To the Editor:
Sex is increasingly factoring into pulmonary medicine research (1–3). This advance reflects a broader trend of considering sex, gender, and gender identity in healthcare practice and policy (4). The impact of gender identity on medical practice is pervasive, particularly in binary (e.g., cisgender) references for laboratory and screening tests (5). Historically, this has contributed to healthcare disparities for transgender and gender nonbinary (TGNB) populations (6). Binary, sex-specific references persist for pulmonary function testing (PFT). The 2019 American Thoracic Society (ATS) spirometry guidelines specify that patients, regardless of gender identity, should “be informed” that “birth sex” is a determinant of predicted lung size (7). Anecdotally, reference choice may impact spirometry interpretation and clinical care (8, 9). In this study, we examined PFT reference choice by providers for TGNB patients and the impact on spirometry. Some of the results of these studies have been previously reported in the form of an abstract (10).
Methods
TGNB patients were identified by note keywords, problem list diagnoses, and gender identity demographic fields (11–13) in the electronic health record (EHR) (Epic Corporation) among patients with a primary care provider in a large academic health system between 2015 and 2019. Only patients with PFTs were selected and TGNB identity was confirmed with chart reviews for gender identity (e.g., past medical history, social history). We extracted the following information for each PFT: age, listed gender, testing indication, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio. Sex recorded at birth, legal sex, race, and any active, concurrent transgender-related hormone therapy prescriptions were captured from the EHR. Obstruction was defined as FEV1/FVC less than the lower limit of normal (LLN). LLN values using a male versus female reference population were calculated post hoc using rspiro: Spirometry equations for R and the Global Lung Function Initiative (GLI)-2012 reference equations (Theodore Lytras (2017), R package version 0.1). In this paper, trans man describes an individual with a male gender identity who was assigned female sex at birth, trans woman describes an individual with a female gender identity who was assigned male sex at birth, and gender nonbinary describes a person whose gender identity does not conform to the binary categories of male or female. For each patient, the first PFT in the EHR with a recorded gender after documented TGNB status was used for the analysis.
Results
Three hundred three patients with PFTs met our initial search criteria. After manual chart review, only 17 patients were confirmed to be transgender. Fifteen patients had full spirometry available including five trans men, eight trans women, and two gender nonbinary individuals (Table 1). Average age at PFT was 44.1 years (range 18.8–67.2 yr) and patients were predominantly white/non-Hispanic (80%). The majority of PFTs (87%) were done in a PFT lab, with primary indications listed as dyspnea (40%), asthma (27%), obstructive sleep apnea (7%), or unlisted (27%).
Table 1.
PFT characteristics for transgender and gender nonbinary patients
| Characteristics | Trans Man (n = 5) | Trans Woman (n = 8) | Gender Nonbinary (n = 2) | |
|---|---|---|---|---|
| Age, yr, average | 38.0 | 49.8 | 36.8 | |
| White, % | 80 | 75 | 100 | |
| Concurrent hormone use, % | 100 | 62.5 | 0 | |
| Female gender reference used for PFTs, % | 60 | 75 | 100 | |
| Performed in PFT lab, % | 80 | 100 | 50 | |
| Primary PFT indication, % | |
|||
| Asthma | 20 | 25 | 50 | |
| Dyspnea | 80 | 25 | — | |
| OSA | — | 12.5 | — | |
| Mean FEV1, L | 2.5 | 3.3 | 2.9 | |
| Mean FVC, L | 2.9 | 4.0 | 3.9 | |
| Mean FEV1/FVC | 0.85 | 0.80 | 0.74 | |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; OSA = obstructive sleep apnea; PFT = pulmonary function testing.
A female reference population was used by providers for 60% of trans men, 75% of trans women, and 100% of gender nonbinary patients. An active sex hormone prescription was concurrent with 67% of PFTs (100% of trans men, 63% of trans women, 0% of gender nonbinary) including estrogens (50%), testosterones (40%), and spironolactone (10%).
