Abstract
Rationale: Mandibular advancement device (MAD) treatment efficacy varies among patients with obstructive sleep apnea.
Objectives:
The current study aims to explain underlying individual differences in efficacy using obstructive sleep apnea endotypic traits calculated from baseline clinical polysomnography: collapsibility (airflow at normal ventilatory drive), loop gain (drive response to reduced airflow), arousal threshold (drive preceding arousal), compensation (increase in airflow as drive increases), and the ventilatory response to arousal (increase in drive explained by arousal). On the basis of previous research, we hypothesized that responders to MAD treatment have a lower loop gain and milder collapsibility.
Methods: Thirty-six patients (median apnea–hypopnea index [AHI], 23.5 [interquartile range (IQR), 19.7–29.8] events/h) underwent baseline and 3-month follow-up full polysomnography, with MAD fixed at 75% of maximal protrusion. Traits were estimated using baseline polysomnography according to Sands and colleagues. Response was defined as an AHI reduction ≥ 50%.
Results: MAD treatment significantly reduced AHI (49.7%baseline [23.9–63.6], median [IQR]). Responders exhibited lower loop gain (mean [95% confidence interval], 0.53 [0.48–0.58] vs. 0.65 [0.57–0.73]; P = 0.020) at baseline than nonresponders, a difference that persisted after adjustment for baseline AHI and body mass index. Elevated loop gain remained associated with nonresponse after adjustment for collapsibility (odds ratio, 3.03 [1.16–7.88] per 1–standard deviation (SD) increase in loop gain [SD, 0.15]; P = 0.023).
Conclusions: MAD nonresponders exhibit greater ventilatory instability, expressed as higher loop gain. Assessment of the baseline degree of ventilatory instability using this approach may improve upfront MAD treatment patient selection.
Clinical trial registered with www.clinicaltrials.gov (NCT 01532050)
Keywords: obstructive sleep apnea, OSA, MAD, personalized medicine, pathophysiology
Obstructive sleep apnea (OSA) is defined as repetitive upper-airway collapse (apnea) or narrowing (hypopnea) during sleep for a period of at least 10 seconds. OSA affects up to 9% of middle-aged women and 17% of middle-aged men (1) and is associated with several comorbidities, including, but not limited to, cardiovascular sequelae (2). OSA severity is quantified using the apnea–hypopnea index (AHI), capturing the frequency of apneas and hypopneas per hour of sleep (3).
Mandibular advancement device (MAD) therapy is the first-line non–continuous positive airway pressure (CPAP) treatment for patients with moderate-to-severe OSA (AHI ≥ 15 events/h). MAD therapy acts by protruding the lower jaw and increasing pharyngeal patency (4, 5). Typically, treatment involves custom-made, titratable MADs that allow individual treatment optimization (6–9). Although CPAP shows high efficacy in unselected patients, adherence is suboptimal (10, 11). In contrast, MAD adherence is high, but response to MAD treatment is highly patient dependent (12). A recent meta-analysis showed complete resolution of OSA, obtaining an AHI < 5 events/h, under MAD therapy in approximately one-third of patients. Another third showed a decrease in AHI of 50% or more, whereas the last one-third of patients showed negligible improvement in OSA severity (13). Therefore, upfront patient selection could be beneficial.
OSA pathophysiology can be subdivided into different characteristics: upper-airway collapsibility, loop gain, upper-airway muscle responsiveness, arousal threshold, and the ventilatory response to arousal (VRA). However, the gold-standard measurement techniques to assess these traits are rigorous and time consuming, involving multiple CPAP drops during sleep overnight with a sealed mask and pneumotach and/or via esophageal catheters to assess ventilatory drive (14, 15). Therefore, pathophysiological OSA traits are not routinely measured in clinical practice. However, recent research showed that pathophysiological OSA traits affect MAD treatment efficacy (16, 17). Specifically, a recent study by Edwards and colleagues (16) showed that patients with lower loop gain, measured using a gold-standard technique, were more likely to respond to MAD treatment; milder collapsibility was also a predictor (16, 18). Furthermore, recent research showed it is possible to derive the pathophysiological OSA traits using baseline polysomnographic signals, avoiding additional invasive measurements (19–25). These techniques already showed their potential in calculating and estimating the OSA traits to differentiate between responders and nonresponders to upper-airway surgery (26). Recently, Bamagoos and colleagues (17) showed that a combination of the different calculated pathophysiological traits explains AHI reduction after MAD treatment.
In the current study, we aimed to use the pathophysiological OSA traits, calculated from the baseline polysomnographic signals, to differentiate between responders and nonresponders to MAD treatment. We hypothesized that MAD responders exhibit lower loop gain (primary) and less severe collapsibility (secondary), as per Edwards and colleagues (16). We also aimed to expand the results to other nonanatomical OSA traits: arousal threshold, VRA, and compensation, as per Bamagoos and colleagues (17). The results described in this article were presented orally at the 2019 American Thoracic Society International Conference on May 20, 2019, and at the 2019 American Academy of Dental Sleep Medicine Annual Meeting in San Antonio on June 7, 2019, and have been published in abstract form (27, 28).
