Abstract
Approximately 2,500 pediatric hematopoietic cell transplants (HCTs), most of which are allogeneic, are performed annually in the United States for life-threatening malignant and nonmalignant conditions. Although HCT is undertaken with curative intent, post-HCT complications limit successful outcomes, with pulmonary dysfunction representing the leading cause of nonrelapse mortality. To better understand, predict, prevent, and/or treat pulmonary complications after HCT, a multidisciplinary group of 33 experts met in a 2-day National Institutes of Health Workshop to identify knowledge gaps and research strategies most likely to improve outcomes. This summary of Workshop deliberations outlines the consensus focus areas for future research.
Keywords: hematopoietic cell transplantation, pulmonary complications, acute respiratory distress syndrome, pediatrics, respiratory failure
Hematopoietic cell transplantation (HCT) is curative for an ever-increasing number of children with malignant and nonmalignant conditions (1, 2). However, successful outcomes are limited by post-transplant complications, most notably pulmonary dysfunction. The prevalence and severity of pulmonary complications impair quality of life and limit the therapeutic scope of HCT. The National Heart, Lung, and Blood Institute in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute organized a multidisciplinary Workshop to address research needs to mitigate pulmonary complications after pediatric HCT. Experts representing a wide range of disciplines participated in this National Institutes of Health (NIH) Workshop on April 25–26, 2018 (Figure 1). The Workshop was organized into six sessions to cover all aspects of this multidimensional problem (Figure 2). Five sessions summarized the current state of the science, key knowledge gaps, and suggestions for future research direction, on which consensus was reached in session 6. This manuscript, following the format in Figure 3, describes the Workshop conclusions, highlights the data that informed them, and provides a broad framework to guide future investigation (Figure 4).
Figure 1.
Geographic distribution of Workshop participants at the 2-day cross-disciplinary trans–National Institutes of Health (NIH) Workshop to elucidate pulmonary complications presented by the pediatric hematopoietic cell transplant recipient. Sponsored by the National, Heart, Lung and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Cancer Institute of the NIH in Bethesda, Maryland, the Workshop was attended by 33 speakers and participants from across the United States and from Vancouver, Canada, and 15 federal participants from across the NIH.
Figure 2.
Organization of Workshop sessions. This figure represents the organization of the 2-day Workshop. Led by a session chair, experts in the various fields succinctly summarized the current state of the art, identified key knowledge gaps, and suggested future research direction for the listed topics addressed in sessions 1–5. In session 6, the chairs presented the summary of their individual sessions, and the group reached consensus on essential knowledge gaps and research strategies. This sixth session was facilitated by the National Heart, Lung, and Blood Institute Program Official, and consensus was attained through candid discussion and informal voting without the use of a more formal consensus building process (e.g., Delphi process). HCT = hematopoietic cell transplant.
Figure 3.
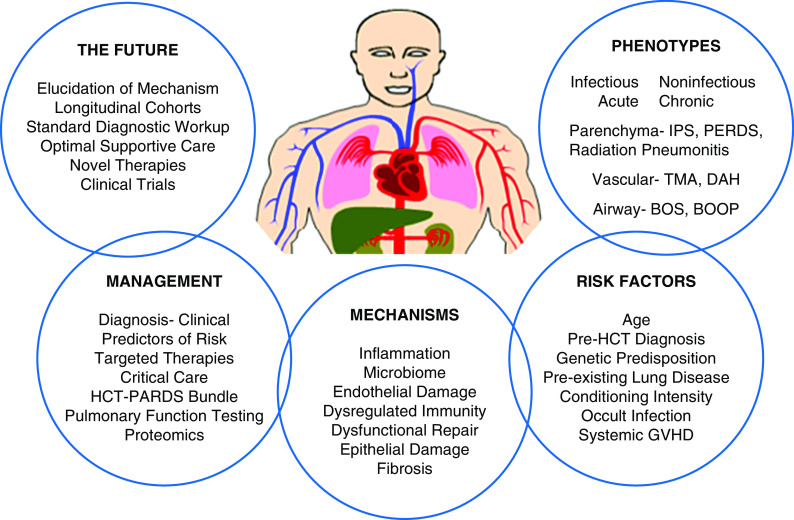
Organization of content centered around the patient receiving hematopoietic cell transplant. This figure represents the broad cross-cutting areas covered during the Workshop, integrated around the transplant recipient. BOOP = bronchiolitis obliterans organizing pneumonia; BOS = bronchiolitis obliterans syndrome; DAH = diffuse alveolar hemorrhage; GVHD = graft-versus-host disease; HCT = hematopoietic cell transplant; IPS = idiopathic pneumonia syndrome; PARDS = pediatric acute respiratory distress syndrome; PERDS = periengraftment respiratory distress syndrome; TMA = thrombotic microangiopathy.
Figure 4.

Knowledge gaps and research opportunities in pulmonary dysfunction after pediatric hematopoietic cell transplantation. *Hematopoietic cell transplant–pediatric acute respiratory distress syndrome bundle includes 1) lung protective strategies, 2) prone positioning, 3) sedation/neuromuscular blockade, 4) aggressive screening and management of cardiac dysfunction, 5) monitoring of renal function and restricted fluid management, 6) antiinflammatory therapies, 7) nutritional supplementation, 8) indications of extracorporeal membrane oxygenation, and 9) palliative care. HCT = hematopoietic cell transplant; PARDS = pediatric acute respiratory distress syndrome.
Overview
Lung injury and dysfunction occur frequently after HCT, contributing significantly to morbidity and mortality both in the immediate post-transplant period and in subsequent months and years (3–6). Lung injury requiring mechanical ventilation after HCT occurs in 10–39% of pediatric, adolescent, and young adult patients and often results in death, underscoring its prevalence and severity (3, 7–9). Allogeneic HCT is increasingly employed for nonmalignant disorders in which pulmonary disease preexists/coexists (sickle cell disease, immune deficiencies, and metabolic disorders), increasing the likelihood of post-transplant lung dysfunction. In addition, chemotherapy, opportunistic infections, conditioning regimens, immunosuppression, and graft-versus-host reactions may contribute to pulmonary dysfunction before and after HCT. In one report of long-term survivors of pediatric hematologic malignancies and allogeneic HCT, 40% had abnormal pulmonary function tests (PFTs) before transplant and nearly two-thirds developed abnormal PFTs after HCT (10). Unfortunately, current retrospective/registry data lack the granularity, accuracy, and/or completeness to identify pretransplant pulmonary phenotypes. Likewise, there are few prospective studies in children characterizing pulmonary risk before HCT or longitudinal studies following pulmonary morbidity afterwards. Clinical definitions for certain conditions unique to adult HCT patients, such as bronchiolitis obliterans syndrome (BOS), may be less applicable in children, likely accounting for reported variation in pediatric incidence and outcomes. Prompt and accurate diagnosis of lung injury in this population is also hindered by the lack of effective, reliable monitoring of lung function in young and/or critically ill children (11–13) and the low sensitivity and specificity of imaging techniques (14). Developing longitudinal prospective cohorts of pediatric HCT recipients with PFTs, markers of individual risk, prognostic biomarkers, and enhanced diagnostic techniques could potentially improve outcomes (15). Incorporation of patient-reported and family-centered outcomes would identify the impact of pulmonary complications on quality of life (Figure 4).
Clinical Phenotypes, Diagnostic Challenges, and Risk Factors
Clinical Phenotypes
Pulmonary diagnoses after HCT can be divided into infectious and noninfectious categories (4, 16). Infectious lung injury remains problematic, particularly in patients with acute or chronic graft-versus-host disease (GVHD). The Workshop included four presentations summarizing the state of the science regarding viral, fungal, and bacterial causes of pulmonary dysfunction, including a discussion on animal models and viral infections. Historically, half of all pulmonary complications after HCT were secondary to infection, but the current use of broad-spectrum antimicrobial agents has tipped the balance toward noninfectious causes (17). Noninfectious lung injury can be acute or chronic depending on the time of onset after HCT and the tempo of disease progression.
The clinical spectrum of acute noninfectious lung dysfunction includes several entities, including idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage (DAH), transfusion-related acute lung injury, and pulmonary thrombotic microangiopathy (TMA) (reviewed in Reference 4) that could ensue from cytotoxicity of antineoplastic therapies, the infusion of blood products, and/or inflammation engendered during HCT (4, 17). IPS is defined as widespread alveolar injury after HCT occurring in the absence of active infection or cardiogenic causes (4, 16). Diagnostic criteria include clinical signs and symptoms of pneumonia, nonlobar radiographic infiltrates, abnormal pulmonary function, and the absence of infection as determined by bronchoalveolar lavage (BAL) or lung biopsy, preferably including multiplex polymerase chain reaction (PCR)–based assays (18). The updated definition of IPS better differentiates infectious from noninfectious etiologies, classifying noninfectious lung disease by anatomical site (4). IPS typically presents within the first 120 days of HCT (4, 16). Historically, the incidence of IPS after myeloablative conditioning ranged from 3% to 15% in adults (4, 16, 19–21) and children (21). The implementation of reduced-intensity conditioning regimens coupled with enhanced contemporary molecular diagnostic techniques has reduced the incidence of IPS in adults (18, 22); pediatric data are sparse. The full impact of stem cell source, conditioning intensity, human leukocyte antigen (HLA) matching, and the emerging techniques of next-generation sequencing to study the pulmonary microbiome in adults and children needs elucidation (23).
