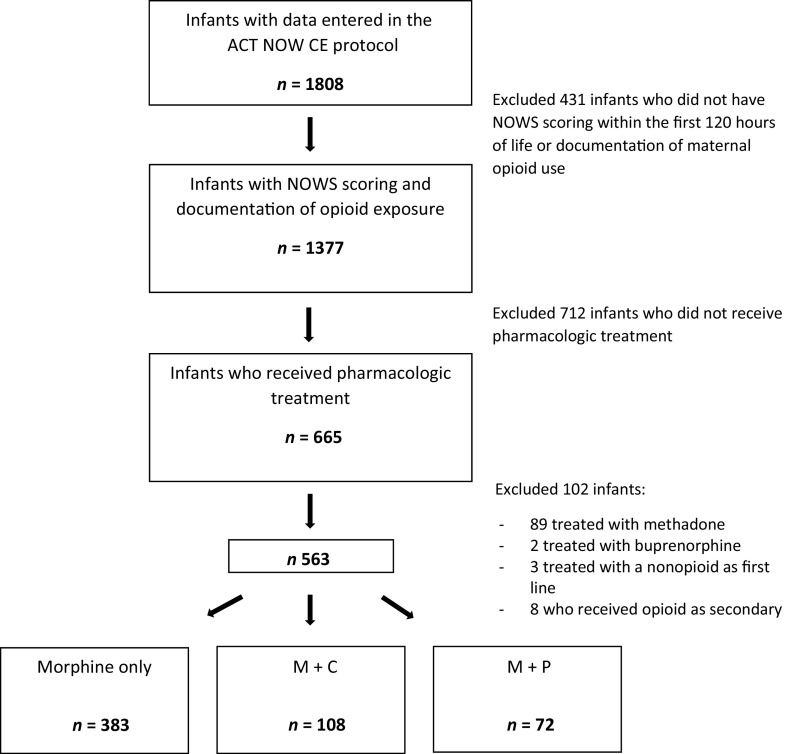
Of the 1808 mother-infant dyads in the ACT NOW Current Experience study, 563 infants with documented prenatal opioid exposure and NOWS scoring received pharmacologic treatment with morphine as first-line therapy and were included in the current study (Fig 1). Of those infants, 180 (32%) also received secondary treatment: 72 (40%) with M + P and 108 (60%) with M + C. No participants received both phenobarbital and clonidine. Only 1 participant received a tertiary medication (methadone) after failure of secondary medication, and this participant was included in the analysis. Four sites used morphine alone or M + P, 7 sites used morphine alone or M + C, 6 sites used all 3 combinations, and 7 sites never used secondary medications, treating infants only with morphine. Sites that only used morphine alone were retained in the analysis to provide greater generalizability and precision of outcome estimates for the group of infants treated with morphine alone. Phenobarbital treatment was continued after discharge in 56 of 72 infants (78%), and 31 of 108 infants (29%) were discharged from the hospital on clonidine, P < .0001. The time of onset of secondary medication was similar in both groups (7 ± 8.5 days after morphine start in the phenobarbital group, 8.5 ± 7.6 days after morphine start in the clonidine group, P = .072). Thirty-five infants were started on secondary medication on the same day as the primary morphine treatment: 14 in the M + P group (19.4%) and 21 in the M + C group (19.4%). Demographics of each group are shown in Table 1. The average birth weight and gestational age at birth were similar across the groups. Approximately 55% of the infants were male. There was a higher proportion of non-Hispanic white infants in the M + C group compared with the morphine alone group. Infants whose mothers only used methadone as opposed to buprenorphine or other and/or multiple opioids were more likely to be treated with phenobarbital versus clonidine. Other maternal measures were similar across groups.
FIGURE 1.
Subject flow diagram. CE, Current Experience.
TABLE 1.
