Using a multicenter administrative database, in this study, we identify risk of in-hospital mortality associated with neurologic involvement among pediatric patients with HUS.
Abstract
BACKGROUND AND OBJECTIVES:
Acute severe neurologic involvement is the most threatening complication in children with hemolytic-uremic syndrome (HUS). Our primary study objectives were to describe the association between acute neurologic manifestations (ANMs) and in-hospital mortality among children with HUS.
METHODS:
Using the Pediatric Health Information System database, in this retrospective multicenter cohort study, we identified the first HUS-related inpatient visit among children ≤18 years (years 2004–2018). Frequency of selected ANMs and combinations of ANMs, as well as the rate of mortality, was calculated. Multivariate logistic regression was used to identify the association of ANMs and the risk of in-hospital mortality.
RESULTS:
Among 3915 patients included in the analysis, an ANM was noted in 10.4% (n = 409) patients. Encephalopathy was the most common ANM (n = 245). Mortality was significantly higher among patients with an ANM compared with patients without an ANM (13.9% vs 1.8%; P < .001). Individuals with any ANM had increased odds of mortality (odds ratio [OR]: 2.25; 95% confidence interval [CI]: 1.29–3.93; P = .004), with greater risk (OR: 2.60; 95% CI: 1.34–5.06; P = .005) among patients with ≥2 manifestations. Brain hemorrhage (OR: 3.09; 95% CI: 1.40–6.82; P = .005), brain infarction (OR: 2.64; 95% CI: 1.10–6.34; P = .03), anoxic brain injury (OR: 3.92; 95% CI: 1.49–10.31; P = .006), and brain edema (OR: 4.81; 95% CI: 1.82–12.71; P = .002) were independently associated with mortality.
CONCLUSIONS:
In this study, the largest systematic assessment of ANMs among children with HUS to date, we identify differences in in-hospital mortality based on the type of ANM, with increased risk observed for patients with multiple ANMs.
What’s Known on This Subject:
Hemolytic-uremic syndrome (HUS) presents in children with microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. Acute neurologic involvement is the most threatening extrarenal complication in children with HUS.
What This Study Adds:
In this study, we describe the largest cohort of pediatric patients with HUS and identify risks associated with specific neurologic manifestations, including having multiple neurologic conditions as well as combinations of those conditions.
Hemolytic-uremic syndrome (HUS) presents in children with a constellation of features that include microangiopathic hemolytic anemia, thrombocytopenia, and varying degrees of renal impairment. This is further classified into typical HUS (postdiarrhea variant) and atypical HUS (no associated diarrhea).1 The kidney and the brain are the 2 primary target organs involved in complications associated with HUS.2
Neurologic involvement, although less common than renal disease, can have a sudden onset and is the most frequent cause of acute mortality and long-term disability among patients with HUS.1 The most frequent initial acute neurologic manifestations (ANMs) and severe neurologic manifestations reported are coma and seizures.3 These clinical findings reflect the sudden occurrence of microvascular injury in the various brain structures and represent the combined effect of toxin-induced vascular injury, endothelial dysfunction, hypertension, and electrolyte disorders.4
Although the effects of ANMs is well described in adults with HUS, less is known about ANMs in children with HUS. Authors of previous studies describing ANMs have reported ANM rates of 3% to 53%,5–10 but these studies are limited by their small sample sizes5–9 and reports from single centers.7–10 We add to this literature by analyzing the Pediatric Health Information System (PHIS) database, a large multicenter validated pediatric database, to investigate ANMs in children with HUS. The large size of the data set allowed for comparisons between different individual ANMs and combinations of ANMs. As such, our primary objective of the study was to describe ANMs in HUS and identify independent neurologic risk factors associated with in-hospital mortality among children with HUS.
Methods
This study was determined as a non–human subject investigation by the first author’s institutional review board.
Data Source
The data for this study come from the PHIS, an administrative database that contains inpatient data from >40 not-for-profit tertiary care children’s hospitals in North America.11 Institutions are affiliated with the Child Health Corporation of America (Shawnee Mission, KS) and account for 20% of all tertiary care children’s hospitals in the United States. Participating hospitals provide hospital discharge data, which include diagnoses and procedural information via the International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, 10th Revision (ICD-10) and procedural information via Current Procedural Terminology codes. Data are deidentified at the time of submission and are subjected to a number of reliability and validity checks before being added into the database.