Methods
Subjects
The current study is a secondary analysis of the parent clinical trial NCT 01532050 (clinicaltrials.gov) that was approved by the local ethical committee at the University of Antwerp and Antwerp University Hospital (11/11/103, Belgian registration number: B300201212961). Written informed consent was obtained from all patients.
Data of 36 patients with moderate-to-severe OSA (all available) were included in this secondary analysis (see Figure E1 in the online supplement). As this is a secondary analysis, only data of patients with a complete data set were selected. In the parent trial, 47 patients with an AHI ≥ 15 events/h consented, but 11 dropped out. Five patients quit the study because of time constraints, two failed to return despite several reminders, one moved abroad, two stopped because of financial reasons, one patient preferred to stop treatment because of an absence of symptomatic relief, and one patient quit the study because of an improvement of OSA after weight reduction. In total, 36 (77%) of the patients with an AHI ≥ 15 events/h completed the parent study.
As described in detail by Verbruggen and colleagues (29), all patients had undergone a baseline clinical type 1 polysomnography, confirming moderate-to-severe OSA (AHI ≥ 15 events/h), at the Antwerp University Hospital. Hypopneas were scored according to the American Academy of Sleep Medicine 1999 guidelines (30). For the present analysis, arousal start and end times were manually rescored using electroencephalography because precise arousal timing was not required in the parent clinical trial. Custom-made MAD treatment (RespiDent Butterfly MAD; Orthodontic Clinics NV) was administered (31). For standardization, the MAD was fixed at 75% of the individual patient’s maximal protrusion without further titration. After 3 months of MAD treatment, a follow-up visit was scheduled, including a type 1 polysomnography with MAD treatment. Response was defined as a change in AHI (ΔAHI) ≥ 50% from baseline.
Pathophysiological Trait Calculation
The pathophysiological traits were calculated from the baseline clinical polysomnography using the previously validated method as described by Sands and colleagues (23–25, 32). The calculated traits included collapsibility (airflow at normal ventilatory drive [Vpassive]), loop gain (drive response to reduced airflow), arousal threshold (drive preceding arousal), compensation (increase in airflow as drive increases), and the VRA (increase in drive explained by arousal).
Briefly, the traits were calculated automatically using overlapping windows of manually scored polysomnographic data during non–rapid eye movement sleep. The nasal pressure signal was linearized (power = 0.67) (23) and used to generate a breath-to-breath ventilation signal (normalized to 100% of local average). A physiologically constrained chemoreflex control model (parameters: loop gain [gain, response time, delay]; response to arousal) was fit to the ventilation data (input: ventilation) so that the output (estimated ventilatory drive) best fit the ventilation signal when the airway was open (between scored respiratory events). Loop gain, defined as the change in ventilatory drive in response to a drop in airflow (25), was calculated directly from the best-fit model (taken at 1 cycle/min; median value for each patient used); an elevated loop gain indicates a hypersensitive ventilatory-control system more prone to cyclic ventilatory instability. The VRA was taken from the best-fit model (median value used) and represents the increase in ventilatory drive that is attributed to arousal from sleep (25, 26) as opposed to the increase in chemical drive (attributed to loop gain) (23). The arousal threshold was taken as the ventilatory drive on breaths preceding arousals (24) (median value used); a lower threshold indicates that sleep is more easily disturbed. Collapsibility was taken as the median value of airflow (lowered because of anatomical deficit) at normal drive and referred to as the Vpassive; a lower Vpassive value indicates a greater degree of pharyngeal collapsibility. Compensation, a measure of upper-airway muscle responsiveness, was taken as the increase in airflow as ventilatory drive rises from normal degrees (Vpassive) to more active conditions (at the arousal threshold). A single value (median) of each trait for each patient was used for statistical analyses.
Statistical Analysis
Statistical analysis was performed using the software packages MATLAB (MATLAB and Statistics Toolbox Release 2018a; MathWorks) and R (R: A language and environment for statistical computing, 2016; R Core Team, R Foundation for Statistical Computing). Descriptive statistics were reported as the mean and 95% confidence interval (CI) or median and interquartile range (IQR). Normality was tested using the Shapiro-Wilk test. To differentiate between responders and nonresponders, normally distributed continuous variables were compared using unpaired t tests, and nonnormally distributed variables were compared using the Wilcoxon signed-rank or Mann-Whitney U test. Loop gain was considered the primary determinant. Associations were also adjusted for potential confounders (baseline AHI and body mass index [BMI]) using multivariable logistic regression (nonresponder = 1; responder = 0). For example, the percentage ΔAHI may depend somewhat on the baseline AHI. The BMI is also a potential confounder between loop gain and MAD response because those with a higher BMI are expected to have not only higher loop gain (i.e., via lowered functional residual capacity) but also a poorer MAD response. Likewise, collapsibility is a potential confounder for loop gain and MAD response (i.e., via possible acquired increases in loop gain over time with more severe recurrent obstruction); thus, further adjustment for collapsibility was also performed to assess the extent to which loop gain is associated with MAD response, independent of collapsibility. The 36 patients were expected to have 80% power to detect a 1–standard deviation (SD) difference (α level = 0.05) in loop gain between responders and nonresponders (logistic regression); inclusion of uncorrelated confounders was expected to lower statistical power by under 1% per variable (simulations: 10,000 iterations). Because of the limited sample size of this study, multivariable analysis with multiple traits was considered exploratory. However, we also expected that the bivariate relationships may be strengthened by concurrent inclusion of loop gain and collapsibility, as seen previously (32). All reported P values are two-sided. Statistical significance was considered to be present at P < 0.05.