A subset of patients with IPS may develop acute DAH, which is characterized by progressive shortness of breath, cough, and hypoxemia with or without fever (24). Classically, the diagnosis of DAH is based on a progressively bloodier return of BAL fluid, but frank hemoptysis is rare. The frequent difficulty of timely BAL in children highlights the importance of a clinical diagnosis. DAH has been reported in 5–12% of adult HCT recipients (17) and generally occurs within the first 2–3 months after transplant (17, 24). A disproportionately higher incidence of DAH after HCT is reported in children with mucopolysaccharidosis (25). DAH can occur with or without infection; either form is associated with poor outcomes after conventional therapy including steroids (26).
TMA is another complication in adults and children receiving HCT and is associated with high mortality. TMA in the lungs presents as pulmonary hypertension, often leading to acute hypoxia, cardiopulmonary compromise, and death (27–29). The role of complement activation and associated genetic susceptibility to the development of TMA was demonstrated in a prospective study in pediatric HCT recipients (30).
Subacute/chronic (occurring or persisting 6–24 mo after HCT) noninfectious pulmonary complications contribute to significant morbidity in adults and likely in children. Pediatric studies are few and small, highlighting a need for inquiry, and are of particular significance in the developing lung. HCT recipients can develop obstructive lung disease (OLD) or restrictive lung disease (RLD) from fibrotic remodeling, either primarily within and around the small airways/bronchioles or within gas exchange regions (e.g., interstitial fibrosis), respectively (31–33). RLD can develop in association with previous chest wall/thoracic surgery and/or radiation therapy and in patients who develop acute lung injury (e.g., IPS). In the subacute setting, RLD may occur with cryptogenic organizing pneumonia/bronchiolitis obliterans organizing pneumonia (34) or in the context of chronic fibrotic interstitial lung disease (17). BOS after HCT is often reflective of pulmonary GVHD. BOS, typically occurring 6–18 months after transplant, is associated with OLD, with air trapping demonstrated by chest radiographs, computed tomographic (CT) imaging and spirometry (reduced forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ratio). BOS is a clinical diagnosis based on a scoring system designed for adult lung transplant patients, predicated on a decline in FEV1 and/or midexpiratory-phase forced expiratory flow (FEF25–75%). A modified version of the NIH criteria for the diagnosis of BOS in HCT recipients was developed for adults but not validated in children (35). A rapid decline in FEV1 has been demonstrated during the 6 months before the diagnosis of chronic GVHD/BOS in two adult cohorts (36), and a 10% decline in FEV1 from baseline increases the risk of developing BOS (37). Early detection of pulmonary dysfunction is believed to be critical to initiate preemptive therapies in HCT recipients, as reversing changes from fibrosis is challenging once BOS is clinically apparent (38–40). Frequent PFTs should be obtained after transplant, particularly in those with baseline abnormalities and/or significant nonpulmonary GVHD. Two pediatric cohorts demonstrated that PFTs are more sensitive than clinical examinations for the early detection and diagnosis of BOS; those investigators recommend monitoring PFTs longitudinally beyond 12 months after HCT in children at risk for BOS (41, 42). Home-based, handheld spirometry correlates with traditional laboratory spirometry in adults after lung transplantation (43) and HCT (36, 37) and warrants similar evaluation in children.
Diagnostic Challenges
The approach to lung dysfunction after HCT requires attention to pulmonary and nonpulmonary causes. Because symptoms of respiratory distress may progress rapidly once identified, the timely initiation of a work-up including pulmonary function assessment, imaging, and procurement of samples to rule out infection is critical for optimizing outcomes. Initial chest imaging may identify the presence of lobar, multilobar, or diffuse pulmonary infiltrates. Echocardiography may reveal pulmonary hypertension or left heart dysfunction. Although such findings impact the decision-making process, they are nondiagnostic. Challenges with effective and reliable monitoring of lung function in young and/or critically ill children (38), including imaging techniques that only offer low sensitivity and specificity (14, 38–41), represent major obstacles to the prompt and accurate diagnosis of lung injury. Although successful spirometry with acceptable results and valid interpretation is reported in patients as young as 3 years (42) and the acceptability of testing is 87% in 5-year-olds (44), consistent measurements of FEV1 in young children present significant challenges (Figure 4). Forced expiratory volume in 0.5 seconds, which was found to be more reproducible in preschool children with cystic fibrosis (45) than FEV1, could be evaluated as an early physiological biomarker of BOS. Forced oscillometry testing is a feasible, yet-to-be-validated technique in preschoolers that detects changes in pulmonary resistance and compliance (46). In addition, the lung clearance index, measured by the multiple breath washout technique, has demonstrated greater sensitivity than standard PFTs in detecting airway changes in adults after HCT (41, 47). The lung clearance index has value in studying ventilation distribution and should be studied further in children. Novel parameters of respiratory function assessed by quantitative CT imaging (e.g., parametric response mapping) (48) and specific gas volume mapping (49) need to be tested prospectively in childhood. Hyperpolarized 129Xe magnetic resonance imaging, feasible in children unable to perform reliable spirometry, can differentiate patients with cystic fibrosis with early lung obstruction from age-matched control subjects (50). The development and validation of novel criteria to predict risk, follow the trajectory of lung function, and diagnose BOS in pediatric HCT populations is needed (51).
In the absence of obvious nonpulmonary causes, bronchoscopy with BAL should be considered to distinguish infectious from noninfectious etiologies of lung dysfunction and to initiate appropriate management. The need to complete BAL in critically ill HCT recipients remains an area of active debate (52). Complication rates from completed bronchoscopies in adults were <2% in three large series (53–55), suggesting that the procedure can be completed safely, but this needs validation in pediatric HCT recipients. The diagnostic yield from BAL fluid ranges from 31% to 67% depending on the timing (after HCT) of respiratory distress, the time elapsed between the onset of symptoms and BAL, and the start of antimicrobial therapy (54–57). BAL within 4 days of the appearance of pulmonary infiltrates increases overall diagnostic yield (56). Recently, the therapeutic and diagnostic impact of lung biopsy in children with pulmonary dysfunction after HCT has been reported (58). The diagnostic utility of serum and lung biomarkers in lung injury among HCT recipients with IPS (59, 60) is described in a consensus statement (4).
Risk Factors
Numerous patient-specific factors influence the risk of developing pulmonary complications after HCT. Indications for transplant, especially in nonmalignant conditions, may include disorders with baseline airway/parenchymal abnormalities or predisposition to pretransplant pulmonary complications. A thorough medical history may reveal factors affecting pretransplant lung function. Previous cancer therapy, underlying genetic variants, particular phyla within the individual microbiome, pretransplant conditioning, and early post-transplant inflammation can all contribute to lung abnormalities and influence the likelihood of long-term complications. For example, 10–15% of pretransplant children demonstrate abnormal forced and static lung volumes, and >50% demonstrate decreased diffusing capacity, placing them at risk of immediate pulmonary complications and abnormal lung function and mortality after transplant (61, 62). Furthermore, a higher pretransplant lung function score based on estimation of expiratory flow (FEV1) and diffusion (diffusing capacity of the lung for carbon monoxide [DlCO]) is associated with increased risk of post-transplant respiratory failure and death. Preexisting impaired lung function and total body irradiation–based regimens correlated with the lowest survival in a retrospective study of adults (63). Similarly, pulmonary disease and lower pretransplant FVC z-scores have been associated with poor recovery (64). The development of a risk score based on pretransplant pulmonary function, stem cell source, donor type, human leukocyte antigen match, conditioning regimen intensity and composition, fecal microbiota diversity, Day +7 GVHD biomarkers, and the presence of comorbidities, including prior infections and preexisting renal and cardiac impairment, may help stratify patients at risk for pulmonary complications after HCT (Figure 4).
