Abstract
Background
The aim of this study was to carry out a descriptive analysis of the somatic genetic profile and co‐occurring mutations of non‐small cell lung cancer (NSCLC) samples from patients tested with comprehensive genomic profiling (CGP).
Methods
This was a retrospective cross‐sectional study of patients diagnosed with NSCLC from 2013 to 2018 in Brazil and whose samples were submitted to CGP (FoundationOne or FoundationACT) using either tumor or circulating tumor DNA (ctDNA) from plasma.
Results
We recovered 513 CGP results from patients, 457 (89.1%) of which were from tumors and 56 (10.9%) from plasma. The median age of patients was 64 years old, of which 51.6% were males. TP53 mutations were identified in 53.6% of tumor samples, KRAS mutations in 24.2%, EGFR activating mutations were detected in 22.5%, STK11 mutations in 11.6%, PIK3CA mutations in 8.8%, ALK rearrangements in 5.4%, BRAF mutations in 5.2%, and ERBB2 alterations in 4.9%. The most commonly comutated gene was TP53. TP53 p.R337H was observed in 4.3% of samples and was associated with somatic mutations in EGFR and ERBB2 (P < 0.00001). Tumor mutational burden (TMB) analysis was available for 80.5% of samples tested, and 5.5% of samples had high TMB (≥ 20 mutations/Mb).
In conclusion, this retrospective analysis of genomic data from NSCLC patients obtained by CGP showed that common abnormalities such as EGFR mutations and ALK rearrangements had similar frequency to those previously described by other groups using others strategies. Additionally, our data confirm an association between TP53 p.R337H, supposedly germline in nature, and somatic mutations in genes of the HER family.
Key points
Significant findings of the study
This is the first report of the prevalence of driver mutations in Brazilian NSCLC patients using comprehensive genomic profiling (CGP).
The frequency of the most common driver mutations in this population was similar to that previously described in Brazil.
What this study adds
TP53 was the most commonly comutated gene across samples. TP53 p.R337H was associated with somatic mutations in EGFR and ERBB2.
Most samples had low TMB; only 5.5% of samples had high TMB.
Keywords: Co‐occurring mutations, genomic profiling, non‐small cell lung cancer, tumor mutational burden
This is the first report of the prevalence of driver mutations in Brazilian NSCLC patients using comprehensive genomic profiling.
Introduction
Lung cancer is the leading cause of cancer‐related mortality worldwide. 1 Cancer driver mutations have been examined extensively and are the basis for modern precision therapy, and in addition patients diagnosed with advanced lung cancer may have multiple and sometimes rare genetic alterations. 2 Non–small cell lung cancer (NSCLC), regardless of histological subtype, is one of the most genetically diverse and deranged cancers, posing challenges for developing effective prevention, diagnostic and treatment strategies. 3 , 4
Treatment selection was previously largely based on lung cancer classification in two broad categories: NSCLC or small cell lung cancer (SCLC). Nowadays, lung cancers are histologically subclassified and some undergo molecular profiling to determine the best treatment option for patients. The first genomic alterations reported to show sensitivity to specific targeted therapies in lung adenocarcinoma were EGFR mutations and ALK rearrangements. 5 , 6 , 7 , 8 More recently, other mutations have been identified as new therapeutic targets such as BRAF mutations or ROS1 and NTRK rearrangements. 9 , 10 , 11 , 12 , 13 , 14 The role of nondisruptive TP53 mutations in EGFR mutated lung adenocarcinoma patients has been previously explored and a prevalence close to 30%–50% has been described with a negative impact on prognosis, especially in those patients with exon 19 deletions. 13 , 14 , 15
The access to comprehensive genomic tests in Brazil is still limited, and the prevalence of driver mutations and co‐occurring genetic alterations among Brazilian NSCLC patients is not well known. Brazil is a country with large territorial extension and some degree of genetic background heterogeneity may exist in the population. Knowledge of the molecular profile of NSCLC in Brazil is extremely important to define better public health strategies. The objective of this study was to carry out a descriptive analysis of the somatic genetic profile of NSCLC in Brazilian patients and describe co‐occurring mutations in samples tested with either tumor or circulating tumor DNA (ctDNA) profiling.