Study Population
The study sample included pediatric patients who were discharged from a PHIS participating hospital between January 1, 2004, and December 31, 2018, with a diagnosis code of HUS. Among 4584 index HUS visits, we excluded 669 patients with renal disease, receiving an organ transplant, or with congenital cardiac disease, resulting in a final sample of 3915 children with HUS (Supplemental Table 5).
Statistical Analysis
We identified common but severe ANMs, including meningitis, seizure disorder, intracranial bleed, brain infarction or hemiplegia, encephalopathy, anoxic brain injury, and brain edema or brain compression. Patients with ≥1 of these conditions were indicated to have any ANM. Descriptive statistics t tests (continuous variables) and Wald χ2 tests (categorical variables) were used to test for differences between demographic and clinical characteristics of patients with ANM and patients without ANM. Descriptive analyses were also conducted to compare in-hospital mortality rate among patients with different combinations of ANMs.
Multivariable logistic regression was used to assess the association of ANMs with in-hospital mortality. Specifically, models were calculated to assess the risk associated with having any ANM, the risk associated with having 1 or having ≥2 ANMs, and the risk associated with specific ANMs. Covariates selected for inclusion in the multivariable models included demographic characteristics and procedures associated with increased risk of in-hospital mortality. Additionally, adjusted models included specific infection types associated with HUS, including Shiga toxin, pneumococcal infection, and enteritis, and we controlled for an annual linear time trend. To adjust for the center effects, we added 2 components to all adjusted regressions. First, we controlled for the number of HUS discharges per hospital per year. Second, we clustered the SEs at the hospital level to control for correlation that might exist between discharges from the same hospital. Corresponding codes for all diagnoses and procedures included in this study are provided in Supplemental Table 6. Additionally, length of stay was censored at the 99th percentile (88 days) for 41 patients (15 patients with ANMs and 25 without ANMs).
Two sensitivity analyses were conducted to assess robustness of the models. Specifically, models were recalculated after we removed patients who transferred out of the hospital (n = 98) as well as patients with missing racial information (n = 180), who were originally included in the “other or missing” racial category. All statistical analyses were conducted by using Stata version 16 (Stata Corp, College Station, TX), and graphs were created by using Tableau 2019.4. All tests were 2 sided, assuming a P value <.05 as statistically significant.
Results
Among 3915 subjects included in the study, ANMs were observed in 10.4% (n = 409) of subjects (Table 1). The median age among patients with ANMs was 3.3 years, with 48.7% of patients being between 1 and 5 years of age. Individuals with ANMs were more likely to have procedures such as mechanical ventilation (P < .001), extracorporeal membrane oxygenation (ECMO) (P < .001), and intracranial drainage (P < .001) compared with patients without ANMs. The in-hospital mortality was significantly higher among patients with ANMs compared with patients without ANMs (13.9% vs 1.8%; P < .001). The average hospital length of stay was also significantly higher among patients with ANMs compared those without ANMs (27.8 vs 13.8 days; P < .001).
TABLE 1.
Demographic Characteristics and Unadjusted Outcomes, by Neurologic Status (N = 3915)
| Overall (N = 3915) | No ANMs (n = 3506) | Had an ANM (n = 409) | Pa | |
|---|---|---|---|---|
| Covariate variables | ||||
| Age, yb | .04 | |||
| Mean | 5.3 | 5.4 | 4.9 | — |
| Median | 3.8 | 3.9 | 3.3 | — |
| IQR | 2.0–7.5 | 2.0–7.5 | 1.6–7.0 | — |
| Age, % | <.001 | |||
| <30 d | 2.9 | 2.6 | 4.9 | |
| 30 d to 1 y | 5.3 | 4.7 | 10.5 | |
| 1–5 y | 52.2 | 52.6 | 48.7 | |
| >5 yc | 39.7 | 40.1 | 35.9 | |
| Sex, % | .13 | |||
| Male | 47.5 | 47.9 | 44.0 | |
| Femalec | 52.5 | 52.1 | 56.0 | |
| Race, % | <.001 | |||
| Whitec | 75.5 | 76.8 | 64.1 | |
| Black | 6.2 | 5.7 | 11.0 | |
| Asian American | 2.5 | 2.4 | 3.2 | |
| Other or missing | 15.8 | 15.2 | 21.8 | |
| No. ANMs, % | ||||
| 0 conditions | 89.6 | 100.0 | — | — |
| 1 condition | 6.7 | — | 63.8 | |
| 2+ conditions | 3.8 | — | 36.2 | |
| Procedures, % | ||||
| ECMO | 0.8 | 0.5 | 4.2 | <.001 |
| Mechanical ventilation | 16.9 | 11.4 | 64.1 | <.001 |
| Brian drainage | 0.6 | 0.2 | 3.9 | <.001 |
| Shiga toxin–positive, % | 18.9 | 18.4 | 22.7 | .04 |
| Enteritis, % | 44.0 | 43.8 | 46.0 | .49 |
| Pneumococcal infection, % | 5.3 | 4.6 | 11.5 | <.001 |
| No. HUS discharges by hospital and y, mean (SD)d | 9.1 (5.1) | 9.2 (5.2) | 8.9 (5.0) | .42 |
| Outcomes | ||||
| Hospital LOS, mean (SD) | 15.3 | 13.8 | 27.8 | <.001 |
| In-hospital mortality, % | 3.0 | 1.8 | 13.9 | <.001 |
IQR, interquartile range; LOS, length of stay; —, not applicable.