Descriptive Summary of Maternal and Infant Characteristics by Group
| All (N = 563) | Morphine Alone (n = 383) | M + P (n = 72) | M + C (n = 108) | P | |
|---|---|---|---|---|---|
| Infant demographics | |||||
| Birth wt, mean (SD), kg | 3.0 (0.5) | 3.0 (0.5) | 3.1 (0.5) | 3.1 (0.5) | .190 |
| Gestational age, mean (SD), wk | 38.3 (1.4) | 38.2 (1.4) | 38.6 (1.5) | 38.5 (1.3) | .054 |
| Male, n (%) | 310 (55.1) | 211 (55.1) | 43 (59.7) | 56 (52.9) | .816 |
| Finnegan score before starting morphine treatment, mean (SD) | 10.6 (2.5) | 10.7 (2.5) | 10.1 (2.5) | 10.9 (2.4) | .782 |
| Maternal measures, n (%) | |||||
| Non-Hispanic white | 389 (69.1) | 252 (65.8) | 53 (73.6) | 84 (77.8) | .040 |
| Buprenorphine only | 145 (25.8) | 99 (25.9) | 13 (18.1) | 33 (30.6) | .171 |
| Methadone only | 84 (14.9) | 52 (13.6) | 20 (27.8) | 12 (11.1) | .004 |
| >1 opioid | 286 (50.8) | 199 (52.0) | 33 (54.2) | 54 (50.0) | .624 |
| Maternal polysubstance use | 351 (62.3) | 240 (62.7) | 52 (72.2) | 59 (54.6) | .068 |
| Any breastfeeding | 216 (38.4) | 156 (40.7) | 23 (33.3) | 36 (33.3) | .141 |
| Hospital NOWS volume during study period, n (%) | |||||
| 1 (<30) | 54 (9.6) | 37 (9.7) | 3 (4.2) | 14 (13.0) | .214 |
| 2 (30–79) | 138 (24.5) | 100 (26.1) | 15 (20.8) | 23 (21.3) | — |
| 3 (≥ 80) | 371 (66.0) | 246 (64.2) | 54 (75.0) | 71 (65.7) | — |
The P values are based on a one-way ANOVA or χ2 test comparing across the 3 groups; pairwise comparisons were performed for non-Hispanic white with statistical significance between morphine alone versus M + C (P = .02); pairwise comparisons were performed for methadone only with statistical significance between morphine alone versus M + P (P = .003) and M + C versus M + P (P = .004). —, not applicable.
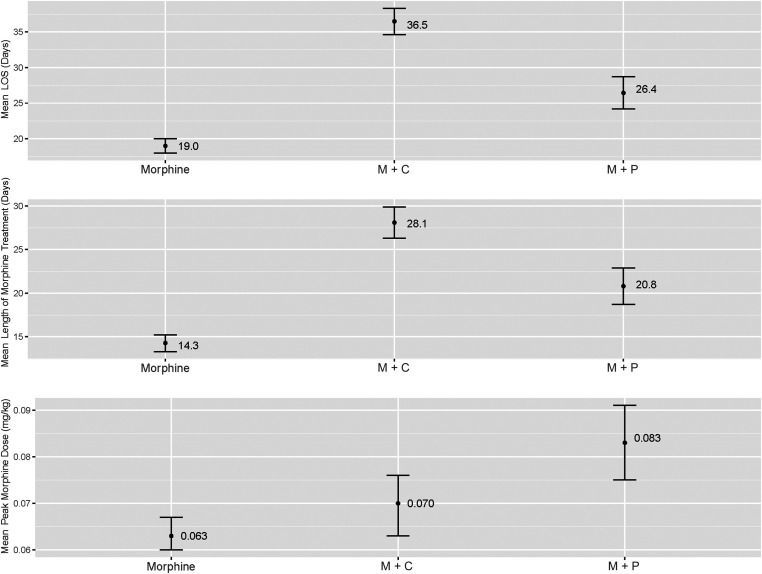
As shown in Fig 2, the unadjusted mean length of stay was shorter (26.4 ± 12.4 days versus 36.5 ± 12.6 days; Δ = −10.04 days; 95% confidence interval [CI]: 7.08 to 12.99 days; P < .0001) and length of morphine treatment shorter (20.8 ± 11.4 days versus 28.1 ± 11.3 days; Δ = −7.3 days; 95% CI: 4.51 to 10.07 days; P < .0001) for the M + P group compared with the M + C group, but the peak morphine dose was higher in the M + P group compared with the M + C group (0.08 ± 0.05 mg/kg versus 0.07 ± 0.03 mg/kg; Δ = 0.01 mg/kg; 95% CI: 0.003 to 0.023 mg/kg; P = .01). The length of stay and duration of morphine treatment were longer for infants receiving either secondary medication than for those treated with morphine only (P < .0001) (Table 2). After adjusting for race and ethnicity, sex, maternal polysubstance use, type of maternal opioid, >1 maternal opioid, and hospital volume, length of stay and length of morphine treatment remained shorter in the M + P group compared with the M + C group (Δlength of stay = −10.26 ± 1.50 days; 95% CI −13.21 to −7.31 days; Δmorphine treatment = −7.51 ± 1.4; 95% CI −10.27 to −4.75 days; P < .0001 for both), but difference in peak morphine dose was no longer statistically significant at the 0.05 level between the 2 groups. Results from the mixed model using site as a random effect are shown in Table 3. In the mixed model, length of stay remained