Abstract
A 78‐year‐old male with renal cell carcinoma was treated with combined immunotherapy of nivolumab and ipilimumab. After four courses of the treatment, a chest computed tomography (CT) revealed newly formed ground‐glass opacities (GGOs) in both the lower lung lobes; drug‐induced pneumonia was speculated. Eosinophil counts were elevated in both peripheral blood and bronchoalveolar lavage fluid. Both the immune checkpoint inhibitors (ICIs) were discontinued, following which the chest CT findings improved. Based on these findings, a diagnosis of ICI‐induced eosinophilic pneumonia was made. Hence, clinicians should be wary of the risk of eosinophilic pneumonia during ICI‐anticancer therapy.
Keywords: eosinophilic pneumonia, immune checkpoint inhibitor, immune‐related adverse event, ipilimumab, nivolumab
This report describes a 78‐year‐old man with renal cell carcinoma treated with nivolumab and ipilimumab immunotherapy and is the first reported case of eosinophilic pneumonia caused by treatment with combined immune checkpoint inhibitors (ICIs).
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have recently been attracting attention for the effective treatment of patients with advanced cancer. ICIs act on immune checkpoint molecules on the surface of both cancer and immune cells. In contrast to the favorable anticancer effects, immune related adverse events (irAEs) induced by ICIs such as nivolumab and ipilimumab have been reported, of which fatigue (16%), decreased appetite (11%), asthenia (10%), nausea (9%), and diarrhea (8%) by nivolumab, 1 and fatigue (45%), diarrhea (37%), pruritus (28%), rash (26%), and nausea (24%) by ipilimumab 2 are prevalent. However, irAE of eosinophilic pneumonia caused by the treatment of combination of nivolumab and ipilimumab has not been previously reported. Here, we present a case of eosinophilic pneumonia as an irAE in a renal cancer patient related to lung complications induced by ICIs.
CASE REPORT
A 78‐year‐old Japanese male, non‐smoker, with a history of liver cirrhosis and chronic renal failure, in maintenance hemodialysis, was diagnosed with a right papillary renal cell carcinoma. He had previously undergone a right nephrectomy without adjuvant chemotherapy or radiotherapy 28 months ago in a different hospital. He was referred as a result of an abnormality found on chest radiograph and relapsing renal cell carcinoma with bone metastasis which had appeared 24 months following the initial surgery. Four cycles of combined nivolumab and ipilimumab therapy were administered. One month later, his chest computed tomography (CT) revealed ground‐glass opacities (GGOs) in both the lower lung lobes (Figure 1). He had no history of allergic or parasitic diseases (caused by the consumption of food infected with parasites) and was not taking any new medication or supplements.
FIGURE 1.
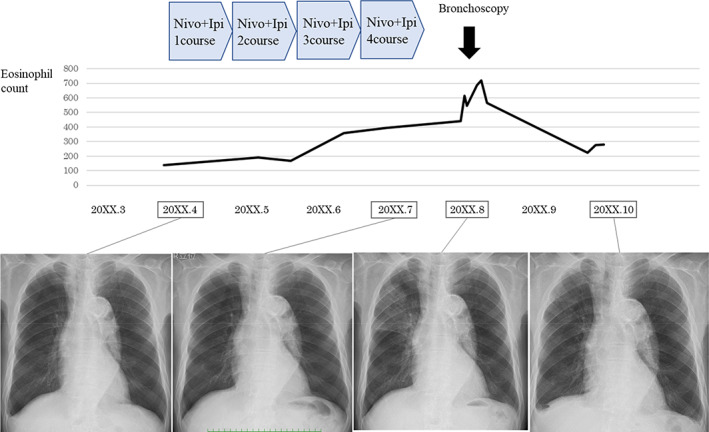
Clinical course of the patient. Abbreviations: Nivo, nivolumab; Ipi, ipilimumab
Upon admission, his physical examination results were as follows: height, 163.6 cm; bodyweight, 52.4 kg; body temperature, 36.4°C; heart rate, 68 bpm; blood pressure, 166/74 mmHg; and oxygen saturation, 98% (room air, at rest). No abnormalities were detected on chest auscultation, neurological examination, or on the skin. Laboratory test results revealed slightly elevated eosinophils (Table 1).
TABLE 1.