Basic Mechanisms of Lung Injury after HCT
The mechanisms contributing to noninfectious pulmonary complications after HCT are complex. The pathophysiology of IPS has been deciphered in part via laboratory investigation (reviewed in References 4 and 17) and ongoing translational research efforts (65–67). Data generated using established preclinical models support a shift in perspective away from considering acute pulmonary dysfunction as an idiopathic clinical syndrome and toward considering heightened susceptibility of the lung to two pathways of immune-mediated injury that include the recruitment of donor-derived immune cells and inflammatory cytokine release (reviewed in References 4 and 17). These distinct but interrelated pathways 1) involve components of the adaptive and the innate immune responses, synergistic interactions between lymphoid (68, 69) and myeloid cells, and the release of soluble inflammatory chemokines (70–73) and cytokines including tumor necrosis factor-α (TNF-α) (19, 74–77), INF-γ (78, 79), and IL-6 (80); 2) orchestrate the sequential recruitment of donor-derived immune cells (T-lymphocytes, macrophages, monocytes, and neutrophils) to the lung; and 3) ultimately contribute to tissue damage and dysfunction (reviewed in Reference 4). Laboratory insights have been translated back to the clinic for pediatric patients with IPS (60, 65, 81). Importantly, proteomic studies from three human clinical studies revealed striking similarities in mechanisms contributing to IPS in humans and mice, underscored a role for the acute-phase response (TNF-α–IL-6) signaling pathway, illuminated a possible role for injury and activation of the pulmonary vascular endothelium in the development of lung dysfunction, and identified several novel pathways for further exploration (65, 66, 80). Moreover, the results identified a set of robust markers predictive of disease progression and response to therapy (66).
Chronic pulmonary dysfunction after allogeneic HCT can manifest as either OLD or RLD (17, 31, 32, 34). The pathophysiology of lung fibrosis after HCT is complex and incompletely understood, highlighting significant knowledge gaps with respect to the biology and approach to therapy for this spectrum of disorders (31, 82, 83) (Figure 4). These limitations stem from the 1) lack of consistent approaches to monitor for respiratory compromise and accurately diagnose the cause of lung dysfunction; 2) absence of correlative data obtained from afflicted HCT recipients, and until recently; 3) paucity of suitable HCT animal models for either form of chronic lung disease (31, 84). Fibrosis (scarring) refers to the excessive deposition of extracellular matrix primarily cross-linked fibrillar collagens by persistently or abnormally activated fibroblasts, usually resulting in architectural distortion and physiological dysfunction of tissues and organs (31, 85, 86). When developing in the context of pulmonary GVHD, fibrosis is believed to involve a persistent or recurrent antigenic stimulus that elicits chronic inflammation (reviewed in References 17, 31, and 32). In this context, a triphasic model recently proposed for the development of chronic GVHD (31) can be applied to the development of chronic pulmonary dysfunction after HCT (32). This model involves acute inflammation, which may be subclinical (phase I) (4), dysregulated immunity (phase II), dysfunctional repair, and the propagation of chronic inflammation resulting in the deposition of collagen and the development of fibrosis (phase III). When this occurs in and around bronchial structures, obliteration of small airways and significant “fixed” OLD ensues. By contrast, fibroblast proliferation and intraseptal collagen deposition may ultimately result in interstitial fibrosis, volume loss, and impaired gas exchange characteristic of severe RLD (31). TGF (Transforming Growth Factor)-β is a central mediator that may be necessary to initiate, but not enough to sustain, fibrosis. Recently, murine systems have revealed that dysregulation of other factors, including aberrant B-cell immunity with associated autoantibody/alloantibody production (87–91), disruption of the balance of M1/M2 macrophage function (92), and the release of proinflammatory cytokines including TNF-α (93, 94), may all be operative during the development of fibrosis (31) (Figure 4).
The role of the microbiome in the development of lung complications after HCT has only recently been studied. Pathogenicity may result from disturbances in microbe–host interaction balance (95). For example, γ-proteobacterial domination of fecal microbiota before transplant predicted pulmonary complications and mortality in a cohort of adults receiving allogeneic HCT (96). The possible effects of the microbiome and the function of the intestinal barrier on the development of BOS are unscored by studies demonstrating that genetic variations in bactericidal/permeability-increasing protein and NOD2/CARD15 gene variants influence the risk of developing airflow decline after allogeneic HCT (97, 98). Hence, interventions to preserve the respiratory and gut–lung microbiome axis before and after transplant may decrease pulmonary risk. Moreover, host responses to a commensal and/or known pathogen may be more deleterious than the pathogen itself. The impact of the lung microbiome and prior infections as triggers of dysregulated immunity and repair contributing to fibrotic lung injury that may appear noninfectious in etiology at later time points is a topic of active investigation (99–101). Newer methods, including metagenomic sequencing, gene-expression profiling, and proteomics, could help clarify these relationships and their effects on disease severity and long-term lung function (102).
Targets for direct alloimmune-mediated damage may include pulmonary endothelial and epithelial cells (84). Epithelial apoptosis, generally ascribed to T cell–mediated injury and considered pathognomonic for acute GVHD in other target tissues, has not been consistently observed in allogeneic HCT recipients with lung injury. Experimental studies have, however, provided evidence for epithelial injury during IPS (68, 103) and BOS (84). Endothelial cell damage has been implicated as a direct contributor to the development of several complications after allogeneic HCT, including DAH and TMA (104). Endothelial cell activation and injury are also observed after clinical and experimental IPS (60, 68, 75, 105) and likely contribute to the development of GVHD (106) and other late effects after HCT (107).
Treatment of Pulmonary Complications in Pediatric HCT Recipients
Conducting clinical trials in relatively small heterogeneous pediatric populations with acute or chronic pulmonary complications after HCT presents unique opportunities and challenges (108). For example, three pediatric reports (21, 109, 110) confirmed that IPS remains a serious complication after HCT with high mortality (50–80%) and poor response to treatment (18–30%). The administration of steroids to HCT patients with noninfectious acute lung injury has produced mixed results. The Pediatric Acute Lung Injury Consensus Conference recommendations state that “future studies are needed to identify specific populations that might benefit from glucocorticoid therapy” (111). To this end, early-phase prospective studies (60, 81) and a retrospective report (112) suggested that treatment of IPS with corticosteroids and etanercept, a soluble TNF-α–binding protein, may improve survival. A subsequent multicenter, open-label, phase II pediatric study, which closed early for clinical efficacy, found that the administration of corticosteroids combined with etanercept was safe and resulted in response rates of 71% with Day +28 and 1-year survival rates of 89% and 63%, respectively (65). Although this trial had uniform eligibility (excluding age), dosing schedules, and assessments with a parallel phase III IPS study in adults (113), the 1-year overall survival was extremely poor (<25%) for adults in both arms compared with the 63% overall survival observed in children. Notably fewer pediatric patients received reduced-intensity conditioning regimens and peripheral blood as the stem cell source compared with adult patients. Overall protocol adherence also differed significantly; 37% of adults on the treatment arm received two or fewer (of eight) etanercept doses, whereas >80% of pediatric patients received all eight scheduled doses. Finally, interpretation of both studies is influenced by the number of patients enrolled; the pediatric trial ended early as an efficacy stopping rule was met, whereas the adult trial was terminated early for poor accrual, with only 34 patients (of a targeted 120) randomized (113). Despite advances in the treatment of pediatric patients with IPS (65), not all patients responded to TNF-α neutralization, and this begs continued investigation. The utility of cytokine analysis and additional targetable protein biomarkers such as Ang-2 (104, 114) in further optimizing the recognition and treatment of IPS requires additional study. For example, a retrospective trial revealed that elevated concentrations of ST2 (suppression of tumorigenicity/stimulation-2), a biomarker implicated in acute lung injury and GVHD, when combined with elevated concentrations of IL-6 and sTNFR1 were most predictive in diagnosing IPS even before clinical signs and symptoms were present (67). Preemptive, combinatorial anticytokine strategies for patients at high risk for IPS may have merit (Figure 4).
DAH is associated with mortality as high as 75% in adult and pediatric HCT patients (4, 17, 24). Studies report minimal benefit with high-dose steroids or aminocaproic acid therapy. A recent single-center retrospective review of 119 adults with DAH treated with varying doses of steroids revealed Day +100 and overall mortality rates of 85% and 95%, respectively. Two-thirds of cases were noninfectious in origin and could be classified as IPS (115). Anecdotal reports of inhaled recombinant factor VIIa therapy suggest benefit, and it appears well tolerated despite a risk of endotracheal tube occlusion from sudden clot formation (116, 117). The Workshop proposed that DAH and IPS be considered together for inclusion in multicenter studies of targetable cytokine biomarkers and novel focused therapies.
TMA typically involves multiple organ systems, so a multidisciplinary approach to diagnosis and treatment is needed (118). Management of TMA includes replacing calcineurin inhibitors with non–calcineurin-based immune suppression and interventions such as total plasma exchange (119). Complement activation is a major contributor to the multiorgan endothelial injury of TMA, and early intervention with the terminal complement blocking agent eculizumab has been effective in treating TMA (120, 121). Eculizumab effectiveness appears to depend on precise personalized drug dosing regimens, and a clinical trial demonstrating the value of this approach would facilitate widespread adoption at transplant centers and insurance approval (122). Other interventions suggested for TMA have included defibrotide and rituximab. In summary, an improved understanding of the basic mechanisms involved in the development of acute noninfectious lung injury after HCT is needed, including those processes contributing to endothelial cell injury and the role of occult infections.