P values were calculated from t tests for continuous variables and χ2 tests for categorical variables. The test for continuous variables was used to evaluate differences in mean values.
Variable was not included in regressions.
The categorical variable that served as the reference level in adjusted regressions.
Number of hospital discharges were calcualted for each year separately. A linear annual time trend was additionally used as a covariate in adjusted analyses.
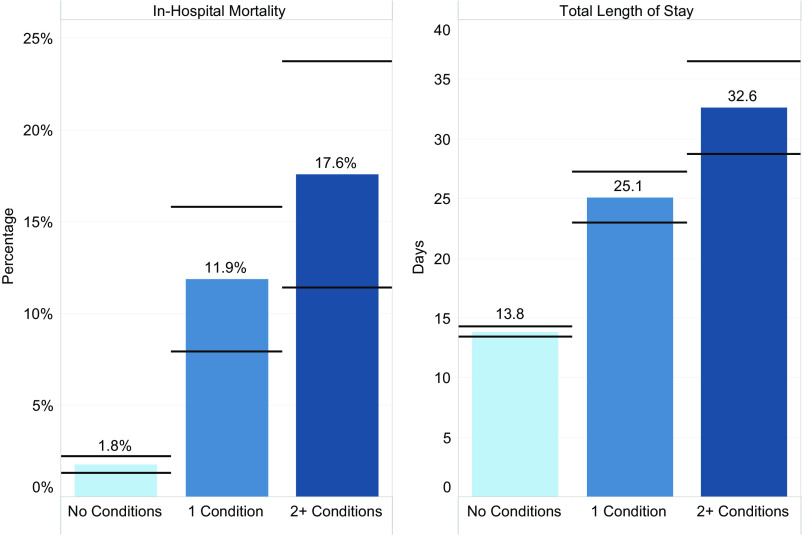
Approximately two-thirds of participants with any ANMs had only 1 ANM, and approximately one-third had multiple ANMs (Table 1). The mean hospital lengths of stay were 13.8, 25.1, and 32.6 days for patients with 0, 1, and ≥2 ANMs, and the percent mortality was 1.8%, 11.9%, and 17.6%, respectively (Fig 1). In Table 2, we provide combinations of various ANMs and associated mortality for combinations of ANMs that included at least 10 patients (information for less frequent ANM combinations are provided in Supplemental Table 7). Encephalopathy was the most common ANM and was reported among 245 patients, with 138 (56.3%) of these patients having isolated encephalopathy with no other concomitant ANM. Seizures were noted in 108 patients, of whom 45 patients (44.4%) had seizures as the sole ANM.
FIGURE 1.
Patient outcomes by the number of ANMs (n = 3915). Horizontal lines indicate 95% CIs based on SEs of the means. The number of conditions indicates the number of neurologic manifestations, including meningitis, seizure disorder, brain bleed, brain infarction or hemiplegia, encephalopathy, anoxic brain, and cerebral edema or brain compression.
TABLE 2.