Results of peripheral blood analysis on admission
| Blood cell counts | Blood chemistry | Serology | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 4400 | /μl | TP | 6.5 | g/dl | CRP | 0.27 | mg/dl |
| Neutrophils | 63.7 | % | Alb | 3.2 | g/dl | |||
| Lymphocytes | 21.4 | % | T‐bil | 0.3 | mg/dl | KL‐6 | 103 | U/ml |
| Eosinophils | 9.0 | % | AST | 29 | IU/l | SP‐D | 99.2 | ng/ml |
| Monocytes | 5.4 | % | ALT | 20 | IU/l | |||
| Basophils | 0.5 | % | LDH | 150 | IU/l | |||
| RBC | 331 × 104 | /μl | ALP | 270 | IU/l | BAL Findings (rt.B2b) | ||
| Hb | 11.0 | g/dl | γ‐GTP | 51 | IU/l | Recovery | 92/150 | ml |
| Ht | 34.0 | % | BUN | 56 | mg/dl | Total cell count | 1.8 × 105 | /ml |
| Platelets | 10.7 × 104 | /μl | Cre | 9.37 | mg/dl | Macrophages | 17.5 | % |
| Neutrophils | 0 | % | ||||||
| Lymphocytes | 11 | % | ||||||
| Eosinophils | 71.5 | % | ||||||
Abbreviations: Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BALF, bronchoalveolar lavage fluid.; BUN, blood urea nitrogen; Cre, creatinine; CRP, C‐reactive protein; Hb, hemoglobin; Ht, hematocrit; KL‐6, sialylated carbohydrate antigen KL‐6; LDH, lactate dehydrogenase; RBC, red blood cell; SP‐D, pulmonary surfactant protein‐D; T‐bil, total bilirubin; TP, total protein; WBC, white blood cell; γ‐GTP, gamma‐glutamyl transferase.
Eosinophilia was not observed before the chemotherapy (130/μl), and elevated peripheral eosinophil count (357/μl) was first observed after two courses of the combined treatment with nivolumab and ipilimumab. Subsequently, eosinophils gradually increased to 720/μl after four courses of the combination chemotherapy (Figure 1), and chest radiography and high‐resolution CT revealed ground‐glass attenuations in the bilateral lower lobes with no lymphadenopathies and pleural effusion (Figure 2).
FIGURE 2.
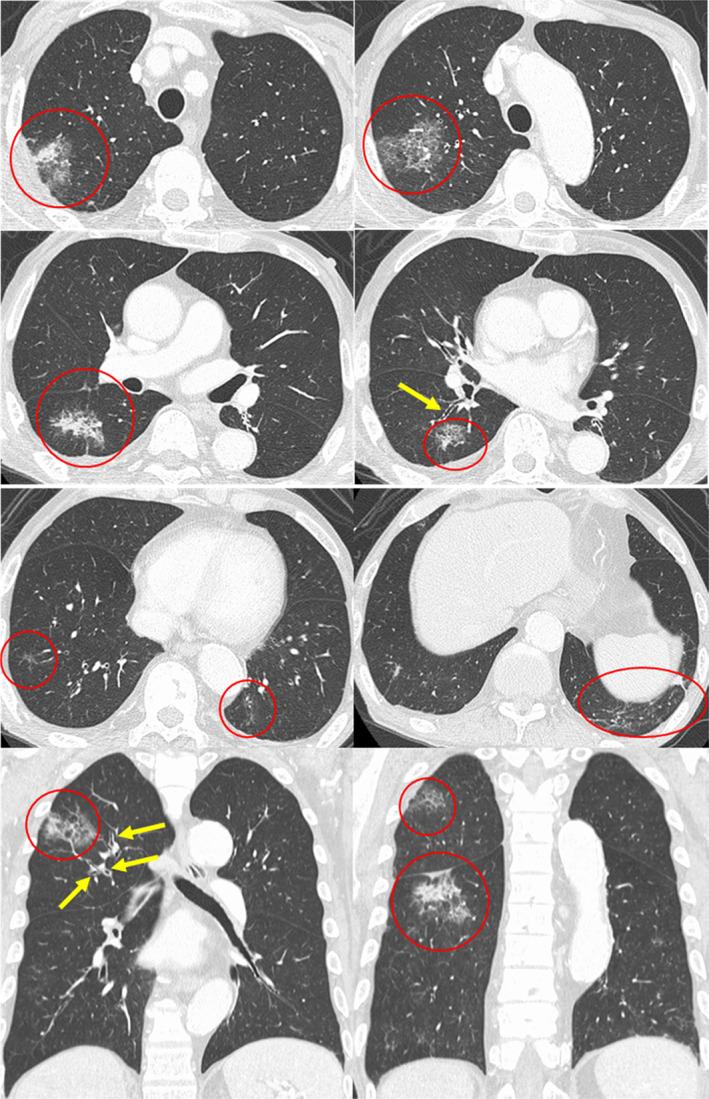
Axial and coronal chest high‐resolution computed tomography shows consolidation with peripheral ground‐glass attenuation (red circles) and thickening of bronchovascular bundles (yellow arrows)
Bronchoalveolar lavage fluid (BALF) obtained from the right B2 b revealed a high cell count (1.8 × 105/ml) with significantly elevated eosinophils (71.5%) and negative results for bacterial/fungal culture (Table 1). The patient refused a transbronchial lung biopsy due to the risk of pneumothorax.