Results
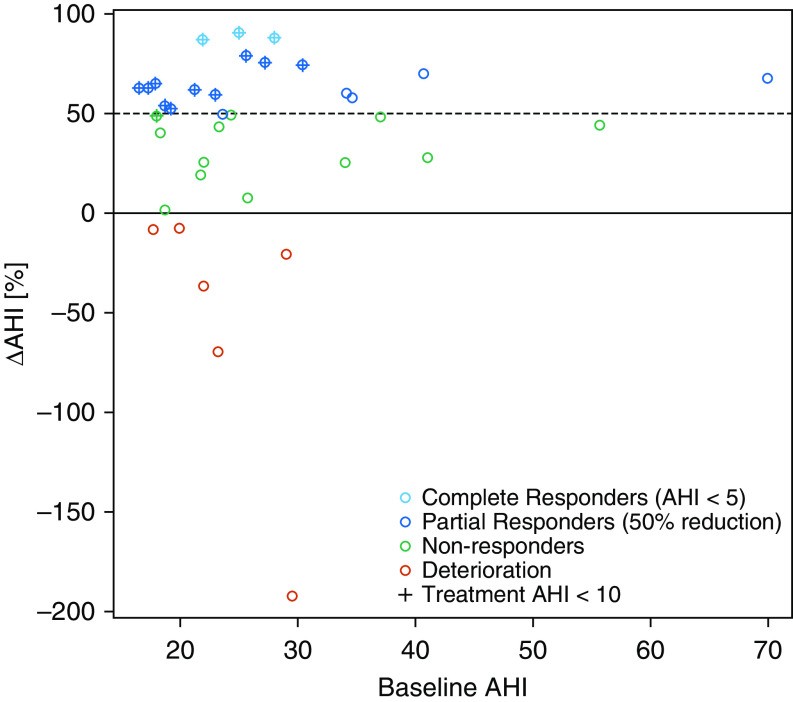
Data from 36 patients were assessed, and all 36 patients were included in the final analysis (median AHI, 23.5 [IQR, 19.7–29.8] events/h; median BMI, 28.8 kg/m2 [95% CI, 27.8–29.7]; 69% male; median age, 48.5 yr [95% CI, 45.8–51.1]). MAD treatment significantly improved AHI, supine AHI, nonsupine AHI, minimum oxygen saturation, oxygen desaturation index, and Epworth sleepiness scale after 3 months of MAD treatment (Table 1). In total, 18 out of the 36 patients (50%) were classified as responders (ΔAHI ≥ 50%) in a nontitrated 75% protrusion, 13 (36%) patients reached an AHI < 10 events/h, and 3 (8%) patients were complete responders (AHI < 5 events/h). Five responders (5 of 18, 28%) had a residual AHI > 10 events/h despite a 50% reduction (baseline AHI = 34.1, 34.6, 23.6, 69.9, and 40.7 events/h; see Figure 1). No significant differences in baseline characteristics, including baseline AHI and BMI, were present between responders (n = 18; 50%) and nonresponders (n = 18; 50%) to MAD treatment, and MAD responders were slightly younger than nonresponders (P = 0.04; Table 2). No patients reported temporomandibular joint problems after treatment start-up.
Table 1.
Baseline and 3-month follow-up patient characteristics
| Outcome Parameter | Baseline PSG (n = 36) | Follow-Up PSG (3 M) (n = 36) |
|---|---|---|
| Apnea–hypopnea index, events/h* | 23.5 (19.7–29.8) | 12.8 (7.9–21.7) |
| Supine apnea–hypopnea index, events/h* | 40.9 (29.0–62.4) | 22.1 (10.4–38.8) |
| Nonsupine apnea–hypopnea index, events/h* | 16.5 (11.03–21.0) | 7.0 (4.4–15.6) |
| Mean SaO2* | 94.6 (93.9–95.7) | 95.2 (94.1–95.7) |
| Minimal SaO2† | 85.3 (83.8–86.8) | 87.5 (86.3–88.7) |
| Oxygen desaturation index, events/h* | 10.6 (6.3–16.6) | 3.7 (1.4–8.0) |
| Body mass index, kg/m2† | 28.8 (27.9–29.7) | 29.0 (28.0–30.0) |
| Epworth sleepiness scale* | 7 (5–14) | 6 (3–9) |
| Visual analog scale* | 7 (6–9) | 7 (5–9) |
| Age, yr† | 48.5 (45.8–51.1) | — |
| Sex, M/F, %M | 31/5, 86% M | — |
Definition of abbreviations: F = female; M = male; PSG = polysomnography; SaO2 = arterial oxygen saturation.
One patient did not have follow-up minimal O2 saturation (n = 35).
Data are presented as medians (interquartile ranges).
Data are presented as means (95% confidence intervals).
Figure 1.