shorter in the M + P group compared with the M + C group (Δ = −4.07 ± 1.93 days; 95% CI: −7.86 to −0.27 days; P = .036), but difference in length of morphine treatment and peak morphine dose were no longer significant. Comparison of the site with the largest number of M + P observations and the site with the largest number of M + C observations were consistent with the results of the main analysis. Directionality of the observed effects among observations in smaller sites were consistent with the main effect, although, because of smaller sample size, statistical significance was not reached. The interaction effects of sex and treatment group were not significant for length of stay or for length of morphine treatment (P = .07 for both). In contrast, for peak morphine dose, the interaction of treatment group and sex was significant (P = .01). Further analysis by sex only identified significant differences in peak morphine dose among male infants treated with M + P compared with boys treated with M + C and boys compared with girls treated with M + P. However, no differences between these groups were >0.04 mg/kg and are therefore likely not of clinical significance.
FIGURE 2.
Comparison of unadjusted group means. Error bars reflect SEs. LOS, length of stay.
TABLE 2.
Results of Multivariable Linear Regression Models
| Outcome and Group Comparison | Mean Difference | P | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Length of hospital stay | ||||
| Morphine versus M + P | −7.22 | <.0001 | −9.70 | −4.75 |
| Morphine versus M + C | −17.48 | <.0001 | −19.59 | −15.38 |
| M + P versus M + C | −10.26 | <.0001 | −13.21 | −7.31 |
| Length of morphine treatment | ||||
| Morphine versus M + P | −6.19 | <.0001 | −8.49 | −3.88 |
| Morphine versus M + C | −13.70 | <.0001 | −15.67 | −11.72 |
| M + P versus M + C | −7.51 | <.0001 | −10.27 | −4.75 |
| Peak morphine dose mg/kg | ||||
| Morphine versus M + P | −0.017 | <.0001 | −0.026 | −0.008 |
| Morphine versus M + C | −0.008 | .037 | −0.015 | −0.0005 |
| M + P versus M + C | 0.010 | .072 | −0.0008 | 0.019 |
Morphine; M + P; M + C; covariates included race and ethnicity, infant sex, maternal polysubstance use; type of maternal opioid, >1 opioid, and hospital volume.
TABLE 3.
Results of Multivariable Linear Mixed Regression Models
| Outcome Group Comparison | Mean Difference | P | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Length of hospital stay | ||||
| Morphine versus M + P | −13.15 | <.0001 | −16.18 | −10.11 |
| Morphine versus M + C | −17.22 | <.0001 | −19.71 | −14.73 |
| M + P versus M + C | −4.07 | .036 | −7.86 | −0.27 |
| Length of morphine treatment | ||||
| Morphine versus M + P | −11.75 | <.0001 | −14.54 | −8.96 |
| Morphine versus M + C | −13.47 | <.0001 | −15.77 | −11.16 |
| M + P versus M + C | −1.71 | .337 | −5.22 | 1.79 |
| Peak morphine dose, mg/kg | ||||
| Morphine versus M + P | −0.022 | <.0001 | −0.032 | −0.011 |
| Morphine versus M + C | −0.015 | .0006 | −0.024 | −0.0007 |
| M + P versus M + C | 0.007 | .324 | −0.007 | 0.020 |
Morphine; M + P; M + C; covariates included race and ethnicity, infant sex, maternal polysubstance use; type of maternal opioid, and >1 opioid.