Combinations of Neurologic Manifestations and Associated Mortality Rates (N = 3915)
| Meningitis (n = 62) | Seizure Disorder (n = 108) | Brain Bleed (n = 28) | Brain Infarction or Hemiplegia (n = 92) | Encephalopathy (n = 245) | Anoxic Brain (n = 32) | Cerebral Edema or Brain Compression (n = 37) | No. Conditions | Total Patients | No. Died | % Died |
|---|---|---|---|---|---|---|---|---|---|---|
| O | O | O | O | O | O | O | 0 | 3506 | 62 | 1.8 |
| O | O | O | O | X | O | O | 1 | 138 | 6 | 4.3 |
| O | X | O | O | O | O | O | 1 | 45 | 4 | 8.9 |
| O | X | O | O | X | O | O | 2 | 37 | 0 | 0.0 |
| O | O | O | X | O | O | O | 1 | 27 | 6 | 22.2 |
| X | O | O | O | O | O | O | 1 | 23 | 5 | 21.7 |
| O | O | O | X | X | O | O | 2 | 21 | 1 | 4.8 |
| O | O | O | O | O | X | O | 1 | 10 | 4 | 40.0 |
| O | O | X | O | O | O | O | 1 | 10 | 4 | 40.0 |
| X | O | O | X | O | O | O | 2 | 10 | 2 | 20.0 |
The table is limited to only those rows with ≥10 patients. Rows with <10 patients are available in Supplemental Table 7. Isolated cerebral edema or brain compression included 8 patients, of whom 2 experienced in-hospital mortality. O, patients included in this row did not have this condition; X, patients included in this row had this condition.
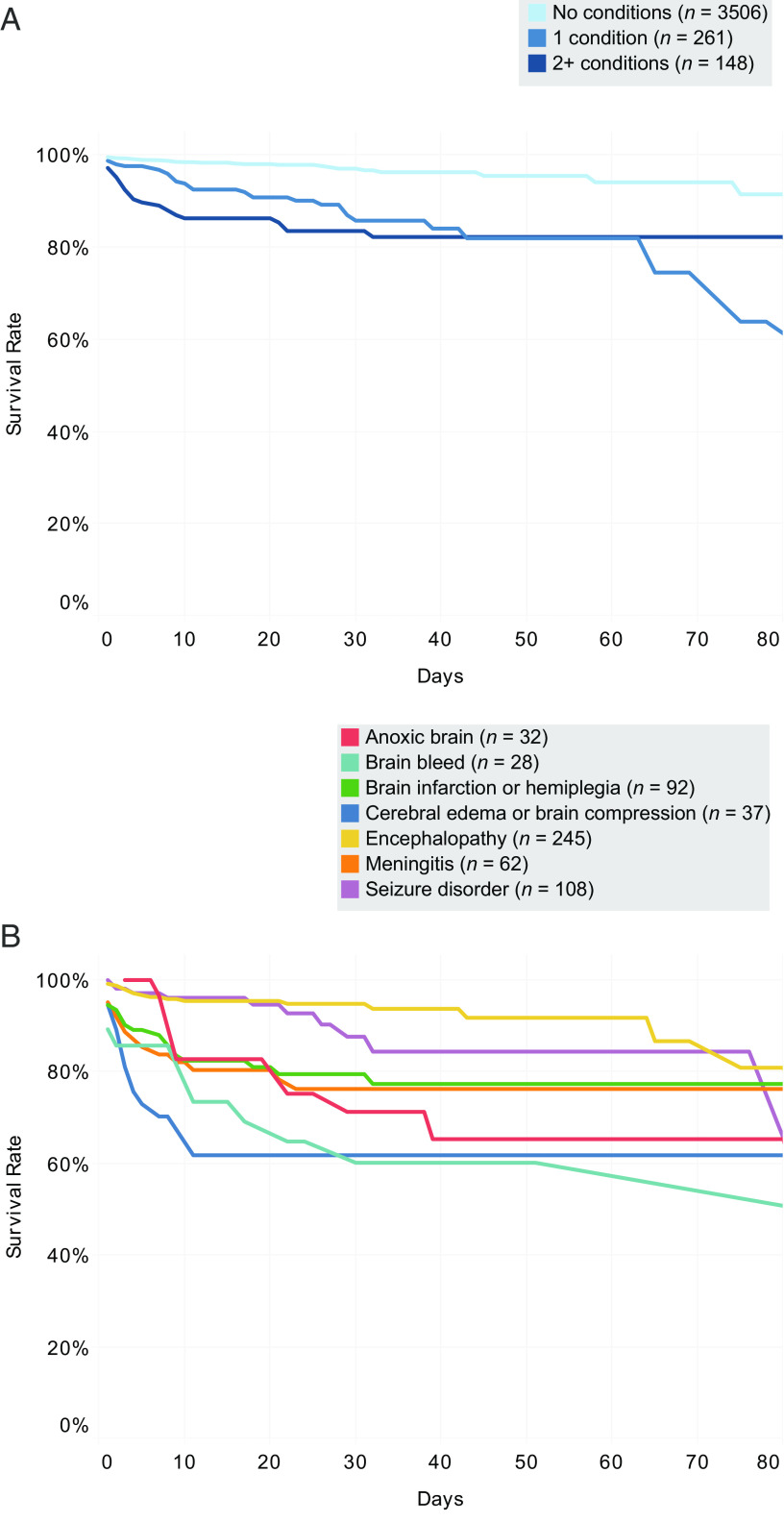
In-hospital mortality differed widely between various ANMs and ANM combinations (Table 2, right most column). Mortality was 4.3%, 8.9%, 21.7%, 22.2%, 40.0%, 25.0%, and 40.0% for isolated encephalopathy, seizures, meningitis, stroke, intracranial hemorrhage, cerebral edema, and anoxic brain injury groups, respectively, compared to only 1.8% among patients with no ANMs. Of note is the increased percentage of in-hospital mortality among patients with 1 ANM (11.9%) or ≥2 ANMs (17.6%) compared with patients with no ANMs (1.8%) (Fig 1). Figure 2 shows the Kaplan-Meier survival estimates for various ANMs, further highlighting the increased risk of mortality among patients with multiple ANMs. Table 3 shows a comparison of length of stay between survivors and nonsurvivors for various ANMs.