Dot plot showing the ΔAHI as a function of the baseline AHI. Deteriorating patients (treatment AHI > baseline AHI) are shown in red, nonresponders (ΔAHI < 50%) are shown in green, partial responders (ΔAHI ≥ 50% but treatment AHI > 5) are shown in dark blue, and complete responders (treatment AHI < 5) are shown in light blue. Patients with a treatment AHI < 10 events/h are depicted with crosses. The dotted line shows the ΔAHI 50% threshold. AHI = apnea–hypopnea index.
Table 2.
Baseline characteristics for responders and nonresponders
| Outcome Parameter | Responders (n = 18) | Nonresponders (n = 18) | P Value |
|---|---|---|---|
| Apnea–hypopnea index, events/h* | 24.3 (19.7–29.8) | 23.3 (20.4–29.5) | 0.9 |
| Supine apnea–hypopnea index, events/h† | 42.4 (28.3–56.5) | 52.2 (41.1–63.3) | 0.3 |
| Nonsupine apnea–hypopnea index, events/h* | 16.4 (12.1–21.0) | 16.5 (9.7–20.7) | 0.5 |
| Mean SaO2* | 94.4 (93.3–95.4) | 94.9 (94.1–95.8) | 0.3 |
| Minimal SaO2† | 85.1 (82.5–87.7) | 85.4 (84.0–86.8) | 0.8 |
| Oxygen desaturation index, events/h* | 8.4 (3.6–14.8) | 10.9 (8.5–18.2) | 0.16 |
| Body Mass index, kg/m2† | 28.8 (27.4–30.2) | 28.7 (27.4–30.0) | 0.9 |
| Age, yr† | 45.7 (42.7–48.8) | 51.2 (47.2–55.3) | 0.04 |
| Sex, M/F‡ | 14/4 | 17/1 | 0.34 |
| Epworth sleepiness scale* | 9 (6–16) | 7 (3–13) | 0.3 |
| Visual analog scale* | 7 (6–9) | 8 (6–9) | 0.9 |
Definition of abbreviations: F = female; M = male; SaO2 = arterial oxygen saturation.
Significant values are shown in bold.
Data are presented as medians (interquartile ranges), and the Mann-Whitney U test was used.
Data are presented as means (95% confidence intervals), and an unpaired t test was used.
Data are presented as ratios, and the Fisher exact test was used.
Pathophysiological traits were calculated in all 36 patients, yielding the following average values for the entire group: loop gain, 0.59 (95% CI, 0.54 to 0.64); Vpassive, 92.7% (95% CI, 91.4% to 94.0%) eupnea; arousal threshold, 122.6% (95% CI, 117.1% to 128.7%) eupnea; compensation, 0.83% (95% CI, −7.1% to 8.8%) eupnea; and VRA, 43.9% (95% CI, 36.7% to 51.2%) eupnea.
Bivariate Associations with MAD Efficacy
Compared with responders, nonresponders to MAD treatment (ΔAHI < 50%) showed a significantly higher loop gain (odds ratio, 2.16 [95% CI, 1.17–3.97] per 1-SD increase in loop gain [SD, 0.15]; P = 0.020) (Table 3 and Figure 2).
Table 3.
Baseline pathophysiological traits for responders and nonresponders
| Endotypic Trait | Responders [Mean (95% CI)] | Nonresponders [Mean (95% CI)] | Difference [Mean (95% CI), P Value] | Adjusted Difference [Mean (95% CI), P Value] |
|---|---|---|---|---|
| Loop gain | 0.53 (0.47 to 0.59) | 0.65 (0.58 to 0.71) | −0.12 (−0.21 to −0.02), 0.019 | −0.12 (−0.21 to −0.03), 0.013 |
| Collapsibility, Vpassive | 94 (93 to 95) | 91 (90 to 93) | 2.5 (0.0 to 4.5), 0.055 | 2.6 (0.7 to 4.3), 0.014 |
| Arousal threshold | 118 (112 to 125) | 128 (121 to 137) | −10 (−19 to 1), 0.074 | −11 (−18 to −1), 0.045 |
| Compensation | 0.74 (−9.9 to 11.4) | 0.90 (−9.7 to 11.5) | −0.5 (−16.1 to 15.0), >0.9 | 0.2 (−14.3 to 14.6), >0.9 |
| Ventilatory response to arousal | 38 (28 to 48) | 50 (40 to 60) | −11.6 (−25.3 to 2.0), 0.104 | −11.9 (−25.4 to 1.6), 0.094 |
Definition of abbreviations: CI = confidence interval; Vpassive = airflow at normal ventilatory drive.
An unpaired t test was used. Adjusted differences and P values take into account the baseline apnea–hypopnea index and body mass index (potential confounders). The primary trait for analysis was loop gain. Vpassive and arousal threshold values were transformed (square-root) to provide normally distributed data for analysis; back-transformed results are shown for presentation. Significant values are in bold, and nearly significant values are in italics.
Figure 2.