Methods
Study design
This cross‐sectional study collected data from NSCLC samples tested in the period from 2013 to 2018. We retrospectively analyzed unidentified data from a Foundation Medicine database that comprised worksheets containing anonymous minimal clinical‐pathological characteristics and comprehensive genomic profiling (CGP) results from either tumor (FoundationOne CDx) or plasma ctDNA from plasma (FoundationACT). The database was provided by Roche Pharmaceuticals and contained all genomic data, including tumor mutational burden (TMB). No patient had duplicated tumor samples analyzed. Patients or their relatives were not directly contacted and the researchers did not have access to the medical records of patients.
Ethical considerations
A waiver for informed consent was requested since all patients had previously signed an authorization for testing, and data were collected retrospectively through pathological reports review. No information capable of identifying patients was collected. This project was approved by the Ethics Committee of PUCRS on July 2020, approval number 3462050.
Statistical analysis
Statistical analyses were performed with information from 513 NSCLC samples. We described the molecular profile by using descriptive statistics of variables. Categorical variables were presented as total frequency and percentages and compared using Pearson's Chi‐square test. All comparisons included two‐tailed tests, with level of significance set at 5%.
All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc. Cary, NC). The graphics were produced using Tableau Desktop version 2019.1.13.
Results
A total of 513 CGP results were analyzed, 457 (89.1%) from tumors and 56 (10.9%) from plasma ctDNA. Adenocarcinoma was the most common histological subtype (83.8%) followed by NSCLC not otherwise specified (NOS) (16.1%). Median age of patients at testing date was 64 years old (16–97) and 51.3% of patients were male.
Median number of mutated genes by sample was three (range 0–14) and most tumors had at least two (range 2–6) different types of mutations. The most common genomic alterations were single nucleotide variations (SNVs) (81.0%) followed by copy number variations (CNVs) (49.7%), frameshift mutations (31.4%), indels (19.3%), splice site mutations (19.1%), and rearrangements/fusions (12.5%).
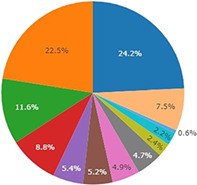
TP53 mutations were identified in 53.6% of tumor samples, KRAS mutations in 24.2%, EGFR activating mutations were detected in 22.5%, STK11 mutations in 11.6%, PIK3CA mutations in 8.8%, ALK rearrangements in 5.4%, BRAF mutations in 5.2%, ERBB2 alterations in 4.9%, MET alterations in 4.7%, RET alterations in 2.4%, ROS1 rearrangements in 2.2% and NTRK rearrangements in 0.6% (Fig 1). We also evaluated the frequency and distribution of mutations according to the available histological classification (adenocarcinoma x NSCLC) (Fig S1). We detected a higher frequency of TP53 (45,45% × 33,59%) and PI3K (8,27% × 3,99%) mutations among the nonspecified NSCLC cancer group when compared to the adenocarcinomas, and in the opposite direction, less frequent mutations in KRAS (9,09% × 17,64%) and ERRB2 (0,83% × 3,83%).
Figure 1.
Frequency of driver mutations in Brazilian non‐small‐cell lung cancer (NSCLC). Mutation description was recovered from FoundationOne CDx and FoundationACT reports and calculated as a percentage of the total number of patients studied (N = 513) ( ) KRAS, (
) KRAS, ( ) EGFR, (
) EGFR, ( ) STK11, (
) STK11, ( ) PI3K, (
) PI3K, ( ) ALK, (
) ALK, ( ) BRAF, (
) BRAF, ( ) ERBB2, (
) ERBB2, ( ) MET, (
) MET, ( ) RET, (
) RET, ( ) ROS, (
) ROS, ( ) NTRK, (
) NTRK, ( ) Others.
) Others.
A total of 55% of patients whose tumors bore an EGFR mutation were female. Among tumor samples with EGFR mutations, 37.0% had exon 19 deletion, 26.0% had exon 21 Leu858Arg substitutions (L858R), 17.6% had exon 20 insertions, 1.7% had T790M mutations, 1.7% had L861Q mutations and 1.7% had G719A mutations (Fig 2a). Two or more concurrent mutations in the EGFR gene were found in 13 tumor samples (10.9%): three samples with p.L858R and p.T790M; two samples with exon 19 deletion and p.T790M; one sample with p.V774M and p.H773L; one patient with p.G779C and p.L747_T751del (exon 19 deletion); one sample with p.L858R and p.E709K; one patient p.L858R and p.R108K, one patient with p.V774M and p.H773L, and one patient with p.L858R and p.L707F mutations. Two tumor samples had three concurrent mutations: one tumor with p.L833V, p.L858R and p.T790M and another tumor with p.C797S, exon 19 deletion, and p.T790M.