Discussion
In this large, multicenter, contemporary, observational study, both in unadjusted analyses and after adjusting for relevant covariates, infants with NOWS given M + P had shorter length of hospital stay and shorter length of morphine treatment than infants treated with M + C. When including site as a random effect, difference in length of morphine treatment was no longer significant, suggesting this is not as robust a finding and could be due to site level differences not accounted for in our main model.
Potential benefits of shorter hospitalization include reduced cost and family stress. NICU hospitalizations cost ∼$2500 per day, making a 10-day reduction in length of stay financially significant for families and insurance providers. To an extent, our findings support a shorter length of morphine exposure for those treated with phenobarbital, and researchers of previous studies have demonstrated potential benefits of shorter morphine exposure. Indeed, in vitro studies reveal apoptosis after morphine exposure in human neurons and microglia,21 animal studies reveal adverse effects of postnatal morphine on neurobehavior,22 and studies in preterm infants reveal an adverse association between neonatal morphine exposure and later neurodevelopment.23
Yet, more infants in our study cohort were sent home on phenobarbital, likely resulting in a longer total pharmacologic treatment period for NOWS. Although we did not examine postdischarge treatment duration or neurodevelopment, previous research suggests that most infants are treated with phenobarbital for several months after discharge.24 Additional or longer exposure to phenobarbital is concerning. Phenobarbital is an inhibitor of the γ-aminobutyric acid type A receptor. Phenobarbital’s potent sedative effects decrease some of the neurologic symptoms of NOWS (hyperirritability, sleep disturbances), but phenobarbital does not directly address the pathophysiology of withdrawal.9 There is concern about side effects of phenobarbital in neonates, particularly oversedation, as well as neuronal apoptosis and long-term neurodevelopmental effects of neonatal phenobarbital treatment seen in both animal25,26 and human27 studies. It is unclear to what extent the neurodevelopmental impact seen in previous studies is due to phenobarbital itself versus the large concentration of alcohol in commercially available solutions.
Clonidine has been used in both monotherapy and as adjunctive therapy for NOWS.28 There has been less concern for clonidine’s side effects, although authors of a recent article reported hypotension and rebound hypertension in infants treated with clonidine for NOWS.13 Clonidine as a secondary therapy to morphine has been shown to reduce the duration of opioid therapy compared with placebo29 in a randomized controlled trial, with no increase in adverse outcomes (hypotension, rebound hypertension, bradycardia, or desaturations). There has also been interest in clonidine as monotherapy versus morphine, with researchers in 1 small study30 showing decreased length of treatment in the clonidine group. The clonidine group also had improved neurobehavior using the NICU Network Neurobehavioral Scale at 40 to 44 weeks postmenstrual age, although Bayley Scales of Infant Development at 1 year of age did not differ between groups. We found that, overall, infants treated with M + C had a lower peak morphine dose than those treated with M + P in unadjusted analysis, but this may have been due to confounding, and the result did not meet statistical significance after adjustment.
Our results align with the 2013 Surran et al12 and 2020 Brusseau et al13 randomized controlled trials of phenobarbital versus clonidine as secondary treatments for NOWS. Although these studies were small (Surran, 68 infants total and Brusseau, 25 infants total) and differed in design (Surran randomly assigned infants to M + P or M + C when initially starting treatment, Brusseau randomly assigned infants to phenobarbital or clonidine after failure of morphine), our study suggests that their results do generalize to a larger and broader population of infants and that using phenobarbital as a secondary medication, although usually continued after discharge, does allow for shorter length of stay. Our results contrast with the 2017 study by Devlin et al14 in which phenobarbital and clonidine were retrospectively compared as adjuvant treatments but with variable dosing intervals of morphine. In their study, they found that infants treated with morphine every 3 hours plus clonidine had shorter length of treatment and hospital stay than infants treated with morphine every 4 hours plus phenobarbital, with all medications weaned off before hospital discharge. It is unclear whether the shorter morphine dosing interval or their practice of weaning off all medications before discharge contributed to their findings, and this raises the question of whether the larger proportion of infants discharged from the hospital on phenobarbital in our study was the primary driver for shorter hospital stay. This underscores the need for a study of secondary NOWS treatments that includes data collection after discharge as well as long-term follow-up.