Standard treatments for noninfectious forms of OLD and RLD are unfortunately suboptimal; no combination of agents is particularly effective. However, a number of new therapeutic strategies have been employed for BOS after HCT (123). The administration of etanercept and combination therapy with fluticasone, azithromycin, and montelukast (FAM) have shown promise in improving (124) and potentially stabilizing (125) lung function, respectively. Both studies were early-phase open-label trials, and therefore, results need to be interpreted in the context of the known natural history of BOS, which can include stabilization of disease with time (126, 127). A very small randomized placebo-controlled study demonstrated no beneficial effect of azithromycin over placebo for severe BOS (128, 129). Moreover, long-term administration of azithromycin, a component of FAM, as prophylaxis against pulmonary injury was recently found to increase the risk of malignancy relapse (130). The impact of FAM on relapse or the development of secondary malignancy when used to treat patients with chronic GVHD and BOS remains to be fully elucidated (131–133). By contrast, treatment with formoterol and budesonide may improve FEV1 in patients with moderately severe BOS when detected early (134). In addition, laboratory insights currently being translated into clinical medicine regarding the role of B cells (89, 135) and JAK pathways (136) may pave the way for novel strategies to treat chronic pulmonary dysfunction. Finally, it is unclear whether established pulmonary fibrosis after HCT in childhood is reversible. Accordingly, the antifibrotic drug nintedanib, which is U.S. Food and Drug Administration approved for idiopathic pulmonary fibrosis, is currently being tested in children with fibrosis from multiple causes. Normalization of lung function after initial impairment improves survival to rates comparable with those of long-term HCT survivors with normal baseline PFTs (137), highlighting the importance of pulmonary surveillance and early intervention.
Intensive Care for Pediatric HCT Recipients with Pulmonary Dysfunction
Critical care interventions are often necessary for pediatric HCT recipients, and mortality for patients requiring intensive cardiopulmonary support for pulmonary dysfunction remains high (138, 139). Noninvasive positive pressure ventilation (NIPPV) in adults suggested more favorable intubation rates and survival with early NIPPV compared with oxygen therapy alone (140–142). However, multicenter, retrospective pediatric data revealed that initial NIPPV use resulted in intubation in the majority of cases and was associated with higher mortality and rates of cardiac arrest at the time of intubation (139, 143). As for other patients with pediatric acute respiratory distress syndrome (PARDS), lung protective strategies are advocated for intubated HCT patients. Although the role of high-frequency oscillatory ventilation remains to be determined, current data suggest that an earlier transition to high-frequency oscillatory ventilation holds the most promise (144). Increasing weight gain (145) and early warning scores (146) may identify HCT patients at risk of respiratory failure and allow increased surveillance and early intervention. In addition to protective lung ventilation strategies, the Pediatric Acute Lung Injury Consensus Conference (147) recommends the study of nutritional support and steroid therapy in the HCT patient. Enteral nutrition, which may modulate inflammation and minimize lung injury while preserving intestinal integrity and decreasing the risk of mucosal barrier infection, improves overall survival (148). The ideal enteral nutrition may include optimized lipid composition (149) and supplemental vitamins and minerals (150–154). The potential impact of prone positioning, inhaled nitric oxide, phosphodiesterase-5 inhibitors, endothelin receptor inhibitors, corticosteroids, combinatorial anticytokine approaches, and strategies to protect the pulmonary vascular endothelium remain to be investigated (155).
The contribution of cardiac dysfunction to PARDS is likely underrecognized. Right heart strain and alterations in pulmonary vascular resistance often accompany mechanical ventilation. Routine echocardiography can reveal abnormalities in 30% of patients as early as HCT Day +7, with 13% having elevated right ventricular pressures. A trend toward decreased survival with any echocardiographic abnormality at Day +7 has been reported (156). Among HCT recipients requiring admission to the pediatric intensive care unit (ICU), one-third were found to have elevated right ventricular pressures (157). In addition, cardiac diastolic dysfunction is now being recognized more frequently in pediatric HCT recipients, as is the appreciation of its associated impact on outcomes (158).
Fluid overload and acute renal failure have long been reported to be associated with worse outcomes among ventilated pediatric HCT recipients (159, 160). The ARDSNet (NHLBI Acute Respiratory Distress Syndrome network) fluid management studies demonstrated that conservative fluid management improved lung function and shortened mechanical ventilation and ICU days, although mortality was not impacted (159). The potential impact of early renal replacement therapies has been recognized (161–163) but remains understudied in PARDS after HCT (164). The role of extracorporeal membrane oxygenation in the treatment of PARDS in the pediatric HCT population is largely unestablished (160, 165, 166). Recent promising survival rates with the use of uncontrolled extracorporeal membrane oxygenation in a small subset of HCT patients (167, 168) warrant further evaluation. Prospective comprehensive data are needed to best inform future use of this modality. Finally, palliative care, symptom control, and family care should be incorporated early in the management strategy for pediatric HCT patients. HCT patients receive more aggressive and less limited care at end of life than non-HCT patients (169). Ideally, palliative care services can, and should, be integrated using various models throughout the illness and not just at end of life (170).
The poor outcomes among HCT recipients with PARDS underscore the need for early identification and mitigation of risk factors for critical illness and consideration of nonconventional therapies. In the first study to match patients from two large databases, the Virtual Pediatric Systems and the Center for International Blood and Marrow Transplant Research aimed to identify pediatric HCT patients who required admission to ICUs (171). Five independent variables were incorporated into a multivariate model to establish quartiles of risk of pediatric ICU mortality. The findings underscored the value of establishing collaborative efforts between pediatric intensive care, HCT, and multiple other subspecialties to identify and follow very high-risk patients. In summary, the development of a “HCT–PARDS bundle” for implementation and future study is suggested (Figure 4).
Conclusions
Pulmonary dysfunction remains common and life threatening after HCT in children. Participants at this Workshop arrived at a consensus on important focus areas. First, there is a need to build, characterize, and study prospective observational cohorts. This effort should use, expand, and link existing databases to enhance deep phenotyping of pulmonary toxicities before and after transplant. Observational studies to evaluate the association of phenotype, genotype, conditioning regimens, and therapies with pulmonary complications before and after transplant are needed, with the goal of developing a practical, robust clinical scoring system for the assessment of pulmonary risk. Second, there is a continued need to enhance the mechanistic understanding of pulmonary disease in HCT recipients and translate this understanding into potential therapies. The role of microorganisms in initiating/exacerbating inflammation, endothelial cell activation, and pathways to fibrosis must be established. Genetic/proteomic predictors that identify patients at risk of pulmonary complications after HCT must be determined, and basic research must be translated into novel strategies and therapies to mitigate pulmonary toxicity in HCT recipients. Finally, there is a critical need to improve clinical outcomes. Recent translational research efforts highlight the value of bench to bedside research through collaborative, multicenter consortium studies (65). The potential role of serum biomarkers, BAL findings, and novel imaging technologies to facilitate early diagnosis, characterize disease processes, and monitor progression of pulmonary disease should continue to be studied. Novel pulmonary function testing in young children needs to be developed and validated. Importantly, this multidisciplinary Workshop concluded that a team approach to research and to clinical care, involving basic scientists, translational scientists, clinician-scientists, and clinicians, is essential to enhance and refine mechanistic understanding, overcome vexing clinical management challenges, and improve outcomes in children after HCT. To support the much needed research discussed during this Workshop, two notices of special interest addressing pulmonary complications of HCT in childhood (NOT-HL-20–761/https://grants.nih.gov/grants/guide/notice-files/NOT-HL-20–761.html and NOT-HL-20–790/https://grants.nih.gov/grants/guide/notice-files/NOT-HL-20–790.html) have been released.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge the valuable contributions from the following speakers and participants at the Workshop:
Pulmonary Complications of Pediatric Hematopoietic Cell Transplantation Workshop Participants: Neil Aggarwal (National Heart, Lung, and Blood Institute, National Institutes of Health); Michael Boeckh, Guang-Shing Cheng, Alpana Waghmare, and Joshua Hill (Fred Hutchinson Cancer Research Center); Larissa Broglie (Columbia University); Jason Chien (Janssen Pharmaceuticals of Johnson and Johnson); Shane Cross and Jennifer McArthur (St. Jude Children’s Research Hospital); Christine N. Duncan, Leslie Lehmann, and Christina Ullrich (Dana-Farber Cancer Institute); Brian Fisher (The Children’s Hospital of Philadelphia); Gerhard Hildebrandt (Markey Cancer Center, Lexington); Robert R. Jenq and Kris M. Mahadeo (University of Texas at MD Anderson Center); Sonata Jodele (University of Southern California Keck School of Medicine); James Kiley (National Heart, Lung, and Blood Institute, National Institutes of Health); Valerie Maholmes, Eunice Kennedy Shriver (National Institute of Child Health and Human Development, National Institutes of Health); Bethany Moore and Gregory Yanik (University of Michigan, Ann Arbor); Lawrence Nogee (Johns Hopkins University School of Medicine); Sophie Paczesny (Medical University of South Carolina); Courtney Rowan (Indiana University School of Medicine); Kirk R. Schultz (BC Children’s Hospital and Research Institute, Vancouver, British Columbia, Canada); Kirsten Williams (Emory University); Jason Woods (Cincinnati Children’s Hospital Medical Center); and Matt S. Zinter (University of California at San Francisco).