Figure 2.
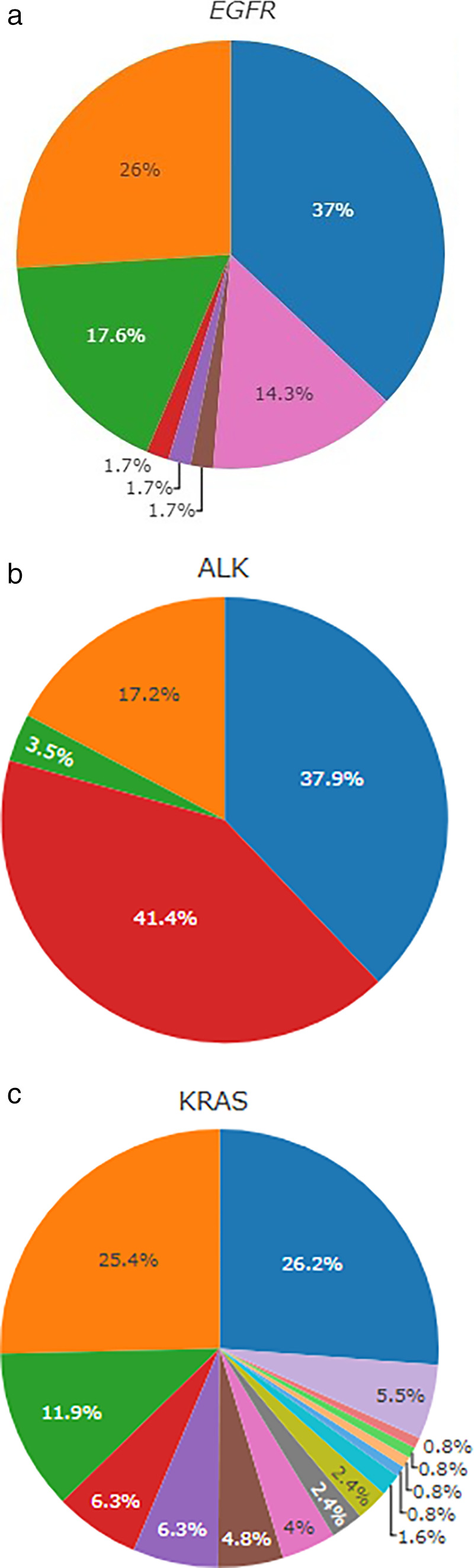
Distribution of EGFR mutations, ALK rearrangements and KRAS mutations subtypes. (a) Frequency of EGFR mutation subtypes (N = 119) ( ) Exon 19 deletion, (
) Exon 19 deletion, ( ) L858R, (
) L858R, ( ) Exon 20 insertion, (
) Exon 20 insertion, ( ) T790M, (
) T790M, ( ) L861Q, (
) L861Q, ( ) G719A, (
) G719A, ( ) Others. (b) Frequency of ALK fusion subtypes (N = 29) (
) Others. (b) Frequency of ALK fusion subtypes (N = 29) ( ) Variant 1, (
) Variant 1, ( ) Variant 3, (
) Variant 3, ( ) Variant 2, (
) Variant 2, ( ) Others. (c) Frequency of KRAS mutation subtypes (N = 126) (
) Others. (c) Frequency of KRAS mutation subtypes (N = 126) ( ) G12V, (
) G12V, ( ) G12C, (
) G12C, ( ) G12D, (
) G12D, ( ) G12A, (
) G12A, ( ) G12S, (
) G12S, ( ) G13D, (
) G13D, ( ) G12R, (
) G12R, ( ) G13C, (
) G13C, ( ) Q61H, (
) Q61H, ( ) Q61L, (
) Q61L, ( ) A146V, (
) A146V, ( ) G12F, (
) G12F, ( ) G13R, (
) G13R, ( ) Q16K, (
) Q16K, ( ) Amplification.
) Amplification.
Among tumor samples with ALK rearrangements, variant 1 was found in 37.9%, followed by variant 3 in 17.2% (Fig 2b).