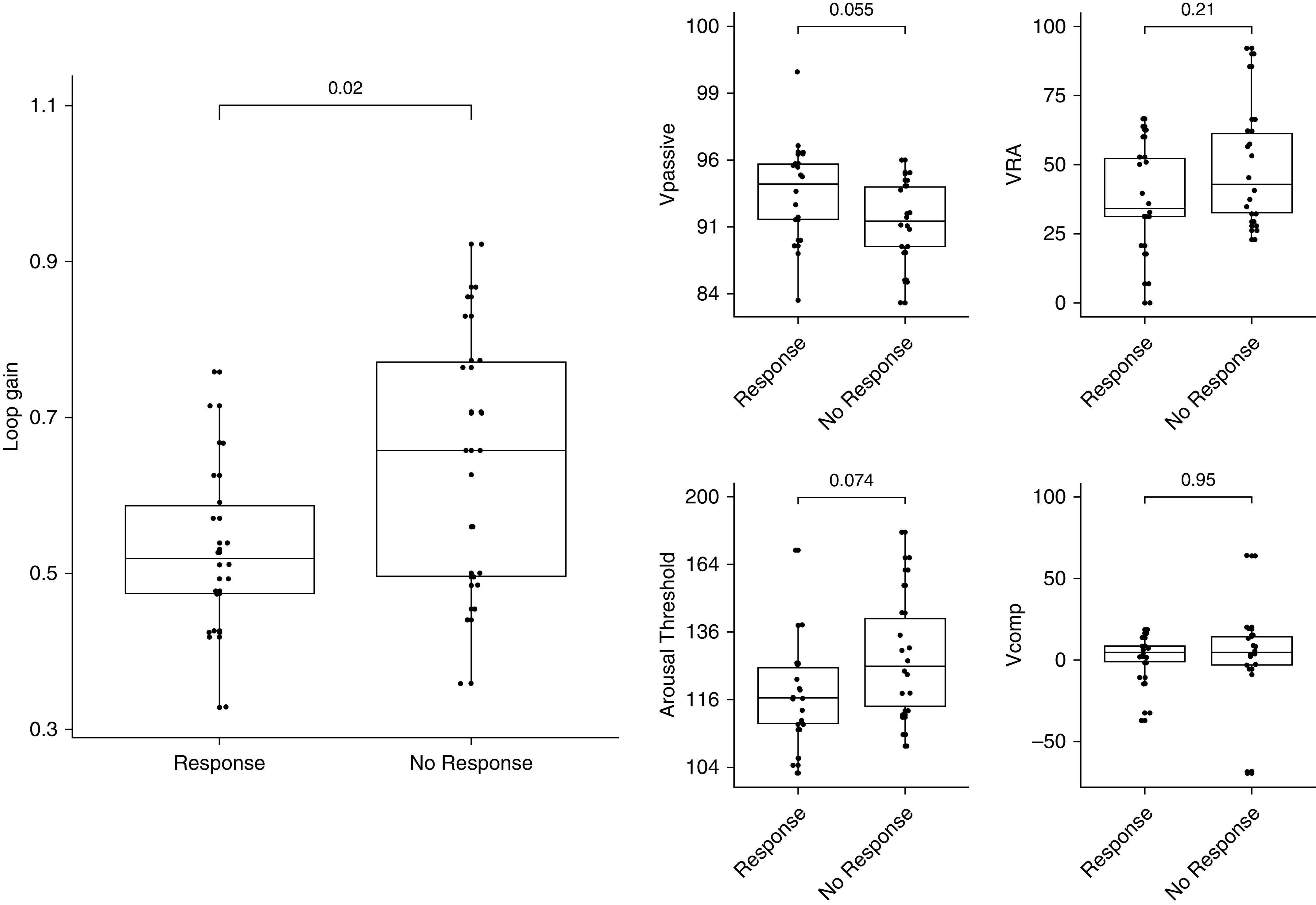
Bivariate analysis showed that nonresponders to mandibular-advancement-device treatment had a significantly higher loop gain than responders. Without adjustment for apnea–hypopnea index and body mass index, there was no significant difference between responders and nonresponders for any of the other traits. After adjustment for apnea–hypopnea index and body mass index, nonresponders exhibited significantly greater collapsibility (lower Vpassive) and a higher arousal threshold. Vpassive and arousal-threshold values were transformed (square-root) to provide normally distributed data for analysis; back-transformed results are shown for presentation. Vcomp = ventilatory compensation; Vpassive = airflow at normal ventilatory drive; VRA = ventilatory response to arousal.
Adjusted Bivariate Associations
After adjusting for baseline AHI and BMI, differences in loop gain were upheld (odds ratio, 2.17 [95% CI, 1.22–3.88] per 1-SD increase in loop gain [SD, 0.15]; P = 0.013; Table 3). Furthermore, greater collapsibility (odds ratio, 1.97 [95% CI, 1.18–3.29] per 1-SD decrease in Vpassive; P = 0.014) and a higher arousal threshold (odds ratio, 1.86 [95% CI, 1.04–3.35] per 1-SD increase in arousal threshold [SD, 18.0%]; P = 0.045) in nonresponders was also significant after adjusting for baseline AHI and BMI.
Multivariable Associations
We further explored whether loop gain remained associated with nonresponder status after additional adjustment for collapsibility (potential confounder; AHI and BMI included). Higher loop gain (odds ratio, 3.03 [95% CI, 1.16–7.88] per 1-SD increase in loop gain [SD, 0.15]; P = 0.023) remained associated with nonresponder status, independent of more severe collapsibility (lower Vpassive; odds ratio, 4.6 [95% CI, 1.1–18.5] per 1-SD decrease in Vpassive [SD, 7.2%]; P = 0.032; R2 = 0.28; χ2 test–derived P = 0.008). Variance inflation factors of the final model were below two. With this model, a sensitivity of 66.7%, a specificity of 72.2%, a positive predictive value of 70.6%, and a negative predictive value of 68.4% were obtained to explain nonresponse.