Our patient fulfilled three of the five criteria for drug‐induced pneumonia, 3 such as a history of consumption of a medication known to cause lung injury, other causes of lung disease had been ruled out, and improvement following discontinuation of the suspected drug, and also four of five criteria for eosinophilic pneumonia 4 (pulmonary parenchymal infiltrates, peripheral blood eosinophilia, a BALF eosinophil fraction >25% or histopathological results from a transbronchial biopsy, and a negative work‐up for other causes of peripheral blood eosinophilia). Hence, the patient was diagnosed with drug‐induced eosinophilic pneumonia.
The patient had no respiratory symptoms; therefore, the manifested side effect of ICI was categorized as class 1 according to the Common Terminology Criteria for Adverse Events version 5.0. The ICIs were discontinued and no treatment was instituted for his eosinophilic pneumonia. Consistent with drug‐induced eosinophilic pneumonia, the pulmonary infiltrations on chest CT and peripheral eosinophilia gradually improved and disappeared three months following discontinuation of ICIs (Figure 1). We could not use other antitumor agents because of the poor general condition of the patient. He was transferred to another nursing home 11 months after first receiving ICIs.
DISCUSSION
To the best of our knowledge, this is the first case report of eosinophilic pneumonia caused by combined ICI treatment with nivolumab and ipilimumab. In our patient, eosinophilic infiltration in the lung with peripheral blood eosinophilia occurred soon after starting combined treatment with nivolumab and ipilimumab. Discontinuation of these two ICIs resulted in the spontaneous improvement of both pulmonary infiltrations and peripheral blood eosinophilia, indicative of nivolumab and ipilimumab‐induced eosinophilic pneumonia.
Nivolumab — an anti‐programmed cell death 1 receptor (PD‐1) antibody, inhibits the interaction of PD‐1 receptors with both programmed cell death receptor ligand 1 (PD‐L1) and PD‐L2 on lung dendritic cells. Jodai et al. reported that this interaction might explain the mechanism of eosinophilic pneumonia. 5 Binding of PD‐1 to PD‐L2 on the dendritic cells in the lung may facilitate pulmonary Th2‐type inflammation. This inflammation is induced by Th2 cells which predominantly produces interleukin (IL)‐4, IL‐5, and IL‐13, eventually resulting in eosinophilic activation and inflammation (Figure 3). 5 The PD‐1/PD‐L2 interaction can initiate a Th1‐type response with increased expression of IFN‐γ and reduce the airway hyperreactivity in allergic asthma. 6
FIGURE 3.
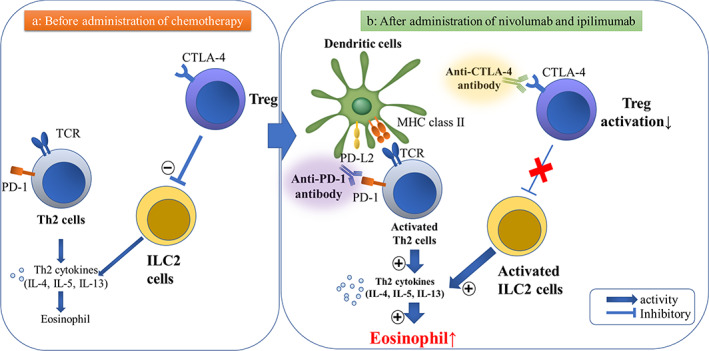
Hypothesized pathogenesis of eosinophilic pneumonia as an irAE in lung cancer patients receiving anti‐PD‐1 and anti‐CTLA‐4 antibodies (nivolumab and ipilimumab, respectively). (a) Before administration of chemotherapy. Th2 cell is not activated, and Tregs in the tumor cells suppress the production of ILC2‐driven proinflammatory cytokines. (b) After administration of nivolumab and ipilimumab. Anti‐PD‐1 antibodies inhibit binding not only of PD‐1 to PD‐L1, but also PD‐1 to PD‐L2 on lung dendritic cells, which facilitates pulmonary Th2‐type inflammation. CTLA‐4 acts as a negative regulator of Treg activation; this promotes the production of ILC2‐driven pro‐inflammatory cytokines IL‐4, IL‐5 and IL‐13, also leading to pulmonary Th2‐type inflammation. Hence, both nivolumab and ipilimumab might play a role in eosinophil recruitment to the lungs. Abbreviations: PD‐1, programmed cell death‐1; PD‐L2, programmed cell death‐1 ligand 2; CTLA‐4, cytotoxic T‐lymphocyte‐associated antigen‐4; MHC, major histocompatibility complex; TCR, T cell receptor; ICL‐2, innate lymphoid cells‐2
Ipilimumab is a fully human monoclonal immunoglobulin (Ig) G1κ antibody against cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4), an immune‐inhibitory molecule expressed in activated T cells and in suppressor T regulatory cells (Treg). It also acts as a negative regulator of T cell activation by binding and preventing the costimulatory interaction between the cluster of differentiation (CD) 28 and B7 ligands. Finally, it blocks the binding of CTLA‐4 to B7 (CD80/CD8) ligands expressed on antigen‐presenting cells, induces the reduction of inhibitory signals generated through this receptor, and enhances T cell activation, leading to anticancer responses. 7