In relation to KRAS mutation, most alterations involved codons 12 and 13 (94.4%). p.G12V was the most frequent variant (26.2%), followed by p.G12C, observed in 25.4% of samples with KRAS mutation (Fig 2c). The majority of patients with KRAS mutation were male (57.1%) and median age was 67 years old.
zThe most common BRAF mutation was p.V600E detected in 50% of BRAF mutated samples. The majority of patients with BRAF mutations were female (53.5%) and median age in this group was 67 years old. ERBB2 alterations were more common among male patients (57.7%), with a median age of 66 years old. The most common genomic alteration affecting the ERBB2 gene was p.A775_G776INSYVMA (exon 20 insertion), corresponding to 26.9% of cases.
TP53 p.R337H mutation was observed in 22 samples (4.3%). They corresponded to 8.0% of all TP53 gene alterations. Two patients harbored other variants in the R337 position (1 p.R337C and 1 p.R337L). 63.6% of patients bearing TP53 p.R337H were 50 years old or younger.
Co‐occurring mutations
In patients with EGFR mutations, the most commonly co‐occurring mutations identified were in TP53 (51.6%), PIK3CA (5.7%) and CDK4 (3.8%) (Fig 3a). Among patients with TP53 and EGFR co‐occurring mutations, 12 patients (2.3%) bore TP53 p.R337H. TP53 p.R337H was also found in four (0.8%) patients whose tumors bore an ERBB2 somatic genomic alteration. In patients with ALK rearrangements, co‐occurring mutations were detected in 55.2% of tumor samples, TP53 being the most prevalent, observed in 31%, followed by PIK3A detected in 6.9% (Fig 3b). Among KRAS‐mutant samples, TP53 co‐occurring mutations were detected in 38.2% and STK11 in 22.2% (Fig 3c).
Figure 3.
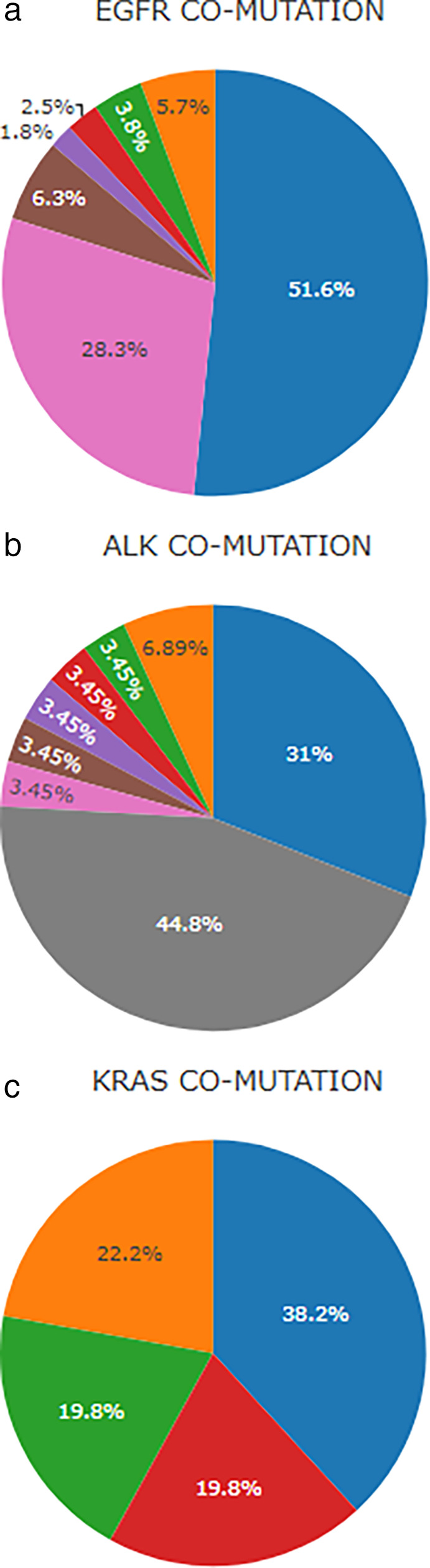
Distribution of co‐occurring mutations in NSCLC bearing EGFR mutations, ALK rearrangements and KRAS mutations. (a) Co‐occurring genomic alterations in patients with EGFR mutation (N = 119) ( ) EGFR + TP53, (
) EGFR + TP53, ( ) EGFR + P13KA, (
) EGFR + P13KA, ( ) EGFR + CDK4, (
) EGFR + CDK4, ( ) EGFR + KRAS, (
) EGFR + KRAS, ( ) EGFR + MET, (
) EGFR + MET, ( ) Others, (
) Others, ( ) No co‐mutation identified. (b) Co‐occurring genomic alterations in patients with ALK mutation (N = 29) (
) No co‐mutation identified. (b) Co‐occurring genomic alterations in patients with ALK mutation (N = 29) ( ) ALK + TP53, (
) ALK + TP53, ( ) ALK + P13KA, (
) ALK + P13KA, ( ) ALK + CDK4, (
) ALK + CDK4, ( ) ALK + ERBB, (
) ALK + ERBB, ( ) ALK + KRAS, (
) ALK + KRAS, ( ) ALK + NTRK, (
) ALK + NTRK, ( ) ALK + STK11, (
) ALK + STK11, ( ) No comutation identified. (c) Co‐occurring genomic alterations in patients with KRAS mutation (N = 126) (
) No comutation identified. (c) Co‐occurring genomic alterations in patients with KRAS mutation (N = 126) ( ) KRAS + TP53, (
) KRAS + TP53, ( ) KRAS + STK11, (
) KRAS + STK11, ( ) Others, (
) Others, ( ) No comutation identified.