Discussion
The current study showed that nonresponders to MAD treatment have a more hypersensitive (less stable) ventilatory-control system, reflected by a higher baseline loop gain observed using routine clinical polysomnography (Figure 2). A higher loop gain was associated with nonresponse, independent of baseline AHI, BMI, and collapsibility (logistic regression), with a 1-SD increase in loop gain yielding a threefold increase in the likelihood of being a nonresponder. Determining the ventilatory-control stability at baseline could thus potentially help predict MAD treatment response.
In previous research, increased collapsibility of the upper airway was found to have an effect on MAD treatment efficacy. Patients with an optimal CPAP pressure of 10.5 cm H2O or more, reflecting a highly collapsible upper airway, are more likely to be nonresponders to MAD therapy (33). Likewise, other studies found greater MAD efficacy in patients with a lower BMI (34), a surrogate of less severe collapsibility (35). Results obtained in the study of Edwards and colleagues (16), using gold-standard measurement techniques to define each pathophysiological trait, showed that patients with lower loop gain have increased probability for a favorable MAD response. In a recent study by Bamagoos and colleagues (17), loop gain and collapsibility were also found to be associated with AHI reduction under MAD treatment (although only in nonlinear multivariable models rather than in bivariate analyses). In the present study, we demonstrated an increased odds for MAD treatment nonresponse in patients with high loop gain, independent of collapsibility, showing that loop gain might be the main trait that can be used to differentiate between responders and nonresponders to MAD treatment.
Furthermore, recently, loop gain was found to be a predictor for response to upper-airway surgery using the same algorithm to calculate pathophysiological traits from the baseline clinical polysomnography (26). As the results in our study show, an association between higher loop gain and an increased likelihood of nonresponse to MAD treatment suggest that similar patients might be suitable for MAD therapy and upper-airway surgery.
In current clinical practice, MAD patient selection, if it is done at all, is mainly based on the site of collapse and surrogates of collapsibility (5, 33, 34, 36–39). However, as discussed, previous and current research shows other traits also play a role in MAD treatment efficacy (16, 17, 33). We showed that it is now feasible to calculate these traits in a clinical setting using baseline polysomnographic data, without the need for invasive, labor-intensive techniques. Pending future validation of our results in a larger sample, we consider that patients at elevated risk of nonresponse to MAD could be identified on the basis of their pathophysiology (higher loop gain), as such reducing the time needed to guide patients toward their optimal treatment. These results highlight that pathophysiological endotyping might be a useful approach for predicting non-CPAP treatment efficacy in general.
Strengths and Limitations
This study has several strengths. First, the results of this study confirm the results of Edwards and colleagues (16) obtained using the gold-standard measurement techniques (n = 14) showing that MAD treatment outcome is associated with a low loop gain at baseline and a less collapsible upper airway. In contrast to that study, the endotypic traits in the current study were derived from a standard baseline clinical polysomnography, avoiding the more invasive and labor-intensive aspects of measuring these traits with the gold-standard techniques.
Second, our results are in line with the results as described by Bamagoos and colleagues (17), in which a greater reduction in AHI was associated with lower loop gain, a higher arousal threshold, a lower response to arousal, moderate collapsibility, and weaker muscle compensation. However, in contrast to this study, our data set showed a bivariate association between baseline loop gain and MAD response that was sustained after correcting for baseline covariates and collapsibility. As in our smaller sample, the loop gain findings were retained; a lower loop gain might be the most important parameter in explaining MAD response.
A third strength is the well-designed study protocol. All patients followed a standardized methodological protocol with fixed study dates. As there is no gold-standard titration protocol available for MAD treatment (7), all MADs were fixed at 75% of the individual patient’s maximal protrusion without further titration. Further titration might have increased response rates, but we argue that this approach was needed to allow for an objective and reproducible comparison among patients.
Fourth, all patients were fitted with the same MAD (RespiDent Butterfly). This MAD type consists of two clips attached to each other via a screw system located in the frontal area of the teeth. As such, it avoids mouth opening during sleep, thereby obviating backward and downward movement of the upper jaw that could reduce treatment efficacy.
Fifth, our laboratory collected high-quality nasal pressure signals, sampled at ≥100 Hz, with true direct-coupled data acquisition (no inherent high-pass “drift-correction” that creates artificial variability in the zero-flow baseline and conflates inspiration and expiration), without digital high-pass filtering (or low-pass “smoothing”) or signal clipping. These data are a rare sample that were able to meet American Academy of Sleep Medicine recommendations and standards for physiological trait estimation.
Our study also has some limitations. First of all, a potential limitation of our study is the rather low sample size of 36 patients. Hence, the multivariable analysis should be considered exploratory. However, it was of particular interest to demonstrate the association between MAD response and loop gain after adjusting for Vpassive, as similar models were published previously (16, 17). Because of this limited sample size, further exploration of effect modification by age and sex was also not possible. Future larger studies, with the current results as the primary outcome, are needed to confirm these findings and enable MAD treatment outcome prediction.