Footnotes
Supported by the National Heart, Lung, Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Cancer Institute, U.S. National Institutes of Health.
A complete list of the Pulmonary Complications of Pediatric Hematopoietic Cell Transplantation Workshop Participants may be found before the beginning of the References.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Pulmonary Complications of Pediatric Hematopoietic Cell Transplantation Workshop Participants, Neil Aggarwal, Michael Boeckh, Guang-Shing Cheng, Alpana Waghmare, Joshua Hill, Larissa Broglie, Jason Chien, Shane Cross, Jennifer McArthur, Christine N. Duncan, Leslie Lehmann, Christina Ullrich, Brian Fisher, Gerhard Hildebrandt, Robert R. Jenq, Kris M. Mahadeo, Sonata Jodele, James Kiley, Valerie Maholmes, Bethany Moore, Gregory Yanik, Lawrence Nogee, Sophie Paczesny, Courtney Rowan, Kirk R. Schultz, Kirsten Williams, Jason Woods, and Matt S. Zinter
References
- 1.Khandelwal P, Millard HR, Thiel E, Abdel-Azim H, Abraham AA, Auletta JJ, et al. Hematopoietic stem cell transplantation activity in pediatric cancer between 2008 and 2014 in the United States: a center for international blood and marrow transplant research report. Biol Blood Marrow Transplant. 2017;23:1342–1349. doi: 10.1016/j.bbmt.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, et al. European Society for Blood and Marrow Transplantation EBMT. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49:744–750. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eikenberry M, Bartakova H, Defor T, Haddad IY, Ramsay NK, Blazar BR, et al. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant. 2005;11:56–64. doi: 10.1016/j.bbmt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, et al. American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 6.Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol. 1995;26:668–675. doi: 10.1016/0046-8177(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 7.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 8.van Gestel JP, Bollen CW, Bierings MB, Boelens JJ, Wulffraat NM, van Vught AJ. Survival in a recent cohort of mechanically ventilated pediatric allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2008;14:1385–1393. doi: 10.1016/j.bbmt.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 9.van Gestel JP, Bierings MB, Dauger S, Dalle JH, Pavlíček P, Sedláček P, et al. Outcome of invasive mechanical ventilation after pediatric allogeneic hematopoietic SCT: results from a prospective, multicenter registry. Bone Marrow Transplant. 2014;49:1287–1292. doi: 10.1038/bmt.2014.147. [DOI] [PubMed] [Google Scholar]
- 10.Inaba H, Yang J, Pan J, Stokes DC, Krasin MJ, Srinivasan A, et al. Pulmonary dysfunction in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem cell transplantation. Cancer. 2010;116:2020–2030. doi: 10.1002/cncr.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkby J, Aurora P, Spencer H, Rees S, Sonnappa S, Stocks J. Stitching and switching: the impact of discontinuous lung function reference equations. Eur Respir J. 2012;39:1256–1257. doi: 10.1183/09031936.00173011. [DOI] [PubMed] [Google Scholar]
- 12.Kirkby J, Bonner R, Lum S, Bates P, Morgan V, Strunk RC, et al. Interpretation of pediatric lung function: impact of ethnicity. Pediatr Pulmonol. 2013;48:20–26. doi: 10.1002/ppul.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson PD, Spencer H, Aurora P. Impact of lung function interpretation approach on pediatric bronchiolitis obliterans syndrome diagnosis after lung transplantation. J Heart Lung Transplant. 2015;34:1082–1088. doi: 10.1016/j.healun.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Walkup LL, Higano NS, Woods JC. Structural and functional pulmonary magnetic resonance imaging in pediatrics-from the neonate to the young adult. Acad Radiol. 2019;26:424–430. doi: 10.1016/j.acra.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broglie L, Fretham C, Al-Seraihy A, George B, Kurtzberg J, Loren A, et al. Pulmonary complications in pediatric and adolescent patients following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25:2024–2030. doi: 10.1016/j.bbmt.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary: idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1601–1606. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 17.Cooke KR, Yanik G.Lung injury following hematopoietic cell transplantation Forman SJ, Negrin RS, Antin JH, Appelbaum F.editors. Thomas’s hematopoietic cell transplantation 5th edHoboken, NJ: Wiley-Blackwell; 20161117–1130. [Google Scholar]
- 18.Seo S, Renaud C, Kuypers JM, Chiu CY, Huang ML, Samayoa E, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood. 2015;125:3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark JG, Madtes DK, Martin TR, Hackman RC, Farrand AL, Crawford SW. Idiopathic pneumonia after bone marrow transplantation: cytokine activation and lipopolysaccharide amplification in the bronchoalveolar compartment. Crit Care Med. 1999;27:1800–1806. doi: 10.1097/00003246-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation. 1997;63:1079–1086. doi: 10.1097/00007890-199704270-00006. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi H, Takahashi Y, Watanabe N, Doisaki S, Muramatsu H, Hama A, et al. Incidence, clinical features, and risk factors of idiopathic pneumonia syndrome following hematopoietic stem cell transplantation in children. Pediatr Blood Cancer. 2012;58:780–784. doi: 10.1002/pbc.23298. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102:2777–2785. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 23.Zinter MS, Dvorak CC, Mayday MY, Iwanaga K, Ly NP, McGarry ME, et al. Pulmonary metagenomic sequencing suggests missed infections in immunocompromised children. Clin Infect Dis. 2019;68:1847–1855. doi: 10.1093/cid/ciy802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26:539–543. doi: 10.1038/sj.bmt.1702546. [DOI] [PubMed] [Google Scholar]
- 25.Kharbanda S, Panoskaltsis-Mortari A, Haddad IY, Blazar BR, Orchard PJ, Cornfield DN, et al. Inflammatory cytokines and the development of pulmonary complications after allogeneic hematopoietic cell transplantation in patients with inherited metabolic storage disorders. Biol Blood Marrow Transplant. 2006;12:430–437. doi: 10.1016/j.bbmt.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12:1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jodele S, Hirsch R, Laskin B, Davies S, Witte D, Chima R. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19:202–207. doi: 10.1016/j.bbmt.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–996. doi: 10.1182/blood-2015-08-663435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: a task force report from the national Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–234. doi: 10.1016/j.bbmt.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43:42–52. doi: 10.1053/j.seminhematol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 34.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401, e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng GS, Campbell AP, Xie H, Stednick Z, Callais C, Leisenring WM, et al. Correlation and agreement of handheld spirometry with laboratory spirometry in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2016;22:925–931. doi: 10.1016/j.bbmt.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loiseau C, Lemonnier F, Randrianarivelo O, Itzykson R, Nguyen S, Becquemin MH, et al. Home spirometry in bronchiolitis obliterans after allogeneic haematopoietic cell transplant. Eur Respir J. 2018;52:1702328. doi: 10.1183/13993003.02328-2017. [DOI] [PubMed] [Google Scholar]
- 38.Lahzami S, Schoeffel RE, Pechey V, Reid C, Greenwood M, Salome CM, et al. Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2011;38:1180–1188. doi: 10.1183/09031936.00018311. [DOI] [PubMed] [Google Scholar]
- 39.Nyilas S, Baumeler L, Tamm M, Halter JP, Savic S, Korten I, et al. Inert gas washout in bronchiolitis obliterans following hematopoietic cell transplantation. Chest. 2018;154:157–168. doi: 10.1016/j.chest.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Nyilas S, Carlens J, Price T, Singer F, Müller C, Hansen G, et al. Multiple breath washout in pediatric patients after lung transplantation. Am J Transplant. 2018;18:145–153. doi: 10.1111/ajt.14432. [DOI] [PubMed] [Google Scholar]
- 41.Uhlving HH, Mathiesen S, Buchvald F, Green K, Heilmann C, Gustafsson P, et al. Small airways dysfunction in long-term survivors of pediatric stem cell transplantation. Pediatr Pulmonol. 2015;50:704–712. doi: 10.1002/ppul.23058. [DOI] [PubMed] [Google Scholar]
- 42.Kerby GS, Rosenfeld M, Ren CL, Mayer OH, Brumback L, Castile R, et al. Lung function distinguishes preschool children with CF from healthy controls in a multi-center setting. Pediatr Pulmonol. 2012;47:597–605. doi: 10.1002/ppul.21589. [DOI] [PubMed] [Google Scholar]
- 43.Robson KS, West AJ. Improving survival outcomes in lung transplant recipients through early detection of bronchiolitis obliterans: daily home spirometry versus standard pulmonary function testing. Can J Respir Ther. 2014;50:17–22. [PMC free article] [PubMed] [Google Scholar]
- 44.Kampschmidt JC, Brooks EG, Cherry DC, Guajardo JR, Wood PR. Feasibility of spirometry testing in preschool children. Pediatr Pulmonol. 2016;51:258–266. doi: 10.1002/ppul.23303. [DOI] [PubMed] [Google Scholar]