) No comutation identified.
A total of 12 samples with TP53 p.R337H had concurrent EGFR (54.5%) and 16 samples had concurrent ERBB2 (72.7%) mutations (Table S1). The association between TP53 p.R337H and EGFR mutations was statistically significant (P = 0.0008) as well as between TP53 p.R377H and EGFR plus ERBB2 mutations combined (P < 0.0001) (Table S1). A total of 63.6% of these patients were 50 years old or younger (P < 0.0001) (Table S1). EGFR or ERBB2 mutations altogether were observed in 28% of samples that bore a non‐R337H TP53 variant. The co‐occurrence of KRAS p.G12C and TP53 p.Y220C was observed in one case. We did not observe other driver mutations frequently associated with TP53 p.R337H (AKT1 p.E17K in one case, NF1 p.N1683FS*1 in one case, PTEN loss and RB1 mutation 1 one case, and KEL, MUTYH, VHL and RB1 mutations in one case).
TMB
TMB analysis was available for 80.5% of samples tested and measured in mutation per megabase. A low TMB (<5 mutations/Mb) was found in 42.7%, an intermediate TMB (5–9 mutations/Mb in 32.4% and only 5.5% of samples had high TMB (≥10 mutations/Mb) The distribution of the tumor mutational burden according to the different driver genes studied is described in Fig 4.
Figure 4.
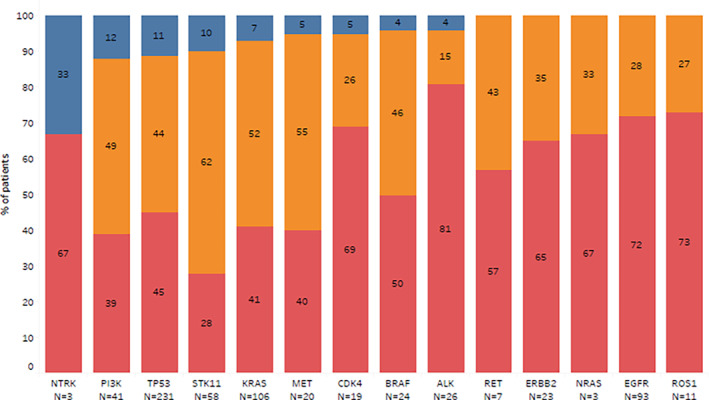
Distribution of TMB subgroups according to the type of driver mutation (N = 413) ( ) high, (
) high, ( ) intermediate, (
) intermediate, ( ) low.
) low.
Discussion
NSCLC is the leading cause of cancer death in Brazil and has a major impact on the public health system. In Brazil, many hurdles impair patients' access to expanded molecular testing, and consequently to the best available treatment. A better characterization of the genomic profile of NSCLC in Brazil is paramount to guide and improve the design of treatment strategies for NSCLC in the country.
This retrospective analysis of genomic data obtained by CGP from samples of Brazilian NSCLC patients showed that common abnormalities had, in general, similar frequency to those previously described by other groups using other strategies such as PCR based tests (EGFR) or immunohistochemistry and FISH (ALK). 16 For example, a study conducted in the South region of Brazil analyzed 619 lung tumor samples and identified EGFR mutations in 120 (19.2%), and ALK expression in 4.0%. 17 Another study that analyzed 262 lung adenocarcinomas samples detected EGFR mutations in 23% and ALK rearrangements in 7%. 18 Nevertheless, CPG‐based strategies may provide more accurate output since they assess all types of genetic mutations, copy number variations, and, in the case of gene fusions and rearrangements, the partner gene which might have predictive implications in the future.