FIGURE 2.
Kaplan-Meier survival estimates by ANM. A, Survival estimate curves stratified by the number of ANMs, including meningitis, seizure disorder, brain bleed, brain infarction or hemiplegia, encephalopathy, anoxic brain, and cerebral edema or brain compression. B, Survival estimate curves for individual ANMs. Note that for panel B, the categories are not mutually exclusive, so a patient may be in >1 category. Graphs were censored at 80 days.
TABLE 3.
Comparison of Length of Stay Between Survivors and Nonsurvivors of Various ANMs
| ANMs | Survivors | Nonsurvivors | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Meningitis | 37 | 24.5–54 | 3.5 | 2–9 |
| Seizure | 19 | 13–32 | 20 | 4–29 |
| Brain bleed | 43 | 24–82 | 10 | 1–22 |
| Brain infarction or hemiplegia | 31 | 18–46 | 4 | 1–9 |
| Encephalopathy with coma | 25.5 | 18–38 | 8 | 3–43 |
| Anoxic brain | 35 | 25–55 | 39 | 9–82 |
| Cerebral edema or brain compression | 27 | 20–41 | 3.5 | 2–7 |
IQR, interquartile range.
Table 4 provides results from the multivariate logistic regression that was used to assess the association of ANMs with in-hospital mortality. Having any ANM was associated with increased risk of in-hospital mortality (odds ratio [OR]: 2.25; 95% confidence interval [CI]: 1.29–3.93; P = .004; Table 3, model 1); however, having ≥2 ANMs was associated with further increased risk of mortality (OR: 2.60; 95% CI: 1.34–5.06; P = .005; Table 3, model 2). Neurologic conditions that were independently associated with increased risk of in-hospital mortality (Table 3, model 3) included brain hemorrhage (OR: 3.09; 95% CI: 1.40–6.82; P = .005), brain infarction (OR: 2.64; 95% CI: 1.10–6.34; P = .03), anoxic brain injury (OR: 3.92; 95% CI: 1.49–10.31; P = .006), and brain edema (OR: 4.81; 95% CI: 1.82–12.71; P = .002). Patients who received mechanical ventilation or ECMO support had an increased risk of mortality across all 3 models. Patients younger than 30 days had an increased risk of mortality in the model for any ANMs and by number of ANMs. A sensitivity analysis indicated that the findings were robust to excluding patients who transferred or who had missing racial information (Supplemental Table 8).
TABLE 4.