- 45.Aurora P, Stocks J, Oliver C, Saunders C, Castle R, Chaziparasidis G, et al. London Cystic Fibrosis Collaboration. Quality control for spirometry in preschool children with and without lung disease. Am J Respir Crit Care Med. 2004;169:1152–1159. doi: 10.1164/rccm.200310-1453OC. [DOI] [PubMed] [Google Scholar]
- 46.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146:841–847. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 47.Subbarao P, Milla C, Aurora P, Davies JC, Davis SD, Hall GL, et al. Multiple-breath washout as a lung function test in cystic fibrosis: a cystic fibrosis foundation workshop report. Ann Am Thorac Soc. 2015;12:932–939. doi: 10.1513/AnnalsATS.201501-021FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aliverti A, Pennati F, Salito C, Woods JC. Regional lung function and heterogeneity of specific gas volume in healthy and emphysematous subjects. Eur Respir J. 2013;41:1179–1188. doi: 10.1183/09031936.00050112. [DOI] [PubMed] [Google Scholar]
- 50.Walkup LL, Myers K, El-Bietar J, Nelson A, Willmering MM, Grimley M, et al. Xenon-129 MRI detects ventilation deficits in paediatric stem cell transplant patients unable to perform spirometry. Eur Respir J. 2019;53:1801779. doi: 10.1183/13993003.01779-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Towe C, Chester Ogborn A, Ferkol T, Sweet S, Huddleston C, White F, et al. Bronchiolitis obliterans syndrome is not specific for bronchiolitis obliterans in pediatric lung transplant. J Heart Lung Transplant. 2015;34:516–521. doi: 10.1016/j.healun.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Chellapandian D, Lehrnbecher T, Phillips B, Fisher BT, Zaoutis TE, Steinbach WJ, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol. 2015;33:501–509. doi: 10.1200/JCO.2014.58.0480. [DOI] [PubMed] [Google Scholar]
- 53.Huaringa AJ, Leyva FJ, Signes-Costa J, Morice RC, Raad I, Darwish AA, et al. Bronchoalveolar lavage in the diagnosis of pulmonary complications of bone marrow transplant patients. Bone Marrow Transplant. 2000;25:975–979. doi: 10.1038/sj.bmt.1702335. [DOI] [PubMed] [Google Scholar]
- 54.Jain P, Sandur S, Meli Y, Arroliga AC, Stoller JK, Mehta AC. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125:712–722. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 55.Yanik G, Maslak J, Connelly J, Peres E, Mineishi S, Levine JE, et al. Impact of Broncho-alveolar lavage on the diagnosis and management of pulmonary complicaitons post transplant Biology of Blood and Marrow Transplantation 2008143318162219 [Google Scholar]
- 56.Shannon VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–655. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 57.Kasow KA, King E, Rochester R, Tong X, Srivastava DK, Horwitz EM, et al. Diagnostic yield of bronchoalveolar lavage is low in allogeneic hematopoietic stem cell recipients receiving immunosuppressive therapy or with acute graft-versus-host disease: the St. Jude experience, 1990-2002. Biol Blood Marrow Transplant. 2007;13:831–837. doi: 10.1016/j.bbmt.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Dieffenbach BV, Madenci AL, Murphy AJ, Weldon CB, Weil BR, Lehmann LE. Therapeutic impact and complications associated with surgical lung biopsy after allogeneic hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2019;25:2181–2185. doi: 10.1016/j.bbmt.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Speck NE, Schuurmans MM, Murer C, Benden C, Huber LC. Diagnostic value of plasma and bronchoalveolar lavage samples in acute lung allograft rejection: differential cytology. Respir Res. 2016;17:74. doi: 10.1186/s12931-016-0391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanik GA, Ho VT, Levine JE, White ES, Braun T, Antin JH, et al. The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:3073–3081. doi: 10.1182/blood-2008-03-143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ginsberg JP, Aplenc R, McDonough J, Bethel J, Doyle J, Weiner DJ. Pre-transplant lung function is predictive of survival following pediatric bone marrow transplantation. Pediatr Blood Cancer. 2010;54:454–460. doi: 10.1002/pbc.22337. [DOI] [PubMed] [Google Scholar]
- 62.Quigg TC, Kim YJ, Goebel WS, Haut PR. Lung function before and after pediatric allogeneic hematopoietic stem cell transplantation: a predictive role for DLCOa/VA. J Pediatr Hematol Oncol. 2012;34:304–309. doi: 10.1097/MPH.0b013e3182346ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172:384–390. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivasan A, Sunkara A, Mitchell W, Sunthankar S, Kang G, Stokes DC, et al. Recovery of pulmonary function after allogeneic hematopoietic cell transplantation in children is associated with improved survival. Biol Blood Marrow Transplant. 2017;23:2102–2109. doi: 10.1016/j.bbmt.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yanik GA, Grupp SA, Pulsipher MA, Levine JE, Schultz KR, Wall DA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome: a joint pediatric blood and marrow transplant consortium and children’s oncology group study (ASCT0521) Biol Blood Marrow Transplant. 2015;21:67–73. doi: 10.1016/j.bbmt.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlatzer DM, Dazard JE, Ewing RM, Ilchenko S, Tomcheko SE, Eid S, et al. Human biomarker discovery and predictive models for disease progression for idiopathic pneumonia syndrome following allogeneic stem cell transplantation. Mol Cell Proteomics. 2012;11:M111.015479. doi: 10.1074/mcp.M111.015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seo S, Yu J, Jenkins IC, Leisenring WM, Steven-Ayers T, Kuypers JM, et al. Diagnostic and prognostic plasma biomarkers for idiopathic pneumonia syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24:678–686. doi: 10.1016/j.bbmt.2017.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM, Wangensteen OD, Bitterman PB, Ingbar DH, et al. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J Clin Invest. 1997;100:1015–1027. doi: 10.1172/JCI119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooke KR, Krenger W, Hill G, Martin TR, Kobzik L, Brewer J, et al. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood. 1998;92:2571–2580. [PubMed] [Google Scholar]
- 70.Hildebrandt GC, Olkiewicz KM, Choi S, Corrion LA, Clouthier SG, Liu C, et al. Donor T-cell production of RANTES significantly contributes to the development of idiopathic pneumonia syndrome after allogeneic stem cell transplantation. Blood. 2005;105:2249–2257. doi: 10.1182/blood-2004-08-3320. [DOI] [PubMed] [Google Scholar]
- 71.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;103:2417–2426. doi: 10.1182/blood-2003-08-2708. [DOI] [PubMed] [Google Scholar]
- 72.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, et al. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173:2050–2059. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 73.Choi SW, Hildebrandt GC, Olkiewicz KM, Hanauer DA, Chaudhary MN, Silva IA, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–3455. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, et al. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation. 2000;70:272–279. doi: 10.1097/00007890-200007270-00006. [DOI] [PubMed] [Google Scholar]
- 75.Gerbitz A, Nickoloff BJ, Olkiewicz K, Willmarth NE, Hildebrandt G, Liu C, et al. A role for tumor necrosis factor-alpha-mediated endothelial apoptosis in the development of experimental idiopathic pneumonia syndrome. Transplantation. 2004;78:494–502. doi: 10.1097/01.tp.0000128839.13674.02. [DOI] [PubMed] [Google Scholar]
- 76.Hildebrandt GC, Olkiewicz KM, Corrion LA, Chang Y, Clouthier SG, Liu C, et al. Donor-derived TNF-alpha regulates pulmonary chemokine expression and the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;104:586–593. doi: 10.1182/blood-2003-12-4259. [DOI] [PubMed] [Google Scholar]
- 77.Hildebrandt GC, Olkiewicz KM, Corrion L, Clouthier SG, Pierce EM, Liu C, et al. A role for TNF receptor type II in leukocyte infiltration into the lung during experimental idiopathic pneumonia syndrome. Biol Blood Marrow Transplant. 2008;14:385–396. doi: 10.1016/j.bbmt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burman AC, Banovic T, Kuns RD, Clouston AD, Stanley AC, Morris ES, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110:1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 79.Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, et al. Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T-cell-mediated graft-versus-host disease. Am J Respir Crit Care Med. 2008;178:379–388. doi: 10.1164/rccm.200711-1648OC. [DOI] [PubMed] [Google Scholar]
- 80.Varelias A, Gartlan KH, Kreijveld E, Olver SD, Lor M, Kuns RD, et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood. 2015;125:2435–2444. doi: 10.1182/blood-2014-07-590232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanik G, Hellerstedt B, Custer J, Hutchinson R, Kwon D, Ferrara JL, et al. Etanercept (Enbrel) administration for idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:395–400. doi: 10.1053/bbmt.2002.v8.pm12171486. [DOI] [PubMed] [Google Scholar]
- 82.Sengsayadeth SM, Srivastava S, Jagasia M, Savani BN. Time to explore preventive and novel therapies for bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1479–1487. doi: 10.1016/j.bbmt.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 83.Martin PJ, Chien JW. What we know and mostly do not know about bronchiolitis obliterans syndrome. Bone Marrow Transplant. 2012;47:1–4. doi: 10.1038/bmt.2011.38. [DOI] [PubMed] [Google Scholar]