Another study evaluated 173 NSCLC samples from patients who lived in the Northeast region of Brazil. ALK expression was detected in 10.4% of the samples, and 22.0% of the tumors harbored EGFR mutations. The most common EGFR mutation was an exon 21 L858R point mutation (in 45.5%), followed by an exon 19 deletion (in 36.3%). In this study, the authors did not describe TMB, mutations in other driver genes or co‐occurring mutations. These results are somehow different from what we found in our study, with a higher than expected prevalence of ALK alterations. 19
The knowledge that co‐occurring mutations in addition to the classic driver mutations can largely modify the biology of the tumors and the response to treatment is rapidly consolidating. Co‐occurring genomic alterations contribute to the heterogeneity of driver oncogene‐defined NSCLC subgroups and can result in biologically important interactions. Selective pressure imposed by previous anticancer therapy can also substantially influence patterns of co‐mutations. 20 No other published cohort from Brazil has described the frequency of genomic alterations in less common mutated genes like RET and HER2 or the frequency of co‐occurring genomic alterations.
Hu et al. evaluated the presence of mutations in KRAS, NRAS, PIK3CA, BRAF, and HER2 as well as ALK, ROS1, and RET gene fusions in 320 patients who harbored EGFR activating mutations and received treatment with EGFR‐tyrosine kinase inhibitor (TKIs). A total of 21 (6.6%) of the EGFR mutant samples had additional gene alterations, mutations in PIK3CA being the most common, followed by EML4‐ALK rearrangements, ERBB2 mutations, RET rearrangements, ROS1 rearrangements and KRAS mutations. Those with isolated EGFR mutations had a significantly longer progression‐free survival (PFS) compared to those with concurrent gene alterations; however, this condition did not have a significant impact in OS. 21
The most frequently comutated gene we observed in association with mutations in EGFR, ALK and KRAS (the most frequently mutated NSCLC driver genes) was TP53, followed by PIK3CA in EGFR‐ and ALK‐mutant samples, and STK11 in KRAS‐mutant genes. TP53 mutations have been reported to be associated with shorter disease progression intervals in patients with EGFR or ALK mutated NSCLC treated with TKI. 14 , 21 Similarly, the comutation of STK11 in KRAS mutated NSCLC has been reported to be associated with resistance to immune checkpoint inhibitors. 22
The current study was based on an anonymized dataset from laboratory reports. Unfortunately, only limited clinical data were available, and we were unable to test the association between the genomic alterations we identified in this population and other variables such as smoking history, racial group, geographic location, stage or previous treatment.
To our knowledge, this is the first report of TMB from a Brazilian NSCLC cohort. We found that most tumors harbored low or intermediate TMB. High TMB was especially frequent among TP53, STK11, PIK3CA and NTRK mutant samples, while ALK and ROS1 had the highest proportion of samples with low TMB. Singal et al. analyzed genomic and clinical data from 4064 NSCLC patients and found the same pattern of association between TMB and driver mutations as described herein. 23
The great population heterogeneity and the diverse genetic background in the Brazilian population can lead to new findings. This is exemplified by the finding of a higher than expected frequency of TP53 p.R337H in the present cohort. This variant represents a prevalent pathogenic germline TP53 mutation frequently found in the South and Southeast of Brazil, 24 which seems to be associated with a higher frequency of somatic EGFR and ERBB2 activating mutations in NSCLC in Brazil. Couto et al. identified germline TP53 R337H mutations in 8.9% of 45 unselected Brazilian NSCLC patients tested for TP53 germline mutations. 25 Barbosa et al. also described a high frequency of EGFR somatic mutations (89%) among adenocarcinomas diagnosed in Brazilian carriers of germline TP53 p.R337H mutation, while the frequency of EGFR mutations among unselected lung adenocarcinomas is around 25% in the country. The same authors described an association between EGFR somatic mutations and TP53 p.R337H among unselected lung adenocarcinoma patients younger than 50 years old. 26 Following this publication, Mezquita et al. evaluated 22 NSCLC patients with Li‐Fraumeni syndrome harboring diverse germline TP53 variants and identified a somatic mutation in a driver gene in 90% (18 EGFR mutations and 1 ROS1 fusion) of 21 samples analyzed. 27 Although we cannot ascertain that the aforementioned mutations identified in these patients are germline for sure based on the FoundationOne tests results, which is designed to assess somatic mutations, the authors inferred that they are probably germline in nature based on the fact that this variant (TP53 p.R337H) is very rarely identified as a somatic mutation in NSCLC 28 and on data showing a high prevalence of this mutation (germline) in Brazil, 24 allied with the previously reported association with EGFR mutated NSCLC in Brazilian patients. 25 , 26 Besides, these mutations fulfill the criteria recently proposed to investigate germline origin from genomic profiling data. 29 , 30 The results of the present study reinforce the need to investigate TP53 germline mutations in EGFR mutant NSCLC patients and to discuss genetic counseling, mainly in individuals younger than 50 years old at diagnosis.