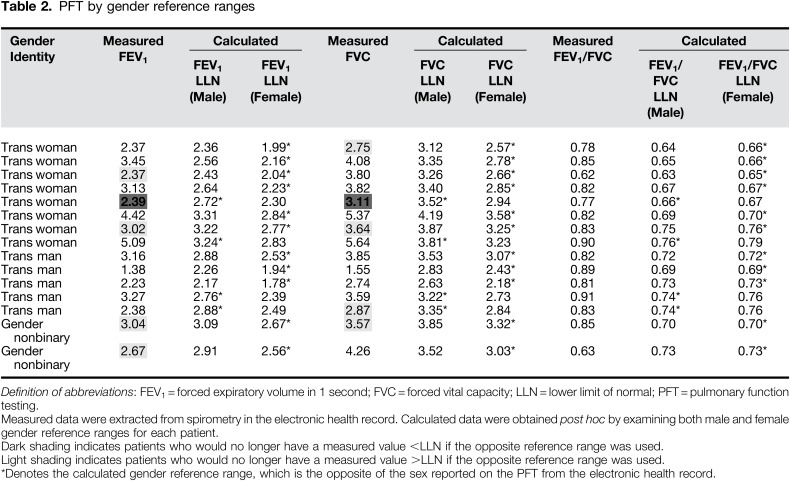
Post hoc calculation of pulmonary function percentiles demonstrated that using the opposite gender as reference did not change the detection of obstruction (FEV1/FVC <LLN) (Table 2). Values above and below LLN changed based on the selected gender reference for FEV1 in five patients and for FVC in five patients; in three patients, both values were impacted by the reference choice (Table 2).
Discussion
Pulmonary function testing uses a cisgender algorithm for lung function estimation. We found that providers inconsistently use female and male reference ranges for transgender patients, independent of gender identity. The predominant selection of a female reference in our small sample was striking and may signal systemic or unconscious provider biases. The error rate in gender allocation and reference selection in the non-TGNB population (e.g., based on patient names or appearance) is unknown and the impact of the recent ATS guidelines on provider behavior remains to be seen.
Use of the opposite gender reference affected interpretation of FEV1 and FVC in our sample. These findings suggest that use of male predicted FEV1 and FVC values, for a female-sized body, could result in a pseudo-restriction and the reverse scenario may mask a true restriction. However, the presence of obstruction based on predicted FEV1/FVC was not affected by gender reference assignment. This provides an important discussion point for pulmonary providers and PFT technologists with TGNB patients. Additionally, as spirometry is increasingly incorporated into bioinformatic algorithms phenotyping pulmonary disease (14), our findings support the importance of extracting and reporting full PFTs particularly for transgender patients.
We used multiple methods to identify potential TGNB patients based on published algorithms and EHR demographic fields. The sensitivity and specificity of these methods ranged widely (11, 13). The resulting small sample size aligns with recently published findings that U.S. health centers have a high percentage of missing sexual orientation and gender identity data (15). Our study supports the need for increased use of sexual orientation and gender identity data collection for clinical application in pulmonary disease.
Accurately measuring lung function is a fundamental step to addressing healthcare disparities in pulmonary disease globally (16). Notably, cigarette (17) and e-cigarette use (18) is more prevalent among transgender individuals than the general population. ATS guidelines reinforce a cisgender approach and favor sex recorded at birth as reference. This contrasts with guidelines in other clinical specialties where providers and patients may consider using both binary reference ranges as an option in shared decision making (19). Finally, the majority of patients in our study were on concurrent hormone therapy at the time of PFTs, yet the impact of hormone therapy—particularly pubertal blockade—and surgical interventions on longitudinal lung function remains unknown. This is a compelling area of future study given the emerging research on the roles of estrogen, progesterone, and testosterone in lung pathophysiology (3). Until then, risks for disparities—despite guidelines—will persist.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health (NIH-T32AI007306) (D.F.). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: D.F. and D.R. wrote the initial draft of the manuscript. A.A., P.G.W., D.W.B., and O.-P.R.H. participated in review of the manuscript and all authors approved its final version. All authors were involved in the design, or acquisition, analysis, or interpretation of data.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Tam A, Churg A, Wright JL, Zhou S, Kirby M, Coxson HO, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:825–834. doi: 10.1164/rccm.201503-0487OC. [DOI] [PubMed] [Google Scholar]
- 2.Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol. 2018;9:2997. doi: 10.3389/fimmu.2018.02997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han YY, Forno E, Celedón JC. Sex steroid hormones and asthma in a nationwide study of U.S. adults. Am J Respir Crit Care Med. 2020;201:158–166. doi: 10.1164/rccm.201905-0996OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill SR, Baker K, Deutsch MB, Keatley J, Makadon HJ. Inclusion of sexual orientation and gender identity in stage 3 meaningful use guidelines: a huge step forward for LGBT health. LGBT Health. 2016;3:100–102. doi: 10.1089/lgbt.2015.0136. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein Z, Corneil TA, Greene DN. When gender identity doesn’t equal sex recorded at birth: the role of the laboratory in providing effective healthcare to the transgender community. Clin Chem. 2017;63:1342–1352. doi: 10.1373/clinchem.2016.258780. [DOI] [PubMed] [Google Scholar]
- 6.Grant JM, Mottet LA, Tanis J. National transgender discrimination survey report on health and healthcare. 2010 [accessed 2019 Jun 17]. Available from: https://cancer-network.org/wp-content/uploads/2017/02/National_Transgender_Discrimination_Survey_Report_on_health_and_health_care.pdf.