Multivariate Logistic Regression to Assess the Association of Neurologic Manifestation With In-Hospital Mortality (N = 3915)
| Variable | Model 1: Any ANM | Model 2: No. ANMs | Model 3: Specific ANMs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Sex | |||||||||
| Male | 0.82 | 0.50–1.34 | .42 | 0.81 | 0.50–1.32 | .40 | 0.75 | 0.46–1.23 | .26 |
| Female | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Age | |||||||||
| <30 d | 2.41 | 1.26–4.62 | .008 | 2.45 | 1.29–4.64 | .006 | 1.78 | 0.89–3.58 | .10 |
| 30 d to 1 y | 0.6 | 0.29–1.49 | .32 | 0.67 | 0.30–1.49 | .33 | 0.56 | 0.24–1.31 | .18 |
| 1–5 y | 0.75 | 0.47–1.18 | .21 | 0.75 | 0.47–1.19 | .22 | 0.68 | 0.42–1.08 | .11 |
| >5 y | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Race | |||||||||
| White | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Black | 0.99 | 0.56–1.77 | .98 | 0.99 | 0.55–1.78 | .98 | 0.96 | 0.51–1.80 | .91 |
| Asian American | 0.23 | 0.04–1.28 | .09 | 0.23 | 0.04–1.29 | .10 | 0.21 | 0.04–1.13 | .07 |
| Other or missing | 0.98 | 0.59–1.63 | .94 | 0.99 | 0.60–1.64 | .97 | 1.06 | 0.62–1.79 | .84 |
| ECMO | 9.19 | 4.55–18.58 | <.001 | 9.56 | 4.69–19.51 | <.001 | 9.97 | 5.19–19.14 | <.001 |
| Mechanical ventilation | 24.39 | 10.67–55.75 | <.001 | 24.13 | 10.58–55.05 | <.001 | 28.84 | 13.32–62.43 | <.001 |
| Brain drainage | 3.64 | 1.26–10.55 | .02 | 3.54 | 1.21–10.41 | .02 | 2.60 | 0.76–8.85 | .13 |
| Shiga toxin–positive | 0.37 | 0.16–0.86 | .02 | 0.37 | 0.16–0.86 | .02 | 0.36 | 0.16–0.83 | .02 |
| Pneumococcal infection | 0.64 | 0.34–1.23 | .18 | 0.63 | 0.34–1.18 | .15 | 0.46 | 0.23–0.92 | .03 |
| Enteritis | 0.75 | 0.45–1.23 | .25 | 0.75 | 0.45–1.24 | .26 | 0.74 | 0.43–1.27 | .27 |
| No. HUS discharges by hospital and y | 1.00 | 0.95–1.05 | .93 | 1.00 | 0.95–1.05 | .90 | 0.99 | 0.94–1.05 | .77 |
| Year of admission datea | 0.98 | 0.93–1.03 | .50 | 0.98 | 0.93–1.03 | .47 | 0.99 | 0.94–1.04 | .72 |
| Any ANMs | 2.25 | 1.29–3.93 | .004 | — | — | — | — | — | — |
| No. ANMs | |||||||||
| 0 conditions | — | — | — | Reference | Reference | Reference | — | — | — |
| 1 condition | — | — | — | 2.01 | (1.06–3.82) | .03 | — | — | — |
| 2+ conditions | — | — | — | 2.60 | (1.34–5.06) | .005 | — | — | — |
| Specific brain condition | |||||||||
| Meningitis | — | — | — | — | — | — | 1.63 | 0.58–4.58 | .34 |
| Seizure | — | — | — | — | — | — | 0.97 | 0.42–2.20 | .94 |
| Brain bleed | — | — | — | — | — | — | 3.09 | 1.40–6.82 | .005 |
| Brain infarction or hemiplegia | — | — | — | — | — | — | 2.64 | 1.10–6.34 | .03 |
| Encephalopathy | — | — | — | — | — | — | 0.45 | 0.24–0.85 | .01 |
| Anoxic brain | — | — | — | — | — | — | 3.92 | 1.49–10.31 | .006 |
| Cerebral edema or brain compression | — | — | — | — | — | — | 4.81 | 1.82–12.71 | .002 |
ANMs include meningitis, seizure disorder, brain bleed, brain infarction or hemiplegia, encephalopathy, anoxic brain, and cerebral edema or brain compression. —, not applicable.
Number of hospital discharges were calculated for each year separately. A linear annual time trend was used as a covariate in adjusted analyses based on the year of the discharge date.
Discussion
This investigation is the largest published systematic assessment of ANMs and outcomes among children with HUS. We identified frequencies of combinations of ANMs and found large differences in in-hospital mortality based on the number of ANMs a patient has as well as the type of ANM, suggesting that specific ANMs serve as independent risk factors for in-hospital mortality.
In our study, the frequency of ANMs in children with HUS was 10.4%. This contrasts to a reported frequency (between 3% and 53%)5–10,12–15 from previous investigations. In an audit analysis of the European Pediatric Study Group for HUS, researchers found an overall frequency of 20% for ANMs among 71 patients with atypical HUS,8 and in a 26-center study from France of children with diarrhea-associated HUS, researchers found a rate of 3% (52 of 1308 patients).7 These differences may in part be related to how ANM was defined in different studies. Some studies with a higher frequency of ANMs have included mild, often transient symptoms, such as headache,3 hallucinations,3 and irritability,8 whereas others with lower ANM frequency have only included severe ANMs.7 Additionally, to have a more homogenous study sample for acute HUS, we excluded patients with codes for other concomitant renal diseases, transplants, or complex cardiac disease, and we only included the first HUS-coded hospitalization in the data set. Furthermore, the large sample size in our study allowed for detailed analysis of specific types of ANMs.