Second, the patients with OSA included in this study are in a narrow range concerning OSA severity, BMI (<35 kg/m2), and age and do not show ethnic diversity. Although these patient characteristics are rather typical of the patients presenting in our sleep clinic for MAD therapy, this limits the generalizability of the current results to patients outside these ranges (e.g., higher BMI and other ethnicities). To allow clinical application outside this patient range, future studies with a broader range in OSA severity and patient characteristics are needed.
Third, the current study does not include an untreated control group, as it was a retrospective analysis of a previous prospective study. Prospective validation of our findings would ultimately require demonstration of greater efficacy (vs. untreated control patients) in patients with favorable endotypes.
Fourth, the fixed 75% protrusive position is an advantage for scientific consistency but may not fully reflect the current clinical practice with titration toward an optimal protrusion in the individual patient. In our clinical practice, most patients end up at around 80% of their maximal comfortable protrusion. Therefore, more optimal results could have been obtained with further titration. However, we advocate that the fixed 75% protrusive position was imperative for a more objective and comparable study design, as it removes a potential confounder or source of unrelated variability.
Fifth, the response definition used (ΔAHI ≥ 50%) is rather liberal. Patients with severe OSA who are classified as responders using this definition might not be complete responders (AHI < 5 events/h). However, we preferred this liberal definition, as we believe a drop in AHI of more than 50% is a clinically meaningful response. For patients without complete response, MAD treatment might be a valuable component for combination therapy.
Another limitation is that the endotyping approach used here does not incorporate site-of-obstruction information. For example, recent research showed that patients with tongue-base collapse show increased odds for being a responder to MAD treatment (39, 40) and that those with complete concentric collapse at the level of the palate or a complete lateral wall collapse at the level of the oropharynx were at risk of an increased AHI with MAD treatment (39). Furthermore, expiratory pinching associated with palatal prolapse was, together with increased event depth, shown to be associated with MAD nonresponse (41). Although positive and negative predictive values of the current study are only moderate, these values are in line with those derived from previously researched methods like cephalometry and phrenic-nerve stimulation (42, 43). Combining different prediction methods and including information on the site of obstruction (e.g., using the airflow signal [19, 20, 22, 41]) may help improve the predictive value to the amounts needed for clinical application.
Conclusions
The current study showed that hypersensitive ventilatory control (higher loop gain) is associated with a greater odds for nonresponse to MAD therapy, even after consideration of baseline AHI, BMI, and collapsibility (Vpassive), as calculated from standard clinical polysomnography.
The current results confirm the results as obtained in previous research using the gold-standard measurement methods. Our findings show that it may be possible to use the pathophysiological OSA traits from signals collected at a standard baseline clinical polysomnography to differentiate between responders and nonresponders to MAD treatment (23). As such, pending validation in a larger study, this technique potentially makes the findings reported previously by Edwards and colleagues (16) available for clinical practice.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the coworkers of the Multidisciplinary Sleep Disorders Center, Specialized Dentistry Care and Ear, Nose and Throat Department of the Antwerp University Hospital.
Footnotes
Supported by a 3-year grant of the Flemish government agency for Innovation by Science and Technology (IWT-090864). M.D. holds a Postdoctoral Fellowship at Research Foundation Flanders (FWO) [12H4520N]. A.A. was supported by the U.S. National Institutes of Health (NIH) NHLBI [R01HL153874], American Heart Association [19CDA34660137] and the American Academy of Sleep Medicine [188-SR-17]. A.W. was supported by the NIH NHLBI [R01HL102321 and R01HL128658]. S.A.S. was supported by the American Heart Association [15SDG25890059] and NIH NHLBI [R01HL146697]. O.M.V holds a Senior Clinical Investigator Fellowship at the Research Foundation Flanders (FWO) [1833517N].