The strengths of our study include a large multicenter cohort of infants with standardized collection of observational data. The limitations of our study include the issues inherent in medical record review and retrospective multicenter studies, including the lack of standardized treatment and care protocols among sites and the lack of randomization. We are also limited by the fact that only a few centers routinely used M + C or M + P, which increases the chance that a nonidentified confounder could have influenced our findings. We did not have information on social barriers to discharge affecting length of stay, potential medication side effects, and postdischarge outcomes (including medication discontinuation, readmissions, and neurodevelopment). It is also possible we were unable to account for practice differences between centers in our models, or that larger centers may have contributed more strongly to the overall effect.
Conclusions
In this study, infants treated with M + P had substantially shorter length of hospital stay and, potentially, fewer days of morphine treatment. However, the benefits of phenobarbital over clonidine as a secondary medication to morphine must be weighed against the possible long-term adverse neurodevelopmental effects of phenobarbital continuation after discharge. Further research should focus on the development of optimal nonpharmacologic care and pharmacologic treatment regimens that reduce length of hospital stay, length of morphine treatment, and peak morphine dosing while protecting long-term neurodevelopmental outcomes.
Acknowledgments
The following investigators, in addition to those listed as authors, participated in this study: IDeA States Pediatric Clinical Trials Network Steering Committee Chair: Jill G. Joseph, MD, PhD. NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011–present). Alaska Native Medical Center (Alaska Native Tribal Health Consortium and Southcentral Foundation) (ISCPTN: UG1OD024944): Rosalyn Singleton, MD; Matthew Hirschfeld, MD, PhD; Jennifer Shaw, PhD; Amy Swango-Wilson, RN, PhD; Mary Herrick, MD; Christine Hallas, PNP. Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (NRN: U10 HD27904; ISCPTN: UG1 OD024951): Abbot Laptook, MD; Martin Keszler, MD; Thomas Chun, MD; Phyllis Dennery, MD; Angelita M. Hensman, PhD, MS, RNC-NIC; Elizabeth Trailburns, RN. Arkansas Children's Research Institute, University of Arkansas for Medical Sciences, and Arkansas Children’s Hospital (ISCPTN: UG1OD024945): Jessica Snowden, MD; Clare Nesmith, MD; Sherry Courtney, MD; Frederick Barr, MD; Laura James, MD; Denise Pearson, RN, CPN; Jana McConnell, RN; Melanie Mason, RN. Case Western Reserve University, University Hospitals Cleveland Medical Center and Rainbow Babies & Children's Hospital (NRN: UG1 HD21364): Michele Walsh, MD; Anna Marie Hibbs, MD; Nancy S. Newman, BA, RN; Leslie Clarke, RN, BSN, MBA. Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (NRN: UG1 HD27853): Cathy Grisby, BSN CCRC; Traci Beiersdorfer, BSN; Greg Muthig, BS. Dartmouth College, Dartmouth-Hitchcock Medical Center, and Children’s Hospital at Dartmouth-Hitchcock (ISCPTN: UG1OD024946): Paul Palumbo, MD; J. Dean Jarvis, BSN, MBA, RN; Mary McNally, BSRT, RRT. Duke University Environmental influences on Child Health Outcomes (Environmental influences on Child Health Outcomes; ECHO) Coordinating Center (1U2COD023375-01): P. Brian Smith, MD. ECHO Program, Office of the Director, National Institutes of Health: Carol J. Blaisdell, MD, MEd; Mary Roary, PhD; Divya Kalaria, MD. Eunice Kennedy Shriver Eunice Kennedy Shriver National Institute of Child Health and Human Development: Andrew Bremer, MD; Stephanie Wilson Archer, MA. Larner College of Medicine at the University of Vermont, University of Vermont Children's Hospital, and University of Vermont Medical Center (ISCPTN: UG1OD024955): Adrienne Pahl, MD; Kelly Cowan, MD; Jerilyn Matayer, RN; Ethan Jones, MPH; Laurie Chassereau, RN. Louisiana State University, Pennington Biomedical