- 7.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update: an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hadidi N, Baldwin JL. Spirometry considerations in trangender patients. J Allergy Clin Immunol. 2017;139:AB198. [Google Scholar]
- 9.Haynes JM, Stumbo RW. The impact of using non-birth sex on the interpretation of spirometry data in subjects with air-flow obstruction. Respir Care. 2018;63:215–218. doi: 10.4187/respcare.05586. [DOI] [PubMed] [Google Scholar]
- 10.Foer DRD, Almazan A, Wickner P, Bates D, Hamnvik O. P200 spirometry use for transgender patients. Ann Allergy Asthma Immunol. 2019;123:S32. [Google Scholar]
- 11.Roblin D, Barzilay J, Tolsma D, Robinson B, Schild L, Cromwell L, et al. A novel method for estimating transgender status using electronic medical records. Ann Epidemiol. 2016;26:198–203. doi: 10.1016/j.annepidem.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blosnich JR, Cashy J, Gordon AJ, Shipherd JC, Kauth MR, Brown GR, et al. Using clinician text notes in electronic medical record data to validate transgender-related diagnosis codes. J Am Med Inform Assoc. 2018;25:905–908. doi: 10.1093/jamia/ocy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foer D, Rubins DM, Almazan A, Chan K, Bates DW, Hamnvik OR. Challenges with accuracy of gender fields in identifying transgender patients in electronic health records. J Gen Intern Med. doi: 10.1007/s11606-019-05567-6. [online ahead of print] 5 Dec 2019; DOI: 10.1007/s11606-019-05567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akgün KM, Sigel K, Cheung KH, Kidwai-Khan F, Bryant AK, Brandt C, et al. Extracting lung function measurements to enhance phenotyping of chronic obstructive pulmonary disease (COPD) in an electronic health record using automated tools. PLoS One. 2020;15:e0227730. doi: 10.1371/journal.pone.0227730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasso C, Goldhammer H, Funk D, King D, Reisner SL, Mayer KH, et al. Required sexual orientation and gender identity reporting by US health centers: first-year data. Am J Public Health. 2019;109:1111–1118. doi: 10.2105/AJPH.2019.305130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Lesbian, gay, bisexual, and transgender persons and tobacco use. 2019 [accessed 2020 Jul 31]. Available from: https://www.cdc.gov/tobacco/disparities/lgbt/index.htm.
- 18.Mirbolouk M, Charkhchi P, Kianoush S, Uddin SMI, Orimoloye OA, Jaber R, et al. Prevalence and distribution of e-cigarette use among U.S. adults: behavioral risk factor surveillance system, 2016. Ann Intern Med. 2018;169:429–438. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen HN, Hamnvik OR, Jaisamrarn U, Malabanan AO, Safer JD, Tangpricha V, et al. Bone densitometry in transgender and gender non-conforming (TGNC) individuals: 2019 ISCD official position. J Clin Densitom. 2019;22:544–553. doi: 10.1016/j.jocd.2019.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.