The most frequent ANM noted was encephalopathy (in 60.0% of all patients with ANMs), followed by seizures (26.4%) and stroke (22.5%). Besides describing the increased risk of mortality associated with a given ANM, this investigation also describes the frequency and mortality outcomes among various combinations of ANMs, which allow providers to more specifically understand a patient’s clinical outcome when the patient presents with multiple ANMs. Furthermore, we intentionally chose the term “encephalopathy,” given the more robust and specific ICD-9 or ICD-10 diagnosis codes that can be used to identify encephalopathy within large administrative databases. As such, our finding that encephalopathy was the most common manifestation was not found across previous studies. For example, the authors of a study of 71 patients with atypical HUS reported seizures as the most common neurologic manifestation (11.3%),8 and the authors of a 52-patient, 26-center study of patients with severe initial neurologic involvement reported coma as the most common manifestation (85%).7 The authors of a second study additionally noted seizure as the most commonly reported ANM (21%) among 169 patients with atypical HUS.3 Although seizure was not the most commonly identified ANM in our study, we found a similar rate of seizure disorder (26%) among the patients in our study.
We found significantly higher rates of mortality among patients with ANMs (13.9%) compared with patients without ANMs (1.8%). Of importance was that the unadjusted risk of mortality among patients with ANMs was higher for every measured isolated ANM relative to the risk among individuals without ANM, with the highest rates among patients with isolated brain hemorrhage (40%) or anoxic brain injury (40%). Relatedly, the risk of in-hospital mortality was amplified among patient with ≥2 ANMs. The larger sample size in this study has allowed us to calculate mortality rates for various ANMs in isolation as well when present in combination, which is a novel analysis. Among specific ANMs, the presence of brain bleed, stroke, anoxic brain injury, and cerebral edema was independently associated with increased risk of in-hospital mortality. In contrast, encephalopathy was associated with a lower risk of mortality. In a fully adjusted model, there was no association of risk of mortality with encephalopathy or any other specific ANM covariates (results not shown). These findings may help clinicians better prognosticate mortality outcomes when patients present with isolated ANMs or with >1 ANM. The mortality rates in previous literature for patients with HUS and ANMs have ranged from 8.7%3 to 28%.16 The rate of mortality in our study aligns with the findings reported by Nathanson et al,7 who found a mortality rate of 17.3% (9 of 52) among children with severe ANMs.
There are a number of limitations to our study. In this study, we used an administrative data set that was not collected for research purposes. The use of the PHIS administrative database offers the advantage of a large samples size, but it is limited by the fact that specific data points are not available, such as laboratory values or mortality outside the acute inpatient setting. As such, our ability to classify HUS into typical and atypical types was limited by the diagnostic coding of various associated etiologies; however, we adjusted for 3 types of infection in all adjusted models. The coding in the database indicates a much lower percentage of patients with Shiga toxin–positive HUS than what is generally found in the literature, so we felt that further investigation into HUS subtypes was not warranted. In the study, we focused only on severe neurologic manifestations and ANMs but not on mild, nonspecific, or chronic ANMs, which may preclude comparison with other studies that include these nonsevere acute or chronic classifications. We could not adjust for annual fixed effects because of the small sample size in each year. All ANM diagnoses are constructed by using ICD-9 or ICD-10 diagnosis codes, which do not have timing indicators in this data set. Therefore, we are unable to specifically identify the ANM timing. We could not follow our study cohort after the primary hospitalization for chronic sequelae given the significant case attrition on follow-up of patients across multiple hospitalizations within the PHIS database and the fact that many sequelae may be observed in settings other than acute inpatient facilities. Relatedly, we were also unable to collect more granular data on the impact of EEG and imaging findings on outcomes among the sample population from this study. Finally, the information used to construct ANM categorization (ie, diagnoses codes) do not have time stamps available within a given discharge. This, along with the observational design of the study, does not allow us to draw causal conclusions regarding the impact of ANM on mortality. Despite these limitations, this study provides the distinctive advantage of evaluating a large-scale sample of pediatric data from the majority of United States metropolitan areas and can serve as a foundation for clinical implications as well as for further investigation through a prospective study design.