Research Center, Tulane University School of Medicine and Tulane Lakeside Hospital for Women and Children (ISCPTN: UG1OD024959); Stacy Drury, MD PhD; Elizabeth Lindsay, MD; Daniel S. Hsia, MD; Kelsey Confreda, MPH; Cade Herman, BS; Tegan Clarke, BA. Nationwide Children’s Hospital, Center for Perinatal Research, The Abigail Wexner Research Institute at Nationwide Children’s Hospital, The Ohio State University Wexner Medical Center, The Ohio State College of Medicine (NRN: UG1 HD68278): Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Nathalie L. Maitre, MD, PhD; Kristina M. Reber, MD; Erin L. Keel, DNP, APRN, NNP-BC; Patricia Luzader, RN; Margaret K. Burns, RN, BSN, MS; Jacqueline McCool; Teri McCarty, PharmD; Pavel Prusakov, PharmD. Nemours Alfred I. duPont Hospital for Children and ChristianaCare (ISCPTN: UG1OD024958): David Paul, MD; Judith Ross, MD; Kelly Gray, RN; Amy Mackley, CNS, MSN; Karen Kowal, PAC. RTI International (U10 HD36790): Carla M. Bann, PhD; Jamie E. Newman, PhD, MPH; Margaret M. Crawford, BS; Jeanette O’Donnell Auman, BS; Marie G. Gantz, PhD; Kristin M. Zaterka-Baxter, RN, BSN CCRP. University of Arkansas for Medical Sciences Data Coordination and Operations Center and Department of Biostatistics (Institutional Development Award States Pediatric Clinical Trials Network: U24OD024957): Jeannette Lee, PhD; Anita Walden, MS; Kimberly Harris, PhD; Amy Doville, MBA, CCRP; Irene Chedjieu, BDS, MPH; Lora Lawrence, RN, CCRP; Emil Seker, MSIQ; Vaishali Thombre, MS; Sunitha Kenchey, MS. University of Hawaii at Manoa and the Kapiolani Medical Center for Women and Children (ISCPTN: UG1OD024948): Akshatha, MD; Charles Neal, MD; Bruce Shiramizu, MD; Moara Palma, PhD; Annette Amiotte, RN, BSN. University of Kansas Medical Center, the University of Kansas Health System, Children’s Mercy Kansas City and Pittsburg State University Irene Ransom Bradley School of Nursing (ISCPTN: UG1OD024943): Krishna Dummula, MD; Barbara Pahud, MD; Kristi Frisbee, DNP; Melissa Lopez, BSN; Barbara McClaskey, MN, PhD; Megan Bledsoe, PhDc, MSc. University of Louisville, Norton Hospital Newborn Nursery, and Norton Children’s Hospital (ISCPTN: UG1OD024954): Sara Watson, MS, MD; Janice Sullivan, MD; Jennifer Nason, RN, BSN; Laura Thomas, RN, BSN; Stephanie Houston, BSN, RNC-NIC; Jackie Perry Boyd, RN, BSN. University of Mississippi Medical Center (ISCPTN: UG10D024942): Robert Annett, PhD; Lauren Tucker, MD; J. Marc Majure, MD; Dana Lindsay, RN, BSN; Takila Keys, MHS, RHIA; Lacy Malloch, BS. University of Montana, Community Medical Center, St. Vincent Healthcare, and Billings Clinic (ISCPTN: UG1OD024952): Paul Smith, DO; Lauren Parks, PharmD; Helen Rusette, MPH; Sara Cox-McClure, RN, BSN; Susan McAtee, RN, BSN; Dawn Hedstrom, RN-NIC. University of Nebraska Medical Center (ISCPTN: UG1OD024953): Ann Anderson Berry, MD, PhD; Russell McCulloh, MD; Kelly Erickson, MPH; Rachel Wellman, RN, BSN, MS. University of New Mexico Health Sciences Center and University of New Mexico School of Medicine (NRN: U10 HD53089; ISCPTN: UG1OD024947; National Center for Advancing Translational Sciences: UL1 TR41): Jessie Maxwell, MD; Heather Pratt-Chavez, MD; Kristi L. Watterberg, MD; Alberta Kong, MD; Hengameh Raissy, PharmD; Grace McCauley, MPH; Sandra Beauman, MSN, RNC-NIC; Sara Sanders, RN, MS; Mary Hanson, RN; Olivia Nunez, BS. University of Oklahoma Health Sciences Center, Comanche County Memorial Hospital, and University of Oklahoma Children’s Hospital (ISCPTN: UG1OD024950): Kimberly Ernst, MD; Abhishek Makkar, MD; Edgardo Szyld, MD; Paul Darden, MD; Christi Madden, MPA; Michael McCoy, MS, APRN; Ashley Anderson, RN, BSN; Erin Bohon, LPN; Erica Doefler, NNP; LaDale Johnson, NNP; Lindsay Cobianchi; Shannon Wilson. University of South Carolina at Columbia, Spartanburg Medical Center, Medical University of South Carolina, McLeod Regional Medical Center, and Pediatrix Medical Group of South Carolina (ISCPTN: UG1OD024956): Andrew Atz, MD; Christine Turley, MD; Jaime Brown, MD; Efrain Sanchez-Rivera, MD; Laura Valleni, MD; Julie Ross, MD; Brian Wood, MD; Lisa Knight, MD; Hanna Sahhar, MD; Devonne Gerstenacker, BS, RN, MSN; Sarah Roland, RN, MSN; Veronia Oakes, BSN, RNC-NIC; Heather Heape, RN; Barbara Thompson, LPN; Sarah Newman-Norland, MA; Mary Freeman, BS; Carolyn Emeneker, BA. West Virginia University (ISCPTN: UG1OD024949): Lesley Cottrell, PhD; Lee Pyles, MD; Phillip Saul, MD; Michelle Schaffer, RN; Kaitlyn Earle, BSN.