In conclusion, detailed analysis of NSCLC samples at the molecular level may provide relevant insights to improve the understanding of this disease and is paramount to establish personalized targeted therapy. The use of CGP‐based testing may grant a deeper understanding of the prevalence of each specific driver mutation in the country and the identification of a larger repertoire of actionable mutations. At the same time, the detection of concurrent mutations may improve the prediction of response to targeted therapies.
This retrospective analysis of genomic data from Brazilian NSCLC patients obtained by CGP showed that common abnormalities such as EGFR mutations and ALK rearrangements had similar frequency to that described previously by other groups; nevertheless, here we describe for the first time the frequency of genomic alterations in other less common driver genes, comutations and the distribution of TMB in NSCLC tumor samples in Brazil. Additionally, we believe that our results strengthen the idea that there is an association between TP53 p.R337H (probably germline) and somatic mutations in genes of the HER family (EGFR and ERBB2).
Disclosure
All authors declare that there are no conflicts of interest.
Supporting information
Table S1. Association of TP53 R337H mutation with EGFR mutation and age.
Table S2. Association between the presence of the TP53 p.R337H mutation and mutations in EGFR, ERBB2 and age. The TP53 p.R337H is a putative germline mutation highly prevalent in the South and Southeast regions of Brazil. Associations between variables were tested with the Pearson's Chi‐squared test.
Acknowledgments
We thank Foundation Medicine, Inc. and Hoffmann La Roche for allowing access to the genomic data, for the scientific review, and financial support with medical writing and statistical analysis.
[Correction added on 19 January 2021, after first online publication: the last author's name has been corrected.
References
- 1. Siegel R, DeSantis C, Virgo K et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62 (4): 220–41 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2. Larsen JE, Minna JD. Molecular biology of lung cancer: Clinical implications. Clin Chest Med 2011; 32 (4): 703–40 10.1016/j.ccm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soda M, Choi Y, Enomoto M et al. Identification of the transforming EML4–ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448 (7153): 561–6 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4. Rikova K, Guo A, Zeng Q et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 13 (16): 1190–203 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 5. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350 (21): 2129–39 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304 (5676): 1497–500 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7. Pao W, Miller V, Zakowski M et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci 2004; 101 (36): 13306–11 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim SM, Kim HR, Lee J‐S et al. Open‐label, multicenter, phase II study of ceritinib in patients with non–small‐cell lung cancer harboring ROS1 rearrangement. J Clin Oncol 2017; 35 (23): 2613–8 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 9. Shaw AT, Ou Sai‐Hong I, Bang Y‐J et al. Crizotinib in ROS1‐rearranged non–small‐cell lung cancer. N Engl J Med 2014; 371 (21): 1963–71 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drilon A, Siena S, Ou Sai‐Hong I et al. Safety and antitumor activity of the multitargeted pan‐TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA‐372‐001 and STARTRK‐1). Cancer Discov 2017; 7 (4): 400–9 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planchard D, Besse B, Groen HJM et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E‐mutant metastatic non‐small cell lung cancer: An open‐label, multicentre phase 2 trial. Lancet Oncol 2016; 17 (7): 984–93 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drilon A, Laetsch TW, Kummar S et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med 2018; 378 (8): 731–9 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina‐Vila MA, Bertran‐Alamillo J, Gasco A et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non–small cell lung cancer. Clin Cancer Res 2014; 20 (17): 4647–59 10.1158/1078-0432.CCR-13-2391. [DOI] [PubMed] [Google Scholar]