Administration of CTLA‐4 immunoglobulin fusion protein costimulates CD28, inhibits recruitment of eosinophils into the lungs by 75%, and suppresses IgE production in BALF. 8 In in vitro and in vivo experiments, the Treg cells in the tumor suppresses production of the innate lymphoid cell (ILC) 2‐driven proinflammatory cytokines IL‐5 and IL‐13. 9 Hence, we hypothesized the pathogenesis that CTLA‐4–mediated inactivation of Treg cells might show that eosinophilic recruitment and activation in the lungs can be induced by IL‐5 and IL‐13 (Figure 3). This is a purported mechanism for eosinophilic pneumonia following the use of ipilimumab. For the above reasons, both nivolumab and ipilimumab may cause eosinophilic pneumonia, although it is difficult to determine if it was the primary cause in this case. There have been two previously reported cases of ipilimumab‐induced eosinophilia after changing from nivolumab, 10 , 11 suggesting that ipilimumab might play a significant role in this case.
The characteristic chest CT findings in acute eosinophilic pneumonia are bilateral reticular opacities and pleural effusion. 12 In contrast, bilateral peripheral segmental infiltrates are typical findings in drug‐induced chronic eosinophilic pneumonia, and few reported cases had pleural effusion (3.3%). 4 Our patient's chest CT findings showed ground‐glass attenuations in bilateral lower lobes with no pleural effusion, which were consistent with previous reports.
In the radiographic findings of PD‐1 inhibitor‐related pneumonitis, the most common pattern is organizing pneumonia, followed by nonspecific interstitial pneumonia, hypersensitivity pneumonitis, and acute interstitial pneumonia/acute respiratory distress syndrome. 13 In ipilimumab‐related pneumonitis, the most common pattern is organizing pneumonia, followed by nonspecific interstitial pneumonia. 14 The reported organizing pneumonia may include EP since both have similar findings on chest CT.
In conclusion, peripheral blood eosinophil counts should be regularly monitored during ICI use. If an irAE due to ICI therapy is considered a possibility, eosinophilic damage to organs, including the lungs, should be evaluated.
CONFLICT OF INTEREST
The authors report that there are no conflicts of interest.
Hara K, Yamasaki K, Tahara M, et al. Immune checkpoint inhibitors‐induced eosinophilic pneumonia: A case report. Thoracic Cancer. 2021;12:720–724. 10.1111/1759-7714.13848
REFERENCES
- 1. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–26. [DOI] [PubMed] [Google Scholar]
- 4. Bartal C, Sagy I, Barski L. Drug‐induced eosinophilic pneumonia: a review of 196 case reports. Medicine (Baltimore). 2018;97:e9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jodai T, Yoshida C, Sato R, Kakiuchi Y, Sato N, Iyama S, et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti‐PD‐1 immune checkpoint antibody in a lung cancer patient. Immun Inflammation Dis. 2019;7:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh AK, Stock P, Akbari O. Role of PD‐L1 and PD‐L2 in allergic diseases and asthma. Allergy. 2011;66:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res. 2012;65:9–22. [DOI] [PubMed] [Google Scholar]
- 8. Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle AJ. Costimulation through B7‐2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med. 1997;185:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T‐cell costimulator‐inducible T‐cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139:1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ariyasu H, Inaba H, Ota T, Yamaoka H, Furukawa Y, Iwakura H, et al. Thyrotoxicosis and adrenocortical hormone deficiency during immune‐checkpoint inhibitor treatment for malignant melanoma. Case Rep. 2018;32:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwatsuka Y, Iwanaga A, Kuwatsuka S, Okubo Y, Murayama N, Ishii N, et al. Bullous pemphigoid induced by ipilimumab in a patient with metastatic malignant melanoma after unsuccessful treatment with nivolumab. Case Rep. 2018;45:e21–2. [DOI] [PubMed] [Google Scholar]
- 12. Jeong YJ, Kim KI, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–37. [DOI] [PubMed] [Google Scholar]
- 13. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6021–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune‐related adverse events in advanced melanoma patients treated with Ipilimumab. Cancer Immunol Res. 2015;3:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