We are indebted to our medical and nursing colleagues who agreed to take part in this study.
Glossary
- ACT NOW
Advancing Clinical Trials in Neonatal Opioid Withdrawal
- ANOVA
analysis of variance
- CI
confidence interval
- M + C
morphine plus clonidine
- M + P
morphine plus phenobarbital
- NOWS
neonatal opioid withdrawal syndrome
- NRN
Neonatal Research Network
Footnotes
The data set supporting the conclusions of this article is available by request to the corresponding author through a data use agreement and will be uploaded into a publicly available repository within 6 months of publication.
Drs Merhar, Das, Devlin, Higgins, Lester, Poindexter, Simon, P. Smith, and Young made substantial contributions to the conception and design of the study and drafting of the manuscript; Drs Fuller, Sanchez, and Whalen contributed to the design of the data collection forms and data acquisition; Dr Ounpraseuth and Ms Thombre conducted statistical analysis, provided interpretation of the data, substantially contributed to the methods and results sections of the manuscript, and created graphics; Drs Berkey, Crowley, Czynski, Kiefer, Newman, and M. Smith helped with acquisition of data, and provided input on data interpretation; and all authors reviewed the manuscript for important intellectual content and have approved the final version for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Advancing Translational Sciences provided support for the Neonatal Research Network and the National Institutes of Health, Office of the Director, Environmental influences on Child Health Outcomes (ECHO) program provided support for the Institutional Development Award States Pediatric Clinical Trials Network. Although NICHD and National Institutes of Health ECHO staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD or the ECHO program, the National Institutes of Health, the Department of Health and Human Services, or the US Government. Data were collected at participating sites of the NICHD Neonatal Research Network and participating sites of the ECHO Institutional Development Award States Pediatric Clinical Trials Network and transmitted to University of Arkansas for Medical Sciences, the Data Coordination and Operations Center for this study. Drs Jeannette Lee and Jessica Snowden (Data Coordination and Operations Center Principal Investigators) had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Whalen is a codeveloper of the ESC Care Tool; Children’s Hospital at Dartmouth-Hitchcock is one of the institutions on the ESC Care Tool copyright; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics. 2019;143(3):e20183801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics . Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 4.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17 [DOI] [PubMed] [Google Scholar]
- 5.Hall ES, Wexelblatt SL, Crowley M, et al.; OCHNAS Consortium . A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemans-Cope L, Holla N, Lee HC, et al. Neonatal abstinence syndrome management in California birth hospitals: results of a statewide survey. J Perinatol. 2020;40(3):463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cober MP, Johnson CE. Stability of an extemporaneously prepared alcohol-free phenobarbital suspension. Am J Health Syst Pharm. 2007;64(6):644–646 [DOI] [PubMed] [Google Scholar]
- 8.McPherson C. Pharmacotherapy for neonatal abstinence syndrome: choosing the right opioid or no opioid at all. Neonatal Netw. 2016;35(5):314–320 [DOI] [PubMed] [Google Scholar]