Conclusions
This study is the largest published systematic assessment of ANMs among children with HUS to date, and identifies differences in risk of in-hospital mortality associated with different specific types of ANMs. Knowledge of these risk factors can help clinicians prognosticate clinical course and appropriately counsel families. With the findings of this analysis, we emphasize the need to conduct a multicenter prospective study to evaluate these outcomes with more granularity.
Acknowledgment
We thank Ms Julie Nick for data abstraction from the PHIS database.
Glossary
- ANM
acute neurologic manifestation
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- HUS
hemolytic uremic syndrome
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, 10th Revision
- OR
odds ratio
- PHIS
Pediatric Health Information System
Footnotes
Dr Brown conducted the analyses and interpretation of data, drafted components of the initial manuscript, and reviewed and revised the manuscript; Drs Garcia, Bhakta, and Sanders drafted and revised the article critically for important intellectual content; Dr Prodhan initiated the research idea, guided its analysis, participated in the interpretation of the data, drafted components of the initial manuscript, and reviewed and revised the manuscript; and all the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflict of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Translational Research Institute through the National Center for Advancing Translational Sciences of the National Institutes of Health (award UL1 TR003107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder did not participate in the work. Funded by the National Institutes of Health (NIH).
References
- 1.Siegler RL. The hemolytic uremic syndrome. Pediatr Clin North Am. 1995;42(6):1505–1529 [DOI] [PubMed] [Google Scholar]
- 2.Walsh PR, Johnson S. Treatment and management of children with haemolytic uraemic syndrome. Arch Dis Child. 2018;103(3):285–291 [DOI] [PubMed] [Google Scholar]
- 3.Fidan K, Göknar N, Gülhan B, et al. Extra-renal manifestations of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2018;33(8):1395–1403 [DOI] [PubMed] [Google Scholar]
- 4.Trachtman H, Austin C, Lewinski M, Stahl RA. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol. 2012;8(11):658–669 [DOI] [PubMed] [Google Scholar]
- 5.Tavasoli A, Zafaranloo N, Hoseini R, Otukesh H, Hooman N, Panahi P. Chronic neurological complications in hemolytic uremic syndrome in children. Iran J Kidney Dis. 2019;13(1):32–35 [PubMed] [Google Scholar]
- 6.Bauer A, Loos S, Wehrmann C, et al. Neurological involvement in children with E. coli O104:H4-induced hemolytic uremic syndrome. Pediatr Nephrol. 2014;29(9):1607–1615 [DOI] [PubMed] [Google Scholar]
- 7.Nathanson S, Kwon T, Elmaleh M, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5(7):1218–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson S, Stojanovic J, Ariceta G, et al. An audit analysis of a guideline for the investigation and initial therapy of diarrhea negative (atypical) hemolytic uremic syndrome. Pediatr Nephrol. 2014;29(10):1967–1978 [DOI] [PubMed] [Google Scholar]
- 9.Ağbaş A, Göknar N, Akıncı N, et al. Outbreak of Shiga toxin-producing Escherichia-coli-associated hemolytic uremic syndrome in Istanbul in 2015: outcome and experience with eculizumab. Pediatr Nephrol. 2018;33(12):2371–2381 [DOI] [PubMed] [Google Scholar]
- 10.Matthies J, Hünseler C, Ehren R, et al. Extrarenal manifestations in Shigatoxin-associated haemolytic uremic syndrome. Klin Padiatr. 2016;228(4):181–188 [DOI] [PubMed] [Google Scholar]
- 11.Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22–26 [PubMed] [Google Scholar]
- 12.Hahn JS, Havens PL, Higgins JJ, O’Rourke PP, Estroff JA, Strand R. Neurological complications of hemolytic-uremic syndrome. J Child Neurol. 1989;4(2):108–113 [DOI] [PubMed] [Google Scholar]
- 13.Bale JF Jr., Brasher C, Siegler RL. CNS manifestations of the hemolytic-uremic syndrome. Relationship to metabolic alterations and prognosis. Am J Dis Child. 1980;134(9):869–872 [DOI] [PubMed] [Google Scholar]
- 14.Cimolai N, Morrison BJ, Carter JE. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics. 1992;90(4):616–621 [PubMed] [Google Scholar]
- 15.Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J Infect Dis. 2002;186(4):493–500 [DOI] [PubMed] [Google Scholar]
- 16.Sheth K J, Swick HM, Haworth N. Neurological involvement in hemolytic-uremic syndrome. Ann Neurol. 1986;19(1):90–93 [DOI] [PubMed] [Google Scholar]