- 84.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med. 2007;176:713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc. 2008;5:305–310. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dubovsky JA, Flynn R, Du J, Harrington BK, Zhong Y, Kaffenberger B, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124:4867–4876. doi: 10.1172/JCI75328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flynn R, Allen JL, Luznik L, MacDonald KP, Paz K, Alexander KA, et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125:4085–4094. doi: 10.1182/blood-2014-08-595470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verghese DA, Chun N, Paz K, Fribourg M, Woodruff TM, Flynn R, et al. C5aR1 regulates T follicular helper differentiation and chronic graft-versus-host disease bronchiolitis obliterans. JCI Insight. 2018;3:e124646. doi: 10.1172/jci.insight.124646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander KA, Flynn R, Lineburg KE, Kuns RD, Teal BE, Olver SD, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis: a mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harris B, Morjaria SM, Littmann ER, Geyer AI, Stover DE, Barker JN, et al. Gut microbiota predict pulmonary infiltrates after allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med. 2016;194:450–463. doi: 10.1164/rccm.201507-1491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chien JW, Zhao LP, Hansen JA, Fan WH, Parimon T, Clark JG. Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood. 2006;107:2200–2207. doi: 10.1182/blood-2005-06-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hildebrandt GC, Granell M, Urbano-Ispizua A, Wolff D, Hertenstein B, Greinix HT, et al. Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:67–74. doi: 10.1016/j.bbmt.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 99.O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol. 2016;196:4839–4847. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1127–1138. doi: 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou X, O’Dwyer DN, Xia M, Miller HK, Chan PR, Trulik K, et al. First-onset herpesviral infection and lung injury in allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med. 2019;200:63–74. doi: 10.1164/rccm.201809-1635OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panoskaltsis-Mortari A, Ingbar DH, Jung P, Haddad IY, Bitterman PB, Wangensteen OD, et al. KGF pretreatment decreases B7 and granzyme B expression and hastens repair in lungs of mice after allogeneic BMT. Am J Physiol Lung Cell Mol Physiol. 2000;278:L988–L999. doi: 10.1152/ajplung.2000.278.5.L988. [DOI] [PubMed] [Google Scholar]
- 104.Cooke KR, Jannin A, Ho V. The contribution of endothelial activation and injury to end-organ toxicity following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:23–32. doi: 10.1016/j.bbmt.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Gerbitz A, Ewing P, Olkiewicz K, Willmarth NE, Williams D, Hildebrandt G, et al. A role for CD54 (intercellular adhesion molecule-1) in leukocyte recruitment to the lung during the development of experimental idiopathic pneumonia syndrome. Transplantation. 2005;79:536–542. doi: 10.1097/01.tp.0000151763.16800.b0. [DOI] [PubMed] [Google Scholar]
- 106.Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 107.Nieder ML, McDonald GB, Kida A, Hingorani S, Armenian SH, Cooke KR, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric blood and marrow transplant consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant. 2011;17:1573–1584. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomas NJ, Spear D, Wasserman E, Pon S, Markovitz B, Singh AR, et al. CALIPSO: a randomized controlled trial of calfactant for acute lung injury in pediatric stem cell and oncology patients. Biol Blood Marrow Transplant. 2018;24:2479–2486. doi: 10.1016/j.bbmt.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant. 2006;38:285–289. doi: 10.1038/sj.bmt.1705436. [DOI] [PubMed] [Google Scholar]
- 110.Sano H, Kobayashi R, Iguchi A, Suzuki D, Kishimoto K, Yasuda K, et al. Risk factor analysis of idiopathic pneumonia syndrome after allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2014;49:38–41. doi: 10.1038/bmt.2013.123. [DOI] [PubMed] [Google Scholar]
- 111.Tamburro RF, Kneyber MC Pediatric Acute Lung Injury Consensus Conference Group. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(Suppl 1):S61–S72. doi: 10.1097/PCC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 112.Tizon R, Frey N, Heitjan DF, Tan KS, Goldstein SC, Hexner EO, et al. High-dose corticosteroids with or without etanercept for the treatment of idiopathic pneumonia syndrome after allo-SCT. Bone Marrow Transplant. 2012;47:1332–1337. doi: 10.1038/bmt.2011.260. [DOI] [PubMed] [Google Scholar]
- 113.Yanik GA, Horowitz MM, Weisdorf DJ, Logan BR, Ho VT, Soiffer RJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20:858–864. doi: 10.1016/j.bbmt.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ueda N, Chihara D, Kohno A, Tatekawa S, Ozeki K, Watamoto K, et al. Predictive value of circulating angiopoietin-2 for endothelial damage-related complications in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1335–1340. doi: 10.1016/j.bbmt.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 115.Rathi NK, Tanner AR, Dinh A, Dong W, Feng L, Ensor J, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant. 2015;50:420–426. doi: 10.1038/bmt.2014.287. [DOI] [PubMed] [Google Scholar]
- 116.Heslet L, Nielsen JD, Nepper-Christensen S. Local pulmonary administration of factor VIIa (rFVIIa) in diffuse alveolar hemorrhage (DAH) - a review of a new treatment paradigm. Biologics. 2012;6:37–46. doi: 10.2147/BTT.S25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park JA, Kim BJ. Intrapulmonary recombinant factor VIIa for diffuse alveolar hemorrhage in children. Pediatrics. 2015;135:e216–e220. doi: 10.1542/peds.2014-1782. [DOI] [PubMed] [Google Scholar]
- 118.Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54:181–190. doi: 10.1016/j.transci.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jodele S, Bleesing JJ, Mehta PA, Filipovich AH, Laskin BL, Goebel J, et al. Successful early intervention for hyperacute transplant-associated thrombotic microangiopathy following pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2012;16:E39–E42. doi: 10.1111/j.1399-3046.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 120.Jodele S. Complement in pathophysiology and treatment of transplant-associated thrombotic microangiopathies. Semin Hematol. 2018;55:159–166. doi: 10.1053/j.seminhematol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 121.Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20:518–525. doi: 10.1016/j.bbmt.2013.12.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:307–315. doi: 10.1016/j.bbmt.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bergeron A, Cheng GS. Bronchiolitis obliterans syndrome and other late pulmonary complications after allogeneic hematopoietic stem cell transplantation. Clin Chest Med. 2017;38:607–621. doi: 10.1016/j.ccm.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Yanik GA, Mineishi S, Levine JE, Kitko CL, White ES, Vander Lugt MT, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1044–1054. doi: 10.1016/j.bbmt.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:710–716. doi: 10.1016/j.bbmt.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng GS, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13:1932–1939. doi: 10.1513/AnnalsATS.201604-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bergeron A, Chevret S, Peffault de Latour R, Chagnon K, de Margerie-Mellon C, Rivière F, et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2018;51:1702617. doi: 10.1183/13993003.02617-2017. [DOI] [PubMed] [Google Scholar]
- 128.Lam DC, Lam B, Wong MK, Lu C, Au WY, Tse EW, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46:1551–1556. doi: 10.1038/bmt.2011.1. [DOI] [PubMed] [Google Scholar]
- 129.Lemonnier F, Rivaud E, Neveu H, Catherinot E, Suarez F, Dhedin N, et al. Azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant. 2012;47:1374. doi: 10.1038/bmt.2012.23. [DOI] [PubMed] [Google Scholar]
- 130.Bergeron A, Chevret S, Granata A, Chevallier P, Vincent L, Huynh A, et al. ALLOZITHRO Study Investigators. Effect of azithromycin on airflow decline-free survival after allogeneic hematopoietic stem cell transplant: the ALLOZITHRO randomized clinical trial. JAMA. 2017;318:557–566. doi: 10.1001/jama.2017.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shamoun M, Braun T, Magenau JM, Choi SW, Reddy P, Pawarode A, et al. The effect of azithromycin on relapse in patients with moderate-severe chronic graft versus host disease. Biol Blood Marrow Transplant. 2019;25:s26–s27. [Google Scholar]
- 132.Cheng GS, Bondeelle L, Gooley T, He Q, Jamani K, Krakow EF, et al. Azithromycin use and increased cancer risk among patients with bronchiolitis obliterans after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26:392–400. doi: 10.1016/j.bbmt.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patel B, Sheshardri A, Saliba RM, Rondon G, Ahmed T, Arain MH, et al. Azithromycin exposure following hematopoietic stem cell transplantation is associated with an increased risk of cancer relapse in acute leukemia. Biol Blood Marrow Transplant. 2019;25:S231–S232. [Google Scholar]