- 9.Farooqi M, Seifert S, Kunkel S, Johnson M, Benson B. Toxicity from a clonidine suspension. J Med Toxicol. 2009;5(3):130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie HG, Cao YJ, Gauda EB, Agthe AG, Hendrix CW, Lee H. Clonidine clearance matures rapidly during the early postnatal period: a population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J Clin Pharmacol. 2011;51(4):502–511 [DOI] [PubMed] [Google Scholar]
- 11.Pacifici GM. Clinical pharmacology of phenobarbital in neonates: effects, metabolism and pharmacokinetics. Curr Pediatr Rev. 2016;12(1):48–54 [DOI] [PubMed] [Google Scholar]
- 12.Surran B, Visintainer P, Chamberlain S, Kopcza K, Shah B, Singh R. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome. A prospective randomized clinical trial. J Perinatol. 2013;33(12):954–959 [DOI] [PubMed] [Google Scholar]
- 13.Brusseau C, Burnette T, Heidel RE. Clonidine versus phenobarbital as adjunctive therapy for neonatal abstinence syndrome. J Perinatol. 2020;40(7):1050–1055 [DOI] [PubMed] [Google Scholar]
- 14.Devlin LA, Lau T, Radmacher PG. Decreasing total medication exposure and length of stay while completing withdrawal for neonatal abstinence syndrome during the neonatal hospital stay. Front Pediatr. 2017;5:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zozus MN, Young LW, Simon AE, et al. Training as an intervention to decrease medical record abstraction errors multicenter studies. Stud Health Technol Inform. 2019;257:526–539 [PMC free article] [PubMed] [Google Scholar]
- 16.Zozus MN, Pieper C, Johnson CM, et al. Factors affecting accuracy of data abstracted from medical records. PLoS One. 2015;10(10):e0138649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh A, Gopalakrishnan M, Azeem A, Booth A, El-Metwally D. Racial association and pharmacotherapy in neonatal opioid withdrawal syndrome. J Perinatol. 2019;39(10):1370–1376 [DOI] [PubMed] [Google Scholar]
- 18.Charles MK, Cooper WO, Jansson LM, Dudley J, Slaughter JC, Patrick SW. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp Pediatr. 2017;7(6):328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isemann BT, Stoeckle EC, Taleghani AA, Mueller EW. Early prediction tool to identify the need for pharmacotherapy in infants at risk of neonatal abstinence syndrome. Pharmacotherapy. 2017;37(7):840–848 [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health . NIH policy on sex as a biological variable. Available at: https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable. Accessed June 10, 2020
- 21.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42(6):829–836 [DOI] [PubMed] [Google Scholar]
- 22.Robinson SA, Jones AD, Brynildsen JK, Ehrlich ME, Blendy JA. Neurobehavioral effects of neonatal opioid exposure in mice: influence of the OPRM1 SNP. Addict Biol. 2020;25(5):e12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwicker JG, Miller SP, Grunau RE, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81–87.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maalouf FI, Cooper WO, Slaughter JC, Dudley J, Patrick SW. Outpatient pharmacotherapy for neonatal abstinence syndrome. J Pediatr. 2018;199:151–157.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Phenobarbital and midazolam increase neonatal seizure-associated neuronal injury. Ann Neurol. 2017;82(1):115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99(23):15089–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures–effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322(6):364–369 [DOI] [PubMed] [Google Scholar]
- 28.Streetz VN, Gildon BL, Thompson DF. Role of clonidine in neonatal abstinence syndrome: a systematic review. Ann Pharmacother. 2016;50(4):301–310 [DOI] [PubMed] [Google Scholar]
- 29.Agthe AG, Kim GR, Mathias KB, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bada HS, Sithisarn T, Gibson J, et al. Morphine versus clonidine for neonatal abstinence syndrome. Pediatrics. 2015;135(2). Available at: www.pediatrics.org/cgi/content/full/135/2/e383 [DOI] [PubMed] [Google Scholar]