- 14. Canale M, Petracci E, Delmonte A et al. Impact of TP53 mutations on outcome in EGFR‐mutated patients treated with first‐line tyrosine kinase inhibitors. Clin Cancer Res 2017; 23 (9): 2195–2 10.1158/1078-0432.CCR-16-0966. [DOI] [PubMed] [Google Scholar]
- 15. VanderLaan PA, Rangachari D, Mockus SM et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: Correlation with clinical outcomes. Lung Cancer 2017; 106: 17–21 10.1016/j.lungcan.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Araujo LH, Baldotto C, de Castro G et al. Lung cancer in Brazil. J Bras Pneumol 2018; 44 (1): 55–64 10.1590/s1806-37562017000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreis TF, Correa BS, Vianna FS et al. Analysis of predictive biomarkers in patients with lung adenocarcinoma from southern Brazil reveals a distinct profile from other regions of the country. J Global Oncol 2019; 5: 1–9 10.1200/JGO.19.00174,5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferreira CG, Zalis M, Reis M, Schluckebier L, Montella T. PUB070 rare actionable mutations in a lung adenocarcinoma cohort in Brazil. J Thorac Oncol 2017; 12 (11): S2388–9 10.1016/j.jtho.2017.09.1933. [DOI] [Google Scholar]
- 19. da Silva Mendes de Oliveira AC, Alves da Silva AV, Alves M et al. Molecular profile of non‐small cell lung cancer in northeastern Brazil. J Bras Pneumol 2019; 45 (3): e20180181 10.1590/1806-3713/e20180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skoulidis F, Heymach JV. Co‐occurring genomic alterations in non‐small‐cell lung cancer biology and therapy. Nat Rev Cancer 2019; 19 (9): 495–509 10.1038/s41568-019-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu W, Liu Y, Chen J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR‐TKIs in Chinese patients with non‐small cell lung cancer. Oncotarget 2017; 8 (15): 25054–46 10.18632/oncotarget.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skoulidis F, Goldberg ME, Greenawalt DM et al. STK11/LKB1 mutations and PD‐1 inhibitor resistance in KRAS‐mutant lung adenocarcinoma. Cancer Discov 2018; 8 (7): 822–35 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singal G, Miller PG, Agarwala V et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non–small cell lung cancer using a clinicogenomic database. JAMA 2019; 321 (14): 1391–9. 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmero EI, Schüler‐Faccini L, Caleffi M et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in southern Brazil. Cancer Lett 2008; 261 (1): 21–5. 10.1016/j.canlet.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 25. Couto PP, Bastos‐Rodrigues L, Schayek H et al. Spectrum of germline mutations in smokers and non‐smokers in Brazilian non‐small‐cell lung cancer (NSCLC) patients. Carcinogenesis 2017; 38 (11): 1112–8. 10.1093/carcin/bgx089. [DOI] [PubMed] [Google Scholar]
- 26. Barbosa MVR, Cordeiro de Lima VC, Formiga MN, Andrade de Paula CA, Torrezan GT, Carraro DM. High prevalence of EGFR mutations in lung adenocarcinomas from Brazilian patients harboring the TP53 p. R337H variant. Clin Lung Cancer 2019; 21: e37–44 10.1016/j.cllc.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 27. Mezquita L, Jové M, Nadal E et al. Brief report: High prevalence of somatic oncogenic driver alterations in non‐small cell lung cancer patients with Li‐Fraumeni syndrome. J Thorac Oncol 2020), 10.1016/j.jtho.2020.03.005; 15: 1232–9. [DOI] [PubMed] [Google Scholar]
- 28. [Cited 20 Jun 2020.] Available from URL: https://www.cbioportal.org/results/mutations.
- 29. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract 2019; 15 (9): 465–73. 10.1200/JOP.19.00201. [DOI] [PubMed] [Google Scholar]
- 30. Awada A, El Saghir NS. Personalized medicine, genomic profiling and germline mutations: Toward more precise decisions. J Oncol Pract 2019; 15 (9): e755–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association of TP53 R337H mutation with EGFR mutation and age.
Table S2. Association between the presence of the TP53 p.R337H mutation and mutations in EGFR, ERBB2 and age. The TP53 p.R337H is a putative germline mutation highly prevalent in the South and Southeast regions of Brazil. Associations between variables were tested with the Pearson's Chi‐squared test.