- 134.Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, Peffault de Latour R, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. doi: 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 135.Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–2250. doi: 10.1182/blood-2017-07-793786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Streiler C, Shaikh F, Davis C, Abhyankar S, Brownback KR. Ruxolitinib is an effective steroid sparing agent in bronchiolitis obliterans due to chronic graft-versus-host-disease. Bone Marrow Transplant. 2020;55:1194–1196. doi: 10.1038/s41409-019-0662-6. [DOI] [PubMed] [Google Scholar]
- 137.Jain NA, Pophali PA, Klotz JK, Ito S, Koklanaris E, Chawla K, et al. Repair of impaired pulmonary function is possible in very-long-term allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant. 2014;20:209–213. doi: 10.1016/j.bbmt.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duncan CN, Lehmann LE, Cheifetz IM, Greathouse K, Haight AE, Hall MW, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med. 2013;14:261–267. doi: 10.1097/PCC.0b013e3182720601. [DOI] [PubMed] [Google Scholar]
- 139.Rowan CM, Smith LS, Loomis A, McArthur J, Gertz SJ, Fitzgerald JC, et al. Pediatric acute respiratory distress syndrome in pediatric allogeneic hematopoietic stem cell transplants: a multicenter study. Pediatr Crit Care Med. 2017;18:304–309. doi: 10.1097/PCC.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 140.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 141.Depuydt PO, Benoit DD, Roosens CD, Offner FC, Noens LA, Decruyenaere JM. The impact of the initial ventilatory strategy on survival in hematological patients with acute hypoxemic respiratory failure. J Crit Care. 2010;25:30–36. doi: 10.1016/j.jcrc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 142.Gristina GR, Antonelli M, Conti G, Ciarlone A, Rogante S, Rossi C, et al. GiViTI (Italian Group for the Evaluation of Interventions in Intensive Care Medicine) Noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: a 5-year multicenter observational survey. Crit Care Med. 2011;39:2232–2239. doi: 10.1097/CCM.0b013e3182227a27. [DOI] [PubMed] [Google Scholar]
- 143.Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: a multicenter study. Pediatr Crit Care Med. 2016;17:294–302. doi: 10.1097/PCC.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 144.Rowan CM, Loomis A, McArthur J, Smith LS, Gertz SJ, Fitzgerald JC, et al. Investigators of the Pediatric Acute Lung Injury and Sepsis Network. High-frequency oscillatory ventilation use and severe pediatric ARDS in the pediatric hematopoietic cell transplant recipient. Respir Care. 2018;63:404–411. doi: 10.4187/respcare.05765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rowan CM, Nitu ME, Moser EAS, Swigonski NL, Renbarger JL. Weight gain and supplemental O2: risk factors during the hematopoietic cell transplant admission in pediatric patients. Pediatr Blood Cancer. 2017;64:e26562. doi: 10.1002/pbc.26561. [DOI] [PubMed] [Google Scholar]
- 146.Cater DT, Tori AJ, Moser EAS, Rowan CM. Modification and assessment of the bedside pediatric early warning score in the pediatric allogeneic hematopoietic cell transplant population. Pediatr Crit Care Med. 2018;19:483–488. doi: 10.1097/PCC.0000000000001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Valentine SL, Nadkarni VM, Curley MA Pediatric Acute Lung Injury Consensus Conference Group. Nonpulmonary treatments for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(Suppl 1):S73–S85. doi: 10.1097/PCC.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 149.Wilson B, Typpo K. Nutrition: a primary therapy in pediatric acute respiratory distress syndrome. Front Pediatr. 2016;4:108. doi: 10.3389/fped.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 151.Carcillo JA, Dean JM, Holubkov R, Berger J, Meert KL, Anand KJS, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN) Interaction between 2 nutraceutical treatments and host immune status in the pediatric critical illness stress-induced immune suppression comparative effectiveness trial. JPEN J Parenter Enteral Nutr. 2017;41:1325–1335. doi: 10.1177/0148607116670377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boudreault F, Pinilla-Vera M, Englert JA, Kho AT, Isabelle C, Arciniegas AJ, et al. MICU Registry. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight. 2017;2:86507. doi: 10.1172/jci.insight.86507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9:43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 154.Wallace G, Jodele S, Howell J, Myers KC, Teusink A, Zhao X, et al. Vitamin D deficiency and survival in children after hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2015;21:1627–1631. doi: 10.1016/j.bbmt.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sipmann FS, Santos A, Tusman G. Heart-lung interactions in acute respiratory distress syndrome: pathophysiology, detection and management strategies. Ann Transl Med. 2018;6:27. doi: 10.21037/atm.2017.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dandoy CE, Davies SM, Hirsch R, Chima RS, Paff Z, Cash M, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21:113–118. doi: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dandoy CE, Jodele S, Paff Z, Hirsch R, Ryan TD, Jefferies JL, et al. Team-based approach to identify cardiac toxicity in critically ill hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26513. [DOI] [PubMed] [Google Scholar]
- 158.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 159.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19:91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 160.Flores FX, Brophy PD, Symons JM, Fortenberry JD, Chua AN, Alexander SR, et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation: a report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol. 2008;23:625–630. doi: 10.1007/s00467-007-0672-2. [DOI] [PubMed] [Google Scholar]
- 161.DiCarlo JV, Alexander SR, Agarwal R, Schiffman JD. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol. 2003;25:801–805. doi: 10.1097/00043426-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 162.Elbahlawan L, West NK, Avent Y, Cheng C, Liu W, Barfield RC, et al. Impact of continuous renal replacement therapy on oxygenation in children with acute lung injury after allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2010;55:540–545. doi: 10.1002/pbc.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raina R, Abusin GA, Vijayaraghavan P, Auletta JJ, Cabral L, Hashem H, et al. The role of continuous renal replacement therapy in the management of acute kidney injury associated with sinusoidal obstruction syndrome following hematopoietic cell transplantation. Pediatr Transplant. 2018;22 doi: 10.1111/petr.13139. [DOI] [PubMed] [Google Scholar]
- 164.Rajasekaran S, Jones DP, Avent Y, Shaffer ML, Elbahlawan L, Henderson N, et al. Outcomes of hematopoietic stem cell transplant patients who received continuous renal replacement therapy in a pediatric oncology intensive care unit. Pediatr Crit Care Med. 2010;11:699–706. doi: 10.1097/PCC.0b013e3181e32423. [DOI] [PubMed] [Google Scholar]
- 165.Di Nardo M, Merli P, Cecchetti C, Pasotti E, Bertaina A, Locatelli F. Progressive increase in D-dimer levels during extracorporeal membrane oxygenation can predict membrane oxygenator failure in children given hematopoietic stem cell transplantation? J Crit Care. 2016;31:262–263. doi: 10.1016/j.jcrc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 166.Maue DK, Hobson MJ, Friedman ML, Moser EA, Rowan CM. Outcomes of pediatric oncology and hematopoietic cell transplant patients receiving extracorporeal membrane oxygenation. Perfusion. 2019;34:598–604. doi: 10.1177/0267659119842471. [DOI] [PubMed] [Google Scholar]
- 167.Klein OR, Bembea MM, McCloskey J, Wilkinson M, Chen AR, Gamper C, et al. Outcomes in pediatric bone marrow transplant patients undergoing extra-corporeal membrane oxygenation. Biol Blood Marrow Transplant. 2017;23:S238. [Google Scholar]
- 168.Steppan DA, Coleman RD, Viamonte HK, Hanson SJ, Carroll MK, Klein OR, et al. Pediatric ECMO (PediECMO) subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Extracorporeal Life Support Organization (ELSO) Outcomes of pediatric patients with oncologic disease or following hematopoietic stem cell transplant supported on extracorporeal membrane oxygenation: the PEDECOR experience. Pediatr Blood Cancer. doi: 10.1002/pbc.28403. [online ahead of print] 9 Jun 2020; DOI: 10.1002/pbc.28403. [DOI] [PubMed] [Google Scholar]
- 169.Ullrich CK, Dussel V, Hilden JM, Sheaffer JW, Lehmann L, Wolfe J. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood. 2010;115:3879–3885. doi: 10.1182/blood-2009-10-250225. [DOI] [PubMed] [Google Scholar]
- 170.Ullrich CK, Lehmann L, London WB, Guo D, Sridharan M, Koch R, et al. End-of-Life care patterns associated with pediatric palliative care among children who underwent hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2016;22:1049–1055. doi: 10.1016/j.bbmt.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zinter MS, Logan BR, Fretham C, Sapru A, Abraham A, Aljurf MD, et al. Comprehensive prognostication in critically ill pediatric hematopoietic cell transplant patients: results from merging the center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) registries. Biol Blood Marrow Transplant. 2020;26:333–342. doi: 10.1016/j.bbmt.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.