Author Contributions: S.O.d.B., A.W., S.A.S., and O.M.V. contributed to the study concept and design. S.O.d.B., M.D., M.W., J.V., M.J.B., and O.M.V. collected the data. A.A. was involved in data interpretation and the critical revision of the article. S.O.d.B. and S.A.S. performed the trait analysis and statistical analysis. All authors interpreted the results, performed a critical revision of the article, and approved the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 4.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 5.Chan AS, Sutherland K, Schwab RJ, Zeng B, Petocz P, Lee RW, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65:726–732. doi: 10.1136/thx.2009.131094. [DOI] [PubMed] [Google Scholar]
- 6.Vanderveken OM, Devolder A, Marklund M, Boudewyns AN, Braem MJ, Okkerse W, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178:197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 7.Dieltjens M, Vanderveken OM, Heyning PH, Braem MJ. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev. 2012;16:177–185. doi: 10.1016/j.smrv.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Vanderveken OM, Van de Heyning P, Braem MJ. Retention of mandibular advancement devices in the treatment of obstructive sleep apnea: an in vitro pilot study. Sleep Breath. 2014;18:313–318. doi: 10.1007/s11325-013-0886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johal A, Haria P, Manek S, Joury E, Riha R. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: a randomized clinical trial. J Clin Sleep Med. 2017;13:175–182. doi: 10.5664/jcsm.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]
- 11.Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10:215–227. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015;11:861–868. doi: 10.5664/jcsm.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamagoos AA, Cistulli PA, Sutherland K, Madronio M, Eckert DJ, Hess L, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc. 2019;16:1422–1431. doi: 10.1513/AnnalsATS.201903-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland K, Phillips CL, Davies A, Srinivasan VK, Dalci O, Yee BJ, et al. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med. 2014;10:943–949. doi: 10.5664/jcsm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azarbarzin A, Marques M, Sands SA, Op de Beeck S, Genta PR, Taranto-Montemurro L, et al. Predicting epiglottic collapse in patients with obstructive sleep apnoea. Eur Respir J. 2017;50:1700345. doi: 10.1183/13993003.00345-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azarbarzin A, Sands SA, Marques M, Genta PR, Taranto-Montemurro L, Messineo L, et al. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J. 2018;51:1–11. doi: 10.1183/13993003.01419-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azarbarzin A, Sands SA, Taranto-Montemurro L, Oliveira Marques MD, Genta PR, Edwards BA, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep (Basel) 2017;40:zsw005. doi: 10.1093/sleep/zsw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genta PR, Sands SA, Butler JP, Loring SH, Katz ES, Demko BG, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152:537–546. doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep (Basel) 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joosten SA, Leong P, Landry SA, Sands SA, Terrill PI, Mann D, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep (Basel) 2017;40:zsx094. doi: 10.1093/sleep/zsx094. [DOI] [PubMed] [Google Scholar]
- 27.Op de Beeck S, Sands SA, Azarbarzin A, Willemen M, Verbraecken J, Braem MJ, et al. Lower loop gain and reduced ventilatory response to arousal are associated with mandibular advancement device treatment efficacy [abstract] Am J Respir Crit Care Med. 2019;199:A4027. [Google Scholar]
- 28.Op de Beeck S, Sands SA, Azarbarzin A, Willemen M, Verbraecken J, Braem MJ, et al. Mandibular advancement device efficacy is associated with ventilatory control stability at baseline [abstract] J Dent Sleep Med. 2019;6:008. [Google Scholar]
- 29.Verbruggen AER, Vroegop AVMT, Dieltjens M, Wouters K, Kastoer C, De Backer WA, et al. Predicting therapeutic outcome of mandibular advancement device treatment in obstructive sleep apnoea (PROMAD): study design and baseline characteristics. J Dent Sleep Med. 2017;4:119–138. [Google Scholar]
- 30.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 31.Dieltjens M, Vanderveken OM, Hamans E, Verbraecken JA, Wouters K, Willemen M, et al. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: a prospective clinical study. Sleep Breath. 2013;17:565–572. doi: 10.1007/s11325-012-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sands SA, Edwards BA, Terrill PI, Butler JP, Owens RL, Taranto-Montemurro L, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J. 2018;52:1800674. doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuiki S, Kobayashi M, Namba K, Oka Y, Komada Y, Kagimura T, et al. Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J. 2010;35:1098–1105. doi: 10.1183/09031936.00121608. [DOI] [PubMed] [Google Scholar]
- 34.Tsuiki S, Ito E, Isono S, Ryan CF, Komada Y, Matsuura M, et al. Oropharyngeal crowding and obesity as predictors of oral appliance treatment response to moderate obstructive sleep apnea. Chest. 2013;144:558–563. doi: 10.1378/chest.12-2609. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 36.Vroegop AV, Vanderveken OM, Dieltjens M, Wouters K, Saldien V, Braem MJ, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2013;22:348–355. doi: 10.1111/jsr.12008. [DOI] [PubMed] [Google Scholar]
- 37.Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35:836–842. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 38.Chung JW, Enciso R, Levendowski DJ, Morgan TD, Westbrook PR, Clark GT. Treatment outcomes of mandibular advancement devices in positional and nonpositional OSA patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:724–731. doi: 10.1016/j.tripleo.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Op de Beeck S, Dieltjens M, Verbruggen AE, Vroegop AV, Wouters K, Hamans E, et al. Phenotypic labelling using drug-induced sleep endoscopy improves patient selection for mandibular advancement device outcome: a prospective study. J Clin Sleep Med. 2019;15:1089–1099. doi: 10.5664/jcsm.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques M, Genta PR, Azarbarzin A, Taranto-Montemurro L, Messineo L, Hess LB, et al. Structure and severity of pharyngeal obstruction determine oral appliance efficacy in sleep apnoea. J Physiol. 2019;597:5399–5410. doi: 10.1113/JP278164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vena D, Azarbarzin A, Marques M, Op de Beeck S, Vanderveken OM, Edwards BA, et al. Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow. Sleep. 2020;43:zsaa004. doi: 10.1093/sleep/zsaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamagoos AA, Sutherland K, Cistulli PA. Mandibular advancement splints. Sleep Med Clin. 2016;11:343–352. doi: 10.1016/j.jsmc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Cistulli PA, Sutherland K. Phenotyping obstructive sleep apnoea-Bringing precision to oral appliance therapy. J Oral Rehabil. 2019;46:1185–1191. doi: 10.1111/joor.12857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.